Abstract
COVID-19 is accompanied by strong inflammatory reaction and is often followed by long-term cognitive disorders. The fragment 674–685 of SARS-Cov-2 spike protein was shown to interact with α7 nicotinic acetylcholine receptor involved in regulating both inflammatory reactions and cognitive functions. Here we show that mice immunized with the peptide corresponding to 674–685 fragment of SARS-Cov-2 spike protein conjugated to hemocyanin (KLH-674-685) demonstrate decreased level of α7 nicotinic acetylcholine receptors, increased levels of IL-1β and TNFα in the brain and impairment of episodic memory. Choline injections prevented α7 nicotinic receptor decline and memory loss. Mice injected with immunoglobulins obtained from the blood of (KLH-674-685)-immunized mice also demonstrated episodic memory decline. These data allow suggesting that post-COVID memory impairment in humans is related to SARS-Cov-2 spike protein-specific immune reaction. The mechanisms of such effect are being discussed.
Keywords: COVID-19, SARS-CoV-2 spike protein, α7 nicotinic acetylcholine receptor, Neuroinflammation, Episodic memory, Antibodies
Abbreviations: nAChR, nicotinic acetylcholine receptor; KLH, keyhole limpet hemocyanine; BSA, bovine serum albumin
1. Introduction
SARS-Cov-2 infection causes severe respiratory disease but also affects multiple organs and tissues including the brain [1]. Moreover, people who recovered from COVID-19 suffer from its consequences for months. Post-COVID patients often possess cognitive disorders like depression, intellectual weakness and memory loss [2]. In spite of several suggested explanations, the reason for such long-lasting complications are not completely understood. Some hypotheses attribute it to immune mechanisms triggered by SARS-CoV-2 [3].
One of the interesting features of SARS-CoV-2 is the homology of its spike protein fragments with the fragments of snake toxins responsible for the interaction with nicotinic acetylcholine receptors (nAChRs). Fragment 375 to 390 is homologous to Neurotoxin homolog NL1 [4], while fragment 674–685 is homologous to fragments of α-cobratoxin and α-bungarotoxin, the well-known blockers of α7 nicotinic acetylcholine receptor, and to a fragment of rabies virus known to infect the cells through nAChRs [5,6]. Although it has been established that the main gate for SARS-CoV-2 penetration into the cell is ACE2 receptor [7], the contribution of α7 nAChR cannot be neglected. The α7 nAChRs are involved in regulating pro-inflammatory cytokines production [8]; therefore, it was logical to suggest that “cytokine storm” observed in COVID-19 patients may be related to the blockade of α7 nAChRs [6]. However, no direct evidence for such mechanism has been obtained by now. Previously we reported that peptide corresponding to 674–685 fragment of SARS-CoV-2 spike protein (further mentioned as 674–685 peptide) competed with the antibody elicited against (179–190) fragment of α7 nAChR subunit for the binding to the α7-containing cell and mitochondria preparations and attenuated cytochrome c release from isolated mitochondria similarly to α7-specific agonists or positive allosteric modulators [9]. This data indicated that peptide 674–685 can bind α7-containing nAChRs and influence their functions.
The α7 nAChRs expressed in the brain are involved in regulating cognitive processes like memory and learning capacity [10]. The decrease of the brain α7 nAChR content caused by neuroinflammation is accompanied by episodic memory loss [11]. Taking into account the established capacity of 674–685 peptide to bind α7 nAChRs, in the present paper we put an aim to investigate if immunization with 674–685 peptide can influence the α7 nAChR expression and functioning in the brain. The results obtained demonstrate that immune reaction elicited by 674–685 peptide results in pro-inflammatory cytokines production, the decrease of α7 nAChR content in the brain and impairment of episodic memory of immunized mice. The mechanisms of such effect, as well as the way to overcome it are being discussed.
2. Materials and methods
2.1. Reagents
All reagents were of chemical grade and were purchased from Sigma-Aldrich (Saint Louis, USA) unless specifically indicated. The peptide corresponding to SARS-Cov-2 spike protein fragment 674–685 modified with N-terminal cysteine (further mentioned as (674–685)), and the peptide corresponding to (179–190) fragment of α7 nAChR subunit (further mentioned as α7(179–190)) were synthesized by JPT Peptide Technologies GmbH (Berlin, Germany). Molecular weight markers, maleimide-activated KLH and BSA and Neutravidin-peroxidase conjugate were from Thermo Fisher Scientific and were purchased by ALT Ukraine Ltd. Kits for determination of IL-1β (Cat.# 88-7261-88) and TNFα (Cat.# 88-7346-88) were from Invitrogen and were purchased by ALT Ukraine Ltd. Antibodies against α7(1–208), α7(179–190), α4(181–192) and β2(190–200) nAChR fragments were obtained, validated and biotinylated previously in our laboratory [[12], [13], [14]].
2.2. Animals
We used C57BL/6J mice of both genders, 3–5 months of age, 20–25 g of weight. Animals were kept in the animal facility of Palladin Institute of Biochemistry. They were housed in quiet, temperature-controlled rooms and provided with water and food pellets ad libitum. Before removing the brain mice were sacrificed by cervical dislocation. All procedures conformed to the guidelines of Palladin Institute's IACUC. Before starting the experiments, the protocols were approved by the IACUC.
2.3. Preparation and characterization of peptide-protein conjugates
Peptide (674–685) conjugation to protein carriers was performed according to the manufacturer (Thermo Fisher Scientific) instructions. Briefly, 2 mg of the peptide was dissolved in 0.5 ml of PBS and mixed with 2 mg of maleimide-activated KLH or BSA in 1.0 ml PBS with 0.1 M EDTA рН 7.2 for 2h at room temperature and then dialized against PBS. Peptide α7(179–190) was conjugated to BSA by glutaraldehyde procedure as previously described [12].
SDS-PAGE of non-conjugated BSA and BSA-674-685 conjugate was performed in 8% polyacrylamide gel [15]. The gel was stained with Thermo Scientific PageBlue Protein Staining Solution. Molecular weight of resulting protein bands was estimated according to resolution of molecular weight markers Thermo Scientific PageRuler Plus Prestained Protein Ladder.
2.4. Immunization procedures and mice treatment
In the first set of experiments, a group of mice (n = 10) was immunized intraperitoneally with KLH-674-685 (50 μg per mouse) emulsified in Complete Freund's Adjuvant; a control group of mice (n = 10) was immunized similarly with non-conjugated KLH. The second immunization was performed with the same dose of antigens emulsified in Incomplete Freund's Adjuvant on day 28 after initial immunization. Mice were examined in behavioral test each week after immunization. The blood (about 100 μl) was taken from the tail vein before each behavioral test. A half of each group of immunized mice (n = 5) was sacrificed by cervical dislocation on day 42, another half (n = 5) on day 63 after immunization and their brains were removed for investigation.
In the next set of experiments, 10 mice were immunized intraperitoneally with KLH-674-685 conjugate and a half of them (n = 5) obtained intraperitoneal injections of choline (0.5 mg per mouse in 0.3 ml of PBS) on days 9, 10, 11, 12 and 13 after initial immunization and on days 5, 7, 9, 11 and 13 after boosting. Mice were examined in behavioral test each week after immunization; the blood (about 100 μl) was taken from the tail vein before each behavioral test. All mice were sacrificed on day 42 and their brains were removed for investigation.
For passive transfer experiment, 10 mice were immunized intraperitoneally with KLH-674-685 conjugate (50 μg per mouse) emulsified in Complete Freund's Adjuvant. Two immunizations with the interval of two months have been performed. The mice were examined in behavioral test two weeks after the second immunization, sacrificed and their blood was collected. The immunoglobulin fraction was obtained from the pooled blood sera by a standard procedure of ammonium sulfate precipitation [16] and was dialized against PBS. The specificity of purified immunoglobulins was tested in ELISA.
Twelve non-immunized mice were examined in behavioral test and then divided into three groups, 4 animals in each. One group was injected intraperitoneally with LPS (E. coli strain 055:B5, 30 μg per mouse), another one obtained (674–685)-specific immunoglobulins (intravenously, in the tail vein, 200 μg per mouse in PBS) and the third group obtained both LPS and immunoglobulin injections. LPS was injected once (on day 0), while immunoglobulins were injected on days 0, 1 and 3 and the mice were examined in behavioral test on days 3 and 7.
2.5. Behavioral experiments
Mice were tested in the “Novel Object Recognition” (NOR) behavioral test as described [17]. Briefly, the mice were suggested to explore two identical objects for 15 min and then, after a 15 min break, one object was replaced by a novel one of similar size but different shape or color and the number of explorations of the two objects was registered in subsequent 15 min session. The results of NOR test are presented as Discrimination Index calculated as the difference in the number of “novel” and “familiar” object explorations divided by the total number of explorations of two identical objects. The lack of novel object preference, expressed as a Discrimination Index decrease, was qualified as episodic memory impairment.
2.6. The brain preparations
The removed brains were used to prepare the detergent lysates as described previously [18]. Briefly, the brains were thoroughly washed with PBS and homogenized in a porcelain mortar with the lysis buffer (0.01 M Tris-HCl, pH 8.0; 0.14 M NaCl; 0.025% NaN3; 1% Tween 20 and protease inhibitors cocktail). The primary homogenate was frozen at −70 °C, thawed, washed by centrifugation at 10 000 rpm and resuspended in the lysis buffer on ice upon intensive stirring for 2h. The resulting lysate was cleared by centrifugation (20 min at 13 000 rpm) and dialyzed against PBS containing 0.025% NaN3 and protease inhibitors. Protein content was measured with the BCA kit (Thermo Scientific, France).
2.7. ELISAs
To determine the level of (674–685)-specific antibodies in the mouse blood sera or immunoglobulin fraction, the immunoplates (Nunc, Maxisorp) were coated with BSA-674-685 (10 μg/ml) and blocked with 1% BSA. The sera or immunoglobulins were diluted in 0.05% Tween 20-containing PBS and applied into the wells for 2h at 37 °C. The plates were washed with water and the bound antibodies were determined with peroxidase-conjugated anti-mouse IgG (Fab-specific) followed by o-phenylendiamine-containing substrate solution.
To determine the level of α7(179–190)-specific antibodies in the mouse blood sera or immunoglobulin fraction, the immunoplates were coated with BSA-α7(179–190) (10 μg/ml) and blocked with 1% BSA. The blood sera or immunoglobulins diluted in 0.05% Tween 20-containing PBS were applied into the wells for 2h at 37 °C. The plates were washed with water and the bound antibodies were determined with peroxidase-conjugated anti-mouse IgG (Fab specific) followed by o-phenylendiamine-containing substrate solution.
To determine the level of (674–685)-specific antibodies in the mouse brains, the immunoplates were coated with BSA-(674–685) (10 μg/ml) and blocked with 1% BSA. The brain detergent lysates diluted in PBS were applied into the wells (1 μg of protein per 0.05 ml per well) for 2h at 37 °C. To study if choline prevents binding of (674–685)-specific antibodies to immobilized BSA-(674–685), the brain detergent lysates were pre-incubated with choline (0.1; 1.0 and 10 μM) for 15 min at room temperature before being applied into the wells. The plates were washed with water and the bound antibodies were determined with peroxidase-conjugated anti-mouse IgG (Fab-specific) followed by o-phenylendiamine-containing substrate solution.
To determine the level of α4, α7 or β2 nAChR subunits within the brain preparations, the immunoplates were coated with rabbit α7(1–208)-specific antibody (20 μg/ml), blocked with 1% BSA, and the detergent lysates of brain tissue were applied into the wells (1 μg of protein per 0.05 ml per well) for 2h at 37 °C. The plates were washed with water and the second biotinylated α4(181–192)-, α7(179–190)-, or β2(190–200)-specific antibody was applied for additional 2 h being revealed with Neutravidin-peroxidase conjugate and o-phenylendiamine-containing substrate solution.
The cytokines (IL-1β and TNFα) were determined in the brain detergent lysates by Sandwich ELISA according to the instructions of the kits manufacturer.
The optical density of chromogenic substrate reactions was read with Stat-Fax 2100 Microplate reader (Awareness Technology, USA).
2.8. Statistical analysis
All experimental schemes have been performed with at least 4–5 mice per group. ELISAs have been performed in triplicates. The mean values were used for statistical analysis using one-way ANOVA test and Origin 9.0 software. The data are presented as Mean ± SD. p < 0.05 was considered a significant difference.
3. Results
3.1. Immunogen preparation and immune response
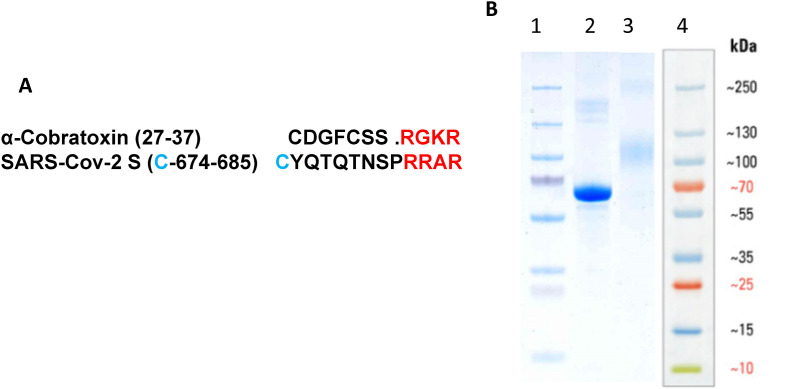
The short peptides are weakly immunogenic by themselves. In order to increase the 674–685 peptide immunogenicity we conjugated it to a protein carrier, keyhole limpet hemocyanin (KLH). To ensure the exposure of an α-cobratoxin-resembling part of this peptide (residues 682–685), we used a modified peptide possessing an N-terminal cysteine residue (Fig. 1 A). This enabled coupling it to maleimide-modified KLH and, therefore, leaved C-terminal arginines available for immune recognition. Similarly, for ELISA studies, the Cys-modified 674–685 peptide was coupled to maleimide-modified BSA. The efficiency of peptide-BSA conjugation was assessed by SDS-PAGE. As shown in Fig. 1B, the estimated molecular weight of BSA-(674–685) conjugate was between 90 and 120 KDa. Taking into account the molecular weight of BSA (68 KDa) and 674–685 peptide (1.58 KDa), this means that the conjugate contained 15–30 mol of the peptide per mole of BSA. Such an approach could not be used for peptide-KLH conjugate because of the high and uneven molecular weight of KLH. However, the ability of antibodies elicited by the peptide-KLH conjugate to recognize the peptide-BSA conjugate (see below) indicates that the peptide coupling to maleimide-modified KLH was also quite efficient.
Fig. 1.
A: Amino acid sequences of (27–37) fragment of α-cobratoxin from Naja kaouthia (UniProtKB P01391) and SARS CoV-2 S-protein (674–685) fragment (PDB 6VXX) modified with an N-terminal cysteine residue (blue). Homologous C-terminal residues are marked by red.B: SDS-PAGE of BSA-(674–685) (line 3) compared to BSA (line 2). Lines 1,4 – molecular weight markers in the gel (1) or as described by the manufacturer (4). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
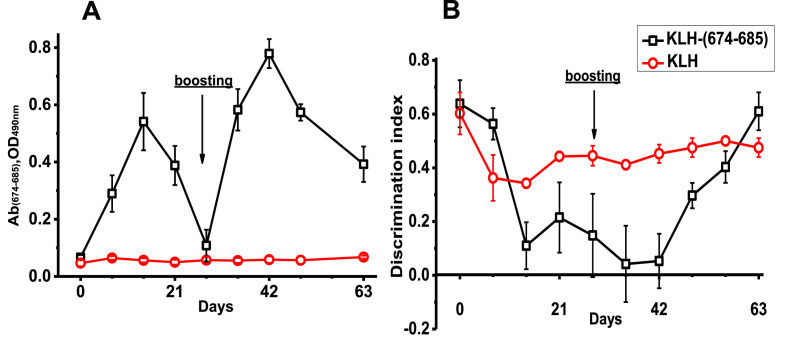
Mice were immunized with KLH-(674–685) and the antibodies capable to recognize BSA-(674–685) were detected in their blood starting from day 7, reached peak on day 14 and went down by day 28 after initial immunization (Fig. 2 A). The second immunization performed on day 28 stimulated more powerful antibody response, which was maximal on day 42 (14 days after boosting) and decreased by day 63 (day 28 after boosting), but the antibody level was still higher than before immunization. Immunization with unconjugated KLH did not stimulate (674–685)-specific antibody production.
Fig. 2.
The level of (674–685)-specific antibodies in the blood sera of mice measured by ELISA (A) and Discrimination index measured in NOR test (B) in mice immunized with either KLH or KLH-(674–685).
3.2. Effects on the brain and behavior
According to behavioral NOR test, discrimination index of mice immunized with KLH-(674–685) was decreased by day 14 after initial immunization and even more after boosting (Fig. 2B). Then it started to grow up and recovered completely by day 63. Therefore, maximal decline of episodic memory (as indicated by discrimination index) coincided with the peaks of (674–685)-specific antibodies in the blood of immunized mice. Mice immunized with KLH demonstrated only slight decrease of discrimination index that did not change during the period of observation.
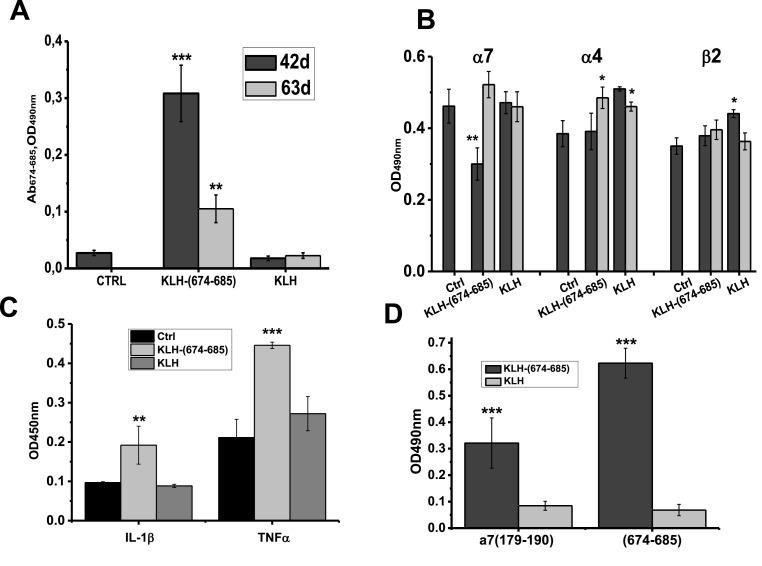
The (674–685)-specific antibodies were also found in the brain preparations of immunized mice. As shown in Fig. 3 A, significant (674–685)-specific signal was detected in the brain detergent lysates on day 42 and, less, on day 63 after initial immunization. Immunization with KLH-(674–685) significantly reduced the level of α7 (but not α4 or β2) nAChR subunits (Fig. 3B) and increased the level of pro-inflammatory cytokines IL-1β and TNFα (Fig. 3C) in the brain of mice on day 42 after immunization. Surprisingly, the blood sera of mice immunized with KLH-(674–685) also contained antibodies capable to bind α7(179–190) (Fig. 3D).
Fig. 3.
The levels of (674–685)-specific antibodies (A), of α7, α4 and β2 nAChR subunits (B) and of pro-inflammatory cytokines IL-1β and TNFα (C, day 42) in the brains of mice immunized with either KLH or KLH-(674–685); the brains were taken on days 42 (n = 5) and 63 (n = 5) after initial immunization. ∗ - p < 0.05; ∗∗ - p < 0.005; ∗∗∗ - p < 0.0005 compared to non-immunized mice (Ctrl). Designations in A and B are similar. D: Antibodies against (674–685) S-protein fragment or α7(179–190) found in the blood sera of mice immunized with either KLH-(674–685) or KLH on day 42 after initial immunization. Each column corresponds to M±SD; ∗∗∗ - p < 0.0005 compared to the data of KLH-immunized mice; n = 5.
3.3. Passive transfer experiments
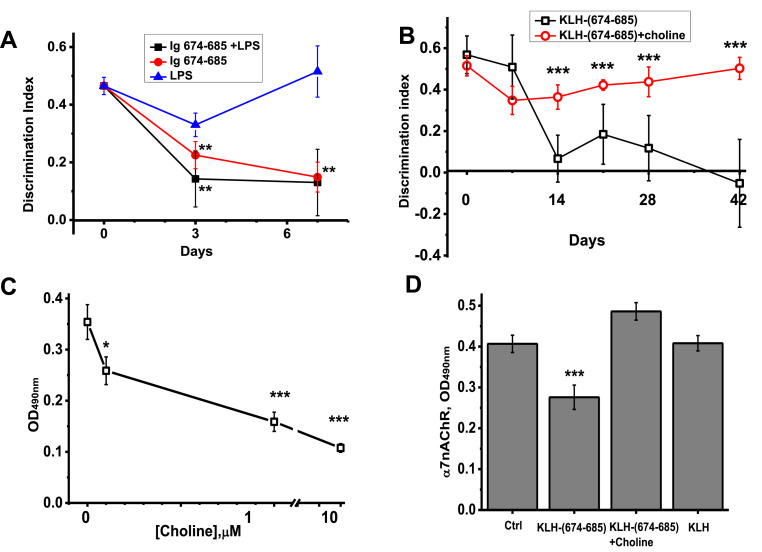
The mice injected intravenously with immunoglobulins obtained from the blood sera of (KLH-674-685)-immunized mice demonstrated episodic memory decline on day 3 after injection already (Fig. 4 A). The immunoglobulins effect was additive to that of LPS. Mice injected with LPS recovered by day 7, while those injected with (KLH-674-686)-specific immunoglobulins, with or without LPS, still demonstrated significantly decreased episodic memory.
Fig. 4.
Episodic memory measured in NOR test (Discrimination Index) of mice injected with LPS, immunoglobulins of (KLH-674-685)-immunized mice (Ig 674–685) or their combination (A, n = 4) and of those immunized with KLH-674-685 in the presence or absence of choline (B, n = 5); the effects of choline on the brain antibody binding to BSA-(674–685) (C), and on the content of α7 nAChR subunits in the brains of mice (day 42, D). ∗ - p < 0.05; ∗∗ - p < 0.005; ∗∗∗ - p < 0.0005 compared to LPS-treated (A), non-treated mice (B, D) or to the antibody binding in the absence of choline (C).
3.4. The effects of choline
The (674–685) SARS-CoV-2 S-protein fragment competed with α7(179–190)-specific antibodies and, therefore, could bind the (179–190) portion of α7 nAChR subunit [9]. The (179–190) fragment is a part of the nAChR agonist-binding site [19] and also a target for snake toxins binding [20]. We hypothesized that positively charged C-terminal arginine residues of 674–685 peptide (Fig. 1A) mimic the charge of the quaternary nitrogen of α7 nAChR agonists choline and acetylcholine and, therefore, antibodies against 674–685 peptide found in the brain could interact with choline. To test this hypothesis, the brain preparations of mice immunized with KLH-(674–685) and containing (674–685)-specific antibodies (Fig. 3A) were pre-incubated with choline before being applied into BSA-(674–685)-coated wells. As shown in Fig. 4C, choline dose-dependently inhibited the antibody binding indicating that (674–685)-specific antibodies were blocked by choline.
Then we asked if choline could prevent the pathogenic effect of (674–685)-specific antibody on the brain and memory of mice. For this purpose, two groups of mice were immunized with KLH-(674–685) and one group was additionally injected with choline on days 9–13 after initial immunization and each second day on days 5–13 after boosting. As shown in Fig. 4B, mice injected with choline did not demonstrate episodic memory decline compared to those which did not obtain choline. In addition, choline treatment prevented α7 nAChR subunits decrease in the brains of mice observed on day 42 after immunization with KLH-(674–685) (Fig. 4D).
4. Discussion
The α7 nAChRs expressed in the brain are involved in regulating cognitive functions and decreasing their content by either inflammation or α7-specific antibodies results in episodic memory impairment in mice (reviewed in Ref. [21]). The (674–685) fragment of SARS-CoV-2 S-protein contains a portion (RRAR), which resembles the fragments of α-cobratoxin and α-bungarotoxin responsible for the interaction with and blocking of α7 nAChRs or, at least, bears similar set of positively charged residues (RGKR). The interaction of (674–685) peptide with α7 nAChR was demonstrated in our experiments where (674–685) peptide competed with α7(179–190)-specific antibodies for the binding to α7-expressing cells and mitochondria and affected mitochondria similarly to α7-specific ligands [9]. The binding of SARS-Cov-2 virus to the target cell is facilitated by the cleavage of its spike protein by serine proteinase just after R685 [22]; therefore, the cluster of positively charged arginines is naked and becomes available for interaction. This cluster has been also suggested as a site of neuropilin1 binding thus assuming an additional entry receptor for SARS-Cov-2 [23]. Similarly, the spike protein fragment with naked RRAR cluster can bind α7 nAChRs including those expressed in the brain. The idea of dysregulation of nicotinergic system upon SARS-CoV-2 infection has already been discussed [6], in particular, in relation to another SARS-CoV-2 spike protein fragment 383–386, which is homologous to Neurotoxin homolog NL1 and was predicted in silico to bind α7 and α9 nAChRs [4]. However, no experimental evidence about the direct SARS-Cov-2 influence on the brain α7 nAChRs has been provided.
The cognitive effects of COVID-19 like depression, memory impairment and difficulties in concentration, are usually found not immediately after infection and often during months after recovery that allows attributing them not to direct effect of the virus but rather to post-COVID immune reaction. Here we show that immune response to 674–685 fragment of SARS-CoV-2 spike protein may be the reason of cognitive deficiency.
The data presented demonstrate that immunization of mice with the peptide corresponding to (674–685) fragment of SARS-CoV-2 spike protein results in the increase of pro-inflammatory cytokines content and the decrease of α7 nAChR content in the brain accompanied by impairment of episodic memory. Similar effect was previously observed in LPS-treated mice and was attributed to neuroinflammation [11]. Using that model it was shown that intraperitoneal injections of α7 nAChR agonist PNU282987 prevented the α7 nAChR decline and memory loss [24]. Here we show that pathogenic effect of (674–685)-specific antibodies can be prevented by choline injections. Choline inhibited the (674–685)-specific antibody binding to BSA-(674–685) in ELISA and, therefore, could neutralize a part of (674–685)-specific antibodies in the brain. In addition, it could stimulate the brain α7 nAChRs providing both anti-inflammatory and pro-cognitive effect, similarly to that of PNU282987 in LPS-treated mice.
The pathogenic effect of antibodies elicited in response to KLH-674-685 immunization was further confirmed in passive transfer experiments where immunoglobulins purified from the blood of (KLH-674-685)-immunized mice impaired episodic memory of mice. In this experiment, we compared the effects of (KLH-674-685)-specific immunoglobulins with that of LPS. In fact, LPS was used to facilitate the antibody penetration into the brain and we expected that immunoglobulins will enhance the effect of suboptimal LPS dose. This appeared to be the case; however, to our surprise, the intravenously injected (KLH-674-685)-specific immunoglobulins decreased episodic memory of mice even without LPS. Probably, the inflammatory effect of these immunoglobulins was sufficient to loosen the blood-brain barrier and to allow the antibodies affecting the brain.
The mechanism by which (674–685)-specific antibodies influence the brain α7 nAChRs and impair memory is not completely understood. One possibility is that such antibodies interfere with acetylcholine signaling underlying learning and memory [25]. However, the selective decrease of α7 nAChRs indicates that the antibody influence is targeted to this receptor subtype. Previously we reported that immunization with α7(1–208) extracellular domain affects mice similarly to LPS injections [11] and α7(179–190)-specific antibodies stimulate pro-inflammatory cytokines production in cultured astrocytoma cells [26]. We did found antibodies capable to bind α7(179–190) peptide in the blood sera, as well as in the immunoglobulin fraction of mice immunized with KLH-(674–685). Their presence could explain the appearance of symptoms observed in KLH-(674–685)-immunized mice, however, their origin is quite intriguing. One version is that such antibodies are formed in response to destroyed α7-bearing cells that seems poorly probable. Alternatively, they could be anti-idiotypic antibodies formed along with (674–685)-specific antibodies generation.
The theory of idiotypic networks suggested by K. Yerne [27] claims that antigen-specific antibody elicited in response to infection stimulates the production of anti-idiotypic antibody directed to the antibody binding site and, therefore, somehow resembling the antigen. This idea has successfully been used for developing anti-idiotypic vaccines for a number of viruses including reovirus, rabies virus, murine mammary tumor virus (MMTV), murine leukemia virus, hepatitis B virus (HBV) and human immunodeficiency virus (HIV) [28]. The generation of auto-anti-idiotypic antibodies during anti-viral immune response has been also confirmed experimentally [29,30]. In the case of (674–685)-specific antibodies, the anti-idiotypic antibodies are expected to mimic 674–685 fragment of SARS-CoV-2 spike protein and, therefore, bind (179–190) fragment of α7 nAChR subunit that was actually observed. We hypothesize that generation of anti-idiotypic α7-specific antibodies can underlie the neuroinflammation-like effects observed upon immunization with SARS S-protein-derived 674–685 peptide. However, this hypothesis needs additional experiments to be proved.
According to our preliminary evidence, both the (674–685)-specific and α7(179–190)-specific antibodies are detected in the blood of people who experienced COVID-19 2–3 months before (data not shown). Additional experiments with larger groups of people are required to elucidate whether the antibody levels correlate with the severity of post-COVID cognitive impairments. However, taking into account the data obtained in mice, it can be suggested that antibodies generated in response to 674–685 fragment of SARS-CoV-2 spike protein contribute to post-COVID memory loss.
5. Conclusions
-
1)
Immune reaction to SARS-CoV-2 S-protein fragment 674–685 causes neuroinflammation, decreases α7 nAChR content in the brain and impairs episodic memory.
-
2)
Such pathogenic effect is due to impairment of the brain α7 nAChR signaling and can be overcome by choline injections.
-
3)
Immune reaction to SARS-CoV-2 S-protein fragment 674–684 can be the reason of memory impairment in post-COVID patients.
Funding
This research was funded by the grant of Alexander von Humboldt Foundation (Research Group Linkage Program).
Credit author statement
Olena Lykhmus – Methodology, Investigation, Visualization, Writing – Review and Editing, Olena Kalashnyk: Investigation, Writing – Review and Editing; Lyudmyla Koval – Investigation, Writing – Review and Editing; Olga Krynina - Investigation, Writing – Review and Editing; Serhiy Komisarenko – Supervision; Maryna Skok – Conceptualization, Writing – Original draft, Review and Editing.
Declaration of competing interest
Olena Lykhmus, Olena Kalashnyk, Lyudmyla Koval, Olga Krynina, Serhiy Komisarenko and Maryna Skok, - declare that there is no conflict of interest.
References
- 1.Ritchie K., Chan D., Watermeyer T. The cognitive consequences of the COVID-19 epidemic: collateral damage? Brain Commun. 2020;2(2) doi: 10.1093/braincomms/fcaa069. fcaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carod-Artal F.J. Post-COVID-19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev. Neurol. 2021;72(11):84–396. doi: 10.33588/rn.7211.2021230. [DOI] [PubMed] [Google Scholar]
- 3.Woodruff M.C., Ramonell R.P., Saini A.S., Haddad N.S., Anam F.A., Rudolph M.E., et al. 2021. Relaxed peripheral tolerance drives broad de novo autoreactivity in severe COVID-19. medRxiv. [Preprint] [DOI] [Google Scholar]
- 4.Farsalinos K., Eliopoulos E., Leonidas D.D., Papadopoulos G.E., Tzartos S., Poulas K. Nicotinic cholinergic system and COVID-19: in silico identification of an interaction between SARS-CoV-2 and nicotinic receptors with potential therapeutic targeting implications. Int. J. Mol. Sci. 2020;21(16):5807. doi: 10.3390/ijms21165807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lentz T. Rabies virus binding to an acetylcholine receptor α-subunit peptide. Molecular recognition. 1990;3(2):82–88. doi: 10.1002/jmr.300030205. [DOI] [PubMed] [Google Scholar]
- 6.Changeux J.-P., Amoura Z., Rey F.A., Miyara M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Comptes Rendus Biol. 2020;343(1):33–39. doi: 10.5802/crbiol.8. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904. doi: 10.1016/j.cell.2020.03.045. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Jonge W.J., Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalashnyk O., Lykhmus O., Izmailov M., Koval L., Komisarenko S., Skok M. SARS-Cov-2 spike protein fragment 674-685 protects mitochondria from releasing cytochrome c in response to apoptogenic influence. Biochem. Biophys. Res. Commun. 2021;56:14–18. doi: 10.1016/j.bbrc.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dineley K.T., Pandya A.A., Yakel J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015;36(2):96–108. doi: 10.1016/j.tips.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lykhmus О., Voytenko L., Koval L., Mykhalskiy S., Kholin V., Peschana K., et al. α7 Nicotinic acetylcholine receptor-specific antibody induces inflammation and amyloid β42 accumulation in the mouse brain to impair memory. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0122706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skok M.V., Voitenko L.P., Voitenko S.V., Lykhmus E.Y., Kalashnik E.N., Litvin T.I., et al. Alpha subunit composition of nicotinic acetylcholine receptors in the rat autonomic ganglia neurons as determined with subunit-specific anti-alpha (181-192) peptide antibodies. Neuroscience. 1999;93(4):1427–1436. doi: 10.1016/s0306-4522(99)00160-8. [DOI] [PubMed] [Google Scholar]
- 13.Koval O.M., Voitenko L.P., Skok M.V., Lykhmus E.Y., Tsetlin V.I., Zhmak M.N., et al. The β-subunit composition of nicotinic acetylcholine receptors in the neurons of the Guinea pig inferior mesenteric ganglion. Neurosci. Lett. 2004;365(2):143–146. doi: 10.1016/j.neulet.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 14.Lykhmus O., Koval L., Pavlovych S., Zouridakis M., Zisimopoulou P., Tzartos S., et al. Functional effects of antibodies against non-neuronal nicotinic acetylcholine receptors. Immunol. Lett. 2010;128:68–73. doi: 10.1016/j.imlet.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Brunelle J.L., Green R. One-dimensional SDS polyacrylamide gel electrophoresis (1D SDS-PAGE) Methods Enzymol. 2014;541:151–159. doi: 10.1016/B978-0-12-420119-4.00012-4. [DOI] [PubMed] [Google Scholar]
- 16.Harlow E., Lane Antibodies D. Cold Spring Harbor Laboratory; New York: 1988. A Laboratory Manual; pp. 341–342. [Google Scholar]
- 17.Antunes M., Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognit. Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lykhmus O. Yu, Koval L.M., Skok M.V., Zouridakis M., Zisimopoulou P., Tzartos S.J., et al. Antibodies against extracellular domains of alpha4 and alpha7 subunits alter the levels of nicotinic receptors in the mouse brain and affect memory: possible relevance to Alzheimer pathology. J. Alz. Dis. 2011;24:693–704. doi: 10.3233/JAD-2011-101842. [DOI] [PubMed] [Google Scholar]
- 19.Middletone R., Cohen J. Mapping of the acetylcholine binding site of the nicotinic acetylcholine receptor: [3H] nicotine as an agonist photoaffinity label. Biochemistry. 1991;30(28):6987–6997. doi: 10.1021/bi00242a026. [DOI] [PubMed] [Google Scholar]
- 20.Bourne Y., Talley T.T., Hansen S.B., Taylor P., Marchot P. Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake alpha-neurotoxins and nicotinic receptors. EMBO J. 2005;24(8):1512–1522. doi: 10.1038/sj.emboj.7600620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skok M., Lykhmus O. The role of α7 nicotinic acetylcholine receptors and α7-specific antibodies in neuroinflammation related to Alzheimer disease. Curr. Pharmaceut. Des. 2016;22(14):2035–2049. doi: 10.2174/1381612822666160127112914. [DOI] [PubMed] [Google Scholar]
- 22.Takeda M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2022;66(1):15–23. doi: 10.1111/1348-0421.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chekol Abebe E., Mengie Ayele T., Tilahun Muche Z., Asmamaw Dejenie T. Neuropilin 1: a novel entry factor for SARS-CoV-2 infection and a potential therapeutic target. Biologics. 2021;15:143–152. doi: 10.2147/BTT.S307352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lykhmus O.Y., Kalashnyk O.M., Uspenska K.R., Skok M.V. Positive allosteric modulation of alpha7 nicotinic acetylcholine receptors transiently improves memory but aggravates inflammation in LPS-treated mice. Front. Ageing Neurosci. 2020;11:359. doi: 10.3389/fnagi.2019.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picciotto M.R., Zoli M., Léna C., Bessis A., Lallemand Y., Le Novère N. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- 26.Kalashnyk O.M., Lykhmus O.Yu, Oliinyk O.A., Komisarenko S.V., Skok M.V. α7 nAChR-specific antibodies stimulate pro-inflammatory reaction in human astrocytes through p38-dependent pathway. Int. Immunopharm. 2014;23(2):475–479. doi: 10.1016/j.intimp.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Jerne K., Cocteau J. Idiotypic networks and other preconceived ideas. Immunol. Rev. 1984;79:5–24. doi: 10.1111/j.1600-065x.1984.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 28.Naveed A., Rahman S.U., Arshad M.I., Sharif M., Saleem A., Aslam B. Recapitulation of the anti-Idiotype antibodies as vaccine candidate. Trans. Med. Commun. 2018;3:1. doi: 10.1186/s41231-018-0021-4. [DOI] [Google Scholar]
- 29.Jiang Z., Zhou E.M., Ameri-Mahabadi M., Zimmerman J.J., Platt K.B. Identification and characterization of auto-anti-idiotypic antibodies specific for antibodies against porcine reproductive and respiratory syndrome virus envelope glycoprotein (GP5) Vet. Immunol. Immunopathol. 2003;92(3–4):125–135. doi: 10.1016/s0165-2427(03)00022-9. [DOI] [PubMed] [Google Scholar]
- 30.Tzioufas A.G., Routsias J.G. Idiotype, anti-idiotype network of autoantibodies: pathogenetic considerations and clinical application. Autoimmun. Rev. 2010;9(9):631–633. doi: 10.1016/j.autrev.2010.05.013. [DOI] [PubMed] [Google Scholar]