Abstract
Objectives
This study aimed to investigate the effect of electronic cigarette vaping and cigarette smoking on the levels of interleukin-1β and transforming growth factor‑β salivary biomarkers compared to non-smokers.
Methods
One hundred and fifty people participated in this study; There were 50 participants who smoked traditional cigarettes, 50 who used electronic cigarettes, and 50 healthy people who had never smoked cigarettes (control group). Furthermore, 5 ml of unstimulated whole saliva was sampled and clarified by centrifugation and frozen until analysis. Interleukin-1β and transforming growth factor‑β concentrations were assessed in saliva samples using ELISA. The duplicate readings average was utilized to interpret the data.
Results
We found that cigarette smokers had significantly higher levels of interleukin-1β and transforming growth factor‑β than non-smokers and electronic cigarette users (p < 0.05). The difference between control participants and electronic cigarette users, as well as that between control participants and traditional cigarette smokers, was statistically significant (p < 0.05).
Conclusion
Electronic cigarette users have higher levels of inflammatory and cancer risk biomarker than non-smokers, suggesting that electronic cigarettes can pose a risk of developing systemic diseases but less than conventional cigarettes. In conclusion, our study could be regarded as new evidence supporting the hazardous effects of e-cigs using a cost-effective, non-invasive method.
Keywords: Cytokine, Inflammation, Biomarker, Vaping
1. Introduction
Cigarette smoking is a significant risk factor for chronic diseases, mainly because of inflammation. It is also a major cause of oral health problems, including increased failure of dental implants, periodontal diseases, and cancer (Xue et al., 2016).
Unlike conventional cigarettes, electronic cigarettes (e-cigs, also known as vaporizer pens or vapor cigarettes) are devices that use a battery heating element that supplies an inhalant containing nicotine and additional additives to give the consumer a feeling of smoking conventional cigarettes but without combustion (McNeil et al., 2015).
In the last decade, the chronic use of e-cigs has increased remarkably between teenagers and young adults, primarily because e-cigs convey nicotine with flavors to be used in a vapor rather than smoke, which is less banned in public places (Stone and Marshall, 2019).
The assumed e-cigs safety due to the removal of combustion toxins attracts new consumers. However, the use of e-cigs has begun to arouse more and more arguments (McNeil et al., 2015).
The aerosol produced during the use of e-cigs is associated with deleterious effects. Cytotoxicity was noted, resulting in the death of periodontal fibroblasts and oral epithelial keratinocytes (Yu et al., 2016, Cichońska et al., 2019). Besides, flavors added to e-cigs accelerate oxidative stress, inflammatory reactions, or DNA damage to periodontal and human lung cells (Sundar et al., 2016). Meanwhile, the impact of e-cigs on systemic inflammatory biomarkers is still understudied (Ye et al., 2020).
Cytokines are a group of signaling molecules that mediate inflammation and immunity (Polz-Dacewicz et al., 2016). Interleukin-1β (IL-1β) is a possible biomarker for estimating smoking and oral inflammatory condition (Mokeem et al., 2018). Moreover, it could be a reliable biomarker for assessing cancer risk and prognosis (Brailo et al., 2012).
Transforming growth factor- β (TGF- β) is a polypeptide cytokine that regulates cell proliferation, differentiation, and apoptosis (Kubiczkova et al., 2012). In malignancy, TGF-β expression increases significantly with increased tumor grade, suggesting a close association between this cytokine and the malignant changes of a tumor (Xue et al., 2016).
To the best of our knowledge, to date, no study has been conducted to compare salivary TGF-β levels between non-smokers (NS), cigarette smokers (CS), and electronic cigarette users (EC). Therefore, we aimed to assess the differences in the salivary levels of IL-1β and TGF-β among CS, EC, and NS to highlight the influence of e-cigs and cigarette smoking on the levels of biomarkers of tissue injury and inflammation.
2. Materials and methods
2.1. Patient population, demographics, smoking, and vaping status
A total of 150 student volunteers from our faculty participated in this study. They were carefully chosen according to their e-cig and traditional cigarette consumption status and classified into three categories of 50 participants each as follows: those smoking traditional cigarettes (daily smokers), those using e-cigs, and healthy persons who had never smoked cigarettes (control group). None of the participants in any patient group smoked both conventional and electronic cigarettes. The study was carried out in 2020–2021 at Ahram Canadian University, Egypt.
“Cigarette-smokers” define persons who had smoked a minimum of 5 cigs/day for at least one year. On the other hand, persons who reported vaping e-cigs exclusively for at least 12 months and had never smoked traditional cigarettes before were categorized as “electronic cigarette users” (Javed et al., 2017).
We excluded from the study individuals that had taken antibiotics within the previous three months, individuals suffering from systemic diseases, and those who underwent periodontal therapy within the previous six months. All study participants gave their written informed consent to participate in the study. Information about age and sex (demographic variables), the daily frequency of cigarette smoking and e-cigs vaping, and the duration of the session was obtained. This study was approved by the ethics committee of our faculty and was carried out following the Declaration of Helsinki principles.
2.2. Collection of unstimulated whole saliva (UWS) samples
UWS samples were utilized for evaluation. They were collected as reported by Navazesh and Kumar, 2008.
About 5 ml of UWS was collected in the morning hours into sterile Corning-type silicone test tubes two hours after the last meal, drink, or smoke had been taken. Samples were collected by expectoration at room temperature. They were clarified by centrifugation at 1500 rpm for 10 min and stored at −80 °C until analysis.
2.3. Assessment of salivary biomarkers via enzyme-linked immunosorbent assay (ELISA)
2.3.1. IL-1β assessment
The IL-1β concentration (pg/ml) in the collected saliva was assessed via ELISA. The ELISA kit determines the concentrations of human IL-1β in saliva in vitro. Per the manufacturer’s instructions, the PicoKine kit (Catalog number: MBS175901, USA) was used. The duplicate readings average for each control and sample was used to interpret the data. Using an ELISA plate reader (Awareness Technologies, Florida, USA), we subtracted the average zero standard O.D. reading. The mean absorbance for each standard was plotted against the concentration. In the sample, the measured concentration can be interpolated by employing a linear regression of each average relative O.D. compared to the generated standard curve using a curve-fitting software.
2.3.1.1. TGF-β assessment
The TGF-β concentration (pg/ml) was evaluated in saliva via ELISA. The Novus Biologicals kit (Catalog number: NBP1-91252, USA) was used per the manufacturer’s instructions. A colored substance was developed in proportion to the sample concentration of TGF-β. An ELISA plate reader was used to assess the reaction color absorbance (450 nm). Using TGF-β standard dilutions, a standard curve was established, and the concentration was detected.
2.4. Statistical analysis
SPSS version 26 was used to code and input the collected data (IBM Corp., Armonk, NY, USA). Quantitative data were presented using the mean and the standard deviation while categorical data were presented using frequencies and percentages. To compare groups, the ANOVA with multiple comparisons post hoc test was utilized. The chi-square (χ2) test was used to compare categorical data. When the anticipated frequency was less than 5, the exact test was used. P-values of less than 0.05 were considered statistically significant.
3. Results
3.1. Demographic and clinical findings
The NS group did not differ significantly in terms of the gender and age distribution from the CS and ES groups (Table 1).
Table 1.
Demographics and characteristics of the study groups.
|
Non-smoker (n = 50) |
Cigarette smokers (n = 50) | E-cigs users (n = 50) | ||
|---|---|---|---|---|
| Age in years | ||||
| (Mean ± SD) | 28.33 ± 8.23 | 29.23 ± 5.83 | 29.36 ± 8.01 | |
| Sex (%) | Female | 24 (48%) | 22 (44%) | 18 (36%) |
| Male | 26 (52%) | 28 (56%) | 32 (64%) | |
| Daily frequency of the habit | ||||
| (Mean number of times per day) | – | 14.7 ± 2.5 | 10.1 ± 1.4 | |
|
Mean duration of a session (in minutes) |
– | 5.2 ± 0.8 | 7.8 ± 0.4 | |
Table 1 shows the daily frequency of cigarette smoking, e-cigarette vaping, and the mean duration of each cigarette smoking and e-cigarette vaping session. This revealed non-significant difference in the overall nicotine exposure duration per day (approximately 76.4 min/day in the CS group and approximately 78.8 min/day in EC group).
3.2. Cytokine levels in saliva among the study groups
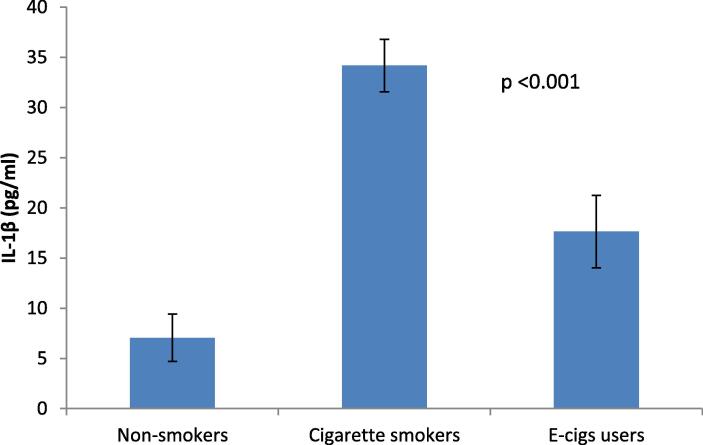
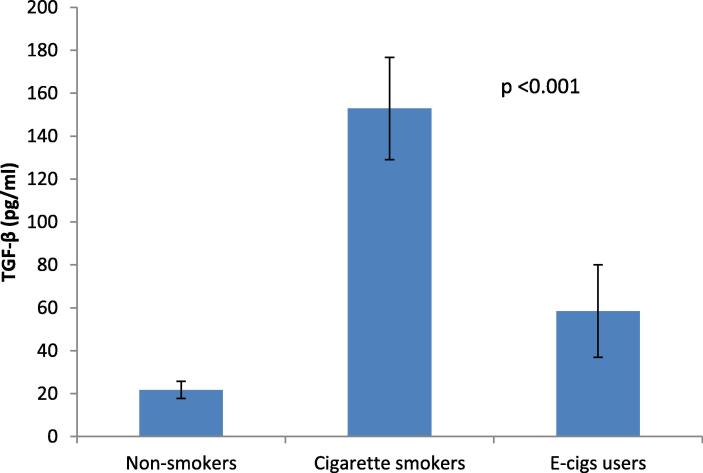
Salivary IL-1β and TGF-β levels were evaluated to assess if the systemic inflammatory response differed between the EC, CS, and NS groups. IL-1β and TGF-β salivary levels were significantly higher in the CS group than in the EC and NS groups (p < 0.05).
Data analysis revealed significant differences between the EC and the NS, as well as between the CS and the NS (p < 0.05) (Table 2, Fig. 1, and Fig. 2).
Table 2.
Observed value difference and P-value of salivary biomarkers between the groups.
| Cigarette smokers | E-cigs users | Cigarette smokers | ||
|---|---|---|---|---|
| –Non-smoker | –Non-smoker | - e-cigs users | ||
| IL-1β (pg/ml) | Mean Difference | 27.12906 | 10.58016 | 16.54890 |
| P-value | <0.001* | <0.001* | <0.001* | |
| TGF-β (pg/ml) | Mean Difference | 131.22308 | 36.75000 | 94.47308 |
| P-value | <0.001* | <0.001* | <0.001* | |
Statistically significant differences.
Fig. 1.
Bar chart showing the mean and standard deviation of IL-1β among the studied groups. Significance level p < 0.05.
Fig. 2.
Bar chart showing the mean and standard deviation of TGF-β among the studied groups. Significance level p < 0.05.
4. Discussion
Many types of growth factors and cytokines that have been found in saliva possess a significant role in carcinogenesis and inflammation (Ye et al., 2020). Many of them such as TGF-β and IL1β are expressed in smoking (Pezzuto et al., 2019). Furthermore, Cruz-Almeida et al., 2017 demonstrated that saliva was a non-invasive, cost-effective serum alternative to cytokine testing.
So, this study aimed to evaluate the effect of e-cigs vaping and cigarette smoking on the levels of IL1-β and TGF-β in human saliva compared to their levels in NS. The selected biomarkers have been associated with tissue injury, systemic inflammation, and cancer risk. To the best of our knowledge, this is the first study that compares the salivary TGF-β levels among CS and EC.
Several studies have identified nicotine as a possible contributor to the pathogenesis of cancer. Previous in vitro experimental studies on cell cultures and in vivo rodent and human studies showed that nicotine itself could stimulate tumorigenesis (Grando, 2014). Unlike traditional cigarettes, there is a paucity of knowledge on the health hazards of e-cigs, including their association with cancer. The ongoing promotion of e-cigs as a safe alternative to conventional tobacco products has several ramifications (Raj et al., 2020).
The analysis of the components of different e-cig brands revealed the presence of a variety of well-established carcinogens (Kadimisetty et al., 2017). In this regard, Yu et al., 2016 studied the impact of e-cig vapor with and without nicotine on cell lines of the normal oral mucosa and head and neck squamous cell cancer. According to their findings, whether nicotine was present or not, e-cig vapor promoted cell death by necrosis or apoptosis. Their findings were in line with those of the study by Kadimisetty et al., 2017 in which flavors added to e-cigs accelerated oxidative stress, inflammatory reactions, or DNA damage to periodontal and human lung cells. The one-time use of e-cigs can cause oxidative stress and endothelial cell dysfunction (Carnevale et al., 2016).
IL-1β is a crucial inflammatory cytokine secreted in response to cellular damage or infection (Idris et al., 2015). It is more abundant in saliva than in serum, suggesting that saliva is a valuable biological fluid for the assessment of IL-1β levels (Brailo et al., 2012). Furthermore, IL-1β levels are elevated in CS, ES, and in different cancer types (Pezzuto et al., 2019). This cytokine promotes inflammation by acting directly on various cell types, either alone or in conjunction with other cytokines. While macrophages are the primary IL-1 β producers, other cells such as epithelial cells, and salivary gland cells may also produce it (Idris et al., 2015).
On the other hand, TGF-β is a cytokine released in inflammatory conditions. Its level is elevated in vitro by exposure to cigarette smoke. Notably, tumor development may be promoted by an altered TGF-β signaling pathway. Some previous studies reported increased TGF-β concentrations in growing tumors and emphasized its prognostic characteristics (Polz-Dacewicz et al., 2016).
Furthermore, cigarette smoking and e-cig consumption have been associated with increased oxidative stress and the development of advanced glycation end products in gingival and periodontal tissue cells (ArRejaie et al., 2019, Cichońska et al., 2021). Advanced glycation end products have been associated with the formation of reactive oxygen species that cause an oxidative burst within the gingival tissue. Consequently, reducing antibody production and enhancing the local and systemic inflammatory load through increased cytokine expression (Katz et al., 2007).
The results of this study support the hypothesis that inflammatory cytokine levels are higher in CS and EC than in NS.
Regarding IL-1β, we found its significantly higher salivary levels in CS than in EC and NS (3.2). Moreover, we found a significant difference in IL-1β levels between the EC and the control groups (3.2). Our findings are consistent with those of previous studies that reported significantly higher levels of salivary IL-1β in CS than in EC and NS (Sundar et al., 2016, Mokeem et al., 2018, ArRejaie et al., 2019). Another study by Singh et al., 2019 reported higher IL-1 β levels in EC than in NS. Additionally, Mokeem et al., 2018, Ye et al., 2020 reported an essential role of IL-1 β in inflammatory pathways, suggesting an association between e-cig use and the development of oral and chronic systemic diseases, though less than that of traditional cigarette consumption.
The processing and release of IL-1-β from cells are controlled by caspase 1 activation pathway, which is mediated by inflammasomes (Wang et al., 2019). Inflammasomes are multiprotein signaling complexes that regulate antimicrobial host defenses and trigger inflammatory responses (Broz and Dixit, 2016). Their activation by cigarette smoke was induced by oxidative stress and boosted the production of inflammatory cytokines in human alveolar epithelial cells, including IL-1β (Wang et al., 2019). In cancer, the most clinically significant IL-1 subtype is IL-1β. This cytokine is suggested to be a promising biomarker of cancer risk and prognosis (Idris et al., 2015).
Previous studies have also reported increased salivary levels of IL-1β in other pathologies such as periodontitis and peri-implant inflammation (Rocha et al., 2014).
This cytokine’s properties are associated with tissue destruction, including the activation of tissue degradation proteinases, and the initiation of bone resorption (ArRejaie et al., 2019).
Regarding TGF-β, few studies have been conducted to investigate the correlation between cigarette smoking and the increased expression of TGF-β, however, there are currently no available data on the effect of e-cigs on TGF-β expression.
Our preliminary results showed significantly increased salivary TGF-β levels in EC than in NS though less than those in CS (3.2).
These results are supported by those of a previous study by Wang et al., 2020 who reported the activation of TGF-β and its receptors in airway inflammation and smoking-induced chronic obstructive pulmonary disease. Cigarette smoke can stimulate several mediators to trigger the release of growth factors by monocytes (Singh et al., 2019).
Another in vitro study by Pezzuto et al., 2019 was conducted on rat lungs exposed to cigarette smoke extracts. Their results demonstrated increased levels of TGF-β. Moreover, another study by Polz-Dacewicz et al., 2016 reported a significant rise in salivary TGF-β levels in people with oral squamous cell carcinoma, which was higher than serum levels.
A limitation of this study is that the data collected on smoking and vaping status depends on patients’ recall abilities, which may have influenced the outcomes.
Furthermore, the primary strength of the current work is the assessment of TGF-β levels in the saliva of CS and EC for the first time. Our clinical study could be regarded as new evidence supporting the hazardous effects of e-cigs using a cost-effective non-invasive method. However, broader longitudinal and cross-sectional clinical studies are needed to assess the deleterious effects of e-cig use on health, especially regarding oral cancer.
5. Conclusions
Our results demonstrated that the type of smoker can influence some of the detectable inflammatory biomarkers, which may be beneficial to future regulatory and translational investigations. More research is required to determine the negative consequences of e-cig vaping on oral health.
CRediT authorship contribution statement
Naglaa M. Kamal: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing, Formal analysis. Noha S. Shams: Validation, Visualization, Writing – original draft, Software, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Naglaa M. Kamal, Email: naglaa.kamal@acu.edu.eg.
Noha S. Shams, Email: Noha.shaban@acu.edu.eg.
References
- ArRejaie A.S., Al-Aali K.A., Alrabiah M., Vohra F., Mokeem S.A., Basunbul G., Alrahlah A., Abduljabbar T. Proinflammatory cytokine levels and peri-implant parameters among cigarette smokers, individuals vaping electronic cigarettes, and non-smokers. J. Periodontol. 2019;90(4):367–374. doi: 10.1002/JPER.18-0045. [DOI] [PubMed] [Google Scholar]
- Brailo V., Vucicevic-Boras V., Lukac J., Biocina-Lukenda D., Zilic-Alajbeg I., Milenovic A., Balija M. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Medicina oral, patología oral y cirugía bucal. 2012;17(1) doi: 10.4317/medoral.17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P., Dixit V.M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16(7):407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- Carnevale R., Sciarretta S., Violi F., Nocella C., Loffredo L., Perri L., Peruzzi M., Marullo A.G., De Falco E., Chimenti I., Valenti V. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150(3):606–612. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Cichońska D., Król O., Słomińska E.M., Kochańska B., Świetlik D., Ochocińska J., Kusiak A. Influence of Electronic Cigarettes on Antioxidant Capacity and Nucleotide Metabolites in Saliva. Toxics. 2021;9(10):263. doi: 10.3390/toxics9100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichońska D., Kusiak A., Kochańska B., Ochocińska J., Świetlik D. Influence of electronic cigarettes on selected antibacterial properties of saliva. Int. J. Environ. Res. Public Health. 2019;16(22):4433. doi: 10.3390/ijerph16224433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Almeida Y., Aguirre M., Sorenson H., Tighe P., Wallet S.M., Riley J.L., III Age differences in salivary markers of inflammation in response to experimental pain: does venipuncture matter? J. Pain Res. 2017;10:2365. doi: 10.2147/JPR.S138460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grando S.A. Connections of nicotine to cancer. Nat. Rev. Cancer. 2014;14(6):419–429. doi: 10.1038/nrc3725. [DOI] [PubMed] [Google Scholar]
- Idris A., Ghazali N.B., Koh D. Interleukin 1β—a potential salivary biomarker for cancer progression? Biomarkers Cancer. 2015 doi: 10.4137/BIC.S25375. 7:BIC-S25375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed F., Abduljabbar T., Vohra F., Malmstrom H., Rahman I., Romanos G.E. Comparison of periodontal parameters and self-perceived oral symptoms among cigarette smokers, individuals vaping electronic cigarettes, and never-smokers. J. Periodontol. 2017;88(10):1059–1065. doi: 10.1902/jop.2017.170197. [DOI] [PubMed] [Google Scholar]
- Kadimisetty K., Malla S., Rusling J.F. Automated 3-D printed arrays to evaluate genotoxic chemistry: e-cigarettes and water samples. ACS Sensors. 2017;26(5):670–678. doi: 10.1021/acssensors.7b00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Yoon T.Y., Mao S., Lamont R.J., Caudle R.M. Expression of the receptor of advanced glycation end products in the gingival tissue of smokers with generalized periodontal disease and after nicotine induction in primary gingival epithelial cells. J. Periodontol. 2007;78(4):736–741. doi: 10.1902/jop.2007.060381. [DOI] [PubMed] [Google Scholar]
- Kubiczkova L., Sedlarikova L., Hajek R., Sevcikova S. TGF-β–an excellent servant but a bad master. Journal of translational medicine. 2012;1;10(1):183 doi: 10.1186/1479-5876-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil A., Brose L.S., Calder R., Hitchman S.C., Hajek P., McRobbie H., E-cigarettes: an evidence update A report commissioned by Public Health England. Public Health England. 2015;111:14–15. [Google Scholar]
- Mokeem S.A., Alasqah M.N., Michelogiannakis D., Al-Kheraif A.A., Romanos G.E., Javed F. Clinical and radiographic periodontal status and whole salivary cotinine, IL-1β and IL-6 levels in cigarette-and waterpipe-smokers and E-cig users. Environ. Toxicol. Pharmacol. 2018;1(61):38–43. doi: 10.1016/j.etap.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Navazesh M., Kumar S.K. Measuring salivary flow: challenges and opportunities. The Journal of the American Dental Association. 2008;1; 139:35S–40S. doi: 10.14219/jada.archive.2008.0353. [DOI] [PubMed] [Google Scholar]
- Pezzuto A., Citarella F., Croghan I., Tonini G. The effects of cigarette smoking extract on cell cycle and tumor spread: novel evidence. Future Sci. OA. 2019;3;5(5):FSO394. doi: 10.2144/fsoa-2019-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polz-Dacewicz M., Strycharz-Dudziak M., Dworzański J., Stec A., Kocot J. Salivary and serum IL-10, TNF-α, TGF-β, VEGF levels in oropharyngeal squamous cell carcinoma and correlation with HPV and EBV infections. Infectious Agents Cancer. 2016;11(1):1–8. doi: 10.1186/s13027-016-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A.T., Sujatha G., Muruganandhan J., Kumar S.S., Bharkavi S.I., Varadarajan S., Patil S., Awan K.H. Reviewing the oral carcinogenic potential of E-cigarettes using the Bradford Hill criteria of causation. Translational. Cancer Res. 2020;9(4):3142. doi: 10.21037/tcr.2020.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha F.S., Jesus R.N., Rocha F.M., Moura C.C., Zanetta-Barbosa D. Saliva versus peri-implant inflammation: quantification of IL-1β in partially and totally edentulous patients, 2014. J. Oral Implantol. 2014;40(2):169–173. doi: 10.1563/AAID-JOI-D-11-00224. [DOI] [PubMed] [Google Scholar]
- Singh K.P., Lawyer G., Muthumalage T., Maremanda K.P., Khan N.A., McDonough S.R., Ye D., McIntosh S., Rahman I. Systemic biomarkers in electronic cigarette users: implications for noninvasive assessment of vaping-associated pulmonary injuries. ERJ Open Res. 2019;5(4) doi: 10.1183/23120541.00182-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone E., Marshall H. Tobacco and electronic nicotine delivery systems regulation. Transl. Lung Cancer Res. 2019;8(Suppl 1):S67. doi: 10.21037/tlcr.2019.03.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar I.K., Javed F., Romanos G.E., Rahman I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget. 2016;22(47):77196. doi: 10.18632/oncotarget.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Meng J, Wang C, Yang C, Wang Y, Li Y, Li Y. Hydrogen Sulfide Alleviates Cigarette Smoke-Induced COPD Through Inhibition of the TGF-β 1/smad Pathway, 2020. Experimental biology and medicine (Maywood, NJ). 245(3):190-200. [DOI] [PMC free article] [PubMed]
- Wang M., Zhang Y., Xu M., Zhang H., Chen Y., Chung K.F., Adcock I.M., Li F. Roles of TRPA1 and TRPV1 in cigarette smoke-induced airway epithelial cell injury model. Free Radical Biol. Med. 2019;1(134):229–238. doi: 10.1016/j.freeradbiomed.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Xue X., Zhao S., Zhang Z., Wang Y., Chang Y., Xu Y., Jiang H., Ma X., Qian J., Guo R., Wang K. The relationship of transforming growth factor-β and lung cancer, Int. J Clin Exp Med. 2016;1;9(6):9766–80 [Google Scholar]
- Ye D., Gajendra S., Lawyer G., Jadeja N., Pishey D., Pathagunti S., Lyons J., Veazie P., Watson G., McIntosh S., Rahman I. Inflammatory biomarkers and growth factors in saliva and gingival crevicular fluid of e-cigarette users, cigarette smokers, and dual smokers: A pilot study. J. Periodontol. 2020;91(10):1274–1283. doi: 10.1002/JPER.19-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V., Rahimy M., Korrapati A., Xuan Y., Zou A.E., Krishnan A.R., Tsui T., Aguilera J.A., Advani S., Alexander L.E., Brumund K.T. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;1(52):58–65. doi: 10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]