Graphical abstract
Keywords: Salicylic acid, Target of rapamycin, Hyphal growth, Pathogenicity, Fusarium oxysporum
Highlights
-
•
Isolating and sequencing the genome of F. oxysporum from potato tubers with dry rot symptoms.
-
•
SA efficiently arrests hyphal growth, sporular production and pathogenicity of F. oxysporum.
-
•
SA inhibits the activity of FoTORC1 via activating FoSNF1 in F. oxysporum.
-
•
Transgenic potato plants with interference of FoTOR1 and FoSAH1 genes prevent the occurrence of Fusarium wilt.
-
•
Providing insights SA into controlling various fungal diseases by targeting the SNF1-TORC1 pathway of pathogens.
Abstract
Introduction
Biofungicides with low toxicity and high efficiency are a global priority for sustainable agricultural development. Phytohormone salicylic acid (SA) is an ancient medicine against various diseases in humans and activates the immune system in plants, but little is known of its function as a biofungicide.
Objectives
Here, Fusarium oxysporum, the causal agent of devastating Fusarium wilt and immunodepressed patients, was used as a model system to explore whether SA can enter the pathogen cells and suppress key targets of the pathogen.
Methods
Oxford Nanopore MinION sequencing and high-throughput chromosome conformation capture (Hi-C) sequencing were used to analyzed the genome of F. oxysporum. In addition, RNA-seq, qRT-PCR, and western blotting were conducted to detect gene and protein expression levels.
Results
We isolated and sequenced the genome of F. oxysporum from potato dry rot, and the F. oxysporum included 12 chromosomes and 52.3 Mb genomic length. Pharmacological assays showed that exogenous application of SA can efficiently arrest hyphal growth, spore production, and pathogenicity of F. oxysporum, whereas endogenous salicylate hydroxylases significantly detoxify SA. The synergistic growth inhibition of F. oxysporum was observed when SA was combined with rapamycin. Kinase assays showed that SA inhibits FoTOR complex 1 (FoTORC1) by activating FoSNF1 in vivo. Transgenic potato plants with the interference of FoTOR1 and FoSAH1 genes inhibited the invasive growth of hyphae and significantly prevented the occurrence of Fusarium wilt.
Conclusion
This study revealed the underlying mechanisms of SA against F. oxysporum and provided insights into SA in controlling various fungal diseases by targeting the SNF1-TORC1 pathway of pathogens.
Introduction
The use of chemical fungicides has substantially increased in the past few decades. Chemical fungicides are necessary to control fungal diseases in agricultural crops. However, health risks, food safety hazards, and water and soil pollution resulting from chemical fungicide residues are a global challenge [1], [2]. Infants and children are particularly vulnerable to chemical fungicide residues. The bioaccumulation of chemical residues can be magnified through the food chain and quickly reaches a harmful level in the human body. Biofungicides with low toxicity and high efficiency are required for food safety, human health and sustainable development.
Phytohormone salicylic acid (SA) is an ancient medicine made from willow bark. It has been used against fever, pain, and inflammation since ancient Egypt [3]. SA can activate adenosine monophosphate-activated protein kinase (AMPK), an important regulator of cell growth and metabolism [4], [5]. Activated AMPK directly phosphorylates RAPTOR at the conserved serine residue (Ser792) and inhibits TORC1 activity in animals and humans [6]. In plants, SA plays a vital role in activating plant disease resistance systems, including pattern-triggered immunity, effector-triggered immunity, and systemic acquired resistance [7], [8]. In addition, previous studies have shown that SA enhances antifungal activity by inhibiting hyphal growth and spore germination in Fusarium oxysporum, Magnaporthe grisea, and Penicillium expansum [9], [10], [11], [12]. However, molecular mechanism of SA function as a biofungicide targeting plant pathogens is unclear.
Fusarium wilt, caused by Fusarium oxysporum, is one of the most devastating plant diseases worldwide [13], [14]. Due to the soil-borne and vascular plant colonization feature of F. oxysporum, a high dosage of chemical fungicides with high toxicity is required to control Fusarium wilt. More than 100 plants, for example, banana, potato, cotton, and tomato, are severely affected by this pathogen [15], [16], [17], globally resulting in tremendous economic losses. However, limited progress has been made in finding effective biofungicides for controlling Fusarium wilt disease.
Target of rapamycin (TOR) is an evolutionarily conserved Ser/Thr protein kinase in eukaryotes. The TOR signaling pathway regulates cell growth, metabolism, and proliferation in response to nutrients, energy, and stresses [18], [19], [20]. Rapamycin (RAP) is a macrolide immunosuppressant produced by Streptomyces hygroscopicus. It can mimic nutrient limitations to arrest cell growth and proliferation. RAP specifically interacts with FKBP12 to prevent TOR from associating with its scaffold protein RAPTOR (regulatory associate protein of TOR) [21], [22], which hinders TOR protein activity and results in irreversibly arresting the G1 phase of the cell cycle [23].
Potato is a primary staple food worldwide, but potato Fusarium wilt and dry rot diseases caused by F. oxysporum are global challenges for potato production. In this study, the potato / F. oxysporum system was employed as a model system to reveal new functions of SA against vascular fungal diseases. We isolated and identified the pathogenic fungus F. oxysporum from potato tubers with dry rot symptoms, and further inoculation assays showed that the pathogen could infect a wider range of crops. Genome sequencing and chromosome assembly showed that the F. oxysporum contained 12 chromosomes and 52.3 Mb genomic length. We found that the phytohormone SA inhibited FoTORC1 signaling by activating FoSNF1 (FoAMPKα) and then phosphorylating FoRAPTOR, resulting in arrested mycelial growth, spore production, and virulence of F. oxysporum. However, endogenous salicylate hydroxylase (FoSAH1) significantly attenuated the toxicity of exogenous SA. Additionally, the synergistic growth inhibition of F. oxysporum was generated when SA was combined with RAP. Transgenic plants with FoTOR1 and FoSAH1 RNA interference significantly prevented the occurrence of Fusarium wilt. Our results demonstrated that SA could function as a novel biofungicide to control fungal diseases such as Fusarium wilt by targeting the FoSNF1-FoTORC1 signaling pathway.
Material and methods
Fungal strains and culture conditions
The wild-type (WT) strain (Fo-4) was isolated from Chongqing local potato with dry rot symptoms. Fusarium oxysporum f. sp. lycopersici (Fol) strain comes from BeNa Culture Collection (BNCC, Beijing, China; BNCC114534) in this study. The Fo-4 strain, deletion mutant strains, and complementary strains were routinely cultured on potato dextrose agar (PDA) at 27 °C. To extract genomic DNA and RNA of F. oxysporum, hyphae were incubated in potato dextrose broth (PDB) for four days at 27 °C with shaking (Incubator shaker, MQT-60R, China) at 160 rpm.
Genome sequencing and assembly
DNA of Fo-4 strain was isolated by CTAB method. Libraries were built using the Ligation Sequencing Kit 1D (SQK-LSK108) from Oxford Nanopore. MinION sequencing was performed following the manufacturer’s standard protocol and sequenced to achieve approximately 170X sequencing coverage. wtdbg2 software was used to assemble the reads after error correction [24]. The genomic assembly completeness of polished contigs was evaluated by BUSCO v2.0 [25].
Hi-C sequencing
According to the Hi-C procedure, nuclear DNA from the mycelium of Fo-4 was cross-linked and then cut with a restriction enzyme. Illumina sequencing (150 bp paired-end) was performed on a HiSeq with ∼100X coverage. After reads filtering, we obtained 12.57 million valid interaction pairs for the chromosome-level assembly of Fo-4. The contigs within the assemblies were separately broken into fragments with a length of 50 Kb and were then clustered by LACHESIS software [26]. Thirty-three contigs with total lengths of 51.7 Mb were anchored and oriented to 12 chromosome-level groups. The mitochondrial DNA was assembled from Illumina sequencing reads using GRAbB software [27].
Construction of vectors for gene deletion and complementation
The primers used to amplify the flanking sequences or CDS of each gene were listed in Table S9. Constructs for gene deletion and complementation of Fo-4 were carried out as described previously [28]. Transgenic F. oxysporum was produced by Agrobacterium-mediated genetic transformation. The Agrobacterium tumefaciens strain AGL-1 containing deletion or complementation vectors was grown at 28 °C in LB liquid medium until the OD600 reached 0.8. The culture was diluted to OD600 = 0.25 in the induction medium contains 200 μM acetosyringone and cultured for 6 h at 28 °C, 180 rpm on a shaker (Incubator shaker, MQT-60R, China). Mixing with an equal volume of the conidial suspension of F. oxysporum (107 conidia mL−1). Then, 300 μL of the mixture was placed onto nitrocellulose filters (pore size, 0.45 μm; diameter, 80 mm) on co-cultivation medium for 48 h. The filters subsequently were transferred to selective medium containing antibiotic and cefotaxime. After seven days, selected transformants were transferred to fresh PDA medium with antibiotic.
Yeast two-hybrid assay
To construct plasmids for yeast two-hybrid analyses, the coding sequences of genes were amplified from the cDNA of Fo-4. The genes were inserted into pGBKT7 and pGADT7 vectors (Clontech, CA, USA). The pairs of yeast two-hybrid plasmids were co-transformed into Saccharomyces cerevisiae strain Y2HGold following the PEG/LiAc transformation protocol (Clontech, Cat. No. 630489). Transformants were grown at 28 °C for five days on a synthetic medium lacking Leu and Trp, and then colonies were transferred to synthetic medium lacking His, Leu, Ade and Trp supplemented with 40 µg/mL X-a-Gal and 200 ng/mL Aureobasidin A.
Western blotting analysis
For western blotting of Fo-4, hyphae were treated with different concentrations of SA and total protein extracts were prepared with RIPA lysis buffer. Primary antibodies were used: rabbit anti-phospho-AMPKα T172 (#2535; Cell signaling technology), mouse anti-AMPKα (MCA2672GA; BIO-RAD), rabbit anti-phospho-RAPTOR S792 (#2083; Cell signaling technology), rabbit anti-RAPTOR (#2280; Cell signaling technology), rabbit anti-phospho-S6 ribosomal protein S235/236 (#2211; Cell signaling technology), rabbit anti-RPS6 (ab40820; Abcam), and mouse anti-yeast β-actin (AT0014; CMCTAG). The integrated optical density values of the indicated bands were quantified using the software Image-Pro Plus.
Expression profiling sequencing and analysis
Hyphae of Fo-4 were grown for four days in PDB medium at 27 °C with shaking at 160 rpm, and then treated with 5 mM SA and DMSO (as a control) for 12 h, respectively. Total RNA of Fo-4 mycelium was isolated using the RNAprep Pure Fungi Kit (TIANGEN, Beijing, China). For each treatment, three independent biological replicates were performed. An Illumina Hiseq 2000 platform was used to sequence the cDNA library. The clean reads were mapped to the reference F. oxysporum genome by using TopHat2 software. Cuffdiff was used to identify differentially expressed genes (DEGs). Gene ontology (GO) enrichment (corrected P value < 0.05) of the differentially expressed genes was performed by using GOseq software. The enrichment of DEGs in Kyoto Encyclopedia of Genes and Genomes pathways (corrected P value < 0.05) was obtained by KOBAS software [29].
Quantitative real-time PCR
Total RNA of Fo-4 mycelium was isolated using the RNAprep Pure Fungi Kit (TIANGEN, Beijing, China). cDNA was synthesized from total RNA using the TransScript® Reverse Transcriptase (TransGen, Beijing, China). Relative transcript levels were tested by the CFX96 real-time PCR system (BIO-RAD, USA). Relative transcript level of target gene was calculated by 2-ΔΔCt. Real-time primers were presented in Table S9. The F. oxysporum translation elongation factor 1 alpha (FoEF1α) and Actin1 (FoACT1) genes were used as internal control. The data represents the mean ± SD of three independent experiments.
Combination index (CI) value measurement
Combination index (CI) values were used to quantitatively measure the interaction between SA and RAP. The degree of reagent interaction is based on synergism (CI < 1), additive effect (CI = 1), or antagonism (CI greater than 1) [30]. Hyphae of Fo-4 were incubated on PDA medium including different concentrations of SA, RAP, and combinations of SA and RAP for six days at 27 °C. Colony diameter was measured to calculate growth inhibition. Experiments were repeated three times. Affected fraction (Fa) represented the percentage of colony diameter affected by drug and was calculated by (1-T/C) × 100%. C: colony diameter of control, T: colony diameter of drug-treatment.
Pathogen inoculation assay
Pathogen inoculation was performed by point-inoculated on the surface of potato leaves and tubers with conidia of Fo-4 (107 conidia mL−1) as described previously [31]. Inoculated leaves and tubers were cultured on moist filter paper at 27 °C in a long day-light condition for four days. At least ten leaves and tubers of each line were treated every time. The experiment was repeated three times.
Statistical analyses
Two-tailed Student’s t-tests and two-way ANOVA were performed using the IBM SPSS statistics software to investigate the statistical differences between control and other samples. Different letters represented significant differences between treatments (Tukey, p < 0.05) in the two-way ANOVA. Significant differences between control and other samples were indicated by one (*P < 0.05) or two (**P < 0.01) asterisks in the two-tailed Student’s t-tests.
Data availability
The RNA-seq and genome data have been deposited in the NCBI Sequence Read Archive under accession numbers PRJNA730676 and PRJNA730382, respectively. The ITS sequences of Fo-2 and Fo-4 have been deposited in the NCBI GenBank accession numbers MZ208819 and MZ223468, respectively. Other relevant data are available in supplemental materials.
Results
Genome sequencing and assembly of F. oxysporum
Fusarium oxysporum, one of the most destructive fungal pathogens resulting in Fusarium wilt and dry rot in potato, was used as a model system to test whether SA can directly inhibit its growth. Two F. oxysporum (Fo) strains were isolated and identified from potato tubers with dry rot symptoms (Fig. S1a). Severe potato Fusarium dry rot infections were observed in Fo-2 and Fo-4 strains when inoculated on potato tubers, indicating that Fo-2 and Fo-4 strains are the causal agents of potato Fusarium dry rot (Fig. S1b). The DNA sequencing results of internal transcribed spacer (ITS) showed that Fo-2 and Fo-4 strains belong to the F. oxysporum, close to Fusarium oxysporum f. sp. tuberosi and Fusarium oxysporum f. sp. lycopersici (Fol) forma specialis (Fig. S1c). Because Fo-2 and Fo-4 strains showed highly consistent growth patterns and genetic features, the Fo-4 strain was selected for subsequent research. To test whether F. oxysporum can infect other crops, Fo-4 and Fol strains were point-inoculated onto the surface of several economically crops. Interestingly, Fo-4 rather than Fol was observed to infect many economically crops ranging from citrus, Chinese yam, tomato, grape, banana, pachyrhizus to cucumber (Fig. S2), indicating that Fo-4 does not show forma specialis as the most of pathological F. oxysporum species [16].
To advance the understanding of Fo-4 and other F. oxysporum species, we sequenced the whole genome of Fo-4 using the Nanopore sequencing platform. A total of ∼8.9 Gb clean reads were obtained with a sequencing depth of 170x, and the total genome length was assembled to 52.3 Mb (Table S1). The genome encodes 17,406 genes, and the GC content of the genome is 47.28%. In contrast with the high content of repetitive sequences in the Fol (4287) genome, approximately 6.37% of the entire Fo-4 genome was identified as repeat sequences, including many retro-elements, short and long interspersed nuclear elements, and DNA transposons.
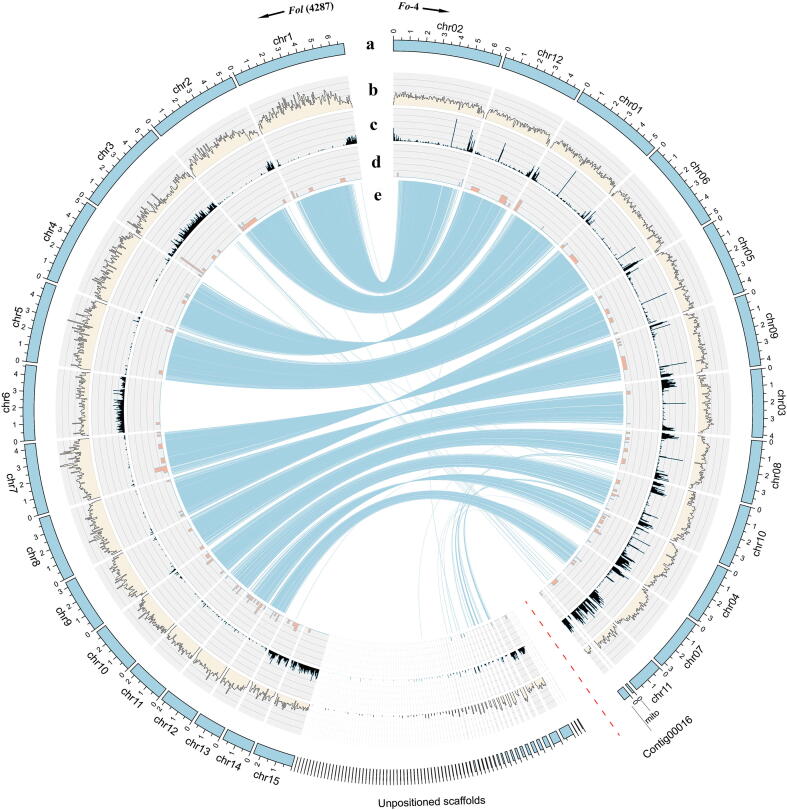
Chromosome level ordering of the scaffolds was achieved by high-throughput chromosome conformation capture (Hi-C). A Hi-C heatmap indicated that the assembled genome of Fo-4 had 12 chromosomes and a mitochondrial chromosome, and 51.7 Mb genomic sequences were mapped to these chromosomes (Fig. S3). Eleven conserved chromosomes were identified through synteny analysis of orthologous genes between Fo-4 and Fol (4287). However, chromosome 11 and contig00016 did not show clear synteny with the Fol (4287) chromosomes (Fig. 1). Furthermore, chromosome 11 and contig00016 contain many repetitive sequences, suggesting that chromosome 11 and contig00016 have greater variability in the Fo-4 strain.
Fig. 1.
Comparison of chromosomes between the Fo-4 genome assembly and Fol (4287). a The Fo-4 genome assembly, b Gene density, c Repeat sequence density, and d Candidate effector genes. Calculated in 50 kb windows. e Indicates nucmer alignments with the Fol (4287) reference genome. Eleven conserved chromosomes were identified through synteny between Fo-4 and Fol (4287), whereas chromosome 11 and contig00016 show reduced synteny.
Exogenous SA significantly inhibits the mycelial growth and pathogenicity of F. oxysporum
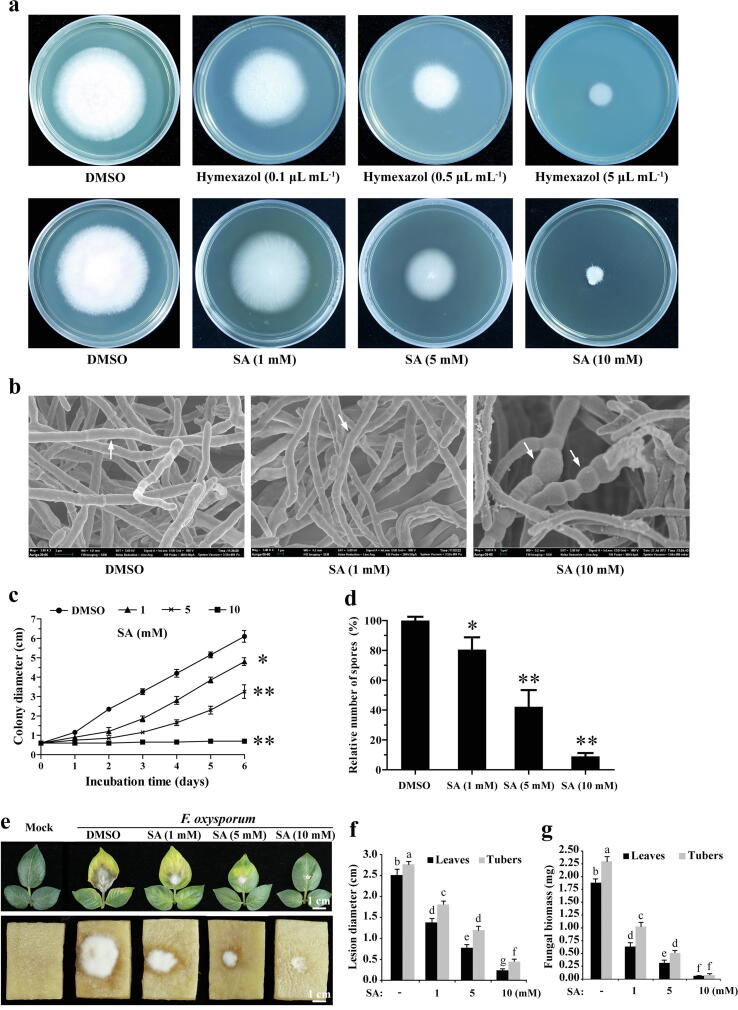
To examine the effects of SA on the growth of F. oxysporum, Fo-4 was treated with different concentrations of SA. Hymexazol fungicide, widely used for Fusarium wilt control, was used as a positive control. The growth inhibition of F. oxysporum positively correlated with SA dose, and the IC50 (half-maximal inhibitory concentration) value of SA was 5 mM (Fig. 2a and c). The hyphal growth of F. oxysporum was completely inhibited when the concentration of SA reached 10 mM, which was similar to hymexazol fungicide at 5 µL mL−1 (Fig. 2a). To examine whether the defective phenotype of hyphal growth can be restored by removing SA and hymexazol from the PDA medium, the SA- and hymexazol-treated F. oxysporum hyphae were transferred to PDA medium plates and grown for four days (Fig. S4). The results showed that 15 mM SA- and 10 µL mL−1 hymexazol-treated F. oxysporum hyphae could not be rescued on the drug free PDA medium, indicating that SA can function as a biofungicide to cause cell death in F. oxysporum. Next, the ability of SA to damage the ultrastructure of F. oxysporum was investigated. The scanning electron microscope (SEM) results showed distorted and swollen hyphae and shorter hyphal septa of F. oxysporum caused by SA treatment (Fig. 2b). This result suggested that SA induced hyphal cell death in F. oxysporum, causing the deficient growth phenotypes. Notably, the spore production of F. oxysporum was also significantly decreased in a SA dose-dependent manner (Fig. 2d). These observations demonstrated that SA could mimic chemical fungicides to cause cell death of F. oxysporum.
Fig. 2.
SA inhibited the hyphal growth and pathogenicity of F. oxysporum in a dose-dependent manner. a SA mimics chemical fungicide to inhibit hyphae growth of F. oxysporum. Hyphae of Fo-4 were incubated on PDA with different concentrations of hymexazol and SA for 6 days. b Growth defects of Fo-4 generated by SA treatment. Hyphae of Fo-4 were incubated on PDA with SA for 6 days and photographed by scanning electron microscopy. c Colony diameter of Fo-4 treated with different concentrations of SA. d Spores production of Fo-4. Hyphae of Fo-4 were incubated on PDA with SA for 6 days and then the spores were eluted with sterile water for counting. The data represents the mean ± SD of n = 3 independent experiments. Asterisks denote student’s t test significant difference compared with that of DMSO (*P < 0.05; **P < 0.01). e SA inhibited the Fusarium wilt lesions caused by F. oxysporum. Spores of Fo-4 mixing with different concentrations of SA were point-inoculated on the surface of potato leaves and tubers for 4 days. Potato leaves and tubers inoculated with water were used as a mock. Bar = 1 cm. f Lesion diameter of potato leaves and tubers as described in e. g Fungal biomass of Fo-4 as described in e. The data represents the mean ± SD of n = 3 independent experiments. Different letters represent significant differences between treatments (Tukey, p < 0.05).
To test the pathogenicity of F. oxysporum treated with SA in potato, spores were mixed with different concentrations of SA and then point-inoculated on the surface of potato leaves and tubers. As expected, SA inhibited Fusarium wilt lesions caused by F. oxysporum in a dose-dependent manner, reducing the fungal biomass, shrinking the necrosis symptoms, and slowing down yellowness and wilting (Fig. 2e-g). These results indicated that SA inhibited the hyphal growth and pathogenicity of F. oxysporum.
SA affects various biological processes in F. oxysporum
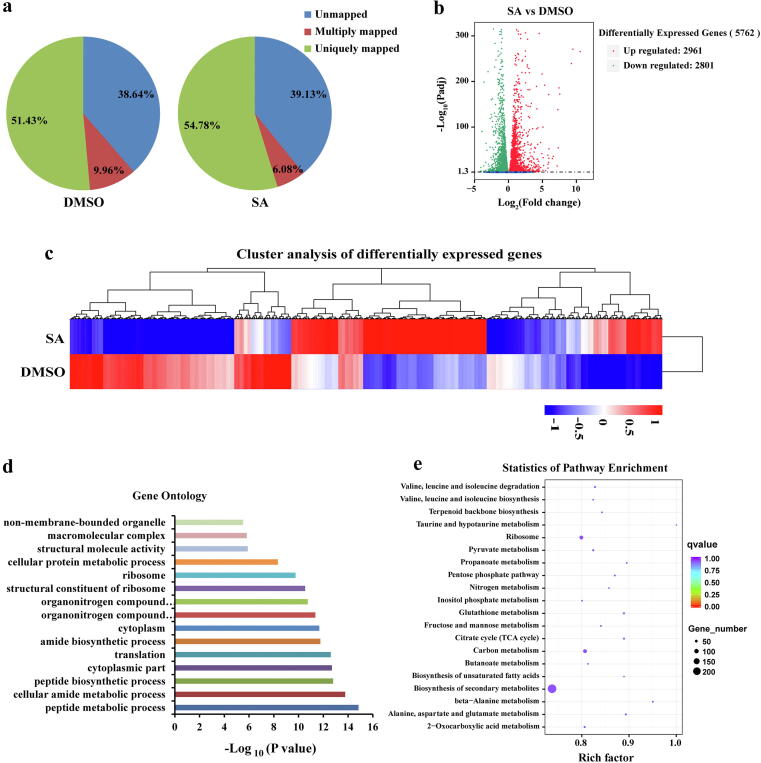
To further elucidate the function of SA on vegetative growth and cell metabolism of F. oxysporum, RNA-seq was performed in F. oxysporum with DMSO and SA treatment (Fig. 3a). A total of 5762 differentially expressed genes (DEGs) were found between DMSO control and SA treatment, of which 2801 DEGs were downregulated and 2961 DEGs were upregulated (Fig. 3b and c). Gene ontology (GO) enrichment analysis was performed to identify the biological functions of the DEGs found between SA and DMSO. A total of 368 upregulated GO terms and 285 downregulated GO terms were enriched in the RNA-seq data (Table S2). Among these GO terms, peptide metabolic process, cellular amide metabolic process, and peptide biosynthetic process were the most significant enrichment (Fig. 3d). Pathway enrichment analysis showed that SA affects multiple metabolic pathways, including carbon metabolism, ribosome, and biosynthesis of secondary metabolites (Fig. 3e). The most significant enrichment pathway was the biosynthesis of secondary metabolites. There were 230 DEGs in the biosynthesis of secondary metabolites, most of which were significantly upregulated (Table S3). Furthermore, these DEGs mainly regulated the biosynthesis of type Ⅱ polyketide backbone and products, polyketides and nonribosomal peptides, and nonribosomal peptide structures in the biosynthesis of secondary metabolites (Fig. S5).
Fig. 3.
RNA-seq analysis of F. oxysporum hyphae treated with DMSO and SA. a Proportions of clean reads of unmapped, multiply mapped, and uniquely mapped. b Number of downregulated and upregulated DEGs between SA and DMSO treatment. c Cluster analysis of DEGs between SA and DMSO treatment. d Significantly enriched gene ontology in SA treatment. Gene ontology was ranked by significance. e Significantly enriched pathways in SA treatment.
Next, we analyzed the effect of SA on the pathogenicity-related genes of F. oxysporum. Through analysis of gene expression profile data, we found 24 DEGs associated with mitogen-activated protein kinase (MAPK) signaling pathway, including 19 downregulated genes and 5 upregulated genes (Fig. S6 and Table S4). Previous studies showed that MAPK signaling pathway had important functions in hyphal infection, appressorium formation, cell wall integrity, stress response, and virulence of phytopathogenic fungi [32]. The MAPKs Fmk1 and Hog1 have been shown to control invasive growth and pathogenicity in F. oxysporum [33], [34]. Transcriptome data showed that the transcription levels of Fmk1 (FOXG_08140) and Hog1 (FOXG_06318) were downregulated 0.57- and 0.48-fold in SA-treated hyphae, respectively (Table S4). In addition, cell wall degrading enzymes (CWDEs) produced by phytopathogenic fungi were proved to be virulence factors involved in fungal infection processes [35], [36]. CWDEs, including cellulases, xylanases and pectinases, were changed under SA treatment in the RNA-seq data (Table S5). Notably, most of the differentially expressed CWDE genes were significantly downregulated. The transcriptome results supported our previous observations in which SA inhibited the hyphal growth and pathogenicity of F. oxysporum.
FoSAH1 significantly attenuates the SA sensitivity of F. oxysporum
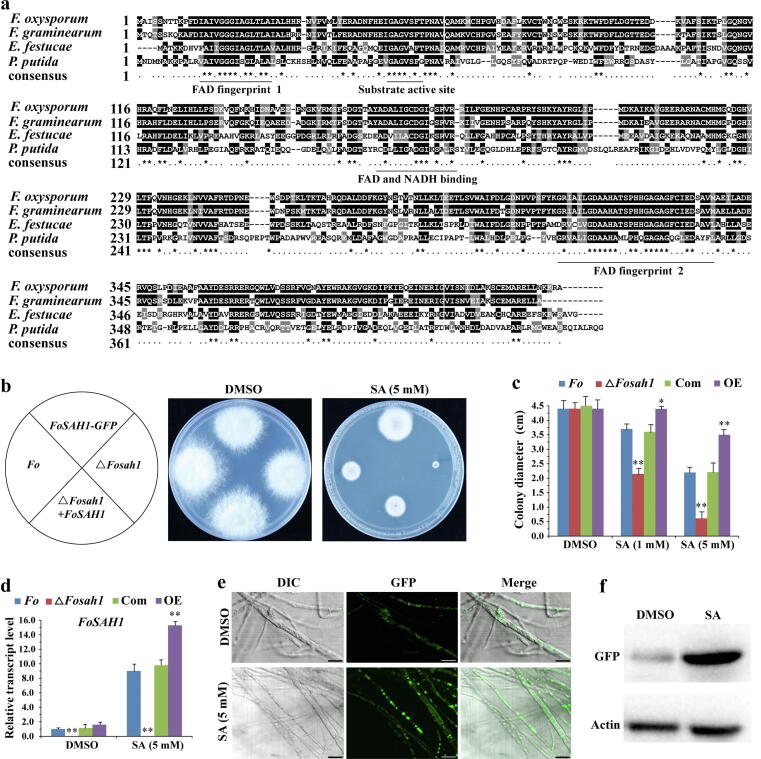
SA is catalyzed by salicylate hydroxylase to generate biologically inactive catechol, resulting in a deficiency in SA accumulation and thus enabling the evasion of host plant defenses in phytopathogenic bacteria and fungi [37], [38]. Because the IC50 of SA for F. oxysporum is much higher than that of chemical fungicide, we hypothesized that SA may be detoxified by salicylate hydroxylase in F. oxysporum. Sixteen putative salicylate hydroxylase (SAH) genes were found in the F. oxysporum genome using Pseudomonas putida NahG (salicylate hydroxylase) amino acid sequence as a reference, and FoSAH1 (FOXG_09708) shared the highest similarity with PpNahG. Therefore, FoSAH1 was selected for further study. The overall amino acid sequence identity was 36% between PpNahG and FoSAH1 proteins. Sequence analysis results showed that FoSAH1 was evolutionarily conserved. The FAD and NADH binding domain and substrate active site of FoSAH1 were identified and highly conserved across various organisms (Fig. 4a). Analysis of transcriptome data showed that the transcription level of FoSAH1 was upregulated 10.15-fold in SA-treated hyphae. To further confirm the function of FoSAH1, FoSAH1 deletion mutants (ΔFosah1) were created (Fig. S7). The ΔFosah1 mutant was more sensitive to SA than Fo and the complementary strain (Fig. 4b). The IC50 value of SA decreased to 1 mM in the ΔFosah1 mutant. Importantly, overexpression of FoSAH1 strain displayed resistance to SA (Fig. 4c), and the transcription and protein levels of FoSAH1 were significantly increased when hyphae were exposed to SA treatment (Fig. 4d and f), indicating that FoSAH1 protein contributes to the detoxification of SA. The subcellular localization showed that FoSAH1 was localized in the cytoplasm (Fig. 4e). Considering that F. oxysporum is an endophytic pathogen with 16 homologs of SAHs, they may play major roles in detoxifying SA to survive during long-term co-evolution between host and pathogens.
Fig. 4.
Salicylate hydroxylase (FoSAH1) can effectively detoxify SA in F. oxysporum.a Comparison of amino acid sequences of FoSAH1 protein with that of representative organisms including F. graminearum, E. festucae, and P. putida. Conserved domains were underlined. b Colony morphology of the ΔFosah1 mutant and overexpression FoSAH1 strains on PDA medium containing DMSO and SA for 4 days. c Colony diameter of Fo, ΔFosah1, Com and OE strains treated with DMSO and SA for 4 days. OE, PFoSAH1::FoSAH1-GFP overexpression line; Com, ΔFosah1 + FoSAH1 complemented strain. d Transcript level of FoSAH1 in F. oxysporum treated with SA. Hyphae of Fo, ΔFosah1, Com and OE were incubated on PDA medium containing DMSO and SA grown for 4 days, respectively. FoEF1α was used as an internal control. The data represents the mean ± SD of n = 3 independent experiments. Asterisks denote student’s t test significant difference compared with that of Fo (*P < 0.05; **P < 0.01). e Subcellular localization of FoSAH1 in F. oxysporum. PFoSAH1::FoSAH1-GFP overexpression line was cultured on PDA medium containing DMSO (control) and SA grown for 4 days, respectively. GFP was photographed by laser confocal scanning microscope (OLYMPUS FLUOVIEW FV1200). Bars = 10 μm. f Analysis of protein level of PFoSAH1::FoSAH1-GFP overexpression line by western blot as described in e. β-actin was used as an internal control.
SA and RAP synergistically inhibit hyphal growth in F. oxysporum.
The growth retardation and deficiency of F. oxysporum resulting from SA treatment show similar features of target of rapamycin (TOR) pathway mutants [18]. It is well-known that TOR, evolutionarily conserved in all eukaryotes, acts as a master regulator of cell growth and proliferation [21], [39], [40]. Rapamycin (RAP) is a well-known TOR specific inhibitor, which negatively regulates TOR kinase activity. To assess the effect of RAP on TOR inhibition in F. oxysporum, we first assayed the sensitivity of the Fo-4 strain to RAP (Fig. S8a). Growth inhibition of F. oxysporum was positively correlated with concentrations of RAP, and the IC50 value of RAP was 0.1 μM. The pairwise combination of SA and RAP displayed more obvious growth inhibition than that treated with SA or RAP alone (Fig. S8b-d), and the IC50 value of single drug (SA: 1 mM; RAP: 10 nM) was drastically reduced when F. oxysporum was treated with SA and RAP combination, implying that potential synergistic effects can be generated by combining SA with RAP. The computer-simulated Fa-CI curve was then assessed using the CompuSyn software. A synergistic effect (CI < 1) was observed when hyphae were treated with the combinations of SA + RAP (Fig. S8e), indicating that the combination of SA + RAP had a strong synergism. These results suggested that SA and RAP could inhibit hyphal growth by simultaneously targeting the TOR signaling pathway in F. oxysporum.
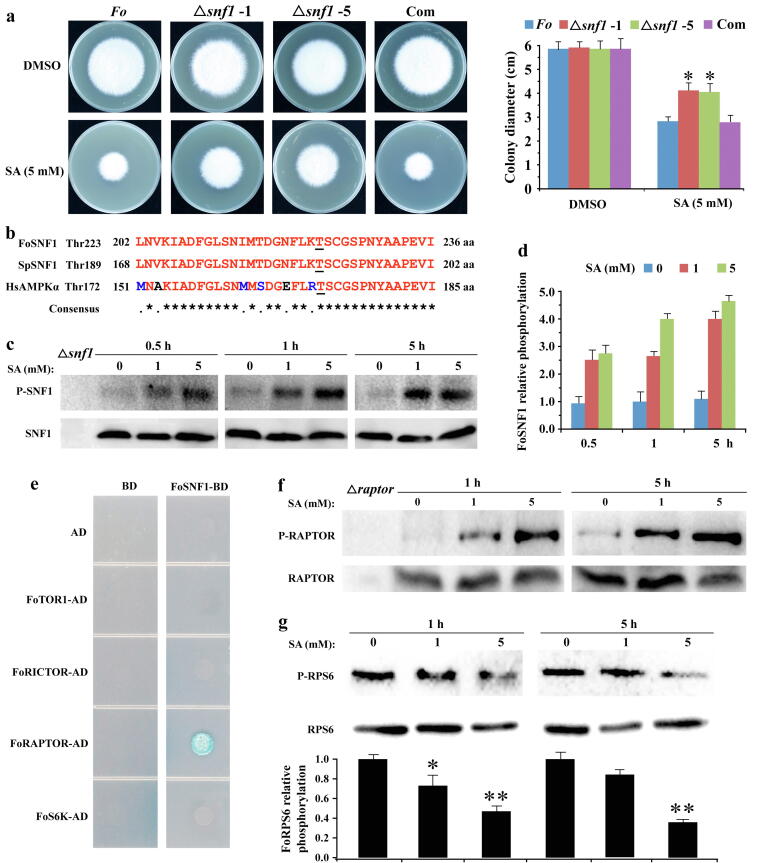
SA activates FoSNF1 to inhibit FoTORC1 signaling through FoRAPTOR phosphorylation
Previous reports have shown that phosphorylation of RAPTOR by AMPK is required for the inhibition of TORC1 in animals and humans [5], [6], [41]. To dissect the possible mechanism between SA and the TOR signaling pathway, we created FoSNF1 (also known as AMPKα) deletion mutants (Δsnf1), an upstream kinase of TORC1 [6]. Pharmacological assays showed that FoSNF1 deletion mutants were insensitive to SA compared with that of Fo (Fig. 5a), implying that FoSNF1 may be a key target of SA. Next, we assessed whether SA could activate FoSNF1. Sequences analysis found the conservation within the activation loops of FoSNF1, SpAMPKαSsp2 and HsAMPKα [42] (Fig. 5b), the anti-Thr172 phospho-AMPKα antibody was thus used to recognize phosphorylated FoSNF1 at Thr223. SA increased the phosphorylation of FoSNF1 at Thr223, which reflected in FoSNF1 activity (Fig. 5c and d). Furthermore, FoSNF1 interacted with FoRAPTOR in yeast two-hybrid assay (Fig. 5e), and phosphorylation of FoRAPTOR was markedly increased in hyphae treated with SA (Fig. 5f). These results indicated that SA mediated the phosphorylation of FoRAPTOR by activating FoSNF1.
Fig. 5.
SA inhibited FoTOR signaling pathway by activating FoSNF1 in F. oxysporum. a Hyphae of Fo-4, Δsnf1, and complemented strain (Com) were incubated on PDA plates with DMSO and SA for 6 days. The data represents the mean ± SD of n = 3 independent experiments. Asterisks denote student’s t test significant difference compared with that of Fo-4 (*P < 0.05). b Sequences alignment of the conserved activation loops of FoSNF1, SpSNF1, and HsAMPKα. Phosphorylated residue of AMPKα was shown with an underline. Sp, Schizosaccharomyces pombe; Hs, Homo sapiens. c SA phosphorylated the T223 of FoSNF1 in F. oxysporum. Hyphae of Fo-4 were treated with different concentrations of SA for 0.5, 1, and 5 h. d Relative phosphorylation level of FoSNF1 in different concentrations of SA treatment. The data represents the mean ± SD of n = 2 independent experiments. e Screening the interaction proteins of FoSNF1 by yeast two-hybrid assay. The full-length CDS of FoSNF1 was inserted into BD vector; other genes were inserted into AD vector. AD: pGADT7, BD: pGBKT7. Vectors BD and AD were used as negative controls. Transformants with the AD and BD constructs were assessed for growth on synthetic medium lacking His, Leu, Ade and Trp medium at 28 °C for 3 days. f Western blot analysis of FoRAPTOR phosphorylation. Hyphae of Fo-4 were treated with different concentrations of SA for 1 and 5 h. g Western blot analysis of FoRPS6 phosphorylation. Hyphae of Fo-4 were treated with different concentrations of SA for 1 and 5 h, and the indicated bands were quantified relative to total FoRPS6 using the software Image-Pro Plus. The data represents the mean ± SD of n = 2 independent experiments. Asterisks denote student’s t test significant difference compared with that of DMSO (*P < 0.05; **P < 0.01).
RAPTOR is known to recruit substrates to TOR kinase and is required for the regulation of TORC1 activity [43]. RAPTOR phosphorylation inhibits TORC1 activity [6], [44]. In order to verify whether phosphorylated FoRAPTOR inhibits the activity of FoTORC1, we detected the phosphorylation of RPS6 (ribosomal protein S6) at Ser 235/236, which is the output of TOR signaling and is essential for protein synthesis and cell cycle progression [45]. As expected, the phosphorylation level of FoRPS6 at Ser 235/236 was decreased in hyphae with SA treatment (Fig. 5g), which indicated a decrease in TORC1 activity. These results suggested that SA exerts an inhibitory effect on TORC1 signaling by activating FoSNF1 in F. oxysporum.
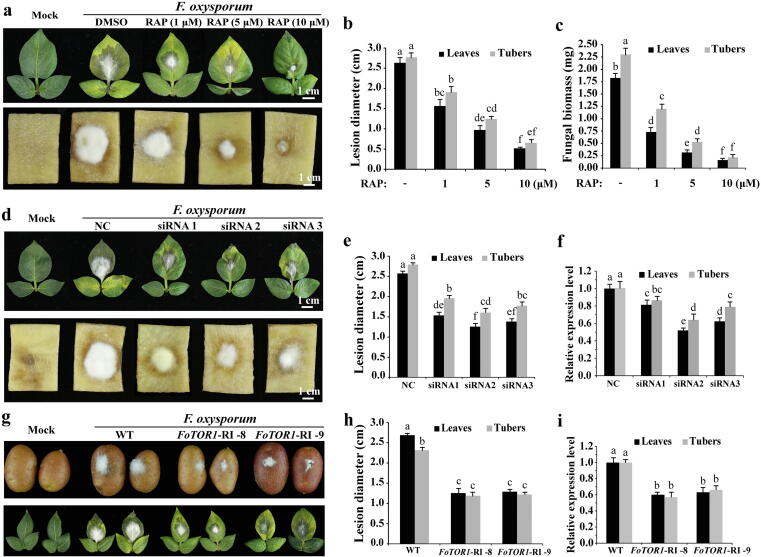
Suppression of FoTOR1 protein activity in vivo and in vitro can block the occurrence of Fusarium wilt in potatoes
We next tested the pathogenicity of F. oxysporum under FoTOR1 inhibition in potato. Spores were mixed with different concentrations of RAP and then point-inoculated on the surface of potato leaves and tubers. Consistently, RAP inhibited Fusarium wilt lesions caused by F. oxysporum in a dose-dependent manner as described in SA, which reflected less biomass of the fungus, smaller necrosis symptoms, and slower yellowing and wilting (Fig. 6a-c). To further confirm that FoTOR1 (FOXG_02818) interference can inhibit the mycelial growth and pathogenicity of F. oxysporum, we synthesized siRNAs that specifically targeted the FoTOR1 gene in vitro (Table S6). All siRNAs of FoTOR1 significantly inhibited the mycelial growth of F. oxysporum (Fig. 6d and e). Furthermore, the FoTOR1 mRNA level of F. oxysporum decreased in response to FoTOR1 siRNAs treatment (Fig. 6f and Fig. S9b). These results suggested that FoTOR1 is a key target for the control of Fusarium wilt in potatoes.
Fig. 6.
Suppression of FoTOR1 protein activity can delay the onset of Fusarium wilt in potato leaves and tubers. a RAP inhibited the Fusarium wilt lesions caused by F. oxysporum. Spores of Fo-4 mixing with different concentrations of RAP were point-inoculated on the surface of potato leaves and tubers for 4 days. Potato leaves and tubers inoculated with water were used as mock. Bar = 1 cm. b Lesion diameter of potato leaves and tubers as described in a. c Fungal biomass of Fo-4 as described in a. d siRNAs of FoTOR1 significantly inhibited the mycelial growth of F. oxysporum. Spores of Fo-4 mixing with FoTOR1 siRNAs were point-inoculated on the surface of potato leaves and tubers for 4 days. Spores of Fo-4 mixing with random siRNA were used as a negative control (NC). e Lesion diameter of potato leaves and tubers as described in d. f Relative expression level of the FoTOR1 gene as described in d. g Transgenic FoTOR1-RI potato plants were resistant to Fusarium wilt. Spores of Fo-4 were point-inoculated on the surface of transgenic FoTOR1-RI potato leaves and tubers for 4 days. Potato (WT) leaves and tubers were point-inoculated with conidial suspension of Fo-4 as a positive control. h Lesion diameter of transgenic FoTOR1-RI potato leaves and tubers as described in g. i Relative expression level of the FoTOR1 gene as described in g. FoEF1α was used as an internal control. The data represents the mean ± SD of n = 3 independent experiments. Different letters represent significant differences between treatments (Tukey, p < 0.05).
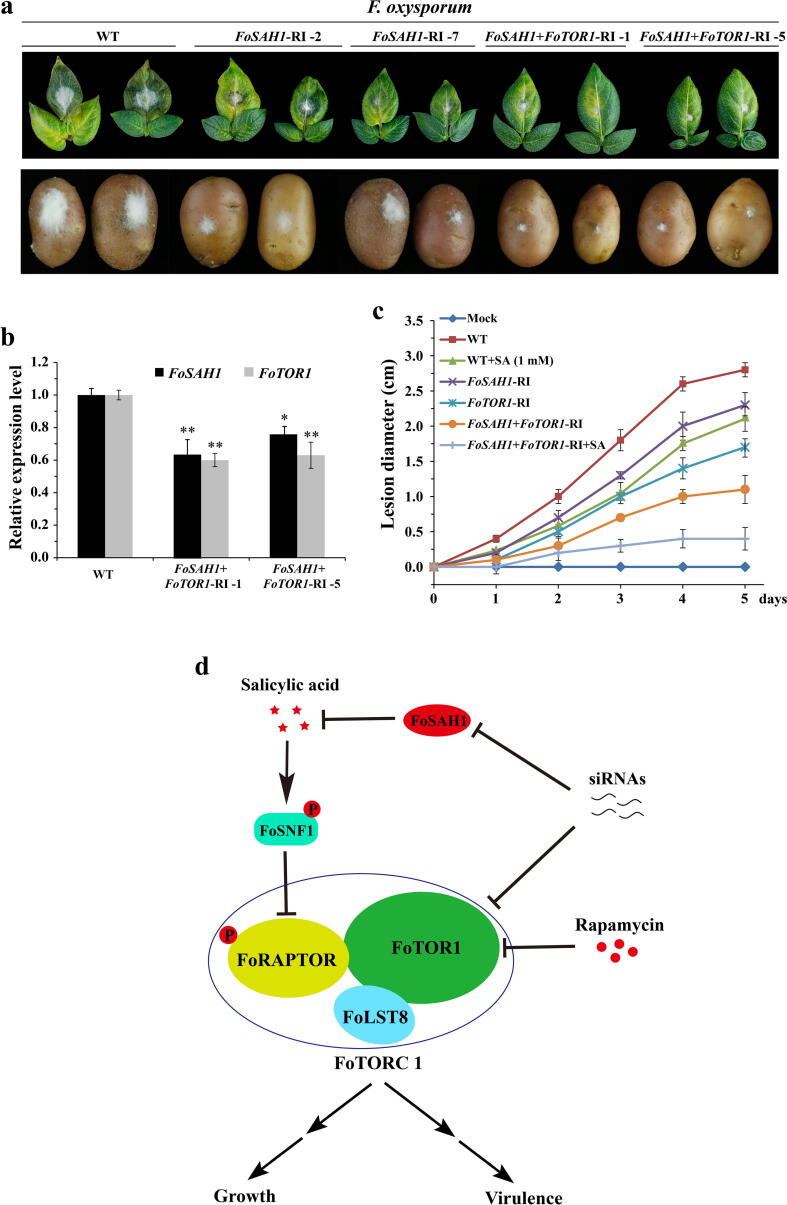
Next, transgenic FoTOR1 and FoSAH1 RNA interference (RNAi) potato plants were generated using agrobacterium-mediated transformation approach. Independent transgenic RNAi potato plants that carry FoTOR1-RI, FoSAH1-RI, and FoTOR1 + FoSAH1-RI fragments were obtained (Table S7). Phenotypic analyses showed that all transgenic potato plants were indistinguishable from wild-type plants. Most of the transgenic potato plants were resistant to Fusarium wilt (Table S7). The transgenic FoTOR1-RI potato plants significantly inhibited mycelial growth and reduced the pathogenicity of F. oxysporum, which conferred resistance against Fusarium wilt on potato leaves and tubers (Fig. 6g and h). Furthermore, the TOR1 mRNA level of F. oxysporum was significantly decreased after incubation with transgenic FoTOR1-RI potato plants (Fig. 6i and Fig. S9c). Consistent with FoTOR1 interference, FoSAH1-RI plants were resistant to F. oxysporum, and FoTOR1 + FoSAH1-RI plants displayed a smaller lesion size than FoTOR1-RI or FoSAH1-RI plants alone (Fig. 7a). The expression levels of FoSAH1 and FoTOR1 genes were significantly decreased in the FoTOR1 + FoSAH1-RI plants (Fig. 7b and Fig. S9d). Additionally, transgenic FoTOR1/FoSAH1-RI RNAi potato plants plus exogenous SA significantly reduced the pathogenicity of F. oxysporum compared with that of FoTOR1/FoSAH1-RI RNAi potato plants or exogenous SA treatment alone (Fig. 7c and Fig. S10). These results suggested that suppression of FoTOR1 protein activity or reduction of FoTOR1 and FoSAH1 mRNA levels by RNAi could inhibit mycelial growth and decrease the pathogenicity of F. oxysporum in potato plants.
Fig. 7.
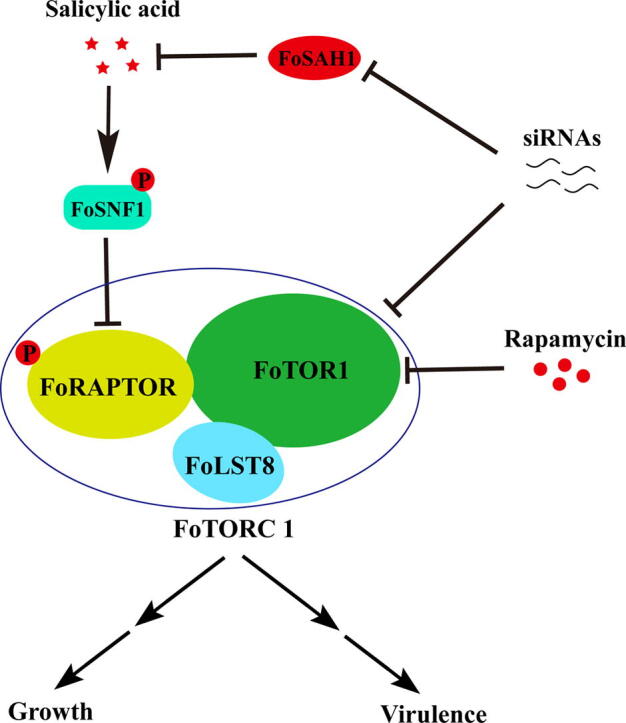
Transgenic potato plants with interference of FoSAH1 and FoTOR1 genes were resistant to Fusarium wilt and dry rot. a Transgenic FoSAH1-RI and FoSAH1 + FoTOR1-RI potato plants were resistant to Fusarium wilt and dry rot. Spores of Fo-4 were point-inoculated on the surface of transgenic FoSAH1-RI and FoSAH1 + FoTOR1-RI potato leaves and tubers for 4 days. Potato (WT) leaves and tubers were point-inoculated with conidial suspension of Fo-4 as a positive control. b Relative expression levels of FoSAH1 and FoTOR1 genes as described in a.FoEF1α was used as an internal control. The data represents the mean ± SD of n = 3 independent experiments. Asterisks denote student’s t test significant difference compared with that of WT (*P < 0.05; **P < 0.01). c Lesion diameter of transgenic FoSAH1-RI, FoTOR1-RI, and FoSAH1 + FoTOR1-RI potato leaves. Spores of Fo-4 mixing with or without SA (1 mM) were point-inoculated on the surface of transgenic potato leaves and tubers for 4 days. Potato (WT) leaves inoculated with water were used as a negative control (Mock). The data represents the mean ± SD of n = 3 independent experiments. d A simplified model shows that SA regulates growth and pathogenicity through FoTORC1 in F. oxysporum. In this model, salicylic acid, rapamycin, and siRNAs suppress the activity of FoTORC1. FoTORC1 positively controls the expression of growth and virulence genes. Arrows and T-bars represent enhancement and inhibition, respectively.
Discussion
Fusarium wilt is a major phytopathogenic disease affecting more than 100 economically important crops, such as potato, tomato, banana, cotton and melon, and many horticultural crops [15], [16], [46]. Fusarium oxysporum is the major shared pathogen in various Fusarium wilt diseases and is ranked fifth in a survey of the Top 10 fungal plant pathogens [47]. Forma specialis of F. oxysporum typically has a narrow host range among species, but a few forma specialis show an extended host range among genera [48]. Expanding the host range by horizontal chromosome transfer between different forma specialis of F. oxysporum makes the pathogen more deadly [16], [48]. In this study, Fo-4 displayed much broader host ranges among families from Solanaceae, Vitaceae, Musaceae, Cucurbitaceae to Dioscoreaceae than other forma specialis of F. oxysporum. As far as we know, Fo-4 is the most infectious pathogenic F. oxysporum. To explore the underlying reasons why Fo-4 had the broader host ranges, we sequenced the Fo-4 genome. The results showed that Fo-4 had 12 chromosomes, of which chromosome 11 included the most repetitive sequences and many pathogenic secreted proteins. However, whether the chromosome 11 is a pathogenicity chromosome that infects more hosts requires further study.
Due to the lack of varieties resistant to Fusarium wilt and efficient biofungicides, the control of this disease largely depends on high dosage and high frequency of chemical fungicide applications. However, these chemical fungicides face increasing challenges, including limited efficacy, environmental contamination, and drug resistance development. Thus, effective, safe, and eco-friendly biological fungicides are urgently needed to replace the chemical fungicides in the agricultural industry and sustainable development. Development of these new biofungicides would promote environmental protection, sustainability, and improve human health. However, so far, few useful biological fungicides have been developed against Fusarium wilt [17]. In this study, we discovered that SA, which was induced by stresses and natively produced by plants [49], [50], can directly inhibit F. oxysporum cells and significantly prevent the occurrence of potato Fusarium wilt. Importantly, we found that SA showed potent inhibitory activity against F. oxysporum by targeting the FoTOR signaling pathway. TOR is a key lethal gene with a large molecular weight (280 KD) and multiple functional domains [20], [51]. TOR signaling operates as a master input/output node that mediates various upstream signal cascades for modulating cell growth, development, proliferation, and death in all examined eukaryotic organisms [40], [52], [53]. We further found that FoSNF1 can mediate SA to inhibit the activity of FoTORC1 by phosphorylating FoRAPTOR. Thus, the FoSNF1-FoTORC1 signaling cascade is an ideal target for high-throughput screening of potential biofungicides such as SA against Fusarium wilt. Besides, the RNA-seq data showed that SA affects multiple metabolic pathways, especially in the MAPK cascade and CWDEs, indicating that SA targets multiple signaling pathways in F. oxysporum. These observations provide new insights into that SA is not only a defensive weapon, but also an offensive weapon against fungal pathogens.
Fusarium oxysporum is a hemibiotrophic and endophytic pathogen feeding on plant cells [54]. In our results, SA can be used as an offensive weapon to damage F. oxysporum cells. To successfully colonize and survive in plant cells, F. oxysporum must spatiotemporally deploy and evolve strong SAHs to degrade SA in plants. More than 16 SAHs were found in F. oxysporum, which was higher than that of other phytopathogenic fungi (Table S8). Diverse and rapid evolution of FoSAHs during long-term co-evolution between host and the pathogen may be the reason why F. oxysporum can successfully infect over 100 plants. Transgenic Arabidopsis with the bacterium Pseudomonas putida salicylate hydroxylase gene can decompose SA, leading to lower resistance to pathogens [37], indicating that salicylate hydroxylase has functions in plants. It is possible that the engineered plants with enough siRNAs of FoSAHs may significantly block the invasion and colonization of F. oxysporum in the vascular part of plants.
We therefore propose a model to highlight the role of exogenous SA in regulating hyphal growth and pathogenicity via the FoSNF1-FoTORC1 signaling pathway in F. oxysporum (Fig. 7d). Phytohormone SA, produced by plants, inhibits the activity of FoTORC1 by phosphorylating FoSNF1, resulting in the arrest of growth and reduction of pathogenicity of F. oxysporum, whereas the endogenous salicylate hydroxylases (FoSAHs) attenuate the toxicity of SA by detoxifying SA. The mycelial growth, cell viability, and virulence of F. oxysporum were synergistically inhibited when SA was combined with TOR inhibitors such as rapamycin and siRNAs of FoTOR1. Thus, a efficient FoTOR signaling inhibition system was established for controlling F. oxysporum. Especially, SA and siRNAs of FoTOR1 and FoSAH1 genes can be engineered and produced by plants [55], [56]. Considering that TOR is a lethal gene in various eukaryotic pathogens from plants to animals and humans, the synergistic effects generated by SA and TOR inhibitors may contribute to therapies for various fungal diseases from plants to animals and humans. Hopefully, F. oxysporum pathogen can be successfully controlled by applying SA and siRNAs of FoTOR1 and FoSAH1 genes in crops in the future. Crop cultivars sustainably resistant to Fusarium wilt may be generated by inducing and enhancing SA and siRNAs of FoTOR1 and FoSAH1 genes in crops.
Compliance with Ethics Requirement
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
MR and LL designed the experiments. LL, TZ, YS and LF performed the experiments. LL, TZ, RD and YS analyzed the data. MR, LL, TZ, YS, PK, RR and MS wrote the manuscript. This work was supported by grants from the National Natural Science Foundation of China (No. 32002105 and 31972469) and Agricultural Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences (34-IUA-02).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.10.014.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Chambers J.E., Greim H., Kendall R.J., Segner H., Sharpe R.M., Van Der Kraak G. Human and ecological risk assessment of a crop protection chemical: a case study with the azole fungicide epoxiconazole. Crit Rev Toxicol. 2014;44(2):176–210. doi: 10.3109/10408444.2013.855163. [DOI] [PubMed] [Google Scholar]
- 2.Stamatis N., Hela D., Konstantinou I. Occurrence and removal of fungicides in municipal sewage treatment plant. J Hazard Mater. 2010;175(1-3):829–835. doi: 10.1016/j.jhazmat.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 3.Norn S., Permin H., Kruse P.R., Kruse E. From willow bark to acetylsalicylic acid. Dan Medicinhist Arbog. 2009;37:79–98. [PubMed] [Google Scholar]
- 4.Klessig D.F., Tian M., Choi H.W. Multiple Targets of Salicylic Acid and Its Derivatives in Plants and Animals. Front Immunol. 2016;7:206. doi: 10.3389/fimmu.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawley S.A., Fullerton M.D., Ross F.A., Schertzer J.D., Chevtzoff C., Walker K.J., et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336(6083):918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Y., Sun T., Ao K., Peng Y., Zhang Y., Li X., et al. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell. 2018;173(6):1454–1467.e15. doi: 10.1016/j.cell.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Radojicic A., Li X., Zhang Y. Salicylic Acid: A Double-Edged Sword for Programed Cell Death in Plants. Front Plant Sci. 2018;9:1133. doi: 10.3389/fpls.2018.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amborabé B.-E., Fleurat-Lessard P., Chollet J.-F., Roblin G. Antifungal effects of salicylic acid and other benzoic acid derivatives towards Eutypa lata: structure–activity relationship. Plant Physiol Bioch. 2002;40(12):1051–1060. [Google Scholar]
- 10.da Rocha Neto A.C., Maraschin M., Di Piero R.M. Antifungal activity of salicylic acid against Penicillium expansum and its possible mechanisms of action. Int J Food Microbiol. 2015;215:64–70. doi: 10.1016/j.ijfoodmicro.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Daw B.D., Zhang L.H., Wang Z.Z. Salicylic acid enhances antifungal resistance to Magnaporthe grisea in rice plants. Australas Plant Path. 2008;37(6):637–644. [Google Scholar]
- 12.Wu H.S., Raza W., Fan J.Q., Sun Y.G., Bao W., Liu D.Y., et al. Antibiotic effect of exogenously applied salicylic acid on in vitro soilborne pathogen, Fusarium oxysporum f.sp.niveum. Chemosphere. 2008;74(1):45–50. doi: 10.1016/j.chemosphere.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Ma L.-J., van der Does H.C., Borkovich K.A., Coleman J.J., Daboussi M.-J., Di Pietro A., et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature. 2010;464(7287):367–373. doi: 10.1038/nature08850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu Rev Phytopathol. 2017;55(1):23–39. doi: 10.1146/annurev-phyto-080615-095919. [DOI] [PubMed] [Google Scholar]
- 15.García Bayona L., Grajales A., Cárdenas M.E., Sierra R., Lozano G., Garavito M.F., et al. Isolation and characterization of two strains of Fusarium oxysporum causing potato dry rot in Solanum tuberosum in Colombia. Rev Iberoam Micol. 2011;28(4):166–172. doi: 10.1016/j.riam.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Husaini A.M., Sakina A., Cambay S.R. Host-pathogen interaction in Fusarium oxysporum infections: where do we stand? Mol Plant Microbe Interact. 2018;31(9):889–898. doi: 10.1094/MPMI-12-17-0302-CR. [DOI] [PubMed] [Google Scholar]
- 17.Raza W., Ling N., Zhang R., Huang Q., Xu Y., Shen Q. Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies. Crit Rev Biotechnol. 2017;37(2):202–212. doi: 10.3109/07388551.2015.1130683. [DOI] [PubMed] [Google Scholar]
- 18.De Virgilio C., Loewith R. Cell growth control: little eukaryotes make big contributions. Oncogene. 2006;25(48):6392–6415. doi: 10.1038/sj.onc.1209884. [DOI] [PubMed] [Google Scholar]
- 19.Yuan H.-X., Xiong Y., Guan K.-L. Nutrient Sensing, Metabolism, and Cell Growth Control. Mol Cell. 2013;49(3):379–387. doi: 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González A., Hall M.N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017;36(4):397–408. doi: 10.15252/embj.201696010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J.L., Bonenfant D., et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10(3):457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 22.Hara K., Maruki Y., Long X., Yoshino K.-I., Oshiro N., Hidayat S., et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110(2):177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 23.Heitman J., Movva N.R., Hall M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 24.Ruan J., Li H. Fast and accurate long-read assembly with wtdbg2. Nat Methods. 2020;17(2):155–158. doi: 10.1038/s41592-019-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simão F.A., Waterhouse R.M., Ioannidis P., Kriventseva E.V., Zdobnov E.M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 26.Burton J.N., Adey A., Patwardhan R.P., Qiu R., Kitzman J.O., Shendure J. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat Biotechnol. 2013;31(12):1119–1125. doi: 10.1038/nbt.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brankovics B., Zhang H., van Diepeningen A.D., Ta V.D.L., Waalwijk C., de Hoog G.S. GRAbB: Selective Assembly of Genomic Regions, a New Niche for Genomic Research. PLoS Comput Biol. 2016;12(6):e1004753. doi: 10.1371/journal.pcbi.1004753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo X., Mao H., Wei Y., Cai J., Xie C., Sui A., et al. The fungal-specific transcription factor Vdpf influences conidia production, melanized microsclerotia formation, and pathogenicity in Verticillium dahliae. Mol Plant Pathol. 2016;17(9):1364–1381. doi: 10.1111/mpp.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao X., Cai T., Olyarchuk J.G., Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21(19):3787–3793. doi: 10.1093/bioinformatics/bti430. [DOI] [PubMed] [Google Scholar]
- 30.Chou T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 31.Thatcher L.F., Manners J.M., Kazan K. Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis. Plant J. 2009;58(6):927–939. doi: 10.1111/j.1365-313X.2009.03831.x. [DOI] [PubMed] [Google Scholar]
- 32.Hamel L.-P., Nicole M.-C., Duplessis S., Ellis B.E. Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. Plant Cell. 2012;24(4):1327–1351. doi: 10.1105/tpc.112.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Pietro A., García-MacEira F.I., Méglecz E., Roncero M.I. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol Microbiol. 2001;39(5):1140–1152. [PubMed] [Google Scholar]
- 34.Segorbe D., Di Pietro A., Pérez-Nadales E., Turrà D. Three Fusarium oxysporum mitogen-activated protein kinases (MAPKs) have distinct and complementary roles in stress adaptation and cross-kingdom pathogenicity. Mol Plant Pathol. 2017;18(7):912–924. doi: 10.1111/mpp.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quoc N.B., Chau N.N.B. The Role of Cell Wall Degrading Enzymes in Pathogenesis of Magnaporthe oryzae. Curr Protein Pept Sci. 2017;18(10):1019–1034. doi: 10.2174/1389203717666160813164955. [DOI] [PubMed] [Google Scholar]
- 36.Ospina-Giraldo M.D., Mullins E., Kang S. Loss of function of the Fusarium oxysporum SNF1 gene reduces virulence on cabbage and Arabidopsis. Curr Genet. 2003;44(1):49–57. doi: 10.1007/s00294-003-0419-y. [DOI] [PubMed] [Google Scholar]
- 37.Delaney T.P., Uknes S., Vernooij B., Friedrich L., Weymann K., Negrotto D., et al. A central role of salicylic Acid in plant disease resistance. Science. 1994;266(5188):1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- 38.Fang Y.i., Bullock H., Lee S.A., Sekar N., Eiteman M.A., Whitman W.B., et al. Detection of methyl salicylate using bi-enzyme electrochemical sensor consisting salicylate hydroxylase and tyrosinase. Biosens Bioelectron. 2016;85:603–610. doi: 10.1016/j.bios.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 39.Dobrenel T., Caldana C., Hanson J., Robaglia C., Vincentz M., Veit B., et al. TOR Signaling and Nutrient Sensing. Annu Rev Plant Biol. 2016;67(1):261–285. doi: 10.1146/annurev-arplant-043014-114648. [DOI] [PubMed] [Google Scholar]
- 40.Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Din F.V.N., Valanciute A., Houde V.P., Zibrova D., Green K.A., Sakamoto K., et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142(7):1504–1515.e3. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davie, Elizabeth, Forte, Gabriella, Nbsp, M.A, et al. Nitrogen Regulates AMPK to Control TORC1 Signaling. Curr Biol 2015;25(4):445–54. [DOI] [PMC free article] [PubMed]
- 43.Kim Do-Hyung, Sarbassov Dos D., Ali Siraj M., King Jessie E., Latek Robert R., Erdjument-Bromage Hediye, et al. MTOR interacts with Raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110(2):163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 44.Dunlop Elaine A., Hunt David K., Acosta-Jaquez Hugo A., Fingar Diane C., Tee Andrew R. ULK1 inhibits mTORC1 signaling, promotes multisite Raptor phosphorylation and hinders substrate binding. Autophagy. 2011;7(7):737–747. doi: 10.4161/auto.7.7.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chauvin C, Koka V, Nouschi A, Mieulet V, Hoareau-Aveilla C, Dreazen A, et al. Ribosomal protein S6 kinase activity controls the ribosome biogenesis transcriptional program. Oncogene. 2014;33(4):474–483. doi: 10.1038/onc.2012.606. [DOI] [PubMed] [Google Scholar]
- 46.Ploetz Randy C. Fusarium Wilt of Banana. Phytopathology. 2015;105(12):1512–1521. doi: 10.1094/PHYTO-04-15-0101-RVW. [DOI] [PubMed] [Google Scholar]
- 47.Dean R., Van Kan J.A., Pretorius Z.A., Hammondkosack K.E., Di P.A., Spanu P.D., et al. The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012;13(4):804. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Dam Peter, Fokkens Like, Ayukawa Yu, van der Gragt Michelle, ter Horst Anneliek, Brankovics Balázs, et al. A mobile pathogenicity chromosome in Fusarium oxysporum for infection of multiple cucurbit species. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-07995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlot A. Corina, Dempsey D'Maris Amick, Klessig Daniel F. Salicylic Acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47(1):177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 50.Liu X., Rockett K.S., Korner C.J., Pajerowska-Mukhtar K.M. Salicylic acid signalling: new insights and prospects at a quarter-century milestone. Essays Biochem. 2015;58:101–113. doi: 10.1042/bse0580101. [DOI] [PubMed] [Google Scholar]
- 51.Yang Haijuan, Rudge Derek G., Koos Joseph D., Vaidialingam Bhamini, Yang Hyo J., Pavletich Nikola P. mTOR kinase structure, mechanism and regulation. Nature. 2013;497(7448):217–223. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu F., Gu Q., Yun Y., Yin Y., Xu J.R., Shim W.B., et al. The TOR signaling pathway regulates vegetative development and virulence in Fusarium graminearum. New Phytol. 2014;203(1):219–232. doi: 10.1111/nph.12776. [DOI] [PubMed] [Google Scholar]
- 53.Li Linxuan, Zhu Tingting, Song Yun, Luo Xiumei, Datla Raju, Ren Maozhi. Target of rapamycin controls hyphal growth and pathogenicity through FoTIP4 in Fusarium oxysporum. Mol Plant Pathol. 2021;22(10):1239–1255. doi: 10.1111/mpp.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyons R., Stiller J., Powell J., Rusu A., Manners J.M., Kazan K. Fusarium oxysporum triggers tissue-specific transcriptional reprogramming in Arabidopsis thaliana. PLoS One. 2015;10(4):e0121902. doi: 10.1371/journal.pone.0121902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang M., Weiberg A., Lin F.M., Thomma B.P.H.J., Huang H.D., Jin H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat Plants. 2016;2(10):16151. doi: 10.1038/nplants.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai Qiang, Qiao Lulu, Wang Ming, He Baoye, Lin Feng-Mao, Palmquist Jared, et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360(6393):1126–1129. doi: 10.1126/science.aar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq and genome data have been deposited in the NCBI Sequence Read Archive under accession numbers PRJNA730676 and PRJNA730382, respectively. The ITS sequences of Fo-2 and Fo-4 have been deposited in the NCBI GenBank accession numbers MZ208819 and MZ223468, respectively. Other relevant data are available in supplemental materials.