Abstract
This study (ReCORD-FL) sought to construct a historical control cohort to augment single-arm trials in relapsed/refractory follicular lymphoma (r/r FL). A retrospective study in 10 centers across North America and Europe was conducted. Adults with grade 1–3A FL were required to be r/r after ≥2 therapy lines including an anti-CD20 and an alkylator. After first becoming r/r, patients were required to initiate ≥1 additional therapy line, which defined the study index date. Endpoints were observed from start of each therapy line (including index line) until death, last follow-up, or December 31, 2020. Endpoints were complete response (CR) rate, overall response rate (ORR), time to next treatment or death (TNT-D), event-free survival (EFS), and overall survival (OS). One hundred eighty-seven patients were identified. Most patients’ (80.2%) index therapy occurred in third line (3L) (range, 3L–6L). Median follow-up from FL diagnosis was 9 years (range, 1–21 years). CR and ORR to the index therapy were 39.0% and 70.6%, respectively. Median (95% confidence interval) EFS from index was 14.6 (11.0-18.0) months; median OS from index was 10.6 years. Outcomes worsened across successive treatment lines and for patients who were double refractory (r/r to both an anti-CD20 monoclonal antibody and an alkylator) or POD24 (progressed ≤24 months after front-line anti-CD20) at index. Findings demonstrate the unmet need of FL patients with multiply relapsed, double refractory, or POD24 disease. Based on robustness of the historical data collected and comparability with a previous study (SCHOLAR-5), ReCORD-FL presents a valuable source of control data for comparative studies in r/r FL.
INTRODUCTION
Standard front-line therapy options for symptomatic follicular lymphoma (FL) includes an anti-CD20 monoclonal antibody (mAb) in combination with chemotherapy (eg, cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP] or bendamustine).1 Despite good effectiveness of standard front-line immunochemotherapy treatment,2-4 many patients will relapse repeatedly with progressively increasing resistance to therapy.5,6 It is also estimated that approximately 20% of patients with FL experience progression of disease within 24 months following the start of front-line immunochemotherapy (POD24)7-9; these patients have been shown to have a substantially poorer prognosis compared with those patients who do not experience early progression.10 Patients with early or multiply relapsed/refractory FL (r/r FL) therefore represent an area of high unmet need where newer treatments with novel mechanisms of action are needed to offer potentially curative options to this population.
Novel treatments for multiply r/r FL, particularly chimeric antigen receptor T-cell (CAR-T) therapies, are typically evaluated in single-arm trials with no comparative data on patients receiving usual care. Recent examples of such trials are DELTA, ZUMA-5, and ELARA.11-15 In the DELTA trial, effectiveness of idelalisib monotherapy was compared with patients’ most recent regimen before study entry in a heavily pretreated population (median of 4 previous therapy lines) with r/r FL. Median (95% confidence interval [CI]) progression-free survival (PFS) with idelalisib was 11.0 (8.0-14.0) months versus 5.1 (4.4-6.0) months for patients’ most recent regimen.11 ZUMA-5 examined the clinical effectiveness of axicabtagene ciloleucel (axi-cel) CAR-T therapy in patients with grade 1–3A r/r FL that relapsed or was refractory after ≥2 prior lines of therapy (including an anti-CD20 mAb and an alkylating agent) and who had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 and no evidence of prior histological transformation.12 On the basis of results from the ZUMA-5 trial showing high overall response rates (ORRs) and durable remissions,14 axi-cel received United States Food and Drug Administration approval for the treatment of r/r FL in March 2021. ELARA is a similarly designed ongoing single-arm trial examining the effectiveness of the CAR-T therapy tisagenlecleucel (tisa-cel) in patients with r/r FL meeting similar inclusion criteria as the ZUMA-5 trial.13 Results from ELARA indicate high and durable efficacy of tisa-cel in r/r FL, where 69% of patients achieved complete remission and 76% maintained response for at least 9 months.13
Despite promising results from these recent trials, findings from these and other single-arm studies require comparison against data from patients receiving usual care, which will help inform treatment decisions by patients and providers among available novel therapies. The study reported here (ReCORD-FL) therefore sought to construct a historical control cohort to augment current and future single-arm trials in r/r FL among patients receiving usual care who meet similar inclusion criteria to these recent trials. The analytic aims were to document patient characteristics, treatment patterns, and clinical outcomes in an r/r FL population treated with usual therapies in routine practice.
MATERIALS AND METHODS
This was a retrospective cohort study via medical record review in 10 oncology centers across North America (United States, Canada) and Europe (United Kingdom, France, Germany, Spain). Adult patients were required to meet at least one of the following criteria defining multiply r/r FL: (1) r/r after ≥2 lines of systemic therapy (including both anti-CD20 mAb and an alkylator [see footnote a in Table 1 for list of alkylating agents]), (2) relapse during or within 6 months after completion of anti-CD20 mAb maintenance therapy following at least two prior lines of therapy including both anti-CD20 mAb and an alkylator, or (3) relapse at any time after autologous hematopoietic stem cell transplantation (HSCT); patients were allowed to have met more than one of these criteria defining multiply r/r status. Patients were also required to have ≥1 line of systemic therapy after first meeting the r/r FL criteria; the date of first systemic therapy after meeting the r/r FL criteria defined the study index date. At index, patients were required to have grade 1-3A FL, ECOG performance status of 0 or 1, and no evidence of prior histological transformation. Relapse/refractoriness for each treatment line was defined as a clinician-documented best response of stable or progressive disease (ie, failure to respond) during the treatment line, or documented disease progression following a best response of complete or partial response at any point during the treatment line or within 6 months after discontinuation/completion of the treatment line. If clinician-documented progression or the progression date was unknown, start of the next treatment line, if it occurred within 6 months after discontinuation/completion of the current treatment line, was considered as relapse/refractoriness.
Table 1.
Baseline Patient Characteristics by Double Refractoriness and POD24 Status
| All Patients (N = 187) | Double Refractory Before the Index Treatment Line | POD24 to Frontline Anti-CD20 mAb Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No (n = 82) | Yes (n = 105) | No (n = 82) | Yes (n = 105) | |||||||
| Age category at index, n (%) | ||||||||||
| ≤60 y | 103 | 55.1 | 46 | 56.10 | 57 | 54.29 | 35 | 47.95 | 68 | 59.65 |
| >60 y | 84 | 44.9 | 36 | 43.90 | 48 | 45.71 | 38 | 52.05 | 46 | 40.35 |
| Age, median (range) | 58 (25–86) | 57.5 (25–82) | 59 (30–86) | 62 (25–82) | 58 (30–86) | |||||
| Time from FL diagnosis to index date, months | ||||||||||
| Mean (SD) | 57.1 (39.9) | 71.3 (38.5) | 46.0 (37.5) | 81.5 (40.1) | 41.5 (31.0) | |||||
| Median (range) | 46.0 (2.8–241.7) | 62.8 (19.3–241.7) | 32.8 (2.8–168.5) | 80.2 (28.0–241.7) | 32.0 (2.8–138.0) | |||||
| Gender, n (%) | ||||||||||
| Male | 106 | 56.7 | 48 | 58.54 | 58 | 55.24 | 47 | 64.38 | 59 | 51.75 |
| Female | 81 | 43.3 | 34 | 41.46 | 47 | 44.76 | 26 | 35.62 | 55 | 48.25 |
| FLIPI score at index, n (%) | ||||||||||
| Low | 25 | 13.4 | 13 | 15.85 | 12 | 11.43 | 10 | 13.70 | 15 | 13.16 |
| Intermediate | 28 | 15.0 | 8 | 9.76 | 20 | 19.05 | 9 | 12.33 | 19 | 16.67 |
| High | 71 | 38.0 | 30 | 36.59 | 41 | 39.05 | 31 | 42.47 | 40 | 35.09 |
| Unknown | 63 | 33.7 | 31 | 37.80 | 32 | 30.48 | 23 | 31.51 | 40 | 35.09 |
| ECOG PS at index, n (%) | ||||||||||
| 0 | 87 | 46.52 | 44 | 53.66 | 43 | 40.95 | 37 | 50.68 | 50 | 43.86 |
| 1 | 100 | 53.48 | 38 | 46.34 | 62 | 59.05 | 36 | 49.32 | 64 | 56.14 |
| Line of index treatment, n (%) | ||||||||||
| 3L | 150 | 80.2 | 67 | 81.71 | 83 | 79.05 | 59 | 80.82 | 91 | 79.82 |
| 4L | 31 | 16.6 | 13 | 15.85 | 18 | 17.14 | 13 | 17.81 | 18 | 15.79 |
| 5L | 4 | 2.1 | 2 | 2.44 | 2 | 1.90 | – | – | 4 | 3.51 |
| 6L | 2 | 1.1 | – | – | 2 | 1.90 | 1 | 1.37 | 1 | 0.88 |
| Index treatment regimen, n (%)a | ||||||||||
| Idelalisib | 10 | 5.4 | 6 | 7.32 | 4 | 3.81 | 4 | 5.48 | 6 | 5.26 |
| Anti-CD20 mAb + alkylator | 97 | 51.9 | 34 | 41.46 | 63 | 60.00 | 37 | 50.68 | 60 | 52.63 |
| Anti-CD20 mAb monotherapy | 15 | 8.0 | 13 | 15.85 | 2 | 1.90 | 8 | 10.96 | 7 | 6.14 |
| Anti-CD20 mAb + nonalkylator | 23 | 12.3 | 13 | 15.85 | 10 | 9.52 | 12 | 16.44 | 11 | 9.65 |
| Alkylator not in combo w/anti-CD20 mAb | 21 | 11.2 | 8 | 9.76 | 13 | 12.38 | 7 | 9.59 | 14 | 12.28 |
| Neither anti-CD20 mAb nor alkylator | 21 | 11.2 | 8 | 9.76 | 13 | 12.38 | 5 | 6.85 | 16 | 14.04 |
| Had prior auto-HSCT, n (%) | ||||||||||
| No | 146 | 78.1 | 55 | 67.07 | 91 | 86.67 | 51 | 69.86 | 95 | 83.33 |
| Yes | 41 | 21.9 | 27 | 32.93 | 14 | 13.33 | 22 | 30.14 | 19 | 16.67 |
| Years from index date until last available follow-up, median (range) | 9.3 (1.0–21.3) | 11.5 (3.8–21.3) | 7.6 (1.0–19.7) | 11.3 (3.8–21.3) | 7.8 (1.0–20.7) | |||||
| Total number of treatment lines received after initial FL diagnosis, median (range) | 5 (3–11) | 5 (3–11) | 4 (3–11) | 5 (3–11) | 5 (3–11) | |||||
aAlkylating agents were defined as bendamustine, carmustine, cyclophosphamide, ifosfamide, busulfan, chlorambucil, melphalan, nitrosoureas, cisplatin, trofosfamide, as well as any alkylator-containing regimen—BR (bendamustine and rituximab), R-CHOP (rituximab, cyclophosphamide, doxorubicin [doxorubicin hydrochloride {hydroxydaunorubicin hydrochloride}], vincristine, and prednisone), R-DHAP (rituximab, dexamethasone, cytarabine, and cisplatin), DHAP (dexamethasone, cytarabine, and cisplatin), R-CVP (rituximab, cyclophosphamide, vincristine, prednisone), R-EPOCH (rituximab, etoposide, prednisone, vincristine [Oncovin], cyclophosphamide, and doxorubicin hydrochloride [hydroxydaunorubicin hydrochloride]), EPOCH (etoposide, prednisone, vincristine [Oncovin], cyclophosphamide, and doxorubicin hydrochloride [hydroxydaunorubicin hydrochloride]), and RICE (rituximab, ifosfamide, carboplatin, and etoposide).
– = no patients in this category; 3L = third line; 4L = fourth line; 5L = fifth line; 6L = six line; auto-HSCT = autologous hematopoietic stem cell transplantation; ECOG = Eastern Cooperative Oncology Group; FL = follicular lymphoma; mAb = monoclonal antibody; POD4 = progression of disease within 24 months following the start of front-line immunochemotherapy.
Clinical outcomes were measured from the start of each observed therapy line (including the index treatment line) until the earliest of death, last available follow-up, or data cutoff (December 31, 2020). The index date was required to occur between January 1, 2000, and December 31, 2018; the index date window was selected to adequately capture the typically extended follow-up available for patients with FL, with a selection end date (December 31, 2018) that facilitates a minimum follow-up opportunity of 2 years (before data cutoff on December 31, 2020) for patients with index dates in 2018. Patients’ initial FL diagnosis was required to occur before index date but not earlier than January 1, 1998.
Endpoints examined were complete response (CR) rate, ORR, time to next treatment or death (TNT-D), event-free survival (EFS), and overall survival (OS). For each patient and each line of therapy, CR and ORR were based on the physician’s assessment and interpretation of information in the patient’s medical record regarding best clinical response, where ORR was derived as the proportion of patients with a best response of complete or partial response. To maintain consistency with ZUMA-5,12 ELARA,13 and other single-arm trials, nonresponders were defined as patients with progressive disease, stable disease, or unknown response status; thus, patients with unknown (ie, missing) response status were included in the denominator of the CR and ORR rates reported here and were not excluded from the analysis. As this was a retrospective study, no predetermined criteria (eg, Lugano) for response assessments were imposed upon the clinicians in determining best response to each therapy line. TNT-D was defined as time (in months) from start of a treatment line until the earliest of start of the next treatment line or death if no new treatment line was initiated; patients who did not have a next treatment line and were still alive at last follow-up were censored at last available follow-up date. EFS was defined as time (in months) from start of a treatment line until the earliest of clinician-documented progression during and up to 2 weeks after completion or discontinuation of the treatment line, start of a new treatment line in the absence of disease progression (start of maintenance therapy was not considered to be the start of a new treatment line), or death due to any cause; patients who did not have an event as defined above were censored at last available follow-up date. In the context of a retrospective chart review, EFS is similar to PFS as measured in a clinical trial, but with the additional consideration of starting a new anticancer treatment as an event in the absence of clinician-documented progression. Overall survival was defined as time (in months) from start of a treatment line until death due to any cause; patients who did not have a death event recorded were censored at last available follow-up date. Finally, although histological transformation before the index date was an exclusion criterion for study entry, the proportion of patients of patients who experienced transformation after the index date was examined. In subgroup analyses, all endpoints were examined by double refractoriness (failure to respond or relapsed to both an anti-CD20 mAb and an alkylator) at index and POD24 status at index. POD24 was defined by patients who, by the time of the index date, had received a first exposure to anti-CD20 mAb treatment and did not respond (stable/progressive disease as best response) or progressed in less than 24 months after starting the anti-CD20 mAb. Progression within less than 24 months after anti-CD20 mAb initiation was based on the date of documented clinical progression or date of starting a new therapy line, whichever occurred first.
Analyses were descriptive, with time-to-event outcomes analyzed via the Kaplan-Meier method. In addition to the primary analyses, to assess the validity of ReCORD-FL as a source for historical controls, EFS and OS were additionally analyzed and compared with results from DELTA for a subset of double-refractory patients in ReCORD-FL who did not receive idelalisib. Because patients in ReCORD-FL could have multiple relapses or refractoriness, it was possible to have more than one qualifying “index” treatment line. To appropriately compare EFS and OS in ReCORD-FL with the DELTA population, ReCORD-FL patients identified for this comparison had a qualifying index treatment line that was randomly selected, as described below, to approximate the index treatment line distribution of DELTA trial enrollees. In ReCORD-FL, 95 patients were double refractory and did not receive idelalisib at any point. Among these patients, 36 had a qualifying index treatment line occurring in one greater than the fifth line (5L) (ie, sixth line [6L]+). The remaining 59 patients had a qualifying index line occurring in the third line (3L), fourth line (4L), or 5L. To adjust the distribution of qualifying index line number in ReCORD-FL to match the line number distribution in DELTA (ie, to achieve a median qualifying index line of 5L in ReCORD-FL), we randomly selected 37 patients (1 more than the number with a qualifying index line in >5L) from the group of 59 who had an index line of ≤5L and, for each of these patients, randomly selected their qualifying index line if they had multiple qualifying lines in the ≤5L setting. Likewise, for the 36 patients with an index line >5L, the index line was randomly selected for each patient for those with multiple qualifying lines. Random selection and survival estimations were performed over 100 replications, and mean data from those replications were used to produce the survival distributions reported here. Each replicate estimation of EFS (using the Kaplan-Meier method) was weighted using inverse probability of treatment weighting based on the probability of the index line selection during the random selection procedure.
In addition to the formal validation exercise described earlier, findings from ReCORD-FL were also compared with a recent study, SCHOLAR-5,16 that was undertaken with the similar aim of constructing an international external control cohort providing comparative evidence in r/r FL. A summary of this comparison is presented later in the discussion. The single-arm DELTA trial and the SCHOLAR-5 real-world study were selected for ReCORD-FL comparison and validity assessment because there have been no randomized trials to date in the r/r FL setting directly comparing usual care with a novel treatment.
This study was subjected to review and approval by country- and site-specific institutional review boards (IRBs), where applicable. Further information on the local IRBs reviewing this study is available upon request.
RESULTS
A total of 187 patients were identified for inclusion (Table 1). Most patients’ (80.2%) index therapy occurred in 3L (range, 3L–6L). Anti-CD20 mAb plus chemotherapy (including alkylating or non-alkylating agents) was the most common index regimen (64.2% of patients); 8% received anti-CD20 mAb monotherapy, 11.2% received alkylator-based chemotherapy alone (ie, an alkylator-containing regimen without anti-CD20 mAb), and 16.6% received other therapies (ie, other regimens containing neither anti-CD20 mAb nor alkylator). Median follow-up from FL diagnosis was 9 years (range, 1–21 years), over which a median of 5 (range, 3–11) lines of therapy were observed per patient.
CR rate and ORR to the index treatment were 39.0% and 70.6%, respectively (Table 2). Median (95% CI) TNT-D and EFS from index were 14.4 (11.8-18.6) and 14.6 (11.0-18.0) months, respectively. Median OS from index was 128 months (10.6 years). Compared with patients who were not double refractory (n = 82), those with double-refractory disease at index (n = 105) had numerically lower CR rate (34.3% versus 45.1%) and ORR (67.6% versus 74.4%) and substantially shorter median (95% CI) TNT-D (11.8 [9.0-15.2] versus 20.9 [14.4-26.2] months), EFS (10.7 [7.7-14.5] versus 20.1 [14.4-25.4] months), and OS (78.1 [45.8-146.7] months versus not reached). Outcomes were similarly less favorable for patients who had POD24 at index, as these patients had a lower CR rate (36.8% versus 42.5%) and ORR (65.8% versus 78.1%) and shorter median (95% CI) TNT-D (11.9 [9.8-15.2] versus 20.9 [14.4-26.8] months), EFS (11.8 [8.5-14.6] versus 19.5 [12.0-26.2] months), and OS (101.9 [59.5-not reached] versus 128.0 [76.0-not reached] months) as compared with patients who did not have POD24 at index.
Table 2.
Clinical Outcomes by Double Refractoriness and POD24 Status
| All Patients (N = 187) | Double Refractory Before the Index Treatment Line | POD24 to Frontline Anti-CD20 mAb Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No (n = 82) | Yes (n = 105) | No (n = 73) | Yes (n = 114) | |||||||
| Best clinical response, n (%) | ||||||||||
| CR | 73 | 39.0 | 37 | 45.1 | 36 | 34.3 | 31 | 42.5 | 42 | 36.8 |
| PR | 59 | 31.6 | 24 | 29.3 | 35 | 33.3 | 26 | 35.6 | 33 | 29.0 |
| ORR [CR + PR], n (%) | 132 | 70.6 | 61 | 74.4 | 71 | 67.6 | 57 | 78.1 | 75 | 65.8 |
| PD | 23 | 12.3 | 5 | 6.1 | 18 | 17.1 | 5 | 6.9 | 18 | 15.8 |
| SD | 17 | 9.1 | 8 | 9.8 | 9 | 8.6 | 5 | 6.9 | 12 | 10.5 |
| Unknown | 15 | 8.0 | 8 | 9.8 | 7 | 6.7 | 6 | 8.2 | 9 | 7.9 |
| TNT-D | ||||||||||
| n, % censored | 37 | 19.8 | 16 | 19.5 | 21 | 20.0 | 18 | 24.7 | 19 | 16.7 |
| Median, mo (95% CI) | 14.6 (11.8–18.6) | 20.9 (14.4–26.2) | 11.8 (9.0–15.2) | 20.9 (14.4–26.8) | 11.9 (9.8–15.2) | |||||
| EFS | ||||||||||
| n, % censored | 36 | 19.3 | 16 | 19.5 | 20 | 19.0 | 18 | 24.7 | 18 | 15.8 |
| Median, mo (95% CI) | 14.4 (11.0–18.0) | 20.1 (14.4–25.4) | 10.7 (7.7–14.5) | 19.5 (12.0–26.2) | 11.8 (8.5–14.6) | |||||
| 18-mo EFS rate, % | 44.2 | 54.9 | 35.8 | 54.7 | 37.4 | |||||
| OS | ||||||||||
| n, % censored | 114 | 61.0 | 58 | 70.7 | 56 | 53.3 | 50 | 68.5 | 64 | 56.1 |
| Median, mo (95% CI) | 128.0 (78.1–NR) | NR (128.0–NR) | 78.1 (45.8–146.7) | 128.0 (70.6–NR) | 101.9 (59.5–NR) | |||||
| 2-y OS rate, % | 92.4 | 90.0 | 75.9 | 88.8 | 77.9 | |||||
| Histological transformation after start of index therapy, n (%) | ||||||||||
| Yes | 32 | 17.1 | 8 | 9.8 | 24 | 22.9 | 32 | 17.1 | 8 | 9.8 |
| No | 151 | 80.8 | 72 | 87.8 | 79 | 75.2 | 151 | 80.8 | 72 | 87.8 |
| Unknown | 4 | 2.1 | 2 | 2.4 | 2 | 1.9 | 4 | 2.1 | 2 | 2.4 |
| Type of histological transformation, among patients with transformation, n (%) | (n = 32) | (n = 8) | (n = 24) | (n = 32) | (n = 8) | |||||
| DLBCL | 25 | 78.13 | 6 | 75.00 | 19 | 79.17 | 8 | 80.00 | 17 | 77.27 |
| BCLU | 1 | 3.13 | 1 | 12.50 | – | – | 1 | 10.00 | – | – |
| Other | 2 | 6.25 | – | – | 2 | 8.33 | – | – | 2 | 9.09 |
| Unknown | 4 | 12.50 | 1 | 12.50 | 3 | 12.50 | 1 | 10.00 | 3 | 13.64 |
| Median (range) time to transformation, months, among patients with transformation | 16.4 (0–103.4) | 27.3 (1.2–72.5) | 12.4 (0–103.4) | 14.2 (0–103.4) | 16.7 (1.2–84.9) | |||||
BCLU = Unclassifiable B-cell lymphoma; CI = confidence intervals; CR = complete response; DLBCL = Diffuse large B-cell lymphoma; EFS = event-free survival; FL = follicular lymphoma; NR = not reached; ORR = overall response rate; OS = overall survival; PD = progression of disease; POD4 = progression of disease within 24 months following the start of front-line immunochemotherapy; PR = partial response; SD = stable disease; TNT-D = time to next treatment or death.
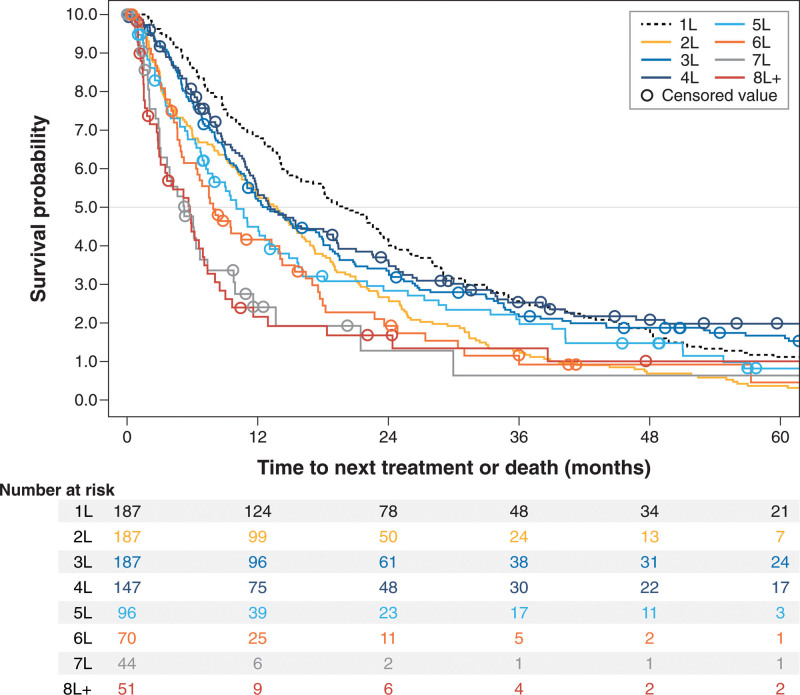
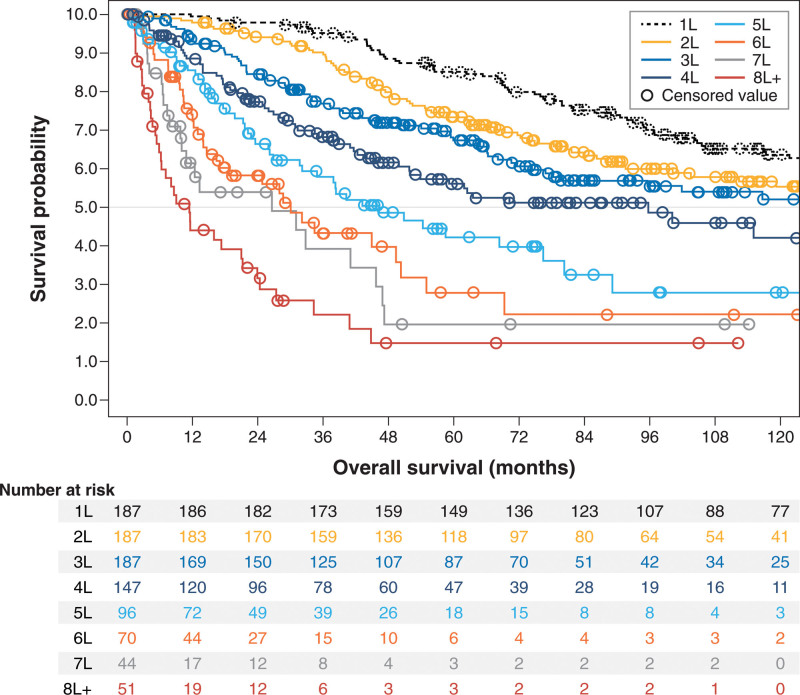
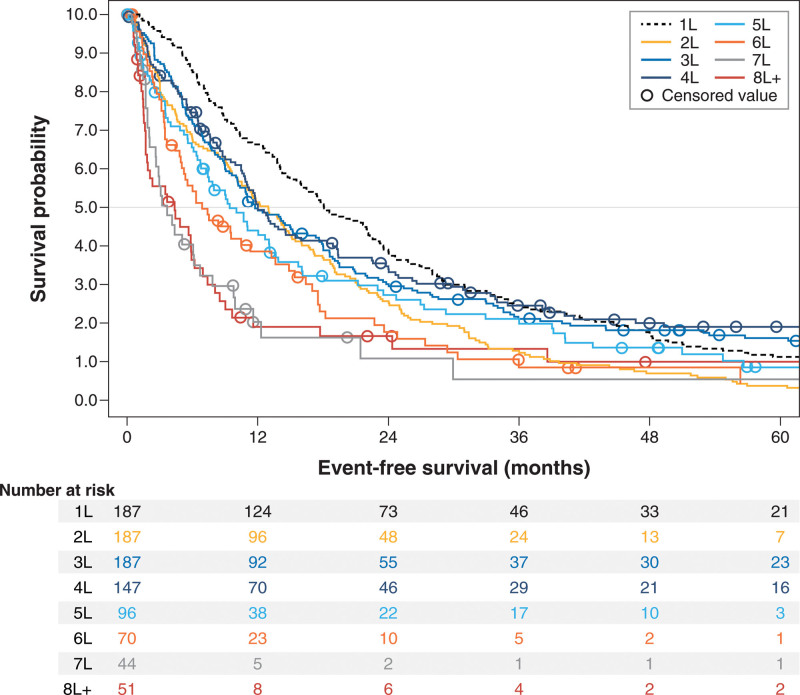
All response and survival outcomes steadily worsened across successive treatment lines (Table 3, Figures 1–3). Overall response rate, for example, decreased from 69.5% in 3L (n = 187) to 45.8% in 5L (n = 96) and 41.2% in ≥eighth line (8L) (n = 51), whereas median (95% CI) EFS decreased from 11.8 (10.1-16.6) months in 3L to 9.4 (6.8-13.1) months in 5L and 4.4 (1.7-5.9) months in ≥8L; median (95% CI) OS had a similar trend: 133.7 (78.1-232.4), 46.3 (31.7-76.5), and 11.4 (5.9-21.2) months in 3L, 5L, and ≥8L, respectively.
Table 3.
Clinical Outcomes by Line of Therapy
| Index Line (N = 187) | All Treatment Lines After Initial FL Diagnosis | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1L (n = 187) | 2L (n = 187) | 3L (n = 187) | 4L (n = 147) | 5L (n = 96) | 6L (n = 70) | 7L (n = 44) | ≥8L (n = 51) a | |||||||||||
| Best clinical response | ||||||||||||||||||
| CR, n (%) | 73 | 39.0 | 71 | 38.0 | 56 | 30.0 | 70 | 37.4 | 47 | 32.0 | 21 | 21.9 | 13 | 18.6 | 7 | 15.9 | 10 | 19.6 |
| PR, n (%) | 59 | 31.6 | 61 | 32.6 | 54 | 28.9 | 60 | 32.1 | 43 | 29.3 | 23 | 24.0 | 21 | 30.0 | 8 | 18.2 | 11 | 21.6 |
| ORR, n (%) | 132 | 70.6 | 132 | 70.6 | 110 | 58.8 | 130 | 69.5 | 90 | 61.2 | 44 | 45.8 | 34 | 48.6 | 15 | 34.1 | 21 | 41.2 |
| TNT-D | ||||||||||||||||||
| n, % censored | 37 | 19.8 | − | − | − | − | 30 | 16.0 | 36 | 24.5 | 16 | 16.7 | 11 | 15.7 | 11 | 25.0 | 10 | 19.6 |
| Median, mo (95% CI) | 14.6 (11.8-18.6) | 19.9 (16.0-23.3) | 13.8 (10.5-16.1) | 12.9 (10.9-17.6) | 13.2 (11.3-19.0) | 10.1 (7.3-13.1) | 7.9 (5.2-14.0) | 5.3 (3.0-7.4) | 5.8 (3.0-7.1) | |||||||||
| EFS | ||||||||||||||||||
| n, % censored | 36 | 19.3 | − | − | − | − | 29 | 15.5 | 34 | 23.1 | 16 | 16.7 | 10 | 14.3 | 9 | 20.5 | 9 | 17.6 |
| Median, mo (95% CI) | 14.4 (11.0-18.0) | 18.1 (14.9-22.2) | 13.0 (10.2-14.9) | 11.8 (10.1-16.6) | 12.0 (10.7-16.0) | 9.4 (6.8-13.1) | 6.9 (4.9-13.3) | 3.7 (2.1-6.1) | 4.4 (1.7-5.9) | |||||||||
| 18-mo EFS rate, % | 44.2 | 51.3 | 36.9 | 40.4 | 41.6 | 32.5 | 23.3 | 16.2 | 16.9 | |||||||||
| OS | ||||||||||||||||||
| n, % censored | 114 | 61.0 | 114 | 61.0 | 114 | 61.0 | 114 | 61.0 | 84 | 57.1 | 48 | 50.0 | 32 | 45.7 | 21 | 47.7 | 15 | 29.4 |
| Median, mo (95% CI) | 128.0 (78.1-NR) | 182.3 (147.9-NR) | 150.5 (103.6-236.8) | 133.7 (78.1-232.4) | 95.8 (51.9-134.9) | 46.3 (31.7-76.5) | 30.1 (16.4-50.3) | 26.6 (10.0-45.7) | 11.4 (5.9-21.2) | |||||||||
| 18-mo OS rate, % | 92.4 | 99.5 | 97.9 | 93.5 | 88.7 | 85.7 | 74.1 | 60.9 | 44.4 | |||||||||
aDue to small sample sizes in later therapy lines, 8L+ consolidates all lines of therapy from 8L or later and includes repeated observations for some patients.
1L = first line; 2L = second line; 3L = third line; 4L = fourth line; 5L = fifth line; 6L = sixth line; 7L = seventh line; 8L = eighth line; CR = complete response; EFS = event-free survival; FL = follicular lymphoma; NR = not reached; ORR = overall response rate; OS = overall survival; PR = partial response; TNT-D = time to next treatment or death.
Figure 1.
Kaplan-Meier curves for time to next treatment or death from start of treatment, by line of therapy after initial follicular lymphoma diagnosis among patients selected for having ≥3 lines of therapy.
Figure 3.
Kaplan-Meier curves for overall survival from start of treatment, by line of therapy after initial follicular lymphoma diagnosis among patients selected for having ≥3 lines of therapy.
Figure 2.
Kaplan-Meier curves for event-free survival from start of treatment, by line of therapy after initial follicular lymphoma diagnosis among patients selected for having ≥3 lines of therapy.
Finally, 32 of the 187 patients (17.1%) included in ReCORD-FL experienced histological transformation following initiation of the index treatment line. Transformation was more common in patients who were double refractory at index (22.9%) versus not double refractory (9.8%); postindex transformation was also higher in patients who were POD24 at index (19.3%) versus not (13.7%). Among the 32 patients with postindex transformation, diffuse large B-cell lymphoma (DLBCL) was the most common type of transformation (n = 25, 78.1%). Among these 32 patients, median time to transformation from the index date was 16.4 months. The type of and time to transformation among those with transformation did not vary substantially by double refractory or POD24 status.
DISCUSSION
Despite the observation that FL is characterized by inevitable and recurrent relapses, there is a major gap in understanding outcomes in the relapsed and refractory setting. Because of both ethical and practical considerations, interventional studies are most often designed as single-arm trials, and there is a growing need for data on r/r FL patients who have received usual care in order to best implement new therapies. Such data help to further contextualize findings from interventional studies and to provide a potentially valuable data source for constructing external, historical control cohorts for single-arm trials. With this in mind, ReCORD-FL is a multicenter, multicountry, natural history study that provides benchmarking data against which therapeutic interventions may be compared.
Our findings reflect the long-term course of FL, as the patients examined here had a median total follow-up duration of 9.3 years from initial diagnosis and treatment pathways involving multiple lines of therapy (median [range]: 5 [3–11] treatment lines). Findings further demonstrate the poorer outcomes of patients with double-refractory disease or POD24, as these patients had lower rates of clinical response and EFS that were approximately half the duration of those who were not double refractory or did not have POD24. Our findings also showed steadily worsening outcomes with successive lines of therapy (median OS, for example, decreased in duration from approximately 11 years at start of 3L treatment to less than 1 year at 8L+), further documenting the diminishing prognosis of patients with multiply r/r FL, even in the modern era of anti-CD20 agents.
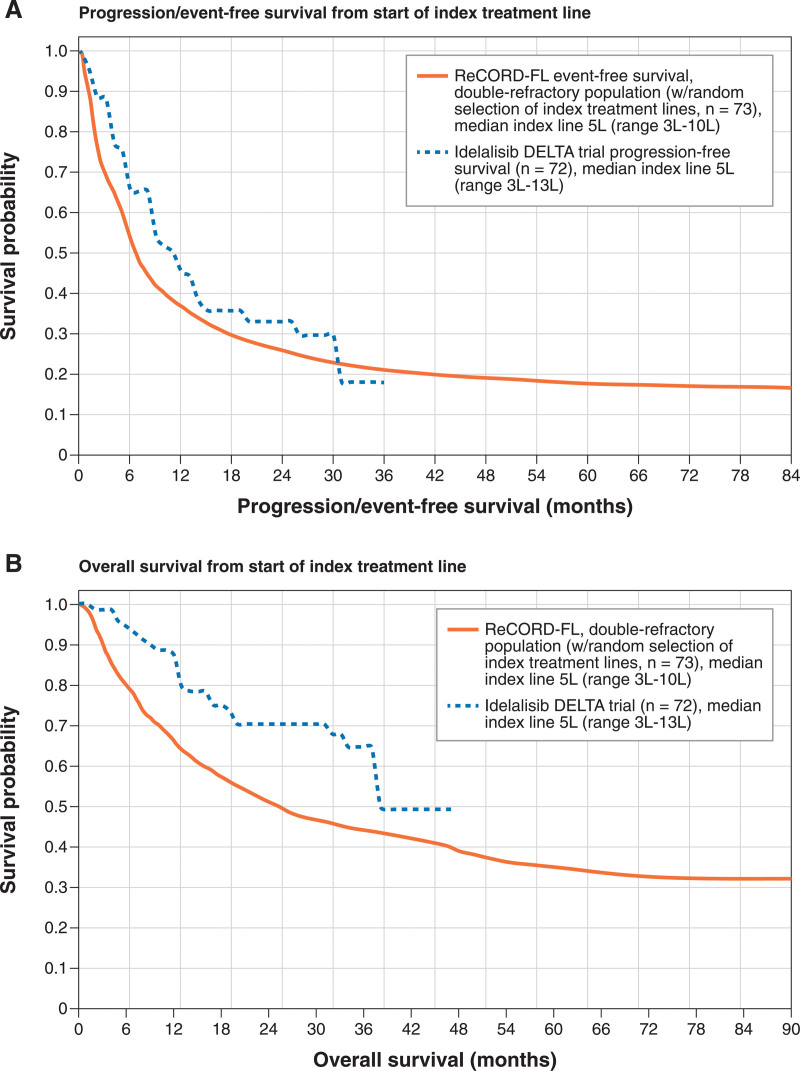
To assess the validity of ReCORD-FL as a comparable source of historical controls, EFS and OS were additionally compared with results from DELTA for double-refractory patients in ReCORD-FL who did not receive idelalisib. After random treatment line selection in ReCORD-FL and obtaining mean data over 100 replications, the median index treatment line for patients in ReCORD-FL was 5L (range, 3L–10L), which is comparable to the DELTA trial (median index line of 5L; range, 3L–13L) (Figure 4A). In DELTA, median (95% CI) PFS was 11.0 (8.0-14.0) months versus 6.8 (4.4-11.8) months for EFS in ReCORD-FL after random treatment line selection. OS in ReCORD-FL after randomized selection of index treatment line tracked substantially lower than OS in the DELTA trial (Figure 4B). These findings, indicating less favorable outcomes in ReCORD-FL for usual care recipients as compared with idelalisib recipients in DELTA, provide some evidence indicating the validity of ReCORD-FL as an external control cohort for r/r FL trials.
Figure 4.
Validation of ReCORD-FL against DELTA trial progression-free survival and overall survival. (A) Progression/event-free survival from start of index treatment line. (B) Overall survival from start of index treatment line.
Besides ReCORD-FL, the most recent study undertaken with similar aims is the SCHOLAR-5 study, which is an international, external control cohort generated to provide comparative evidence in r/r FL patients meeting the inclusion criteria of the ZUMA-5 trial.16 Key eligibility criteria are consistent between SCHOLAR-5 and ReCORD-FL (ie, ≥2 prior lines of therapy, grades 1-3A FL, ECOG of 0 or 1, and no histological transformation allowed before start of follow-up [ie, before the index treatment line]). Similar to the validation exercises described earlier involving comparison of ReCORD-FL to DELTA, SCHOLAR-5 employed randomization of index treatment line selection as well as weighting techniques to achieve comparability with ZUMA-5. After weighting, the mean number of therapy lines before the index line in SCHOLAR-5 was 3.5, indicating that, on average, patients in the weighted SCHOLAR-5 comparison with ZUMA-5 had an index line of 4L or 5L. When examining 4L and 5L outcomes in ReCORD-FL, generally comparable results to SCHOLAR-5 were observed for most endpoints (Table 4) except for 4L OS, which was longer in ReCORD-FL (95.8 months) than in SCHOLAR-5 (59.8 months). This discrepancy in OS may be the result of a somewhat younger population captured in ReCORD-FL (median age at index of 58 years, as compared with 62 years in SCHOLAR-5) and a substantially longer historical follow-up for ReCORD-FL patients (median 9.3 years as compared with 2.2 years in SCHOLAR-5).
Table 4.
ReCORD-FL Versus SCHOLAR-5
| Endpoint | SCHOLAR-5 | ReCORD-FL | |
|---|---|---|---|
| 4L Treatment (n = 85) | 4L Treatment (n = 147) | 5L Treatment (n = 96) | |
| CR | 29.9% | 32.0% | 21.9% |
| ORR | 49.9% | 61.2% | 45.8% |
| TNT-D, median (95% CI), months | 14.4 (6.2-25.8) | 13.2 (11.3-19.0) | 10.1 (7.3-13.1) |
| PFS/EFS, median (95% CI), months | 12.7 (6.2-14.7) | 12.0 (10.7-16.0) | 9.4 (6.8-13.1) |
| OS, median (95% CI), months | 59.8 (21.9-NR) | 95.8 (51.9-134.9) | 46.3 (31.7-76.5) |
4L = fourth line; 5L = fifth line; CR = complete response; EFS = event-free survival; NR = not reached; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; TNT-D = time to next treatment or death.
ReCORD-FL was subject to several limitations inherent in retrospective medical record review studies, and these limitations should be considered when drawing conclusions from or interpreting the study findings. First, as the purpose of this study was to create an external control cohort for comparison with single-arm interventional trials in r/r FL, the study was designed with stricter trial-like inclusion criteria to ensure that the patients captured in ReCORD-FL were clinically similar to patients in relevant trials. Although this aspect of the study design achieves comparability with the trials, it diminishes generalizability to all real-world r/r FL patients, many of whom may not receive treatment in the types of academic centers from which ReCORD-FL patients were identified. Additionally, all patients were required to have histologically confirmed FL, whereas some practices outside the academic settings of ReCORD-FL may not routinely perform histological assessment, thereby further reducing generalizability. Second, in retrospective studies of cancer patients receiving usual care, clinical assessments of disease progression are not performed on a predetermined schedule or according to a predefined set of progression criteria as would typically be required in a prospective interventional trial. As such, assessments for progression in usual care studies tend to be less frequent and more subjective than those performed within the stricter protocols of clinical trials. Moreover, in retrospective studies, the methods and information upon which clinical assessments were made within the same patient across different lines of therapy may also be heterogeneous, reducing intrapatient comparability of treatment response at each line of therapy. One implication of this limitation is that progression events may be detected and recorded later than they would have been otherwise under a more frequent schedule, as typical in a clinical trial, which may introduce some overestimation of PFS or EFS. There is also evidence in the literature that clinical assessments of treatment response in routine practice, which typically do not adhere to a predefined set of progression criteria (eg, the Lugano criteria), may overestimate treatment benefit.17 In ReCORD-FL, while radiographic imaging was frequently documented in determining treatment response (79.1% of all index treatment responses), standard assessment criteria such as Lugano were rarely documented (only 7% of all index treatment responses). Although it is reassuring for clinical assessment accuracy that radiographic imaging was most prominent, data were not collected on the specific type of imaging (eg, computed tomography and magnetic resonance imaging) utilized. With no randomized trials available comparing usual care to novel therapies in r/r FL, and considering that retrospective application of formal response criteria such as Lugano is difficult in this setting, further studies are needed to confirm or more accurately benchmark outcomes for patients with r/r FL receiving usual care. Despite these limitations, as shown in the validation exercise summarized earlier, endpoint data collected in ReCORD-FL appear to be reasonably consistent with that for the r/r FL populations in the DELTA and SCHOLAR-5 studies. Third, the clinical endpoints in ReCORD-FL were examined separately for all lines of therapy observed from initial FL diagnosis. However, findings on outcomes for first-line (1L) and second-line (2L) treatment should be interpreted with caution, as the ReCORD-FL study design required all patients to have at least 3 lines of therapy. Because most FL patients (>80%) will not require 3 or more lines of treatment within 10 years after initial diagnosis,18 selection of multiply relapsed patients with at least 3 lines of therapy implies that ReCORD-FL (like ELARA) includes patients with more aggressive disease and a worse-than-average baseline prognosis (as indicated by the proportion of patients who experience POD24 at index: 56% in ReCORD-FL and 61% in ELARA) as compared with the general FL population. Therefore, 1L and 2L endpoints in ReCORD-FL should not be directly compared with 1L and 2L endpoints from a general, unselected FL population, where 1L and 2L outcomes are likely to be markedly better. Fourth, as with all retrospective studies, loss to follow-up limited the precision of endpoint estimations for patients’ final line of therapy. In ReCORD-FL, the data cutoff date was December 31, 2020. For patients with a final line of therapy occurring closer to the data cutoff date, it is not possible to definitively determine whether a lack of a progression or death event for EFS and OS analyses was due to follow-up loss or to true censoring resulting from a durable therapy response. Nonetheless, EFS and OS survival curves by line of therapy still followed expected patterns, with survival times steadily decreasing as treatment line increased. Finally, although constructing historical control data such as ReCORD-FL for comparison with prospective interventional trials is a valuable and often necessary research tool (particularly in areas such as r/r FL where most trials are singe-arm), such comparisons should never replace randomized, controlled phase III clinical studies. Opportunities to increase the number of such trials in r/r FL should be explored in future research efforts.
CONCLUSIONS
Follicular lymphoma is characterized by a prolonged disease course that nevertheless requires repeated therapies, with no clear guidance on optimal sequencing or type of intervention. Data collected in ReCORD-FL reflect this pattern, as the patients examined had, at the median, a follow-up duration exceeding 9 years from initial diagnosis spanning multiple lines of therapy. Findings from ReCORD-FL further demonstrate the poor outcomes and limited survival in patients with FL with multiply r/r disease, double-refractory disease, or POD24, which reaffirms the need for particular consideration of these subpopulations in the development of novel treatments. Findings from ReCORD-FL also reaffirm an increasing impact of disease progression (as evidenced by waning clinical response, EFS, and OS) as patients become multiply relapsed, with all outcomes examined having steadily worsened across successive treatment lines. Despite the study limitations noted earlier, based on comparability of results with another similar study (SCHOLAR-5), validation of results with the DELTA trial, the robustness of the data collected, and a lack of randomized trials in r/r FL, ReCORD-FL provides valuable historical control data from a multinational population for new r/r FL therapies in development. Future studies should consider including an even broader geographic scope and larger sample size to more fully capture the variability between institutions and countries in how FL treatment is approached.
ACKNOWLEDGMENTS
We would like to acknowledge the critical contributions of Qiufei Ma, PhD, to the conception, design, and conduct of this study.
AUTHOR CONTRIBUTIONS
All authors provided critical review and revision of each article draft and provided approval for submission of the final draft. GS, JZ, and BKL provided additional contributions in the conception and design of the study. KLD and SN provided additional contributions in the design of the study and conducted all data analyses. GS, SJS, LF, JK, PEMP, BvT, SS, AJU, YW, and BKL provided additional contributions in the collection and provision of study data.
DISCLOSURES
GS received consultancy and honoraria from AbbVie, Allogene, Beigene, BMS/Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Ipsen, Janssen, Kite-Gilead, Loxo, Miltneiy, Morphosys, Novartis, Rapt, Regeneron, Takeda, Velosbio. SJS received consultancy, honoraria, patents and royalties, or research funding from Celgene, Nordic, Nanovector, Novartis, Abbvie, Acerta Pharma/AstraZeneca, Alimera Sciences, BeiGene, Juno Therapeutics, Loxo Oncology, Tessa Therapeutics, Genentech/Roche, Merck, Pharmacyclics, Adaptive Biotechnologies, lncyte, TG Therapeutics. LF received travel assistance from Roche. JK received honoraria or research funding from Abbvie, Amgen, Antengene, AstraZeneca, BMS, Gilead, Incyte, Janssen, Karyopharm, Medison Ventures, Merck, Novartis, Pfizer, Roche, Seattle Genetics, TG Therapeutics. PEMP received honoraria or research funding from Roche, Gilead Sciences, Abbvie, AstraZeneca, Janssen, Novartis. BvT received consultancy, research funding, honoraria, or congress/travel support from AbbVie, Amgen, AstraZeneca, BMS-Celgene, Kite-Gilead, MSD, Novartis, Pentixafarm, Pfizer, Roche, Takeda. SS received research funding and other support from Adaptive, ADC Therapeutics, BMS, Morphosys, Janssen, Karyopharm, Genentech, TGT, Bayer, Portola, Acerta, Pharmacyclics, Forty Seven/Gilead, Merck, Novartis. AJU has no relevant financial relationship to disclose. KLD received research funding from Novartis, Vertex, Pfizer, Eisai, Eli Lilly and Company, AstraZeneca. SPN received research funding from Novartis, Eisai, AstraZeneca. JZ received current employment and equity holder with Novartis. VB received current employment and equity holder with Novartis. EJ received current employment and equity holder with Novartis. RR received current employment and equity holder with Novartis. YW received research funding from Incyte, InnoCare, LOXO Oncology, Novartis, Genentech, MorphoSys; board membership with Eli Lilly and Company, TG Therapeutics, LOXO Oncology, Incyt. BKL received consultancy or research funding from Genentech/Roche, MEI, Jannsen, Novartis.
SOURCES OF FUNDING
This study and the preparation of this article was funded by Novartis Pharmaceuticals Corporation.
REFERENCES
- 1.Matasar MJ, Luminari S, Barr PM, et al. Follicular lymphoma: recent and emerging therapies, treatment strategies, and remaining unmet needs. Oncologist. 2019;24:e1236–e1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salles G, Mounier N, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the gela-goelams fl2000 study. Blood. 2008;112:4824–4831. [DOI] [PubMed] [Google Scholar]
- 3.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–4586. [DOI] [PubMed] [Google Scholar]
- 4.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the german low-grade lymphoma study group. Blood. 2005;106:3725–3732. [DOI] [PubMed] [Google Scholar]
- 5.Montoto S, López-Guillermo A, Ferrer A, et al. Survival after progression in patients with follicular lymphoma: analysis of prognostic factors. Ann Oncol. 2002;13:523–530. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher CJ, Gregory WM, Jones AE, et al. Follicular lymphoma: prognostic factors for response and survival. J Clin Oncol. 1986;4:1470–1480. [DOI] [PubMed] [Google Scholar]
- 7.Maurer MJ, Bachy E, Ghesquières H, et al. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol. 2016;91:1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federico M, Luminari S, Dondi A, et al. R-cvp versus r-chop versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: results of the follo5 trial conducted by the fondazione italiana linfomi. J Clin Oncol. 2013;31:1506–1513. [DOI] [PubMed] [Google Scholar]
- 9.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. [DOI] [PubMed] [Google Scholar]
- 10.Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the national lymphocare study. J Clin Oncol. 2015;33:2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salles G, Schuster SJ, de Vos S, et al. Efficacy and safety of idelalisib in patients with relapsed, rituximab- and alkylating agent-refractory follicular lymphoma: a subgroup analysis of a phase 2 study. Haematologica. 2017;102:e156–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson C, Chavez JC, Sehgal AR, et al. Primary analysis of zuma-5: a phase 2 study of axicabtagene ciloleucel (Axi-Cel) in patients with relapsed/refractory (R/R) indolent non-hodgkin lymphoma (iNHL). Blood. 2020;136(Suppl 1):40–41. [Google Scholar]
- 13.Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 elara trial. Nat Med. 2022;28:325–332. [DOI] [PubMed] [Google Scholar]
- 14.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor t cells in refractory b-cell lymphomas. N Engl J Med. 2017;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghione P, Patel AR, Bobillo S, et al. A comparison of clinical outcomes from ZUMA-5 (axicabtagene ciloleucel) and the international SCHOLAR-5 external control cohort in relapsed/refractory follicular lymphoma (r/r FL). P205-4. Presented at the Annual Congress of the European Hematology Association; June 9–17, 2021; virtual. [Google Scholar]
- 17.Feinberg BA, Bharmal M, Klink AJ, Chadi Nabhan, Hemant Phatak. Using response evaluation criteria in solid tumors in real-world evidence cancer research. Future Oncol. 2018;14:2841–2848. [DOI] [PubMed] [Google Scholar]
- 18.Link BK, Day BM, Zhou X, et al. Second-line and subsequent therapy and outcomes for follicular lymphoma in the united states: data from the observational national lymphoCare study. Br J Haematol. 2019;184:660–663. [DOI] [PubMed] [Google Scholar]