Abstract
Introduction:
The clinical significance and treatment recommendations for an unexpected positive Cutibacterium acnes (C acnes) culture remain unclear. The purpose of our study was to evaluate the clinical effect of a C acnes positive culture in patients undergoing open orthopaedic surgery.
Methods:
Patients with a minimum of one positive C acnes intraoperative culture were retrospectively reviewed over a 7-year period. True C acnes infection was defined as culture isolation from ≥1 specimens in the presence of clinical or laboratory indicators of infection.
Results:
Forty-eight patients had a positive intraoperative C acnes culture. 4.2% had a C acnes monoinfection, and 12.5% of the patients had a coinfection. The remainder was classified as indeterminate. Significant differences were identified between the indeterminate and true C acnes infection groups, specifically in patients with surgery history at the surgical site (P = 0.04), additional antibiotic therapy before surgery (P < 0 .001), and postoperative clinical signs of infection (P < 0 .001).
Discussion:
Suspicion for true C acnes infection should be raised in patients with surgery site history, antibiotic therapy before surgery, and clinical infectious signs. The indeterminate unexpected positive culture patients had a low risk of developing a true clinical infection that required antibiotic therapy.
Propionibacterium acnes (P acnes) is an anaerobic, slow-growing, non–spore-forming, gram-positive organism part of the normal skin flora and is most notable in orthopaedic literature for its pathogenicity in deep and superficial surgical site infections.1 Cutibacterium acnes (C acnes) is able to colonize the acidic, anaerobic environment of the dermal sebaceous glands and the epidermis.2 Furthermore, C acnes can form a resistant biofilm, especially on orthopaedic implants, to elude the human immune response.3 Recently, reclassification of P acnes to C acnes has been suggested on the basis of genomic and metagenomic investigations.3,4
Regardless of nomenclature, one positive C acnes culture may indicate true infection that is clinically significant or relatively asymptomatic. Postoperative infections after orthopaedic surgery can result in notable long-term sequelae including long-term antibiotic therapy and possible return to the operating room for irrigation and débridement, removal of implants, or revision surgery.1,3 Most of the current literature focuses on shoulder surgeries given the proposed increased C acnes burden around the shoulder girdle.5,6,7,8,9,10,11,12 In the shoulder arthroplasty literature, C acnes represents the causative organism in 12% to 51.3% of prosthetic joint infections.12,13
The clinical diagnosis and relevance of unexpected positive culture (UPC) C acnes continues to be heavily researched.9,10,14,15,16,17,18 C acnes infections are characteristically difficult to diagnose because of their indolent nature, and they usually lack clinical signs common to many infective processes.13,16 In the shoulder arthroplasty literature, standard infectious laboratory markers, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), were found to be elevated in only 10% of patients.19 To complicate the clinical picture further, C acnes contamination has been reported between 7% and 13%.15,20 Criteria for the diagnosis of a true C acnes infection has been suggested by Lutz et al. and Asseray et al.17,18 Our study uses similar criteria, combining clinical features in the presence of one positive culture.
Although there is a growing recognition of the effect of C acnes in the general orthopaedic population, the literature involving a wide range of orthopaedic patients is lacking. The diagnosis of a true C acnes infection versus an indeterminate culture-positive patient continues to be a topic of interest and controversy given its frequent isolation in deep tissue infections.16,17,21 We conducted a retrospective study aiming to determine the clinical significance of a single sterile culture-positive C acnes sample after an open orthopaedic surgery case. We hypothesized that in patients with a positive C acnes culture, in the setting of no clinical or laboratory signs of infection, does not correlate with true postoperative infection. We also hypothesized that patients who fail to meet the diagnostic criteria of true infection do not require antibiotic therapy.
Methods
Patients
A retrospective cohort study was conducted at the Cedar Sinai Medical Center, a tertiary trauma care center (886 beds), from January 1, 2013, to May 1, 2020. International Review Board committee approval was received before beginning the investigation. All patients who were surgically treated by the primary surgeon (E.B.) were identified through the hospital computer electronic medical record database. Any patient with at least one positive culture for C acnes, in any intraoperative orthopaedic tissue sample, was included in this study. Relevant patient data were extracted from medical records. There were no exclusion criteria. All antibiotics used were reviewed and characterized by purpose (prophylactic or therapeutic), antibiotic class, route of administration, and duration. All orthopaedic surgeries were reviewed and characterized by anatomical site and type.
Clinical records were reviewed for patient medical and surgical history. Information collected included radiographic, laboratory, microbiologic data, along with surgical, treatment, and follow-up information. For the purpose of this study, postoperative antibiotic administration was classified as “antibiotic prophylaxis” when less than 24 hours of antibiotics was administered and as “antibiotic treatment” when treatment lasted more than 24 hours.
Before incision, patients were prepared and draped according to standard hospital protocols, which involve thorough washing of the surgical site with 70% isopropyl alcohol. The surgical site is then prepared in an aseptic manner with 2% chlorhexidine gluconate in 70% isopropyl alcohol (ChloraPrep; Becton, Dickinson and Company) in two layers. After sterile draping, an adhesive antimicrobial clear drape (Ioban; 3M) was placed over the surgical site. In addition, if working near the shoulder, the axilla was isolated by means of adhesive drapes.
All culture swabs were obtained from the deep tissue in a sterile fashion without making contact to the epidermis or dermal layer of the skin. It should be noted that the skin knife was discarded from the surgical field after initial skin incision. The primary surgeon ensured sterility was maintained, avoiding any contact with the superficial structures, to obtain each intraoperative culture. All orthopaedic specimens were transported under anaerobic conditions and inoculated on routine bacteriological media, including a pre-reduced CDC anaerobic blood agar (CDC) and chopped meat medium broth. Incubation in anaerobic conditions was done for a minimum of 14 days. C acnes was identified either by rapid identification (gram-positive coryneform rods, positive 15% catalase, and positive indole spot test) or by routine culture.
We examined all patients with positive intraoperative cultures obtained during open orthopaedic surgery. Suspected true C acnes infection criteria used were one positive culture with associated elevated laboratory markers (CRP, ESR, and white blood cell count) and/or clinical infection manifestations (local inflammatory signs [swelling, erythema, and pain], implant loosening, nonunion, and bone erosion). Patients with a positive C acnes culture and none of the above findings were classified into an indeterminate group (subclinical infection or contaminant). C acnes monoinfection was defined as an infection solely caused by C acnes. A coinfection was defined as an infection caused by ≥2 microorganisms both of which were considered clinically significant.16-18,22
Statistical Analysis
Nonparametric analyses were conducted. The relationship between a true C acnes infection and interval levels of measurement (ie, age and body mass index) were analyzed using the Wilcoxon rank sum test. The relationship between a true C acnes infection and nominal levels of measurement (ie, ethnicity) was analyzed using the chi square test. A P-value of 0.05 or less was considered to be statistically significant. Statistical analyses were conducted using IBS SPSS Statistics 26.
Results
Description of the Studied Cohort
During the study period, 48 patients matched our inclusion and exclusion criteria (Table 1). There were 31 male and 17 female patients. The mean age of patients with a suspected C acnes infection was 57 years, and the mean age for patients in the indeterminate group was 55 years. The ethnicity of the study participants was 36 White, three Hispanic, three African American, three Asian, and three Middle Eastern.
Table 1.
Comparison of Those Who met the Criteria for a C acnes Infection Versus Those Within the Indeterminate Group
| C acnes Infection (n = 8) | Did not meet C acnes Infection Criteria (n = 40) | P-Value | |
| Mean (SD) | Mean (SD) | ||
| Age, yr | 57.13 (16.89) | 54.78 (18.51) | 0.75 |
| BMI | 25.66 (4.23) | 27.87 (6.39) | 0.37 |
| Frequency (%) | Frequency (%) | ||
| Sex | 0.50 | ||
| Female (n = 17) | 2 (11.76%) | 15 (88.23%) | |
| Male (n = 31) | 6 (19.35%) | 25 (80.64%) | |
| Ethnicity | 0.11 | ||
| White (n = 36) | 5 (13.89%) | 31 (86.11%) | |
| Hispanic (n = 3) | 0 (0%) | 3 (100%) | |
| Black (n = 3) | 2 (66.67%) | 1 (33.33%) | |
| Asian (n = 3) | 1 (33.33%) | 2 (66.67%) | |
| Middle Eastern (n = 3) | 0 (0%) | 3 (100%) | |
| Surgery type | 1.00 | ||
| With implants (n = 6) | 1 (16.67%) | 5 (83.33%) | |
| Without implants (n = 42) | 7 (16.67%) | 35 (83.33%) | |
| Location of surgery | 0.76 | ||
| Shoulder (n = 15) | 2 (13.33%) | 13 (86.67%) | |
| Hip (n = 12) | 4 (33.33%) | 8 (66.67%) | |
| Arm (n = 6) | 1 (16.67%) | 5 (83.33%) | |
| Thigh (n = 6) | 0 (0%) | 6 (100%) | |
| Back (n = 4) | 1 (25%) | 3 (75%) | |
| Hand (n = 1) | 0 (0%) | 1 (100%) | |
| Neck (n = 1) | 0 (0%) | 1 (100%) | |
| Immunosuppressed | 0.07 | ||
| Yes (n = 12) | 4 (33.3%) | 8 (66.67%) | |
| No (=38) | 4 (10.5%) | 32 (84.21%) | |
| Previous surgery on the surgical site | 0.04* | ||
| Yes (n = 11) | 4 (36.36%) | 7 (63.63%) | |
| No (n = 37) | 4 (10.81%) | 33 (89.18%) | |
| Previous biopsy on the surgical site | 0.79 | ||
| Yes (n = 22) | 4 (18.2%) | 18 (81.8%) | |
| No (n = 26) | 4 (15.4%) | 22 (84.6%) | |
| Additional antibiotics before surgery | 0.00* | ||
| Yes (n = 3) | 3 (100%) | 0 (0%) | |
| No (n = 45) | 5 (11.1%) | 40 (88.9%) | |
| Clinical postoperative symptoms of infection | 0.00* | ||
| Yes (n = 8) | 8 (100.0%) | 0 (0%) | |
| No (n = 40) | 0 (0%) | 40 (100.0%) |
Altogether, the cases included 14 lipoma excisions, 10 soft-tissue sarcoma resections, 2 implant removals, 2 elastofibromas, 2 neurogenic tumor excisions, revision joint arthroplasty, impending long bone fracture fixation, enchondroma protuberans excision, osteochondroma excision, chondrosarcoma resection, desmoid tumor excision, enchondroma curettage, chondroblastoma curettage, dermatofibrosarcoma protuberans, bone cyst excision and curettage, metastatic renal cell carcinoma resection, pigmented villonodular synovitis excision, hip arthroplasty, and myxoma excision. Antimicrobial prophylaxis received at the time of the surgery consisted mainly of a first-generation cephalosporin (n = 46, 96%). The average follow-up period was of 11.5 months (range 8 to 101 months).
True C acnes Infection Cohort
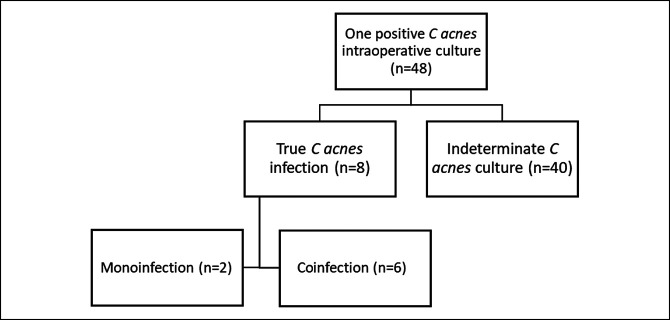
According to our definition, C acnes was considered to be a causative pathogen in eight patients who had at least one positive C acnes culture, whereas the other 40 culture-positive patients were considered indeterminate (subclinical infection or contaminated) by C acnes. In the subset of true C acnes infections, two were monoinfections and six were coinfections with an additional organism (Figure 1). The additional organisms identified included Staphylococcus aureus, Staphylococcus lugdunensis, Group B Streptococcus, and Peptostreptococcus.
Figure 1.
Patient flowchart.
The true C acnes infections occurred in patients undergoing the following procedures: two thigh sarcoma resections, two lipoma resections, two groin sarcoma resections, one total hip revision, and one implant removal. In the true infection group, six of the eight patients underwent repeat surgery for irrigation and débridement for infection treatment. All patients who underwent a second surgery for infection treatment were classified as coinfections. The two true C acnes monoinfections were in patients undergoing removal of implants and lipoma excision. Both were treated with a short course (10 days) of doxycycline and required no additional surgical intervention.
The postoperative complications identified in the true infection group of patients included revision total hip arthroplasty due to coinfection of S aureus and C acnes, cellulitis after lipoma resection that subsequently resolved with oral antibiotics, and abscess formation after soft-tissue sarcoma and lipoma resections.
Local inflammatory signs were present in 6 of the 8 patients (75%) meeting the criteria for true C acnes infections. The white blood cell count, CRP, and ESR were elevated in only 2 of the 8 patients with true C acnes infection (25%). Of the two true monoinfections, one patient showed local incisional cellulitis postoperatively; however, neither patient had elevated inflammatory laboratory findings.
Comparison of True C acnes Infection and Indeterminate (Subclinical Infection or Contaminant) Cohorts
Implants
In total, 42 surgeries were conducted with orthopaedic implants that included either implantation or removal of implant. In both cohorts, a total of six procedures (12.5%) included orthopaedic implants. In patients classified as suspected true C acnes infection, 1 of the 8 patients (12.5%) underwent surgery that included orthopaedic implants. In the indeterminate group, 5 of the 40 patients (12.5%) underwent a procedure with implants involved. No notable difference was found between the two groups.
Location
Surgery was conducted in the following locations: shoulder (15), hip (12), arm (6), thigh (6), back (4), hand (1), and neck (1). In patients with a true C acnes infection, the most frequent locations were the hip and shoulder, four and two patients, respectively. Overall, in patients with positive C acnes culture from the hip, 4 of the 12 patients (33.33%) resulted in a suspected true infection while 8 of the 12 patients (66.67%) resulted in indeterminate classification. Similarly, with the location of the surgery involving the shoulder, 2 of the 15 patients (13.33%) had a suspected true infection while 13 of the 15 patients (86.67%) were classified as indeterminate. No notable difference was identified between the two cohorts.
Immunosuppressed
Twelve patients met the criteria for the immunosuppressed cohort, 33.3% of whom were diagnosed with a true infection. The criteria included history of receiving chemotherapy or radiation, chronic steroid use, immunodeficiency disorders, and taking immunosuppressive drugs. No notable difference was identified between the two cohorts.
Previous surgery on the surgical site
History of surgery on the same surgical site was found to be statistically significant for an increase in true C acnes infection classification (P = 0.04). Eleven of the 48 patients had previous surgical intervention on the same surgical site. Of those who had undergone previous surgery, 4 of 11 patients (36.36%) fit the criteria for a true C acnes infection. By contrast, 4 of 37 patients (10.81%) who did not have any documented history of prior surgery met the criteria for a true C acnes infection.
Previous biopsy on the surgical site
A total of 22 of the 48 patients (45.83%) had undergone previous CT-guided biopsy on the surgical site for preoperative oncologic diagnosis and surgical planning. No statistically significant correlation was observed between previous biopsy and true C acnes infection diagnosis. Of the eight patients classified as true C acnes infection, 4 of the 8 (50%) had a previously done biopsy.
Additional antibiotics before surgery
Patients with a history of antibiotics use before surgery had a statistically significant increase in suspected true C acnes infections (P < 0.001). All three patients who received preoperative antibiotics were diagnosed with a true C acnes infection. In patients who did not receive preoperative antibiotics, 5 of 45 (11.1%) were diagnosed with a suspected C acnes infection.
Postoperative antibiotic use
Patients receiving postoperative antibiotics were all within the true C acnes infection cohort. Postoperative antibiotics included Zosyn, cefepime, Keflex, Bactrim, Levaquin, and cefazolin. No postoperative therapeutic antibiotics were given to the indeterminate culture-positive group.
Clinical postoperative symptoms of infection
As expected, patients with clinical signs and symptoms of infection were found to be more frequently classified as a true C acnes infection (P < 0.001). Eight of the eight patients (100%) who displayed the symptoms of postoperative infection (fever, erythema, swelling, and wound issues) were diagnosed with a true C acnes infection. By contrast, no patients in the indeterminate group showed clinical signs of infection. Postoperative laboratory markers were found to be elevated in only three patients in the entire cohort, two of whom were diagnosed with coinfections. One patient in the indeterminate group had a mildly elevated ESR without other clinical signs of infection.
Discussion
The clinical significance, optimal treatment strategy, and outcomes in patients with positive intraoperative C acnes cultures and no overt signs of infection are largely debated.8,12,23 The incidence of UPC has been reported in the shoulder literature as high as 17%; however, only 5.9% to 12.1% of the UPC patient population went on to progress to a true infection postoperatively.8,10,24 In our cohort, the presence of a positive C acnes culture was associated with a diagnosis of true infection in 8 of the 48 cases (16.6%). Removing patients with coinfections, 2 of the 48 patients with UPC (4.2%) were diagnosed with a true C acnes monoinfection. The monoinfection patients were treated with a 10-day course of doxycycline with no further surgical treatment. 40 of the /48 patients (83.3%) in the indeterminate cohort were treated with close surveillance and no antibiotic therapy and required no additional surgical treatment.
Although an accepted definition of a C acnes infection remains a topic of debate, we used simplified criteria to define suspected true C acnes infection.16-18 One positive culture and perioperative clinical and/or laboratory signs of infection placed the patient in the true infection group. Our criteria was adapted from the guidelines of Asseray et al18 that had a diagnostic probability of >90% for a C acnes infection: ≥2 positive cultures in addition to one of the following criteria: perioperative findings, local signs of infection, ≥2 previous operations, or orthopaedic devices or one positive culture in addition to three of the following criteria among perioperative findings, local signs of infection, ≥2 previous surgical operations, orthopaedic devices, and inflammatory syndrome.
Skin flora contains many native bacteria. However, controversy remains where exactly C acnes inhabits.14,25,26 Lee et al2 revealed that C acnes is common on the epidermal surface of unprepared skin of normal patients and is found more frequently and in greater numbers in male patients with increased sebaceous glands. Their observations support the overarching concept that the epidermis, dermis, and hair follicles are potential contamination sources of the C acnes culture positivity.12,14,20,27-29
In the case of C acnes positivity in one or more deep surgical samples, the question for the surgeon is whether there is an infection, possible indeterminate infection, or sample contamination. Probable infection should be made based on associating bacteriological and clinical findings.16-18 In cases of indeterminate infection or suspected contamination, simple surveillance without antibiotic initiation has been argued in the literature.9,10,17,21 Grosso et al. retrospectively reviewed the results of 17 patients undergoing revision shoulder arthroplasty with at least one UPC who were not treated for infection. Their patients had no clinical symptoms or laboratory signs of infection. They found a low clinical recurrence rate (5.9%) of infection for this untreated revision arthroplasty group and concluded that prolonged antibiotic therapy may not be necessary.10 Similarly, Dramis et al9 identified 50 patients with prosthetic joints from whom C acnes was isolated at least once. Patients with UPC were treated with surveillance with no antibiotics. Only one patient had additional revision surgery for infection. Our findings support clinical surveillance with a close follow-up for 2 years in the UPC indeterminate group.
In the shoulder arthroplasty literature, Kim et al investigated treatment strategies regarding patients with an UPC, including C acnes, without overt signs of infection in revision shoulder arthroplasty. In their systematic review of 1402 patients undergoing revision shoulder arthroplasty, 16.7% of the patients had an UPC. Occurrence of a true infection from an UPC after revision shoulder arthroplasty was seen in 24 shoulders (10.2%). They concluded that there is a low risk of having a true infection from an UPC after revision shoulder arthroplasty without clinical signs of perioperative infection.8
Additional research evaluating C acnes UPCs in open shoulder surgery was conducted by Mook et al.15. Patients with a history of shoulder surgery or any concern for active or previous shoulder infection were excluded. In their study, three soft-tissue samples were obtained from the shoulder, as well as a sterile sponge. Overall, 20.5% of the surgeries yielded at least one specimen removed for culture that was positive for bacterial growth, and 13.0% of the sterile control sponge specimens had positive culture growth. C acnes represented 83.0% of all positive cultures. They identified male sex and preoperative corticosteroid injections as risk factors for bacterial growth on culture. They concluded that C acnes is isolated through culture at a substantial rate from clinically noninfected shoulders; however, there is a substantial level of culture contamination.15 Similarly, the infections in our cohort occurred mostly in male patients (5/8, 71.4%); however, they failed to reach significance.
A recent study by Lavergne et al examined the clinical difference between C acnes infection and contamination. A total of 68 patients had at least one positive C acnes culture, 35 of whom were considered to be infected. The infections were mostly found in males and located in the shoulder girdle. Ninty-one percent of the infections occurred at a site already containing an orthopaedic implant. Local inflammatory signs were present in half of the cases when an infection was diagnosed. Coinfection with other pathogens was present in 31% of the patients. This study demonstrates the importance of clinical correlation and the importance of accurate diagnosis of a C acnes infection.16 Our study seeks to further Lavergne et al work by applying a modified diagnostic protocol in patients where only one positive C acnes culture is found. Orthopaedic implants were involved in only 14.5% of our study group, which lacks power to compare directly with the work of Lavergne et al.
UPCs in patients without clinical signs or symptoms of infection continue to confuse the clinical picture and may lead to overdiagnosis and treatment. The rate of contamination from the native microbiome has been recorded ranging from 7% to 15% and has varied depending on the institution conducting this study.14,15,20,27,28,29,30,31 Currently, there is no definitive benchmark skin surface preparation proven to prevent inoculation of bacteria from the epidermal and dermal structures into the deep tissues at the time of the skin incision; however, hydrogen peroxide shows promise.14,20 Additional research into preoperative skin preparation may aid in decreasing culture contamination and provide clarity to the C acnes UPC picture. The C acnes UPC conflict demonstrates the importance of clinically correlating a positive culture in an otherwise healthy patient.17,18 Which patients go on to a clinical infection seems to depend on the size of inoculum, suitability of the microenvironment for growth, relative proportions of various pathogenic strains, and the host response to Cutibacterium.7,28 Other important factors to consider when examining the risk of a C acnes infection include male patients who previously underwent surgical intervention and implant-associated procedures.3,8,15,16
In our study, the presence of a positive C acnes culture was associated with a diagnosis of true infection in only 17% of the cases. The indeterminate patients truly encompass the definition of an UPC cohort. The patients in this group failed to show any clinical or laboratory signs of infection perioperatively and did not require additional postoperative antibiotics or surgical treatment. Close clinical monitoring of these patients was found to be the appropriate management. We did not find any notable true infection association between sex, ethnicity, immunosuppression, and surgery location. Interestingly, we also failed to find any statistically significant connection between previous CT-guided biopsy and diagnosis of a true C acnes infection. This suggests that the sterile biopsy may not increase the risk of future C acnes culture positivity and infection. Our results add to the existing literature that patients who underwent previous surgery on the planned surgical site are at an increased risk of developing a true C acnes infection. In addition, we found that antibiotic use before surgery and clinical signs of infection postoperatively were associated with the diagnosis of a true C acnes infection.
Our study has several limitations. It is retrospective, and complete data may not have been available for all patients. We identified suspected true C acnes infections as those patients with at least one positive culture and one perioperative infectious sign. It could be argued that the second positive culture is necessary to confirm the suspected infection. The arthroplasty literature recommends three tissue samples be sent for culture to improve the yield and to decrease the likelihood of a false-negative or positive culture.32
In patients with an UPC, we did not differentiate between contaminant and subclinical C acnes colonization. Increasing the number of cultures and having a control arm would have aided in separating the two groups. We lacked notable power with many categories, including orthopaedic implant. Although our study may allow us to apply the conclusions to a general orthopaedic practice involving soft-tissue procedures, we are unable to conclude on implant-related C acnes positive cultures.
To decrease selection bias, we did not exclude any cases. The patients were identified directly from the hospital database, and collection of data was done by two independent evaluators. There was a wide range of follow-up length for each patient, which did not allow for consistent long-term follow-up data on all patients. This limits our findings to applying to only short-term outcomes. Future long-term studies with a sterile control group and an increased number of sterile culture swabs per patient should be undertaken to identify the containment group from the indeterminate culture-positive patients. Additional prospective multicenter studies with increased patient numbers are necessary to better define the optimal treatment management strategy.
Conclusion
Orthopaedic surgeons often encounter positive cultures obtained from presumably uninfected cases during the course of their career. We found that a single positive C acnes culture rarely represents a true infection in patients who lack clinical signs of infection. Given the inherently high false-positive rate with long-hold cultures, clinical correlation in patients presenting with culture-positive C acnes is recommended. In this study, we found that patients undergoing open orthopaedic surgery with at least one positive C acnes intraoperative culture, in the absence of clinical or laboratory findings suggesting infection, have a low likelihood of requiring treatment with antibiotics or revision surgery. By contrast, patients who present with C acnes coinfections underwent previous surgery on the surgical site or those who display clinical signs of postoperative infection should be treated with organism-specific antibiotics.
References
- 1.Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N: Propionibacterium acnes: An agent of prosthetic joint infection and colonization. J Infect 2007;55:119-124. [DOI] [PubMed] [Google Scholar]
- 2.Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA, III: Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am 2014;96:1447-1450. [DOI] [PubMed] [Google Scholar]
- 3.Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME: Cutibacterium acnes: From commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev 2014;27:419-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholz CF, Kilian M: The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol 2016;66:4422-4432. [DOI] [PubMed] [Google Scholar]
- 5.Topolski MS, Chin PY, Sperling JW, Cofield RH: Revision shoulder arthroplasty with positive intraoperative cultures: The value of preoperative studies and intraoperative histology. J Shoulder Elbow Surg 2006;15:402-406. [DOI] [PubMed] [Google Scholar]
- 6.Athwal GS, Sperling JW, Rispoli DM, et al. : Deep infection after rotator cuff repair. J Shoulder Elbow Surg 2007;16:306-311. [DOI] [PubMed] [Google Scholar]
- 7.Kelly JD, II, Hobgood ER: Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res 2009;467:2343-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SJ, Kim JH: Unexpected positive cultures including isolation of Propionibacterium acnes in revision shoulder arthroplasty. Chin Med J 2014;127:3975-3979. [PubMed] [Google Scholar]
- 9.Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O'Connell N, Elliott T: What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop 2009;33:829-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosso MJ, Sabesan VJ, Ho JC, Ricchetti ET, Iannotti JP: Reinfection rates after 1-stage revision shoulder arthroplasty for patients with unexpected positive intraoperative cultures. J Shoulder Elbow Surg 2012;21:754-758. [DOI] [PubMed] [Google Scholar]
- 11.Titécat M, Senneville E, Wallet F, et al. : Bacterial epidemiology of osteoarticular infections in a referent center: 10-year study. Orthop Traumatol Surg Res 2013;99:653-658. [DOI] [PubMed] [Google Scholar]
- 12.Patel A, Calfee RP, Plante M, Fischer SA, Green A: Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg 2009;18:897-902. [DOI] [PubMed] [Google Scholar]
- 13.Levy O, Iyer S, Atoun E, et al. : Propionibacterium acnes: An underestimated etiology in the pathogenesis of osteoarthritis? J Shoulder Elbow Surg 2013;22:505-511. [DOI] [PubMed] [Google Scholar]
- 14.Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL: Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am 2009;91:1949-1953. [DOI] [PubMed] [Google Scholar]
- 15.Mook WR, Klement MR, Green CL, Hazen KC, Garrigues GE: The incidence of propionibacterium acnes in open shoulder surgery: A controlled diagnostic study. J Bone Joint Surg Am 2015;97:957-963. [DOI] [PubMed] [Google Scholar]
- 16.Lavergne V, Malo M, Gaudelli C, et al. : Clinical impact of positive Propionibacterium acnes cultures in orthopedic surgery. Orthop Traumatol Surg Res 2017;103:307-314. [DOI] [PubMed] [Google Scholar]
- 17.Lutz MF, Berthelot P, Fresard A, et al. : Arthroplastic and osteosynthetic infections due to propionibacterium acnes: A retrospective study of 52 cases, 1995-2002. Eur J Clin Microbiol Infect Dis 2005;24:739-744. [DOI] [PubMed] [Google Scholar]
- 18.Asseray N, Papin C, Touchais S, et al. : Improving diagnostic criteria for propionibacterium acnes osteomyelitis: A retrospective analysis. Scand J Infect Dis 2010;42:421-425. [DOI] [PubMed] [Google Scholar]
- 19.Mook WR, Garrigues GE: Diagnosis and management of periprosthetic shoulder infections. JBJS 2014;96:956‐965. [DOI] [PubMed] [Google Scholar]
- 20.Chalmers PN, Beck L, Stertz I, Tashjian RZ: Hydrogen peroxide skin preparation reduces Cutibacterium acnes in shoulder arthroplasty: A prospective, blinded, controlled trial. J Shoulder Elbow Surg 2019;28:1554-1561. [DOI] [PubMed] [Google Scholar]
- 21.Boisrenoult P: Cutibacterium acnes prosthetic joint infection: Diagnosis and treatment. Orthop Traumatol Surg Res 2018;104:S19-S24. [DOI] [PubMed] [Google Scholar]
- 22.Osmon DR, Berbari EF, Berendt AR, et al. ; Infectious Diseases Society of America: Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the infectious diseases Society of America. Clin Infect Dis 2013;56:e1-e25. [DOI] [PubMed] [Google Scholar]
- 23.Barrack RL, Aggarwal A, Burnett RS, et al. : The fate of the unexpected positive intraoperative cultures after revision total knee arthroplasty. J Arthroplasty 2007;22:94-99. [DOI] [PubMed] [Google Scholar]
- 24.Foruria AM, Fox TJ, Sperling JW, Cofield RH: Clinical meaning of unexpected positive cultures (UPC) in revision shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:620-627. [DOI] [PubMed] [Google Scholar]
- 25.Qiu B, Al K, Pena-Diaz AM, et al. : Cutibacterium acnes and the shoulder microbiome. J Shoulder Elbow Surg 2018;27:1734-1739. [DOI] [PubMed] [Google Scholar]
- 26.Long DR, Hsu JE, Salipante SJ, Bumgarner RE: Letter to the Editor regarding Qui et al: “Cutibacterium acnes and the shoulder microbiome”. J Shoulder Elbow Surg 2019;28:e275-e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauzenberger L, Heller V, Ostermann RC, Laky B, Heuberer PR, Anderl W: Cutibacterium acnes (formerly propionibacterium acnes) contamination of the surgical field during shoulder arthroscopy. Arthroscopy 2019;35:1750-1757. [DOI] [PubMed] [Google Scholar]
- 28.Hsu JE, Matsen FA, III, Whitson AJ, Bumgarner RE: Cutibacterium subtype distribution on the skin of primary and revision shoulder arthroplasty patients. J Shoulder Elbow Surg 2020;29:2051-2055. [DOI] [PubMed] [Google Scholar]
- 29.Falconer TM, Baba M, Kruse LM, et al. : Contamination of the surgical field with propionibacterium acnes in primary shoulder arthroplasty. J Bone Joint Surg Am 2016;98:1722-1728. [DOI] [PubMed] [Google Scholar]
- 30.Namdari S, Nicholson T, Parvizi J: Cutibacterium acnes is isolated from air swabs: Time to doubt the value of traditional cultures in shoulder surgery? Arch Bone Joint Surg 2020;8:506-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudek R, Sommer F, Kerwat M, Abdelkawi AF, Loos F, Gohlke F: Propionibacterium acnes in shoulder surgery: True infection, contamination, or commensal of the deep tissue? J Shoulder Elbow Surg 2014;23:1763-1771. [DOI] [PubMed] [Google Scholar]
- 32.Atkins BL, Athanasou N, Deeks JJ, et al. ; OSIRIS Collaborative Study Group: Prospective evaluation of criteria for microbiological diagnosis of prosthetic joint infection at revision arthroplasty. J Clin Microbiol 1998;36:2932-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]