Abstract
Introduction
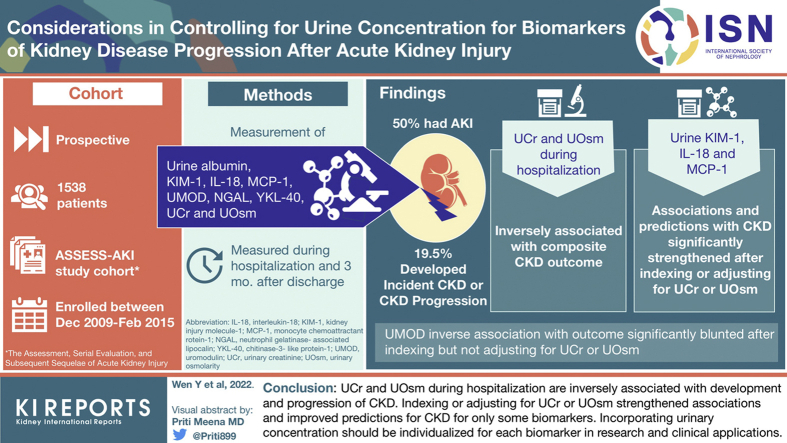
Biomarkers of acute kidney injury (AKI) are often indexed to urine creatinine (UCr) or urine osmolarity (UOsm) to control for urine concentration. We evaluated how these approaches affect the biomarker-outcome association in patients with AKI.
Methods
The Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury Study was a cohort of hospitalized patients with and without AKI between 2009 and 2015. Using Cox proportional hazards regression, we assessed the associations and predictions (C-statistics) of urine biomarkers with a composite outcome of incident chronic kidney disease (CKD) and CKD progression. We used 4 approaches to account for urine concentration: indexing and adjusting for UCr and UOsm.
Results
Among 1538 participants, 769 (50%) had AKI and 300 (19.5%) developed composite CKD outcome at median follow-up of 4.7 years. UCr and UOsm during hospitalization were inversely associated with the composite CKD outcome. The associations and predictions with CKD were significantly strengthened after indexing or adjusting for UCr or UOsm for urine kidney injury molecule-1 (KIM-1), interleukin-18 (IL-18), and monocyte chemoattractant protein-1 (MCP-1) in patients with AKI. There was no significant improvement with indexing or adjusting UCr or UOsm for albumin, neutrophil gelatinase-associated lipocalin (NGAL), and chitinase 3-like 1 (YKL-40). Uromodulin’s (UMOD) inverse association with the outcome was significantly blunted after indexing but not adjusting for UCr or UOsm.
Conclusion
UCr and UOsm during hospitalization are inversely associated with development and progression of CKD. Indexing or adjusting for UCr or UOsm strengthened associations and improved predictions for CKD for only some biomarkers. Incorporating urinary concentration should be individualized for each biomarker in research and clinical applications.
Keywords: acute kidney injury, biomarker, chronic kidney disease, urine concentration, urine creatinine, urine osmolarity
Graphical abstract
See Commentary on Page 1458
AKI represents an acute decline in kidney function and is often caused by ischemic or toxic injury.1 AKI is common in hospitalized patients and is associated with high inpatient mortality and the development or progression of CKD and end-stage kidney disease.2,3 Significant effort in the past decade has centered on identifying specific markers that may serve as early indicators for tubular injury or represent specific pathophysiological processes in the kidney during AKI.
Multiple biomarkers involved in injury, inflammation, and adaptive and maladaptive repair were identified from animal models and evaluated for clinical application in AKI.4, 5, 6, 7 Because biomarkers are often measured from spot urine collection rather than collection during a predefined time interval, biomarker values are often indexed to (i.e., divided by) UCr concentration to account for the variation of urine concentration to estimate its excretion rate. However, there is no consensus on how urine concentration should be accounted for in AKI. In patients with CKD, urine albumin-to-creatinine ratio is often used to estimate 24-hour urine albumin excretion.8,9 This is based on the assumption of an average UCr excretion of approximately 1 g/d in normal persons (thus division by 1 results in the ratio taking on the numerator’s value). However, UCr excretion varies greatly across individuals and is affected by sex, muscle mass, diet, and proximal tubular function.10 In addition, UCr concentration decreases as glomerular filtration rate (GFR) falls during AKI progression and increases when GFR improves during AKI recovery.11 The biomarker creatinine ratio may thus overestimate or underestimate the true biomarker excretion rate.11
Given these limitations of UCr, some authors have suggested controlling for urine concentration using UOsm as an alternative approach. Indexing a biomarker to UOsm would effectively account for concentration variations in spot urine samples.12,13 This approach relies on an assumption of stable daily osmolyte excretion and deserves scrutiny in the setting of AKI, because osmolyte excretion not only varies across individuals in steady state but also changes dynamically during the course of AKI.14 Urine urea nitrogen contributes to a large proportion of UOsm. Its excretion decreases when GFR suddenly drops and increases during the recovery phase of AKI.
Considering the variations of UCr and UOsm across individuals, it is not surprising that the association between urine albumin concentration and mortality alters with different approaches to control for urine concentration in patients with patients with CKD.15 However, this has not been thoroughly investigated in the field of AKI. Considering the dynamic nature of UCr and UOsm excretion in AKI, we evaluated how different approaches to account for urine concentration affect the biomarker-outcome associations in patients with and without AKI. We hypothesized that indexing to or adjusting for UCr versus UOsm will have similar effects on strengthening the biomarker-outcome association, but such strengthening may differ between patients with and without AKI.
Methods
Study Population
The Assessment, Serial Evaluation, and Subsequent Sequelae of AKI study is a prospective cohort study consisted of 1538 hospitalized adults enrolled between December 2009 and February 2015 from 4 North American clinical centers involving various hospital settings.16 The study was approved by institutional review boards in the participating sites. Written informed consent was obtained from the participants.
Details of the study design have been described previously.16,17 Briefly, 769 participants who developed AKI were recruited and a control group of participants who did not develop AKI were matched in 1:1 ratio based on preadmission CKD status and an integrated score, including age, history of cardiovascular disease, diabetes mellitus, baseline estimated GFR (eGFR), and treatment in an intensive care unit. AKI was defined as an increase of serum creatinine concentration of 0.3 mg/dl or more or at least 50% from the nearest serum creatinine concentration value obtained from an outpatient, nonemergency department setting within 365 days before hospitalization (baseline serum creatinine concentration). Both groups of participants were enrolled during hospitalization and had their first outpatient research study visits 3 months after discharge. Follow-up study visits were conducted annually thereafter with telephone contacts conducted at 6-month intervals.
Urine Measurement and Outcome
Urine albumin, KIM-1, IL-18, MCP-1, UMOD, NGAL, YKL-40, UCr, and UOsm were measured using samples collected during hospitalization and 3 months after discharge. For patients with AKI, urine samples were collected within 48 to 96 hours of diagnosis of AKI during hospitalization. The samples were collected and processed using a standard protocol. After collection, the samples were placed on ice if they were not processed within 30 minutes. The samples could be processed up to 6 hours after collection, were spun for 10 minutes at 1000g, aliquoted, frozen, and stored at −70 °C until measurement.18 Assays for these measurements are listed in Supplementary Table S1.18
We chose incident CKD or CKD progression as a composite CKD outcome.16 We calculated eGFR using the CKD-Epidemiology Collaboration equation.19 We defined incident CKD as ≥25% reduction in eGFR (compared with eGFR calculated using baseline serum creatinine concentration) and achieving eGFR <60 ml/min per 1.73 m2 among participants without preexisting CKD. In participants with preexisting CKD, we defined CKD progression as ≥50% reduction in eGFR compared with baseline, eGFR <15 ml/min per 1.73 m2, or receiving kidney replacement therapy or kidney transplant.
Statistical Analysis
We reported median, interquartile range (quartile 1–quartile 3), and proportion of baseline characteristics, including age, sex, race, ethnicity, and urine measures stratified by AKI status. All analyses were performed in patients with AKI and those without AKI separately. We first assessed the linear correlation (Pearson) of urine biomarkers (albumin, KIM-1, IL-18, MCP-1, UMOD, NGAL, and YKL-40), UCr and UOsm, using their log 2-transformed normally distributed Z score. We chose this method to gain insights on how adjusting or indexing UCr of UOsm may change the exposure–outcome associations. We considered correlation to be strong, moderate, and weak based on correlation coefficients ≥0.6, 0.4 to 0.59, and <0.4.
We converted urine measures (biomarker, UCr, UOsm, biomarker-to-UCr ratio, and biomarker-to-UOsm ratio) to log 2-transformed normally distributed Z scores and assessed the association between urine biomarkers, UCr and UOsm, collected during initial hospitalization or 3 months after discharge, with the composite CKD outcome using Cox proportional hazards regression. We then assessed the association of each urine biomarker at these 2 time points, with the composite CKD outcome when biomarker-to-UCr ratio or biomarker-to-UOsm ratio was used, and when UCr or UOsm was used as covariates in Cox proportional hazards regression models. For all models, participants were censored if they died, lost to follow-up, or withdrew from the study. We considered a 2-tailed P < 0.05 as statistically significant.
For model comparisons, we produced 1000 bootstrap samples and compared the coefficients of biomarker-to-UCr ratio, biomarker-to-UOsm ratio, and biomarker after adjusting for UCr or UOsm against the coefficients of biomarker without indexing or adjusting UCr or UOsm. We also compared the coefficients of biomarker-to-UCr ratio versus biomarker-to-UOsm ratio, coefficients of biomarkers when adjusting for UCr versus adjusting UOsm. We tested the null hypothesis that accounting for urine concentration by these 4 approaches does not strengthen the biomarker-outcome association and null hypotheses that using biomarker-to-UOsm ratio or adjusting UOsm do not strengthen the biomarker-outcome association compared with biomarker-to-UCr ratio or adjusting UCr, respectively. We chose a P < 0.01 as statistically significant to account for multiple comparisons. Therefore, if we observed strengthening of the biomarker-outcome association in >99% of the bootstrap samples, we considered the strengthening statistically significant.
In addition, we assessed the change of predictive performance of biomarkers for biomarkers for the composite CKD outcomes at 3 years after hospital discharge, using these 4 approaches to account for urine concentration, from the predictive performance of biomarkers alone. We constructed 1000 bootstrap samples and compare the C-statistics of logistic regression models with indexing or adjusting UCr or UOsm against with biomarker alone. We considered a P < 0.01 as statistically significant to account for multiple comparisons. We performed all analyses using R version 4.0.2.
Results
Characteristics and Biomarker Measurement in Study Participants
Table 1 summarizes the baseline characteristics and biomarker measurements during hospitalization and 3 months after discharge in participants with and without AKI. The median age of all participants was 65.9 (quartile 1– quartile 3, 56.7–73.9) years. Of the participants, 37.3% were female and 12.7% were African American. Among 1538 participants, 769 (50%) had AKI and 216 (14%) had stage 2 or stage 3 AKI. After 4.7 years (median) of follow-up, 300 (19.5%) participants developed incident CKD or experienced CKD progression. The kidney function at baseline, 3 months after hospital discharge, at end point, or censoring in patients is shown in Supplementary Table S2.
Table 1.
Baseline characteristics and biomarker measurement in ASSESS-AKI study participants stratified by AKI status
| Patient characteristics and urine measurements | AKI patients (n = 769) | Non-AKI patients (n = 769) |
|---|---|---|
| Patient characteristics | ||
| Age | 64.9 (55.7–72.9) | 67.3 (57.4–74.7) |
| Sex, n (%) | ||
| Female | 250 (32.5) | 324 (42.1) |
| Male | 519 (67.5) | 445 (57.9) |
| Race, n (%) | ||
| White | 607(78.9) | 653 (84.9) |
| African American | 117 (15.2) | 78 (10.1) |
| Other | 45 (5.9) | 38 (5) |
| Hispanic, n (%) | 21 (2.7) | 17 (2.2) |
| Urine measurements during hospitalization | ||
| Creatinine, mg/dl | 82 (52–124) | 83 (46.5–137) |
| Osmolarity, mOsm/dl | 441.5 (345–570.8) | 483 (353–659) |
| KIM-1, pg/ml | 2798 (1194.8–6088.3) | 2150 (801–5077) |
| IL-18, pg/ml | 40.8 (18.4–93) | 31.95 (15.2–62.7) |
| MCP-1, pg/ml | 486 (235.8–1146.7) | 279.2 (126.1–648.3) |
| Albumin, mg/dl | 42 (14–113) | 18 (7–56) |
| NGAL, ng/ml | 66.7 (28.8–187) | 31.5 (14.2–73.7) |
| YKL-40, pg/ml | 1259.5 (395.8–5271.9) | 919.4 (322.3–2523.7) |
| UMOD, pg/ml | 2245.6 (1354.2–4220.8) | 2634.1 (1611.9–4407.2) |
| Urine measurements at 3 mo after discharge | ||
| Creatinine, mg/dl | 83 (50–135) | 82 (43–137) |
| Osmolarity, mOsm/dl | 487 (354–656) | 483.5 (333.3–686.8) |
| KIM-1, pg/ml | 1486 (661.5–3131) | 1235.5 (390–2704.5) |
| IL-18, pg/ml | 28.6 (15.1–53.9) | 22.79 (11–44.5) |
| MCP-1, pg/ml | 274.2 (129.2–545.7) | 206.6 (81.8–449.8) |
| Albumin, mg/dl | 19 (7–87) | 11 (4–29) |
| NGAL, ng/ml | 27 (12.5–65.1) | 21.5 (10.4–49.5) |
| YKL-40, pg/ml | 559.5 (233.1–1305.7) | 446.9 (193.9–947.3) |
| UMOD, pg/ml | 2346.6 (1384.1–3787.9) | 2736.8 (1699.2–4119) |
ASSESS-AKI, Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury; AKI, acute kidney injury; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; NGAL, neutrophil gelatinase-associated lipocalin; Q, quartile; UMOD, uromodulin; YKL-40, chitinase 3-like 1.
Results are presented as n (%), or median (Q1–Q3).
Association Between UCr, UOsm, and Composite CKD Outcome
Table 2 shows associations between UCr, UOsm, and the composite CKD outcome in patients with AKI, patients with different stages of AKI, and patients without AKI. During hospitalization, UCr and UOsm were inversely associated with the composite CKD outcome in both patients with and without AKI (for patients with AKI, hazard ratio [HR] 0.84, 95% CI 0.73–0.96 for UCr; HR 0.81, 95% CI 0.71–0.93 for UOsm; for patients without AKI, HR 0.78, 95% CI 0.64–0.95 for UCr; HR 0.71, 95% CI 0.6–0.85 for UOsm). The HR represents change per 1 SD difference of UCr or UOsm on their log-2 scale. When stratifying patients with AKI by AKI severity, this inverse association remained significant only in patients with stage 1 AKI.
Table 2.
Association between urine creatinine, urine osmolarity, and composite CKD outcome in patients based on AKI status
| Patient subgroups | Hazard ratio (95% CI) for composite CKD outcome |
||||
|---|---|---|---|---|---|
| During hospitalization | 3 mo after discharge | ||||
| Subgroup | n | Urine creatinine | Urine osmolarity | Urine creatinine | Urine osmolarity |
| AKI | 769 | 0.84 (0.73–0.96)a | 0.81 (0.71–0.93)a | 0.9 (0.78–1.04) | 0.78 (0.68–0.9)a |
| Stage 1 AKI | 553 | 0.83 (0.71–0.98)a | 0.81 (0.68–0.95)a | 0.9 (0.76–1.06) | 0.8 (0.68–0.95)a |
| Stage 2 AKI | 118 | 0.86 (0.63–1.17) | 0.81 (0.58–1.13) | 1 (0.68–1.46) | 0.84 (0.62–1.13) |
| Stage 3 AKI | 98 | 0.87 (0.61–1.24) | 0.98 (0.66–1.45) | 0.8 (0.56–1.15) | 0.54 (0.34–0.85)a |
| Stages 2–3 AKI | 216 | 0.85 (0.68–1.08) | 0.86 (0.67–1.1) | 0.9 (0.69–1.17) | 0.75 (0.59–0.94)a |
| No AKI | 769 | 0.78 (0.64–0.95)a | 0.71 (0.6–0.85)a | 0.98 (0.81–1.19) | 1.02 (0.85–1.23) |
AKI, acute kidney injury; CKD, chronic kidney disease; UCr, urine creatinine; UOsm, urine osmolarity.
Hazard ratios with P < 0.05.
At 3 months after discharge, UCr was no longer associated with the composite CKD outcome (for patients with AKI, HR 0.9, 95% CI 0.78–1.04; for patients without AKI, HR 0.98, 95% CI 0.81–1.19). UOsm remained inversely associated with the composite CKD outcome in patients with AKI but not in those without AKI (for patients with AKI, HR 0.78, 95% CI 0.68–0.9; for patients without AKI, HR 1.02, 95% CI 0.85–1.23). When stratifying patients with AKI by AKI severity, UCr’s inverse association with CKD outcome remained significant in patients with stages 1 and 3 AKI.
Correlation Between Urinary Biomarkers, UCr and UOsm
Correlations of biomarkers with UCr or UOsm at hospitalization and 3 months after discharge in patients with AKI and those without AKI are shown in Table 3. We observed strong correlation between UCr and UOsm in patients without AKI at both time points. For patients with AKI, this correlation was moderate at both time points, but appeared stronger at 3 months after discharge. During hospitalization, urine KIM-1, IL-18, and MCP-1 had moderate-to-strong correlation with UCr in patients with AKI and strong correlation with UCr in patients without AKI. Urine albumin, NGAL, and YKL-40 were weakly correlated with UCr in patients with AKI, but these correlations were moderate in patients without AKI. Urine UMOD was weakly correlated with UCr in patients with and without AKI. Correlation between these urine biomarkers and UOsm was weaker than UCr in both patients with and without AKI. After 3 months postdischarge, the correlations between biomarker and UCr or UOsm followed a similar pattern but were stronger than those during hospitalization in both patients with and without AKI.
Table 3.
Correlation between urine biomarkers, UCr and UOsm, during hospitalization and at 3 mo after discharge
| Biomarkers | Pearson correlation coefficient |
|||||||
|---|---|---|---|---|---|---|---|---|
| Hospitalization |
3 months after discharge |
|||||||
| AKI |
Non-AKI |
AKI |
Non-AKI |
|||||
| UCr | UOsm | UCr | UOsm | UCr | UOsm | UCr | UOsm | |
| UCr | N/A | 0.65 | N/A | 0.77 | N/A | 0.74 | N/A | 0.82 |
| KIM-1 | 0.61 | 0.41 | 0.7 | 0.58 | 0.66 | 0.47 | 0.79 | 0.69 |
| IL-18 | 0.44 | 0.33 | 0.66 | 0.54 | 0.61 | 0.46 | 0.75 | 0.65 |
| MCP-1 | 0.45 | 0.18 | 0.75 | 0.65 | 0.65 | 0.47 | 0.8 | 0.7 |
| Albumin | 0.22 | 0.08 | 0.43 | 0.38 | 0.32 | 0.21 | 0.5 | 0.43 |
| NGAL | 0.2 | 0.03a | 0.43 | 0.31 | 0.25 | 0.1 | 0.43 | 0.36 |
| YKL-40 | 0.25 | 0.13 | 0.43 | 0.36 | 0.28 | 0.12 | 0.44 | 0.36 |
| UMOD | 0.38 | 0.11 | 0.17 | −0.08 | 0.27 | 0.06a | 0.14 | −0.05a |
AKI, acute kidney injury; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; N/A, not applicable; NGAL, neutrophil gelatinase-associated lipocalin; UCr, urine creatinine; UMOD, uromodulin; UOsm, urine osmolarity; YKL-40, chitinase 3-like 1.
 Strong correlation (coefficient ≥0.6).
Strong correlation (coefficient ≥0.6).
 Moderate correlation (coefficient 0.4–0.59).
Moderate correlation (coefficient 0.4–0.59).
 Weak or no correlation (coefficient <0.4).
Weak or no correlation (coefficient <0.4).
P values for all correlation are statistically significant at level of 0.05 unless indicated by a footnote symbol a.
Not significant.
Biomarker Association and Prediction for the Composite CKD Outcome Using Different Approaches to Account for Urine Concentration
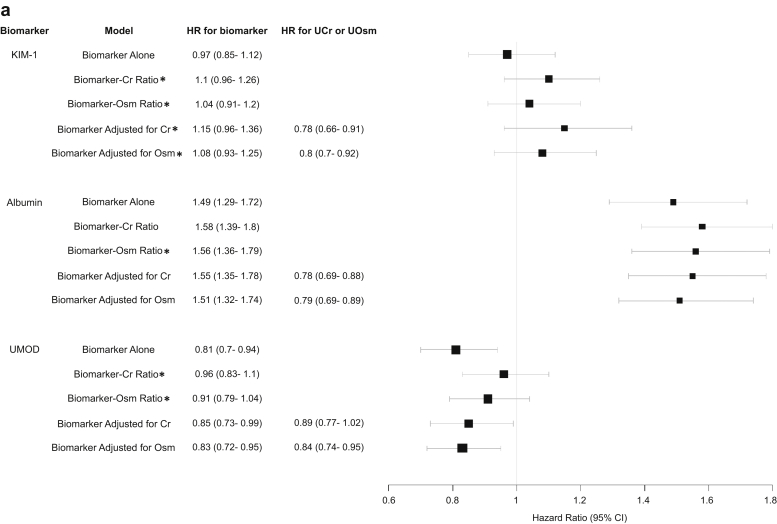
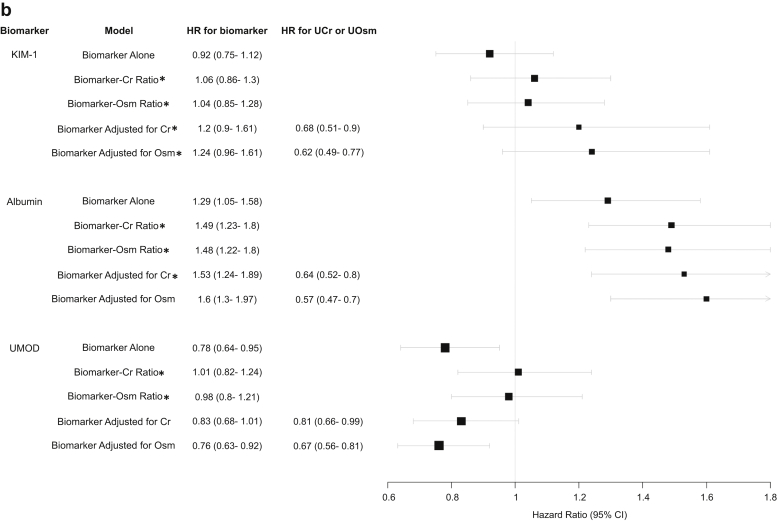
Figure 1a and b demonstrates the HRs of KIM-1, albumin, and UMOD collected during hospitalization with the composite CKD outcome in patients with and without AKI using univariable model with biomarker alone, biomarker-to-UCr ratio, and biomarker-to-UOsm ratio and using multivariable model with biomarker adjusted for UCr or UOsm. The HR represents a comparison per 1 SD difference in the biomarker, biomarker-to-UCr ratio, biomarker-to-Osm ratio, UCr, and UOsm on their log-2 scale.
Figure 1.
(a) HR of urine KIM-1, albumin, and UMOD collected during hospitalization in patients with AKI with composite CKD outcome using different approaches to account for urine concentration. (b) HR of urine biomarkers collected during hospitalization in patients without AKI with composite CKD outcome using different approaches to account for urine concentration. ∗P < 0.01 compared with biomarker’s association with composite CKD outcome when urine creatinine or osmolarity is accounted for versus biomarker alone. #The difference between biomarker’s association with composite CKD outcome when using urine creatinine versus urine osmolarity was insignificant in any model (P > 0.01 for all comparisons). All urine measurements were converted to log-2 base normally distributed Z score. HR therefore represents change per 1 SD increase of each biomarker on its log-2 scale. AKI, acute kidney injury; CKD, chronic kidney disease; Cr, creatinine; HR, hazard ratio; KIM-1, kidney injury molecule-1; Osm, osmolarity; UCr, urine creatinine; UMOD, uromodulin; UOsm, urine osmolarity.
For urine KIM-1 and albumin, the biomarker-outcome associations are strengthened after indexing or adjusting UCr or UOsm when it has moderate-to-strong correlation with UCr and UOsm. This was consistent in both patients with and without AKI. Specifically, urine KIM-1 had moderate-to-strong correlation with UCr and UOsm, and the association between urine KIM-1 with the composite CKD outcome strengthened significantly after indexing or adjusting UCr or UOsm. For urine albumin, which had weak correlation with UCr and UOsm in patients with AKI, its association with the composite CKD outcome changed only minimally after indexing or adjusting UCr or UOsm. However, urine albumin was moderately correlated with UCr and UOsm in patients without AKI, and its associations with composite CKD outcome strengthened significantly after indexing or adjusting UCr or UOsm. The changes in other biomarker-outcome associations (with the exception of UMOD) after indexing or adjusting UCr or UOsm followed the same principle and were similar between urine KIM-1, IL-18, and MCP-1, including between urine albumin, NGAL, and YKL-40 (Supplementary Figure S1A and B).
We observed significant improvement in the predictive performance (C-statistics) of urine KIM-1, IL-18, and MCP-1 for the CKD outcome after adjusting or indexing UCr or UOsm compared with biomarker alone, particularly for patients with AKI (Supplementary Table S3). Although there was some improvement in the predictive performance of urine albumin, NGAL, and YKL-40 after adjusting or indexing UCr or UOsm, most were not statistically significant.
UMOD is only weakly or not correlated with UCr or UOsm. In both patients with and without AKI, it was inversely associated with the composite CKD outcome in univariate model with biomarker alone or multivariable model with adjustment for UCr or UOsm, but its associations were attenuated significantly when biomarker-to-UCr ratio or biomarker-to-UOsm ratio was used, becoming statistically insignificant. The predictive values of UMOD after indexing to UCr or UOsm were also attenuated (Supplementary Table S3).
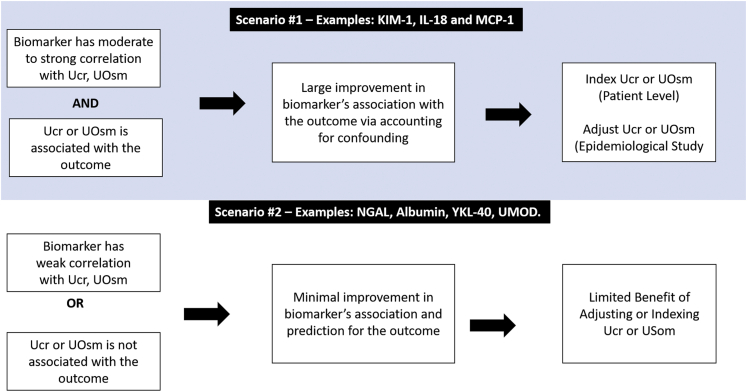
For biomarkers measured 3 months after hospital discharge in both patients with and without AKI, the HR and C-statistic changes followed the same principle as previously observed. These results are shown in Supplementary Figure S2A and S2B and Supplementary Table S3. We also performed additional analyses in patients with stages 2 to 3 AKI, and the results were similar to overall patients with AKI (Supplementary Figure S3A and S3B). On the basis of the results, a step-by-step approach to determine how to apply UCr or UOsm to account for confounding when investigating etiologic relationships or to enhance risk prediction for urine biomarkers is illustrated in Figure 2.
Figure 2.
Recommended approach to adjust or index UCr or UOsm to assess biomarkers’ associations with outcome. IL-18, interleukin-18; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; NGAL, neutrophil gelatinase-associated lipocalin; UMOD, uromodulin; UCr, urine creatinine; UOsm, urine osmolarity; YKL-40, chitinase 3-like 1.
Finally, for most biomarkers in patients with and without AKI, the biomarker-outcome associations were similar whether UCr or UOsm was used to index or adjust the biomarkers.
Discussion
In this study, we provide insights regarding how the associations between urine biomarkers and occurrence of a composite CKD outcome changed based on various methods of accounting for urine concentration variation in patients with and without AKI. We measured urine biomarkers and 2 common methods of accounting for urine concentration, UCr and UOsm, in urine samples collected from patients during and 3 months after hospitalization. We ascertained the outcome of CKD at an average of 4 years after hospitalization. We found that UCr and UOsm during hospitalization were inversely associated with the composite outcomes both in patients with stage 1 AKI and without AKI. We did not see any associations with more severe stages of AKI, likely because the number of events was inadequate for increased variation in UCr and UOsm, especially when UCr and UOsm excretion were not in steady states.
The biomarker-outcome association strengthened significantly after indexing or adjusting UCr or UOsm when the biomarker was moderately or strongly correlated with UCr or UOsm and UCr or UOsm was associated with the outcome, suggesting the mechanism is via controlling for confounding. Specifically, biomarkers’ associations with and predictive performance for CKD were significantly strengthened for urine KIM-1, IL-18, and MCP-1 in patients with AKI, but not for urine albumin, NGAL, or YKL-40. In addition, UMOD’s inverse association and predictive performance for CKD were attenuated when indexing UCr or UOsm. Finally, there was no significant difference in using UCr versus UOsm to account for urine concentration, either by indexing or adjusting. These results were consistent in both patients with and without AKI.
In kidney disease research, timed urine collection is often cumbersome, but it provides the most accurate information on the daily rate of biomarker excretion. Although the excretion rate of different biomarkers may be of interest in patients with AKI, 24-hour studies are rarely conducted owing to practicality issues. To account for variation in urine concentration (i.e., water content in the urine), the biomarker-to-UCr ratio and biomarker-to-UOsm ratio are often used. However, our study suggests that both UCr and UOsm themselves may be prognostic. Creatinine is mostly filtered from the glomeruli, and only a small proportion is secreted from the proximal tubules; thus, a decrease in UCr excretion mainly represents a decrease in the GFR. UOsm is largely determined by both tubular water reabsorption and electrolytes and urea nitrogen handling, which is freely filtered, but reabsorbed and secreted along the distal nephron. A decrease in UOsm may directly reflect the severity of GFR decline and impairment of urea cycling from tubular injury. Moreover, both UCr and UOsm are affected by the nephron’s capacity to reabsorb water at all tubular segment levels and may also represent general health of the tubule. Therefore, UCr and UOsm serve as indicators of disease severity in patients with AKI, both in terms of glomerular filtration and tubular function. This may be particularly important in patients with stage 1 AKI because UCr and UOsm may provide additional information regarding patients’ tubular health that is not sensitively captured by the transient, mild increase in serum creatinine. It may also provide important prognostication for long-term kidney outcomes in patients who otherwise may be considered to be at low risk for AKI to CKD transition. After 3 months postdischarge, only UOsm was still inversely associated with the composite CKD outcome in patients with AKI. This may be due to incomplete recovery of tubular injury in these patients with AKI. As both dietary sodium and protein intake can influence osmolyte excretion, lower UOsm levels may also serve as an indicator for frailty or malnutrition in patients with AKI. The inverse association between UOsm and kidney function decline has also been observed in patients with CKD, possibly owing to impaired concentration capacity from distal nephron dysfunction.20,21
In addition, the correlation of UCr and UOsm with various biomarkers differs among biomarkers, between patients with and without AKI, and between hospitalization and 3 months after discharge. The correlations were in general stronger in biomarkers originating from proximal tubules (e.g., KIM-1, IL-18, and MCP-1) than biomarkers that are mainly filtered through the glomeruli (e.g., albumin) or originating from the distal nephron (e.g., NGAL and UMOD), although urine albumin and NGAL may also originate from the proximal tubule after injury based on a recent transcriptomic study of human AKI.22 Water reabsorption throughout the nephron may have similar impacts on the concentration of UCr, UOsm, and biomarkers originating from proximal tubules, but not those from the distal nephron. Albumin is not filtered as freely as UCr or urea. The correlations were stronger in patients without AKI than those with AKI and stronger at 3 months after discharge than during hospitalization. This is possibly owing to the up-regulation of injury biomarkers and decreased glomerular filtration during AKI hospitalization and persistent generation of injury and inflammation markers from recovering tubules 3 months after clinical AKI has resolved.23
Owing to the correlation of UCr and UOsm with biomarkers and inverse associations with the CKD outcome, the strengthening of biomarker-outcome associations after adjustment may reflect the contribution of controlling for confounding from UCr and UOsm, beyond correcting for urine concentration. In the conceptual framework of accounting for urine concentration to establish associations and etiologic relationships between urine biomarkers and outcomes (Supplementary Figure S4), urine concentration can be viewed as a strong instrumental variable. In other words, its association with the outcome occurs largely through the strong effect on the exposure (e.g., biomarkers). Adjusting the instrumental variable was previously shown to result in bias and larger variance in the exposure–outcome association, especially in the presence of unmeasured confounders, arguing against adjusting urine concentration for urine biomarkers in studies investigating biomarker-outcome associations.24 The inverse associations of UCr and UOsm with CKD are possibly owing to their associations with frailty and tubular function, which are both associated with risk of kidney injury and CKD progression. Therefore, UCr and UOsm may be viewed as indicators for these confounders rather than simply for urine concentration.
Nevertheless, the inclusion of UCr or UOsm as covariates, or as biomarker-to-UCr ratio or biomarker-to-UOsm ratio, to achieve better predictions of the outcome is a distinct goal compared with investigating etiologic relationships. Our study suggests that adjusting UCr or UOsm may be particularly important for urine KIM-1, MCP-1, and IL-18, whose predictions for CKD were significantly strengthened. A previous study demonstrated the strengthening of urine albumin-outcome associations in the general population after accounting for urine concentration using the same 4 approaches.15 However, whether this is due to controlling for confounding is unknown. Our study suggests that for these 3 biomarkers, their predictive performance is significantly improved after indexing or adjusting UCr or UOsm, which should be considered in future risk-prediction research studies and clinical practice.
Although adjusting UCr or UOsm as covariates may be statistically more robust in regression models for risk prediction, biomarker-to-UCr ratio and biomarker-to-UOsm ratio may still have their roles because the ratios can be calculated at the individual patient level to guide clinical practice. However, there are several assumptions underlying the use of these ratios to represent biomarker excretion rates in patients with AKI that deserve thorough consideration. Creatinine and osmolyte excretion decreases with the decline of GFR and tubular injury during AKI progression and increases during AKI recovery.11,25,26 Dividing the biomarker by UCr or UOsm may result in overestimating or underestimating the true production rate of a biomarker.11 At the individual patient level, the dynamic trajectory of UCr and UOsm excretion may result in significant alterations in interpreting the biomarker-to-UCr ratio or biomarker-to-UOsm ratio even when biomarker excretion rates do not change. Despite these concerns, we showed that the strengthening of biomarker-outcome associations after indexing to UCr or UOsm was often similar to the strengthening of these associations after statistically adjusting them. Given the concern for the overestimation or underestimation of the true biomarker excretion rates in nonsteady states, indexing to UCr and UOsm should be only considered for clinical application of a subset of urine biomarkers, such as KIM-1, IL-18, and MCP-1.
For UMOD, indexing to UCr and UOsm significantly blunted its association with CKD. This may be caused by mathematical issues in the regression model when forcing 2 markers into 1 ratio, or, in other words, including an interaction term (i.e., biomarker times 1/UCr or 1/UOsm).27,28 This is particularly problematic when the numerator (i.e., biomarker) is inversely associated with the outcome. The attenuation of UMOD-outcome association when using the UMOD-to-UCr ratio or UMOD-to-UOsm ratio was regardless of AKI status, highlighting the importance of careful consideration of the relationship between biomarker, UCr, or UOsm, and outcome, before using biomarker-to-UCr ratio or biomarker-to-UOsm ratio. With adjusting for UCr or UOsm, UMOD’s inverse association with and prediction for CKD are unchanged, suggesting adjustment may not be necessary for this biomarker in risk-prediction research and clinical practice.
During AKI, when steady-state creatinine or osmolyte excretion cannot be assumed, timed urine collection may be another approach to estimate the biomarker excretion rate, although this requires more coordination, may obscure rapidly changing levels, and may delay time-sensitive decision-making.29,30 The stability of biomarkers will also need to be ascertained.11 Another approach is to perform repeated biomarker measurements and account for urine volume within short periods of time; however, this would likely require an accurate measurement of urine volumes and the assumption of a constant biomarker production rate. In addition, it is not clear which metric (e.g., biomarker production rate calculated from timed collection or biomarker-to-UCr ratio or biomarker-to-UOSm ratio from spot samples) has a stronger association with the outcome or which should be used as a reference or gold standard.
Our study provided important insights regarding how and why different approaches to account for urine concentration result in the strengthening of urine biomarkers’ associations with CKD outcome. One of the limitations of our analysis was the lack of timed collection or repeated measurements of urine biomarkers; therefore, we are unable to compare our approaches to account for urine concentration against this as the reference. Another limitation was the lack of multivariable analysis with the adjustment of other covariates. This is because the goal of our study is to determine how different approaches to account for urine concentration alter the biomarker-outcome association, rather than to determine individual biomarkers’ associations with CKD. Biomarkers were collected within 96 hours of AKI diagnosis, which is relatively late considering the rapid increase of injury biomarkers within hours after insult. This may explain why the difference in biomarker levels in patients with and without AKI was not prominent. However, this would be unlikely to affect the interpretation of our results, as we showed that the strengthening of the biomarker-outcome association is largely from controlling for confounding from UCr or UOsm, which should be determined on a case-by-case basis for each biomarker-outcome combination. Owing to the variation in collection time, we cannot ensure homogeneity in patients with AKI regarding whether they were in the progressive or recovery phase of AKI. However, the wide window after AKI diagnosis suggests a generalizability of the findings to the clinical situation where ascertainment of AKI may be delayed. The associations of UCr, UOsm, biomarkers, and outcomes and the changes in these associations with different adjustment approaches may also not be generalizable to other clinical scenarios or biomarkers and would require validation. Last, whether other metrics, such as free water clearance, could serve as a surrogate for urine concentration, can be explored in future studies.
In conclusion, lower UCr and UOsm during hospitalizations of patients with AKI are associated with higher risk of developing CKD after discharge. This suggests that they may serve as indicators for other confounders associated with CKD, rather than surrogates for urine concentration. Ultimately, indexing or adjusting UCr or UOsm could be considered for certain biomarkers, such as urine KIM-1, MCP-1, and IL-18 to strengthen the biomarker-outcome association and enhance risk prediction, especially for patients with AKI.
Disclosure
JSK reports ownership interest from Amgen outside the submitted work; WBR reports ownership interest from Amgen outside the submitted work; ASK reports research funding from National Institute of Health, Amarin Pharmaceuticals, Bristol Myers Squibb, grants from CSL Behring, iRhythm Technologies, Janssen Research and Development, Novartis, outside the submitted work; EDS reports personal fees from Akebia Therapeutics, outside the submitted work; JH reports personal fees from Akebia Therapeutics, Chinook Therapeutics, Maze Therapeutics, Pfizer, RenalytixAI, Seattle Genetics, outside the submitted work; KDL reports research funding from National Institute of Health, personal fees from AM-Pharma, Biomerieux, BOA Medical, Durect, Seastar Medical, Amgen, outside the submitted work; PLK reports royalties from Elsevier - Co-Editor of Chronic Renal Disease and Co-Editor of Psychosocial Aspects of Chronic Kidney Disease; TAI reports research funding from National Institute of Health, personal fees from Abbott Renal Care, Fresenius Kabi, La Renon, Nestle, International Society of Nephrology, outside the submitted work; WBR reports ownership interest from Amgen outside the submitted work; CYH reports research funding from National Institute of Health, Satellite Healthcare, outside the submitted work; CRP is a member of the advisory board of and owns equity in RenalytixAI and serves as a consultant for Genfit and TriCeda. DGM and CRP are named co-inventors on pending patent “Methods and Systems for Diagnosis of Acute Interstitial Nephritis.” All the other authors declared no competing interests.
Acknowledgments
Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury study was supported by cooperative agreements from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (U01DK082223, U01DK082185, U01DK082192, U01DK082183, R01DK098233, R01DK101507, R01DK114014, K23DK100468, R03DK111881). DGM is also supported by National Institutes of Health grants K23DK117065 and R01DK12681. CRP was supported by the National Institutes of Health grants R01DK-93770 and R01HL-085757. CYH was supported by the National Institutes of Health grant R01 DK114014. DGM is also supported by K23DK117065 and R01DK12681. CRP, JH, and DGM also participate in the National Institute of Diabetes and Digestive and Kidney Diseases–sponsored Kidney Precision Medicine Project UH3DK114866. The opinions expressed herein do not necessarily represent those of the National Institute of Diabetes, Digestive and Kidney Diseases, the National Institutes of Health, the Department of Health and Human Services, or the government of the United States of America.
Author Contributions
YW, CYH, HTP, and CRP designed the study. YW and HTP carried out statistical analyses. YW, CYH, HTP, PLK, JSK, WBR, NG, TAI, ASG, KDL, EDS, JH, DGM, and CRP drafted and revised the manuscript. All authors approved the final version.
Footnotes
Figure S1. (A) Hazard ratio of urine IL-18, MCP-1, NGAL and YKL-40 collected during hospitalization in AKI patients with composite CKD outcome using different approaches to control for urine concentration. (B) Hazard ratio of urine IL-18, MCP-1, NGAL and YKL-40 collected during hospitalization in non- AKI patients with composite CKD outcome using different approaches to control for urine concentration.
Figure S2. (A) Hazard ratio of urine biomarkers collected three months after discharge in AKI patients with composite CKD outcome using different approaches to control for urine concentration. (B) Hazard ratio of urine biomarkers collected three months after discharge in non-AKI patients with composite CKD outcome using different approaches to control for urine concentration.
Figure S3. (A) Hazard ratio of urine biomarkers collected during in subgroup of stage 2-3 AKI patients with composite CKD outcome using different approaches to control for urine concentration. (B) Hazard ratio of urine biomarkers collected three months after discharge in subgroup of stage 2-3 AKI patients with composite CKD outcome using different approaches to control for urine concentration.
Figure S4. Direct Acyclic Graph Depicting the Conceptual Framework of Urine Creatinine and Urine Osmolarity as Confounders rather than Surrogates for Urine Concentration in Investigating Etiological Relationship between Urine Biomarkers and Outcomes.
Table S1. Biomarker Measurement Details.
Table S2. Kidney Function at Different Study Timepoints in Participants Stratified by AKI and Baseline CKD Status.
Table S3. Predictive Performance of biomarkers for 3-year composite CKD outcomes using different approaches to account for urine concentration.
STROBE statement.
Supplementary Material
Figure S1. (A) Hazard ratio of urine IL-18, MCP-1, NGAL and YKL-40 collected during hospitalization in AKI patients with composite CKD outcome using different approaches to control for urine concentration. (B) Hazard ratio of urine IL-18, MCP-1, NGAL and YKL-40 collected during hospitalization in non- AKI patients with composite CKD outcome using different approaches to control for urine concentration.
Figure S2. (A) Hazard ratio of urine biomarkers collected three months after discharge in AKI patients with composite CKD outcome using different approaches to control for urine concentration. (B) Hazard ratio of urine biomarkers collected three months after discharge in non-AKI patients with composite CKD outcome using different approaches to control for urine concentration.
Figure S3. (A) Hazard ratio of urine biomarkers collected during in subgroup of stage 2-3 AKI patients with composite CKD outcome using different approaches to control for urine concentration. (B) Hazard ratio of urine biomarkers collected three months after discharge in subgroup of stage 2-3 AKI patients with composite CKD outcome using different approaches to control for urine concentration.
Figure S4. Direct Acyclic Graph Depicting the Conceptual Framework of Urine Creatinine and Urine Osmolarity as Confounders rather than Surrogates for Urine Concentration in Investigating Etiological Relationship between Urine Biomarkers and Outcomes.
Table S1. Biomarker Measurement Details.
Table S2. Kidney Function at Different Study Timepoints in Participants Stratified by AKI and Baseline CKD Status.
Table S3. Predictive Performance of biomarkers for 3-year composite CKD outcomes using different approaches to account for urine concentration.
STROBE statement.
References
- 1.Hsu Cy, McCulloch C.E., Fan D., Ordoñez J.D., Chertow G.M., Go A.S. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca S.G., Yusuf B., Shlipak M.G., Garg A.X., Parikh C.R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla L.S., Kimmel P.L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 4.Han W.K., Bailly V., Abichandani R., Thadhani R., Bonventre J.V. Kidney injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 5.Moledina D.G., Isguven S., McArthur E., et al. Plasma monocyte chemotactic protein-1 is associated with acute kidney injury and death after cardiac operations. Ann Thorac Surg. 2017;104:613–620. doi: 10.1016/j.athoracsur.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puthumana J., Hall I.E., Reese P.P., et al. YKL-40 associates with renal recovery in deceased donor kidney transplantation. J Am Soc Nephrol. 2017;28:661–670. doi: 10.1681/ASN.2016010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belcher J.M., Sanyal A.J., Peixoto A.J., et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622–632. doi: 10.1002/hep.26980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49:S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg J.M., Chang B.S., Matarese R.A., Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309:1543–1546. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- 10.Carter C.E., Gansevoort R.T., Scheven L., et al. Influence of urine creatinine on the relationship between the albumin-to-creatinine ratio and cardiovascular events. Clin J Am Soc Nephrol. 2012;7:595–603. doi: 10.2215/CJN.09300911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waikar S.S., Sabbisetti V.S., Bonventre J.V. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486–494. doi: 10.1038/ki.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagshaw S.M., Langenberg C., Bellomo R. Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48:695–705. doi: 10.1053/j.ajkd.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Noto A., Cortegiani A., David A. NephroCheck: should we consider urine osmolality? Crit Care. 2019;23:48. doi: 10.1186/s13054-019-2341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadkarni G.N., Patel A., Simoes P.K., et al. Dialysis-requiring acute kidney injury among hospitalized adults with documented hepatitis C virus infection: a nationwide inpatient sample analysis. J Viral Hepat. 2016;23:32–38. doi: 10.1111/jvh.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koopman J.J.E., Scherzer R., Ix J.H., Shlipak M.G., Waikar S.S. A comparison of different estimates of albuminuria in association with mortality in epidemiologic research. Clin J Am Soc Nephrol. 2020;15:1814–1816. doi: 10.2215/CJN.07290520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu C.Y., Chinchilli V.M., Coca S., et al. Post-acute kidney injury proteinuria and subsequent kidney disease progression: the assessment, serial evaluation, and subsequent sequelae in acute kidney injury (ASSESS-AKI) study. JAMA Intern Med. 2020;180:402–410. doi: 10.1001/jamainternmed.2019.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go A.S., Parikh C.R., Ikizler T.A., et al. The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: design and methods. BMC Nephrol. 2010;11:22. doi: 10.1186/1471-2369-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu K.D., Siew E.D., Reeves W.B., et al. Storage time and urine biomarker levels in the ASSESS-AKI study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M.J., Chang T.I., Lee J., et al. Urine osmolality and renal outcome in patients with chronic kidney disease: results from the KNOW-CKD. Kidney Blood Press Res. 2019;44:1089–1100. doi: 10.1159/000502291. [DOI] [PubMed] [Google Scholar]
- 21.Hebert L.A., Greene T., Levey A., Falkenhain M.E., Klahr S. High urine volume and low urine osmolality are risk factors for faster progression of renal disease. Am J Kidney Dis. 2003;41:962–971. doi: 10.1016/s0272-6386(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 22.Wen Y., Parikh C.R. Current concepts and advances in biomarkers of acute kidney injury. Crit Rev Clin Lab Sci. 2021;58:354–368. doi: 10.1080/10408363.2021.1879000. [DOI] [PubMed] [Google Scholar]
- 23.Kirita Y., Wu H., Uchimura K., Wilson P.C., Humphreys B.D. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci U S A. 2020;117:15874–15883. doi: 10.1073/pnas.2005477117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers J.A., Rassen J.A., Gagne J.J., et al. Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol. 2011;174:1213–1222. doi: 10.1093/aje/kwr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cirillo M., Laurenzi M., Mancini M., Zanchetti A., De Santo N.G. Low muscular mass and overestimation of microalbuminuria by urinary albumin/creatinine ratio. Hypertension. 2006;47:56–61. doi: 10.1161/01.HYP.0000197953.91461.95. [DOI] [PubMed] [Google Scholar]
- 26.Levey A.S., Berg R.L., Gassman J.J., Hall P.M., Walker W.G. Creatinine filtration, secretion and excretion during progressive renal disease. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney Int Suppl. 1989;27:S73–S80. [PubMed] [Google Scholar]
- 27.Curran-Everett D. Explorations in statistics: the analysis of ratios and normalized data. Adv Physiol Educ. 2013;37:213–219. doi: 10.1152/advan.00053.2013. [DOI] [PubMed] [Google Scholar]
- 28.Jasienski M., Bazzaz F. The fallacy of ratios and the testability of models in biology. Oikos. 1999;84:321–326. [Google Scholar]
- 29.Han W.K., Wagener G., Zhu Y., Wang S., Lee H.T. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang K.W.A., Toh Q.C., Teo B.W. Normalisation of urinary biomarkers to creatinine for clinical practice and research—when and why. Singapore Med J. 2015;56:7–10. doi: 10.11622/smedj.2015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.