See Clinical Research on Page 1565
Anemia in a patient with chronic kidney disease is a multifactorial disorder associated with impaired quality of life, reduced energy, decreased exercise capacity, neurocognitive decline, and increased mortality risk. Anemia can be successfully managed with adequate oral or i.v. iron (Fe) supplementation and erythropoiesis-stimulating agent (ESA) administration.
Routine use of ESA, targeting hemoglobin (Hb) level between 10 and 12 g/dl, has markedly improved quality of life of patients with chronic kidney disease. However, a wide variation in individual response to ESA is often observed. Some patients do not respond well and need high dose of ESA. This subgroup of patients is considered as resistant or ESA hyporesponders. Administration of high doses of ESAs is a real concern as it had been associated with an increased risk of hypertension, arteriovenous access thrombosis, neoplasia, and mortality.1
The Kidney Disease: Improving Global Outcomes defines ESA hyporesponsiveness as the presence of at least 1 of the following: (i) when the recommended Hb target (10.0–11.5 g/dl) is not reached whatever the ESA molecule, whatever the dose, and whatever the route of administration; (ii) a significant decrease in the Hb level with a constant ESA dose; (iii) an increase in the ESA dose which leads to a dose >50% of that with which the Hb value was obtained within the recommended target; or (iv) failure of the Hb level to rise >11.0 g/dl despite an ESA dose that is equivalent to a recombinant human erythropoietin dose >500 IU/kg/wk or >30,000 IU</wk.2 The erythropoietin resistance index (ERI), calculated by dividing the weekly adjusted dose of recombinant human erythropoietin (expressed in IU/kg/wk) by the Hb level, is an alternative method to assess the degree of ESA hyporesponsiveness, but it is not used in current clinical practice.3 Despite recent advances, the pathogenesis of ESA hyporesponsiveness is not completely understood and involves numerous factors, including demographic variables such as age and sex distribution, morbidity pattern such as inadequate dialysis, absolute or functional Fe deficiency, malnutrition, renal osteodystrophy, chronic inflammation, and dialysis modalities.2 Other factors with less strong but with promising clinical implications had also been evocated. The role of non-Fe micronutrient such as folates, vitamin C, and vitamin B12 deficiency is frequently underestimated. For example, vitamin C increases Hb synthesis by facilitating incorporation of Fe into protoporphyrins and Fe availability by the reticuloendothelial system.4 Moreover, the use of vitamin C may reduce oxidative stress and inflammation. Most of these micronutrients are eliminated to a various range during hemodialysis and, thus, could exacerbate the anemia and the hyporesponsiveness to ESA.5
A special interest is given to a trace element, in particular selenium. As a matter of fact, selenium is an essential trace element with antioxidative properties, being a component of selenoproteins, including selenoenzymes such as glutathione peroxidase, selenoprotein-P, and thioredoxin reductase. In nonchronic kidney disease population, low selenium levels had been associated with various neurologic disorders, a compromised immune function, and an increased risk of mortality and in pregnant women with pre-eclampsia and preterm delivering.6 As the thyroid is the gland with the highest selenium concentration of all tissues, low selenium levels are associated with higher incidence of thyroid cancer and Hashimoto’s thyroiditis.1 Of interest, selenium deficiency may also lead to left ventricular dilation and heart failure, especially in patients with AIDS.4
Evidence from animal and human studies suggests a link between circulating low selenium levels and anemia. Selenium regulates erythropoiesis by preventing oxidative stress-mediated hemolysis as found in a mouse model.7 In elderly people and in patients with chronic kidney disease, several studies have found an association between low selenium levels and anemia.8 The link of selenium deficiency and ESA hyporesponsiveness is worth studying as not many evidences are available.
In this current issue of the KI Reports, Yasukawa et al.9 investigated the association between serum selenium levels and the ERI in a cross-sectional study of hemodialysis patients. They performed a single measurement of selenium levels and Fe parameters in 173 patients from 4 hemodialysis facilities in Japan. Most patients were on hemodiafiltration and had stable dose of ESA during the previous 6 months of the study. Consistently with previous studies, serum selenium levels were low (<10.5 μg/dl for normal values ranging from 70 to 150 μg/dl) in half of the patients. Only 33 patients (19%) had serum selenium levels > 12.2 μg/dl, which is associated with good prognosis and does not require any supplementation.1
The authors observed a significant inverse correlation between serum selenium levels and ERI but not with Hb, transferrin saturation, or ferritin. The independent association between serum selenium levels and ERI (>9.44 in this cohort) was then confirmed by multiple regression analyses, after adjustment by potential confounding factors (gender, cardiovascular disease, dialysis vintage, ferritin, transferrin saturation, albumin, C-reactive protein, zinc, and parathyroid hormone levels). A similar trend was observed when the authors repeated these tests with a higher ERI threshold (>15) that is associated with the worst clinical outcomes in a European population. Finally, by performing sensitivity analysis using distinct criteria for Fe status (transferrin saturation <30% and ferritin <500 ng/ml), the authors consistently found significant difference across 4 groups divided by the Fe status and serum selenium levels.
The principal limitation of this study is its cross-sectional design, and even though the reported within-person coefficient of variance for selenium was similar to that for calcium and potassium, the evolution of serum selenium levels and ERI kinetics was not assessed. Second, Fe deficiency management in Japan differs from that of the European and the Kidney Disease: Improving Global Clinical Practice Guidelines. Even if this study took into account these differences, the results must still be confirmed in an independent Western cohort.
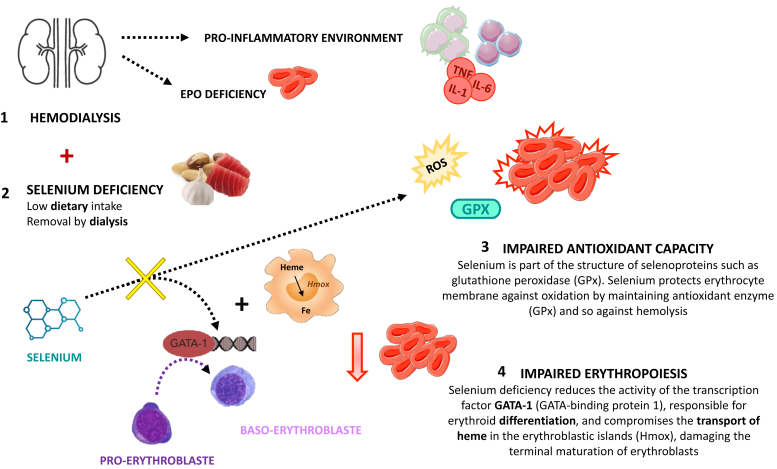
Finally, the mechanism that leads to ESA hyporesponsiveness could only be hypothesis driven (Figure 1). Previous data from hemodialysis patients suggest that low serum selenium levels contribute to increased oxidative stress and inflammation. Salehi et al.10 revealed in a controlled randomized trial that selenium supplementation in hemodialysis patients, probably mediated by inhibiting oxidative stress and inflammation, improved their nutritional status. In the present study, there was no measurement of oxidative stress markers, and thus, the potential pathophysiologic mechanisms remain unclear.
Figure 1.
Hypothesis-driven mechanism that leads to ESA hyporesponsiveness. ESA, erythropoiesis-stimulating agent; Fe, iron; IL, interleukin; ROS, reactive oxygen species.
This study is of clinical importance because it highlights a new therapeutic intervention for ESA hyporesponsiveness management. Tonelli et al.11 proved that selenium supplementation is feasible and safe among patients on hemodialysis, but that low or medium doses (50 and 75 μg/d, respectively) did not fully correct selenium status, suggesting higher doses (>100 μg/d) may be necessary. Nonetheless, selenium supplementation does not have a wide window of therapeutic index, and a U-shaped curve describes the link between selenium status and outcomes. Thus, selenium intake for people with poor nutritional status may benefit from supplementation, but people of high status (more specifically with selenium levels >122 μg/l) should not take selenium supplementation. Adverse effects of selenium toxicity include brittle hair and nails and their loss, dermatitis, and type 2 diabetes.2 This finding may indicate that serum selenium levels should be monitored in case of selenium supplementation. Further studies are necessary to find the safer and more effective way to selenium supplementation in hemodialysis patients.
Disclosure
All the authors declare no competing interests.
References
- 1.Phrommintikul A., Haas S.J., Elsik M., Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369:381–388. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 2.Bamgbola O.F. Pattern of resistance to erythropoietin-stimulating agents in chronic kidney disease. Kidney Int. 2011;80:464–474. doi: 10.1038/ki.2011.179. [DOI] [PubMed] [Google Scholar]
- 3.Panichi V., Rosati A., Bigazzi R., et al. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: results from the RISCAVID study. Nephrol Dial Transplant. 2011;26:2641–2648. doi: 10.1093/ndt/gfq802. [DOI] [PubMed] [Google Scholar]
- 4.Constans J., Sire S., Sergeant C., et al. Cardiomyopathie dilatée et déficit en sélénium au cours du SIDA. A propos d'un cas [Dilated cardiomyopathy and selenium deficiency in AIDS. Apropos of a case] Rev Med Intern. 1997;18:642–645. doi: 10.1016/s0248-8663(97)82466-6. [DOI] [PubMed] [Google Scholar]
- 5.Tarng D.C. Novel aspects of vitamin C in epoetin response. J Chin Med Assoc. 2007;70:357–360. doi: 10.1016/S1726-4901(08)70020-0. [DOI] [PubMed] [Google Scholar]
- 6.Eze S.C., Ododo N.A., Ugwu E.O., et al. Serum selenium levels of pre-eclamptic and normal pregnant women in Nigeria: A comparative study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur R., Ghanghas P., Rastogi P., Kaushal N. Protective role of selenium against hemolytic anemia is mediated through redox modulation. Biol Trace Elem Res. 2019;189:490–500. doi: 10.1007/s12011-018-1483-y. [DOI] [PubMed] [Google Scholar]
- 8.Semba R.D., Ricks M.O., Ferrucci L., et al. Low serum selenium is associated with anemia among older adults in the United States. Eur J Clin Nutr. 2009;63:93–99. doi: 10.1038/sj.ejcn.1602889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasukawa M, Arai S, Nagura M, et al. Selenium associates with response to erythropoiesis-stimulating agents in hemodialysis patients. Kidney Int Rep. 2022;7:1565–1574. doi: 10.1016/j.ekir.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salehi M., Sohrabi Z., Ekramzadeh M., et al. Selenium supplementation improves the nutritional status of hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Nephrol Dial Transplant. 2013;28:716–723. doi: 10.1093/ndt/gfs170. [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M., Wiebe N., Thompson S., et al. Trace element supplementation in hemodialysis patients: a randomized controlled trial. BMC Nephrol. 2015;16:52. doi: 10.1186/s12882-015-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]