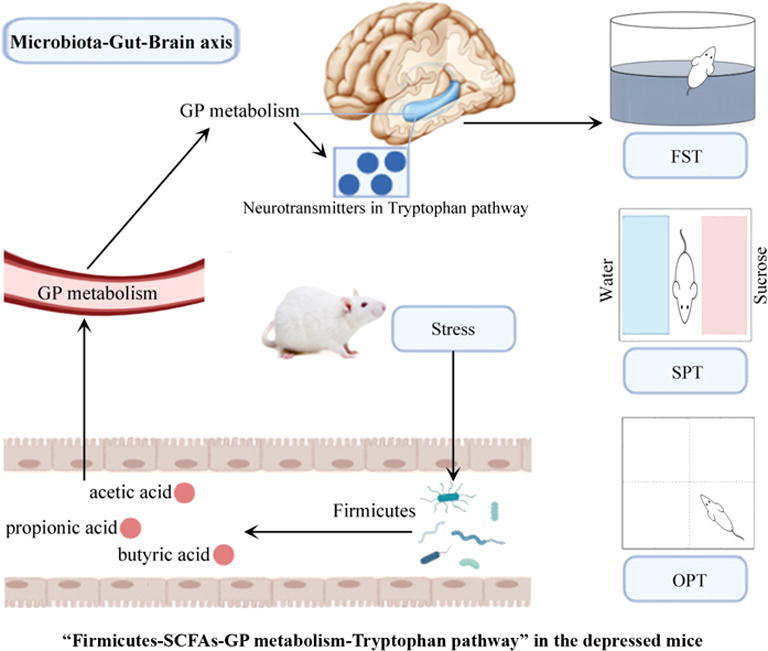
Graphical abstract
Keywords: Gut microbiota, Glycerophospholipids, Neurotransmitter, Depression
Highlights
-
•
Three important ”metabolite type-bacterial genus” correlated pairs were identified.
-
•
Peripheral and central GP metabolism was disordered in depressed mice.
-
•
Four differential NEs from tryptophan pathway in hippocampus were found.
-
•
“Firmicutes-SCFAs-GP metabolism-Tryptophan pathway” was possible way in gut-brain.
Abstract
Introduction
Although researchers have done intensive research on depression, its pathogenesis is still not fully explained. More and more evidence suggests that gut microbiota is closely related to the onset of depression; but its specific functional ways are not clearly identified.
Objectives
The purpose of our work was to find out how the gut microbiota was involved in the onset of depression, and to identify the potential ways to link the gut and brain in mice with depressive-like behaviors (DLB).
Methods
We used the chronic restraint stress (CRS)-induced depression model here. Gut microbiota compositions in fecal samples, lipid metabolism (in fecal, serum and hippocampus samples) and neurotransmitters in hippocampus samples were detected.
Results
We found that the 7 of 13 differential genera that significantly correlated with DLB belonged to phylum Firmicutes. The differential lipid metabolites in fecal samples mainly belonged to glycerophospholipids (GP) and fatty acids (FA) metabolism, and three important “metabolite type-bacterial taxa” correlated pairs were identified: “FA/GP-Firmicutes”, “FA/GP-Akkermansia”, and “FA/GP-Bifidobacterium”. The key differential lipid metabolites significantly correlated with DLB mainly belonged to FA and GP, and the DLB-related metagenomic genes were consistently enriched in GP metabolism and FA metabolism. Three significantly changed short-chain fatty acids (SCFAs) were significantly correlated with the majority of differential genera. Meanwhile, we found that the differential lipid metabolites in serum and hippocampus samples were mainly mapped into the GP metabolism, and there were four differential neurotransmitters from the tryptophan pathway in hippocampus samples.
Conclusion
Together, our findings could provide novel insights into the role of “microbiota-gut-brain” (MGB) axis in depression, and indicate that the gut microbiota might have a vital role in the onset of DLB by affecting the peripheral/central GP metabolism and tryptophan pathway. The “Firmicutes-SCFAs-GP metabolism-Tryptophan pathway” might be a possible way to link the gut and brain in depressed mice.
Introduction
Depression is a common mental disease that causes heavy economic burdens to individuals and society [1]. Although many researchers have done intensive investigation on depression [2], [3], [4], its pathophysiological mechanisms still remain mostly obscure, and an objectively diagnostic method is also unavailable. Currently, most of relevant researches mainly emphasize the role of molecular brain dysfunction in depression [4]. However, based on these theories, no definitive biomarkers for depression have been found so far; and only half of depression patients show a good response to medication treatment [5], [6]. Thus, more researches are necessary for a comprehensive understanding in the pathogenesis of depression.
Gut microbiota is important to the maintenance of host homeostasis. It has been extensively investigated for its effect on the host’s behaviors and brain function through “microbiota-gut-brain” (MGB) axis [7], [8]. For example, it plays a role in the onset of depressive states by interacting with external environmental signals and internal systems [9]; and there is evidence that the disruption of gut microbiota is associated with the pathophysiology of depression [10]. Comprehensive literature analyses showed that compared to healthy subjects, depression patients had the significant differences in gut microbiota composition [11], [12]. In our previous studies, the disturbance of gut microbiota was found in depression patients [13], [14], [15], and these changes were different to the differential gut microbiota compositions in both schizophrenia and bipolar disorder patients [14], [15]. Moreover, after being transplanted with gut microbiota from depressed individuals, the germ-free mice present depressive- and anxiety-like behaviors [14], [18]. These findings suggest a causal role of gut microbiota in the onset of depression [7].
Nonetheless, some crucial questions have not been clearly demonstrated yet. There are knowledge gaps between the disordered gut microbiota and the onset of depression. Previous studies suggested that gut microbiota might be involved in the onset of depression through activating immune system and vagus nerve [19], or by producing metabolites and compounds with neuroactive properties [20]. Lipids make up > 50% of the brain's dry weight, and lipid metabolism is very important to brain functions [21]. As a key regulator of lipid metabolism, gut microbiota could significantly affect the peripheral and central lipid metabolism of the host [22]. Our previous findings showed that it could affect the lipid metabolism of brain tissues (hippocampus, prefrontal cortex) in mice [23], [24]. Meanwhile, we also identified some differential molecules closely related to lipid metabolism in the blood and urine of depression patients [25], [26]. Therefore, alterations in lipid metabolism may explain the link between microbiota alteration and depression.
To further explore the possible functions of lipid metabolism in the onset of depression, 16S rRNA gene sequencing analysis was firstly used here to identify the differential gut microbiota in depressed mice (DM). Then, the whole-genome shotgun metagenomics analysis was conducted to find out the potential functions of the differential gut microbiota. Fecal metabolome is viewed as the functional readout of the gut microbiota [27]. Thus, the lipidomics analysis of fecal samples was conducted here using liquid chromatography-mass spectrometry (LC-MS). In addition, central and peripheral lipids play an important role in depression. Hence, the lipidomics analysis of serum and hippocampus was also performed using LC-MS. Since neurotransmitters have an important role in depression, we analyzed the changes of neurotransmitters in the hippocampus of DM. Integrating these multi-omics data, we sought to find out how the gut microbiota was involved in the onset of depression, and identify the potential way to link the gut and brain in DM.
Methods and materials
Ethics statement
All experiments involving mice were performed in accordance with the ethical policies and procedures approved by the Animal Care Welfare Committee of Guizhou Medical University (No. 2001159).
Experimental animal
Guizhou Medical University offered the male adult C57 black 6 (C57BL/6) mice (age, 8–12 weeks). The mice were isolated and housed under the standard conditions. As the food is one of the main drivers of gut microbiome changes; thus, all the mice received the same food (laboratory mice diet) to rule out the potential effects of food type on the gut microbiota. We used the chronic restraint stress (CRS) model of depression in this study. The mice were randomly assigned to the control group or experimental group. The procedure of CRS was conducted according to our previous studies [28], [29], [30]: in the control group, the mice (n = 10) could freely access to food and water. In the experimental group, the mice (n = 10) were exposed to CRS for four hours per day by placing them in 50 ml-plastic tubes with several holes on the tubes to keep air flow. They were under food and water deprivation during the restraint time. The total process continued for 28 consecutive days.
Behavioral tests
All behavioral tests were conducted according to our previous studies [28], [29], [30]. Depressive-like behaviors (DLB) were evaluated. Briefly, we calculated the total distance, center distance (CD), center time (CT) and number of rearing (RN) in the open field test (OFT), the immobility time (IT) in the forced swim test (FST), the sucrose preference (SPF) in the sucrose preference test (SPT). Both OFT and FST continued for six minutes, and data from the last five minutes was collected. In SPT, the test continued for 24 h. At the endpoint of model building, the fecal samples of the two groups were collected and immediately stored at −80 °C under sterile conditions.
16S rRNA gene sequence and metagenomic analysis
The 16S rRNA gene sequence analysis is conducted according to our previous studies [14], [28]. The alpha diversity is assessed using four parameters (simpson, phylogenetic diversity, shannon, and chao), and the beta diversity is assessed using principal coordinate analysis (PCoA). The microbial communities’ diversity and the difference of bacterial communities between the control mice (CM) and DM are assessed using alpha diversity and beta diversity, respectively. The linear discriminant analysis Effective Size (LEfSe) is conducted to identify the statistically and significantly different genus. Meanwhile, the metagenomic analysis is also conducted according to our previous studies [14], [16]. Briefly, we used the Bayesing model averaging to remove the reads belonging to the mice’s reference genome. The metagenomic genes are aligned into Kyoto Encyclopedia of Genes and Genomes (KEGG) genes using BLASTP (expectation value, 1e−5), and then KEGG is used to annotate the function of nonredundant genes.
Lipid metabolites identification and neurotransmitters detection
The lipid metabolites in three kinds of biological samples (fecal, serum and hippocampus) are detected. The detailed information of metabolites detections is described in Supplementary File 1. The orthogonal partial least-squares discriminant analysis (OPLS-DA) is conducted here to find the differential lipid metabolites between CM and DM. According to the number of samples used to build the model, the metabolites with the absolute value of regression coefficient > 0.632 (equivalent to a p-value < 0.05) are identified as the key differential metabolites responsible for the discrimination between CM and DM. Meanwhile, 15 neurotransmitters in the tryptophan pathway in hippocampus samples are detected: 5-hydroxytryptophan, Tryptophan, 5-hydroxyindoleacetic acid, 3-hydroxyanthranilic acid, Tryptamine, Tryptopholle, Kynurenine, Kynurenic acid, N-acetyl serotonin, Homovanillic acid, 5-hydroxytryptamine (5-HT), Indole-3-carboxaldehyde, Indolelactic acid, Melatonine and Histamine. The detailed information of neurotransmitters detections is described in Supplementary File 1.
Statistical analysis
The SPSS version 20 and R software 3.6 are used to do all the statistical analyses. The regularized canonical correlation analysis (rCCA) is conducted here to find the “metabolite type-bacterial taxa” correlation pairs. To identify key DLB-related metabolic modules, the weighted correlation network analysis (WGCNA) is conducted here. The Benjamini and Hochberg False Discovery method is conducted here to adjust the p-value in the multiple statistical tests. The adjusted p-value < 0.05 is considered as statistically significant. The investigators are blinded to the group allocations and outcomes assessments.
Results
Behavioral characteristics in depressed mice
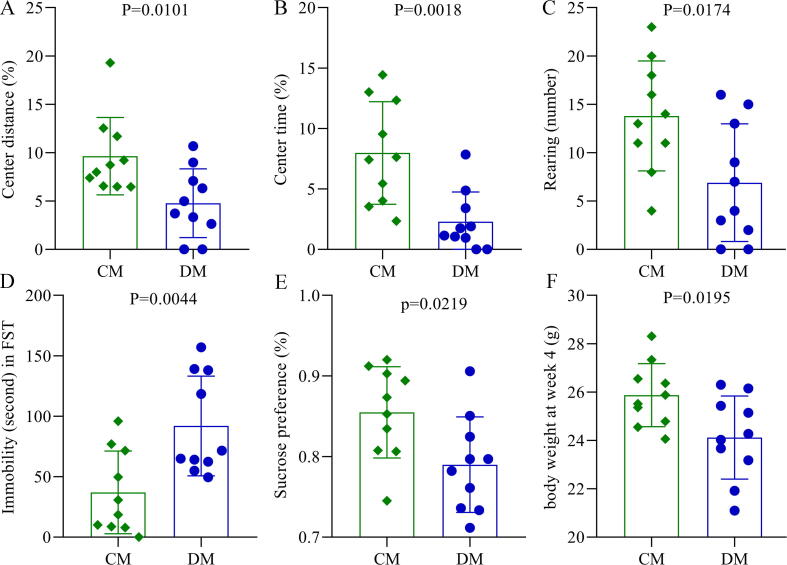
During the period of model building, the food consumption was not significantly different between the two groups (p = 0.57), although the depressed mice had the lower food consumption. In the OFT, we found that the total distances between the two groups were similar, but there were significant differences in CD (p = 0.0101, Fig. 1A), CT (p = 0.0018, Fig. 1B) and RN (p = 0.0174, Fig. 1C) between the two groups. The results of FST showed that compared to CM, DM had a significantly increased IT (p = 0.0044, Fig. 1D). At baseline, both SPF and body weight (BW) were similar between the two groups; but in the end, both SPF (p = 0.0219, Fig. 1E) and BW (p = 0.0195, Fig. 1F) were significantly lower in DM than in CM. These phenomenon were consistent with our previous findings [28], [29], [30], confirming the effectiveness of CRS in building the model of depression.
Fig. 1.
DLB in chronic restraint stress-induced depressed mice. A-C) DM showed the significantly decreased CD (%) (A), CT (%) (B) and RN (C) in OFT; D) the IT in FST was significantly higher in DM than in CM; E) the SPF in SPT was significantly decreased in DM than in CM; F) the body weight at 4 week was significantly lower in DM than in CM. DM, depressed mice; CM, control mice; CD, center distance; CT, center time; RN, the number of rearing; IT, immobility time; SPF, sucrose preference; OFT, open field test; FST, forced swimming test; SPT sucrose preference test.
Differential gut microbiota compositions
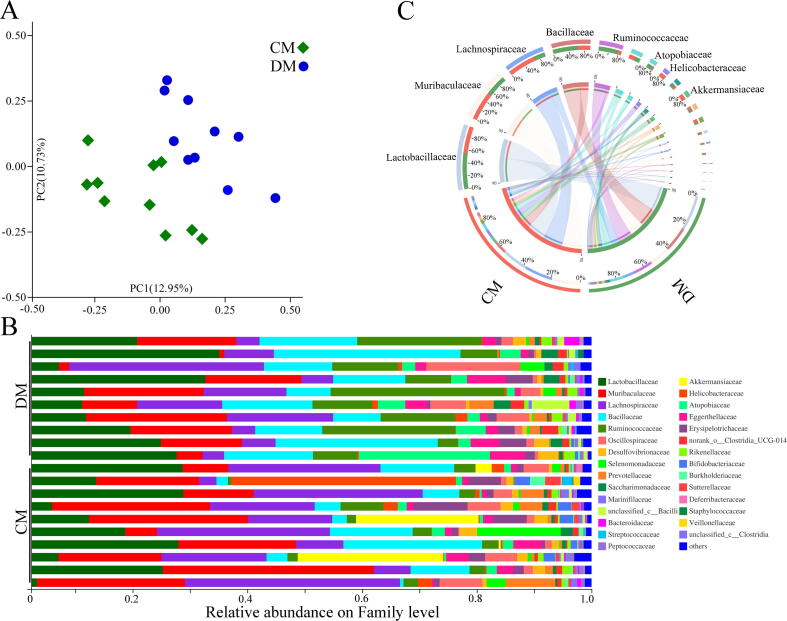
Firstly, the alpha diversity was assessed using four parameters, and no significant difference was observed between DM and CM (Supplementary Fig. 1). However, the PCoA found that the gut microbial community compositions were significantly different between DM and CM (p = 0.0030) (Fig. 2A). The relative abundance on the Family level was described in Fig. 2B. There were three significantly increased bacteria taxa (Ruminococcaceae, p = 0.0004; Atopobiaceae, p = 0.011; Bacillaceae, p = 0.025) and seven significantly decreased bacteria taxa (Butyricicoccaceae, p = 0.006; Veillonellaceae, p = 0.006; Bifidobacteriaceae, p = 0.022; f_norank_o_Rhodospirillales, p = 0.006; Sutterellaceae, p = 0.026; Akkermansiaceae, p = 0.043; Mitochondria, p = 0.030) on Family level in DM. The four of 10 differential bacteria taxa on the Family level belonged to phylum Firmicutes. As shown in Fig. 2C, the dominant bacteria taxa on Family level in CM were Muribaculaceae (19.46%), Lachnospiraceae (19.16%), Lactobacillaceae (17.73%), Bacillaceae (8.28%), Akkermansiaceae (5.42%), and Helicobacteraceae (5.17%); the dominant bacteria taxa on Family level in DM were Lactobacillaceae (23.76%), Lachnospiraceae (7.57%), Bacillaceae (17.20%), Muribaculaceae (14.07%), Ruminococcaceae (13.62%) and Atopobiaceae (5.14%). The differences of gut microbiota on other levels were displayed in Supplementary Fig. 2.
Fig. 2.
Disordered gut microbiota in depressed mice. A) The principal coordinate analysis showed the obvious differences in the gut microbial compositions between CM and DM; B) the relative abundance on Family level between CM and DM; C) the dominant bacteria taxa on Family level in the two groups. DM, depressed mice; CM, control mice.
Differential lipid-related metagenomic genes
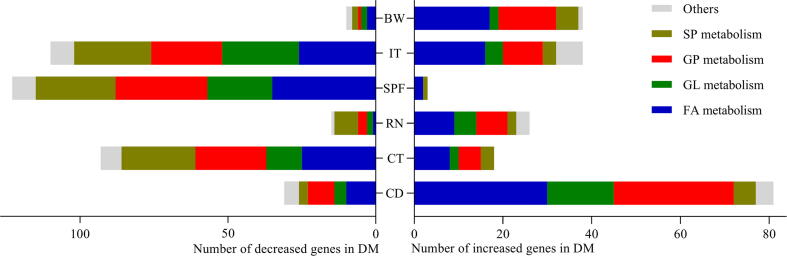
12 fecal samples (n = 6 from DM, n = 6 from CM, randomly) were used for metagenomic analysis. In total, we identified 350 differential lipid-related metagenomic genes: 187 down-regulated genes and 163 up-regulated genes in DM. Among these genes, there were 118 FA-related genes, 61 GL-related genes, 92 GP-related genes, 50 SP-related genes, 10 ST-related genes and 19 other lipid-related genes. The correlation analyses between differential genes and DLB showed that there were 112, 111, 41, 126, 148 and 48 differential genes significantly correlated with CD, CT, RN, SPF, IT and BW, respectively. Functional analysis suggested that these DLB-related genes mainly clustered on GP metabolism, FA metabolism, SP metabolism and GL metabolism (Fig. 3).
Fig. 3.
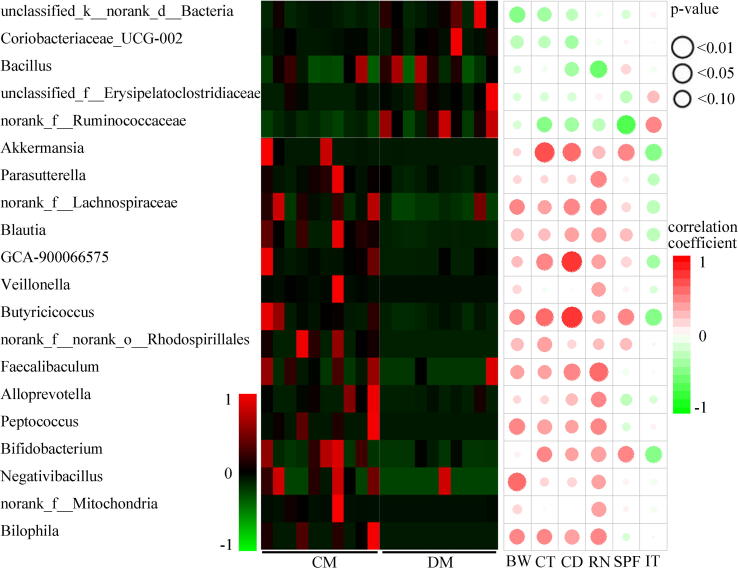
Correlations between differential genera and DLB. DM, depressed mice; CM, control mice; CD, center distance; IT, immobility time; SPF, sucrose preference; CT, center time; BW, body weight; RN, rearing number.
Differential fecal lipid metabolites
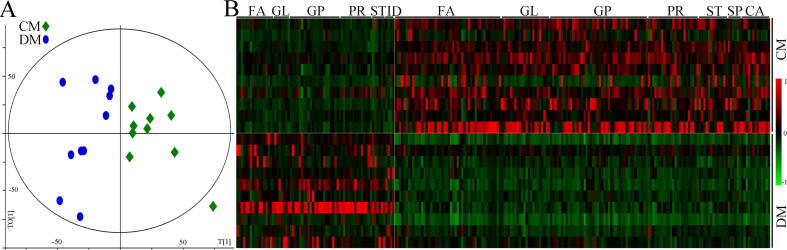
The discrimination model built with fecal metabolites showed a clear separation between CM and DM (Fig. 4A). According to the coefficient loading plot of the built model, 240 differential metabolites with absolute value of regression coefficient > 0.632 were identified. These differential metabolites belonged to Glycerophospholipids (GP) (n = 66, 27.5%), Fatty acids (FA) (n = 66, 27.5%), Prenol lipids (PR) (n = 36, 15%) and Glycerolipids (GL) (n = 30, 12.5%), Steroids and steroid derivatives (ST) (n = 19, 7.92%), Carboxylic acids and derivatives (CA) (n = 13, 5.42%), Sphingolipids (SP) (n = 6, 2.5%), Indoles and derivatives (ID) (n = 4, 1.67%). Heat map of these differential metabolites showed a clear distinction between CM and DM (Fig. 4B). The detailed information of these differential metabolites was described in Supplementary Table 3.
Fig. 4.
Disturbance of fecal lipid metabolism in DM. A) The orthogonal partial least-squares discriminant analysis showed the divergent metabolic phenotypes between CM and DM; B) heatmap of the identified differential lipid metabolites. DM, depressed mice; CM, control mice; GP, Glycerophospholipids; ID, Indoles and derivatives; GL, Glycerolipids; ST, Steroids and steroid derivatives; FA, Fatty acids; CA, Carboxylic acids and derivatives; SP, Sphingolipids; PR, Prenol lipids.
Correlations between differential genera and DLB
20 genera responsible for discriminating CM and DM using LEfSe (Fig. 3 and Supplementary Table 1) were identified. Compared with CM, DM were characterized by 5 increased genera (unclassified_f_Erysipelatoclostridiaceae, norank_f_Ruminococcaceae, Coriobacteriaceae_UCG-002, Bacillus, unclassified_k_norank_d_Bacteria), and 15 decreased genera (Akkermansia, Blautia, Parasutterella, norank_f_Lachnospiraceae, GCA-900066575, Veillonella, Butyricicoccus, Bilophila, Alloprevotella, norank_f_norank_o_Rhodospirillales, Faecalibaculum, Peptococcus, Bifidobacterium, Negativibacillus, norank_f_Mitochondria) (LDA > 2.0 and p-value < 0.05). Interestingly, 55% of the genera (11/20) belonged to the phylum Firmicutes. Among the significantly changed genera, there were 13 genera significantly linked with DLB, especially BW, SPF, RN and CT (Fig. 5). The 7 of 13 genera that significantly correlated with at least one behavioral phenotype belonged to phylum Firmicutes. In addition, 80 differential OTUs (11 increased OTUs and 69 decreased OTUs in DM) between the two groups were identified, and the majority of the OTUs (50/80, 62.5%) also belonged to the phylum Firmicutes (Supplementary Table 2).
Fig. 5.
Differential lipid-related metagenomic genes correlated with DLB. CD, center distance; IT, immobility time; SPF, sucrose preference; CT, center time; BW, body weight; RN, rearing number; GP, Glycerophospholipids; FA, Fatty acids; GL, Glycerolipids; SP, Sphingolipids.
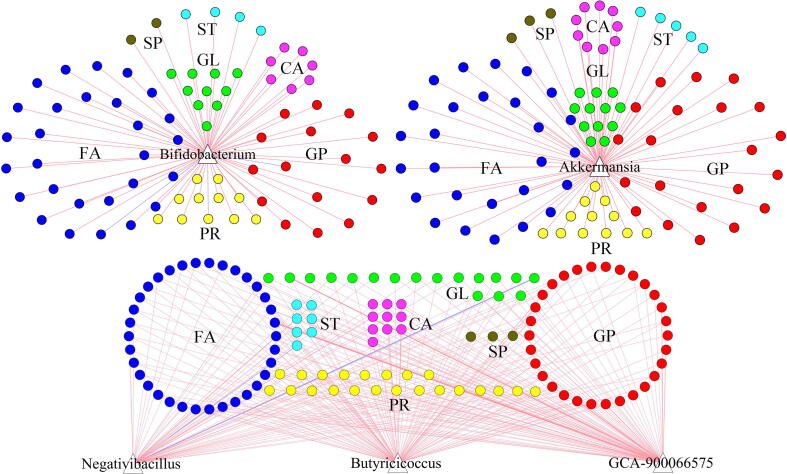
“Metabolite type-bacterial taxa” correlation pairs
The rCCA results found that the contributions to the overall correlations between these differential fecal lipid metabolites and differential genera were mainly from the FA, GP and five differential genera. These five differential genera significantly correlated with most differential metabolites were Bifidobacterium, Akkermansia, GCA-900066575, Negativibacillus and Butyricicoccus (Fig. 6). The genus GCA-900066575, Negativibacillus and Butyricicoccus were under phylum Firmicutes. In total, 37 differential FA and 35 differential GP were significantly correlated with genera under phylum Firmicutes. In addition, 27 differential FA and 21 differential GP were significantly correlated with Akkermansia; 28 differential FA and 16 differential GP were significantly correlated with Bifidobacterium. Considering the biological significance and variable abundance, “FA/GP-Firmicutes”, “FA/GP-Akkermansia”, and “FA/GP-Bifidobacterium” were viewed as the key ”metabolite type-bacterial taxa” correlated pairs.
Fig. 6.
“Metabolite type-bacterial taxa” correlation pairs. GP, Glycerophospholipids; ID, Indoles and derivatives; GL, Glycerolipids; ST, Steroids and steroid derivatives; CA, Carboxylic acids and derivatives; FA, Fatty acids; SP, Sphingolipids; PR, Prenol lipids.
Perturbed lipid metabolic modules in DM
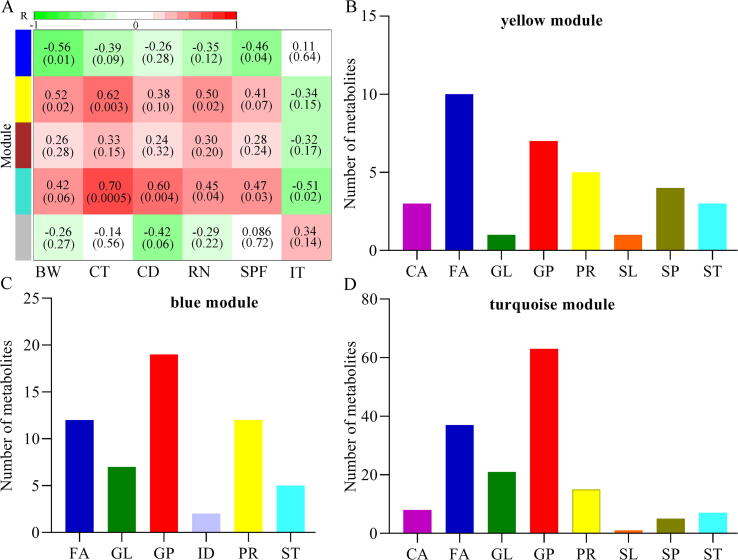
LC-MS was used here to identify differential lipid metabolites in serum and hippocampus. We found that the metabolic signatures in these samples were also significantly different between CM and DM (Supplementary Fig. 3), and the identified differential lipid metabolites in both serum and hippocampus samples mainly belonged to GP (Supplementary Table 3). To explore the possible DLB-related metabolic modules of the MGB axis, we used all identified differential lipid metabolites from fecal, serum and hippocampus samples to conduct WGCNA analysis. This method clustered these differential metabolites into five different modules (Fig. 7A), in which three modules (yellow module, blue module and turquoise module, Fig. 7B-D) were significantly correlated with at least one kind of DLB. The turquoise module, the largest one, was significantly associated with five DLB; the lipid metabolites that comprised this module mainly belonged to peripheral and central FA and GP metabolism. For the other modules, the primary lipid metabolites also mainly belonged to those metabolic pathways.
Fig. 7.
Metabolomic correlations with DLB. A) Heatmap of the correlation coefficients between DLB and metabolomic modules. Green and red squares indicated positive and negative correlation, respectively; B-D) the number of metabolites in each module that was significantly correlated with at least one kind of DLB. CD, center distance; IT, immobility time; SPF, sucrose preference; CT, center time; BW, body weight; RN, rearing number. GP, Glycerophospholipids; ID, Indoles and derivatives; GL, Glycerolipids; ST, Steroids and steroid derivatives; FA, Fatty acids; CA, Carboxylic acids and derivatives; SP, Sphingolipids; PR, Prenol lipids; SL, Saccharolipids.
Correlations between differential neurotransmitters, genera and behaviors
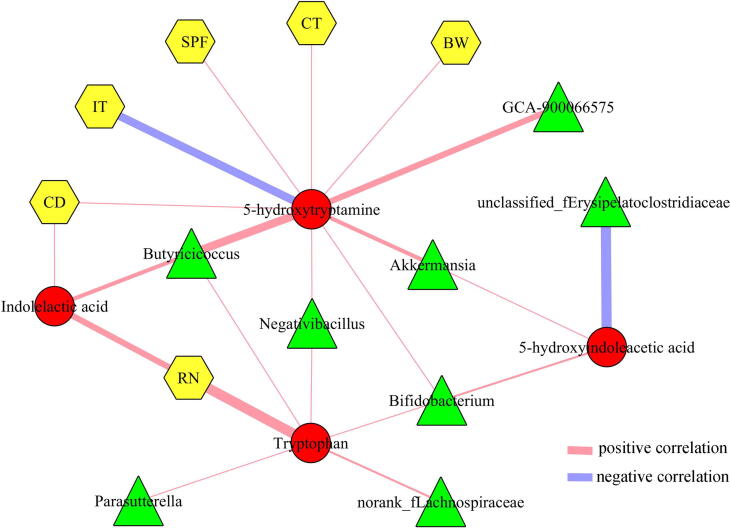
Neurotransmitters in brain could provide valuable information concerning the pathogenesis of depression. In this study, we found that four neurotransmitters in the Tryptophan pathway were significantly decreased in the hippocampus of DM: Tryptophan, 5-hydroxyindoleacetic acid, 5-HT, and Indolelactic acid. The correlations between differential neurotransmitters, genera and DLB were described in Fig. 8. The results showed that the Tryptophan was significantly correlated with five differential genera (Parasutterella, Butyricicoccus, Negativibacillus, Bifidobacterium, norank_f_Lachnospiraceae) and RN; 5-hydroxyindoleacetic acid was significantly correlated with three differential genera (Akkermansia, Bifidobacterium, unclassified_f_Erysipelatoclostridiaceae); 5-HT was significantly correlated with five differential genera (GCA-900066575, Butyricicoccus, Negativibacillus, Bifidobacterium, Akkermansia) and five kinds of DLB (CD, IT, SPF, BW, CT); Indolelactic acid was significantly correlated with Butyricicoccus and two kinds of DLB (CD, RN).
Fig. 8.
Correlations between differetial neurotransmitters, genera and behaviors. CD, center distance; IT, immobility time; SPF, sucrose preference; CT, center time; BW, body weight; RN, rearing number.
Possible ways connecting gut and brain in depressed mice
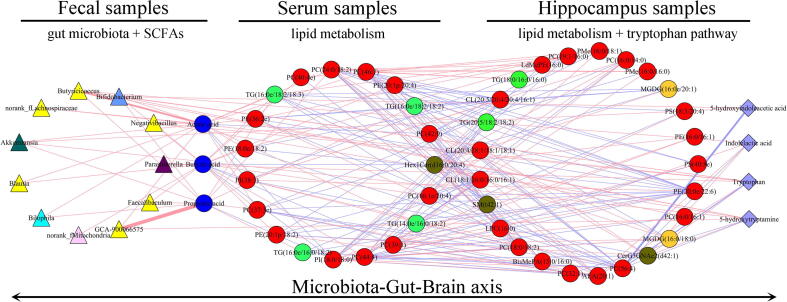
The short-chain fatty acids (SCFAs) might act as a bridge between gut and brain. Three SCFAs (acetic acid, propionic acid and butyric acid) were found here to be significantly changed in depressed mice. To identify potential ways connecting gut and brain in depressed mice, we performed an integrated analysis using these multi-omics data. As shown in Fig. 9, 11 of 20 differential genera were significantly correlated with those differential SCFAs, and these SCFAs were mainly correlated with differential GP metabolites in serum. The differential serum metabolites that significantly correlated with those SCFAs were mainly correlated with differential GP metabolites in hippocampus, and the differential hippocampus metabolites that significantly correlated with differential serum metabolites were significantly correlated with these differential neurotransmitters in Tryptophan pathway. Based on these results, we concluded that “Firmicutes-SCFAs-GP metabolism-Tryptophan pathway” might a possible way to link gut and brain in depressed mice.
Fig. 9.
Possible ways connecting gut and brain in depressed mice.
Discussion
More and more evidence suggests that the disturbed gut microbiota has a close relationship with the depression pathology, but the specific mechanisms are still not identified. In this study, we observed the obvious DLB in DM, and DM was characterized by the alternations of gut microbiota compositions, metabolic products, and neurotransmitters in the MGB axis. Our findings outlined the landscapes and interactions networks of differential gut microbiota, fecal lipid metabolites, peripheral/central lipid metabolism and neurotransmitters in DM. These results would be useful in exploring the role of gut microbiota on the onset of depression.
Loss of appetite is one of the most common symptoms of depression. In our previous findings, although receiving the same food type, the depressed mice had significantly lower weight compared to the control mice, along with non-significantly lower food consumption [14], [28], [29]. Other studies exploring the relationships between depression and gut microbiota also reported the similar results [31], [32], [33]. Nowadays, we tend to think that depression is the cause of loss of appetite, but no causal connection between food consumption and depression has yet been proven. The food consumption makes some effects on the gut microbiota, but the contribution of the lower food consumption to the gut micriobiota alterations is still unclear. Madison et al. reported that gut microbiota could modulate the host’s mood and food cravings, and the diet and stress could also jointly or independently shape the gut microbiota [34]. Here, we found that compared to the control mice, the depressed mice had the significantly lower weight and non-significantly lower food consumption. Accordingly, we inferred that the disordered gut microbiota could cause depression, and then the depression caused the loss of appetite of host. Meanwhile, the loss of appetite might further cause the dysbiosis of the gut microbiota.
Gut homeostasis has a significant role in maintaining the host’s health. In this study, we found that four of 10 differential bacteria taxa on Family level (Bacillaceae, Ruminococcaceae, Butyricicoccaceae and Veillonellaceae) belonged to phylum Firmicutes. Ruminococcaceae and Veillonellaceae were found to be closely related to gut health [35]; thus, the significantly changed Ruminococcaceae and Veillonellaceae indicated the abnormal microbial status in DM. Interestingly, we found that the majority of the differential genera (55%) and differential OTUs (62.5%) belonged to phylum Firmicutes. Consistent with these findings, our previous study found that 76.7% of differential OTUs in patients with depression also belonged to phylum Firmicutes [14]. Meanwhile, Jiang et al. reported that the phylum Firmicutes was decreased in depressed patients [36]. Therefore, these results highlighted the importance of phylum Firmicutes gut population as a possible hallmark of depression.
Chronic stress has a close relationship with the onset and development of depression. The continual stress could result in functional and structural alterations in some brain regions [37]. A systematic review reported that the childhood maltreatment experiences could increase the risk of adult depression and anxiety [38]. Consistent with clinical findings, Zheng et al. found that normal monkeys imposed continual stress on depressed monkeys, which finally resulted in the differential microbial compositions of depressed monkeys [35]. Moreover, researchers found disturbances of the gut microbiota in stress-induced depressed rats [39]. Here, using CRS-induced depression model, we also observed the significantly different gut microbiota compositions in DM. These findings suggested that stress might take a part in the onset of depression by affecting gut microbiota.
A change in diet is the visualized representation from stress to disordered gut microbiota. Even the mild stressors can change the dietary habit, in most cases, change to the unhealthy eating (usually referred to the emotional eaters’ hedonic, taste-based eating). The unhealthy eating will cause the disturbance of gut microbiota, and finally result in depression. Matison et al. reported that the Western diet and fruit/vegetable intakes were associated with an increased and decreased, respectively, risk of depression [40]. Kazemi et al. found a significant relation between red meat consumption and depression in normal-weight male participants [41]. Meanwhile, depression can influence food choices. Previous study reported that the unpleasant or unhappy emotions could increase the taste-based eating [42]. Another study reported that stress could slow the host's response to food cues and result in the bias to comfort foods [43]. Therefore, considering the effects of stress on both dietary habits and gut microbiota, there might be synergistic effects between diet changes and the disordered gut microbiota in causing depression.
Previous studies reported that the disorder of lipid compositions in the brain might be closely related to the onset of neuropsychiatric diseases, such as depression and autism [44], [45]. Many studies, including our work, have found that the peripheral and central lipid metabolism in depressed patients was significantly disordered [46], [47]. However, the possible roles of lipid metabolism in depression are still unclear. In this study, the disordered metabolic modules involving in GP and FA metabolism were found to be significantly correlated with DLB. The DLB-related metagenomic genes also mainly clustered on GP metabolism and FA metabolisms. Consistent with our results, Zhang et al. reported that the chronic unpredictable mild stress could cause the disturbance of lipid metabolism in the hippocampus of depressed rats [48]. Meanwhile, our previous findings showed that gut microbiota could significantly affect the lipid metabolism, especially GP metabolism in the brain tissues of mice [23], [24]. Considering the differential lipid metabolites in serum and hippocampus in this study mainly belonged to GP, we concluded that the disturbances of gut microbiota might be a root factor in the onset of DLB through shaping peripheral and central GP metabolism in DM.
SCFAs, as the main gut microbiota-derived metabolites, could serve as signaling molecules between other organs and gut microbiota. Recent evidence suggested that the changes in gut microbiota compositions and SCFAs might directly or indirectly be involved in the onset of depression [49], [50]. Evans et al. reported that the secretion of 5-HT was partly regulated by SCFAs [51]. Our previous animal study also found that gut microbiota, SCFAs and neurotransmitters had close relationships in depressed mice [28]. However, the precise mechanism of how gut microbiota derived SCFAs play a role in the onset of depression is still unclear. In this study, we found that the disordered gut microbiota (especial differential genera from phylum Firmicutes) could alter the levels of acetic acid, propionic acid and butyric acid, which could caused the disturbance of peripheral and central GP metabolism. As a result, Tryptophan, 5-hydroxyindoleacetic acid, 5-HT, and Indolelactic acid were decreased. Our findings provided possible ways connecting gut and brain in DM, and they were worthy of further explorations.
The 5-HT, as a neurotransmitter, plays an important role in the MGB axis. There is a close relationship between 5-HT and depression, and 5-HT is widely accepted as target by selective serotonin reuptake inhibitors. About 90% of 5-HT is produced in the gut, and the metabolic pathway leading to 5-HT from Tryptophan is under the control of gut microbiota [52]. Meanwhile, gut microbial-derived metabolites, such as SCFAs, have a close relationship with the production of 5-HT. Our previous study found that the disordered gut microbiota could result in the decreased level of 5-HT in the hypothalamus of depressed mice [28]. Rao et al. reported that the level of 5-HT in the depressed mice was significantly increased after improving the disturbance of gut microbiota [53]. In this study, we found that the decreased level of 5-HT in the hippocampus of depressed mice was significantly positively correlated with five differential genera with decreased abundance levels. These results showed that 5-HT might be an agent of gut microbiota, especially Firmicutes, in modulating the brain functions.
Combination analysis of metagenomic and metabolic data is a good method to explore how gut microbiota influences the host’s health [16], [17]. In this study, we used this two-level strategy to reduce the data complexity and identify the principal “metabolite-bacterium “correlation pairs. Finally, we obtained three key” metabolite type-bacterial taxa” correlated pairs. However, this strategy may ignore the “metabolite-bacterium” correlation pairs with high biological meaning, which did not reach statistical significance. These pairs included “GL/PL-Akkermansia”, “GL/PL-Bifidobacterium”, “GL/PL-GCA-900066575”, “GL-Butyricicoccus”, and “FA-Bilophila”. The combined performances of these bacteria and metabolite types did not reaching statistical significance, but these bacteria and metabolites might have important roles in the communications of brain and gut microbiota [54], [55]. Therefore, those pairs might also provide novel insights on the interactions between gut microbiota and brain, and were worthy of further investigations.
Small limitations of our studies should be mentioned here: i) only one emotion-related brain area (hippocampus) was used here; thus, future studies should take other brain areas into consideration to identify more novel clues on the interactions between gut microbiota and depression; ii) only the neurotransmitters in Tryptophan pathway was analyzed in this study. Further studies are needed to find out whether or not the gut microbiota could influence other neurotransmitters systems, such as GABAergic or Catecholaminergic pathway; and iii) the level of calories consumed in the two groups was not accurately calculated, although the food consumption was similar between the two groups during the whole process of model building; thus, future studies should further explore the potential effects of the different level of calories consumed on gut microbiota in the depressed mice.
In conclusion, integrating these multi-omics findings, we characterized the landscapes of altered gut microbiota, lipid metabolites in fecal, serum and hippocampus and neurotransmitters in the tryptophan pathway, and discovered how these disturbed signatures were involved in the onset of depression. Finally, we found that the gut microbiota might contribute to the onset of DLB by affecting the peripheral/central GP metabolism and tryptophan pathway. The “Firmicutes-SCFAs-GP metabolism-Tryptophan pathway” might a potential way to link the gut and brain in DM. Our findings would advance our understanding of the relationship between gut microbiota and depression.
Compliance with Ethics Requirements
The Ethics Committee of Guizhou Medical University carefully reviewed the protocol of this study and then approved our study (Approval No. 2001159), and all the tests were performed referring to the National Institutes of Health Guidelines for Animal Research (Guide for the Care and Use of Laboratory Animals, NIH Publication No.8023, revised 1996).
CRediT authorship contribution statement
Tian Tian: Methodology, Data curation, Formal analysis. Qiang Mao: Data curation, Writing – original draft. Jing Xie: Formal analysis, Writing – review & editing. Ying Wang: Supervision. Wei-hua Shao: Visualization. Qi Zhong: . Jian-jun Chen: Conceptualization, Methodology, Writing – review & editing, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Natural Science Foundation Project of China (81701360, 82160273), the Natural Science Foundation of Chongqing, China (cstc2021jcyj-msxmX0084), the Natural Science Foundation of Guizhou Province, China (Grant No. Qianke He Foundation-ZK [2021] General 416), the Science and Technology Research Program of Chongqing Municipal Education Commission, China (Grant No. KJQN202100420) and the Chongqing Yuzhong District Science & Technology Commission, China (20190115). The 16s data analysis and metagenomic analysis were performed using the free online platform of Majorbio Cloud Platform, and the metabolites data were detected in Shanghai Bioclouds Biotechnology Co., Ltd.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.10.002.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Mitchell A.J., Chan M., Bhatti H., Halton M., Grassi L., Johansen C., et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 2.Chen J.-J., Xie J., Zeng L.i., Zhou C.-J., Zheng P., Xie P. Urinary metabolite signature in bipolar disorder patients during depressive episode. Aging (Albany NY) 2019;11(3):1008–1018. doi: 10.18632/aging.101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Averina O.V., Zorkina Y.A., Yunes R.A., Kovtun A.S., Ushakova V.M., Morozova A.Y., et al. Bacterial Metabolites of Human Gut Microbiota Correlating with Depression. Int J Mol Sci. 2020;21(23):9234. doi: 10.3390/ijms21239234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yano J., Yu K., Donaldson G., Shastri G., Ann P., Ma L., et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warden D., Rush A.J., Trivedi M.H., Fava M., Wisniewski S.R. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449–459. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- 6.Ruelaz AR. Treatment-resistant depression: strategies for management 2006;23:34–7.
- 7.Kelly J.R., Borre Y., O' Brien C., Patterson E., El Aidy S., Deane J., et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Cryan J.F., O'Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., et al. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 9.Makris A.P., Karianaki M., Tsamis K.I., Paschou S.A. The role of the gut-brain axis in depression: endocrine, neural, and immune pathways. Hormones (Athens) 2021;20(1):1–12. doi: 10.1007/s42000-020-00236-4. [DOI] [PubMed] [Google Scholar]
- 10.Karl J.P., Hatch A.M., Arcidiacono S.M., Pearce S.C., Pantoja-Feliciano I.G., Doherty L.A., et al. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front Microbiol. 2013;2018:9. doi: 10.3389/fmicb.2018.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barandouzi Z.A., Starkweather A.R., Henderson W.A., Gyamfi A., Cong X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front Psychiatry. 2020;11:541. doi: 10.3389/fpsyt.2020.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanada K., Nakajima S., Kurokawa S., Barceló-Soler A., Ikuse D., Hirata A., et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J Affect Disord. 2020;266:1–13. doi: 10.1016/j.jad.2020.01.102. [DOI] [PubMed] [Google Scholar]
- 13.Chen J.J., Zheng P., Liu Y.Y., Zhong X.G., Wang H.Y., Guo Y.J., et al. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr Dis Treat. 2018;14:647–655. doi: 10.2147/NDT.S159322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 15.Chen J.-J., He S., Fang L., Wang B., Bai S.-J., Xie J., et al. Age-specific differential changes on gut microbiota composition in patients with major depressive disorder. Aging (Albany NY) 2020;12(3):2764–2776. doi: 10.18632/aging.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng P., Zeng B., Liu M., Chen J., Pan J., Han Y.u., et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2) doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng P., Yang J., Li Y., Wu J., Liang W., Yin B., et al. Gut Microbial Signatures Can Discriminate Unipolar from Bipolar Depression. Adv Sci (Weinh) 2020;7(7):1902862. doi: 10.1002/advs.v7.710.1002/advs.201902862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B.o., Guo K., Zeng L.i., Zeng B., Huo R., Luo Y., et al. Metabolite identification in fecal microbiota transplantation mouse livers and combined proteomics with chronic unpredictive mild stress mouse livers. Transl Psychiatry. 2018;8(1) doi: 10.1038/s41398-017-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacFabe D.F., Cain N.E., Boon F., Ossenkopp K.-P., Cain D.P. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav Brain Res. 2011;217(1):47–54. doi: 10.1016/j.bbr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Yu Q., He Z., Zubkov D., Huang S., Kurochkin I., Yang X., et al. Lipidome alterations in human prefrontal cortex during development, aging, and cognitive disorders. Mol Psychiatry. 2020;25(11):2952–2969. doi: 10.1038/s41380-018-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghazalpour A., Cespedes I., Bennett B.J., Allayee H. Expanding role of gut microbiota in lipid metabolism. Curr Opin Lipidol. 2016;27(2):141–147. doi: 10.1097/MOL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J.-J., Xie J., Zeng B.-H., Li W.-W., Bai S.-J., Zhou C., et al. Absence of gut microbiota affects lipid metabolism in the prefrontal cortex of mice. Neurol Res. 2019;41(12):1104–1112. doi: 10.1080/01616412.2019.1675021. [DOI] [PubMed] [Google Scholar]
- 24.Chen J.-J., Zeng B.-H., Li W.-W., Zhou C.-J., Fan S.-H., Cheng K.e., et al. Effects of gut microbiota on the microRNA and mRNA expression in the hippocampus of mice. Behav Brain Res. 2017;322:34–41. doi: 10.1016/j.bbr.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H.-P., Liu X.-L., Chen J.-J., Cheng K.e., Bai S.-J., Zheng P., et al. Circulating microRNA 134 sheds light on the diagnosis of major depressive disorder. Transl Psychiatry. 2020;10(1) doi: 10.1038/s41398-020-0773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J.-J., Bai S.-J., Li W.-W., Zhou C.-J., Zheng P., Fang L., et al. Urinary biomarker panel for diagnosing patients with depression and anxiety disorders. Transl Psychiatry. 2018;8(1) doi: 10.1038/s41398-018-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zierer J., Jackson M.A., Kastenmüller G., Mangino M., Long T., Telenti A., et al. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet. 2018;50(6):790–795. doi: 10.1038/s41588-018-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu M., Tian T., Mao Q., Zou T., Zhou C.-J., Xie J., et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry. 2020;10(1) doi: 10.1038/s41398-020-01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong X., Huang C., Yang X., Mao Q., Zeng L.i., Zheng P., et al. Proteomic analysis of the intestine reveals SNARE-mediated immunoregulatory and amino acid absorption perturbations in a rat model of depression. Life Sci. 2019;234:116778. doi: 10.1016/j.lfs.2019.116778. [DOI] [PubMed] [Google Scholar]
- 30.Chen X.i., Lan T., Wang Y., He Y., Wu Z., Tian Y.u., et al. Entorhinal cortex-based metabolic profiling of chronic restraint stress mice model of depression. Aging (Albany NY) 2020;12(3):3042–3052. doi: 10.18632/aging.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L., Ma L., Zhang H., Cao Y., Wang C., Hou N., et al. Fto Deficiency Reduces Anxiety- and Depression-Like Behaviors in Mice via Alterations in Gut Microbiota. Theranostics. 2019;9(3):721–733. doi: 10.7150/thno.31562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y., Wan M., Zhong Y.i., Gao T., Zhang Y., Yan F., et al. Partially Hydrolyzed Guar Gum Modulates Gut Microbiota, Regulates the Levels of Neurotransmitters, and Prevents CUMS-Induced Depressive-Like Behavior in Mice. Mol Nutr Food Res. 2021;65(16):2100146. doi: 10.1002/mnfr.v65.1610.1002/mnfr.202100146. [DOI] [PubMed] [Google Scholar]
- 33.Hao W., Wu J., Yuan N., Gong L., Huang J., Ma Q., et al. Xiaoyaosan Improves Antibiotic-Induced Depressive-Like and Anxiety-Like Behavior in Mice Through Modulating the Gut Microbiota and Regulating the NLRP3 Inflammasome in the Colon. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.619103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madison A., Kiecolt-Glaser J.K. Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr Opin Behav Sci. 2019;28:105–110. doi: 10.1016/j.cobeha.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng P., Wu J., Zhang H., Perry S.W., Yin B., Tan X., et al. The gut microbiome modulates gut-brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression. Mol Psychiatry. 2021;26(6):2380–2392. doi: 10.1038/s41380-020-0744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 37.McEwen B.S., Bowles N.P., Gray J.D., Hill M.N., Hunter R.G., Karatsoreos I.N., et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M., D'Arcy C., Meng X. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta-analysis, and proportional attributable fractions. Psychol Med. 2016;46(4):717–730. doi: 10.1017/S0033291715002743. [DOI] [PubMed] [Google Scholar]
- 39.Pearson-Leary J., Zhao C., Bittinger K., Eacret D., Luz S., Vigderman A.S., et al. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry. 2020;25(5):1068–1079. doi: 10.1038/s41380-019-0380-x. [DOI] [PubMed] [Google Scholar]
- 40.Matison A.P., Mather K.A., Flood V.M., Reppermund S. Associations between nutrition and the incidence of depression in middle-aged and older adults: A systematic review and meta-analysis of prospective observational population-based studies. Ageing Res Rev. 2021;70:101403. doi: 10.1016/j.arr.2021.101403. [DOI] [PubMed] [Google Scholar]
- 41.Kazemi S, Keshteli AH, Saneei P, Afshar H, Esmaillzadeh A, Adibi P. Red and White Meat Intake in Relation to Mental Disorders in Iranian Adults. Front Nutr 2021;8:710555. [DOI] [PMC free article] [PubMed]
- 42.Reichenberger J., Kuppens P., Liedlgruber M., Wilhelm F.H., Tiefengrabner M., Ginzinger S., et al. No haste, more taste: An EMA study of the effects of stress, negative and positive emotions on eating behavior. Biol Psychol. 2018;131:54–62. doi: 10.1016/j.biopsycho.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Tryon M.S., Carter C.S., DeCant R., Laugero K.D. Chronic stress exposure may affect the brain's response to high calorie food cues and predispose to obesogenic eating habits. Physiol Behav. 2013;120:233–242. doi: 10.1016/j.physbeh.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Ancelin M.-L., Carrière I., Boulenger J.-P., Malafosse A., Stewart R., Cristol J.-P., et al. Gender and genotype modulation of the association between lipid levels and depressive symptomatology in community-dwelling elderly (the ESPRIT study) Biol Psychiatry. 2010;68(2):125–132. doi: 10.1016/j.biopsych.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Kim E.-K., Neggers Y.H., Shin C.-S., Kim E., Kim E.M. Alterations in lipid profile of autistic boys: a case control study. Nutr Res. 2010;30(4):255–260. doi: 10.1016/j.nutres.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Liu X., Li J., Zheng P., Zhao X., Zhou C., Hu C., et al. Plasma lipidomics reveals potential lipid markers of major depressive disorder. Anal Bioanal Chem. 2016;408(23):6497–6507. doi: 10.1007/s00216-016-9768-5. [DOI] [PubMed] [Google Scholar]
- 47.Bai S., Fang L., Xie J., Bai H., Wang W., Chen J.J. Potential Biomarkers for Diagnosing Major Depressive Disorder Patients with Suicidal Ideation. J Inflamm Res. 2021;14:495–503. doi: 10.2147/JIR.S297930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Yuan S., Pu J., Yang L., Zhou X., Liu L., et al. Integrated metabolomics and proteomics analysis of hippocampus in a rat model of depression. Neuroscience. 2018;371:207–220. doi: 10.1016/j.neuroscience.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 50.Silva Y.P., Bernardi A., Frozza R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne) 2020 Jan;31(11):25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans J.M., Morris L.S., Marchesi J.R. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol. 2013;218(3):R37–R47. doi: 10.1530/JOE-13-0131. [DOI] [PubMed] [Google Scholar]
- 52.Agus A., Planchais J., Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23(6):716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Rao J., Qiao Y., Xie R., Lin L., Jiang J., Wang C., et al. Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. J Psychiatr Res. 2021;137:147–157. doi: 10.1016/j.jpsychires.2021.02.057. [DOI] [PubMed] [Google Scholar]
- 54.Collins S.M., Surette M., Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 55.Mayer E.A. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.