Abstract
Background
Paxlovid is recognized as an effective medication in preventing the progression of coronavirus disease of 2019 (COVID-19) to severe form in adults; however, its efficacy has remained unknown in pediatric cases. This study aimed to analyze the feasibility, safety, and efficacy of Paxlovid treatment in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected children aged 6–14 years.
Methods
We conducted a cohort study based on prospectively collected clinical data. We recruited 5 pediatric cases with underlying diseases treated with Paxlovid from 7 April 2022 to 26 May 2022 and 30 age-matched patients with underlying diseases who were not treated with Paxlovid as controls. The safety and efficacy of Paxlovid were primarily assessed by inter-group comparisons.
Results
Of the 5 Paxlovid-treated cases, including 1 male and 4 females, 3 and 2 cases were mildly and moderately ill, respectively. The underlying diseases included congenital heart defects, cerebral palsy, Down syndrome, and leukemia. Only 1 patient had received 1 dose of an inactivated SARS-CoV-2 vaccine. Paxlovid was initiated within 5 days after the onset of symptoms in all cases. Comedications were used in 2 cases. In the safety analyses, after Paxlovid initiation, 1 patient had transient diarrhea, and 1 patient had transiently elevated liver enzymes [alanine transaminase (ALT), 125 U/L; aspartate transaminase (AST), 83 U/L; normal range, <40 U/L]. In the efficacy analyses, all 5 Paxlovid-treated cases recovered, with the respective viral shedding times of 11, 4, 10, 9, and 9 days. Compared with age-matched controls, the viral shedding times were not significantly different between groups.
Conclusions
Based on the current small sample size study, Paxlovid is a feasible option for treating SARS-CoV-2-infected children aged 6–14 years with underlying diseases. However, the safety and efficacy of Paxlovid warrant further large-scale studies.
Keywords: Coronavirus disease of 2019 (COVID-19), children, Paxlvoid, viral shedding
Introduction
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has spread globally, with detrimental consequences (1). From November 2021, the Omicron variant has been prevalent, characterized by multiple mutations and high transmissibility (2,3). Children with underlying medical conditions are at risk of severe diseases (4). There is a need for safe and effective oral medications that can prevent the progression of SARS-CoV-2 infection to more severe stages, shorten the time to clinical recovery, and reduce the transmission rate.
Nirmatrelvir (PF-07321332), as an oral antiviral agent targeting the SARS-CoV-2 3-chymotrypsin-like cysteine protease enzyme (Mpro), exhibits potent inhibition of Mpro activity and viral replication across a wide spectrum of coronaviruses in vitro (5). Ritonavir, a CYP3A4 inhibitor, enhances nirmatrelvir pharmacokinetics (6). Paxlovid is a co-packaged combination of nirmatrelvir and ritonavir. A double-blind, randomized, controlled trial in phases II and III with a large sample size in symptomatic, unvaccinated, nonhospitalized adults at high risk of progression to severe coronavirus disease of 2019 (COVID-19) demonstrated that Paxlovid treatment resulted in an 89% lower risk of progression to severe COVID-19 than that with placebo, without evident safety concerns (7).
However, the efficacy of Paxlovid in COVID-19 pediatric cases is unknown. The metabolism of Paxlovid in children is potentially different from that in adults due to their immature organ function. The concomitant use of nirmatrelvir plus ritonavir and certain drugs may result in important drug interactions leading to detrimental adverse effects. From March 2022 to May 2022, the Omicron variant BA.2 was prevalent in Shanghai, China (8). Children with underlying diseases account for about 10% of all hospitalized pediatric cases and are at risk of severe infection. Early antiviral therapy is potentially beneficial. Therefore, we planned a clinical study to explore the efficacy of Paxlovid in pediatric cases with underlying diseases. However, with the current epidemic being under control, a total of just 5 cases were treated with Paxlovid. This paper intended to analyze the clinical data of the 5 cases and preliminarily analyze the clinical feasibility, safety, and efficacy of Paxlovid, using pediatric cases without Paxlovid treatment as controls. The results potentially provide a valuable clinical experience for Paxlovid treatment in Children. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2791/rc).
Methods
Patients and setting
This cohort study was conducted in a designated hospital, Renji Hospital (South branch), School of Medicine, Shanghai Jiao Tong University, for the treatment of pediatric COVID-19 cases. All except one admitted pediatric cases were in mild to moderate conditions. From 7 April 2022 to 26 May 2022, a total of 870 children aged 1 month to 14 years were admitted, and 87 cases had underlying diseases. The pre-decided indications for Paxlovid treatment included all the following factors: (I) aged 6–14 years old, polymerase chain reaction (PCR) test-confirmed COVID-19 cases with any symptoms, such as fever, cough, vomiting, diarrhea, and so on; (II) within 5 days since the onset of symptoms; (III) underlying diseases, such as congenital heart disease, bronchopulmonary dysplasia, neurological dysfunction, genetic abnormalities, immunodeficiency or immunosuppression condition, and so on; (IV) not taking medications which were contraindicated for concomitant use with Paxlovid, such as amiodarone and clozapine, referring to drug instructions; and (V) parents were informed and consent was granted.
Paxlovid treatment
The dosages of Paxlovid were as follows: for children aged 12–14 years with body weight higher than 40 kilograms, nirmatrelvir/ritonavir 300 mg/100 mg orally twice daily was given; for children aged 6–14 years with bodyweight 20–40 kilograms, 150 mg/100 mg twice daily was given; and for children aged 6–14 years with bodyweight less than 20 kilograms, 150 mg/100 mg once daily was given. The duration of treatment was 5 days.
Matched patients as controls
To primarily analyze the safety and efficacy of Paxlovid in children, SARS-CoV-2-infected children with underlying diseases aged 6–14 years who were not treated with Paxlovid were selected as controls. Since the sample size of the Paxlovid treatment group was small, the 30 control group cases were matched at a ratio of 6:1.
Data collection and definitions
Clinical data such as clinical symptoms, vaccination status, laboratory tests (COVID-19 nucleic acid PCR test, blood cell counts, chemistry), severity, treatment process, and prognosis were prospectively collected. The SARS-CoV-2 viral load was detected and quantified by reverse transcription (RT)-PCR using nasopharyngeal swabs every day since admission. The PCR test was validated with a sensitivity of 99.72% and specificity of 98.21%. A cycle threshold (Ct) value >35 of PCR test for both ORF1ab and N gene was considered negative. The nucleic acid test negative conversion was defined as 2 consecutive negative tests (Ct value >35 for the ORF1ab and N gene). The viral shedding time was defined as the first positive nucleic acid test or onset of symptoms (whichever earlier) to the date of the first negative test (in 2 consecutive tests).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of the Children’s Hospital of Fudan University (IRB No. 2022-83). Informed consent was obtained from all 5 legal guardians of Paxlovid-treated cases before enrollment and drug administration. Matched cases were recruited retrospectively, therefore the individual informed consent was not required by the ethics committee.
Statistics
Continuous variables were shown as median (range) or median (P25, P75). Wilcoxon rank-sum test was performed to compare these continuous variables. Categorical variables were presented as numbers (percentages), and when the number was less than 5, the percentages were not calculated. Fisher’s exact test was used to detect the inter-group differences of categorical variables. All statistical tests were two-tailed, and a P value of <0.05 was considered statistically significant. Statistical analysis was conducted using the software R 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Paxlovid-treated cases
After the recruiting process and collection of informed consent from caregivers, 5 patients received Paxlovid treatment. The clinical characteristics of these patients are displayed in Table 1. There was 1 male and 4 females, with an average age of 11.7 years. All 5 cases had underlying diseases, including 2 cases of cerebral palsy, 1 congenital heart defect with postoperative cardiac dysfunction, 1 Down syndrome, and 1 leukemia after chemotherapy, and all were mild-to-moderately ill. The main clinical manifestations included fever and cough. Except for 1 child who had received 1 dose of an inactivated COVID-19 vaccine, all of the children were unvaccinated.
Table 1. Clinical manifestations of 5 cases treated with Paxlovid.
| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Female | Female | Female | Female | Male | ||||||
| Age (y/m) | 10y9m | 9y11m | 13y | 13y2m | 11y5m | ||||||
| Weight (kg) | 18 | 15 | 24 | 18 | 44 | ||||||
| BMI (kg/m2) | 11.52 | 10.42 | 15.36 | 16.33 | 19.56 | ||||||
| Underlying diseases | Congenital heart defect | Down syndrome | Cerebral palsy | Cerebral palsy | Leukemia, chemotherapy | ||||||
| Post-cardiac surgery | Delayed growth | Epilepsy | |||||||||
| Delayed growth | |||||||||||
| Vaccination | No | No | 1 dose inactivated vaccine | No | No | ||||||
| Symptoms | |||||||||||
| Fever | Yes | No | No | Yes | No | ||||||
| Cough | Yes | Yes | Yes | Yes | Yes | ||||||
| Vomiting | No | Yes | No | No | No | ||||||
| Diarrhea | No | Yes | No | No | No | ||||||
| Severity | Moderate | Mild | Moderate | Mile | Mild | ||||||
| Paxlovid (nirmatrelvir/ritonavir) (mg) | 150/100 Qd, 5 days | 150/100 Qd, 5 days | 150/100 q12h, 5 days | 150/100 Qd, 5 days | 300/100 Q12h, 5 days | ||||||
| Co-medications | IV Cefoperazone | Oral fluconazole | None | None | None | ||||||
| Viral clearance (days) | 11 | 4 | 10 | 9 | 9 | ||||||
| Laboratory tests (pre-during-post Paxlovid treatment) | |||||||||||
| WBC (109/L) | 7.7, 5.5, 9.9 | 3.5, 4.5, 5.0 | 12.2, 11.3, 12.7 | 6.1, 6.8, 7.5 | 6.9, 5.6, 6.2 | ||||||
| PLT (109/L) | 165, 262, 386 | 178, 202, 274 | 352, 368, 429 | 237, 324, 331 | 221, 306, 239 | ||||||
| ALT (U/L) | 32, 125, 78 | 7, 12, 15 | 10, 15, 13 | 57, 38, 20 | 66, 43, 38 | ||||||
| AST (U/L) | 54, 83, 67 | 22, 17, 19 | 25, 27, 24 | 50, 43, 32 | 43, 31, 30 | ||||||
| Creatine (μmol/L) | 37, 26, 29 | 51, 35, 49 | 35, 32, 36 | 29, 25, 27 | 31, 37, 33 | ||||||
y, years; m, months; BMI, body mass index; WBC, white blood cell; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Qd, daily per day; Q12h, every 12 hours.
Safety analysis of Paxlovid in children
During the Paxlovid treatment process, 1 patient developed diarrhea on the second day after taking the medicine, and the symptoms improved 1 day later without treatment. No other adverse effects, such as allergic reactions, muscle aches, or others, were reported. One patient had transiently elevated liver enzymes for laboratory tests and recovered after completing the 5 days of Paxlvoid, with no need for further treatment (Table 1). The viral shedding times of the 5 patients were 11, 4, 10, 9, and 9 days, respectively. All patients were discharged to their homes without abnormal findings on the second-week follow-ups.
Comparisons of Paxlovid-treated cases and matched controls
To generate a primary analysis of the safety and efficacy of Paxlovid in treating COVID-19-infected children, 30 age-matched controls were selected. The underlying diseases in the controls included neurologic disorders (cerebral palsy, epilepsy, etc.), asthma, cancer or leukemia, congenital heart defects, post-bone marrow transplantation status, hyperthyroidism, and so on. Among all controls, 8 had received 2-doses of vaccine (full vaccination), and 10 were on long-term medications (sodium valproate, inhaled corticosteroids, diuretics, levothyroxine, warfarin, oxcarbazepine, methimazole, etc.). The basic clinical characteristics were comparable, and there were no differences in symptom spectrum, or disease severity. There were no significant differences in incidences of diarrhea and elevated liver enzymes (Table 2). Using viral shedding times as an outcome, there were no significant differences in Paxlovid-treated cases compared with controls {median [interquartile range (IQR)]: 9 (9 to 10) vs. 11 (9 to 12); P=0.152} (Figure 1).
Table 2. Comparisons of clinical characteristics between Paxlovid-treated cases and matched controls.
| Variables | Paxlovid-treated cases (n=5) | Matched controls (n=30) | P value |
|---|---|---|---|
| Gender, n [%] | |||
| Male | 1 | 8 [27] | 1.000 |
| Age (years) | |||
| Median [min, max] | 11.6 [10.0, 13.3] | 10.1 [8.3, 14.4] | 0.127 |
| Median [P25, P75] | 11.6 [10.9, 12.0] | 10.1 [9.25, 10.9] | 0.127 |
| Vaccination (1 or 2 doses), n [%] | 1 | 8 [27] | 1.000 |
| 0 | 0 | 0 | 1.000 |
| 1 | 1 | 0 | 0.143 |
| 2 | 0 | 8 [27] | 0.315 |
| Comedications, n [%] | 2 | 10 [33] | 1.000 |
| Symptoms, n [%] | |||
| Fever | 4 | 28 [93] | 0.902 |
| Cough | 5 | 20 [67] | 0.321 |
| Vomiting | 1 | 4 | 1.000 |
| Diarrhea | 1 | 4 | 1.000 |
| Severity, n [%] | |||
| Mild | 4 | 21 [70] | 1.000 |
| Moderate | 1 | 9 [30] | 1.000 |
| Laboratory tests, n [%] | |||
| Any WBC <4×109/L | 1 | 5 [17] | 1.000 |
| Any PLT <100×109/L | 0 | 1 | 1.000 |
| Any ALT or AST >2× normal range | 1 | 1 | 0.269 |
| Creatine >73 μmol/L | 0 | 0 | N/A |
WBC, white blood cell; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; N/A, not applicable.
Figure 1.
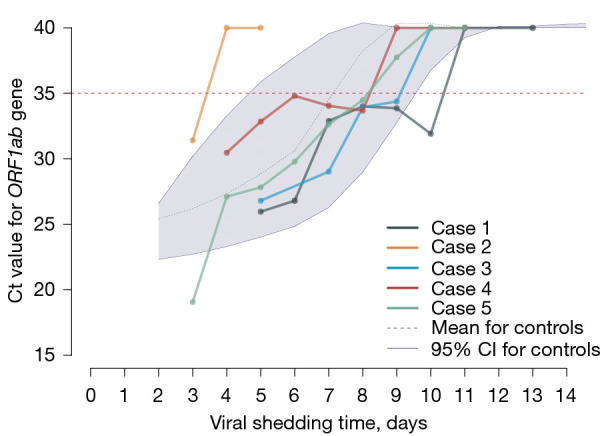
Viral shedding time in 5 Paxlovid-treated cases and matched controls. Ct, cycle threshold; CI, confidence interval.
Discussion
In this study, we reported 5 SARS-CoV-2-infected pediatric cases treated with Paxlovid in the early stage of the disease. As these patients had underlying diseases and none of them were fully vaccinated, they were at risk of progressing to a severe form of COVID-19. According to the diagnostic criteria of COVID-19 and the inclusion criteria for antiviral treatment (9), oral Paxlvoid was initiated in 5 cases. The 5-day course of treatment was completed in all cases, which implies the feasibility of Paxlvoid treatment in children aged 6–14 years.
Vaccination is the most valuable and effective strategy in preventing severe COVID-19 in children with underlying diseases (10). However, vaccination rates in recruited Paxlovid-treated patients and controls were much lower (20% in cases vs. 27% in controls) than in the whole population (6–14 years, >90%). In addition, with the prevalence of the SARS-CoV-2 Omicron variant, vaccine breakthrough infection has become more common (11), and it has been well studied that patients with underlying diseases are at risk of severe COVID-19, even after vaccination (12,13). In addition, in immunocompromised patients, SARS-CoV-2 may persist and cause subsequent resurgence or re-positivity (14,15). As such, early treatment may be beneficial to COVID-19 patients with underlying diseases (16). Compared with placebo, early initiation of Paxlvoid can significantly reduce the risk of hospitalization or death in mild-to-moderate cases compared with placebo (7). On 22 December 2021, Paxlovid received emergency use authorization in the United States for the treatment of mild-to-moderate COVID-19 infection in adults and children (≥12 years, weighing ≥40 kg) who are at risk of progressing to a severe form of infection (17). Similarly, in the updated edition of the COVID-19 drug treatment guidelines by the World Health Organization (WHO) on 22 April 2022, Paxlovid is recommended for patients with suitable indications (18), to prevent the disease progression as soon as possible by early implementation of antiviral therapy to inhibit viral replication. However, there are no clinical data on its use in children under the age of 12 and weighing less than 40 kg.
In this study, we treated 5 pediatric cases with underlying diseases. Regarding the dose of Paxlovid, we divided it into 3 dose ranges according to age and body weight, and these doses were adopted after a thorough discussion with clinical pharmacology experts. During Paxlovid treatment, all children were closely monitored, 1 patient had a day of diarrhea without dehydration and 1 patient had elevated liver enzymes [alanine transaminase (ALT), 125 U/L; aspartate transaminase (AST), 83 U/L; normal range, <40 U/L]. According to the previous adult clinical data, Paxlovid appears to be generally well tolerated; mild adverse events may include gastrointestinal upset, nausea and diarrhea, headache, and myalgia. The liver enzyme elevation was concerning; since it occurred after Paxlovid initiation and recovered after completion of the medication, it was potentially caused by the medication based on the timeline. Prelicensure studies have demonstrated there were no reported episodes of clinically apparent liver injury (19); however, the studies were mainly conducted in adults, thus whether Paxlovid causes liver injury in children warrants further study.
All 5 paxlovid-treated cases in our report were mild-to-moderately ill, no patients progressed to severe conditions. All patients recovered and were discharged home within 2 weeks. Since no cases in the Paxlovid-treated and control groups had reached severe disease stages, the efficacy of Paxlovid in preventing severe COVID-19, intensive care unit (ICU) admission, and mortality were not detectable. As such, viral shedding times were primarily compared between Paxlovid-treated and age-matched cases who did not receive Paxlovid. The differences were insignificant. The efficacy of Paxlovid in children was inconclusive based on our cohort study.
Of note, long-term medication use is common in children with chronic underlying diseases, and the interactions of those medications with Paxlovid could be complex. In this report, 2 Paxlovid-treated cases had comedications, antibiotics in case 1 and fluconazole in case 2. The viral shedding time in case 2 was significantly comparatively shorter, whether fluconazole enhanced the efficacy of Paxlovid was not revealed and has not been reported in previously published literature. Even though the patients with underlying conditions were the population most likely to benefit from antiviral therapy after SARS-CoV-2 infections, the inter-drug interactions are a major concern. The blood concentration of Paxlovid could be up- or down-regulated by long-term medication or vice versa. The chances of drug adverse effects or deterioration of underlying diseases could be high and warrants close monitoring.
The limitations of the current study were obvious. Firstly, as a small size observational study, only 5 cases were included in the Paxlovid-treatment group, and the comparisons of efficacy and safety lack significant power and were inconclusive. However, a 30-case control group did provide a valuable background reference for primarily analyzing the safety and efficacy of Paxlovid in children. Secondly, the blood concentration levels of Paxlovid were not tested, therefore the optimal dosage of Paxlovid in children was not ascertained. Thirdly, even though the primary target population for the study was children aged 6–14 years, all 5 cases were 9–13 years old, the result should not be generalized to all lower-age children, especially, children aged 6–8 years. Well-designed, large-sample clinical trials with controls are expected to be conducted in the future.
In conclusion, for children aged 6–14 years with underlying diseases infected with SARS-CoV-2, Paxlovid antiviral therapy is a potential option for preventing disease deterioration. However, it should be initiated within the framework of clinical trials with close monitoring of adverse effects.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was funded by the National Key Research and Development Program of China (Nos. 2021YFC2701800 and 2021YFC2701801) and the Shanghai Municipal Science and Technology Major Project (No. ZD2021CY001).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of the Children’s Hospital of Fudan University (IRB No. 2022-83). Informed consent was obtained from all 5 legal guardians of Paxlovid-treated cases before enrollment and drug administration. Matched cases were recruited retrospectively, therefore the individual informed consent was not required by the ethics committee.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2791/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2791/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2791/coif). The authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020;324:782-93. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 2.Al-Qahtani AA. Mutations in the genome of severe acute respiratory syndrome coronavirus 2: implications for COVID-19 severity and progression. J Int Med Res 2022;50:3000605221086433. 10.1177/03000605221086433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyer O. Covid-19: Omicron is causing more infections but fewer hospital admissions than delta, South African data show. BMJ 2021;375:n3104. 10.1136/bmj.n3104 [DOI] [PubMed] [Google Scholar]
- 4.Graff K, Smith C, Silveira L, et al. Risk Factors for Severe COVID-19 in Children. Pediatr Infect Dis J 2021;40:e137-45. 10.1097/INF.0000000000003043 [DOI] [PubMed] [Google Scholar]
- 5.Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021;374:1586-93. 10.1126/science.abl4784 [DOI] [PubMed] [Google Scholar]
- 6.Sevrioukova IF, Poulos TL. Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci U S A 2010;107:18422-7. 10.1073/pnas.1010693107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med 2022;386:1397-408. 10.1056/NEJMoa2118542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Zhang W, Chen S. Shanghai's life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet 2022;399:2011-2. 10.1016/S0140-6736(22)00838-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med 2022;54:516-23. 10.1080/07853890.2022.2034936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creech CB, Anderson E, Berthaud V, et al. Evaluation of mRNA-1273 Covid-19 Vaccine in Children 6 to 11 Years of Age. N Engl J Med 2022;386:2011-23. 10.1056/NEJMoa2203315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alcendor DJ, Matthews-Juarez P, Smoot D, et al. Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic. Vaccines (Basel) 2022;10:755. 10.3390/vaccines10050755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta RK, Topol EJ. COVID-19 vaccine breakthrough infections. Science 2021;374:1561-2. 10.1126/science.abl8487 [DOI] [PubMed] [Google Scholar]
- 13.Khoury J, Najjar-Debbiny R, Hanna A, et al. COVID-19 vaccine - Long term immune decline and breakthrough infections. Vaccine 2021;39:6984-9. 10.1016/j.vaccine.2021.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi B, Choudhary MC, Regan J, et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med 2020;383:2291-3. 10.1056/NEJMc2031364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baang JH, Smith C, Mirabelli C, et al. Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient. J Infect Dis 2021;223:23-7. 10.1093/infdis/jiaa666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bierle DM, Ganesh R, Tulledge-Scheitel S, et al. Monoclonal Antibody Treatment of Breakthrough COVID-19 in Fully Vaccinated Individuals with High-Risk Comorbidities. J Infect Dis 2022;225:598-602. 10.1093/infdis/jiab570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marzolini C, Kuritzkes DR, Marra F, et al. Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid®) and comedications. Clin Pharmacol Ther 2022. [Epub ahead of print]. doi: . 10.1002/cpt.2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A living WHO guideline on drugs for covid-19. BMJ 2022;377:o1045. [DOI] [PubMed] [Google Scholar]
- 19.Paxlovid. (Updated 2022 Jan 31). In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK577815/ (Accessed May 27, 2022). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


