Graphical abstract
Keywords: Polymeric nanocarriers, Colorectal cancer, Targeted delivery, Early diagnosis, Superior therapeutic outcomes
Abbreviations: CRC, colorectal cancer; PNCs, polymeric nanocarriers; 5-FU, 5-Fluorouracil; NPs, nanoparticles; LNCs, lipid-based nanocarriers; PLA, poly lactic acid; PLGA, poly lactic-co-glycolic acid; EPR, enhanced permeability and retention; PMs, polymeric micelles; CHO, Chinese hamster ovary; Qu, Quercetin; CUR, curcumin; DOX, doxorubicin; α-TOS, alpha tocopheryl succinate; SN38, 7-ethyl-10-hydroxy camptothecin; CPT, camptothecin; OXA, oxaliplatin; TQ, thymoquinone; GA, gallic acid; MPEG–PCL, monomethoxy poly(ethylene glycol)–poly(ε-caprolactone); Qu-M, Quercetin nano-micelles; IC50, half-maximal inhibitory concentration; PTH, poly (ethylene glycol)-b-poly[(hydroxypropyl methacrylamide)-g-α-tocopheryl succinate-g-histidine)]; EE%, percentage encapsulation efficicnecy, PEG, polyethylene glycol; TfR, transferrin receptor; TBP, transferrin-binding peptide; MMP, matrix metalloproteinase; MPS, mononuclear phagocyte system; AL, alginate; CD, β-cyclodextrin; HA, hyaluronic acid; AA, anisamide; Pr NCs, Protamine nanocapsules; PAMAM, polyamidoamine; EDAC, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride; TRAIL, Tumor necrosis factor-related apoptosis inducing ligand; Apt, aptamer; NIRF, near-infrared fluorescence; PPI, polypropylenimine; QBD, quality by design
Highlights
-
•
Stages of development of CRC and the current recommended diagnostic tools and treatment involved are introduced.
-
•
Applications of different types of PNCs in the specific diagnosis and treatment of CRC are highlighted.
-
•
Important latest in vitro and in vivo models used in the analysis of safety and efficacy of PNCs are described.
-
•
Possible related toxicity and biocompatibility issues are discussed.
Abstract
Background
Colorectal cancer (CRC) is the third most prevalent type of cancer for incidence and second for mortality worldwide. Late diagnosis and inconvenient and expensive current diagnostic tools largely contribute to the progress of the disease. The use of chemotherapy in the management of CRC significantly reduces tumor growth, metastasis, and morbidity rates. However, poor solubility, low cellular uptake, nonspecific distribution, multiple drug resistance and unwanted adverse effects are still among the major drawbacks of chemotherapy that limit its clinical significance in the treatment of CRC. Owing to their remarkable advantages over conventional therapies, the use of nanotechnology-based delivery systems especially polymeric nanocarriers (PNCs) has revolutionized many fields including disease diagnosis and drug delivery.
Aim of Review
In this review, we shed the light on the current status of using PNCs in the diagnosis and treatment of CRC with a special focus on targeting strategies, surface modifications and safety concerns for different types of PNCs in colonic cancer delivery.
Key Scientific Concepts of Review
The review explores the current progress on the use of PNCs in the diagnosis and treatment of CRC with a special focus on the role of PNCs in improvement of cellular uptake, drug targeting and co-delivery of chemotherapeutic agents. Possible toxicity and biocompatibility issues related to the use of PNCs and imitations and future recommendation for the use of those smart carriers in the diagnosis and treatment of CRC are also discussed.
Introduction
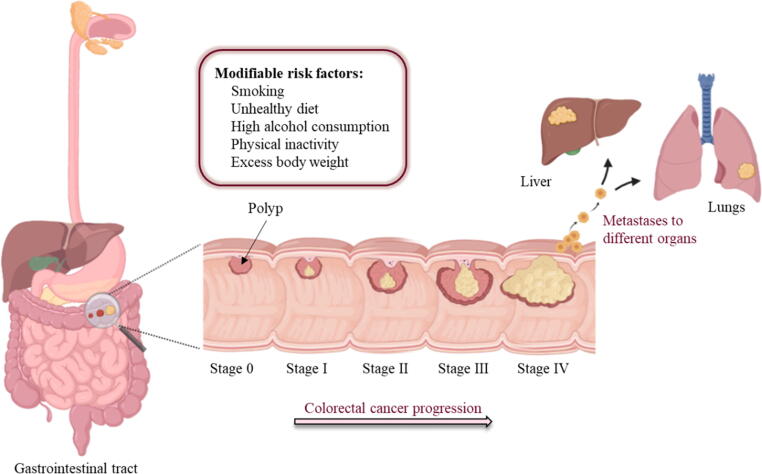
CRC is the third most prevalent type of cancer worldwide accounting for 11% of all cancer diagnoses [1], [2]. In 2020, about 1.9 million new cases of CRC were diagnosed with estimated 900,000 deaths [3]. The incidence of CRC continues to rise steadily with death rate expected to reach 60.0% and 71.5% to both colon and rectal cancers, respectively, in 2035 [4]. Despite the continuous advances in the diagnosis and therapeutic modalities of primary and metastatic CRC, the cure rates and long-term survival in this malignancy is still limited. The mortality rate due to CRC is higher in males compared to females and it may increase due to various risk factors such as ageing, poor dietary habits, smoking, obesity and lack of physical activity [5], [6].
CRC usually starts with abnormal noncancerous growths in the inner lining of the bowl called colorectal polyps [7]. However, less than 10% of polyps have been evidenced to advance to the invasive cancer [5], [8], [9]. This transition process is very slow and occurring gradually over more than 10 to 20 years and is more probable as polyps increase in size [10], [11], [12]. Later, the malignant cells start to invade the lymph and muscular nodes before spreading to other organs e.g. liver and lungs [13], [14]. The American Joint Committee on Cancer classified CRC into five stages (Fig. 1) [15], [16]. Stage 0 is characterized by the presence of abnormal colon cells or polyps on the mucosa and is 100% curable following a surgical resection upon early detection. Surgical resection is also considered as the standard treatment for stages I–II and are usually associated with 5-year survival rate in 37–74% patients. Unfortunately, the survival rate in the advanced stages of CRC drops to 6% due to the high risk of recurrence and metastasis to distant organs [15], [17], [18]. Therefore, adjuvant drug therapies such as chemotherapy are usually recommended to downregulate the recurrence and metastasis in the advanced stages of CRC following the surgical resection [6], [19].
Fig. 1.
Graphical representation for CRC progression showing the different stages of tumor development and associated modifiable risk factors.
The use of adjuvant chemotherapy such as 5-Fluorouracil (5-FU), oxaliplatin (OXA), doxorubicin (DOX), cisplatin and other agents in stages III-IV, aims to slowdown the tumor growth and improve life expectancy. Nevertheless, direct administration of those agents has not shown the desired efficacy at those stages and has been associated with several dose-limiting side effects including hair loss, vomiting and nausea [20]. In order to minimize these side effects, chemotherapeutics are administered in a series of cycles where the number of doses, frequency and duration of each cycle is based on the status of each patient [15], [21]. Further drawbacks associated with the use of anticancer drugs for the treatment of CRC include their hydrophobic nature, poor aqueous solubility, limited biodistribution and vulnerability to multiple drug resistance [22], [23], [24]. This has encouraged researchers to pursue different approaches such as using nanotechnology-based drug delivery systems either to enhance the physicochemical and pharmacological properties of standard chemotherapeutics or to achieve target-specific delivery of cytotoxic drugs to different types of tumor tissues to improve therapeutic efficacy and minimized off-target effects [15], [25], [26], [27].
Nanoparticles (NPs) are one of the promising tools used for management of CRC among other types of cancers since their properties are perceived as an asset to overcome the systemic drug delivery issues of diagnostic agents and anticancer medication [28], [29], [30]. NPs range in size from 1 to 100 nm and exhibit a large surface area-to-volume ratio that permits for greater drug loading via encapsulation within the core and adsorption on the surface of the NPs. The ultra-small size of the NPs and high drug loading and encapsulation efficiency allow them for extended circulation time and reduced clearance rates from the excretory organs [28], [31], [32]. In addition, the physical entrapment of diagnostic agents and anticancer drugs in NPs improves their pharmacokinetic properties and decreases the number of doses administered as they provide sustained release or on-demand release. This mediated release can overcome multiple drug resistance as the cells actively uptake higher concentrations of the drug intracellularly [28], [30], [32], [33]. In addition, the encapsulation enhances the navigation of the anticancer drug in the systemic circulation, improves their cellular uptake and provides advantages over their bulk counterpart [13], [27], [34].
Another promising feature of using NPs is the flexibility of functionalization of their surface with targeting ligands (i.e., peptides or small-molecule ligands) to increase their selectivity towards the targeted cells/tissues and the simultaneous delivery of cytotoxic drugs and/or diagnostic agents to their target site for improved therapeutic efficacy [35]. Moreover, some NPs such as micelles are amphiphilic with a hydrophilic surface and a hydrophobic core and therefore can significantly improve the solubility of hydrophobic drugs [28], [30]. Furthermore, structural features of nanoshells, quantum dots, nanosomes and paramagnetic NPs are used for imaging and diagnosis [36], [37].
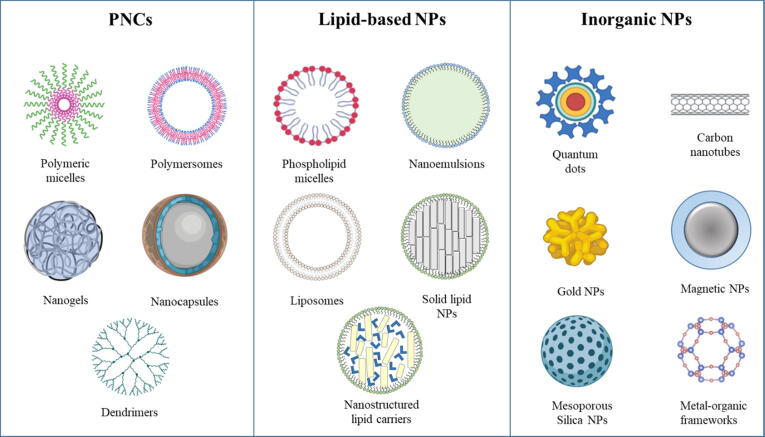
Nanotechnology can be classified to nanodevices, nanocrystalline and nanomaterials [36], [37], [38]. The nanomaterials are divided into polymeric, lipid-based and inorganic nanocarriers (Fig. 2). Polymeric nanocarriers (PNCs) include polymeric micelles, polymersomes, polymeric nanogels, polymeric nanocapsules and dendrimers. Lipid-based nanocarriers (LNCs) include nanoemulsions, phospholipid micelles, liposomes, solid lipid nanocarriers, and nanostructured lipid carriers [26]. Inorganic nanocarriers include quantum dot, carbon nanotubules, gold NPs, magnetic NPs, and silicon NPs [39]. PNCs and LNCs are also known as soft NPs while inorganic nanocarriers are also known as hard NPs [28], [30].
Fig. 2.
Examples of the three divisions of nanomaterials used in therapeutic management of different types of cancer.
PNCs can be prepared using natural polymers such as cellulose and chitosan; and synthetic polymers including poly lactic acid (PLA), poly amides, poly anhydrides and poly lactic-co-glycolic acid (PLGA) [27], [36], [40]. From all the above mentioned polymers, chitosan and PLGA are the most leading natural and synthetic polymers used, respectively [36], [41], [42]. Currently, synthetic polymers are used more frequently due to purity and stability issues associated with natural polymers. In addition, synthetic polymers can be designed to have better biodegradability and protein binding for enhanced cellular uptake [36], [41].
A comprehensive literature search revealed that most of the literature concentrated on the general applications of PNCs in delivery of chemotherapeutic agents for treatment of all types of cancer or the use of different types of NPs in management of CRC. This review confers a special focus on the latest advances in the use of PNCs for the treatment and diagnosis of CRC. We have critically evaluated and summarized a plethora of studies exploring the use various forms of PNCs to enhance the therapeutic efficiency and minimize the side effects of chemotherapeutic agents. In this article, PNCs are classified according to their structural features, method of preparation and physicochemical properties with special emphasis on their ability to enhance target-specific delivery and cellular uptake and minimize toxicity when used for treatment and diagnosis of CRC. Different combinations of the following keywords were used for database search: colorectal cancer, nanomedicine, polymeric nanoparticles, polymeric micelles, polymersomes, nanogels, nanocapsules, nanovesicles, dendrimers, controlled release, sustained release targeted delivery, cancer diagnosis, nanotoxicity and toxicity of nanoparticles. The extracted information was collected from PubMed, ScienceDirect, Scopus and Google Scholar databases through August 2021 with more focus on publications issued during the last decade.
Polymeric nanocarriers
The use of polymers in formulation of NPs as carriers for the delivery of chemotherapeutic agents has significantly improved the therapeutic effectiveness by site-specific targeting of these drugs and minimizing their side effects [13], [43]. Polymers are used alone or in combination with inorganic nanomaterials for production of multifunctional drug delivery systems [13], [44]. The use of block copolymers in the preparation of drug-loaded NPs or the introduction of surface modification improve the interaction between the NPs and target tissues and result in better biodistribution, longer circulation time and improved cellular uptake [13], [45].
The ability to synthesize biodegradable polymers that can be easily eliminated uplifted the use of PNCs in the delivery of anticancer drugs and diagnostic agents. Polymer degradation pathways include thermal, mechanical, photo and chemical degradation. Therefore, it is significantly important to consider the most predominant degradation pathway when selecting a polymer for a specific application [13], [46].
When used as drug carriers, PNCs use different strategies to improve the delivery of their payload. These include enhancing extracellular penetration, intracellular drug release and cellular uptake [13], [47], [48]. Tumors have leaky vasculatures and impaired lymphatic system, which causes enhanced permeability and retention (EPR) effect. Many drug-loaded PNCs use EPR effect to passively target and accumulate into the tumor microenvironment [13], [33], [49], [50]. Once at the target site, the release of drug from PNCs can occur by diffusion through the polymer matrix or the water-filled pores, erosion of the polymeric matrix and/or osmotic pumping [13], [51].
According to their structural features and method of formation, PNCs commonly used for delivery of therapeutic and diagnostic agents can be classified to polymeric micelles (PMs), polymersomes, nanogels, nanocapsules and dendrimers (Fig. 2). PMs are prepared using amphiphilic block copolymers that can self-assembles into a core–shell structure. The core is composed of hydrophobic polymers while the shell is composed of hydrophilic polymers [13], [52]. Polymersomes are polymeric self-assembled vesicles prepared using amphiphilic block copolymers and have a hydrophobic bilayer encapsulating an aqueous core [53], [54]. They are similar to liposomes and are used to encapsulate and protect sensitive molecules [13], [55], [56]. Nanogels are NPs of crosslinked networks of different hydrophilic natural or synthetic polymers [57], [58]. They share some characteristics with hydrogels, their pores are filled with various macromolecules in order to control some properties like chemical behavior, swellability and degradation rate [57]. PNCs can be self-assembled into solid spherical structures in which the drug can be dispersed or dissolved such as nanocapsules or nanospheres depending on the different mechanisms of formation. Finally, dendrimers are polymeric structure prepared using branched monomer molecules. They have a three-dimensional spherical shape that is symmetrical around the core. [13], [59].
Due to their characteristic features, PNCs have been studied for delivery and targeting of chemotherapeutic agents to different types of cancer. Examples for the use of PNCs in treatment of CRC are outlined in in Table 1.
Table 1.
Examples of PNCs used for treatment of CRC.
| Type of PNCs | Anticancer drug | Polymer | Model (in vitro and/or in vivo) | Effect of using PNCs | References |
|---|---|---|---|---|---|
| Polymeric micelles | Quercetin (Qu) | Monomethoxy poly(ethylene glycol)–poly(ε-caprolactone) | CT26 mouse colon carcinoma cell line Sprague Dawley (SD) rats and Female BALB/c mice |
Sustained the release of the drug in vitro Significant 37% reduction in IC50 and enhanced cellular uptake Enhanced t1/2 and Cmax of the drug after IV administration Reduction in tumor volumes in vivo after 3 weeks and increased apoptotic index after treatment with Qu-loaded PMs to 62.7%, versus 28.23% in free drug |
[63] |
| Curcumin (CUR) |
Monomethyl poly (ethylene glycol)-poly (ε-caprolactone)-poly (trimethylene carbonate) | CT26 cell line. Female BALB/c mice. |
Sustained release of CUR in vitro 12% reduction in IC50, enhanced cellular uptake, and increase in induction of apoptosis of cells treated with CUR-loaded PMs by 81% compared to free drug Significant in vivo tumor growth inhibition and reduction in tumor proliferation and angiogenesis effects. Increase in apoptosis index after treatment with CUR-loaded PMs 16.05% compared to free drug 9.07% |
[64] | |
| DOX and α -tocopheryl succinate (α-TOS) | Methoxypoly(ethylene glycol)-b-poly[(hydroxypropyl methacrylamide)-g-α-tocopheryl succinate-g-histidine)] |
HCT 116 human colon cancer cells and L929 mouse fibroblasts BALB-c/nude mice |
Increase in release of DOX and α-TOS from PMs when pH decreased from 7.4 to 4.5 Increase in accumulation of drug-loaded PMs in tumor in vivo after 24 h Increase in IC50 of drug-loaded PMs for L929 mouse fibroblasts and HCT 116 cells by 136% and 52% respectively compared to free DOX Drug-loaded PMs showed similar tumor inhibition to free drug and less systemic toxicity during 22 days of treatments |
[65] | |
| Cabazitaxel (CTX) | Polyethylene glycol monomethyl ether- poly(ε-caprolactone) (MPEG2k-PCL10k) | HCT 15 human colon cancer cells and HCT 15/Taxol cells Balb/c-nu mice |
CTX-MPEG2k-PCL10k showed a stronger inhibitory effect an increased cellular uptake on HCT 15 and HCT 15/Taxol compared with the free drug CTX-MPEG2k-PCL10k showed more significant tumor inhibition and less reduction in body weight than the control group in mice bearing HCT 15 tumor |
[66] | |
| Polymersomes |
DOX | Mixture of TBP functionalized (Tf@TBP-Ps) and non-functionalized (Ps) poly (ethylene glycol)-b-poly(trimethylene carbonate-co-dithiolane trimethylene carbonate), | HCT 116 cells Female Balb/c nude mice |
Increase in cellular uptake of polymersomes in TfR overexpressing cells with increase in TBP densities IC50 of the Tf@TBP-Ps-DOX was 2.5 folds more potent against HCT 116 cells than Ps-DOX In vivo tumor accumulation of Tf@TBP-Ps-DOX was 2-fold higher than Ps-DOX after 24 h Increase in t1/2 and AUC of Tf@TBP-Ps-DOX by 6.7 and 15%, respectively Tf@TBP-Ps-DOX instigated significantly more effective tumor inhibition at 8 mg DOX/kg than Ps-DOX |
[67] |
| 7-ethyl-10-hydroxy camptothecin (SN38) | AS1411 aptamer- PEG-peptide-PLA triblock copolymer (peptide = PVGLIG) |
C26 mouse colon adenocarcinoma and Chinese hamster ovary (CHO) Female BALB/c mice |
SN38 release was controlled by MMP-2 enzyme Apt-SN38-polymersomes were significantly more cytotoxic compared with SN38-polymerosmes at low SN38 concentrations (0.15–1.25 μg/mL) for C26 cells while showing no effect on CHO cells Apt-SN38-polymersomes showed significant tumor growth regression and slight loss of body weight |
[68] | |
| Camptothecin (CPT) |
Tetraiodothyroacetic acid (tetrac)-PEG-PLGA | C26, HT 29 human colon cancer cells and CHO cells Female BALB/C mice |
Increase in cytotoxicity in HT 29 and C26 tumor cells of tet-PEG-PLGA-CPT polymersomes tet-PEG-PLGA-CPT polymersomes showed no cytotoxic effect towards CHO cells tet-PEG-PLGA polymersomes increased the uptake of CPT into HT 29 and C26 tumor cells compared to non-targeted polymersomes tet-PEG-PLGA polymersomes increased in ratumoral accumulation and tumor inhibitory effect of CPT Targeted-polymersomes showed longer circulation time, faster plasma clearance and lower systematic toxicity |
[54] | |
| Paclitaxel (PTX) |
iRGD functionalized- Poly(oligoethylene glycol methacrylate)-poly(2-(diisopropylamino)ethyl methacrylate) (P[(OEG)10MA]20-PDPA90; POEGMA-PDPA) | CT26 cell line Balb/c mice |
PTX-loaded iRGD-polymersomes improved drug targeting, internalization and cytotoxicity in cells expressing neuropilin-1 Intraperitoneal administration of iRGD-polymersomes in mice bearing CT26 peritoneal tumors showed higher tumor-selective accumulation and penetration and tumor growth inhibition than untargeted polymersomes. |
[69] | |
| Nanogels |
5-FU | Alginate - β-Cyclodextrin (AL-CD) | HT 29 cells | The release of 5-FU was controlled by diffusion and degradation of polymer Drug-loaded AL-CD Nanogels showed more cytotoxicity, increase in cellular uptake and 2-folds enhanced apoptosis in HT 29 cells compared to free 5-FU |
[70] |
| Oxaliplatin (OXA) |
Alginate nanogels coasted with hyaluronic acid (HA) and functionalized with folic acid (F/HA/AL) | HT 29 and HEK293 cells | Controlled release of OXA from F/HA/AL nanogels for 7 days Drug-loaded-F/HA/AL showed 42% reduction in IC50 and induced higher apoptosis rate compared to free OXA |
[71] | |
| DOX | Dextrin crosslinked with formaldehyde (FDNG) and glyoxal (GDNG) | CT26 and HT 29 cells Male Balb/c mice |
pH-controlled release of DOX release from nanogels at pH range 5–7.4 Cytotoxicity of DOX-loaded FDNGs was higher than free DOX and GDNGs against both CT26 and HT 29 cells. DNGs effectively delivered DOX to the nucleus due to pH-responsive release. DOX-loaded FDNGs showed significant inhibition of tumor proliferation and induction apoptosis. |
[72] | |
| DOX | Chitosan and carboxymethyl-chitosan (CS/CMCS) crosslinked with tripolyphosphate (TPP) and calcium chloride | Caco-2 human colon adenocarcinoma cell lines Adult male SD rats |
DOX:CS/CMCS/Ca2+ NGs exhibited 2.2 folds increase in cellular uptake and cytotoxicity compared to DOX:CS/CMC/TPP NGs and free DOX in Caco-2 cells DOX:CS/CMCS/Ca2+ NGs showed better binding capacity to mucin and higher in vivo mucoadhesion and limited permeation compared to DOX:CS/CMC/TPP NGs |
[73] | |
| Nanocapsules |
CUR | PEGylated PLGA | CT26 cells Female Balb/c mice |
Nanocapsules controlled the release of CUR compared to free drug Nanocapsules improved the dose-dependent cellular uptake, significantly reduced cell viability of CUR at low concentrations (10 nM) in compared to cells treated with free drug Animal treated with CUR-loaded nanocapsules showed increase in tumor accumulation and 2 folds decrease in tumor volume compared to free drug |
[74] |
| Thymoquinone (TQ) |
Anisamide-Eudragit S100 | HT 29, HCT 116 and Caco-2 cells | Encapsulation of TQ improved solubility of the drug Decrease in IC50 of TQ by 37% after treatment of HT 29 with targeted drug-loaded nanocapsules for 48 h compared to free TQ No significant decrease in IC50 was observed after treatment of HCT 116 and Caco-2 cells with targeted drug-loaded nanocapsules for 48 h compared to free TQ |
[75] | |
| CUR and miR-145 |
Protamine | SW480 human CRC cells | A significant reduction in cell proliferation rate and migration capacity Increased intracellular uptake of miR-145 Increased cytotoxicity of CUR |
[76] | |
| Celecoxib | Hydroxyapatite-chitosan | HCT 15 and HT 29 cells Nude mice |
Significant reduction in cell proliferation and increased apoptosis HCT 15 Time-dependent cytoplasmic uptake for both HCT 15 and HT 29 cells Significant inhibition of tumor growth following treatment with celecoxib loaded nanoparticles. |
[77] | |
| Dendrimers |
Gallic acid (GA) |
Gallic acid-modified PAMAM | HCT 116 and HT 29, CRC, MCF7 human breast carcinoma and NIH 3 T3 mouse embryonic fibroblast cells | Internalization of PAMAM- GA conjugate inside HCT 116 cells within 6 h in dose-dependent manner Significant reduction in IC50 for PAMAM-GA conjugate 3.68 mM compared to 69 mM for GA in HCT 116 cells with less toxicity to normal NIH 3 T3 cells at the same dose Significant inhibition in cell proliferation of HCT 116, HT 29 and MCF7 treated with PAMAM-GA Significant inhibition in cell migration and induction of cell death via activating apoptosis signaling of HCT 116 treated with PAMAM-GA |
[78] |
| DOX and TRAIL plasmid DNA |
PAMAM modified with cholesteryl chloroformate and alkyl-PEG | C26 cells Male BALB/c mice |
PAMAM DOX/TRAIL showed much stronger in vitro cytotoxicity and higher percentage of apoptotic cells in C26 cells compared to free drug and monotherapies PAMAM DOX/TRAIL exhibited lower tumor growth rate in mice compared to control without significant alterations in the morphologies of major organs |
[79] | |
| CPT | AS1411 aptamer- PEG-PAMAM | C26, HT 29 and CHO cells Female Balb/c mice |
Drug-loaded Apt-PEG PAMAM showed slower release of CPT lower hemolytic activities compared to CPT-PEG PAMAM CPT-loaded Apt-PEG PAMAM showed an increase in cellular uptake, a 5, and 3.5 folds reduction in IC50compared to free drug and non-targeted dendrimers, respectively with no significant higher cytotoxicity in CHO cells. In vivo studies showed that drug-loaded Apt-PEG-PAMAM resulted in better survival and tumor growth-inhibition effects and significantly lower systemic toxicity |
[80] | |
| OXA | PEG-PAMAM-Folic acid (PEG-PAMAM G4-FA) | SW480 and MSC human mesenchymal stem cells | After treatment with PEG-PAMAM G4-FA-OXA, SW480 showed 84% cellular uptake compared to 40% in MSC For SW480 cell lines, PEG-PAMAM G4-FA-OXA was 1.47 and 5 times cytotoxic than free drug and PAMAM, respectively Significant increase un cytotoxicity and apoptosis after treatment with PEG-PAMAM G4-FA-OXA compared to pure OXA and PAMAM |
[81] |
PNCs as carriers for anticancer drugs for treatment of CRC
Polymeric micelles
PMs are nanostructures with a hydrophilic shell and a hydrophobic core prepared using amphiphilic copolymers (Fig. 2). In recent years, PMs have attracted increasing attention as nanocarriers for controlled delivery of anticancer drugs. Due to their distinct features, they are considered as potential carriers for hydrophobic drugs that can enhance their solubility in aqueous environment, extend their blood circulation time in vivo, improve their cellular uptake and passively target tumors by the EPR effect [57], [60], [61], [62].
Quercetin (Qu) is a natural flavonoid compound with remarkable anticancer activity in various tumors. Many studies showed that Qu can effectively induce apoptosis and downregulate proliferation of tumor cells and inhibit angiogenesis as well as various other essential signaling pathways [82], [83], [84]. However, the therapeutic efficacy of Qu is limited by its poor water solubility and low bioavailability [63]. In order to increase the aqueous solubility of Qu and improve its bioavailability, nano-sized biodegradable amphiphilic block copolymers of monomethoxy poly(ethylene glycol)–poly(ε-caprolactone) (MPEG–PCL) were used to encapsulate the drug. The obtained Qu-loaded MPEG–PCL nano-micelles (Qu-M) had a particle size of 34.8 ± 3.2 nm, a very low size distribution, a surface charge of −1.56 ± 0.15 mV and high encapsulation efficiency percentage (EE%) of 97.8%. In vitro drug release studies showed that the encapsulated Qu is slowly released from Qu-M, while in vivo pharmacokinetic studies using SD rats showed that encapsulation of Qu in PMs enhanced the t1/2 and Cmax of the drug following intravenous injection. Moreover, Qu-loaded PMs could remarkably suppress the tumor growth in vitro and in vivo when compared with free drug. In vitro anticancer activity studies showed that the small size and high-capacity characteristics of Qu-M significantly decreased the required half-maximal inhibitory concentration (IC50) of Qu-M at 48 h to 15.67 μg/mL, compared to 24.87 μg/mL for free Qu. In addition, the encapsulation of Qu in MPEG–PCL nano-micelles enhanced the uptake of the drug and allowed more efficient induction of cell apoptosis in CT26 colorectal cancer cell lines in vitro than free Qu. In vivo studies using female BALB/c mice inoculated subcutaneously with suspensions of CT26 cells showed a significant reduction in tumor volumes and weights after 3 weeks of treatment with multiple intravenous injection of Qu-M compared to free Qu. Further investigations showed that the anticancer effect of Qu-M in vivo was due to a combination of induction of the tumor cell apoptosis and inhibition of cell proliferation and tumor angiogenesis. [63].
PMs have also been used to develop injectable formulation and enhance the stability of curcumin (CUR), a chemical compound with broad anticancer activity derived from turmeric [64], [85], [86]. The use of CUR as chemotherapeutic agent is limited by its poor aqueous solubility, low stability and oral bioavailability and its rapid metabolism and elimination [64], [87], [88], [89]. CUR-loaded PMs with particle size of 27.6 nm, surface charge of 0.11 mV and EE% of 96% were prepared using monomethyl poly (ethylene glycol)-poly (ε-caprolactone)-poly (trimethylene carbonate) (MPEG-P(CL-co-TMC)) following a single-step solid dispersion method [64]. The growth of CT26 cells was efficiently suppressed in vitro after treatment with free CUR and CUR-loaded micelles for 24 h with IC50 of 16.67 μg/mL and 14.22 μg/mL, respectively. This was accompanied by an increase in cellular uptake and induction of apoptosis that was more significant using CUR-loaded PMs compared to free CUR. In vivo antitumor activity investigated by subcutaneous CT26 colon tumor model showed that CUR micelles were more efficient in inhibiting tumor growth in female BALB/c mice. The results also showed a significant reduction in tumor proliferation and angiogenesis and an increase in tumor cell apoptosis with no observed side effects [64].
In addition to their ability to improve the aqueous solubility and bioavailability of poorly soluble anticancer drugs, PMs were also used to control the release and to deliver combination of chemotherapeutic agents for treatment of colon cancer. A pH-sensitive biocompatible copolymer methoxy poly (ethylene glycol)-b-poly[(hydroxypropyl methacrylamide)-g-α-tocopheryl succinate-g-histidine)] (PTH) were synthesized and used in preparation of micelles for the co-delivery of DOX and α-TOS for treatment of colon cancer. The prepared micelles aimed to improve the targeting efficiency and alleviate adverse effects associated with DOX such as nephrotoxicity, cardiotoxicity and myelosuppression [65], [90], [91]. Furthermore, the rationale behind the use of NPs for combination therapy was to mitigate the limitations associated with the use of a single chemotherapeutic agent such as the need for a higher dose, which eventually also causes side effects. The spontaneous self-assembly of PTH copolymers micellar-type NPs was associated with π-π stacking between DOX/α-TOS and imidazole rings of PTH copolymers which allowed for a regular and tight arrangement of copolymers and drugs to form rod-like micelles of mean size of 166 nm and drug loading and percentage EE % of 9.6% and 32% for DOX, respectively, whereas they were 13.1% and 47.2% for α-TOS, respectively. PMs enabled both DOX and α-TOS to be rapidly released when pH decreased from 7.4 to 4.5 indicating the sensitivity of the drug–loaded micelles to changes in pH. The cytotoxic effect of DOX/α-TOS-micelles on HCT 116 human colon cancer cells was higher than normal cells. In vivo biodistribution studies after intravenous injection of 0.1 ml of micelles modified by Cy5.5 fluorescent dye into HCT 116 cell-bearing BALB-c/nude mice showed a significant accumulation of micelles in tumor tissues after 24 h. In vivo studies also showed that drug-loaded PMs had similar anti-tumor efficacy to free DOX with lower systemic toxicity. Overall, both in vitro and in vivo experimental findings have confirmed that synthesized PTH-based PMs could be used as alternative carriers for co-delivery of various chemotherapeutic agents to improve their therapeutic efficacy in management of CRC [65].
Polymersomes
Polymersomes are tiny hollow spheres prepared using synthetic amphiphilic block copolymers. They are composed of an aqueous core surrounded by a bilayer membrane. The bilayer membrane consists of three areas: an outer and inner hydrophilic and hydrated sublayers enclosing a hydrophobic sublayer (Fig. 2). Accordingly, polymersomes can carry either hydrophilic or hydrophobic drugs as the aqueous core can encapsulate hydrophilic therapeutic molecules while more hydrophobic therapeutic molecules can be loaded onto the lipophilic part of the membrane [53]. Polymersomes are very stable due to their thick and rigid membrane. Their composition usually involves the inclusion of a polyethylene glycol (PEG) hydrophilic surface layer that provides a long circulation time. They are considered versatile by changing the block copolymers thus leading to changing their drug release profiles and overall properties [92].
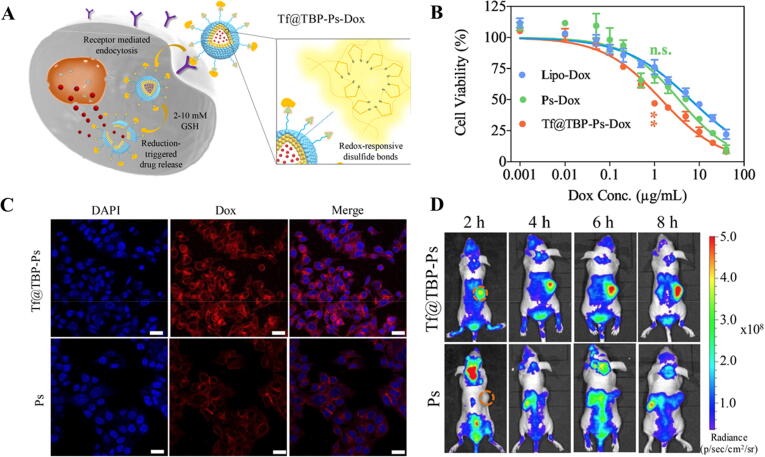
In addition to their use as carrier for chemotherapeutic agents in treatment of breast cancer [93] and lung cancer [94], some studies have reported their use as drug delivery systems for treatment of CRC. Wei et al. prepared polymersomes functionalized with transferrin (Tf)-binding peptide (TBP) by co-assembling poly(ethylene glycol)-b-poly(trimethylene carbonate-co-dithiolane trimethylene carbonate) (mPEG-P(TMC-DTC)) and TBP-PEG-P(TMC-DTC) [67]. Transferrin receptor (TfR) could be a very good target when treating CRC as it has been reported that these receptors are overexpressed in HCT 116 human colorectal carcinoma cell line [67], [95], [96]. The NPs were then loaded with DOX using the pH-gradient method [67], [97]. The particle size of the drug-loaded polymersomes was 72 nm with narrow polydispersity indexes and drug loading efficiency of 52% at different TBP molar ratios. The delivery system was stable for 48 h against dilution with phosphate buffer, in serum and in mouse whole blood. In vitro TfR targeting and antitumor activity of TBP-PEG-P(TMC-DTC) following Tf binding (Tf@TBP-Ps-DOX) showed that the system could efficiently mediate targeted delivery of DOX to CRC cells having over-expressed TfR in vitro and in vivo and that Tf@TBP-Ps-DOX increased the cellular uptake in TfR-overexpressing cells due to the stable bond to Tf on the surface of the polymersomes (Fig. 3). Moreover, in vivo pharmacokinetics studies using healthy BALB/c mice injected intravenously with TBP-Ps-DOX following Tf binding (Tf@TBP-Ps-DOX) exhibited a long circulation time, enhanced inhibition and reduced side effects compared to Ps-DOX [67]. The good stability profile, specificity and selectivity of those polymersomes formulae suggest they could substitute liposomes to deliver DOX. However, the use of Tf@TBP-Ps-Dox is limited by the presence of abundant endogenous Tf in circulation which can competitively inhibit the system toward TfR after intravenous administration, an issue that requires further investigation. [67], [98], [99].
Fig. 3.
Treatment of HCT-116 cell lines with TBP-Ps-Dox. A) Schematic diagram showing the accumulation and retention and cellular uptake of Tf@TBP-Ps-Dox in HCT-116 cells over-expressing transferrin receptor. B) Dependence of HCT-116 cell viability on concentrations of functionalized Ps with significant decrease in IC50 compared to Dox-loaded polymersome (Ps-Dox) and Dox-loaded liposomes (Lipo-Dox); C) confocal laser scanning microscope images showing intracellular release of Dox from Tf@TBP-Ps-Dox and Ps-Dox (Scale Bar: 25 μm) and D) In vivo imaging of HCT-116 tumor-bearing mice after intravenous injection of Cy5-labeled Tf@TBP-Ps and Ps. The orange circles represent the tumor regions. Adapted with permission from Wei et al. 2020, Elsevier [67].
Targeted matrix metalloproteinase 2 (MMP-2)-responsive chimeric polymersomes provided ideal characteristics for controlled release drug delivery against C26 mouse CRC cells [68]. MMP-2 and MMP-9 are effective cancer biomarkers due to their function in the regulation of cell receptor activity and growth factors. MMP-2 has been overexpressed in many tumor tissues thus becoming a good target in cancer treatment [68], [100], [101]. Polymersome loaded with SN38, the active metabolite of irinotecan, significantly increased the efficiency of the drug by overcoming its poor stability and low water solubility which limited its activity under physiological conditions [68], [102], [103], [104]. These polymersomes were prepared by coupling hetero-functional PEG (MAL-PEG-COOH, Mol wt. 3500 Da) with PLA using PVGLIG peptide. PVGLIG peptide was chosen due to its ability to be selectively cleaved by MMP-2 enzyme. The resulting PEG-peptide-PLA triblock copolymer was then loaded with SN38 using a single emulsion method yielding polymersomes with particle size of 172 nm, surface charge of −18.9 mV and 70% EE% [68]. Furthermore, in order to specifically target CRC cells, a DNA aptamer, AS1411, was conjugated on the surface of the polymersome to give Apt-SN38-pep-NPs to increase binding of the polymersomes to nucleolin that is upregulated in many cancer cells including CRC [68], [105]. The results showed that that Apt-SN38-pep-NPs significantly increased the cytotoxic effect of SN38 at low concentrations (0.15–1.25 μg/mL) in C26 cells compared to SN38-pep-NPs and free drug. In addition, in vivo studies using female BALB/c mice showed that Apt-SN38-pep-NPs improved tumor inhibition efficacy compared to SN38-pep-NPs as AS1411 delays clearance of the formulation by binding to nucleolin on C26 cells after intravenous injection [68]. The presence of PEG on the surface increased the circulation time of the drug-loaded polymersomes as it prevented the detection by mononuclear phagocyte system (MPS) and reinforced EPR effect leading to passive targeting [68], [106], [107]. Overall, these targeted polymersomes resulted in better cellular uptake and accumulation in tumor via receptor-mediated endocytosis when compared to SN38-pep-NPs and free drug [68].
CPT is an alkaloid compound having a potent cytotoxic activity that inhibits DNA topoisomerase I. However, its clinical use as chemotherapeutic agent is limited by its low specificity and associated side effects. [54], [108]. Targeted delivery of CPT to colon adenocarcinoma using polymersomes has been studied using tetraiodothyroacetic acid (tetrac) conjugated PEG-PLGA polymersomes [54]. The delivery system aimed at inhibiting tumor growth by targeting the integrin receptors α vß3 largely expressed on the surface of C26 mouse CRC cells and HT 29 human CRC cells and involved in the angiogenesis process [54], [109]. PEG-PLGA NPs were first prepared using double emulsion (W/O/W) method followed by conjugation of aminated tetrac using amide linkage. The encapsulation of CPT in the PEG-PLGA polymersome bilayer was carried out using single emulsion solvent technique in order to increase the efficiency of encapsulation of the hydrophobic drug in the polymer. The synthesized tet-PEG-PLGA-CPT polymersomes had a particle size and EE% of 172 nm of 89%, respectively. They sustained the release of the drug due to the rigid bilayer of the polymersomes and its stable structure leading to a significant increase in cytotoxicity and tumor inhibitory effect compared to free CPT due to their high affinity and selectivity. Furthermore, tetrac binding to integrin on the cell surface provided enhanced cell penetration because of the improved attachment of the polymersomes to C26 and HT 29 cells. In vivo pharmacokinetic studies using healthy female BALB/C mice showed a significant increase in plasma half-life and area under the curve and a considerable reduction in plasma clearance of CPT in polymersomal formulae resulting in a longer residence time after intravenous injection. It was suggested that the prolonged residence time in combination with the small particle size (less than200 nm) resulted in accumulation of the drug-loaded polymersomes in tumor site through EPR. This was confirmed by the increase in the intratumoral accumulation of the drug in BALB/c female C26 tumor-bearing mice especially from targeted polymersomes tet-PEG-PLGA-CPT due to an increase in their cellular uptake. Moreover, the targeted drug-loaded polymersomes showed improved tumor inhibitory effects, longer survival times and reduced systemic toxicity in vivo compared to non-targeted polymersomes and free drug [54].
Nanogels
Nanogels are networks of hydrophilic polymers with a three-dimensional (3D) porous structure (Fig. 2). They are used for the encapsulation of hydrophilic or hydrophobic therapeutic and diagnostic agents in their internal network and thus increase their stability, protect them from premature degradation in the plasma, increase their circulation time and control their release [110], [111]. Nanogels can be prepared using natural polymers, synthetic polymers or combinations that support the encapsulation of small molecules, oligonucleotides, and even proteins. They can be used in the delivery of therapeutic, diagnostic and imaging agents [112]. Nanogels have the ability to maintain their highly hydrated nature and shrinking-swelling properties under various environmental conditions [113]. In addition, the size and morphology, sensitivity to external stimuli such as pH, temperature, ionic strength, redox conditions, and surface properties can all be tuned for more effective circulation time and controlled drug release [114], [115].
5-FU is an effective chemotherapeutic agent used in the therapeutic management of various human malignancies. However, its clinical use is restricted due to various drawbacks that lead to insufficient cellular uptake and cell death. In order to overcome these limitations, pressure – responsive hydrogel NPs were prepared by crosslinking alginate (AL) with β-Cyclodextrin (CD) at various ratios [70]. The resulting AL-CD nanogels were then loaded with 5-FU by mixing the drug in an aqueous solution with the nanogels. The obtained drug-loaded AL‐CD nanogels were cytocompatible and had a suitable drug EE% of 82.1%. The in vitro release studies showed that nanogels controlled the release of the drug in conditions that simulate the intravascular pressure conditions. The nanogels were rapidly taken up by HT 29 cells in vitro followed by a significant intracellular accumulation and cell death by apoptosis when compared with free drug which shows the potential of using AL-CD nanogels to overcome the inefficiency of 5-FU in treatment of cancer [70].
AL-based nanogels were also used for the delivery of OXA in treatment of CRC to reduce its side effects such as nausea, diarrhea, numbness, tiredness and fatigue [71]. In this study, OXA was encapsulated in hyaluronic acid (HA)-coated AL nanogels (HA/AL/OXA) functionalized with folic acid to target folate receptors highly expressed on CRC cells. The obtained nanogels were spherical, negatively charged with a particle size and EE% of 200.3 nm and 85%, respectively. The release of OXA was both pH- and temperature-dependent with optimum release profiles obtained at pH 5 and 42 °C, respectively. OXA-loaded nanogel showed a significant cell toxicity against HT 29 cells when compared to free drug. This high cell toxicity was due to the greater affinity of the nanogels to bind to CD44 receptors followed by an increase their uptake in HT 29 cells due to the presence of HA. Moreover, OXA-loaded nanogels showed a dose- and time-dependent cytotoxicity and the IC50 values were 5.44, 4.6 and 3.76 μM for 24, 48 and 72 h, respectively. In addition, HT 29 cells treated with OXA-loaded nanogels induced higher apoptosis rate compared with the free nanogels as demonstrated by the up-regulation in the level of Bax gene expression as apoptotic gene indicating the greater efficacy of nanogels in treatment of CRC [71].
Dextrin-based nanogels have also been studied as carriers for DOX to decrease its non-specific delivery and other serious side-effects. The nanogels were prepared via emulsion cross-linking of dextrin with formaldehyde (FDNG) and glyoxal (GDNG) in presence of DOX to create a pH-responsive drug-loaded nanogel. After intraperitoneal administration, DOX-loaded nanogels would be absorbed into the systemic circulation, accumulated in tumor tissues via the EPR effect then released by breakage of the pH-sensitive linkages. The results showed that DOX-loaded FDNG were smaller in size (146.6 nm) than GDNG (317 nm) while both nanogels had similar charge and EE% of −5 mV and 23%, respectively. In vitro drug release was pH-dependent where the drug release was slightly higher at more acidic pH due to the increase in rate of hydrolysis of acetal linkage in acidic environment. The cell viability of drug free nanogels and DOX-loaded FDNGs was lower than free DOX and GDNGs, against CT26 and HT 29 CRC cells which suggests that some of the toxicity could be due to residues of cross-linking agent, which could be a drawback for the delivery system. Nevertheless, it was proposed that the pH-responsive ability of DNGs should prevent these residues having effects on normal tissues and release the drug at tumor sites characterized by their lower pH, increasing drug efficacy. DOX-loaded FDNGs substantially enhanced anti-tumor efficacy in orthotopic mouse model of CRC compared to free DOX. Mice weight shifts, tumor weight, tumor volume and histological assessment indicated a significant inhibition of tumor proliferation and induction apoptosis showing the potential of FDNGs as promising delivery vehicle for CRC therapy [72].
Polymeric nanocapsules
Polymeric nanocapsules are nanovesicular structures composed of a hydrophobic core holding lipophilic drugs and surrounded by a polymeric membrane or coating forming a reservoir (Fig. 2). Nanospheres are matrix systems in which the drug is dispersed throughout the whole matrix. The structural features of nanocapsules allow for an increase in hydrophobic drug loading, improved water solubility, enhanced intratumoral distribution, protection from premature degradation and better control over the release of chemotherapeutic agents [74]. In addition, targeted drug delivery could also be achieved through surface tagging of nanocapsules using the suitable targeting moieties [116].
The use of nanocapsules as carriers for chemotherapeutic agents in the treatment of different types of cancers has been the focus of many studies [117], [118]. Nanocapsules prepared using PEGylated PLGA and loaded with CUR were tested for their ability to efficiently delivery the drug to CRC cells in vitro and in vivo via passive targeting. CUR was dissolved in castor oil, soybean oil or miglyol812 oil and loaded on nanocapsules by nanoprecipitation methods. CUR-loaded nanocapsules prepared using castor oil showed the smallest size (150 nm) and highest EE% (92%) compared to other oils. This may be due to the higher solubility of the drug in castor oil as well as the high viscosity and surface tension of castor oil which improved the EE% of the drug in the nanocapsules [74], [119], [120]. In vitro release studies showed that nanocapsules were able to control the release of CUR where 66% and 80% of drug were released in 24 h in the absence or presence of serum, respectively, compared to the release of free CUR that exhibited a burst release of 50% and 100% of the drug in 30 min and 4 h, respectively. In addition, CUR-loaded nanocapsules showed an effective dose-dependent cell killing and apoptosis against CT26 cells causing some morphological changes within 48 hrs by making the cells more round and smaller in size while having no significant toxicity on healthy normal cells [74].
Avoiding intestinal drug absorption after oral administration to deliver a high concentration of the chemotherapeutic agents to tumor sites with minimal toxicity is another added challenge in the treatment of CRC. Ramzy et al. prepared nanocapsules of thymoquinone (TQ) dissolved in an oily core and surrounded by Eudragit-100 shell conjugated to anisamide (AA) to provide targeted delivery to colon cancer associated with sigma receptors overexpression. Sigma 2 receptors in CRC among other cancers are necessary for cell morphology, differentiation, and survival [75], [121]. The encapsulation of TQ in the oily core improved the poor solubility of the drug while the use of Eudragit 100 which dissolves at pH 7 enhanced the specific delivery of the drug to the colon as it hinders drug release in the stomach and small intestine [122], [123]. TQ-loaded AA-nanocapsules were prepared using the nanoprecipitation method. In vitro characterization showed a particle size >200 nm that is suitable to lower the diffusion through intestinal mucosa, a zeta potential of −36 to −39 mV which is needed to avoid aggregation and prolong physical stability of the NPs, a high EE% (>90%) and delayed cumulative drug release. The results of the in vitro cytotoxicity assays using three types of human CRC cell lines, namely HT 29, Caco-2 and HCT 116 cells with different levels of expression of sigma 2 receptors, showed that TQ-loaded AA-nanocapsules exhibited higher cytotoxicity than free TQ or TQ-loaded nanocapsules due to the enhanced cellular uptake of the conjugated nanocapsules. This was also proven by the resistance of HT 29 cells, with highest level of sigma 2 receptors expression, to uptake free TQ. Moreover, the high cell viability of HT 29 treated with blank AA-nanocapsules suggested suitable safety with minimal side effect [75].
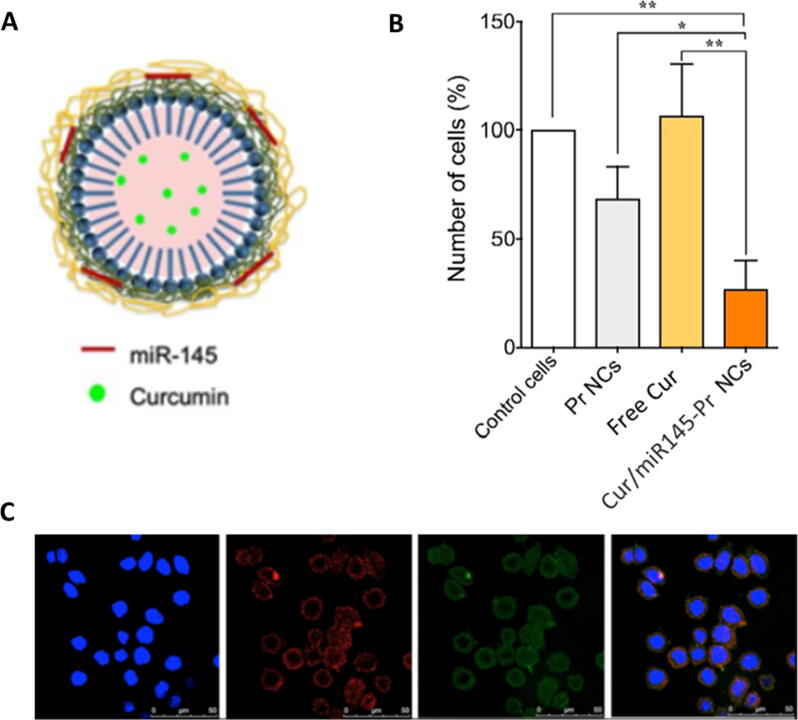
Reimondez-Troitiño et al. conducted a study to test the use of nanocapsules as carriers for co-administration of miR-145 and CUR to restore miR-145 levels as well as interfering CRC tumor growth using versatile protamine (Pr) nanocapsules (Fig. 4). It was shown that the upregulation of oncosuppressor miR-145 was associated with a reduction in tumor growth and migration as well as inhibition of drug resistance both in vitro and in vivo in certain cancers including CRC [76], [124]. Moreover, CUR has also shown anticancer activity by apoptosis induction, modulation of the expression of miRNAs, cell sensitization towards radiotherapy and chemotherapy and inhibition of proliferation [76], [125]. Protamine nanocapsules (Pr NCs) were prepared by solvent displacement method followed by loading miR-145. Then a layer by layer coating was carried out where an extra Pr layer was added. Finally, CUR was encapsulated in the vitamin E core of the nanocapsules [76]. The Pr double layer was beneficial as the first layer provided strong interaction with the cancerous cells while the second layer above the miRNA layer prevented its degradation [76], [126], [127]. The prepared CUR/miR145-Pr NCs had a particle size of 228 nm and EE% of 90% for CUR. In vitro cellular uptake studies were carried out on SW480 human CRC cells and the results showed an efficient increase in the intracellular levels of miR-145 with a 33-fold increase in the transfection values thus stronger inhibition of migration and proliferation. The results of in vitro cytotoxicity assays carried out using CUR-loaded Pr NCs showed significant increase in cytotoxicity to SW480 cells compared to free CUR. The overall results suggested that both CUR and miR-145 could be co-encapsulated in Pr NCs giving a synergistic anticancer effect; as CUR increases the intracellular levels of the oncosuppressor miR-145 along with the miRNA mimics that are being delivered using the nanocapsules leading to restoration of the normal levels and a decrease in the proliferation rate and cell migration [76].
Fig. 4.
Representation of the activity of CUR/miR145-Pr NCs in SW480 CRC cells showing A) a schematic diagram of CUR/miR145-Pr NCs B cell proliferation after treatment of with different nanocapsules; B) the Interaction of CUR and miR-145- loaded protamine nanocapsules with SW480 CRC cells; and C) confocal laser scanning microscope images showing of cells treated with CUR- (green channel) and Cy5-miR- (red channel) loaded into Pr NCs. Adapted with permission from Reimondez-Troitiño et al. 2019, Elsevier [76].
Dendrimers
Dendrimers are highly branched, functionalized polymers with well-defined structure and high density of functional groups (Fig. 2). They can be synthesized by two different approaches, the divergent and the convergent methods [128]. Recently, dendrimer-based drug delivery has been the focus of many studies in cancer research due to the characteristic branched structure and high number of functional group ending of those polymers which increase their drug encapsulation efficiency and conjugation capacity [17], [129], [130]. As a result, dendrimers showed to be useful as a platform for the delivery of many biologically active molecules such as antibodies, folic acid, DNA, chemotherapeutic drugs and MRI contrast agents [17], [131], [132], [133].
Among all dendrimers, polyamidoamine (PAMAM) dendrimers have attracted attention on the development of the drug delivery system due to their distinctive and multilateral attractive features [134]. PAMAM dendrimers have free amine groups on their surface, which contribute to their improved cytoplasmic distribution via the proton-sponge pathway. In addition to these unique features, their nano-sized and extremely aqueous nature imparts an excellent drug delivery properties [78], [135]. Surface modification of PAMAM dendrimers using gallic acid (GA) was carried out aiming to downregulate cell proliferation and migration, inhibit inflammatory response and enhance apoptotic cell death in human CRC cells [78]. GA (3,4,5-trihydroxy benzoic acid) is a natural polyphenolic compound commonly found in a variety of plants. GA is proven to have a wide spectrum of biological functions, such as antioxidant, anti-inflammatory, anti-microbial, anti-allergic, anti-melanogenic and anti-mutagenic effects and has also demonstrated a chemo-preventive and anti-proliferative actions against various types of cancer [78], [136], [137], [138]. However, the therapeutic application of GA is limited by its low bioavailability, limited distribution to tissues and rapid degradation [78], [139]. In order to avoid these limitations and enhance its therapeutic efficacy, GA was chemically conjugated with 4.0 G PAMAM dendrimers via coupling reaction using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDAC) as a cross-linking agent [78], [140]. The therapeutic impact of this chemical conjugation was validated by investigating the inhibition of cell proliferation and apoptosis in HCT 116 cells [78]. The results showed that the chemical conjugation of GA to PAMAM dendrimers resulted in sustained release of GA maintaining a steady-state therapeutic concentration in the plasma over a longer time interval. In addition, in vitro cell viability assay using HCT 116 cells showed that PAMAM-GA conjugate prevented cell proliferation, increased cellular uptake of GA, inhibited colonogenic cell survival, limited cancer cell migration by down regulating the MMP-9 expression, inhibiting the NF-kB activation and declining the release of pro-inflammatory cytokines. Moreover, PAMAM-GA conjugates induced mitochondria-mediated apoptotic cell death in HCT 116 cells instead of necrotic cell death while displaying a marginal cytotoxic effect compared to free GA in normal cells. The findings of this study evidenced that PAMAM-GA conjugate enhances the bioavailability of GA and directs the specific uptake into cancer cells to trigger apoptotic cell death [78].
Tumor necrosis factor-related apoptosis inducing ligand (TRAIL), a crucial factor in the apoptosis pathway, has drawn extensive attention in therapeutic management of many types of cancer due to its ability to bind to death receptors 4 and 5 (DR4 and DR5) which are overexpressed by a variety of tumor cells [79], [141]. TRAIL plasmid triggers cell apoptosis by extrinsic pathway through cell surface death receptors. PAMAM dendrimers were used for the co-delivery of DOX and TRAIL plasmid into CRC cells as DOX induces apoptosis by intrinsic pathways like hypoxia, severe DNA damage, or other cell stresses [79], [142], [143]. In this study, PAMAM (G 5.0) was modified by substituting the primary amine surface with cholesteryl chloroformate and alkyl PEG. The surface modified dendrimers (M-PAMAM) showed an EE% of about 90% indicating adequate encapsulation of DOX. The complexation of the drug-loaded M-PAMAM with TRAIL plasmid resulted in a more significant antitumor effect compared to M-PAMAM containing DOX or TRAIL plasmid only. Moreover, in vitro cytotoxicity and in vivo studies using mice bearing C26 colon carcinoma showed that the treatment with this established co-delivery system significantly increased cellular apoptosis and suppressed the growth rate of the tumor, respectively [79].
As mentioned earlier, CPT is a potent anticancer drug. However, its clinical use is limited by its poor selectivity and associated side effects [54]. AS1411 ssDNA aptamer (Apt) conjugated PAMAM dendrimers were prepared to selectively transport CPT to nucleolin-positive HT-29 and CT26 CRC cells [80]. In vitro drug release studies showed that the release of CPT in pH 5.5 and 7.4 was sustained due to its confinement in the hydrophobic cavities of the dendrimer. MTT cytotoxicity assay showed that IC50 of Apt-PEG-PAMAM-CPT and non-targeted PEG-PAMAM-CPT were 3 μg/mL and 10 μg/mL for HT29 cell line and 1.5 μg/mL and 8 μg/mL and for C26 cell line, respectively. While flow cytometry assays showed that targeted Apt-PEG-PAMAM-CPT complex enhanced cellular uptake and hindered the growth of nucleolin-positive cancer cells more effectively than non-targeted PEG-PAMAM-CPT. In vivo pharmacokinetic studies showed that the conjugation of the AS1411 ssDNA aptamer to the surface of the CPT-loaded pegylated PAMAM dendrimers improved the residence time of CPT. Treatment of BALB/C mice bearing mice colorectal carcinoma cell line C26 cell tumor with nontargeted PEG-PAMAM-CPT showed a significant decrease in tumor size and improved survival compared to animal treated with CPT. This increase in efficiency after intravenous administration is due to rapid distribution of the prepared drug-loaded PAMAM in the body and their enhanced uptake into tumor vessels via EPR effect. The further decrease in tumor volume and increase in survival after administration of Apt-PEG-PAMAM-CPT can be attributed to their ability to selectively bind with the nucleolin protein on the tumor cells surfaces and enhanced uptake into the cytoplasm via receptor-mediated endocytosis pathway. The obtained results suggests a way for the production of an efficient PNC that can provide an optimal therapeutic index for CPT [80].
Application of PNCs in diagnosis of CRC
One of the critical reasons of high mortality in CRC patients is the late diagnosis of this malignancy, eventually resulting in its progression to advanced stages (stages III-IV) and uncontrolled metastasis involving the vital organs of the body. Therefore, the mortality rate associated with CRC can be significantly reduced or prevented by the early and accurate diagnosis of adenomatous colonic polyps during endoscopy followed by their surgical removal [144], [145]. Unfortunately, the available conventional endoscopic technique based on white-light imaging holds a missing rate of up to 24% making it less efficient in early diagnosis of CRC [146]. Failure in noticing invasive flat or depressed lesions leads to late diagnosis of CRC in the advanced stages [147]. Thus, it is extremely crucial to come up with improved methods for early detection of premalignant lesions through novel techniques such as narrow-band imaging and chromoendoscopy to improve visualization [148], [149].
Many studies have focused on imaging and diagnosis of CRC using inorganic NPs such as quantum dots, iron and gold NPs [150], [151], [152]. Interestingly, some PNC-based probes have been engineered and evaluated as non-invasive tumor imaging agents for the early diagnosis and monitoring of therapeutic outcomes owing to their large surface area-to-volume ratio, exceptional pharmacokinetics and accumulation in highly angiogenic tumor tissues by EPR effect [153], [154], [155], [156]. Molecular imaging techniques, like near-infrared fluorescence (NIRF) imaging, have shown promising results in overcoming the drawbacks of invasive and morphological diagnoses [157], [158], [159]. NIRF imaging has high ability to penetrate into the deeper tissues and thus detect lesions more accurately compared to white-light imaging [160], [161]. Fluorescence-based probes are considered a prerequisite for NIRF imaging used for detection of specific target molecules [162]. NIRF imaging combined with an active probe targeting a specific peptide can increase the specificity and sensitivity and lead to better tumor detection [160], [163], [164].
The use of polymers as carriers for fluorescence-based probes improved the stability and targeting of NIRF and reduced their nonspecific binding [165]. A study on the diagnostic effect and monitoring of cancer therapy by NIRF imaging was conducted using a NP-based MMPs activable probe in animal colon cancer and human CRC xenograft model. MMPs are responsible for the development and progression of human cancers, mainly CRC [166], [167], and their expression increases during tumor progression [168], [169], [170]. Exposure of the probe to the protease resulted in cleavage of NIR-dye substrate due to specific substrate recognition and was followed by the emission of fluorescent light in the NIR range. PNCs showed that they can effectively deliver the probe to the target tumor lesions due to the EPR effect. In addition, NIRF imaging using the protease-activable probe showed noticeable fluorescence signal in the developed colorectal tumors compared with those obtained using a scramble control probe and saline. It has the ability to differentiate between normal tissues, adenomas, and adenocarcinomas where NIRF signal substantially increased from normal tissue to adenomas to adenocarcinomas. These results suggest that NIRF imaging combined with a PNC-based probes can support the early assessment and detection of the tumor lesions and thus enhance endoscopic diagnosis and characterization of those lesions instead of invasive lesions like biopsy. This strategy can also be used to assess and monitor the response of the patients to chemotherapeutic agents [156].
Intrinsic fluorescent nanocarriers where the fluorescent probes are part of the molecular structure of the NPs were proposed as an alternative to those prepared by conjugation or encapsulation in the nanocarriers [171]. For instance, pH-sensitive intrinsic anionic fluorescent nanogels (AFN) were prepared using fluorescein O,O′-diacrylate as crosslinker with 2-methacryloyloxy benzoic acid (2-MBA); while N,N-(diethylamino) ethyl methacrylate (DEAEMA) with PEG-methacrylate (PEGMA) were used for the preparation of cationic fluorescent nanogels (CFN) suing the same fluorescent crosslinker. The prepared NGs were loaded with cis-diamino-dichloroplatinum (CDDP) and 5-FU for use as theranostics in management of CRC and other types of cancer. The results showed that the particle size and surface charge of AFN were in the range of 75–150 nm and −13.5 mV, respectively. Loading of AFN with CDDP resulted EE% of 94% and an increase in both the size and surface charge of the particles to 206 nm and −10.5 mV, respectively. In vitro release studies showed sustained release of the drug over 50 h. No initial burst release was observed indicating strong interaction between AFN and the drug. AFN showed a pH–dependent fluorescence behavior where the fluorescence intensity increased upon increasing pH of the medium from 5.5 to 7.4. In vitro cell viability assay using HCT 116 cells and human lung cancer cell line (NCI-H1437), showed no significant cytotoxicity after treatment with blank AFN after 48 h while those treated with CDDP-AFN exhibited significant reduction in % cell viability. Cellular internalization of blank-AFN was confirmed by fluorescent microscopy showing a time-dependent increase in fluorescence intensity in the cytoplasm. Similarly, the particle size, surface charge of the prepared CFN and drug EE% were in the range of 99–120 nm, 19.7 mV and 73%, respectively, and were also affected by drug loading. In addition to its sensitivity to pH, CFN also showed temperature responsiveness where an increase in temperature from 30 to 40 °C resulted in contraction of the NG particles. Cell internalization assay also showed the presence of CFN in the cytoplasm and cell membrane. However, unlike AFN, viability tests showed a reduction in % cell viability due to combined effect of the drug and the nanocarriers [171]. Although this study did not include any in vivo data, it showed that intrinsic fluorescent nanocarriers are more homogenous and less susceptible to leaching and degradation; and represent promising bioimaging and therapeutic capabilities.
Toxicity of polymeric nanoparticles
PNCs offers unique physicochemical properties such as nanoscale size, massive surface area-to-volume ratio, and high reactivity. Their use in nanomedicine has significantly changed the therapeutic and diagnostic methods as they are precisely engineered materials with specific features at a molecular level [172].
However, some polymeric NPs impose serious toxicities that can hinder their use. For example, dendritic scaffold has been found to be an effective carrier for a various number of drugs, including anti-cancer, anti-viral, anti-bacterial, anti-tubercular, with the potential to increase the solubility and bioavailability of poorly soluble drugs [173], [174], [175], [176]. Despite their significant role improving the bioavailability and biodistribution, the clinical use of dendrimers is limited due to their associated inherent toxicity caused by the interaction of their surface cationic charge with negatively charged biological membranes in vivo [174], [177], [178], [179]. Those surface interactions result in nanohole formation, membrane thinning and erosion followed by membrane damage and leakage of cytosolic enzymes and cell death [174].
Accordingly, dendrimers result in different toxicities mainly characterized by hemolytic toxicity due to free cationic terminal groups of dendrimers interacting with RBCs causing hemolysis and cytotoxicity [174], [178], [180], [181]. For example, the cytotoxicity of polypropylenimine (PPI) and PAMAM dendrimers was reported using B16F10 murine melanoma, CCRF human leukemic T-lymphoblast, HepG2 human liver hepatocellular carcinoma and Caco-2 cell lines and found substantial cytotoxicity with these dendrimers owing to the polycationic nature of uncoated dendrimers, which influences various hematological parameters such as white blood and red blood corpuscles, hemoglobin, hematocrit and mean corpuscular hemoglobin [182], [183].
In order to overcome the toxicities associated with dendrimers, two strategies have been utilized. The first involved designing a biodegradable and/or biocompatible dendrimers [174], [184] while the other focused on the synthesis of surface engineered dendrimers to mask peripheral charge of dendrimers [174], [182], [185]. Biocompatible dendrimers can be synthesized using biodegradable core and branching units or using intermediates of different metabolic pathways. A variety of dendrimers with biocompatible materials building blocks have been developed such as citric acid, and peptide dendrimers [186], [187]. Other biodegradable dendrimers with reduced toxicity include polyester, polyether and triazine dendrimers [188], [189], [190]. Along with biodegradable dendrimers, surface engineering was studied as an alternative strategy for the development of less toxic dendrimers. In this approach, the surface of the dendrimers is modified with different functional groups to mask the cationic charge of dendrimers. This could be achieved either by neutralization of charge by PEGylation, acetylation, carbohydrate coating, peptide conjugation or by introducing negative charge using partially anionic dendrimers [174], [180], [182], [187], [191].
Many of the drug-free formulation of other polymeric NPs including PMs, polymersomes, polymeric nanogels, and nanocapsules have not shown significant in vitro toxicities when tested as control group on cancer and normal cell lines as they are prepared using biocompatible polymers (Table 1). For example, no cytotoxic effect was shown upon treatment of CT26 cell with blank dextrin-based NGs [72]. Caco-2 cells, mouse embryonic fibroblasts (MEFs) and human umbilical vein endothelial cells (HUVEC) retained their viability after treatment with DOX free CS/CMCS/TPP and CS/CMCS/Ca2+ NGs for 72 h, showing the biocompatibility of the NGs [73]. Similarly, treatment of CHO cell lines with different types of polymersomes confirmed their biocompatibility as drug carriers [54], [68].
Unfortunately, there are only few reports on the in vivo toxicity of these carriers. However few studies, showed that the administration of drug-loaded targeted-PNCs resulted in a significant reduction in systemic toxicity of the anticancer drugs in vivo [65], [67]. The encapsulation of niclosamide in CD44v6-targeted PM prepared using Pluronic® F127 increased the in vitro efficacy in HCT 116 CD44v6 + and CD44v6– cells. It also improved the in vivo biodistribution and hemocompatiblity of the drug in male NOD-SCID mice; and allowed to increase its intravenous dosage without causing toxicity [192]. Rapamycin-loaded peptide-labeled pegylated octadecyl lithocholate micelles significantly reduced renal toxicity for rapamycin in mice [193]. The in vivo toxicity of drug-free di-block co-polymers micelles intended for controlled release of DOX and prepared using a hydrophilic block of methoxypolyethylene glycol (mPEG) and a polyaminoacid block with different ratios of glutamic acid-γ-hydrazide and leucine was tested in healthy mice after intravenous administration. The results showed that the treatment did not lead to a significant loss in animal body weight and wellbeing for 2 weeks [194]. Similar results were obtained when female BALB/c mice were treated with SN38-loaded AS1411 aptamer- PEG-peptide-PLA triblock copolymer polymersomes as they showed minimal body weight loss and higher therapeutic index when compared to those treated with free SN38 [68]. Treatment of C26 tumor bearing female BALB/c mice bearing tet-PEG-PLGA-CPT showed improved tumor inhibitory effects, longer survival times and reduced systemic toxicity in vivo compared to non-targeted polymersomes and free drug [54]. It is expected that more systematic studies should be performed to test for any potential acute or chronic toxicity after in vivo administration PNCs.
Challenges in using PNCs for management of CRC
The clinical use of PNCs as delivery systems for management of CRC is still in the early stages despite the plethora of preclinical studies in this field (Table 2). The formulation of PNCs is yet limited by possible toxicity due to utilization of organic solvents as well as by the lack of reproducibility and batch to batch variation. Further processes of optimization and upscaling of PNCs for large scale manufacturing are complex and need proper standardization [195]. In addition, many PNCs suffers from long term stability issues when stored at room temperature which makes their practical use more challenging. Other barriers include the biodistribution of PNCs, their uptake by the reticuloendothelial system, their trafficking through the complex the tumour microenvironment and cellular internalization [22].
Table 2.
Examples of clinical trials for using PNCs in treatment and diagnosis of CRC.*
| Title | Identifier | Treatment | Stage |
|---|---|---|---|
| Targeted polymeric nanoparticles loaded with cetuximab and decorated with somatostatin analogue to colon cancer | NCT03774680 | Cetuximab NPs/Oral approved anticancer drug | Phase I |
| Pharmacokinetic, safety and efficacy study of nanoparticle paclitaxel in patients with peritoneal cancer/A Phase I study of intraperitoneal nanoparticle paclitaxel in patients with peritoneal malignancies | NCT00666991 | Nanoparticulate paclitaxel | Phase I |
| Neoadjuvant chemoradiotherapy with CRLX-101 and capecitabine for rectal cancer | NCT02010567 | CRLX101 (a nanopharmaceutical formulation of camptothecin)/Capecitabine/Radiotherapy/surgery | Phase I/Phase II |
| Study of CRLX101 (NLG207) in the Treatment of Advanced Solid Tumors | NCT00333502 | CPT conjugated to a linear, cyclodextrin-based polymer | Phase I/Phase II |
| A Study to Evaluate ONM-100, an Intraoperative Fluorescence Imaging Agent for the Detection of Cancer | NCT03735680 | ONM-100 (A polymer micelle covalently conjugated to indocyanine green) | Phase 2 |
*Source: https://clinicaltrials.gov/.
The use of 2D cell cultures for in vitro testing of the safety and efficiency of loaded PNCs is limited by its inability to simulate the actual tumor microenvironment, cellular interactions and gene and protein expression in response to the applied treatment [196]. 3D tumor spheroids emerged as an alternative in vitro cell culture technique that can mimic different aspects of real colorectal tumors and narrow the gap between in vitro and in vivo studies for antitumor drug delivery [197]. Likewise, the current animal models used for in vivo evaluation of antitumor efficacy are not consistent with the clinical characteristics of human CRC tumors. Humanized animal model and animal models of species with more human-like immune system, such as pigs and monkeys, should be considered as alternative for in vivo evaluation of PNCs in CRC management [197], [198].
Finally, the current guidelines provided by regulatory bodies on using NPs as carriers in cancer management are not sufficient and limit the preclinical to clinical translation of CRC management via PNCs, especially for theranostics intended for both cancer therapy and diagnosis. In fact, most of the clinically approved nanomedicines demonstrated reduced toxicity rather than improved efficacy [195], [199]. Accordingly, there is a pressing need for universal standardized protocols for preclinical development and characterization of PNCs to understand and predict drug release, biodistribution and possible interactions between nanomaterials and biological systems. The Food and Drug Administration (FDA) has the lead the worldwide efforts for standardization of nanomaterial use for management of cancer and other diseases by publishing guidelines about the importance of nanomaterial characterization regarding their toxicity [200]. Likewise, the Nanotechnology Characterization Laboratory (NCL) has also developed regulatory guidelines for the development of nanomedicine for treatment of cancer and is offering testing and validation services according to a series of emerging for new for nanomaterials [199], [201].
Conclusion
The increasing prevalence and high mortality rate associated with CRC are mainly due to late diagnosis and potential failure of conventional therapeutic strategies that drove the research towards the development of more convenient diagnostic tools and efficient therapeutic solutions for CRC. Nanotechnology has gained remarkable recognition in various fields, including cancer diagnosis and treatment, owing to several intrinsic characteristic features such as ultra-small particle size, high EE%, flexibility of functionalization, simultaneous delivery of drugs and ability to specifically delivery their load to targeted sites. Amongst, PNCs have emerged as one of the most promising tools in the efficient treatment of CRC as well as in mitigating the challenges associated with cytotoxic therapies. A critical analysis of the literature revealed that PNCs guided delivery of chemotherapeutic agents has significantly enhanced the aqueous solubility, bioavailability, plasma circulation time, target-specific biodistribution, intracellular internalization, and anticancer efficacy while mitigating their off-target effects due to non-specific accumulation of free/unloaded chemotherapeutic agents. The success of PNCs-supported delivery of cytotoxic agents has been evidenced in reducing tumor volume, downregulating the expression of specific tumor markers, and upregulating the apoptosis and cancer cell death. Although, EPR has been established as one of the main driving forces for the passive accumulation of PNCs into the tumor tissues; the surface modification of these NPs with specific targeting ligands has shown significant improvement in the targeted delivery and reduction in off-target accumulation of cytotoxic drugs. In addition, the specific functionalization of polymeric NPs has also resulted in achieving sustained release of the encapsulated chemotherapeutic agent payload which ultimately resulted in reduction of dose and frequency of administration, leading to an improvement in patient compliance and reduction in dose-dependent toxicity. Moreover, the application of PNCs as non-invasive nano-imaging probes has improved the imaging efficiency for early diagnosis and monitoring of therapeutic outcomes of CRC via signal amplification and better resolution for surface and deep tissue imaging. With all these findings, we anticipate that PNCs could be a promising tool for early diagnosis and efficient treatment of CRC.
Future directions
Most of the research on using PNCs in the management of CRC has focused on developing delivery systems for diagnostic agents or anticancer drug and testing their efficacy using quality by testing approach. This procedure is expensive, time consuming and may result in variations that decrease the safety of the final products. Future studies should focus on the optimization of PNCs formulations using quality by design (QBD) approach that links product quality to the desired clinical performance, prevents variability, improves process design and allows for the design of a quality formulation and upscaling process.
It is also clear that there is an imbalance between the amount of data available for the application of PNCs in drug delivery for treatment of CRC in comparison to their use for delivery of diagnostic agents for efficient imaging or early diagnosis of CRC. This area of research deserves more focus especially with current advancement in theranostics and development of medicinal agents with dual diagnostic and therapeutic effects for management of CRC.
In addition, more research should be conducted on the in vitro and in vivo toxicity of different types of PNCs in order to be able to better analyze the advantages and limitations of each and work on methods to eliminate these toxicities thus providing not only efficient but also safe delivery systems for management of CRC. A novel approach should be used to investigate the modification that occurs in the proteome and the metabolome profiles of normal and CRC cells following the administration PNCs loaded with anticancer drugs and diagnostic agents. The results of such work should shed more light on the biological processes that are affected when PNCs interact with living systems at the molecular level.
Compliance with ethics requirements
This manuscript does not contain any studies performed on human or animal subjects.
CRediT authorship contribution statement
Mohamed Haider: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. Khalid Zaki Zaki: Methodology, Data curation, Writing – original draft, Project administration. Mariam Rafat El Hamshary: Methodology, Data curation, Writing – original draft. Zahid Hussain: Validation, Formal analysis, Writing – review & editing. Gorka Orive: Validation, Formal analysis, Writing – review & editing. Haidy Osama Ibrahim: Methodology, Data curation, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was supported by Univeristy of Sharjah (UOS) targeted research project funds, 2101110345 to MH.
Biographies

Mohamed Haider received his PhD in gene and drug targeting from theCollege of Pharmacy, University of Maryland at Baltimore, Baltimore, Maryland, USA and a Master of Business Administration from the Merrick school of Business University of Baltimore, Baltimore Maryland, USA. He is an Associate Professor and cofounder of the Drug Delivery research group at the College of Pharmacy, University of Sharjah. His professional teaching and research interests focus on the biomedical applications of nanotechnology, sustained release systems and hydrogels for drug delivery and cell-based therapy. In addition, he serves as a member of the medical campus interdisciplinary education committee, the head of the quality assurance and assessment committee of the College of Pharmacy and as a member of the Biomedical Engineering master program coordination committee at the University of Sharjah. Dr. Mohamed is the leading author for this manuscript where he significantly participated in conceptualization, methodology, validation, formal analysis, resources, writing the original draft, reviewing and editing the final draft, visualization, supervision, project administration and funding acquisition.

Haidy Osama Ibrahim received her bachelor degree in pharmaceutical sciences from the College of Pharmacy, University of Sharjah, UAE and is currently studying for her master’s degree in the same college. She has a strong interest in further pursuing the nanotechnology topic and discovering the other benefits this field still holds to enhance the drug delivery for patients and ensure a safer and more successful treatment. As for her contribution in the review article, she gathered and written all the critical data for the review article regarding the introduction as well as the polymeric nanocarriers subsection. She has also searched the literature for data regarding the use of polymersomes and polymeric nanocapsules as carriers for anticancer drugs for treatment of CRC and wrote their subsections in an organized and orderly manner. Finally, she has reviewed the work of her colleagues and organized the references according to the required format.

Khalid Zaki Mohamed Zaki received his bachelor’s degree in pharmacy from College of Pharmacy, University of Sharjah, UAE and currently enrolled as a masters student in pharmaceutics specialization. His main interests include nanoparticles, nanotechnology and cancer. Regarding his contribution in the review article, he was involved in gathering the critical information regarding the epidemiology of the colorectal cancer and what is colorectal cancer in the introduction part. In addition, he gathered information from several research articles about nanogels. Besides, he was responsible for creating initial illustrations used in the manuscripts and collecting information about the application of PNCs in diagnosis of CRC. He has also reviewed and double checked the information gathered by his colleagues as well as making sure that the references and format are correct.

Mariam Raafat Elhamshary is a graduate of the College of Pharmacy, University of Sharjah and currently studying for her Masters in pharmaceutical sciences. In regards to her involvement in the review article, she was responsible for searching the literature and collecting the data regarding the polymeric micelles and dendrimers-based nanoparticles, in terms of their potential as nanocarriers in the early detection and effective therapy of colorectal cancer (CRC). She was also responsible for identifying the major challenges and toxicities related to the use of NP technology in CRC. In addition, she has reviewed the overall format of the article in terms of the data and information gathered by her colleagues, as well as all related references utilized in the review article.

Gorka Orive is Associate Professor of Pharmacy (University of the Basque Country, UPV/EHU; Spain). He grew up in Vitoria, Spain, attending the public Basque school there. He moved to UPV/EHU where he received a B.S. in pharmacy and then a PhD in pharmaceutical technology. He joined the same University as Assistant Professor in 2002. His research interests are in drug-delivery systems, biomaterials, tissue engineering, regenerative medicine and biomarkers for CNS diseases. He is advisor of several biotech and pharma companies and he is also an entrepreneur with the company Geroa Diagnostics. Gorka was involved in conceptualization of the article, validation of the collected data and revision of the final draft. He has published 320 international papers, 70 national articles, an H-index of 73 and more than 19200 citations. Lectured in more than 200 conferences and acted as supervisor of 13 PhD Thesis.

Zahid Hussain is presently serving as assistant professor at Department of Pharmaceutics and Pharmaceutical Technology, College of Pharmacy, University of Sharjah, United Arab Emirates. He has authored more than 95 peer-reviewed articles in high impact factor international journals and 5 book chapters with renowned publishers including Elsevier, Taylor & Francis, and Spring Nature. He has established research collaborations with many renowned researchers and scientists around the globe. His current total impact factor is 400+ with total number of citations 2055, H-index 28, and i-10 index 52 (Source: Google scholar). He is recipient of several prestigious honors and awards including Best Researcher Award (Universiti Teknologi MARA, Malaysia) in 2019, Outstanding Scientist Award (VGGOODTM Technology Factor, India) in 2020, and a number of Gold and Silver awards in Research and innovation. He has been featured among Top 2% Scientists in the world based on Scopus Citation Index published by Standford University USA in 2020. He is the editorial board member of 4 prestigious international journals and is official reviewer of more than 30 well-reputed peer-reviewed international journals. In this interesting and state-of-the-art review article, Dr. Zahid Hussain has contributed in reviewing, editing, content analysis and data curation.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Astolfi M., Rispoli G., Anania G., Artioli E., Nevoso V., Zonta G., et al. Tin, Titanium, Tantalum, Vanadium and Niobium Oxide Based Sensors to Detect Colorectal Cancer Exhalations in Blood Samples. Molecules. 2021;26:466. doi: 10.3390/molecules26020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawla P., Sunkara T., Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xi Y., Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14 doi: 10.1016/j.tranon.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araghi M., Soerjomataram I., Jenkins M., Brierley J., Morris E., Bray F., et al. Global trends in colorectal cancer mortality: projections to the year 2035. Int J Cancer. 2019;144:2992–3000. doi: 10.1002/ijc.32055. [DOI] [PubMed] [Google Scholar]
- 5.Papamichael D. Colorectal cancer. ESMO Handb Cancer Sr Patient. 2010:109–113. doi: 10.3109/9781841847481. [DOI] [Google Scholar]
- 6.Siegel R., DeSantis C., Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R.L., Miller K.D., Goding Sauer A., Fedewa S.A., Butterly L.F., Anderson J.C., et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 8.Levine J.S., Ahnen D.J. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355:2551–2557. doi: 10.1056/NEJMcp063038. [DOI] [PubMed] [Google Scholar]
- 9.Risio M. The natural history of adenomas. Best Pract Res Clin Gastroenterol. 2010 doi: 10.1016/j.bpg.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Winawer S.J., Zauber A.G. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am. 2002;12:1–9. doi: 10.1016/S1052-5157(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 11.Pickhardt P.J., Kim D.H., Pooler B.D., Hinshaw J.L., Barlow D., Jensen D., et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: A longitudinal study of natural history. Lancet Oncol. 2013 doi: 10.1016/S1470-2045(13)70216-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stryker S.J., Wolff B.G., Culp C.E., Libbe S.D., Ilstrup D.M., MacCarty R.L. Natural history of untreated colonic polyps. Gastroenterology. 1987 doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 13.You X., Kang Y., Hollett G., Chen X., Zhao W., Gu Z., et al. Polymeric nanoparticles for colon cancer therapy: overview and perspectives. J Mater Chem B. 2016;4:7779–7792. doi: 10.1039/c6tb01925k. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham D., Atkin W., Lenz H.J., Lynch H.T., Minsky B., Nordlinger B., et al. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 15.Cisterna B.A., Kamaly N., Wl Choi, Tavakkoli A., Farokhzad O.C., Vilos C. Targeted nanoparticles for colorectal cancer. Nanomedicine. 2016;11:2443–2456. doi: 10.2217/nnm-2016-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner H., Kloor M., Pox C.P. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee A., Pathak S., Subramanium V.D., Dharanivasan G., Murugesan R., Verma R.S. Strategies for targeted drug delivery in treatment of colon cancer: current trends and future perspectives. Drug Discov Today. 2017;22:1224–1232. doi: 10.1016/j.drudis.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Chandran S.P., Natarajan S.B., Chandraseharan S., Mohd Shahimi M.S.B. Nano drug delivery strategy of 5-fluorouracil for the treatment of colorectal cancer. J Cancer Res Pract. 2017;4:45–48. doi: 10.1016/j.jcrpr.2017.02.002. [DOI] [Google Scholar]
- 19.Sundaramoorthy P., Ramasamy T., Mishra S.K., Jeong K.Y., Yong C.S., Kim J.O., et al. Engineering of caveolae-specific self-micellizing anticancer lipid nanoparticles to enhance the chemotherapeutic efficacy of oxaliplatin in colorectal cancer cells. Acta Biomater. 2016;42:220–231. doi: 10.1016/j.actbio.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Chabner B.A., Roberts T.G. Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 21.Field K.M., Kosmider S., Jefford M., Jennens R., Green M., Gibbs P. Chemotherapy treatments for metastatic colorectal cancer: Is evidence-based medicine in practice? J Oncol Pract. 2008;4:271–276. doi: 10.1200/JOP.0852002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhaskaran N.A., Kumar L. Treating colon cancers with a non-conventional yet strategic approach: An overview of various nanoparticulate systems. J Control Release. 2021;336:16–39. doi: 10.1016/j.jconrel.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Tummala S., Satish Kumar M.N., Prakash A. Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm J. 2015;23:308–314. doi: 10.1016/j.jsps.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D.Y., Shen X.Z., Wang J.Y., Dong L., Zheng Y.L., Wu L.L. Preparation of chitosan-polyaspartic acid-5-fluorouracil nanoparticles and its anti-carcinoma effect on tumor growth in nude mice. World J Gastroenterol. 2008 doi: 10.3748/wjg.14.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelbary G., Haider M. In vitro characterization and growth inhibition effect of nanostructured lipid carriers for controlled delivery of methotrexate. Pharm Dev Technol. 2013;18:1159–1168. doi: 10.3109/10837450.2011.614251. [DOI] [PubMed] [Google Scholar]
- 26.Haider M., Abdin S.M., Kamal L., Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haider M., Elsherbeny A., Jagal J., Hubatová-Vacková A., Ahmed I.S. Optimization and evaluation of poly(Lactide-co-glycolide) nanoparticles for enhanced cellular uptake and efficacy of paclitaxel in the treatment of head and neck cancer. Pharmaceutics. 2020 doi: 10.3390/pharmaceutics12090828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Field L.D., Nag O.K., Sangtani A., Burns K.E., Delehanty J.B. The role of nanoparticles in the improvement of systemic anticancer drug delivery. Ther Deliv. 2018;9:527–545. doi: 10.4155/tde-2018-0015. [DOI] [PubMed] [Google Scholar]
- 29.Nag O.K., Field L.D., Chen Y., Sangtani A., Breger J.C., Delehanty J.B. Controlled actuation of therapeutic nanoparticles: An update on recent progress. Ther Deliv. 2016;7:335–352. doi: 10.4155/tde-2016-0003. [DOI] [PubMed] [Google Scholar]
- 30.Sangtani A., Nag O.K., Field L.D., Breger J.C., Delehanty J.B. Multifunctional nanoparticle composites: progress in the use of soft and hard nanoparticles for drug delivery and imaging. Wiley Interdiscip Rev Nanomedicine. NanoBiotechnology. 2017;9 doi: 10.1002/wnan.1466. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed I.S., El Hosary R., Hassan M.A., Haider M., Abd-Rabo M.M. Efficacy and Safety Profiles of Oral Atorvastatin-Loaded Nanoparticles: Effect of Size Modulation on Biodistribution. Mol Pharm. 2018;15:247–255. doi: 10.1021/acs.molpharmaceut.7b00856. [DOI] [PubMed] [Google Scholar]
- 32.Wong P.T., Choi S.K. Mechanisms of Drug Release in Nanotherapeutic Delivery Systems. Chem Rev. 2015;115:3388–3432. doi: 10.1021/cr5004634. [DOI] [PubMed] [Google Scholar]
- 33.Bahman F., Pittalà V., Haider M., Greish K. Enhanced Anticancer Activity of Nanoformulation of Dasatinib against Triple-Negative Breast Cancer. J Pers Med. 2021;11 doi: 10.3390/jpm11060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prakash S., Malhotra M., Shao W., Tomaro-Duchesneau C., Abbasi S. Polymeric nanohybrids and functionalized carbon nanotubes as drug delivery carriers for cancer therapy. Adv Drug Deliv Rev. 2011;63:1340–1351. doi: 10.1016/j.addr.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Edis Z., Wang J., Waqas M.K., Ijaz M., Ijaz M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int J Nanomedicine. 2021;16:1313–1330. doi: 10.2147/IJN.S289443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatia S. Natural polymer drug delivery systems: Nanoparticles, plants, and algae; 2016. 10.1007/978-3-319-41129-3. [DOI]
- 37.Martin C.R. Welcome to nanomedicine. Nanomedicine. 2006;1:5. doi: 10.2217/17435889.1.1.5. [DOI] [Google Scholar]
- 38.Nahar M., Dutta T., Murugesan S., Asthana A., Mishra D., Rajkumar V., et al. Functional polymeric nanoparticles: An efficient and promising tool for active delivery of bioactives. Crit Rev Ther Drug Carrier Syst. 2006;23:259–318. doi: 10.1615/CritRevTherDrugCarrierSyst.v23.i4.10. [DOI] [PubMed] [Google Scholar]
- 39.Arora D., Jaglan S. Nanocarriers based delivery of nutraceuticals for cancer prevention and treatment: A review of recent research developments. Trends Food Sci Technol. 2016;54:114–126. doi: 10.1016/j.tifs.2016.06.003. [DOI] [Google Scholar]
- 40.Ghosh S. Recent research and development in synthetic polymer-based drug delivery systems. J Chem Res. 2004:241–246. doi: 10.3184/0308234041209158. [DOI] [Google Scholar]
- 41.Lim T.Y., Poh C.K., Wang W. Poly (lactic-co-glycolic acid) as a controlled release delivery device. J Mater Sci Mater Med. 2009;20:1669–1675. doi: 10.1007/s10856-009-3727-z. [DOI] [PubMed] [Google Scholar]
- 42.Nafee N., Schneider M., Schaefer U.F., Lehr C.M. Relevance of the colloidal stability of chitosan/PLGA nanoparticles on their cytotoxicity profile. Int J Pharm. 2009;381:130–139. doi: 10.1016/j.ijpharm.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 43.Nicolas J., Mura S., Brambilla D., Mackiewicz N., Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42:1147–1235. doi: 10.1039/c2cs35265f. [DOI] [PubMed] [Google Scholar]
- 44.Oh J.K., Park J.M. Iron oxide-based superparamagnetic polymeric nanomaterials: Design, preparation, and biomedical application. Prog Polym Sci. 2011;36:168–189. doi: 10.1016/j.progpolymsci.2010.08.005. [DOI] [Google Scholar]
- 45.Kamaly N., Yameen B., Wu J., Farokhzad O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem Rev. 2016;116:2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M.H., Gu Z.P., Zhang X., Fan M.M. PH-sensitive ternary nanoparticles for nonviral gene delivery. RSC Adv. 2015;5:44291–44298. doi: 10.1039/c5ra04745e. [DOI] [Google Scholar]
- 47.Meng F., Cheng R., Deng C., Zhong Z. Intracellular drug release nanosystems. Mater Today. 2012;15:436–442. doi: 10.1016/S1369-7021(12)70195-5. [DOI] [Google Scholar]
- 48.Deng C., Jiang Y., Cheng R., Meng F., Zhong Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today. 2012;7:467–480. doi: 10.1016/j.nantod.2012.08.005. [DOI] [Google Scholar]
- 49.Haider M., Elsherbeny A., Pittalà V., Fallica A.N., Alghamdi M.A., Greish K. The Potential Role of Sildenafil in Cancer Management through EPR Augmentation. J Pers Med. 2021;11 doi: 10.3390/jpm11060585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang A.Z., Langer R., Farokhzad O.C. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 51.Fredenberg S., Wahlgren M., Reslow M., Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems - A review. Int J Pharm. 2011;415:34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 52.Cabral H., Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Release. 2014;190:465–476. doi: 10.1016/j.jconrel.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 53.Mohammadi M., Ramezani M., Abnous K., Alibolandi M. Biocompatible polymersomes-based cancer theranostics: Towards multifunctional nanomedicine. Int J Pharm. 2017;519:287–303. doi: 10.1016/j.ijpharm.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 54.Alibolandi M., Rezvani R., Farzad S.A., Taghdisi S.M., Abnous K., Ramezani M. Tetrac-conjugated polymersomes for integrin-targeted delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int J Pharm. 2017;532:581–594. doi: 10.1016/j.ijpharm.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed F., Discher D.E. Self-porating polymersomes of PEG-PLA and PEG-PCL: Hydrolysis-triggered controlled release vesicles. J Control Release. 2004;96:37–53. doi: 10.1016/j.jconrel.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 56.Levine D.H., Ghoroghchian P.P., Freudenberg J., Zhang G., Therien M.J., Greene M.I., et al. Polymersomes: A new multi-functional tool for cancer diagnosis and therapy. Methods. 2008;46:25–32. doi: 10.1016/j.ymeth.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiwari A., Saraf S., Jain A., Panda P.K., Verma A., Jain S.K. Basics to advances in nanotherapy of colorectal cancer. Drug Deliv Transl Res. 2020;10:319–338. doi: 10.1007/s13346-019-00680-9. [DOI] [PubMed] [Google Scholar]
- 58.Abouelmagd SA, Ahmad SA, Ai X, Asiri SMM, Athanasiou V, Banerjee R, et al. List of contributors. Stimuli Responsive Polym. Nanocarriers Drug Deliv. Appl., 2019, p. xix–xxiii. 10.1016/b978-0-08-101995-5.09990-6. [DOI]
- 59.Gillies E.R., Fréchet J.M.J. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 60.Croy S., Kwon G. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12:4669–4684. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 61.Gong C., Wang C., Wang Y., Wu Q., Zhang D., Luo F., et al. Efficient inhibition of colorectal peritoneal carcinomatosis by drug loaded micelles in thermosensitive hydrogel composites. Nanoscale. 2012;4:3095–3104. doi: 10.1039/c2nr30278k. [DOI] [PubMed] [Google Scholar]
- 62.Maeda H., Nakamura H., Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 63.Xu G.Y., Shi H.S., Bin R.L., Gou H.F., Gong D.Y., Gao X., et al. Enhancing the anti-colon cancer activity of quercetin by self-assembled micelles. Int J Nanomedicine. 2015;10:2051–2063. doi: 10.2147/IJN.S75550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang X., Li Z., Wang N., Li L., Song L., He T., et al. Curcumin-encapsulated polymeric micelles suppress the development of colon cancer in vitro and in vivo. Sci Rep. 2015;5:10322. doi: 10.1038/srep10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Debele T.A., Lee K.Y., Hsu N.Y., Chiang Y.T., Yu L.Y., Shen Y.A., et al. A pH sensitive polymeric micelle for co-delivery of doxorubicin and α-TOS for colon cancer therapy. J Mater Chem B. 2017;5:5870–5880. doi: 10.1039/c7tb01031a. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y., Lu Y., Hu D., Peng J., Xiao Y., Hao Y., et al. Cabazitaxel-loaded MPEG-PCL copolymeric nanoparticles for enhanced colorectal cancer therapy. Appl Mater Today. 2021;25 doi: 10.1016/j.apmt.2021.101210. [DOI] [Google Scholar]
- 67.Wei Y., Gu X., Sun Y., Meng F., Storm G., Zhong Z. Transferrin-binding peptide functionalized polymersomes mediate targeted doxorubicin delivery to colorectal cancer in vivo. J Control Release. 2020;319:407–415. doi: 10.1016/j.jconrel.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Ramezani P., Abnous K., Taghdisi S.M., Zahiri M., Ramezani M., Alibolandi M. Targeted MMP-2 responsive chimeric polymersomes for therapy against colorectal cancer. Colloids Surfaces B Biointerfaces. 2020;193 doi: 10.1016/j.colsurfb.2020.111135. [DOI] [PubMed] [Google Scholar]
- 69.Simón-Gracia L., Hunt H., Scodeller P., Gaitzsch J., Kotamraju V.R., Sugahara K.N., et al. iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes. Biomaterials. 2016;104:247–257. doi: 10.1016/j.biomaterials.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hosseinifar T., Sheybani S., Abdouss M., Hassani Najafabadi S.A., Shafiee A.M. Pressure responsive nanogel base on Alginate-Cyclodextrin with enhanced apoptosis mechanism for colon cancer delivery. J Biomed Mater Res - Part A. 2018;106:349–359. doi: 10.1002/jbm.a.36242. [DOI] [PubMed] [Google Scholar]
- 71.Shad P.M., Karizi S.Z., Javan R.S., Mirzaie A., Noorbazargan H., Akbarzadeh I., et al. Folate conjugated hyaluronic acid coated alginate nanogels encapsulated oxaliplatin enhance antitumor and apoptosis efficacy on colorectal cancer cells (HT29 cell line) Toxicol Vitr. 2020;65 doi: 10.1016/j.tiv.2019.104756. [DOI] [PubMed] [Google Scholar]
- 72.Manchun S., Dass C.R., Cheewatanakornkool K., Sriamornsak P. Enhanced anti-tumor effect of pH-responsive dextrin nanogels delivering doxorubicin on colorectal cancer. Carbohydr Polym. 2015;126:222–230. doi: 10.1016/j.carbpol.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 73.Feng C., Li J., Kong M., Liu Y., Cheng X.J., Li Y., et al. Surface charge effect on mucoadhesion of chitosan based nanogels for local anti-colorectal cancer drug delivery. Colloids Surf B Biointerfaces. 2015;128:439–447. doi: 10.1016/j.colsurfb.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 74.Klippstein R., Wang J.T.W., El-Gogary R.I., Bai J., Mustafa F., Rubio N., et al. Passively Targeted Curcumin-Loaded PEGylated PLGA Nanocapsules for Colon Cancer Therapy in Vivo. Small. 2015;11:4704–4722. doi: 10.1002/smll.201403799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramzy L., Metwally A.A., Nasr M., Awad G.A.S. Novel thymoquinone lipidic core nanocapsules with anisamide-polymethacrylate shell for colon cancer cells overexpressing sigma receptors. Sci Rep. 2020;10:1–15. doi: 10.1038/s41598-020-67748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reimondez-Troitiño S., González-Aramundiz J.V., Ruiz-Bañobre J., López-López R., Alonso M.J., Csaba N., et al. Versatile protamine nanocapsules to restore miR-145 levels and interfere tumor growth in colorectal cancer cells. Eur J Pharm Biopharm. 2019;142:449–459. doi: 10.1016/j.ejpb.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 77.Venkatesan P., Puvvada N., Dash R., Prashanth Kumar B.N., Sarkar D., Azab B., et al. The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials. 2011;32:3794–3806. doi: 10.1016/j.biomaterials.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 78.Priyadarshi K., Shirsath K., Waghela N.B., Sharma A., Kumar A., Pathak C. Surface modified PAMAM dendrimers with gallic acid inhibit, cell proliferation, cell migration and inflammatory response to augment apoptotic cell death in human colon carcinoma cells. J Biomol Struct Dyn. 2020:1–17. doi: 10.1080/07391102.2020.1802344. [DOI] [PubMed] [Google Scholar]
- 79.Pishavar E., Ramezani M., Hashemi M. Co-delivery of doxorubicin and TRAIL plasmid by modified PAMAM dendrimer in colon cancer cells, in vitro and in vivo evaluation. Drug Dev Ind Pharm. 2019;45:1931–1939. doi: 10.1080/03639045.2019.1680995. [DOI] [PubMed] [Google Scholar]
- 80.Alibolandi M., Taghdisi S.M., Ramezani P., Hosseini Shamili F., Farzad S.A., Abnous K., et al. Smart AS1411-aptamer conjugated pegylated PAMAM dendrimer for the superior delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int J Pharm. 2017 doi: 10.1016/j.ijpharm.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 81.Narmani A., Kamali M., Amini B., Salimi A., Panahi Y. Targeting delivery of oxaliplatin with smart PEG-modified PAMAM G4 to colorectal cell line: In vitro studies. Process Biochem. 2018;69:178–187. doi: 10.1016/j.procbio.2018.01.014. [DOI] [Google Scholar]
- 82.Cheng S., Gao N., Zhang Z., Chen G., Budhraja A., Ke Z., et al. Quercetin induces tumor-selective apoptosis through downregulation of Mcl-1 and activation of bax. Clin Cancer Res. 2010;16:5679–5691. doi: 10.1158/1078-0432.CCR-10-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Psahoulia F.H., Drosopoulos K.G., Doubravska L., Andera L., Pintzas A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther. 2007;6:2591–2599. doi: 10.1158/1535-7163.MCT-07-0001. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X.A., Zhang S., Yin Q., Zhang J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharmacogn Mag. 2015;11:404–409. doi: 10.4103/0973-1296.153096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin J.K., Lin-Shiau S.Y. Mechanisms of cancer chemoprevention by curcumin. Proc Natl Sci Counc Repub China B. 2001;25:59–66. [PubMed] [Google Scholar]
- 86.Kunnumakkara A.B., Anand P., Aggarwal B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 87.Safavy A., Raisch K.P., Mantena S., Sanford L.L., Sham S.W., Krishna N.R., et al. Design and development of water-soluble curcumin conjugates as potential anticancer agents. J Med Chem. 2007;50:6284–6288. doi: 10.1021/jm700988f. [DOI] [PubMed] [Google Scholar]
- 88.Shehzad A., Ul-Islam M., Wahid F., Lee Y.S. Multifunctional polymeric nanocurcumin for cancer therapy. J Nanosci Nanotechnol. 2014;14:803–814. doi: 10.1166/jnn.2014.9103. [DOI] [PubMed] [Google Scholar]
- 89.Sun M., Su X., Ding B., He X., Liu X., Yu A., et al. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine. 2012;7:1085–1100. doi: 10.2217/nnm.12.80. [DOI] [PubMed] [Google Scholar]
- 90.Ichikawa Y., Ghanefar M., Bayeva M., Wu R., Khechaduri A., Naga Prasad S.V., et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124:617–630. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrans V.J., Clark J.R., Zhang J., Yu Z.X., Herman E.H. Pathogenesis and prevention of doxorubicin cardiomyopathy. Tsitologiya. 1997;39:928–936. [PubMed] [Google Scholar]
- 92.Lee J.S., Feijen J. Polymersomes for drug delivery: Design, formation and characterization. J Control Release. 2012;161:473–483. doi: 10.1016/j.jconrel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Shahriari M., Taghdisi S.M., Abnous K., Ramezani M., Alibolandi M. Synthesis of hyaluronic acid-based polymersomes for doxorubicin delivery to metastatic breast cancer. Int J Pharm. 2019;572 doi: 10.1016/j.ijpharm.2019.118835. [DOI] [PubMed] [Google Scholar]
- 94.Yang W., Yang L., Xia Y., Cheng L., Zhang J., Meng F., et al. Lung cancer specific and reduction-responsive chimaeric polymersomes for highly efficient loading of pemetrexed and targeted suppression of lung tumor in vivo. Acta Biomater. 2018;70:177–185. doi: 10.1016/j.actbio.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 95.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G.S., Barzi A., et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 96.Makwana H., Mastrotto F., Magnusson J.P., Sleep D., Hay J., Nicholls K.J., et al. Engineered Polymer-Transferrin Conjugates as Self-Assembling Targeted Drug Delivery Systems. Biomacromolecules. 2017;18:1532–1543. doi: 10.1021/acs.biomac.7b00101. [DOI] [PubMed] [Google Scholar]
- 97.Fang Y., Yang W., Cheng L., Meng F., Zhang J., Zhong Z. EGFR-targeted multifunctional polymersomal doxorubicin induces selective and potent suppression of orthotopic human liver cancer in vivo. Acta Biomater. 2017;64:323–333. doi: 10.1016/j.actbio.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 98.Zhang N., Xia Y., Zou Y., Yang W., Zhang J., Zhong Z., et al. ATN-161 Peptide Functionalized Reversibly Cross-Linked Polymersomes Mediate Targeted Doxorubicin Delivery into Melanoma-Bearing C57BL/6 Mice. Mol Pharm. 2017;14:2538–2547. doi: 10.1021/acs.molpharmaceut.6b00800. [DOI] [PubMed] [Google Scholar]
- 99.Zou Y., Xia Y., Meng F., Zhang J., Zhong Z. GE11-Directed Functional Polymersomal Doxorubicin as an Advanced Alternative to Clinical Liposomal Formulation for Ovarian Cancer Treatment. Mol Pharm. 2018;15:3664–3671. doi: 10.1021/acs.molpharmaceut.8b00024. [DOI] [PubMed] [Google Scholar]
- 100.Mi Y., Wolfram J., Mu C., Liu X., Blanco E., Shen H., et al. Enzyme-responsive multistage vector for drug delivery to tumor tissue. Pharmacol Res. 2016;113:92–99. doi: 10.1016/j.phrs.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ke W., Li J., Zhao K., Zha Z., Han Y., Wang Y., et al. Modular Design and Facile Synthesis of Enzyme-Responsive Peptide-Linked Block Copolymers for Efficient Delivery of Doxorubicin. Biomacromolecules. 2016;17:3268–3276. doi: 10.1021/acs.biomac.6b00997. [DOI] [PubMed] [Google Scholar]
- 102.Alibolandi M., Abnous K., Anvari S., Mohammadi M., Ramezani M., Taghdisi S.M. CD133-targeted delivery of self-assembled PEGylated carboxymethylcellulose-SN38 nanoparticles to colorectal cancer. Artif Cells, Nanomedicine Biotechnol. 2018;46:1159–1169. doi: 10.1080/21691401.2018.1446969. [DOI] [PubMed] [Google Scholar]
- 103.Bala V., Rao S., Boyd B.J., Prestidge C.A. Prodrug and nanomedicine approaches for the delivery of the camptothecin analogue SN38. J Control Release. 2013;172:48–61. doi: 10.1016/j.jconrel.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 104.Ebrahimnejad P., Dinarvand R., Sajadi A., Jaafari M.R., Nomani A.R., Azizi E., et al. Preparation and in vitro evaluation of actively targetable nanoparticles for SN-38 delivery against HT-29 cell lines. Nanomed Nanotechnol, Biol Med. 2010;6:478–485. doi: 10.1016/j.nano.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Wu D.M., Zhang P., Liu R.Y., Sang Y.X., Zhou C., Xu G.C., et al. Phosphorylation and changes in the distribution of nucleolin promote tumor metastasis via the PI3K/Akt pathway in colorectal carcinoma. FEBS Lett. 2014;588:1921–1929. doi: 10.1016/j.febslet.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 106.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotech. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fang J., Nakamura H., Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 108.Bégué J.P., Bonnet-Delpon D.G. Biological Impacts of Fluorination: Pharmaceuticals Based on Natural Products. Fluor. Heal. 2008 doi: 10.1016/B978-0-444-53086-8.00013-8. [DOI] [Google Scholar]
- 109.Brooks P.C., Clark R.A.F., Cheresh D.A. Requirement of vascular integrin $α$v$β$3 for angiogenesis. Science (80-) 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 110.Raemdonck K., Demeester J., De Smedt S. Advanced nanogel engineering for drug delivery. Soft Matter. 2009;5:707–715. doi: 10.1039/b811923f. [DOI] [Google Scholar]
- 111.Yin Y., Hu B., Yuan X., Cai L., Gao H., Nanogel Y.Q. A versatile nano-delivery system for biomedical applications. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soni K.S., Desale S.S., Bronich T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J Control Release. 2016;240:109–126. doi: 10.1016/j.jconrel.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y., Maciel D., Rodrigues J., Shi X., Tomás H. Biodegradable polymer nanogels for drug/nucleic acid delivery. Chem Rev. 2015;115:8564–8608. doi: 10.1021/cr500131f. [DOI] [PubMed] [Google Scholar]
- 114.Sahiner N., Godbey W.T., McPherson G.L., John V.T. Microgel, nanogel and hydrogel-hydrogel semi-IPN composites for biomedical applications: Synthesis and characterization. Colloid Polym Sci. 2006;284:1121–1129. doi: 10.1007/s00396-006-1489-4. [DOI] [Google Scholar]
- 115.Vinogradov S.V., Bronich T.K., Kabanov A.V. Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54:135–147. doi: 10.1016/S0169-409X(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 116.Nagaich U. Polymeric nanocapsules: An emerging drug delivery system. J Adv Pharm Technol Res. 2018;9:73–79. doi: 10.4103/japtr.JAPTR_325_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nicolas S., Bolzinger M.A., Jordheim L.P., Chevalier Y., Fessi H., Almouazen E. Polymeric nanocapsules as drug carriers for sustained anticancer activity of calcitriol in breast cancer cells. Int J Pharm. 2018;550:170–179. doi: 10.1016/j.ijpharm.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 118.Rata D.M., Cadinoiu A.N., Atanase L.I., Popa M., Mihai C.T., Solcan C., et al. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil - An innovative concept for the skin cancer therapy. Mater Sci Eng C. 2021;119 doi: 10.1016/j.msec.2020.111591. [DOI] [PubMed] [Google Scholar]
- 119.Bouchemal K., Briançon S., Perrier E., Fessi H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. Int J Pharm. 2004;280:241–251. doi: 10.1016/j.ijpharm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 120.Mosqueira V.C.F., Legrand P., Pinto-Alphandary H., Puisieux F., Barratt G. Poly(D, L-Lactide) nanocapsules prepared by a solvent displacement process: Influence of the composition on physicochemical and structural properties. J Pharm Sci. 2000;89:614–626. doi: 10.1002/(SICI)1520-6017(200005)89:5<614::AID-JPS7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 121.Sun Y.T., Wang G.F., Yang Y.Q., Jin F., Wang Y., Xie X.Y., et al. Synthesis and pharmacological evaluation of 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline derivatives as sigma-2 receptor ligands. Eur J Med Chem. 2018;147:227–237. doi: 10.1016/j.ejmech.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 122.Subudhi M.B., Jain A., Jain A., Hurkat P., Shilpi S., Gulbake A., et al. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials (Basel) 2015;8:832–849. doi: 10.3390/ma8030832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoo J.-W., Giri N., Lee C.H. pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int J Pharm. 2011;403:262–267. doi: 10.1016/j.ijpharm.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 124.Wang W, Ji G, Xiao X, Chen X, Qin WW, Yang F, et al. Epigenetically regulated miR-145 suppresses colon cancer invasion and metastasis by targeting LASP1. Oncotarget 2016;7:68674–87. 10.18632/ONCOTARGET.11919. [DOI] [PMC free article] [PubMed]
- 125.Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. 2017;174:1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ledo A.M., Sasso M.S., Bronte V., Marigo I., Boyd B.J., Garcia-Fuentes M., et al. Co-delivery of RNAi and chemokine by polyarginine nanocapsules enables the modulation of myeloid-derived suppressor cells. J Control Release. 2019;295:60–73. doi: 10.1016/j.jconrel.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 127.González-Paredes A., Sitia L., Ruyra A., Morris C.J., Wheeler G.N., McArthur M., et al. Solid lipid nanoparticles for the delivery of anti-microbial oligonucleotides. Eur J Pharm Biopharm. 2019;134:166–177. doi: 10.1016/j.ejpb.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 128.Carvalho M.R., Reis R.L., Oliveira J.M. Dendrimer nanoparticles for colorectal cancer applications. J Mater Chem B. 2020;8:1128–1138. doi: 10.1039/c9tb02289a. [DOI] [PubMed] [Google Scholar]
- 129.Baker J.R. Why I believe nanoparticles are crucial as a carrier for targeted drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:423–429. doi: 10.1002/wnan.1226. [DOI] [PubMed] [Google Scholar]
- 130.Kolhe P., Misra E., Kannan R.M., Kannan S., Lieh-Lai M. Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int J Pharm. 2003;259:143–160. doi: 10.1016/S0378-5173(03)00225-4. [DOI] [PubMed] [Google Scholar]
- 131.Konda S.D., Wang S., Brechbiel M., Wiener E.C. Biodistribution of a 153Gd-folate dendrimer, generation = 4, in mice with folate-receptor positive and negative ovarian tumor xenografts. Invest Radiol. 2002;37:199–204. doi: 10.1097/00004424-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 132.Baker J.R. Dendrimer-based nanoparticles for cancer therapy. Hematology Am Soc Hematol Educ Program. 2009:708–719. doi: 10.1182/asheducation-2009.1.708. [DOI] [PubMed] [Google Scholar]
- 133.Thomas T.P., Majoros I.J., Kotlyar A., Kukowska-Latallo J.F., Bielinska A., Myc A., et al. Targeting and inhibition of cell growth by an engineered dendritic nanodevice. J Med Chem. 2005;48:3729–3735. doi: 10.1021/jm040187v. [DOI] [PubMed] [Google Scholar]
- 134.Hao M., Kong C., Jiang C., Hou R., Zhao X., Li J., et al. Polydopamine-coated Au-Ag nanoparticle-guided photothermal colorectal cancer therapy through multiple cell death pathways. Acta Biomater. 2019;83:414–424. doi: 10.1016/j.actbio.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 135.Esfand R., Tomalia D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6:427–436. doi: 10.1016/S1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 136.Badhani B., Sharma N., Kakkar R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015 doi: 10.1039/c5ra01911g. [DOI] [Google Scholar]
- 137.Kahkeshani N., Farzaei F., Fotouhi M., Alavi S.S., Bahramsoltani R., Naseri R., et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran J Basic. Med Sci. 2019;22:225–237. doi: 10.22038/ijbms.2019.32806.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Verma S., Singh A., Mishra A. Gallic acid: Molecular rival of cancer. Environ Toxicol Pharmacol. 2013 doi: 10.1016/j.etap.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 139.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81 doi: 10.1093/ajcn/81.1.230s. [DOI] [PubMed] [Google Scholar]
- 140.Sharma A., Gautam S.P., Gupta A.K. Surface modified dendrimers: Synthesis and characterization for cancer targeted drug delivery. Bioorganic Med Chem. 2011;19:3341–3346. doi: 10.1016/j.bmc.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 141.Guimarães P.P.G., Gaglione S., Sewastianik T., Carrasco R.D., Langer R., Mitchell M.J. Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano. 2018;12:912–931. doi: 10.1021/acsnano.7b05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: Recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and He. J Nanobiotechnology 2018;16:1–33. 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed]
- 143.Golshan M., Salami-Kalajahi M., Roghani-Mamaqani H., Mohammadi M. Synthesis of poly(propylene imine) dendrimers via homogeneous reduction process using lithium aluminium hydride: Bioconjugation with folic acid and doxorubicin release kinetics. Appl Organomet Chem. 2017;31 doi: 10.1002/aoc.3789. [DOI] [Google Scholar]
- 144.Winawer S.J., Zauber A.G., Ho M.N., O’Brien M.J., Gottlieb L.S., Sternberg S.S., et al. Prevention of Colorectal Cancer by Colonoscopic Polypectomy. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/nejm199312303292701. [DOI] [PubMed] [Google Scholar]
- 145.Issa I.A., Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol. 2017;23:5086–5096. doi: 10.3748/wjg.v23.i28.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Akarsu M, Akarsu C. Evaluation of New Technologies in Gastrointestinal Endoscopy. JSLS J Soc Laparoendosc Surg 2018;22:e2017.00053. 10.4293/JSLS.2017.00053. [DOI] [PMC free article] [PubMed]
- 147.Rembacken B.J., Fujii T., Cairns A., Dixon M.F., Yoshida S., Chalmers D.M., et al. Flat and depressed colonic neoplasms: A prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211–1214. doi: 10.1016/S0140-6736(00)02086-9. [DOI] [PubMed] [Google Scholar]
- 148.Barbeiro S., Libânio D., Castro R., Dinis-Ribeiro M., Pimentel-Nunes P. Narrow-Band Imaging: Clinical Application in Gastrointestinal Endoscopy. GE - Port J Gastroenterol. 2019;26:40–53. doi: 10.1159/000487470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Machida H., Sano Y., Hamamoto Y., Muto M., Kozu T., Tajiri H., et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: A pilot study. Endoscopy. 2004;36:1094–1098. doi: 10.1055/s-2004-826040. [DOI] [PubMed] [Google Scholar]
- 150.Gazouli M., Bouziotis P., Lyberopoulou A., Ikonomopoulos J., Papalois A., Anagnou N.P., et al. Quantum dots-bevacizumab complexes for in vivo imaging of tumors. In Vivo (Brooklyn) 2014;28:1091–1096. [PubMed] [Google Scholar]
- 151.Guo R., Zhang L., Qian H., Li R., Jiang X., Liu B. Multifunctional nanocarriers for cell imaging, drug delivery, and near-IR photothermal therapy. Langmuir. 2010;26:5428–5434. doi: 10.1021/la903893n. [DOI] [PubMed] [Google Scholar]
- 152.Pavitra E., Dariya B., Srivani G., Kang S.M., Alam A., Sudhir P.R., et al. Engineered nanoparticles for imaging and drug delivery in colorectal cancer. Semin Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 153.Portney N.G., Ozkan M. Nano-oncology: Drug delivery, imaging, and sensing. Anal Bioanal Chem. 2006;384:620–630. doi: 10.1007/s00216-005-0247-7. [DOI] [PubMed] [Google Scholar]
- 154.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65:271–284. doi: 10.1016/S0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 155.Sajja H., East M., Mao H., Wang Y., Nie S., Yang L. Development of Multifunctional Nanoparticles for Targeted Drug Delivery and Noninvasive Imaging of Therapeutic Effect. Curr Drug Discov Technol. 2009;6:43–51. doi: 10.2174/157016309787581066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yoon S.M., Myung S.J., Kim I.W., Do E.J., Ye B.D., Ryu J.H., et al. Application of near-infrared fluorescence imaging using a polymeric nanoparticle-based probe for the diagnosis and therapeutic monitoring of colon cancer. Dig Dis Sci. 2011;56:3005–3013. doi: 10.1007/s10620-011-1685-z. [DOI] [PubMed] [Google Scholar]
- 157.Weissleder R. Molecular imaging in cancer. Science (80-) 2006;312:1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 158.Weissleder R., Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 159.Mahmood U., Wallace M.B. Molecular Imaging in Gastrointestinal Disease. Gastroenterology. 2007;132:11–14. doi: 10.1053/j.gastro.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 160.Hilderbrand S.A., Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol. 2010;14:71–79. doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 161.Weissleder R., Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 162.Lee S., Park K., Kim K., Choi K., Kwon I.C. Activatable imaging probes with amplified fluorescent signals. Chem Commun. 2008;36:4250–4260. doi: 10.1039/b806854m. [DOI] [PubMed] [Google Scholar]
- 163.Marten K., Bremer C., Khazaie K., Sameni M., Sloane B., Tung C.H., et al. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology. 2002;122:406–414. doi: 10.1053/gast.2002.30990. [DOI] [PubMed] [Google Scholar]
- 164.Weissleder R., Tung C.H., Mahmood U., Bogdanov A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 165.Yang Z., Leon J., Martin M., Harder J.W., Zhang R., Liang D., et al. Pharmacokinetics and biodistribution of near-infrared fluorescence polymeric nanoparticles. Nanotechnology. 2009;20 doi: 10.1088/0957-4484/20/16/165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mysliwiec A.G., Ornstein D.L. Matrix metalloproteinases in colorectal cancer. Clin Colorectal Cancer. 2002;1:208–219. doi: 10.3816/CCC.2002.n.002. [DOI] [PubMed] [Google Scholar]
- 167.Coussens L.M., Fingleton B., Matrisian L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science (80-) 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 168.Kirimlioglu H., Kirimlioglu V., Yilmaz S., Sagir V., Coban S., Turkmen E., et al. Role of matrix metalloproteinase-7 in colorectal adenomas. Dig Dis Sci. 2006;51:2068–2072. doi: 10.1007/s10620-005-9070-4. [DOI] [PubMed] [Google Scholar]
- 169.Scherer R.L., McIntyre J.O., Matrisian L.M. Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 2008;27:679–690. doi: 10.1007/s10555-008-9152-9. [DOI] [PubMed] [Google Scholar]
- 170.Zucker S., Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/A:1025867130437. [DOI] [PubMed] [Google Scholar]
- 171.González-Urías A., Manzanares-Guevara L.A., Licea-Claveríe Á., Ochoa-Terán A., Licea-Navarro A.F., Bernaldez-Sarabia J., et al. Stimuli responsive nanogels with intrinsic fluorescence: Promising nanovehicles for controlled drug delivery and cell internalization detection in diverse cancer cell lines. Eur Polym J. 2021;144 doi: 10.1016/j.eurpolymj.2020.110200. [DOI] [Google Scholar]
- 172.Vasile C. Polymeric Nanomaterials: Recent Developments, Properties and Medical Applications. Polym Nanomater Nanotherapeutics. 2018:1–66. doi: 10.1016/B978-0-12-813932-5.00001-7. [DOI] [Google Scholar]
- 173.Lim J., Simanek E.E. Synthesis of water-soluble dendrimers based on melamine bearing 16 paclitaxel groups. Org Lett. 2008;10:201–204. doi: 10.1021/ol7024907. [DOI] [PubMed] [Google Scholar]
- 174.Jain K., Kesharwani P., Gupta U., Jain N.K. Dendrimer toxicity: Let’s meet the challenge. Int J Pharm. 2010;394:122–142. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 175.Jain N.K., Gupta U. Application of dendrimer-drug complexation in the enhancement of drug solubility and bioavailability. Expert Opin Drug Metab Toxicol. 2008;4:1035–1052. doi: 10.1517/17425255.4.8.1035. [DOI] [PubMed] [Google Scholar]
- 176.Yiyun C., Tongwen X., Rongqiang F. Polyamidoamine dendrimers used as solubility enhancers of ketoprofen. Eur J Med Chem. 2005;40:1390–1393. doi: 10.1016/j.ejmech.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 177.Malik N., Wiwattanapatapee R., Klopsch R., Lorenz K., Frey H., Weener J.W., et al. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–148. doi: 10.1016/S0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 178.Hussain Z., Thu H.E., Haider M., Khan S., Sohail M., Hussain F., et al. A review of imperative concerns against clinical translation of nanomaterials: Unwanted biological interactions of nanomaterials cause serious nanotoxicity. J Drug Deliv Sci Technol. 2020;59 doi: 10.1016/j.jddst.2020.101867. [DOI] [Google Scholar]
- 179.Janaszewska A., Lazniewska J., Trzepiński P., Marcinkowska M., Klajnert-Maculewicz B. Cytotoxicity of Dendrimers. Biomolecules. 2019;9:330. doi: 10.3390/biom9080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bhadra D., Bhadra S., Jain S., Jain N.K. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257:111–124. doi: 10.1016/S0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 181.Asthana A, Chauhan AS, Diwan PV, Jain NK. Poly(amidoamine) (PAMAM) dendritic nanostructures for controlled site-specific delivery of acidic anti-inflammatory active ingredient. AAPS PharmSciTech 2005;6. 10.1208/pt060367. [DOI] [PMC free article] [PubMed]
- 182.Jevprasesphant R., Penny J., Jalal R., Attwood D., McKeown N.B., D’Emanuele A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm. 2003;252:263–266. doi: 10.1016/S0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 183.Bodewein L., Schmelter F., Di Fiore S., Hollert H., Fischer R., Fenske M. Differences in toxicity of anionic and cationic PAMAM and PPI dendrimers in zebrafish embryos and cancer cell lines. Toxicol Appl Pharmacol. 2016;305:83–92. doi: 10.1016/j.taap.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 184.Agrawal P., Gupta U., Jain N.K. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials. 2007;28:3349–3359. doi: 10.1016/j.biomaterials.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 185.Roberts J.C., Bhalgat M.K., Zera R.T. Preliminary biological evaluation of polyamidoamine (PAMAM) StarburstTM dendrimers. J Biomed Mater Res. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 186.Wegmann K.W., Wagner C.R., Whitham R.H., Hinrichs D.J. Synthetic peptide dendrimers block the development and expression of experimental allergic encephalomyelitis. J Immunol. 2008;181:3301–3309. doi: 10.4049/jimmunol.181.5.3301. [DOI] [PubMed] [Google Scholar]
- 187.Namazi H., Adeli M. Dendrimers of citric acid and poly (ethylene glycol) as the new drug-delivery agents. Biomaterials. 2005;26:1175–1183. doi: 10.1016/j.biomaterials.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 188.Gillies E.R., Dy E., Fréchet J.M.J., Szoka F.C. Biological evaluation of polyester dendrimer: Poly(ethylene oxide) “bow-tie” hybrids with tunable molecular weight and architecture. Mol Pharm. 2005;2:129–138. doi: 10.1021/mp049886u. [DOI] [PubMed] [Google Scholar]
- 189.Krishna T.R., Jain S., Tatu U.S., Jayaraman N. Synthesis and biological evaluation of 3-amino-propan-1-ol based poly(ether imine) dendrimers. Tetrahedron. 2005;61:4281–4288. doi: 10.1016/j.tet.2005.02.045. [DOI] [Google Scholar]
- 190.Zhang W., Jiang J., Qin C., Pérez L.M., Parrish A.R., Safe S.H., et al. Triazine Dendrimers for Drug Delivery: Evaluation of Solubilization Properties, Activity in Cell Culture, and In Vivo Toxicity of a Candidate Vehicle. Supramol Chem. 2003;15:607–616. doi: 10.1080/10610270310001605197. [DOI] [Google Scholar]
- 191.Agashe H.B., Dutta T., Garg M., Jain N.K. Investigations on the toxicological profile of functionalized fifth-generation poly(propylene imine) dendrimer. J Pharm Pharmacol. 2006;58:1491–1498. doi: 10.1211/jpp.58.11.0010. [DOI] [PubMed] [Google Scholar]
- 192.Andrade F., Rafael D., Vilar-Hernández M., Montero S., Martínez-Trucharte F., Seras-Franzoso J., et al. Polymeric micelles targeted against CD44v6 receptor increase niclosamide efficacy against colorectal cancer stem cells and reduce circulating tumor cells in vivo. J Control Release. 2021;331:198–212. doi: 10.1016/j.jconrel.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 193.Khondee S., Rabinsky E.F., Owens S.R., Joshi B.P., Qiu Z., Duan X., et al. Targeted therapy of colorectal neoplasia with rapamycin in peptide-labeled pegylated octadecyl lithocholate micelles. J Control Release. 2015;199:114–121. doi: 10.1016/j.jconrel.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Brunato S., Mastrotto F., Bellato F., Bastiancich C., Travanut A., Garofalo M., et al. PEG-polyaminoacid based micelles for controlled release of doxorubicin: Rational design, safety and efficacy study. J Control Release. 2021;335:21–37. doi: 10.1016/j.jconrel.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 195.Operti M.C., Bernhardt A., Grimm S., Engel A., Figdor C.G., Tagit O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int J Pharm. 2021;605 doi: 10.1016/j.ijpharm.2021.120807. [DOI] [PubMed] [Google Scholar]
- 196.Kapałczyńska M., Kolenda T., Przybyła W., Zajączkowska M., Teresiak A., Filas V., et al. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14:910–919. doi: 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ginghină O, Hudiță A, Zaharia C, Tsatsakis A, Mezhuev Y, Costache M, et al. Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients. Materials (Basel) 2021;14. 10.3390/ma14092440. [DOI] [PMC free article] [PubMed]
- 198.Wei G., Wang Y., Yang G., Wang Y., Ju R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics. 2021;11:6370–6392. doi: 10.7150/thno.57828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ventola C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T. 2017;42:742–755. [PMC free article] [PubMed] [Google Scholar]
- 200.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 201.Sainz V., Conniot J., Matos A.I., Peres C., Zupanǒiǒ E., Moura L., et al. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468:504–510. doi: 10.1016/j.bbrc.2015.08.023. [DOI] [PubMed] [Google Scholar]