Abstract
Background
Patients with critical limb ischemia (CLI) still have a high rate of lower limb amputation, which is associated with not only a decrease in quality of life but also poor life prognosis. Implantation of adipose-derived regenerative cells (ADRCs) has an angiogenic potential for patients with limb ischemia.
Objectives
We investigated safety, feasibility, and efficacy of therapeutic angiogenesis by cell transplantation (TACT) of ADRCs for those patients in multicenter clinical trial in Japan.
Methods
The TACT-ADRC multicenter trial is a prospective, interventional, open-labeled study. Patients with CLI (Fontaine class III–IV) who have no other option for standard revascularization therapy were enrolled in this study. Thirty-four target ischemic limbs of 29 patients were received freshly isolated autologous ADRCs implantation.
Results
The overall survival rate at a post-operative period and at 6 months follow-up was 100% at any time points. As a primary endpoint for efficacy evaluation, 32 limbs out of 34 (94.1%) were free from major amputation for 6 months. Numerical rating scale (from 6 to 1) as QOL score, ulcer size (from 317 mm2 at to 109 mm2), and 6-min walking distance (from 255 to 369 m) improved in 90.6%, 83.3%, and 72.2% patients, respectively.
Conclusions
Implantation of autologous ADRCs could be safe and effective for the achievement of therapeutic angiogenesis in the multicenter settings, as a result in no major adverse event, optimal survival rate, and limb salvage for patients with no-conventional option against critical limb ischemia.
TRN: jRCTb040190118; Date: Nov. 24th, 2015.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10456-022-09844-7.
Keywords: Adipose-derived regenerative cells, Therapeutic angiogenesis, Critical limb ischemia, Multicenter clinical trial
Introduction
Critical limb ischemia (CLI) is the most severe stage of ischemic limbs. Although multidisciplinary treatment including lifestyle modification, pharmacotherapy, and revascularization is indicated for CLI, it is still an intractable disease that often leads to limb amputations [1]. Patients with CLI who undergo amputation not only have a significantly reduced quality of life (QOL), but also have an increased mortality rate due to cardiovascular complications [1].
Ever since the therapeutic angiogenesis using cell transplantation (TACT) trial with bone marrow mononuclear cells (BM-MNCs) was first reported in 2002 [2], therapeutic angiogenesis by cell transplantation has demonstrated efficacy against various ischemic diseases over the world [3–5]. Initial results of the bone marrow TACT trial revealed that the estimated time to walk on a treadmill for CLI patients increased from 1.3 to 3.6 min, and resting pain was relieved in 80% of patients during a 6-month follow-up period [2]. However, subsequent examination of the long-term analysis revealed that the existence of certain non-responder group was evident [6–8]. In addition, the protocol of collecting about 1000 mL of autologous bone marrow was an invasive procedure for these patients although the residual red blood cells were re-infused.
To overcome these issues, we have been working on the development of therapeutic angiogenesis using additional novel cell source. The human subcutaneous adipose tissue contains mesenchymal stem cells (MSCs) with properties similar to those of BM-mesenchymal stem cells (BM-MSCs) [9]. These cells, called adipose-derived stem/regenerative cells (ADSCs or ADRCs), can differentiate into multiple-lineage cells [9] and which have prompted us to use ADRCs as a new cell source for TACT in patients with critical limb ischemia [10–13]. It is possible to obtain large numbers of ADRCs as either primary cells or multiple passage cells in culture. In addition, harvesting subcutaneous adipose tissue can be performed with established techniques such as liposuction under local or general anesthesia and is considered a less invasive procedure than bone marrow harvesting.
Previous three pilot studies have demonstrated that therapeutic angiogenesis with ADRC implantation for CLI might be safe in limited conditions of small sample size and single-center trials [14–16]. However, there have been no trials that resolved clinical concerns about whether autologous ADRC implantation would be safe, feasible, and effective for CLI patients in a setting of multicenter trial. Around 2015, when this clinical study began, the concept of chronic limb-threatening ischemia (CLTI) was not yet common, and CLI was initially used to define a severe case of limb ischemia in contemporary practice. Recently, as a novel concept, the new term CLTI has been proposed to include a broader and more heterogeneous group of patients with varying degrees of ischemia that can often delay wound healing and increase amputation risk [1].
Accordingly, the TACT-ADRC multicenter trial was conducted to test the safety, feasibility and potential efficacies of the treatment with autologous freshly isolated ADRC implantation in CLI (or CLTI) patients in eight hospitals in different regions in Japan.
Patients and methods
Study patients
We performed a multicenter, prospective, interventional, single-arm, open-label, phase 2 trial at 8 hospitals in Japan [17]. This study was initially planned to enroll patients from November 2015 to June 2020 for up to 40 patients. Patients with CLI (Fontaine class III–IV) caused by atherosclerotic peripheral artery disease (PAD), Buerger’s disease, or connective tissue disease (CTD)who have no other option for standard revascularization therapy were judged eligible if they were aged 25–79 years. Combined treatment team of cardiologists and vascular surgeons at each institute jointly reviewed the PAD/CLI stage, and a consensus is reached based on the history interview, objective physical findings, and various laboratory findings. We excluded patients if they had (1) an insufficient amount of adipose tissue to isolate, (2) withholding informed consent, (3) comorbidities with a life expectancy <1 year, (4) previous (within 5 years) or current history of neoplasm or clinically significant abnormality on prescreening examination, (5) ischemic heart disease without revascularization, (6) untreated diabetic retinopathy, (7) severe infection, (8) severe liver and/or renal dysfunction (except for patients under hemodialysis), (9) severe hematologic disease, (10) pregnancy, and (11) physician considers patient to be unsuitable for trial inclusion. Audits were periodically conducted by the monitoring committee members to ensure that these diagnostic and implementation criteria were in proper compliance with the protocol.
After passing all pre-screening examinations, candidates finally be able to receive therapeutic angiogenesis with autologous ADRCs transplantation. Details of the screening assessment required before enrolment and follow-up are provided in the protocol manuscript [17].
TACT-ADRC procedures
The TACT-ADRC procedures were performed as previously described in the protocol paper [17]. Briefly, about 300 mL volume of adipose tissue was harvested during standard manual liposuction using an aspiration syringe and cannula from the subcutaneous abdominal, breech and/or femoral regions under general or partial anesthesia [16]. ADRCs were separated and extracted from adipose tissue following the fully automated isolation steps using the Celution Systems (Cytori Therapeutics, Inc., Austin, TX, USA) [16] [18, 19]. The total cell number and their viability were measured using an automated cell counter (NucleoCounter NC-100, M&S TechnoSystems, Inc., Osaka, Japan) [16]. Subsequently, freshly isolated ADRCs were diluted with lactated Ringer’s solution up to a total volume of 50 mL, and the remaining 90% of the cells excluding 10% for the specimen storage were implanted into the ischemic muscles of the target limb using needle and syringe (Supplemental Fig. 1).
Endpoint measures
We assessed overall survival rate at several post-operative periods and at 6 months’ follow-up, any adverse events at post-operative period of ADRCs implantation procedure, development of malignant neoplasm during 6 months’ follow-up after the ADRCs implantation, and exacerbation of proliferative retinopathy or arthritis during 6 months’ follow-up after ADRCs implantation as the primary endpoints for the safety assessment [2]. In addition, the major amputation-free survival rate after 6 months’ follow-up following ADRC implantation was also evaluated for efficacy assessment [2] [16]. We also evaluated ischemic skin ulcer size, Quality of life (QOL) score using numerical rating scale (NRS), ankle-brachial pressure index (ABI), skin perfusion pressure (SPP), angiogenesis as assessed by digital subtraction angiography (DSA), 6-min walking distance as the secondary endpoints [16].
This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, domestic ethics guidelines issued by the Ministry of Health, Labour and Welfare in Japan, and the Clinical Trials Act in Japan.
All protocols were approved by the Nagoya University certified committee for regenerative medicine (#NA8150011) and the Tokai-Hokuriku Regional Bureau of Health and Welfare, Japan. This clinical trial is registered in the UMIN Clinical Trials Registry (#UMIN000010143) and the Japan Registry of Clinical Trials (#jRCTb040190118). All adverse events were reported to the committee based on the Japan Clinical Trial Registry (#jRCTb040190118) for verification of causality and discussion and approval of whether or not to continue the study.
Statistical analysis
Results are all expressed as mean ± SEM unless otherwise specified. The Shapiro–Wilk normality test was performed to evaluate data distribution. Homogeneity of variance was evaluated by a F test. Normally distributed data with 1 variable were analyzed by the paired Student’s t-test. The GraphPad Prism software version 8.0 (GraphPad Software Inc) was used. Values of p < 0.05 were considered statistically significant [20].
Results
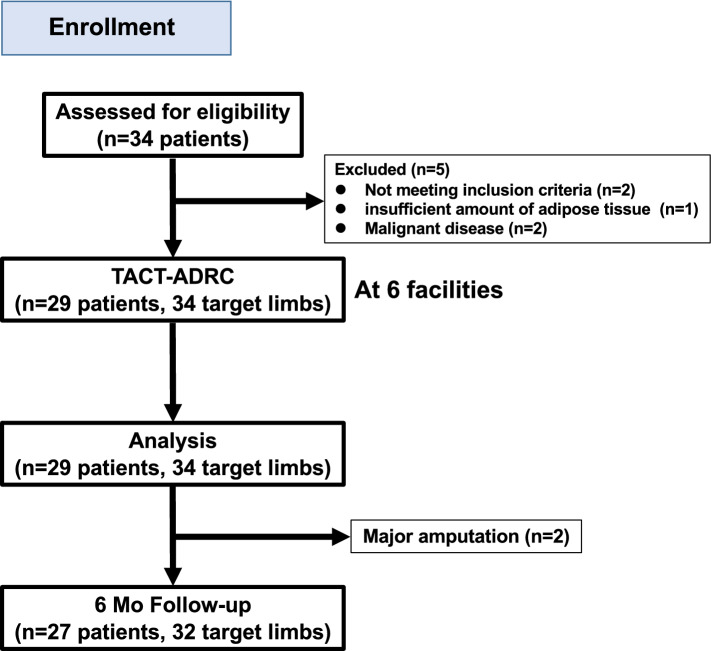
From June 8, 2016 through May 8, 2020, total of 34 CLI patients were registered into the study. Two patients who didn’t meet inclusion criteria (Age = > 80, current smoker), two patients who were found to have silent malignant diseases by the screening examination protocols, and one who had an insufficient amount of adipose tissue, were excluded. The remaining 29 patients with 34 target ischemic limbs were enrolled and received the TACT-ADRC procedures in 6 hospitals finally (Fig. 1). The average age was 57.9 years old, and male patients were 15 (51%). Mean BMI was 21.7 (minimum 14.1), indicating that this treatment was not necessarily administered only to obese patients. In terms of risk factors, the prevalence of hypertension, dyslipidemia, diabetes mellitus, and CKD was 41%, 52%, 31%, and 21%, respectively. Five patients (17%) were on dialysis due to end-stage renal disease. The details of the past history of conventional treatment and current medications are shown in Table 1.
Fig. 1.
Trial profile. TACT: therapeutic angiogenesis by cell transplantation, ADRC: adipose-derived regenerative cell
Table 1.
Patient characteristics at treatment
| All | ||
|---|---|---|
| Available data | (n = 29) | |
| Age, years | 29 | 57.9 (26–77) |
| Male, n (%) | 29 | 15 (51.7) |
| Body mass index, kg/m2 | 29 | 21.7 (14.1–35.0) |
|
Risk factors, n (%) HT |
29 | 12 (41.4) |
| DL | 29 | 15 (51.7) |
| DM | 29 | 9 (31.0) |
| insulin | 29 | 4 (13.8) |
| CKD | 29 | 6 (20.7) |
| HD | 29 | 5 (17.2) |
| ex-smoke | 29 | 17 ( 58.6) |
|
Complications, n (%) IHD |
29 | 6 (20.7) |
| CVD | 29 | 4 (13.8) |
| other vascular complication | 29 | 4 (13.8) |
| Past therapy, n (%) medication | 29 | 29 (100) |
| bypass | 29 | 6 (20.7) |
| EVT | 29 | 11 (37.9) |
|
Drugs, n (%) Antiplatelet drugs |
29 | 24 (82.8) |
| Peripheral vasodilator | 29 | 20 (70.0) |
| Antihypertensive agent | 29 | 12 (41.4) |
| statin | 29 | 14 (48.3) |
| Oral hypoglycemic agent | 29 | 5 (17.2) |
Data are presented as median (interquartile range) or number (percentage).
HT hypertension; DL dyslipidemia; DM diabetes mellitus; CKD chronic kidney disease; HD hemodialysis; IHD ischemic heart disease; CVD cerebrovascular disease; EVT endovascular treatment
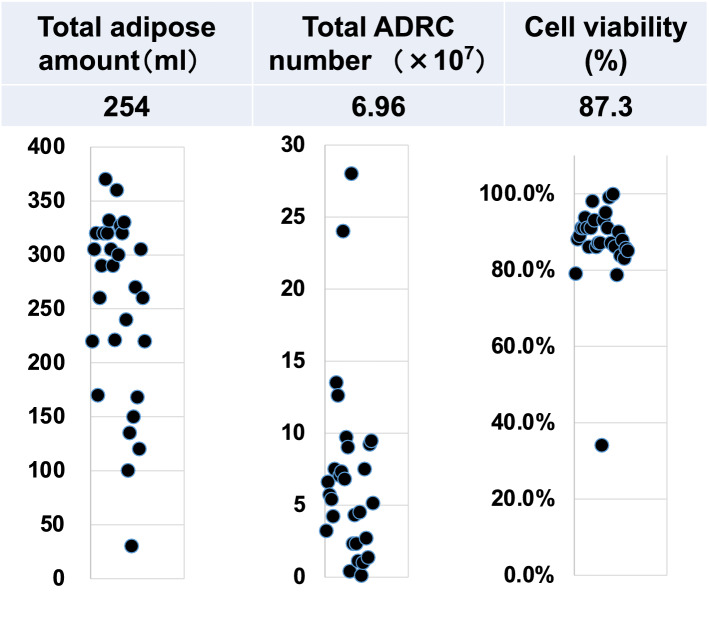
The breakdown of the underlying diseases that led to the ischemic limb was 7 patients (24%) in atherosclerotic PAD, 6 (21%) in TAO, and 16 (55%) in CTD (Fig. 2A). This treatment was administered to 11 upper limbs and 23 lower limbs (Fig. 2B). In the Fontaine class classification, 4 patients had 3rd degree and 25 patients had 4th degree (Fig. 2C). As a result of procedures, mean volume of harvested adipose tissue was 254 mL. The average total number of ADRCs was 6.96 × 107, cell viability was 87.3% (Fig. 3).
Fig. 2.
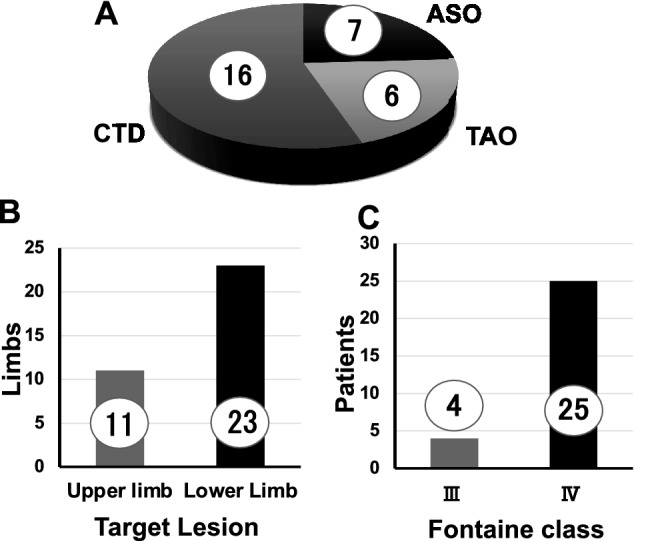
Patients’ characteristics (A) etiologies of critical limb ischemia and (B) target limbs, and (C) breakdown of the Fontaine class in participants
Fig. 3.
Procedural results. Figures indicate the quantity of total adipose tissue collection, the total cell number of ADRCs, and the cell viability rate, respectively, for all individuals
Outcomes measures
Twenty-seven patients (93%) except for 2 patients with major amputation, 32 target limbs (94%) completed the 6-month follow-up (Fig. 1). Although adverse events were observed in 5 cases; 2 anemias with blood transfusion, 1 relative adrenal crisis, 1 renal cancer, and 1 cerebral hemorrhage, all events were judged not to be associated with the TACT-ADRC procedure by the accreditation committee of an external organization.
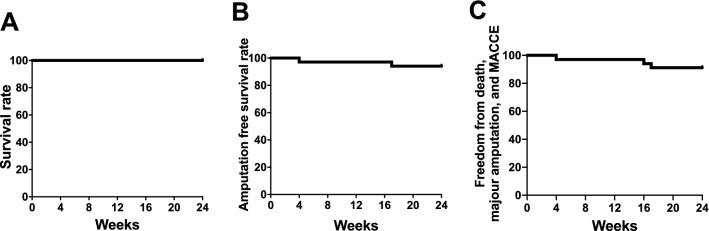
Primary endpoint
As a primary endpoint, the overall survival rate at a post-operative period and at 6 months follow-up was 100% at any time points (Fig. 4A). No adverse events at the operative procedures of ADRCs implantation were observed, and neither exacerbation of proliferative retinopathy nor arthritis was observed for 6 months. Although 1 case was observed the development of a malignant neoplasm (renal cancer) during 6 months follow-up, that was adjudicated not to be associated with the TACT-ADRC procedure by the accreditation committee.
Fig. 4.
Primary outcomes in terms of safety evaluation for the TACT-ADRC procedure. Figures indicate (A) survival rate from all-cause death, (B) evasion rate of major limb amputation, and (C) the composition of survival, the evasion of major limb amputation, and the freedom from MACCE. MACCE: major adverse cardiac and cerebrovascular events
As a primary endpoint for efficacy evaluation, 32 limbs out of 34 (94.1%) were free from major amputation for 6 months (Fig. 4B). Six of the patients with refractory ulcers or gangrene underwent debridement or minor toe amputation at the time of cell transplantation. Kaplan–Meier curve of freedom rate from death, major limb amputation, and major adverse cardiac and cerebrovascular event (MACCE) are shown in Fig. 4C.
Secondary endpoints
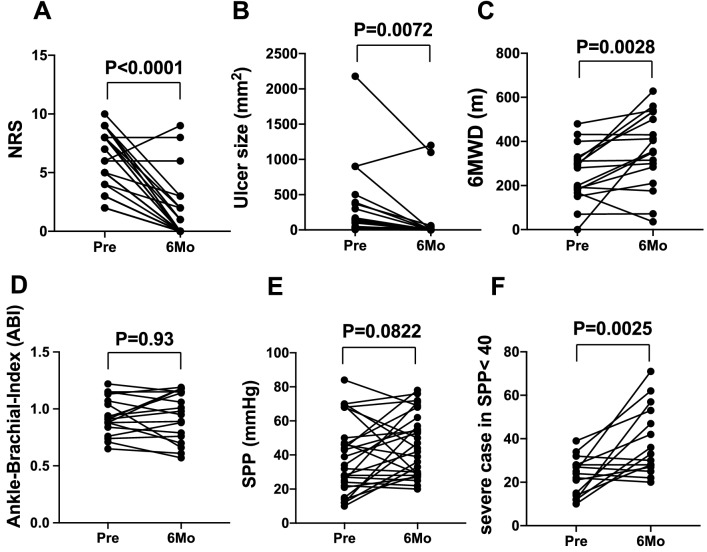
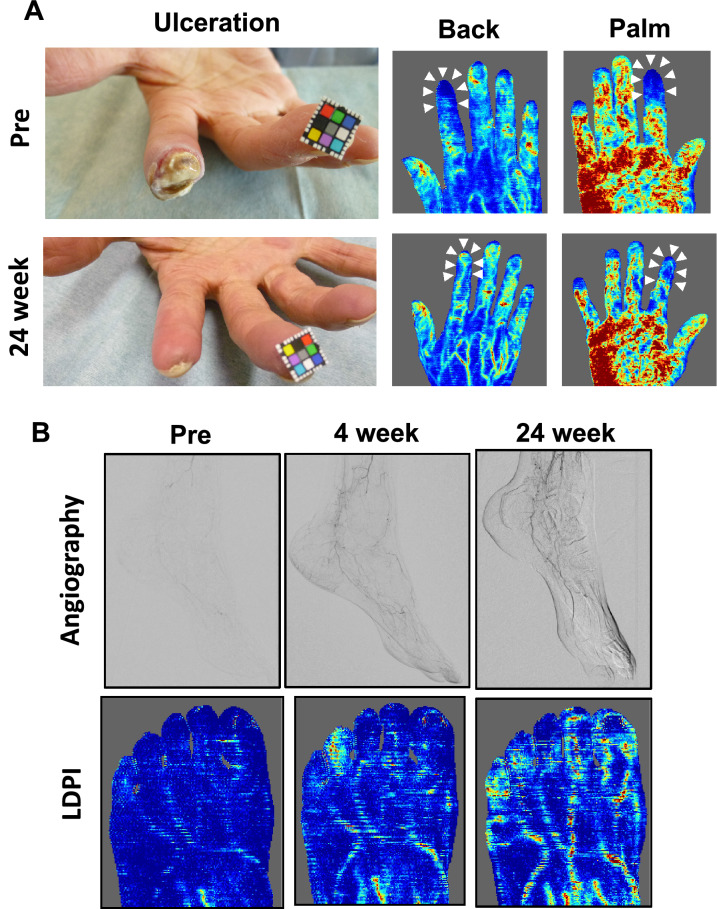
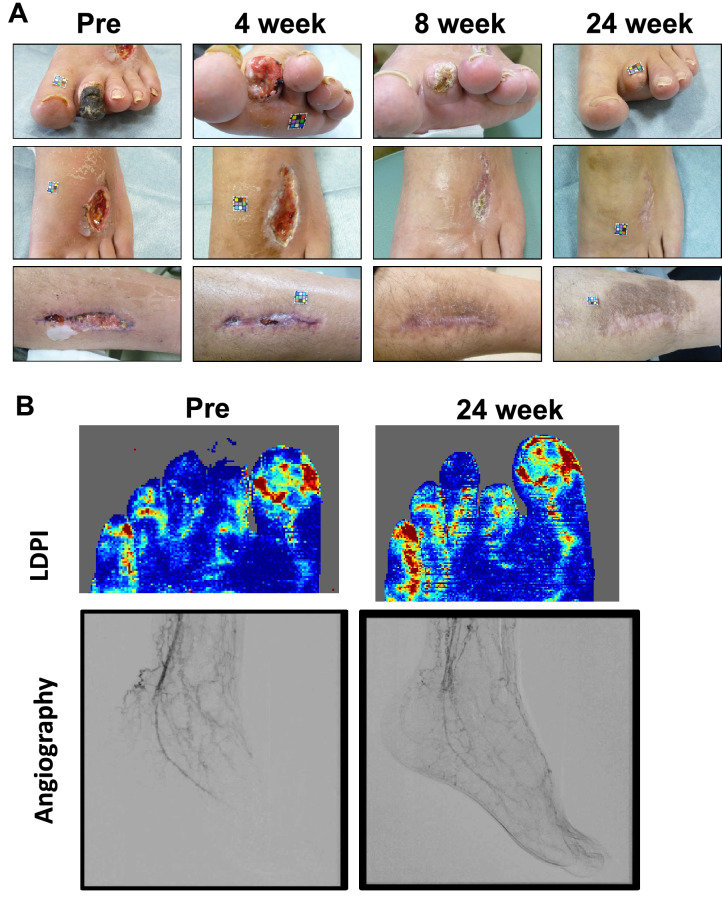
As the secondary endpoint for efficacy evaluation, the rest pain scale improved from 6 at the baseline to 1 at 6 months after the treatment in NRS (Fig. 5A). The responder rate was 90.6% for those patients (29 lesions out of 32). Ulcer size regressed 317 mm2 at baseline to 109 mm2 at 6 months (Fig. 5B). The representative images show a case of striking healing of ulcers after the ADRC implantation and the blood perfusion recovery (Figs. 6A and 7). The responder rate was 83.3% for those patients (20 lesions out of 24). The 6-min walking distance (6MWD) in patients having lower limb ischemia improved from 255 m at baseline to 369 m at 6 months after the treatment. The responder rate as judged by > 10 m increase in the 6MWD was 72.2% for patients with lower limb critical ischemia (13 limbs out of 18) (Fig. 5C). Although we could not confirm the improvement in the parameter of the Ankle-Brachial Index (ABI) at 6 months after the treatment (0.93 at baseline and 0.94 at 6 months) (Fig. 5D), SPP had a trend to be improved in those patients (38.8 at baseline and 46.1 at 6 months) (p = 0.0822) (Fig. 5E). When we analyzed only severe cases in SPP at baseline, such as < 40 mmHg, the TACT-ADRC protocol seemed to be effective to improve SPP (p = 0.0025) (Fig. 5F). Representative results of angiography in a case who had unhealed ulcer at baseline but had healed ulcer at 6 months of ADRC implantation are shown in Fig. 6B and Supplemental Fig. 2.
Fig. 5.
Secondary outcomes of the TACT-ADRC procedure. A Pain scale evaluated as per the NRS at the baseline and 6 months after ADRC implantation, (B) ulcer size was calculated as the grand total of major axis length times the minor axis length at the baseline and 6 months after ADRC implantation, (C) walking distance covered in 6-min for the patients with lower limb ischemia at the baseline and 6 months after ADRC implantation, (D) Tissue blood perfusion indicated by ABI, and (E) SPP in all cases, (F) SPP results only in severe cases as less than 40 mmHg at baseline. NRS numerical rating scale, 6 min walking distance: 6MWD, ABI ankle-brachial index, and SPP skin perfusion pressure
Fig. 6.
Time courses after ADRC implantation (A) improvements in non-healing ulcer and blood perfusion recovery in a case of the 63 years’ upper limb ischemia patient. B Laser Doppler blood perfusion analysis and angiography in the 74-years-old CLI patient due to CTD
Fig. 7.
The case of toe necrosis with multiple non-healing operative scars in a patient of TAO. All non-healing scars were completely healed (A), following the recovery of blood perfusion and angiographical improvement (B) at 24 weeks after ADRC implantation
Discussion
Critical limb ischemia (CLI) is one of the most severe forms of peripheral artery disease with symptoms of rest pain, unhealed skin ulcers, and gangrene of the extremities [21]. The rate of lower limb amputation in the first six months after diagnosis of CLI is as high as 10–40%, and limb amputation is associated with not only a decrease in quality of life but also poor life prognosis since the survival rate was reported to be estimated to 80% at 6 months, 50% at 5 years for those patients [22] [23] [24]. Although surgical or percutaneous catheter-mediated revascularization is the first-line treatment according to the guidelines [25, 26], it is often not effective in cases with peripheral lesions at below the knee, highly calcified lesions, or long lesion morphology. In cases to whom revascularization is impossible, lower limb amputation would be considered as a final strategy to save life.
The SPINACH trial [27], a landmark historical study, showed that the major amputation-free survival rate was 85% at 6 months after revascularization in patients with critical limb ischemia. In addition, less than 77% of patients were free from lower limb amputation and/or other interventions during the same period, suggesting that the number of patients who responded well to the treatment was very limited. Furthermore, the survival rate at 6 months after the treatment was less than 90%. In the present TACT-ADRC study, we have recruited more severe CLI cases as compared to those who were recruited in the SPINACH trial because our patients had no further option for the conventional revascularization therapy. Despite severer clinical background, the TACT-ADRC patients showed 94.1% in the major amputation-free rate at 6 months, and 100% of the survival rate at 6 months after the treatment. Since patients’ age and/or disease status might be different, we cannot make a direct comparison between the SPINACH and the TACT-ADRC trials. However, we strongly believe that our present study demonstrates a great potential as a novel additional therapy for CLI patients with no-option for revascularization.
Our study demonstrated a good survival rate as compared to past clinical studies. It is not clear, however, whether this treatment truly leads to an improvement in the total mortality or not. We believe that the life prognosis might be increased due to the avoidance of the amputation of lower limbs and thereby an increase in the level of daily activity such as walking. Previously, we reported that the TACT-bone marrow (BM) trial [2], that was an initial landmark study of therapeutic angiogenesis using autologous BM mononuclear cells, demonstrated a total mortality rate of 4% (2 deaths out of 47) in patients receiving autologous BM mononuclear cells (MNCs) implantation with the toe amputation salvage rate of 75% (15 out of 20 patients). More recently, the JUVENTAS trial [28], in which BM-MNCs were administered trans-arterially to the ischemic limb, reported that the 6-month total mortality rate in the intervention group was 5%, and the major amputation-free rate was 81%. Therefore, as comparing the past results of cell-based therapeutic angiogenesis, the total mortality rate of 0% at 6 months and the major limb amputation-free rate of 94.1% in the TACT-ADRC procedure would be a promising treatment strategy for CLI patients in the future.
In 2001, Zuk et al. reported that human subcutaneous adipose tissues contain progenitor cells with properties similar to those of BM-derived mesenchymal stem cells (MSCs), which were termed adipose-derived stem cells of ADRCs having an ability to differentiate into multiple cell types [9]. Subsequent studies demonstrated that ADRC can secrete multiple growth factors and chemokines such as VEGF, SDF-1, HGF, etc., promoting tissue regeneration in injured organs by a paracrine mechanism [10, 11, 13]. In addition, harvesting subcutaneous adipose tissue can be performed with established techniques such as liposuction under local or general anesthesia, and is considered to be a less invasive than bone marrow isolation in the clinical settings. Therefore, ADRCs are now expected to be a new source of somatic stem cells instead of bone marrow in the field of regenerative medicine. Furthermore, compared to embryonic stem (ES) cell [29] and induced pluripotent stem (iPS) cells [30], autologous cell transplantation is considered more feasible because these somatic stem cells have less ethical problems, and are less infectious and allergic, with free of immune rejection. These ADRCs are currently used in various regenerative medicine such as breast reconstruction, skin burns, and radiation skin injuries, urinary incontinence, osteoarthritis, and cardiac diseases. For example, three studies [14, 15] [16] have demonstrated that the therapeutic angiogenesis with ADRC implantation was safe and potentially effective for CLI patients. Two studies used cultured ADRCs for 15 and 7 CLI patients in a single center, respectively [14, 15]. Another pilot study from our group referred same protocol as the current TACT-ADRC trial to test initial 5 cases as a phase 1 trial [16]. Now, the present study demonstrated safety, feasibility, and efficacy with the freshly isolated ADRC to those patients in the 6 multicenter studies.
Since cell-based therapeutic angiogenesis may promote angiogenesis in not only ischemic limbs but also potential tumor-associated nutritional vessels [31], a systemic patient screening was performed in all cases before the TACT-ADRC. In addition, at 6 months after the ADRC treatment, the similar systemic screening tests were performed to examine whether any adverse events, including new malignancies or worsening of retinopathy, had occurred or not. As a result, we detected one new case of renal cancer during the 6-month post-treatment observation period. We consulted this case to the Accreditation Committee, and they determined that this event was unlikely to be related to the provision of regenerative strategy. In addition, our recent basic study has demonstrated that ADRC transplantation for a hind limb ischemia model in mice does not augment tumor-related angiogenesis or lymphangiogenesis for distant tumors, nor does it exacerbate tumor growth or metastasis [32]. Therefore, we consider that additional systemic screening tests for potential tumors will not be necessary for the TACT-ADRC protocols in the future.
There are several limitations in our current study. First, during the enrollment period, the pandemic of COVID-19 spread around the world [33], and the number of cases (which did not reach the original target of 40 patients) ended up being 29 patients and 34 target limbs. Second, although past cell therapies have suggested that treatment response may vary by disease, the small number of cases in this study alone does not allow comparison of the treatment outcomes by underlying disease (PAD, TAO and CTD). Future phase-3 trial would be warranted.
In conclusion, the TACT-ADRC multicenter clinical trial demonstrated the safety, feasibility, and effectiveness of autologous ADRCs implantation for therapeutic angiogenesis, resulting in an improvement in major amputation-free survival rates in no-option CLI patients in the multicenter settings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Medical institutions participating in the clinical trial implementation are as follows: Nagoya University Hospital, Kurume University Hospital, Kanazawa University Hospital, Dokkyo Medical University Hospital, St. Marianna University Hospital, Fukuoka Tokushukai Hospital, Shinshu University Hospital, Chiba University Hospital. The authors wish to express their sincere appreciation to all the patients, collaborating physicians, and other medical staff for their important contribution to the TACT-ADRC multicenter trial. TACT-ADRC multicenter trial co-investigators: Akio Kodama, Keisuke Takanari, Yuzuru Kamei, Kimihiro Komori (Nagoya University Graduate School of Medicine); Yuta Ishizaki, Takahiro Yoshikawa, Kensuke Kiyokawa, Hideaki Rikimaru, Hiroyuki Otsuka (Kurume University School of Medicine); Takashi Kudo, Hideki Shimomura (Fukuoka Tokushukai Medical Center); Yoshihide Fujimoto, Takashi Nakayama, Hideki Kitahara, Yoshitaka Kubota, Nobuyuki Mitsukawa, Shinsuke Akita (Chiba University Graduate School of Medicine), Soichiro Ebisawa (Shinshu University School of Medicine).
Abbreviations
- ABI
Ankle-brachial pressure index
- ADRC
Adipose-derived regenerative cell
- ASO
Arterio-sclerosis obliterans
- CLI
Critical limb ischemia
- CTD
Connective tissue disease
- NRS
Numerical rating scale
- SPP
Skin perfusion pressure
- TAO
Thromboangiitis obliterans
- QOL
Quality of life
Appendix
Toyoaki Murohara supervises the TACT-ADRC multicenter trial as the principal investigator.
The Study Monitoring Board
Kazumasa Unno from Japanese Red Cross Nagoya Daini Hospital, Nagoya, Japan.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YS, RH, K-iS, MO, OI, S-IT, MS, TN, and YY. The first draft of the manuscript was written by YSand TM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported, in part, by the Japanese Circulation Society Translational Research Grant 2010-13, Japan Agency for Medical Research and Development (AMED) 2014-15, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 26293184 to T.M.).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the Nagoya University (No. NA8150011).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
The members of the TACT-ADRC multicenter trial Group are listed in the Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuuki Shimizu, Email: shimi123@med.nagoya-u.ac.jp.
Toyoaki Murohara, Email: murohara@med.nagoya-u.ac.jp.
the TACT-ADRC multicenter trial Group:
Akio Kodama, Keisuke Takanari, Yuzuru Kamei, Kimihiro Komori, Yuta Ishizaki, Takahiro Yoshikawa, Kensuke Kiyokawa, Hideaki Rikimaru, Hiroyuki Otsuka, Takashi Kudo, Hideki Shimomura, Yoshihide Fujimoto, Takashi Nakayama, Hideki Kitahara, Yoshitaka Kubota, Nobuyuki Mitsukawa, Shinsuke Akita, and Soichiro Ebisawa
References
- 1.Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH et al: Corrigendum to 'Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia' [European Journal of Vascular & Endovascular Surgery 58/1S (2019) 1–109]. Eur J Vasc Endovasc Surg 2020, 59(3):492–493. [DOI] [PubMed]
- 2.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 3.Povsic TJ, Henry TD, Traverse JH, Fortuin FD, Schaer GL, Kereiakes DJ, Schatz RA, Zeiher AM, White CJ, Stewart DJ, et al. The RENEW trial: efficacy and safety of intramyocardial autologous CD34(+) Cell Administration in Patients With Refractory Angina. JACC Cardiovasc Interv. 2016;9(15):1576–1585. doi: 10.1016/j.jcin.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Mathur A, Fernandez-Aviles F, Bartunek J, Belmans A, Crea F, Dowlut S, Galinanes M, Good MC, Hartikainen J, Hauskeller C, et al. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J. 2020;41(38):3702–3710. doi: 10.1093/eurheartj/ehaa651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolli R, Perin EC, Willerson JT, Yang PC, Traverse JH, Henry TD, Pepine CJ, Mitrani RD, Hare JM, Murphy MP, et al. Allogeneic mesenchymal cell therapy in anthracycline-induced cardiomyopathy heart failure patients: the CCTRN SENECA Trial. JACC CardioOncol. 2020;2(4):581–595. doi: 10.1016/j.jaccao.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (therapeutic angiogenesis by cell transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156(5):1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Kajiguchi M, Kondo T, Izawa H, Kobayashi M, Yamamoto K, Shintani S, Numaguchi Y, Naoe T, Takamatsu J, Komori K, et al. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J. 2007;71(2):196–201. doi: 10.1253/circj.71.196. [DOI] [PubMed] [Google Scholar]
- 8.Kondo K, Yanishi K, Hayashida R, Shintani S, Shibata R, Murotani K, Ando M, Mizuno M, Fujiwara T, Murohara T, et al. Long-term clinical outcomes survey of bone marrow-derived cell therapy in critical limb ischemia in Japan. Circ J. 2018;82(4):1168–1178. doi: 10.1253/circj.CJ-17-0510. [DOI] [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 10.Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29(1):61–66. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 11.Hao C, Shintani S, Shimizu Y, Kondo K, Ishii M, Wu H, Murohara T. Therapeutic angiogenesis by autologous adipose-derived regenerative cells: comparison with bone marrow mononuclear cells. Am J Physiol Heart Circ Physiol. 2014;307(6):H869–879. doi: 10.1152/ajpheart.00310.2014. [DOI] [PubMed] [Google Scholar]
- 12.Pu Z, Shimizu Y, Tsuzuki K, Suzuki J, Hayashida R, Kondo K, Fujikawa Y, Unno K, Ohashi K, Takefuji M, et al. Important role of concomitant lymphangiogenesis for reparative angiogenesis in hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2021;41(6):2006–2018. doi: 10.1161/ATVBAHA.121.316191. [DOI] [PubMed] [Google Scholar]
- 13.Kato T, Kato K, Shimizu Y, Takefuji M, Murohara T. Treatment with adipose-derived regenerative cells enhances ischemia-induced angiogenesis via exosomal microRNA delivery in mice. Nagoya J Med Sci. 2021;83(3):465–476. doi: 10.18999/nagjms.83.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012;76(7):1750–1760. doi: 10.1253/circj.CJ-11-1135. [DOI] [PubMed] [Google Scholar]
- 15.Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA, Fleury S, Gadelorge M, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16(2):245–257. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Katagiri T, Kondo K, Shibata R, Hayashida R, Shintani S, Yamaguchi S, Shimizu Y, Unno K, Kikuchi R, Kodama A, et al. Therapeutic angiogenesis using autologous adipose-derived regenerative cells in patients with critical limb ischaemia in Japan: a clinical pilot study. Sci Rep. 2020;10(1):16045. doi: 10.1038/s41598-020-73096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu Y, Kondo K, Fukumoto Y, Takamura M, Inoue T, Nagata T, Akashi YJ, Yamada Y, Kuwahara K, Kobayashi Y, et al. Rationale and design of therapeutic angiogenesis by cell transplantation using adipose-derived regenerative cells in patients with critical limb ischemia- TACT-ADRC Multicenter Trial. Circ Rep. 2020;2(9):531–535. doi: 10.1253/circrep.CR-20-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin K, Matsubara Y, Masuda Y, Togashi K, Ohno T, Tamura T, Toyoshima Y, Sugimachi K, Toyoda M, Marc H, et al. Characterization of adipose tissue-derived cells isolated with the celution system. Cytotherapy. 2008;10(4):417–426. doi: 10.1080/14653240801982979. [DOI] [PubMed] [Google Scholar]
- 19.Aronowitz JA, Ellenhorn JDI. Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. Plast Reconstr Surg. 2013;132(6):932e–939e. doi: 10.1097/PRS.0b013e3182a80652. [DOI] [PubMed] [Google Scholar]
- 20.Tsuzuki K, Shimizu Y, Suzuki J, Pu Z, Yamaguchi S, Fujikawa Y, Kato K, Ohashi K, Takefuji M, Bando YK, et al. Adverse effect of circadian rhythm disorder on reparative angiogenesis in hind limb ischemia. J Am Heart Assoc. 2021;10(16):e020896. doi: 10.1161/JAHA.121.020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uccioli L, Meloni M, Izzo V, Giurato L, Merolla S, Gandini R. Critical limb ischemia: current challenges and future prospects. Vasc Health Risk Manag. 2018;14:63–74. doi: 10.2147/VHRM.S125065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB, Conte MS, Murad MH. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62(6):1642. doi: 10.1016/j.jvs.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 23.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Group TIW: Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007, 45 Suppl S:S5–67. [DOI] [PubMed]
- 24.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366(9501):1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 25.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American college of cardiology/American heart Association task force on clinical practice guidelines. Circulation. 2017;135(12):e726–e779. doi: 10.1161/CIR.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European stroke organization (ESO)the task force for the diagnosis and treatment of peripheral arterial diseases of the European society of cardiology (ESC) and of the European society for vascular surgery (ESVS) Eur Heart J. 2018;39(9):763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 27.Iida O, Takahara M, Soga Y, Kodama A, Terashi H, Azuma N, Investigators S. Three-year outcomes of surgical versus endovascular revascularization for critical limb ischemia: the SPINACH study (surgical reconstruction versus peripheral intervention in patients with critical limb ischemia) Circ Cardiovasc Interv. 2017;10(12):E005531. doi: 10.1161/CIRCINTERVENTIONS.117.005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teraa M, Sprengers RW, Schutgens RE, Slaper-Cortenbach IC, van der Graaf Y, Algra A, van der Tweel I, Doevendans PA, Mali WP, Moll FL, et al. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled rejuvenating endothelial progenitor cells via transcutaneous Intra-arterial supplementation (JUVENTAS) trial. Circulation. 2015;131(10):851–860. doi: 10.1161/CIRCULATIONAHA.114.012913. [DOI] [PubMed] [Google Scholar]
- 29.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Gimbrone MA, Jr, Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974;52(2):413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki J, Shimizu Y, Tsuzuki K, Pu Z, Narita S, Yamaguchi S, Katagiri T, Iwata E, Masutomi T, Fujikawa Y, et al. No influence on tumor growth by intramuscular injection of adipose-derived regenerative cells: safety evaluation of therapeutic angiogenesis with cell therapy. Am J Physiol Heart Circ Physiol. 2021;320(1):H447–H457. doi: 10.1152/ajpheart.00564.2020. [DOI] [PubMed] [Google Scholar]
- 33.Paules CI, Marston HD, Fauci AS. Coronavirus infections-more than just the common cold. JAMA. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.