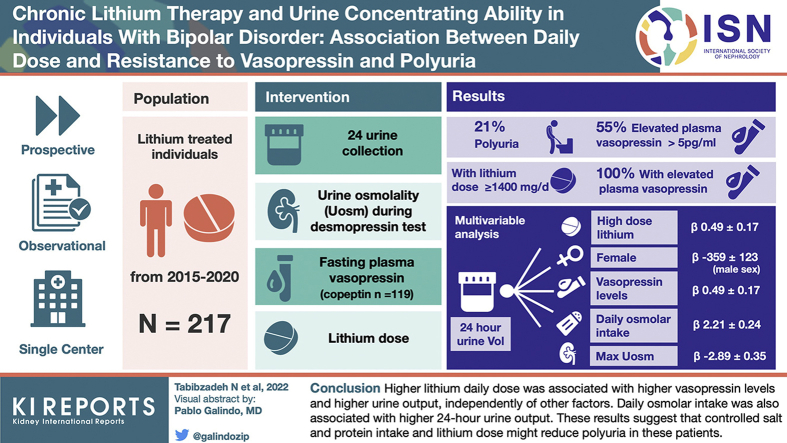
Abstract
Introduction
Lithium treatment can induce nephrogenic diabetes insipidus (NDI), but no consensus intervention is offered to date. We evaluated in these patients patterns of urine concentration and the correlates of 24-hour urine output.
Methods
Prospective, single-center, observational study of 217 consecutive lithium-treated individuals, with 24-hour urine collection, desmopressin (1-deamino-arginine vasopressin [DDAVP]) concentrating test, fasting plasma vasopressin measurement (copeptin measurement in n = 119), and measured glomerular filtration rate (mGFR). Maximal urine osmolality (MaxUosm) was the highest level during the DDAVP test.
Results
Of the individuals, 21% displayed polyuria (>3 l/d), but 55% displayed elevated fasting vasopressin level (>5 pg/ml). Uosm was significantly lower and urinary output and free water clearance were significantly higher in individuals treated for >10 years. MaxUosm was >600 mOsm/KgH2O in 128 patients (59%), among which vasopressin was increased in 51%, associated with higher lithium dose (950 [750–1200] vs. 800 [500–1000] mg/d, P < 0.001). All patients with lithium daily dose ≥1400 mg/d had high vasopressin levels. In multivariable analysis, 24-hour urine output was associated with higher lithium daily dose (β 0.49 ± 0.17, P = 0.005), female sex (β −359 ± 123, P = 0.004), daily osmolar intake (β 2.21 ± 0.24, P < 0.001), MaxUosm (β −2.89 ± 0.35, P < 0.001), and plasma vasopressin level (β 10.17 ± 4.76, P = 0.03).
Conclusion
Higher lithium daily dose was associated with higher vasopressin levels and higher urine output, independently of other factors. Daily osmolar intake was also associated with higher 24-hour urine output. These results suggest that controlled salt and protein intake and lithium dose might reduce polyuria in these patients.
Keywords: bipolar disorder, diabetes insipidus, lithium, nephrotoxicity
Graphical abstract
Lithium carbonate represents the cornerstone treatment of bipolar disorder. Its effectiveness to reduce the risk of manic or depressive relapse has been demonstrated in several studies.1, 2, 3 In addition, lithium has demonstrated its efficacy in suicide prevention.4 This efficacy is however balanced by short- and long-term side effects, including renal and metabolic complications.5 One of the most frequent renal side effects is NDI, resulting in polydipsia and polyuria.6,7 NDI has been described in humans and in experimental animal models and develops shortly after the initiation of the treatment. In healthy volunteers, NDI appears 8 days after lithium treatment initiation and resolves shortly after discontinuation.8 In previous studies, 20% to 70% of lithium-treated individuals had NDI depending on the population and on the definition of NDI.9
The mechanism of lithium-induced NDI is partially known. It implicates the down-regulation of type 2 aquaporin expression and addressing to the apical membrane of the principal cells in the collecting duct of the nephron.10,11 How lithium induces this decrease in type 2 aquaporin expression is still a matter of debate. In individuals treated with lithium, risk factors for developing lithium-induced polyuria are still poorly known. We thus aimed at evaluating the concentrating ability and determining the factors associated with a decrease in urine-concentrating ability in a cohort of 217 individuals treated with lithium.
Methods
Study Design and Population
From March 2015 to December 2020, a total of 230 consecutive adult patients receiving lithium for a bipolar disorder were referred for a systematic assessment to the Department of Renal Physiology by psychiatrists and nephrologists. Among these patients, we excluded 13 individuals who had discontinued lithium treatment at the time of admission, leaving 217 individuals. Eligible patients were ≥18 years of age at inclusion, with all durations of lithium treatment, and had neither started dialysis nor underwent kidney transplantation.
The local ethics committee approved the study (APHP.Nord Institutional Review Board CER-2021-74). All individuals provided written informed consent before inclusion in the study cohort.
Data Collection and Measurements
During a 5-hour in-person visit, a large set of clinical and laboratory data was collected, including GFR measurement using renal clearance of a radioisotope, as previously described.12 Individuals were instructed to fast (not to eat or drink) from 8 pm on the day before the admission. Individuals were also asked to collect their 24-hour urine on the day before the admission. A written information document was provided, and instructions to discard the first morning void on the first day and then to collect all urine including the first void on the next morning were given by a trained nurse. In addition to this information, 24-hour urine collection was verified by interrogation of the patient and corrected using the fractionated creatinine clearance during the test.13 A kidney magnetic resonance imaging was performed in a subset of patients (n = 108). Along with these tests, a urine 2-step concentrating test was performed. First, fasting urine and plasma osmolality were measured on the first morning urine sample, along with natremia, plasma copeptin level (B·R·A·H·M·S Copeptin proAVP KRYPTOR, 1.7 < normal value < 11.25 pmol/l, performed in a subset of 119 individuals), and plasma vasopressin level (in-house radioimmunology assay, 1 < normal value < 5 pg/ml, Tenon Hospital Laboratories), as previously described.14 Urine output, ionogram, urea, creatinine, and osmolality were determined on 24-hour urine collection. The 24-hour osmolar intake was defined by the 24-hour urinary osmolar output. Thirst score was determined at each time point by trained nurses using a numerical scale from 0 (not thirsty at all) to 10 (the worst thirst imaginable). A challenging test with DDAVP was performed, which was injected 2 hours after the admission of the patient who remained in fasting. Briefly, urine and blood samples were collected every 30 minutes over a 4-hour period after 4 μg i.v. administration of DDAVP to measure natremia and plasma and urine osmolality. Individuals were allowed to eat a breakfast after DDAVP injection and to drink 1 glass of water at each time point. The highest value of urine osmolality among all measures was used to define the maximal urinary osmolality. Normal concentrating ability was defined as a MaxUosm >600 mOsm/kgH2O, as previously described.15 Free water clearance was calculated as follows: free water clearance = urinary output × (1 − [Osm]U/[Osm]P).
Polyuria was defined as a 24-hour urinary output >3 liters.16
Statistical Analyses
Categorical variables were described as numbers and percentage and continuous variables as mean ± SD or median (quartile [Q]1–Q3), as appropriate. Baseline individuals’ characteristics were compared according to tertiles of lithium treatment duration and among subgroups according to urine concentration features using analysis of variance (or Kruskal–Wallis test, as appropriate) and the χ2 test, for quantitative and qualitative variables, respectively. The 4 subgroups according to urine-concentrating features were the following: group 1, normal urine-concentrating ability, defined as MaxUosm >600 mOsm/kgH2O and normal vasopressin level <5 pg/ml; group 2, partial resistance to vasopressin, defined by a vasopressin level >5 pg/ml but normal MaxUosm; group 3, complete resistance to vasopressin, defined by a vasopressin level >5 pg/ml and an impaired urine-concentrating ability defined by a MaxUosm <600 mOsm/kgH2O; and group 4, vasopressin level <5 pg/ml and impaired maximal urine concentrating ability <600 mOsm/kgH2O consistent with primary polydipsia or mild concentrating defect. Simple and multiple linear regression analyses were performed to investigate the determinants of 24-hour urinary output. Models were compared using log-likelihood statistics, to select the statistically better multiple linear regression model. A stepwise variable selection method was used (nested models). The absence of multicollinearity was verified by calculating the variance inflation factor.
Validity conditions of the multiple linear regression model were checked. First, the model’s suitability was tested using the Rainbow test.17 We then studied the residuals. Their independence was checked using the Durbin-Watson test associated with a graphical method.18 A quantile-quantile plot was made to check their normal distribution. The distribution’s homogeneity has been checked graphically by representing the square root of standardized residuals versus fitted values. Covariates were selected a priori, including maximal urinary osmolality, mGFR, 24-hour osmolar intake, fasting thirst score, lithium treatment duration, lithium dose, lithium formulation, sex, ionized calcium, extracellular volume, hypertension (defined as treated hypertension or blood pressure >140/90 mm Hg), plasma vasopressin, age, and body mass index.
A 2-sided P < 0.05 indicated statistical significance. Statistical analyses were performed with the R software, using the R Studio interface (version 1.2.5019).19
Results
General Description of the Population
Individuals’ characteristics in the total population and according to lithium treatment duration tertiles (tertile 1: <2.5 years; 2.5 ≤ tertile 2 < 10 years; tertile 3 ≥ 10 years) are reported in Table 1. Median age was 51 (37–62) years, 38% of the individuals were males, median mGFR was 78 (63–90) ml/min/1.73 m2, and median lithium treatment duration was 5 (2–14) years. Median 24-hour urine output was 1932 (1448–2835) ml/d, and 21% of the patients displayed polyuria defined as urine output >3 l/d. Median fasting plasma copeptin and vasopressin levels were elevated at 13 (8–23) pmol/l and 5.8 (3.9–10.3) pg/ml, respectively. Compared with individuals in the first and second tertiles of lithium treatment duration, individuals in the third tertile of lithium treatment duration were significantly older, had a lower daily dose of lithium, a higher 24-hour urine output (Figure 1), a lower MaxUosm, and higher copeptin and vasopressin levels, with no difference in 24-hour osmolar intake or plasma lithium level. mGFR was also significantly lower, and renal microcysts on magnetic resonance imaging were significantly more frequent (Table 1).
Table 1.
Characteristics of the population
| Variables | Overall | Treatment duration |
|||
|---|---|---|---|---|---|
| Tertile 1 <2.5 yr |
Tertile 2 2.5–10 yr |
Tertile 3 >10 yr |
|||
| n | 217 | 68 | 70 | 79 | P value |
| Age, yrs | 51 [37–62] | 38 [30–53] | 45 [36–58] | 60 [52–66] | <0.001 |
| Male sex, n (%) | 83 (38) | 31 (46) | 25 (36) | 27 (34) | 0.3 |
| Hypertension, n (%) | 56 (26) | 20 (29) | 17 (25) | 19 (24) | 0.7 |
| BMI, kg/m2 | 26 [23–29] | 26 [23–28] | 26 [23–30] | 26 [23–29] | 0.6 |
| Lithium treatment duration, yrs | 5 [2–14] | 1 [0.5–2] | 4 [3–6] | 18 [13–26] | <0.001 |
| Sustained-release formulation | 139 (65) | 49 (74) | 48 (71) | 42 (53) | 0.02 |
| Lithium daily dose, mg/d | 800 [575–1000] | 1000 [688–1200] | 800 [500–1000] | 750 [500–888] | 0.004 |
| Serum lithemia, mmol/l | 0.7 [0.6–0.9] | 0.8 [0.6–0.9] | 0.7 [0.6–0.8] | 0.7 [0.6–0.9] | 0.8 |
| 24-hour urine output, ml/d | 1932 [1448–2835] | 1662 [1227–2238] | 1765 [1460–2443] | 2516 [1794–3265] | <0.001 |
| Polyuria >3 l/d | 46 (21.4) | 9 (13) | 12 (17) | 25 (32) | 0.01 |
| 24-hour osmolar intake, mOsm/24 h | 659 [516–847] | 645 [506–887] | 669 [507–837] | 663 [551–829] | 1 |
| Fasting thirst score, on a 0–10 scale | 5 [4–7] | 6 [4–7] | 5 [4–7] | 5.00 [4.00, 7.00] | 0.3 |
| Fasting natremia, mmol/l | 141 [140–142] | 141 [140–141] | 141 [140–142] | 142 [141–144] | <0.001 |
| Fasting Uosm, mOsm/kgH2O | 578 [408–719] | 689 [545–757] | 621 [500–737] | 406 [295–562] | <0.001 |
| Maximal Uosm, mOsm/kgH2O | 716 [547–853] | 823 [706–898] | 789 [636–870] | 529 [385–683] | <0.001 |
| Plasma copeptin, pmol/l | 13 [8–23] | 11 [8–16] | 11 [7–14] | 24 [14–42] | <0.001 |
| Plasma vasopressin, pg/ml | 5.8 [3.9–10.3] | 5 [3.3–7.8] | 5.7 [3.9–8.5] | 7.7 [4.6–13.5] | 0.005 |
| mGFR, ml/min per 1.73 m2 | 78 [63–90] | 88 [76–96] | 84 [71–94] | 63 [46–77] | <0.001 |
| Renal microcysts, n (%) | N = 99 | <0.001 | |||
| 0 | 49 (50) | 19 (76) | 23 (74) | 7 (16) | |
| 1–10 | 30 (30) | 6 (24) | 8 (26) | 16 (37) | |
| 11–50 | 10 (10) | 0 (0) | 0 (0) | 10 (23) | |
| >50 | 10 (10) | 0 (0) | 0 (0) | 10 (23) | |
BMI, body mass index; mGFR, measured glomerular filtration rate; Q, quartile; Uosm, urine osmolality.
Data are expressed as median (Q1–Q3) or n (%). Comparisons were performed using Kruskal–Wallis test or χ2 test.
Figure 1.
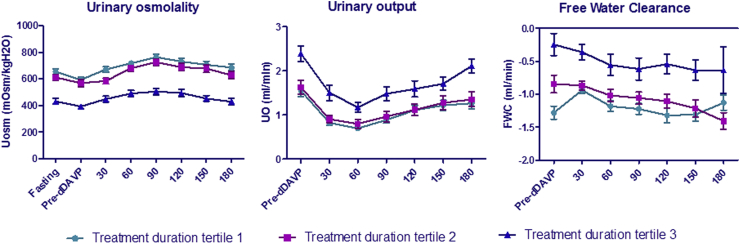
The 24-hour urine output according to treatment duration. Tertile 1 of lithium treatment duration: <2.5 years; tertile 2 of lithium treatment duration: 2.5 to 10 years; tertile 3 of lithium treatment duration: >10 years. ∗∗∗P < 0.001.
DDAVP Test According to Lithium Treatment Duration Tertiles
Throughout the DDAVP challenging test, individuals in the highest lithium treatment duration tertile (>10 years) displayed lower mean urine osmolality, higher urine output, and higher free water clearance throughout the test, compared with the first 2 tertiles. Urinary output decreased significantly 60 minutes after DDAVP injection in all tertiles and tended to increase afterward, but with no significant increase in urine osmolality or decrease in free water clearance (Figure 2). There was no difference in patterns of urine osmolality, urine output, and free water clearance along the test in the first 2 tertiles of lithium treatment duration.
Figure 2.
Water restriction and DDAVP test. Urinary osmolality, urinary output, and free water clearance were assessed along the DDAVP test every 30 minutes. Tertile 1 of lithium treatment duration: <2.5 years; tertile 2 of lithium treatment duration: 2.5 to 10 years; tertile 3 of lithium treatment duration: >10 years. DDAVP, 1-deamino-arginine vasopressin; Uosm, urine osmolality.
Individuals’ Characteristics According to Subgroups of Urine-Concentrating Features: Impact of Lithium Daily Dose on Vasopressin Levels and Polyuria
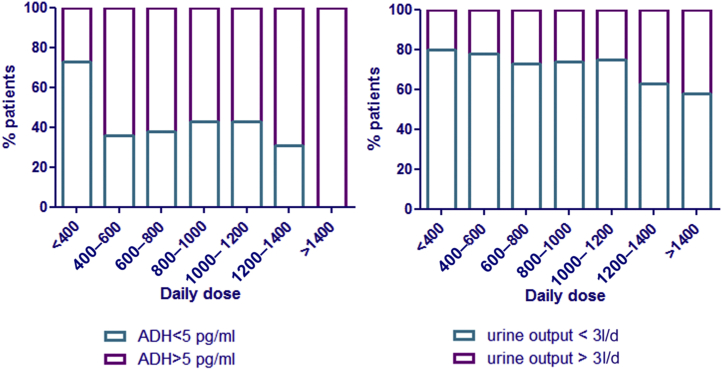
The population was divided in 4 groups according to serum vasopressin levels and maximal urine-concentrating ability as defined in the Methods section. This allowed differentiating between (i) individuals with normal urine-concentrating pattern (normal MaxUosm and normal vasopressin level) (n = 63), (ii) individuals with increased vasopressin level but still normal urine-concentrating ability, suggesting early renal resistance to vasopressin (n = 65), (iii) individuals with impaired urine-concentrating ability and elevated vasopressin level (confirmed NDI [n = 54]), and (iv) individuals with decreased MaxUosm but no elevation of vasopressin level (n = 12) suggesting primary polydipsia (Table 2). When compared with group 1, individuals in group 2 were significantly younger (P = 0.03), with a significantly higher daily dose of lithium (P < 0.001), higher plasma lithium concentrations (P = 0.04), and higher copeptin levels (P < 0.001) (Table 2). There was no significant difference between the 2 groups in terms of lithium treatment duration. In the same line, the percentage of individuals with polyuria and elevated vasopressin levels increased as lithium daily dose increased (Figure 3), with 100% of individuals with a daily dose >1400 mg/d displaying elevated vasopressin levels.
Table 2.
Patients’ characteristics according to urine-concentrating features
| Variables | Group 1 |
Group 2 |
P value group 1 vs. group 2 | Group 3 |
Group 4 |
P value group 3 vs. group 4 | P value all |
|---|---|---|---|---|---|---|---|
| ADH <5 pg/ml Max Uosm ≥ 600 mOsm/kgH2O |
ADH ≥5 pg/ml Max Uosm ≥ 600 mOsm/kgH2O |
ADH ≥5 pg/ml Max Uosm < 600 mOsm/kgH2O |
ADH <5 pg/ml Max Uosm < 600 mOsm/kgH2O |
||||
| n | 63 | 65 | 54 | 12 | |||
| Age, yrs | 50 [38–63] | 40 [31–58] | 0.03 | 60 [48–66] | 51 [41–57] | 0.05 | <0.001 |
| Male sex, n (%) | 25 (39.7) | 27 (41.5) | 1 | 15 (27.8) | 4 (33.3) | 1 | 0.4 |
| Lithium treatment duration, yrs | 3 [2–7] | 3 [1–7] | 0.4 | 18 [10–28] | 13 [4–17] | 0.06 | <0.001 |
| Sustained-release formulation n (%) | 42 (68.9) | 50 (78.1) | 0.3 | 25 (46.3) | 8 (66.7) | 0.3 | 0.003 |
| Daily dose, mg/d | 800 [500–1000] | 950 [750–1200] | <0.001 | 750 [500–1000] | 800 [575–1000] | 0.5 | 0.003 |
| Serum lithemia, mmol/l | 0.65 [0.50–0.85] | 0.77 [0.64–0.87] | 0.04 | 0.72 [0.60–0.89] | 0.76 [0.60–0.88] | 0.8 | 0.2 |
| 24-hour urinary output, ml/d | 1812 [1471–2312] | 1558 [1337–2060] | 0.08 | 2883 [2022–3513] | 3026 [1755–3786] | 1 | <0.001 |
| Fasting thirst score, 0–10 scale | 6 [4–7] | 6 [4–8] | 0.6 | 5 [3–7] | 5 [4–5] | 0.1 | 0.04 |
| Fasting natremia, mmol/l | 141 [140–142] | 141 [140–142] | 0.9 | 143 [141–144] | 141 [141–142] | 0.1 | <0.001 |
| Maximal Uosm, mOsm/kgH2O | 814 [709.50–898] | 826 [739–885] | 1 | 435 [318–516] | 541 [483–560] | 0.03 | <0.001 |
| Plasma vasopressin, pg/ml | 3.39 [2.90–4.20] | 7.80 [5.80–12.50] | <0.001 | 10.55 [6.81–16.73] | 3.69 [2.48–4.38] | <0.001 | <0.001 |
| Plasma copeptin, pmol/l | 8.12 [6.73–10.89] | 13.30 [9.92–19.25] | <0.001 | 25.87 [18.30–45.18] | 9.73 [3.04–16.80] | 0.03 | <0.001 |
| mGFR, ml/min per 1.73 m2 | 78.0 [72.4–90.4] | 88.0 [76.8–94.2] | 0.1 | 54.4 [43.1–72.2] | 81.1 [58.6–89.4] | 0.02 | <0.001 |
ADH, antidiuretic hormone; mGFR, measured glomerular filtration rate; Q, quartile; Uosm, urine osmolality.
Data are expressed as median (Q1–Q3) or number (percentage). Comparisons were performed using Mann–Whitney test when 2 groups were compared and Kruskal–Wallis test when comparing all the groups for quantitative variables. χ2 test was performed for categorical variables.
Figure 3.
Polyuria and plasma vasopressin levels according to lithium daily dose. Daily dose is expressed in mg/d. ADH, antidiuretic hormone.
Determinants of Urinary Output
When considering multivariable analysis, 24-hour urinary output was significantly and independently associated with a lower maximal urinary osmolality (β ± SE −2.89 ± 0.35, P < 0.001), a higher osmolar intake (β ± SE 2.21 ± 0.24, P < 0.001), a higher lithium daily dose (β ± SE 0.49 ± 0.17, P = 0.005), and a higher vasopressin level (β ± SE 10.17 ± 4.76, P = 0.03). It was also positively associated with female sex (β ± SE for male sex −358.74 ± 122.92, P = 0.004). There was no association with lithium formulation (sustained release or immediate release). There was no association between lithium treatment duration and urine output when MaxUosm was in the model, with no added value to the R-squared. Urine output was negatively associated with fasting thirst score (β ± SE −63.45 ± 23.61, P = 0.008) (Table 3).
Table 3.
Multivariable analysis of the correlates of 24-hour urinary output
| Adjusted R-squared of the model = 0.62 | β ± SE | P-value |
|---|---|---|
| Maximal Uosm | −2.9 ± 0.34 | <0.001 |
| Osmolar intake | 2.2 ± 0.24 | <0.001 |
| Thirst score | −62.54 ± 23.48 | 0.009 |
| Lithium daily dose | 0.5 ± 0.17 | 0.004 |
| Male sex | −369.25 ± 121.67 | 0.003 |
| Plasma vasopressin | 10.5 ± 4.74 | 0.03 |
mGFR, measured glomerular filtration rate; Uosm, urine osmolality.
Variables included in the consecutive models were the following and in the following order: maximal urinary osmolality, mGFR, 24-hour osmolar intake, fasting thirst score, lithium treatment duration, lithium dose, lithium formulation, sex, ionized calcium, extracellular volume, hypertension, plasma vasopressin, age, and body mass index. In the last model remained 3 variables that were not significantly associated with urine output: mGFR, lithium formulation, and extracellular volume (P = 0.5, P = 0.2, and P = 0.9, respectively).
Discussion
Our study conducted in a population of patients treated with lithium salts found a relatively low prevalence of polyuria defined as >3 l/d (21%), but most patients displayed a high concentration of serum vasopressin even with no overt concentrating ability defect. This was associated with a higher daily lithium dose. We also found that the major correlates of urine output in these patients were daily osmolar intake, MaxUosm, and lithium daily dose, independently of other factors such as treatment duration.
Although the exact prevalence remains unknown, lithium-induced polyuria has been reported to reach 20% to 70% depending on the studied populations, treatment duration, and definition of polyuria.5,9,20, 21, 22, 23 In our population, the prevalence was relatively low. It is however important to mention that patients were systematically referred to the unit, whether they had symptoms or not.
Results of urine-concentrating tests revealed significant modifications of urine-concentrating test features in patients belonging to the third tertile of treatment duration (i.e., >10 years). In contrary, no significant difference was observed between the 2 first tertiles of treatment duration.
More than half of the individuals had increased levels of serum vasopressin, a finding that was more frequent than overt polyuria. These levels were consistent with levels of serum copeptin in the subset of individuals for which they were available, accordingly with previously reported correlation between the 2 measurements.24 This suggests a certain degree of renal resistance to vasopressin, even in individuals not presenting yet a polyuria or altered urine-concentrating ability. Another feature of vasopressin resistance was the low response to DDAVP test even though most individuals did not reach hypernatremia at the time of DDAVP injection. Elevated levels of vasopressin have been reported in individuals with confirmed NDI associated with lithium.25 This feature of near normal urine-concentrating ability and elevated vasopressin or copeptin (the surrogate marker of vasopressin) levels has been reported in other conditions, such as polycystic kidney disease26,27 and during chronic kidney disease,28 during which a decrease in urine-concentrating ability is predictive of a steeper decline in mGFR and end-stage kidney disease.29 As it is known to play an important role in the pathophysiology of cyst growth in polycystic kidney disease and some authors suggest a deleterious role in polycystic kidney disease as well,30,31 one could hypothesize that it might also be an important mediator of lithium-induced tubulointerstitial and microcystic kidney disease. This increase might also preclude overt NDI. In the same line, serum vasopressin was independently associated with a texture index that we recently developed based on kidney magnetic resonance imaging radiomics analyses of a subset of the studied cohort.32
Interestingly, although treatment duration affected urine-concentrating ability after 10 years, we also found significantly lower doses of lithium in individuals treated >10 years. This result is related to the observational design of our study, as lithium dose might be lowered in patients with a stabilized disorder as recommended by good practice, or for a nephroprotective purpose. Interestingly, plasma lithium level was not correlated with treatment duration suggesting a dose adjustment to renal function.
Nevertheless, we found a significant relationship between urine output and lithium daily dose, independently of other factors including lithium treatment duration, as described by Kinahan et al.33 As expected, MaxUosm was also a major determinant of urine output, which might be at least partly related to lithium treatment duration, explaining the absence of association between treatment duration and urinary output in the multivariable analysis.20 Daily osmolar intake, estimated by the 24-hour urine osmolality, was also a determinant of urine output, suggesting lowering salt and protein intake in individuals with lithium-induced NDI may be advised to lower urine output in clinical practice.34 Female sex was associated with a higher urine output, consistent with a previous report by Schoen et al.35 in a population-based cohort of healthy subjects, but in contrast with a meta-analysis from Perucca et al.36 gathering studies in healthy subjects and in populations with CKD.
We evaluated the association between the galenic formulation of lithium therapy and diuresis and found no significant association. This is often a clinical practice issue when facing lithium-induced polyuria, as data from the literature are conflicting. Some authors have reported a lower risk of polyuria with the sustained-release formulation based on a low level of evidence,37, 38, 39 whereas others have hypothesized the contrary on the basis of a decreased renal parenchyma cumulative exposure time to lithium allowing renal cells to regenerate.40 Our study does not support any of these hypotheses; hence, we could not recommend a specific formulation to prevent lithium-induced NDI.
We also analyzed thirst patterns in the population. We surprisingly found a negative association between fasting thirst score and diuresis, disproving the hypothesis of a central stimulation of thirst by lithium.41 In contrast, these results suggest that these individuals tend to adapt to the thirst distress in a certain degree.
It should be highlighted that the determinants of mGFR were different from those of urinary output in our studied cohort.12 A functional effect of lithium on urine-concentrating ability might thus result from various causes, including daily dose, whereas lithium treatment duration, hence cumulative dose, was associated with a lower mGFR, suggesting 2 different mechanisms inducing these 2 renal side effects of lithium treatment.
Our study displays some limitations. First, the cross-sectional and observational design of the study does not allow directly confirming the direct causal effect of lithium daily dose on 24-hour urinary volume. Second, missing data regarding plasma lithium levels prevented us from analyzing quantitative renal exposure to lithium. Third, we did not perform a hyperosmotic test to evaluate the spontaneous MaxUosm before DDAVP injection. However, the occurrence of central diabetes insipidus is rare in this population42 and DDAVP injection evidences the MaxUosm. Moreover, our protocol was standardized to establish the same time points of DDAVP injection and urine and plasma measurements in all individuals.
In conclusion, our study suggests that lowering lithium daily dose might decrease 24-hour urine output and that it should be considered in individuals affected by the disorder. Controlling salt and protein intake might also help decreasing 24-hour urine volume as it affects 24-hour urine osmolar output. We also found an early increase in vasopressin level in most individuals probably even before the development of polyuria, suggesting an early resistance to the action of vasopressin, hence the development of NDI, that might have potential deleterious role in the pathophysiology of CKD associated with chronic lithium treatment.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors wish to thank all the staff that was involved in the patients’ care and the patients who accepted to be part of this study. This study was partly funded by a research grant from the Société Francophone de Néphrologie, Dialyse et Transplantation and from the Agence Nationale de la Recherche (ANR-21-CE14-0040-01).
References
- 1.Geddes J.R., Burgess S., Hawton K., Jamison K., Goodwin G.M. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;161:217–222. doi: 10.1176/appi.ajp.161.2.217. [DOI] [PubMed] [Google Scholar]
- 2.BALANCE investigators and collaborators. Geddes J.R., Goodwin G.M., Rendell J., et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375:385–395. doi: 10.1016/S0140-6736(09)61828-6. [DOI] [PubMed] [Google Scholar]
- 3.Miura T., Noma H., Furukawa T.A., et al. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2014;1:351–359. doi: 10.1016/S2215-0366(14)70314-1. [DOI] [PubMed] [Google Scholar]
- 4.Cipriani A., Hawton K., Stockton S., Geddes J.R. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646. [DOI] [PubMed] [Google Scholar]
- 5.Tabibzadeh N., Vrtovsnik F., Serrano F., Vidal-Petiot E., Flamant M. Chronic metabolic and renal disorders related to lithium salts treatment. Rev Med Intern. 2019;40:599–608. doi: 10.1016/j.revmed.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Grünfeld J.-P., Rossier B.C. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5:270–276. doi: 10.1038/nrneph.2009.43. [DOI] [PubMed] [Google Scholar]
- 7.Trepiccione F., Christensen B.M. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23(suppl 16):S43–S48. [PubMed] [Google Scholar]
- 8.Walker R.J., Weggery S., Bedford J.J., McDonald F.J., Ellis G., Leader J.P. Lithium-induced reduction in urinary concentrating ability and urinary aquaporin 2 (AQP2) excretion in healthy volunteers. Kidney Int Janv. 2005;67:291–294. doi: 10.1111/j.1523-1755.2005.00081.x. [DOI] [PubMed] [Google Scholar]
- 9.Boton R., Gaviria M., Batlle D.C. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10:329–345. doi: 10.1016/s0272-6386(87)80098-7. [DOI] [PubMed] [Google Scholar]
- 10.Alsady M., Baumgarten R., Deen P.M., de Groot T. Lithium in the kidney: friend and foe? J Am Soc Nephrol. 2016;27:1587–1595. doi: 10.1681/ASN.2015080907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen J., Hoffert J.D., Knepper M.A., Agre P., Nielsen S., Fenton R.A. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A. 2008;105:3634–3639. doi: 10.1073/pnas.0800001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabibzadeh N., Faucon A.-L., Vidal-Petiot E., et al. Kidney function and long-term treatment with lithium salts for bipolar disorder determinants of mGFR and accuracy of kidney microcysts detection in the diagnosis of CKD. Front Pharmacol. 2022 doi: 10.3389/fphar.2021.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidal-Petiot E., Joseph A., Resche-Rigon M., et al. External validation and comparison of formulae estimating 24-h sodium intake from a fasting morning urine sample. J Hypertens. 2018;36:785–792. doi: 10.1097/HJH.0000000000001609. [DOI] [PubMed] [Google Scholar]
- 14.Devuyst O., Chapman A.B., Gansevoort R.T., et al. Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 trial. J Am Soc Nephrol. 2017;28:1592–1602. doi: 10.1681/ASN.2016040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuovo S., Fuiano L., Micalizzi A., et al. Impaired urinary concentration ability is a sensitive predictor of renal disease progression in Joubert syndrome. Nephrol Dial Transplant. 2020;35:1195–1202. doi: 10.1093/ndt/gfy333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christ-Crain M., Bichet D.G., Fenske W.K., et al. Diabetes insipidus. Nat Rev Dis Primer. 2019;5:54. doi: 10.1038/s41572-019-0103-2. [DOI] [PubMed] [Google Scholar]
- 17.Zeileis A., Hothorn T. 2015. Diagnostic checking in regression relationships. [Google Scholar]
- 18.Sage Publications . Sage Publications; Published 2019. An R companion to applied regression third edition.https://socialsciences.mcmaster.ca/jfox/Books/Companion/ [Google Scholar]
- 19.The R Foundation The R Project for Statistical Computing. The R Foundation. https://www.r-project.org/
- 20.McKnight R.F., Adida M., Budge K., Stockton S., Goodwin G.M., Geddes J.R. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721–728. doi: 10.1016/S0140-6736(11)61516-X. [DOI] [PubMed] [Google Scholar]
- 21.Bendz H., Aurell M., Balldin J., Mathé A.A., Sjödin I. Kidney damage in long-term lithium patients: a cross-sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9:1250–1254. [PubMed] [Google Scholar]
- 22.Wallin L., Alling C., Aurell M. Impairment of renal function in patients on long-term lithium treatment. Clin Nephrol Juill. 1982;18:23–28. [PubMed] [Google Scholar]
- 23.Singer I. Lithium and the kidney. Kidney Int. 1981;19:374–387. doi: 10.1038/ki.1981.28. [DOI] [PubMed] [Google Scholar]
- 24.Roussel R., Fezeu L., Marre M., et al. Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J Clin Endocrinol Metab. 2014;99:4656–4663. doi: 10.1210/jc.2014-2295. [DOI] [PubMed] [Google Scholar]
- 25.Padfield P.L., Park S.J., Morton J.J., Braidwood A.E. Plasma levels of antidiuretic hormone in patients receiving prolonged lithium therapy. Br J Psychiatry. 1977;130:144–147. doi: 10.1192/bjp.130.2.144. [DOI] [PubMed] [Google Scholar]
- 26.Ho T.A., Godefroid N., Gruzon D., et al. Autosomal dominant polycystic kidney disease is associated with central and nephrogenic defects in osmoregulation. Kidney Int. 2012;82:1121–1129. doi: 10.1038/ki.2012.225. [DOI] [PubMed] [Google Scholar]
- 27.Meijer E., Bakker S.J.L., van der Jagt E.J., et al. Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:361–368. doi: 10.2215/CJN.04560510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ettema E.M., Heida J., Casteleijn N.F., et al. The effect of renal function and hemodialysis treatment on plasma vasopressin and copeptin levels. Kidney Int Rep. 2017;2:410–419. doi: 10.1016/j.ekir.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabibzadeh N., Wagner S., Metzger M., et al. Fasting urinary osmolality, CKD progression, and mortality: a prospective observational study. Am J Kidney Dis. 2019;73:596–604. doi: 10.1053/j.ajkd.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Bankir L., Bouby N., Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol. 2013;9:223–239. doi: 10.1038/nrneph.2013.22. [DOI] [PubMed] [Google Scholar]
- 31.Torres V.E. Vasopressin in chronic kidney disease, an elephant in the room? Kidney Int. 2009;76:925–928. doi: 10.1038/ki.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beunon P., Barat M., Dohan A., et al. MRI based kidney radiomics analysis during chronic lithium treatment: validation of a texture index associated with decreased kidney function. Eur J Clin Invest. 2022 doi: 10.1111/eci.13756. [DOI] [PubMed] [Google Scholar]
- 33.Kinahan J.C., NiChorcorain A., Cunningham S., et al. Risk factors for polyuria in a cross-section of community psychiatric lithium-treated patients. Bipolar Disord. 2015;17:50–62. doi: 10.1111/bdi.12235. [DOI] [PubMed] [Google Scholar]
- 34.Bockenhauer D., Bichet D.G. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol. 2015;11:576–588. doi: 10.1038/nrneph.2015.89. [DOI] [PubMed] [Google Scholar]
- 35.Schoen T., Blum J., Paccaud F., et al. Factors associated with 24-hour urinary volume: the Swiss salt survey. BMC Nephrol. 2013;14:246. doi: 10.1186/1471-2369-14-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perucca J., Bouby N., Valeix P., Bankir L. Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am J Physiol Regul Integr Comp Physiol. 2007;292 doi: 10.1152/ajpregu.00500.2006. [DOI] [PubMed] [Google Scholar]
- 37.Bowen R.C., Grof P., Grof E. Less frequent lithium administration and lower urine volume. Am J Psychiatry. 1991;148:189–192. doi: 10.1176/ajp.148.2.189. [DOI] [PubMed] [Google Scholar]
- 38.Ljubicic D., Letica-Crepulja M., Vitezic D., Bistrovic I.L., Ljubicic R. Lithium treatments: single and multiple daily dosing. Can J Psychiatry Rev Can Psychiatr. 2008;53:323–331. doi: 10.1177/070674370805300507. [DOI] [PubMed] [Google Scholar]
- 39.Wallin L., Alling C. Effect of sustained-release lithium tablets on renal function. Br Med J. 1979;2:1332. doi: 10.1136/bmj.2.6201.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter L., Zolezzi M., Lewczyk A. An updated review of the optimal lithium dosage regimen for renal protection. Can J Psychiatry. 2013;58:595–600. doi: 10.1177/070674371305801009. [DOI] [PubMed] [Google Scholar]
- 41.Galla J.N., Forrest J.N., Hecht B., Kashgarian M., Hayslett J.P. Effect of lithium on water and electrolyte metabolism. Yale J Biol Med. 1975;48:305–314. [PMC free article] [PubMed] [Google Scholar]
- 42.Baylis P.H., Heath D.A. Water disturbances in patients treated with oral lithium carbonate. Ann Intern Med. 1978;88:607–609. doi: 10.7326/0003-4819-88-5-607. [DOI] [PubMed] [Google Scholar]