Abstract
Background
Growth incidents usually progress in a fairly expected sequence; however, their timing and pattern vary across individual subjects. Biochemical biomarkers have an association with bone metabolism and produce signals which stimulate the growth and development of various craniofacial structures. Steroid dehydroepiandrosterone sulfate (DHEA-S) plays a major role in the initiation of growth hormone actions which has a significant role in promoting and accelerating skeletal maturation during puberty. The aim of this study was to investigate the correlation between salivary DHEA-S levels and cervical vertebral maturation (CVM) stages throughout the circumpubertal developmental period.
Methods
One hundred forty-one participants (70 males and 71 females), between 7 and 23years of age, were categorized into six cervical stages (CS) based on lateral cephalograms. Using a passive drooling technique, unstimulated whole saliva samples were collected from all enrolled subjects. DHEA-S levels were analyzed using the enzyme-linked immunosorbent assay (ELISA) and correlated with the six CVM stages.
Results
One-way analysis of variance ANOVA showed that the mean salivary DHEA-S levels at CS 3 and CS 4 were significantly different from the values recorded at other stages, and the two stages were statistically significant from each other. Pearson linear correlation of mean salivary DHEA-S levels from CS 1 to CS 6 showed a significant positive correlation.
Conclusion
Salivary DHEA-S can be used as a non-invasive indicator for detecting the pubertal growth spurt.
Keywords: Saliva, Biomarker, Dehydroepiandrosterone sulfate DHEA-S, Skeletal maturity indicator, Growth assessment, Cephalometric radiograph
1. Introduction
Growth and development of craniofacial structures is a complex phenomenon. Growth incidents usually possess an expected sequential progression, with variations in their timing and pattern among individual subjects (Santa Maria et al., 2021). This is especially true with regard to several factors that can affect the individual onset of puberty, such as genetics, ethnicity, nutrition, and socioeconomic status (Mehta et al., 2022). Patel et al. describe physiological age as “the maturation degree of different tissue systems of an individual“ which can be estimated by sexual maturation characteristics (puberty), somatic or morphologic age, and skeletal maturation and dental development (Patel et al., 1980). Studies have shown that the skeletal maturation assessment based on cervical vertebral examination is the most widely used indicator (Amasya et al., 2020). The cervical vertebral maturation method relies on inter-stage comparisons of the shape and depth of the inferior concavity and the height and shape of the cervical vertebrae bodies to different growth phases (Baccetti et al., 2005). However, the disadvantage of this method is the challenge of visualizing the subtle changes in the vertebrae, due to an improper neck posture while taking the radiograph, or blocking out of the cervical vertebrae structures by the thyroid collar (Rainey et al., 2016, Dhiman et al., 2015). Therefore, detecting skeletal maturation using biochemical markers has recently become the focus of an increasing number of studies, especially since they overcome the previously mentioned limitations (Tsagkari et al., 2022). Biomarkers have been associated with bone metabolism and cartilage development and production of signals that stimulate growth and development of craniofacial structures and mandibular condyles (Gv and Tripathi, 2021).
The 19 carbon (C19) steroids dehydroepiandrosterone (DHEA) and DHEA- sulfate (DHEA-S) are the most abundant steroid hormones in the body, they are produced and secreted from the adrenal cortex into the circulation (Remer et al., 2004). These sex steroids play major roles in stimulating reproductive glands to produce the sex steroid hormones: testosterone and estrogen (Rosenfield, 2021). In growing individuals, the progressively increasing level of DHEA-S can lead to an increase in linear growth velocity and advanced bone age (Klinge et al., 2018).
DHEA sulfate (DHEA-S) is the sulfated form and immediate metabolite of dehydroepiandrosterone (DHEA) (Temerdashev et al., 2021). Although DHEA and DHEA-S possess similar biological behaviors and both can be easily assayed, DHEA-S provides a more integrative measurement and is always preferred to be measured than DHEA (Maninger et al., 2009, Srinivasan and Premkumar, 2012). This is mainly due to the higher circulating level of DHEA-S than DHEA: approximately 100–1000 times higher (Rosenfeld et al., 1972).
Several studies have been conducted to assess the DHEA-S levels in serum as a skeletal maturation indicator based on hand and wrist or cephalometric radiographic techniques (Anusuya et al., 2020, Srinivasan and Premkumar, 2012, Venkatagiriappa et al., 2016). The majority of these studies found that for both genders DHEA-S levels progressively increase from pre-puberty through adulthood as skeletal maturation progressed (Srinivasan and Premkumar, 2012, Venkatagiriappa et al., 2016). Only one study has been conducted to measure the relationship between the level of salivary DHEA and cervical vertebral maturation (Ganesan, 2016). Nevertheless, the findings reported in the literature highlight the fact that DHEA-S can be a more valuable tool for assessing skeletal maturation than DHEA (Temerdashev et al., 2021). In addition, several studies have proposed that DHEA-S can be accurately and reliably measured from saliva (Dismukes et al., 2016, Heaney et al., 2012, Whetzel and Klein, 2010). Another study examined the correlation between serum and saliva levels of DHEA-S and reported a positive correlation between the two techniques, with higher sensitivity in the salivary approach (El-Gharib and El-Din Hazaa, 2014). The aim of the current study was to investigate the correlation between salivary dehydroepiandrosterone sulfate levels and cervical vertebral maturation stages throughout the circumpubertal developmental period.
2. Material and method
2.1. Sample description
This cross-sectional clinical study involved one hundred and forty-one participants (70 males and 71 females), between 7 and 23 years of age, who were categorized into six groups according to their cervical vertebral maturation stage. The distribution between the groups was matched for sex (Table 1). The study was conducted at the Orthodontic Clinic in the Dental University Hospital of King Saud University, Riyadh, Saudi Arabia. The protocol was approved by the Ethics Committee of College of Dentistry, King Saud University (PR 0113) and the Institutional Review Board approval (IRB #E-20-5285). Informed consent to participate in the study was obtained from all subjects; for those who were under 18 years of age, the informed consent was signed by the accompanying legal guardian. The inclusion criteria were Saudi subjects requiring orthodontic treatment with normal periodontal conditions (generalized probing depth < 3 mm, plaque and gingival indices of 1, with no radiographic evidence of periodontal bone loss) (Löe, 1967). Any subjects diagnosed with systemic diseases, craniofacial abnormalities, oral infections, and/or under medications that affect bone growth and metabolism including bisphosphonates and antiepileptic drugs were excluded. In addition, smokers and those with self-reported pregnancy or lactation were excluded. After determining the eligibility, subjects were scheduled for saliva samples and standardized lateral cephalometric radiographs.
Table 1.
Sample distribution by sex and cervical maturation stages.
| Cervical maturation stages |
||||||
|---|---|---|---|---|---|---|
| CS 1 | CS 2 | CS 3 | CS 4 | CS 5 | CS 6 | |
| Male | 13 | 12 | 11 | 13 | 11 | 12 |
| Female | 11 | 12 | 11 | 11 | 12 | 12 |
2.2. Salivary samples and biochemical assays
All of the scheduled subjects were instructed to refrain from tooth-brushing, food, and drinks for at least 1.5 h prior to saliva collection (Alhazmi, 2017, Henson and Wong, 2010). Using a passive drooling technique, unstimulated whole saliva was collected during the second visit by asking the subjects to rinse their mouths with distilled water, wait for 5 min, and then sit quietly and drool saliva into a 15 ml collection tube after swallowing. A sample volume of 1–5 ml was collected, labeled with subject’s initials, and a serial number. All samples were collected during the period from 8:00 am to 11:00 am, then transported in an ice container to the Molecular and Cell Biology laboratory in the College of Dentistry, King Saud University.
Samples were centrifuged using a centrifuge machine (Eppendorf Centrifuge 5427 R, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) and stored at −80 °C (Voegtline and Granger, 2014). DHEA-Sulfated analysis was performed in triplicate using enzyme-linked immunosorbent assay (ELISA) analysis (MyBioSource, Southern California, San Diego (USA) (MBS288469, 2021).
2.3. Cervical vertebral maturation stages classification
All lateral cephalometric radiographs were taken by the same operator using the same machine (Planmeca ProMax, Planmeca Oy, Helsinki, Finland) in a natural head position with settings of 68 kV, 13 mA, and an exposure time of 16 s. Assessment of the cervical vertebral maturation stage was carried out by two examiners using the Baccetti et al. method (Baccetti et al., 2005).
2.4. Statistical analyses
The sample size estimation performed at the 5% level of significance (α = .05) with a power of 90% showed that a minimum of 10 subjects per CS group is necessary.
The statistical analysis was performed using GraphPad Prism software (version 9.3.1; Sigma Software Distribution, Ashburton, Devon, United Kingdom). Descriptive statistics were presented as percentages, tables, ranges, means, medians, and standard deviations. One-way analyses of variance (ANOVA) was used to measure the difference in concentration mean for salivary DHEA-S among CVM stages and post hoc Bonferroni analysis was done to detect the differences between the groups. Pearson correlation analysis was performed to determine the relationship between mean salivary DHEA-S levels and the cervical skeletal maturational stages. Intra-observer and inter-observer reliabilities were measured using the kappa statistic.
3. Results
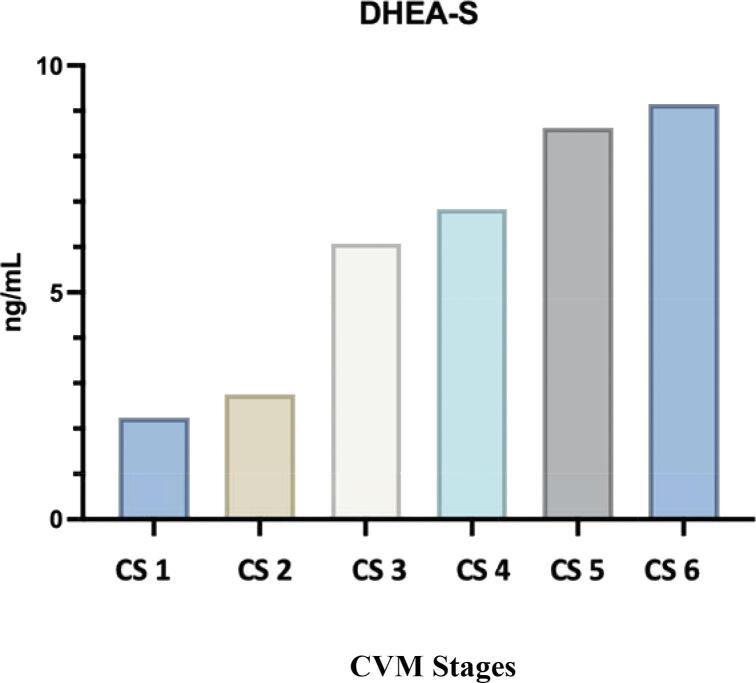
Mean (SD) values of salivary DHEA-S were 2.2 (0.4) ng/mL in CS 1, 2.8 (0.5) ng ng/mL in CS 2, 6 (0.6) ng/mL in CS 3, 6.9 (0.6) ng/mL in CS 4, 8.6 (0.5) ng/mL in CS 5, and 9.2 (0.7) ng/mL in the CS 6 (Table 2). The salivary DHEA-S level increased progressively from its lowest value at CS 1 and sharply increased in the transition stage at CS 3, followed by a gradual increase till reached its peak at CS 6. The highest mean DHEA-S value was observed in CS 6 (9.2 ng/mL), whereas the lowest mean was observed in CS 1 (2.2 ng/mL). The mean salivary DHEA-S levels are plotted against the cervical vertebral maturation stages (Fig. 1).
Table 2.
Descriptive statistics of DHEA-S for each cervical stage and P value of comparisons (one way ANOVA).
| n | Mean | SD | Median | Maximum | Minimum | F* | P | |
|---|---|---|---|---|---|---|---|---|
| CS 1 | 24 | 2.2 | 0.4 | 2.5 | 2.9 | 1.2 | ||
| CS 2 | 24 | 2.8 | 0.5 | 2.8 | 3.4 | 1.6 | ||
| CS 3 | 22 | 6 | 0.6 | 6 | 7 | 5 | 430.7 | .0001 |
| CS 4 | 24 | 6.9 | 0.6 | 6.9 | 8 | 5.7 | ||
| CS 5 | 23 | 8.6 | 0.5 | 8.7 | 9.7 | 7.8 | ||
| CS 6 | 24 | 9.2 | 0.7 | 9.4 | 10 | 8 |
*Calculated using the repeated measures ANOVA.
Significant at P ≤ 0.05.
Fig. 1.
Mean DHEA-S (ng/mL) for each cervical stage.
One-way ANOVA post-hoc Bonferroni analysis showed that the mean salivary DHEA-S level at CS 3 was significantly different from the values recorded at the other stage and similarly DHEA-S level was statistically significant at CS 4 from the other CVM stages (P ≤ 0.05), (Table 2). There was an insignificant difference in the DHEA-S values between CS 1 and CS 2, and between CS 5 and CS 6 (P ≤ 0.05), (Table 3).
Table 3.
Bonferroni multiple comparison test for mean DHEA-S levels at the different cervical stages.
| Bonferroni multiple comparison test for DHEA-S | Mean difference between 2 groups (ng/mL) | P value |
|---|---|---|
| CS 1 vs CS 2 | −0.5111 | .1091 |
| CS 1 vs CS 3 | −3.843 | <.0001 |
| CS 1 vs CS 4 | −4.596 | <.0001 |
| CS 1 vs CS 5 | −6.390 | <.0001 |
| CS 1 vs CS 6 | −6.913 | <.0001 |
| CS 2 vs CS 3 | −3.332 | <.0001 |
| CS 2 vs CS 4 | −4.085 | <.0001 |
| CS 2 vs CS 5 | −5.879 | <.0001 |
| CS 2 vs CS 6 | −6.402 | <.0001 |
| CS 3 vs CS 4 | −0.7527 | .0039 |
| CS 3 vs CS 5 | −2.547 | <.0001 |
| CS 3 vs CS 6 | −3.070 | <.0001 |
| CS 4 vs CS 5 | −1.794 | <.0001 |
| CS 4 vs CS 6 | −2.317 | <.0001 |
| CS 5 vs CS 6 | −0.5229 | .0948 |
P ≤ 0.05 is significant.
Pearson linear correlation of mean salivary DHEA-S levels from CS 1 to CS 6 showed a strong positive correlation with a coefficient of 0.94, the correlation coefficient was statistically significant at P ≤ 0.05. Inter- and intra-examiner reliability tests between the examiners’ readings showed good agreement (kappa, 0.91–0.94) (Table. 4).
Table 4.
Pearson correlation coefficients of DHEA-S with cervical vertebral maturation stages (CVMS).
| CVMS vs DHEA-S | CS 1–6 |
|---|---|
| R | +.94 |
| Strength of relationship % R2 | .89 |
| P value (2-tailed) | .004 |
| Correlation significance | Yes |
P ≤ 0.05 is significant.
4. Discussion
Assessment of skeletal maturation plays an important role in optimal orthodontic diagnosis and treatment planning (Santa Maria et al., 2021). According to Baccetti et al, the ideal biologic indicator of an individual’s skeletal maturity should be categorized by the following characteristics: efficiency in identifying the peak of mandibular growth, reliability of the data interpretation, simplicity, and non-requisite of additional radiation exposure (Baccetti et al., 2005). Recent studies have emphasized the potential of using biomarkers as a useful diagnostic tool for predicting the skeletal maturation stages (Gv and Tripathi, 2021). DHEA and DHEA-S biomarkers have been reported to play a major role in stimulating growth and proliferation of epiphyseal cartilage and initiation of growth hormone action (Srinivasan and Premkumar, 2012). This study demonstrated a positive correlation between the salivary DHEA-S levels and CVM stages throughout the circumpubertal growth phases.
The current study assessed the DHEA-S levels in saliva among Saudi population grouped into 6 stages based on cephalometric radiographs. The data clearly display that there was a progressive rise in salivary DHEA-S levels as skeletal maturation progressed. The results also showed that DHEA-S levels were low in the initiation stage at CS 1 and sharply increased in the transition stage at CS 3 followed by a gradual increase as skeletal maturation progressed, almost reaching the highest value in the completion stage at CS 6 with a mean value of 9.2 ng/mL. Among all the CVM stages, there was a statically significant difference in the means of salivary DHEA-S levels, except between CS 1 and 2 and between CS 5 and 6. Multiple previous studies have reported a similar trend of DHEA and DHEA-S levels in serum, where they found a sharp increase just before puberty followed by a progressive increase until the early twenties (Srinivasan and Premkumar, 2012, Venkatagiriappa et al., 2016). Srininvasan and Premkumar assessed the DHEA-S levels in serum as a skeletal maturation indicator among three groups: pre-pubertal, pubertal, and post-pubertal based on hand and wrist radiographs. Their findings confirmed the outcomes of the current study, as they found a statistically significant increase in serum DHEA-S levels from pre-pubertal to post-pubertal groups (Srinivasan and Premkumar, 2012). Venkatagiriappa et al. used the blood spot collection technique to evaluate the level of DHEA-S based on hand and wrist maturity index and they observed a similar increase pattern, but this increase was statistically insignificant. They explained the difference in their results could be attributed to the extensive variation of the DHEA-S values found in all their study groups (Venkatagiriappa et al., 2016). Only one study has gone beyond previous reports and showed a different pattern of DHEA-S levels in serum. They found that the highest mean values of DHEA-S were observed in pubertal phases at CS 4 and 3 in males and females, respectively, with a statistically significant difference among each CVM stage except between CS 5 and 6 (Anusuya et al., 2020). Sangeeth, K et al. found a positive correlation between salivary non-sulfated DHEA levels and CVM stages. Although their results were in accordance with those of the current study, their study subjects were allocated to only three groups only: pre-pubertal, pubertal, and adult groups (Ganesan, 2016). In addition, several studies have shown that the DHEA-S biomarker is considered a more precious measure for evaluating skeletal maturation than DHEA (Maninger et al., 2009, Rosenfeld et al., 1972, Srinivasan and Premkumar, 2012), as DHEA-S has a great abundance and a long terminal half-life compared to DHEA (Valenti et al., 2009). Only one study assessed the relationship between salivary DHEA-S levels and skeletal maturation (Sultana et al., 2021), however, their sample included only children which doesn’t represent the complete pattern and duration of the circumpubertal growth phases.
The discrepancy between the studies could be attributed to the differences in sample populations and ethnicity. Moreover, the present study is the first to exclude subjects with periodontal problems or smokers. As several studies have found a significant positive correlation between the aforementioned subjects and DHEA-S activity (Brand et al., 2011, Cakmak et al., 2014, Mudrika et al., 2014, Van Voorhees et al., 2013).
Although the diurnal rhythm of DHEA-S is much more stable than DHEA (Dismukes et al., 2016). Several studies have demonstrated diurnal rhythm of salivary DHEA-S levels (El-Gharib and El-Din Hazaa, 2014, Whetzel and Klein, 2010). Whetzel et al have studied the secretory pattern of DHEA-S and they found early morning saliva collection contains high levels of DHEA-S compared with the rest of the day (Whetzel and Klein, 2010). Therefore, in order to overcome the diurnal variations among our subjects, saliva samples were collected early in the morning.
The current study has performed the analysis using the ELISA technique that was advocated by other previous studies, due to the analytic sensitivity, specificity, and availability of the DHEA-S ELISA kit (El-Gharib and El-Din Hazaa, 2014). Furthermore, the alternative assay method is the radioimmunoassay technique (IR) which needs special laboratories for radiation control, which is not simply obtainable (El-Gharib and El-Din Hazaa, 2014).
The current study confirms the possibility of using salivary DHEA-S as a noninvasive indicator for detecting the pubertal growth spurt. There were a few limitations of the study; the sample included only Saudi subjects and was conducted within a departmental clinic at a single University, thus the results cannot be generalized. Although the study included a large number of subjects, future studies with a population of more diverse ethnicity are necessary to validate the potential use of salivary DHEA-S as a biomarker for skeletal maturity, in addition to determining whether a particular salivary biomarker is superior to another.
5. Conclusion
-
•
There was a progressive rise in salivary DHEA-S levels as skeletal maturation progressed.
-
•
Among all the cervical maturation stages, there was a statistically significant difference in the means of salivary DHEA-S levels, except between CS 1 and 2 and between CS 5 and 6.
-
•
The mean salivary DHEA-S levels showed a strong positive correlation with the cervical maturation stage.
-
•
Salivary DHEA-S can be used as a noninvasive indicator for detecting the pubertal growth spurt.
CRediT authorship contribution statement
Sarah Z. Al-Meshari: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Visualization, Writing – original draft. AlJazi H. Aldweesh: Supervision, Project administration.
Acknowledgments
Acknowledgments
We acknowledge Professor Adel Alhadlaq for his valuable contributions and assistance to the present study. A special thanks to Dr. Moneera Alhussain and Dr. Nouf Almeshari for their help during various stages of the experiment.
Also, the authors would like to thank Deanship of scientific research in King Saud University for funding and supporting this research through the initiative of DSR Graduate Students Research Support (GSR).
Funding
This research received no external funding.
Ethical Approval and IRB
Institutional Review Board (IRB) Ethical approval was obtained from the Scientific Research Ethics Committee, King Saud University, Riyadh, Saudi Arabia (IRB #E-20-5285. Another ethical approval was obtained from the College of Dentistry Research Center (CDRC), King Saud University, Riyadh, Saudi Arabia (Reference # PR0113).
Place of Research
The Molecular Cell Biology (MCB) Laboratory, in collaboration with the Prince Naif bin Abdulaziz Health Research Center, a core research facility of the King Saud University College of Dentistry in Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alhazmi, Nora, 2017. Salivary Parathyroid Hormone-related Protein (PTHrP) and Alkaline Phosphatase (ALP) as biomarkers for skeletal maturity.
- Amasya H., Cesur E., Yıldırım D., Orhan K. Validation of cervical vertebral maturation stages: Artificial intelligence vs human observer visual analysis. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. its Const. Soc. Am. Board Orthod. 2020;158:e173–e179. doi: 10.1016/j.ajodo.2020.08.014. [DOI] [PubMed] [Google Scholar]
- Anusuya V., Nagar A., Tandon P., Singh G.K., Singh G.P., Mahdi A.A. Serum DHEA-S levels could be used as a comparable diagnostic test to assess the pubertal growth spurt in dentofacial orthopedics. Prog. Orthod. 2020;21:15. doi: 10.1186/s40510-020-00317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccetti T., Franchi L., McNamara J. The Cervical Vertebral Maturation (CVM) Method for the Assessment of Optimal Treatment Timing in Dentofacial Orthopedics. Semin. Orthod. 2005;11 doi: 10.1053/j.sodo.2005.04.005. [DOI] [Google Scholar]
- Brand J.S., Chan M.-F., Dowsett M., Folkerd E., Wareham N.J., Luben R.N., van der Schouw Y.T., Khaw K.-T. Cigarette smoking and endogenous sex hormones in postmenopausal women. J. Clin. Endocrinol. Metab. 2011;96:3184–3192. doi: 10.1210/jc.2011-1165. [DOI] [PubMed] [Google Scholar]
- Cakmak O., Alkan B.A., Ozsoy S., Sen A., Abdulrezzak U. Association of gingival crevicular fluid cortisol/dehydroepiandrosterone levels with periodontal status. J. Periodontol. 2014;85:e287–e294. doi: 10.1902/jop.2014.130787. [DOI] [PubMed] [Google Scholar]
- Dhiman, S., Maheshwari, S., Verma, S.K., 2015. Assessment of maturity in orthodontics: A review. J. Adv. Clin. Res. Insights. https://doi.org/10.15713/ins.jcri.54
- Dismukes A.R., Meyer V.J., Shirtcliff E.A., Theall K.P., Esteves K.C., Drury S.S. Diurnal and stress-reactive dehydroepiandrosterone levels and telomere length in youth. Endocr. Connect. 2016;5:107–114. doi: 10.1530/EC-16-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gharib M., El-Din Hazaa S. Salivary versus serum approaches in assessment of biochemical hyperandrogenemia. J. Basic Clin. Reprod. Sci. 2014 doi: 10.4103/2278-960x.129283. [DOI] [Google Scholar]
- Ganesan, S., 2016. Evaluation and comparison of salivary dehydroepiandrosterone (DHEA) levels and cervical vertebral maturation stages at pre- pubertal, pubertal and post-pubertal stages of growth.
- Gv V., Tripathi T. Non-invasive methods for the assessment of biomarkers and their correlation with radiographic maturity indicators - a scoping review. Prog. Orthod. 2021;22:26. doi: 10.1186/s40510-021-00372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney J.L.J., Phillips A.C., Carroll D. Ageing, physical function, and the diurnal rhythms of cortisol and dehydroepiandrosterone. Psychoneuroendocrinology. 2012;37:341–349. doi: 10.1016/j.psyneuen.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Henson B.S., Wong D.T. Collection, storage, and processing of saliva samples for downstream molecular applications. Methods Mol. Biol. 2010;666:21–30. doi: 10.1007/978-1-60761-820-1_2. [DOI] [PubMed] [Google Scholar]
- Klinge C.M., Clark B.J., Prough R.A. Dehydroepiandrosterone Research: Past, Current, and Future. Vitam. Horm. 2018;108:1–28. doi: 10.1016/bs.vh.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- Patel M.I., Machado V., Mascarenhas P., Botelho J., Mendes J.J., Delgado A.S. Chronological age range estimation of cervical vertebral maturation using Baccetti method: a systematic review and meta-analysis. Eur. J. Orthod. 1980 doi: 10.1093/ejo/cjac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N., Wolkowitz O.M., Reus V.I., Epel E.S., Mellon S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front. Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S., Arqub S.A., Sharma R., Patel N., Tadinada A., Upadhyay M., Yadav S. Variability associated with mandibular ramus area thickness and depth in subjects with different growth patterns, gender, and growth status. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. its Const. Soc. Am. Board Orthod. 2022;161:e223–e234. doi: 10.1016/j.ajodo.2021.10.006. [DOI] [PubMed] [Google Scholar]
- Mudrika S., Muthukumar S., Suresh R. Relationship between salivary levels of cortisol and dehydroepiandrosterone levels in saliva and chronic periodontitis. J. Int. Clin. Dent. Res. Organ. 2014;6:92–97. doi: 10.4103/2231-0754.143488. [DOI] [Google Scholar]
- Rainey B.-J., Burnside G., Harrison J.E. Reliability of cervical vertebral maturation staging. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. its Const. Soc. Am. Board Orthod. 2016;150:98–104. doi: 10.1016/j.ajodo.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Remer T., Boye K.R., Hartmann M.F., Neu C., Schoenau E., Manz F., Wudy S.A. Adrenal steroid hormones and metaphyseal bone in children. Horm. Res. 2004;62:221–226. doi: 10.1159/000081349. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R.S., Hellman L., Gallagher T.F. Metabolism and interconversion of dehydroisoandrosterone and dehydroisoandrosterone sulfate. J. Clin. Endocrinol. Metab. 1972;35:187–193. doi: 10.1210/jcem-35-2-187. [DOI] [PubMed] [Google Scholar]
- Rosenfield R.L. Normal and Premature Adrenarche. Endocr. Rev. 2021;42:783–814. doi: 10.1210/endrev/bnab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Maria F.D., Barros S.E., Chiqueto K., Mariath L.M., Schüler-Faccini L., Kiszewski A.E. Development of dentofacial characteristics related to Incontinentia Pigmenti syndrome: A repeated cross-sectional study. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. its Const. Soc. Am. Board Orthod. 2021;160:66–76. doi: 10.1016/j.ajodo.2020.03.033. [DOI] [PubMed] [Google Scholar]
- Srinivasan B., Premkumar S. Assessment of serum dehydroepiandrosterone sulphate in subjects during the pre-pubertal, pubertal, and adult stages of skeletal maturation. Eur. J. Orthod. 2012;34:447–451. doi: 10.1093/ejo/cjr041. [DOI] [PubMed] [Google Scholar]
- Sultana, T., Sarada, P., Namineni, S., Reddy, C., KUMAR, S., Shaik, H., 2021. The Relationship Between Salivary Dehydroepiandrosterone Sulphate (DHEAS) Levels and Skeletal Maturation Parameters Before and During Pubertal Growth Spurt in Children. Iran. J. Orthod. 15. https://doi.org/10.5812/ijo.116370.
- Temerdashev A., Dmitrieva E., Podolskiy I. Analytics for Steroid Hormone Profiling in Body Fluids. Microchem. J. 2021;168 doi: 10.1016/j.microc.2021.106395. [DOI] [Google Scholar]
- Tsagkari, E., Deda, O., Krokos, A., Gika, H., Papadopoulos, M.A., Chatzigianni, A., 2022. Investigation of salivary biomarkers as indicators of skeletal and dental maturity in children. Orthod. Craniofac. Res. https://doi.org/10.1111/ocr.12572. [DOI] [PubMed]
- Valenti G., Ferrucci L., Lauretani F., Ceresini G., Bandinelli S., Luci M., Ceda G., Maggio M., Schwartz R.S. Dehydroepiandrosterone sulfate and cognitive function in the elderly: The InCHIANTI Study. J. Endocrinol. Invest. 2009;32:766–772. doi: 10.1007/BF03346534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhees E.E., Dennis M.F., McClernon F.J., Calhoun P.S., Buse N.A., Beckham J.C. The association of dehydroepiandrosterone and dehydroepiandrosterone sulfate with anxiety sensitivity and electronic diary negative affect among smokers with and without posttraumatic stress disorder. J. Clin. Psychopharmacol. 2013;33:556–560. doi: 10.1097/JCP.0b013e3182968962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatagiriappa S., Raman A., Sharma A. The Role of Blood Spot Dehydroepiandrosterone Sulfate Levels in Adjunct to Hand Wrist Radiographs as Skeletal Maturity Indicator. Turkish J. Orthod. 2016;29:69–72. doi: 10.5152/TurkJOrthod.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegtline K.M., Granger D.A. Dispatches from the interface of salivary bioscience and neonatal research. Front. Endocrinol. (Lausanne) 2014;5:25. doi: 10.3389/fendo.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetzel C.A., Klein L.C. Measuring DHEA-S in saliva: Time of day differences and positive correlations between two different types of collection methods. BMC Res. Notes. 2010;3:204. doi: 10.1186/1756-0500-3-204. [DOI] [PMC free article] [PubMed] [Google Scholar]