Abstract
Background
Osteochondral lesions of the talus are common, particularly after trauma. Arthroscopic bone marrow stimulation has emerged as the first-choice surgical treatment for small primary lesions less than 100 mm2. Individual studies on the topic are small and heterogeneous, and they have differed in their main findings; for this reason, systematically reviewing the available evidence seems important.
Questions/purposes
In this systematic review, we asked: (1) What patient-reported outcomes and pain scores have been observed after arthroscopic bone marrow stimulation for secondary osteochondral lesions of the talus? (2) What complications were reported? (3) What demographic and clinical factors were reported to be associated with better patient-reported outcome scores?
Methods
We performed a systematic review according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines using Embase, EmCare, PubMed, CINAHL, and Scopus (databases last searched June 23, 2021). A two-stage title/abstract and full-text screening process was performed independently by two reviewers. Randomized control trials, cohort studies, and observational studies published in English that evaluated the outcome of arthroscopic bone marrow stimulation for secondary osteochondral lesions of the talus were included. Case reports, review articles, commentaries, abstracts, and letters to the editor were excluded. A total of 12 articles (10 case series and two retrospective comparative studies) involving 446 patients were included. Of these, 111 patients with a mean age of 33 years (range 20 to 49) received arthroscopic bone marrow stimulation for a secondary osteochondral lesion of the talus. The Methodological Index for Non-randomized Studies (MINORS) criteria were used to assess the methodologic quality of included studies. The MINORS is a numerical score ranging from 0 to 16 for studies with no comparison group and 0 to 24 for comparative studies, with higher quality studies receiving higher scores. Of the 10 noncomparative case series, the highest score was 10 of 16, with a median (range) score of 7.5 (4 to 10), while the two comparative studies scored 22 of 24 and 19 of 24, respectively.
Results
Studies varied widely in terms of patient-reported outcome measures such as the American Orthopaedic Foot and Ankle Society score (AOFAS), with inconsistent reporting across studies regarding whether or how much patients improved; there was variation in some effect sizes with regard to improvement seeming close to or below the minimum clinically important difference (MCID). Although no perioperative complications were reported in any included studies, 34% (26 of 77, in seven studies that reported on this endpoint) of patients who underwent a revision procedure. One study found a negative association between lesion size and AOFAS and VAS score. No other studies reported on factors associated with patient-reported outcome scores, and most studies were far too small to explore relationships of this sort.
Conclusion
We found that arthroscopic bone marrow stimulation for secondary osteochondral lesions of the talus yielded inconsistent and often small improvements in patient-reported outcomes, with approximately one in three patients undergoing a revision procedure. Reported outcomes likely represent a best-case scenario, inflated by low-level study designs and major sources of bias that are known to make treatment effects seem larger than they are. Therefore, the use of arthroscopic bone marrow stimulation in such patients cannot be recommended, unless we are able to refine selection criteria to effectively identify patients who show a substantial clinical benefit.
Level of Evidence
Level IV, therapeutic study.
Introduction
Osteochondral lesions of the talus occur relatively commonly, with recent research suggesting an increasing incidence [22]. These lesions often occur after ankle trauma, and they occur most often in people between 20 and 30 years of age [18, 47]. Authors agree that nonoperative treatment should be the initial strategy; however, with this approach, symptoms persist in up to 55% of patients [32, 46]. Various surgical interventions have been described, including bone marrow stimulation (drilling, microfracture, and debridement), autologous chondrocyte implantation, osteochondral transplantation, autologous matrix-induced chondrogenesis, vascular bone grafting, ankle replacement, and arthrodesis [8,31,32]. Arthroscopic bone marrow stimulation is often the treatment of choice in small lesions less than 100 mm2, with good improvements in clinical outcome scores such as the American Orthopaedic Foot and Ankle Society (AOFAS) score and Foot and Ankle Ability Measure [8].
However, although there have been many studies on primary lesions, studies focusing on secondary lesions (primary lesions that remain painful after surgery to a degree that necessitates further surgical intervention) are often small, highly heterogenous, and report contrasting findings. Because of this, it is important to systematically review the best available evidence to develop a more coherent understanding of the support for arthroscopic bone marrow stimulation for secondary osteochondral lesions of the talus. Important factors to be considered in clinical decision making and managing patient expectations include outcomes in terms of patient-reported outcome measures, complications, and clinical and demographic factors that may be associated with improved patient-reported outcome measures.
Although a systematic review of arthroscopic bone marrow stimulation for secondary lesions has recently been performed [7], we believe there is sufficient justification for another review. The previous review pooled outcomes from five studies (one randomized controlled trial, one retrospective comparative study, and three case series studies), finding that the overall success proportion of arthroscopic bone marrow stimulation for nonprimary osteochondral lesions of the talus was 61%. Pooling results from such heterogenous studies is not recommended because of the inherent risk of bias [13]. Furthermore, studies with fewer than five patients were excluded from the previous review. We believe it is important to include these smaller studies as they add further data to what is currently an under-researched field [10, 35, 42].
The current systematic review therefore aimed to answer the following questions: (1) What patient-reported outcomes and pain scores have been observed after arthroscopic bone marrow stimulation for secondary osteochondral lesions of the talus? (2) What complications were reported? (3) What demographic and clinical factors were reported to be associated with better patient-reported outcome scores?
Materials and Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews and meta-analyses [19].
Search Strategy
An electronic search was performed using PubMed (from inception to June 2021), Embase (from inception to June 2021), EmCare (from inception to June 2021), CINAHL (from inception to June 2021), and Scopus (from inception to June 2021). Free text and MeSH terms including “osteochondral,” “transchondral,” “chondral,” “cartilage,” “defect,” “lesion,” “talus,” “talar,” “osteochondritis dissecans,” “BMS,” “bone marrow stimulation,” “debridement,” “dril*,” and “microfracture” were combined using Boolean operations (“and” and “or”). All electronic searches were performed on June 23, 2021. Manual hand searching of the reference lists of included studies was performed, and the grey literature (including preprints, dissertations, and theses) was not included.
Study Selection and Data Collection
Studies were imported into Mendeley reference management software (Elsevier) to detect and remove duplicates, and studies were screened using the Rayyan Systematic Reviews internet application (Rayyan Systems Inc) [23]. Two reviewers (ZA, AA) independently screened titles, abstracts, and full-text articles, according to predetermined selection criteria (Table 1):
Population: Patients with osteochondral defects of the talus were included. Studies exclusively reporting the treatment of patients with juvenile osteochondritis dissecans of the talus were excluded.
Intervention: Any arthroscopic bone marrow stimulation technique was included.
Comparators: The presence of a control group was not a criterion for inclusion in this review. Studies with any or no control groups were included.
Outcomes: Studies reporting any patient-reported outcome measure or pain score, such as the AOFAS score and VAS score.
-
Language: Only studies published in English were included.
Date of publication: No date of publication restriction was imposed.
Study design: Randomized control trials, cohort studies, and observational studies (case series or case control) were included. Case reports, review articles, commentaries, abstracts, and letters to the editor were excluded.
Table 1.
Results of the MINORS critical appraisal process
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Total |
| Anders et al. [1] | 2 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | NA | NA | NA | NA | 6 of 16 |
| Becher and Thermann [3] | 2 | 2 | 2 | 2 | 0 | 0 | 1 | 0 | NA | NA | NA | NA | 9 of 16 |
| Chuckpaiwong et al. [6] | 2 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | NA | NA | NA | NA | 7 of 16 |
| Desai [10] | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | NA | NA | NA | NA | 4 of 16 |
| Ogilvie-Harris and Sarrosa [21] | 0 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | NA | NA | NA | NA | 8 of 16 |
| Rikken et al. [30] | 2 | 0 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 19 of 24 |
| Robinson et al. [33] | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | NA | NA | NA | NA | 5 of 16 |
| Savva et al. [34] | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | NA | NA | NA | NA | 10 of 16 |
| Schimmer et al. [35] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | NA | NA | NA | NA | 8 of 16 |
| Schuman et al. [36] | 0 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | NA | NA | NA | NA | 8 of 16 |
| van Bergen et al. [42] | 2 | 0 | 1 | 2 | 0 | 2 | 0 | 0 | NA | NA | NA | NA | 7 of 16 |
| Yoon et al. [44] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 22 of 24 |
Numbers 1 to 12 in the first row refer to the equivalent item numbers in the MINORS criteria; MINORS involves 12 items to be assessed, of which the first eight apply to noncomparative studies with four additional items applicable to comparative studies; each item receives a score of 0 (not reported), 1 (reported but not adequate) or 2 (reported and adequate), which leaves a maximum score of 16 for noncomparative studies and 24 for comparative studies, with higher scores representing better study quality.
When studies did not specify whether included patients were treated for primary or secondary osteochondral lesions of the talus, we contacted the corresponding author and asked them to provide specific data for patients with a secondary osteochondral lesion of the talus. We also contacted authors for further information regarding the characteristics of patients with secondary lesions if this was not reported separately from primary osteochondral lesions of the talus.
Any differences in opinion regarding study inclusion were raised and discussed. When there were discrepancies or when no consensus was reached, a third author (MB) was consulted.
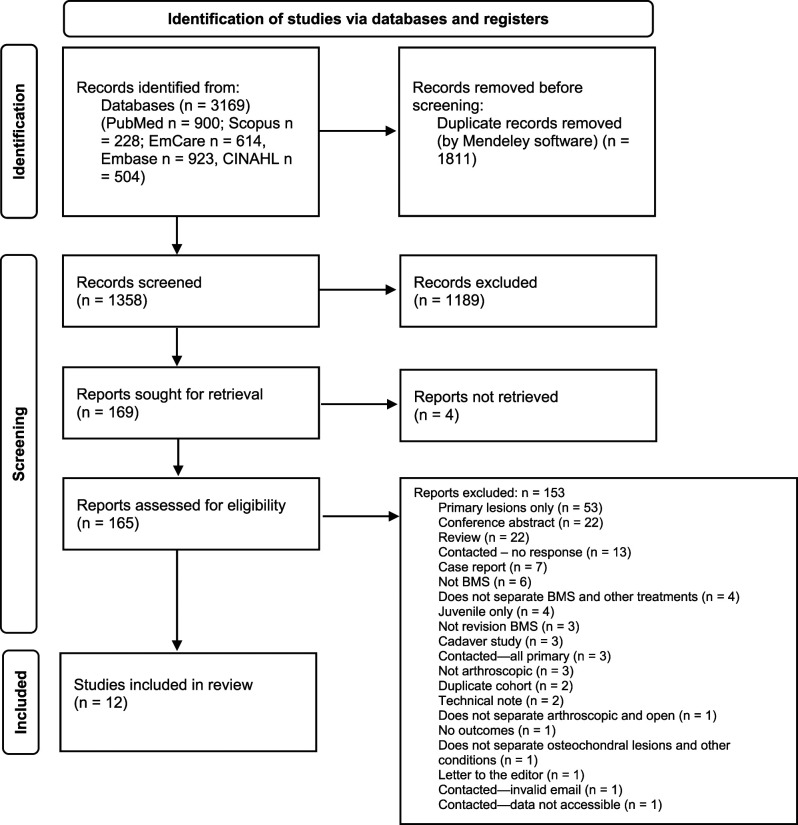
A total of 1358 articles were identified, 0.88% (12) of which were included in the final review [1, 3, 6, 10, 21, 30, 33–36, 42, 44] (Fig. 1). The total number of patients was 446 (450 ankles); 111 patients (111 ankles) received arthroscopic bone marrow stimulation for a secondary osteochondral lesion of the talus (Table 2).
Fig. 1.
This PRISMA flow diagram shows the total number of studies retrieved and excluded at each screening stage.
Table 2.
Summary of the characteristics of all studies
| Authors | Year | Design | Total number of lesions | Number of secondary lesions | Age, in years | Men:women | Size, in mm2 | Follow-up, in months |
| Anders [1]ab | 2012 | Case series | 38 (41 feet) | 7 | 25 (18-38) | 3:4 | 9 (7-14) (diameter) | 29 (12-54) |
| Becher and Thermann [3]a | 2005 | Case series | 30 | 2 | 23.5 (20-27) | 1:1 | 24 (22-27) | |
| Chuckpaiwong et al. [6]c | 2008 | Case series | 105 | 17 | 44.1 ± 9.7 | 21:10 | 21.6 ± 3.0 | 31.6 ± 12.1 |
| Desai [10]a | 2014 | Case series | 9 | 4 | 36.3 (23-49) | 115.8 (42-190) | 12 (7-15) | |
| Ogilvie-Harris and Sarrosa [21] | 1999 | Case series | 8 | 8 | 33 (22-42) | 4:4 | 38 (24-63) | |
| Rikken et al. [30] | 2021 | Retrospective comparative | 34 | 12 | 31.3 ± 8.3 | 8:4 | 102.8 | 12 |
| Robinson et al. [33]c | 2003 | Case series | 65 | 8 | 34.3 (14.1-72.4) | 46:19 | 44 (6-99) | |
| Savva et al. [34] | 2007 | Case series | 12 | 12 | 31.3 (21-49) | 7:5 | 78.6 (25-120) | 80.5 (36-95) |
| Schimmer et al. [35]c | 2001 | Case series | 35 (36 feet) | 3 | 41 (19-78) | 24:11 | 153.6 (120-192) | |
| Schuman et al. [36] | 2002 | Case series | 38 | 16 | 24.3 (16-38) | 7:9 | 66 (24-129) | |
| van Bergen et al. [42] | 2014 | Case series | 50 | 4 | 29.8 (21-34) | 2:2 | 144 (96-240) | |
| Yoon et al. [44] | 2014 | Retrospective comparative | 44 | 44 (22 BMS) | 41.6 ± 13.2 | 18:4 | 138.5 | 50 (24-116) |
Where possible, age, men:women ratio, lesion size, and follow-up period are reported specifically for secondary procedures.
In these studies, the mean follow-up value was derived from the whole cohort, rather than patients with secondary lesions specifically.
In this study, the mean lesion size was derived from the whole cohort, rather than patients with secondary lesions specifically.
These studies did not separate these study characteristics according to the nature of the lesion (primary or secondary); all data presented in these studies therefore refer to the whole study group, rather than patients with secondary lesions specifically. The data presented for the study of Chuckpaiwong et al. [6] are derived from 31 patients with an unsuccessful initial outcome, 17 of whom underwent secondary bone marrow stimulation.
Study Characteristics
Of the 12 included studies, 10 [1, 3, 6, 10, 21, 33–36, 42] were Level IV case series and two [30, 44] were Level III retrospective comparative studies. One retrospective comparative study [30] derived its study population from a previous randomized controlled trial [29]. Three studies [21, 34, 44] exclusively reported the outcomes of secondary lesions, and the remaining nine [1, 3, 6, 10, 30, 33, 35, 36, 42] described the treatment of both primary and secondary lesions. Nine studies reported the mean age of patients receiving secondary bone marrow stimulation [1, 3, 10, 21, 30, 34, 36, 42, 44], in patients with a mean age of 32.2 years (16 to 49).
Various bone marrow stimulation techniques were described in included studies (Table 3). All data provided in this table refer to patients with secondary lesions only. However, in several instances, it was not possible to separate data between primary and secondary lesions, despite attempts to contact authors. The study of Rikken et al. [30] is derived from a previous randomized control trial [29] comparing bone marrow stimulation with and without the addition of pulsed electromagnetic fields. No difference in outcome was reported in these two groups, and so patients from both were merged to form the cohort of Rikken et al. [30]. Three studies [10, 30, 34] reported a mean lesion area between 25 mm2 and 190 mm2 specifically for secondary lesions (Table 3). Included studies described an active rehabilitation regimen involving a short period of partial or nonweightbearing followed by physiotherapy ROM and strengthening exercises (Table 3).
Table 3.
Further study characteristics including etiology, primary and secondary treatment, postoperative rehabilitation protocol, lesion classification, lesion size, time between primary and secondary procedure, symptom duration, and number of cystic lesions
| Study | Etiology | Primary treatment | Secondary treatment | Postoperative rehabilitation protocol | Lesion classification | Location | Time between procedures, in months | Symptom duration, in months | Cysts |
| Anders et al. [1] | NR | Arthroscopic drilling and cancellous bone grafting | Arthroscopic drilling and cancellous bone grafting | 6 weeks partial weightbearing | Berndt-Hardy 5 grade 1, 2 grade 2 |
6 medial, 1 central | NR | NR | NR |
| Becher and Thermann [3] | 1/2 traumatic | Anterograde drilling | Arthroscopic microfracture | Continuous passive motion 6-8 hours a day for 4-6 weeks. Physiotherapy for isometric exercises and weightbearing limited to 15 kg for 4 weeks. | Berndt-Hardy: 1 Grade 3, 1 Grade 4 |
1 lateral, 1 medial | NR | NR | NR |
| Chuckpaiwong et al. [6] | NR | Arthroscopic microfracture | Arthroscopic microfracture | Splint for 1-2 weeks and then followed by full weightbearing in boot. ROM exercises after 2 weeks | NR | NR | 7.2 | NR | NR |
| Desai [10] | NR | NR | Arthroscopic debridement and unspecified bone marrow stimulation technique with micronized allograft cartilage matrix | Nonweightbearing for 6 weeks. 1 week in splint followed by 2 weeks in cast and 3 weeks in boot | Ferkel: 3 Grade F, 1 Grade E | 2 medial talar dome, 2 lateral talar dome | NR | NR | 2 |
| Ogilvie-Harris and Sarrosa [21] | NR | Open arthrotomy | Arthroscopic debridement and abrasion | Compression bandage for 1 week Weightbearing as tolerated with physiotherapy No impact activities for 12 weeks |
NR | NR | 35 (12-62) | NR | NR |
| Rikken et al. [30] | 8/12 traumatic | NR | Arthroscopic debridement and microfracture Unspecified number also had pulsed electromagnetic field | Partial weightbearing as tolerated, followed by full weightbearing at 6 weeks Physiotherapy provided | Berndt-Hardy: 1 Grade 1, 2 Grade 2, 9 Grade 5 | 1 anteromedial, 2 centeromedial, 3 posteromedial, 1 anterolateral, 2 centerolateral, 1 posterolateral, 1 anterocentral, 1 centerocentral | 31.9 ± 22.8 | NR | 7/12 |
| Robinson et al. [33] | NR | Arthroscopic debridement and curettage | Arthroscopic debridement and curettage | Partial weight bearing for 6 weeks, with physiotherapy to mobilise ankle and subtalar joint. | NR | NR | NR | NR | NR |
| Savva et al. [34] | 8/12 traumatic | Arthroscopic debridement | Arthroscopic debridement | Partial weightbearing for 10 days, then weightbearing as tolerated Active rehabilitation with physiotherapy |
Berndt-Hardy: 12 Grade 2 | 6 medial, 6 lateral | 21.8 (8-58) | NR | 0 |
| Schimmer et al. [35] | NR | Arthroscopic drilling | Arthroscopic drilling | Partial weightbearing for 3-5 days followed by weightbearing as tolerated Active exercise program |
NR | NR | NR | NR | NR |
| Schumann et al. [36] | 10/16 traumatic | NR | Arthroscopic debridement and drilling | Nonweightbearing for 6 weeks | NR | 12 medial, 4 lateral | NR | 22.8 | NR |
| van Bergen et al. [42] | 2/4 traumatic | Arthroscopic debridement and drilling | Arthroscopic debridement and drilling | Partial or nonweightbearing for 2-8 weeks, Active ankle motion exercises | Berndt-Hardy: 4 Stage 5 | 3 medial,1 lateral | 51.8 (6-108) | 72.3 (30-144) | NR |
| Yoon et al. [44] | 14/22 traumatic | 9 microfracture 13 abrasion arthroplasty |
Microfracture and abrasion arthroplasty | Partial weightbearing for 4 weeks Physiotherapy for range of motion and strengthening exercised No impact activities for 12 weeks |
Ferkel: majority Grade D-F | 18 medial, 4 lateral 6 anterior, 8 middle, 8 posterior |
NR | 82.2 ± 44.6 | 14/22 |
NR = not reported.
Data Extraction
We created a data extraction spreadsheet to collect the following data: authors, year, total number of patients, number of secondary lesions, age, men:women ratio, etiology, lesion size, classification, lesion location, secondary treatment, time between the first and second procedure, cysts, comorbidities, previous procedures, symptom duration, outcome scores, pain score, complications or revision, and follow-up period.
Assessment of Methodologic Quality and Risk of Bias
We used the Methodological Index for Non-randomized Studies (MINORS) instrument to assess the methodologic quality and internal and external validity of all included studies (Table 1) [40]. MINORS involves 12 items to be assessed, of which the first eight apply to noncomparative studies with four additional items applicable to comparative studies. Each item receives a score of 0 (not reported), 1 (reported but not adequate) or 2 (reported and adequate). This leaves a maximum score of 16 for noncomparative studies and 24 for comparative studies, with higher scores representing better study quality. One reviewer (ZA) assessed quality. Of the 10 noncomparative case series, the highest score was 10 of 16 (Table 1), with a median (range) score of 7.5 (4 to 10), and the two comparative studies scored 22 of 24 and 19 of 24, respectively (Table 1).
Primary and Secondary Study Outcomes
We used a qualitative thematic results synthesis, focusing separately on key study outcomes related to the three review questions. The primary goal of this review was to systematically identify and review patient-reported outcomes measures reported after arthroscopic bone marrow stimulation for secondary osteochondral lesions of the talus. To assess this, we extracted data regarding patient-reported outcome measures and pain scores, comparing postoperative scores with preoperative scores where possible (Table 4).
Table 4.
Outcomes described by included studies, according to various scoring systems
| Study | Outcome metric | Preoperative score | Postoperative score | p value |
| Anders et al. [1] | AOFAS | 43.3 ± 16.1 | 64.6 ± 19.3 | NR |
| VAS | 8 | 8 | NR | |
| Becher and Thermann [3] | VAS | NR | 8.5 (7-10) | NA |
| HSS | NR | Good in one patient, excellent in one | NA | |
| Chuckpaiwong et al. [6] | AOFAS | NR | Increased by 17.2 points, values not given | NR |
| Desai [10] | Subjective pain and functional limitation | NR | Three patients had excellent score, with no pain or functional limitation; one patient had a good score, with some pain | NA |
| Ogilvie-Harris and Sarrosa [21] | Ogilvie-Harris four-point scale: 1 = poor, 4 = excellent | Pain: 1.75 Swelling: 2.3 Stiffness: 2.5 Limp: 2.1 Activity: 1.88 |
Pain: 3.25 Swelling: 3.25 Stiffness: 3.5 Limp: 3.25 Activity: 3.5 |
Pain: 0.01 Swelling: 0.04 Stiffness: 0.01 Limp: 0.03 Activity: 0.02 |
| Rikken et al. [30] (All scores reported in this study are medians rather than means)a | AOFAS | 67 (46-69) | 87 (79.5-100) | < 0.01 |
| FAOS | 60.7 (50-71.4) | 67.9 (48.2-82.1) | 0.03 | |
| AAS | 7.5 (4-9) | 5 (4-8) | > 0.05 | |
| NRS | 8.5 (8-10) | 3 (1-4) | < 0.01 | |
| Robinson et al. [33] | Berndt-Hardy scale: good (no symptoms), fair (improved, but some disability), and poor (symptoms unchanged or resulted in a revision) | NR | Four patients had a fair score; four patients had a poor score | NA |
| Savva et al. [34] | AOFAS | Median 39.5 (28-67) | Median 85 (36-95) | NR |
| Schimmer et al. [35] | Subjective pain | NA | Patients continued to have pain and discomfort | NA |
| Schuman et al. [36] | Tegner score | 2.7 ± 1.1 | 5.6 ± 2.4 | NR |
| Ogilvie-Harris | NR | Three patients had an excellent score, nine patients had a good score, 1 had a fair score, three had a poor score | NR | |
| van Bergen et al. [42] | AOFAS | NR | 80 (75-88) | NA |
| Ogilvie-Harris | NR | Three patients had a good score, one had a fair score | NA | |
| Yoon et al. [44] | VAS | 5.86 ± 1.55 | 3.18 ± 1.05 (6 months); 5.27 ± 1.22 (final) | NR |
| AOFAS | 50.41 ± 83.7 | 83.7 ± 2.65 (6 months); 69.6 ± 10.59 (latest) | < 0.001 at latest follow-up |
The study of Rikken et al. [30] only reports specific p values in situations where the p value is < 0.05; in all other cases the authors wrote “not significant”, which according to their definition of statistical significance, indicates a p value > 0.05; AOFAS = American Orthopaedic Foot and Ankle Society score; HSS = Hospital for Special Surgery score; FAOS = Foot and Ankle Outcome Score; AAS = Ankle Arthritis Score; NRS = numeric rating scale; NR = not reported; NA = not applicable.
Secondary goals involved identifying complications reported after the procedure and clinical and demographic factors that may be associated with improved patient-reported outcomes measures. To assess this, we extracted data concerning perioperative complications and revision rate (Table 5), and prognostic factors are outlined in a narrative synthesis. For the purposes of our review, a revision was considered to be a complication; however, revisions have been reported separately to other perioperative complications (Table 5).
Table 5.
Complication and revision proportions reported by the included studies
| Study | Number of patients | Complications | Revision | Time to revision | Total complication and revision proportion |
| Anders et al.[1] | 7 | NR | 0 of 7 | NA | 0 of 7 |
| Chuckpaiwong et al. [6] | 17 | NR | 7 of 17 with ankle arthrodesis | NR | 7 of 17 |
| Desai [10] | 4 | 0 of 4 | NR | NA | 0 of 4 |
| Ogilvie-Harris and Sarrosa [21] | 8 | 0 of 8 | NR | NA | 0 of 8 |
| Rikken et al. [30] | 12 | 0 of 12 | 1 of 12 with a metal resurfacing implant | Within 1 year | 1 of 12 |
| Savva et al. [34] | 12 | NR | 1 of 12 with cartilage transplantation | NR | 1 of 12 |
| Schimmer et al. [35] | 3 | NR | 3 of 3 with ankle arthrodesis or replacement | NR | 3 of 3 |
| van Bergen et al. [42] | 4 | 0 of 4 | 0 of 4 | NR | 0 of 4 |
| Yoon et al. [44] | 22 | 0 of 22 | 14 of 22 with osteochondral autologous transplantation | NR | 14 of 22 |
NA = not applicable; NR = not reported.
Statistical Analysis
Unfortunately, we were unable to perform a meta-analysis, assessment of heterogeneity through calculation of the I2 statistic, or assessment of publication bias (using tools like funnel plots) because of small study sizes and inconsistent data reporting across included articles.
Results
Patient-reported Outcome Measures and Pain Scores
Studies varied widely in terms of patient-reported outcome measures such as AOFAS with inconsistent reporting across studies in terms of whether or how much patients improved, and some effect sizes in terms of improvement seeming close to or below the minimum clinically important difference (MCID). Of the five studies [1, 30, 34, 42, 44] describing postoperative mean or median AOFAS scores, four [30, 34, 42, 44] reported a mean or median score of at least 80 of 100 (with higher scores representing less pain and improved function), and one [1] reported a mean score of 64.6 [4] (Table 4). Three studies described a postoperative increase in the AOFAS of 20 points or more [1, 30, 34], and two studies reported increases of 19.2 and 17.2 points, respectively [6, 44]. Although the MCID for the AOFAS score has not been defined in this specific clinical context, values between 17 to 30.2 (depending on the statistical method used) have been established in hallux valgus surgery [5, 9]. When comparing this to the results reported here, most improvements were similar to or below the MCID.
A similar trend was seen with respect to VAS pain scores. Only two studies compared pre- and postoperative VAS scores [1, 44]: one reported no improvement [1] and the other reported a reduction of 2.68 points at 6 months postoperatively, worsening to a reduction of only 0.59, 12 months after the procedure [44] (where lower scores represent less pain) (Table 4). Again, this is very similar to or below the proposed MCIDs of between 1.4 to 3 points [16, 41]. Conversely, another study reported a 5.5 point decrease in numerical rating scale (NRS) pain score [30], which is higher than the suggested MCID of 2 [14].
Of the 12 included studies, only six [1, 21, 30, 34, 36, 44] describe both preoperative and postoperative patient-reported outcome measures. This makes it challenging to understand the impact of the intervention with respect to postoperative changes in these outcome measures in several studies.
Complications
No complications were reported in any of the five studies (comprising 50 patients in total) that evaluated perioperative complications in those with secondary lesions [10, 21, 30, 42, 44] (Table 5). Meanwhile, 34% (26 of 77) of patients in seven studies that reported on this endpoint [1, 6, 30, 34, 35, 42, 44] underwent a revision procedure (Table 5).
Factors Associated with Better Patient-reported Outcomes
One retrospective, comparative study [44] found that as lesion size increased, patients generally had poorer AOFAS and VAS scores at most recent follow-up (r = 0.46; p = 0.03). In that same study [44], no correlation was found with respect to patient age, gender, BMI, symptom duration, time between procedures, and type of defect (contained versus uncontained) against clinical outcomes. Ogilvie-Harris and Sarrosa [21] reported that all three patients with preoperative Kellgren-Lawrence Grade 2 osteoarthritis had restricted postoperative sports activity, and they concluded that “the finding of degenerative changes carries an adverse prognostic significance.” No other studies reported on factors associated with patient-reported outcomes scores, and most were far too small to explore relationships of this sort.
Discussion
Although arthroscopic bone marrow stimulation is the first-choice surgical treatment for small osteochondral lesions of the talus (100 mm2) in patients with severe, bothersome symptoms that persist despite reasonable nonsurgical interventions, studies reporting outcomes of the procedure in secondary lesions are small and heterogenous in terms of factors such as endpoints used, duration of follow-up and study methodology. We therefore performed a systematic review of the best-available evidence, with three clearly defined research questions, to facilitate a better understanding of the use of arthroscopic bone marrow stimulation in secondary lesions. We found that published studies have reported inconsistent results regarding whether or how much patients improved in terms of patient-reported outcome measures such as AOFAS and VAS pain scores. Any improvement described in terms of these scores was close to or below the MCID. We therefore argue against the use of arthroscopic bone marrow stimulation in patients with secondary osteochondral lesions of the talus.
Limitations
The main limitations of this review are because of the quality of the included studies, which were all Level IV case series or Level III retrospective, comparative studies, with small numbers of patients included (Table 2). Use of the MINORS criteria revealed a wide spectrum of methodologic quality, with most studies scoring poorly. The 10 comparative studies scored a median of 8, with only two of these studies scoring above 10 of 16 (Table 1), and the two comparative studies scored 22 of 24 and 19 of 24, respectively (Table 1). Several included studies suffered from important kinds of bias. For example, a large degree of assessment bias was introduced through the lack of a blinded evaluation of study outcomes. Yoon et al. [44] was the only study to describe the use of a blinded outcome evaluation. Selection bias was also present because the criteria used to determine which patients received secondary arthroscopic bone marrow stimulation and why they received this as opposed to alternative treatments or no treatment was often not reported. Furthermore, several studies did not report the follow-up period for patients specifically receiving secondary arthroscopic bone marrow stimulation. Among the studies that reported this information, short follow-up periods were noted, which may not be sufficient to detect all relevant harms or benefits associated with treatment. Only four of the included studies reported no loss to follow-up [10, 21, 34, 44], and the remaining studies either did not report this information or described loss to follow-up rates between 7.7% (1 of 13) and 44% (16 of 36). These follow-up related factors introduced transfer bias. Assessment bias, selection bias, and transfer bias all tend to make reported results appear better than they are, and so the findings here probably represent a best-case scenario of how this procedure performs.
Although the AOFAS score was by far the most commonly reported measure in the studies we included (Table 4), use of this score is associated with several shortcomings. First, the AOFAS score has not been validated for use in patients with osteochondral lesions of the talus. There is also a lack of a detailed theoretical framework for how and why the score was developed [27]. However, there is currently no validated outcome score for osteochondral lesions of the talus and due to the lack of reporting of other patient-reported outcome scores, authors, readers, and clinicians have limited choice regarding the use of this score in evaluation of treatment outcome. Furthermore, the AOFAS score has been shown to have inherently limited precision due to a small number of response intervals, which often result in skewed distributions with little resemblance to the underlying distribution of patient states [12]. Because of these limitations, the AOFAS now recommends against the continued use of this outcome measure [27]. Although effort has been made to include discussion of other patient-reported outcome scores, as mentioned, these are rarely reported. Therefore, despite these flaws, clinicians lack a better outcome score to use in the evaluation of treatment outcome, hence the inclusion of the AOFAS score in this review. However, readers should be aware that the AOFAS score may not provide the most precise representation of a patient’s level of pain and function and that it involves a combination of multiple concepts such as pain, gait abnormality, and alignment, some of which may not be entirely relevant to the treatment being evaluated.
This review includes some studies that describe fewer than five patients each with secondary bone marrow stimulation. Such small cohorts are prone to overestimating the true treatment benefit and hence, as described, care must be taken when interpreting increases in the patient-reported outcomes scores they describe. It was felt that in such an under-researched clinical context, with just over 100 patients’ reports in total described in published papers, inclusion of these smaller studies was justified to make readers aware of all patients whose data have been reported.
Finally, studies reporting on mixed cohorts of both male and female patients are included in this review. Findings derived from such mixed cohort studies may not be equally applicable to both studies. Unfortunately, it was not possible to compare results according to patient gender due to small study sizes and lack of studies separating results by gender.
Patient-reported Outcome Measures and Pain Scores
Included studies were heterogenous in terms of the specific patient-reported outcome measures reported and varied in terms of how much improvement was seen in these scores (Table 4). Half of the included studies did not report both preoperative and postoperative outcome measures, making it impossible to understand the effect of the intervention. Those that reported both scores described improvements which were mostly either very close to or below the MCID for the outcome measures used. It is important to consider these results in light of the low-quality studies from which they were derived. Because of the substantial biases involved, it is likely that the inconsistent and generally sub-MCID improvements represented an overestimation of the benefits of treatment, and therefore should be viewed as an optimistic best-case scenario. The improvements seen in patient-reported outcomes scores might also represent a placebo effect, since the treatment effect sizes often were small, the treatment is somewhat invasive, and the studies lacked control groups. The beneficial effect of placebo surgery is well documented in various surgical and medical conditions [11, 15, 17, 43]. For example, two randomized controlled trials reported that patients receiving placebo or sham surgery did not show a greater clinical improvement than those receiving arthroscopic partial meniscectomy and arthroscopic debridement for degenerative meniscal tears and knee osteoarthritis [20, 39]. Similar results were described in research comparing subacromial decompression and diagnostic arthroscopy (placebo) for shoulder impingement [2, 24, 25]. Furthermore, a systematic review of 53 trials found that 51% of these studies (27 trials) did not show actual surgery to be superior to a placebo procedure [43]. The minor improvements reported in this review might represent a placebo surgery effect, rather than a true benefit of the specific surgical procedures involved.
Although most of the inconsistent improvements reported by included studies are likely inflated by the factors described, there may be some patients who genuinely gain benefit from arthroscopic bone marrow stimulation for a secondary osteochondral lesion of the talus. Interestingly, a comparative study [44] found that patients in the bone marrow stimulation group showed fair results at 6 months of follow-up, with a mean AOFAS score of 83.7 ± 2.65, slightly higher than the score in the osteochondral transplantation group. However, results in the bone marrow stimulation group declined such that the mean AOFAS at the final follow-up of 50 months was 69.64 ± 10.59, a lower score than in the osteochondral transplant group. This implies a wide range of final scores and suggests that a specific subgroup of patients in the bone marrow stimulation group, rather than the whole group, showed a decline in outcomes over time. Identification of patient demographic and clinical factors that might be associated with outcomes is crucial, as this would allow physicians to effectively identify and treat patients for whom the procedure is likely to confer a real benefit.
Complications
No perioperative complications were reported by any studies included in this review. This compares well to more invasive alternative surgical treatments such as osteochondral autologous transplantations. For example, one study that compared bone marrow stimulation and osteochondral autologous transplantation [44] reported no perioperative complications in the former group, but reported pain at the harvest site (the knee), crepitation, and wound infection in 18% (4 of 22), 9% (2 of 22), and 4.5% (1 of 22) of patients in the latter group, respectively [44]. These complications are comparable to those assessed in the complications section of this review. However, our analysis reveals a very high proportion of patients underwent revision surgery (approximately 1 in 3), despite the fact that the follow-up was short. Again, because of the low-level evidence and high degree of bias involved, the actual proportion of patients who undergo revision is likely to be even higher. Given the inconsistent-to-modest improvements in patient-reported outcomes scores, the small (or absent) improvements in pain scores, and the high risk of revision surgery, we recommend against the use of arthroscopic bone marrow stimulation in patients with secondary lesions of the talus. Future studies may seek to further clarify and shed light on these concerns through the use of larger comparative studies.
Factors Associated with Better Patient-reported Outcomes
Only one study [44] demonstrated an association between any patient-related or clinical factor and treatment outcome, with that study reporting poorer outcomes in patients with larger lesions. Most research investigating factors associated with outcomes focused on primary lesions, and found an association between poorer outcomes and an increased lesion size, patient BMI, patient age, presence of cysts, deep lesions, uncontained lesions, the presence of a medial lesion uncovered by the medial malleolus, and syndesmosis widening [26, 28, 37, 38, 45]. Although primary and secondary lesions may share common prognostic factors, this is yet to be supported by comprehensive evidence.
Conclusion
This comprehensive systematic review found that arthroscopic bone marrow stimulation for secondary osteochondral lesions of the talus yielded inconsistent improvements in outcomes measures, generally below the MCID, with about one of three patients undergoing a revision procedure. Any apparent benefit reported after this procedure was likely to represent a best-case scenario, with results inflated by low-level study designs, a high degree of bias, and a possible surgical placebo effect. Therefore, the use of arthroscopic bone marrow stimulation cannot be recommended. Despite the likely inflation of treatment outcomes, a small subgroup of patients might show a genuine, substantial clinical improvement because of the procedure. However, arthroscopic bone marrow stimulation cannot be recommended until we can refine the selection criteria to effectively identify such patients.
Footnotes
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was not sought.
This work was performed at the University of Cambridge School of Clinical Medicine, Cambridge, UK.
Contributor Information
Aiman Aslam, Email: aa948@cam.ac.uk.
Adil M. Iqbal, Email: ami26@cam.ac.uk.
Maneesh Bhatia, Email: maneeshbhatia@yahoo.com.
References
- 1.Anders S, Lechler P, Rackl W, Grifka J, Schaumburger J. Fluoroscopy-guided retrograde core drilling and cancellous bone grafting in osteochondral defects of the talus. Int Orthop. 2012;36:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard DJ, Rees JL, Cook JA, et al. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): a multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet. 2018;391:329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becher C, Thermann H. Results of microfracture in the treatment of articular cartilage defects of the talus. Foot Ankle Int. 2005;26:583-589. [DOI] [PubMed] [Google Scholar]
- 4.Ceccarelli F, Calderazzi F, Pedrazzi G. Is there a relation between AOFAS Ankle-Hindfoot Score and SF-36 in evaluation of Achilles ruptures treated by percutaneous technique? J Foot Ankle Surg. 2014;53:16-21. [DOI] [PubMed] [Google Scholar]
- 5.Chan HY, Chen JY, Zainul-Abidin S, Ying H, Koo K, Rikhraj IS. Minimal clinically important differences for American Orthopaedic Foot & Ankle Society Score in hallux valgus surgery. Foot Ankle Int. 2017;38:551-557. [DOI] [PubMed] [Google Scholar]
- 6.Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24:106-112. [DOI] [PubMed] [Google Scholar]
- 7.Dahmen J, Hurley ET, Shimozono Y, et al. Evidence-based treatment of failed primary osteochondral lesions of the talus: a systematic review on clinical outcomes of bone marrow stimulation. Cartilage. 2021;13:1411s-1421s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahmen J, Lambers KTA, Reilingh ML, van Bergen CJA, Stufkens SAS, Kerkhoffs GMMJ. No superior treatment for primary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26:2142-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson J, Doll H, Coffey J, Jenkinson C. Responsiveness and minimally important change for the Manchester-Oxford foot questionnaire (MOXFQ) compared with AOFAS and SF-36 assessments following surgery for hallux valgus. Osteoarthritis Cartilage. 2007;15:918-931. [DOI] [PubMed] [Google Scholar]
- 10.Desai S. Treatment of osteochondral lesions of the talus with marrow stimulation and micronized allograft cartilage matrix. Tech Foot Ankle Surg. 2014;13:167-173. [Google Scholar]
- 11.Gu AP, Gu CN, Ahmed AT, et al. Sham surgical procedures for pain intervention result in significant improvements in pain: systematic review and meta-analysis. J Clin Epidemiol. 2017;83:18-23. [DOI] [PubMed] [Google Scholar]
- 12.Guyton GP. Theoretical limitations of the AOFAS scoring systems: an analysis using monte carlo modeling. Foot Ankle Int. 2001;22:779-787. [DOI] [PubMed] [Google Scholar]
- 13.Harris JD, Brand JC, Cote MP, Dhawan A. Research pearls: the significance of statistics and perils of pooling. part 3: pearls and pitfalls of meta-analyses and systematic reviews. Arthroscopy. 2017;33:1594-1602. [DOI] [PubMed] [Google Scholar]
- 14.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form Mcgill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS) and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63:S240-S252. [DOI] [PubMed] [Google Scholar]
- 15.Jonas WB, Crawford C, Colloca L, et al. To what extent are surgery and invasive procedures effective beyond a placebo response? A systematic review with meta-analysis of randomised, sham controlled trials. BMJ Open. 2015;5:e009655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JS, Hobden E, Stiell IG, Wells GA. Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med. 2003;10:1128-1130. [DOI] [PubMed] [Google Scholar]
- 17.Leopold SS. A conversation with … Ted J. Kaptchuk, expert in placebo effects. Clin Orthop Relat Res. 2021;479:1645-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullough CJ, Venugopal V. Osteochondritis dissecans of the talus: the natural history. Clin Orthop Relat Res. 1979:264-268. [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88. [DOI] [PubMed] [Google Scholar]
- 21.Ogilvie-Harris DJ, Sarrosa EA. Arthroscopic treatment after previous failed open surgery for osteochondritis dissecans of the talus. Arthroscopy. 1999;15:809-812. [DOI] [PubMed] [Google Scholar]
- 22.Orr JD, Dawson LK, Garcia EJ, Kirk KL. Incidence of osteochondral lesions of the talus in the United States military. Foot Ankle Int. 2011;32:948-954. [DOI] [PubMed] [Google Scholar]
- 23.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paavola M, Kanto K, Ranstam J, et al. Subacromial decompression versus diagnostic arthroscopy for shoulder impingement: a 5-year follow-up of a randomised, placebo surgery controlled clinical trial. Br J Sports Med. 2021;55:99-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paavola M, Malmivaara A, Taimela S, et al. Subacromial decompression versus diagnostic arthroscopy for shoulder impingement: randomised, placebo surgery controlled clinical trial. BMJ. [Published online July 19, 2018]. DOI: 10.1136/bmj.k2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JH, Park KH, Cho JY, Han SH, Lee JW. Bone marrow stimulation for osteochondral lesions of the talus: are clinical outcomes maintained 10 years later? Am J Sports Med. 2021;49:1220-1226. [DOI] [PubMed] [Google Scholar]
- 27.Pinsker E, Daniels TR. AOFAS position statement regarding the future of the AOFAS clinical rating systems. Foot Ankle Int. 2011;32:841-842. [DOI] [PubMed] [Google Scholar]
- 28.Ramponi L, Yasui Y, Murawski CD, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2017;45:1698-1705. [DOI] [PubMed] [Google Scholar]
- 29.Reilingh ML, van Bergen CJA, Gerards RM, et al. Effects of pulsed electromagnetic fields on return to sports after arthroscopic debridement and microfracture of osteochondral talar defects. Am J Sports Med. 2016;44:1292-1300. [DOI] [PubMed] [Google Scholar]
- 30.Rikken QGH, Dahmen J, Reilingh ML, van Bergen CJA, Stufkens SAS, Kerkhoffs GMMJ. Outcomes of bone marrow stimulation for secondary osteochondral lesions of the talus equal outcomes for primary lesions. Cartilage. 2021;13;1429s-1437s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rikken QGH, Dahmen J, Stufkens SAS, Kerkhoffs GMMJ. Satisfactory long-term clinical outcomes after bone marrow stimulation of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2021;29:3525-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rikken QGH, Kerkhoffs GMMJ. Osteochondral lesions of the Talus. Foot Ankle Clin. 2021;26:121-136. [DOI] [PubMed] [Google Scholar]
- 33.Robinson DE, Winson IG, Harries WJ, Kelly AJ. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Br. 2003;85:989-993. [DOI] [PubMed] [Google Scholar]
- 34.Savva N, Jabur M, Davies M, Saxby T. Osteochondral lesions of the talus: results of repeat arthroscopic debridement. Foot Ankle Int. 2007;28:669-673. [DOI] [PubMed] [Google Scholar]
- 35.Schimmer RC, Dick W, Hintermann B. The role of ankle arthroscopy in the treatment strategies of osteochondritis dissecans lesions of the talus. Foot Ankle Int. 2001;22:895-900. [DOI] [PubMed] [Google Scholar]
- 36.Schuman L, Struijs PAA, van Dijk CN. Arthroscopic treatment for osteochondral defects of the talus. J Bone Joint Surg Br. 2002;84:364-368. [DOI] [PubMed] [Google Scholar]
- 37.Shim DW, Park KH, Lee JW, Yang Y, Shin J, Han SH. Primary autologous osteochondral transfer shows superior long-term outcome and survival rate compared with bone marrow stimulation for large cystic osteochondral lesion of talus. Arthroscopy. 2021;37:989-997. [DOI] [PubMed] [Google Scholar]
- 38.Shimozono Y, Dankert JF, Deyer TW, Mercer NP, Kennedy JG. Predictors of outcomes of microfracture with concentrated bone marrow aspirate for osteochondral lesions of the talus. Journal of Cartilage & Joint Preservation. 2021;1:100008. [Google Scholar]
- 39.Sihvonen R, Paavola M, Malmivaara A, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515-2524. [DOI] [PubMed] [Google Scholar]
- 40.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-randomized Studies (Minors): development and validation of a new instrument. ANZ J Surg. 2003;73 712-716. [DOI] [PubMed] [Google Scholar]
- 41.Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18:927-932. [DOI] [PubMed] [Google Scholar]
- 42.Van Bergen CJA, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GMMJ, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus. J Bone Joint Surg. 2013;95:519-525. [DOI] [PubMed] [Google Scholar]
- 43.Wartolowska K, Judge A, Hopewell S, et al. Use of placebo controls in the evaluation of surgery: systematic review. BMJ. 2014;348:g3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon HS, Park YJ, Lee M, Choi WJ, Lee JW. Osteochondral autologous transplantation is superior to repeat arthroscopy for the treatment of osteochondral lesions of the talus after failed primary arthroscopic treatment. Am J Sports Med. 2014;42:1896-1903. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimura I, Kanazawa K, Takeyama A, et al. Arthroscopic bone marrow stimulation techniques for osteochondral lesions of the talus: prognostic factors for small lesions. Am J Sports Med. 2013;41:528-534. [DOI] [PubMed] [Google Scholar]
- 46.Zengerink M, Struijs PAA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2010;18:238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zengerink M, Szerb I, Hangody L, Dopirak RM, Ferkel RD, van Dijk CN. Current concepts: treatment of osteochondral ankle defects. Foot Ankle Clin. 2006;11:331-359. [DOI] [PubMed] [Google Scholar]