Abstract
Introduction
Coronavirus disease 2019 (COVID-19) has been shown to affect outcomes among surgical patients. We hypothesized that COVID-19 would be linked to higher mortality and longer length of stay of trauma patients regardless of the injury severity score (ISS).
Methods
We performed a retrospective analysis of trauma registries from two level 1 trauma centers (suburban and urban) from March 1, 2019, to June 30, 2019, and March 1, 2020, to June 30, 2020, comparing baseline characteristics and cumulative adverse events. Data collected included ISS, demographics, and comorbidities. The primary outcome was time from hospitalization to in-hospital death. Outcomes during the height of the first New York COVID-19 wave were also compared with the same time frame in the prior year. Kaplan–Meier method with log-rank test and Cox proportional hazard models were used to compare outcomes.
Results
There were 1180 trauma patients admitted during the study period from March 2020 to June 2020. Of these, 596 were never tested for COVID-19 and were excluded from the analysis. A total of 148 COVID+ patients and 436 COVID− patients composed the 2020 cohort for analysis. Compared with the 2019 cohort, the 2020 cohort was older with more associated comorbidities, more adverse events, but lower ISS. Higher rates of historical hypertension, diabetes, neurologic events, and coagulopathy were found among COVID+ patients compared with COVID− patients. D-dimer and ferritin were unreliable indicators of COVID-19 severity; however, C-reactive protein levels were higher in COVID+ relative to COVID− patients. Patients who were COVID+ had a lower median ISS compared with COVID− patients, and COVID+ patients had higher rates of mortality and longer length of stay.
Conclusions
COVID+ trauma patients admitted to our two level 1 trauma centers had increased morbidity and mortality compared with admitted COVID− trauma patients despite age and lower ISS. C-reactive protein may play a role in monitoring COVID-19 activity in trauma patients. A better understanding of the physiological impact of COVID-19 on injured patients warrants further investigation.
Keywords: COVID-19, Outcomes, Trauma
Introduction
Globally, a staggering 248 million cases and 5 million deaths have occurred since severe acute respiratory syndrome coronavirus-2 spread worldwide in January of 2020.1 , 2 Coronavirus disease 2019 (COVID-19) affected all patient populations, including patients presenting to the hospital with traumatic injuries, adding an extra burden to patients’ health and possibly changing their outcomes. Data on COVID-19 and trauma patients are scarce with few published studies comparing outcomes between trauma patients with and without COVID-19.
Several studies have shown the deleterious effects of COVID-19 on patients with orthopedic trauma.3, 4, 5 Other studies on the impact of the pandemic on trauma centers and outcomes of trauma patients revealed an increased risk of mortality, longer length of stay [LOS], and pulmonary complications in COVID-19–positive (COVID-19+) patients, along with fewer traumatic blunt injury encounters.6 , 7 Increased complications have also been seen in other surgical COVID-19+ patients compared with COVID-19–negative (COVID-19−) patients; mainly pulmonary in nature, followed by multiorgan complications.8 To our knowledge, none of these studies identified a biochemical marker associated with the risk of worse outcomes for COVID-19+ trauma patients. In the present study, in addition to analysis of COVID-19+/COVID-19− trauma patient outcomes, we performed a further analysis on the usual inflammatory markers monitored during the management of COVID-19+ patients. We hypothesized that COVID-19+ trauma patients would have a higher mortality rate and LOS and that the use of inflammatory biomarkers could help guide the management of trauma patients with COVID-19.
Materials and Methods
Approval from our institutional review board was obtained, and a waiver of informed consent granted. We then performed a retrospective analysis of the prospectively accrued trauma registries of two level 1 trauma centers (one suburban and the other urban) from January 3, 2020, to June 30, 2020, and January 3, 2019, to June 30, 2019, following the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines. We compared outcomes and baseline characteristics of the 2020 cohort to trauma patients from the same period in 2019, demonstrating that the groups were comparable before and after the pandemic. Patients from 2020 were categorized into subgroups of COVID-19+ or COVID-19− based on diagnosis codes and COVID-19 testing results found in the electronic health record. Inflammatory biomarkers including C-reactive protein (CRP), D-dimer, and ferritin were interrogated to identify any patterns in COVID19+ versus COVID-19− injured patients. Comorbidity status was determined using Elixhauser's algorithm based on ICD-9-CM and ICD-10 diagnosis codes.9 , 10 R-package “comorbidity” (https://cran.r-project.org/) was applied to compute comorbidity indices.
Demographic characteristics were summarized by groups and presented using the median (interquartile range) or frequency (percentage) as appropriate. Continuous variables were assessed for normality using the Kolmogorov–Smirnov test, histogram, and Q-Q plot. The baseline characteristics were compared between groups using the Wilcoxon rank-sum test for continuous variables and chi-square test or Fisher's exact test for categorical variables.
Survival estimates were compared using the Kaplan–Meier method, and the log-rank test was used to compare the survival curves between the treatment groups. Adjusted models were fit using Cox proportional hazards; model selection was performed using a stepwise approach. Model fit was assessed using the Akaike information criterion. Proportional hazard assumptions were evaluated using graphical analysis, martingale residuals, and the Kolmogorov-Type Supremum test.11 , 12 Time to hospital discharge was also analyzed using similar techniques using death or end of follow-up as censored. A logistic regression model was used to determine the predictive ability of CRP for death. Area under the curve was computed, and Hosmer and Lemeshow test was used to assess the model fit. SAS 9.4 and R 4.0.4 were used to perform all analyses, and P values of ≤0.05 were considered to indicate statistical significance.
Results
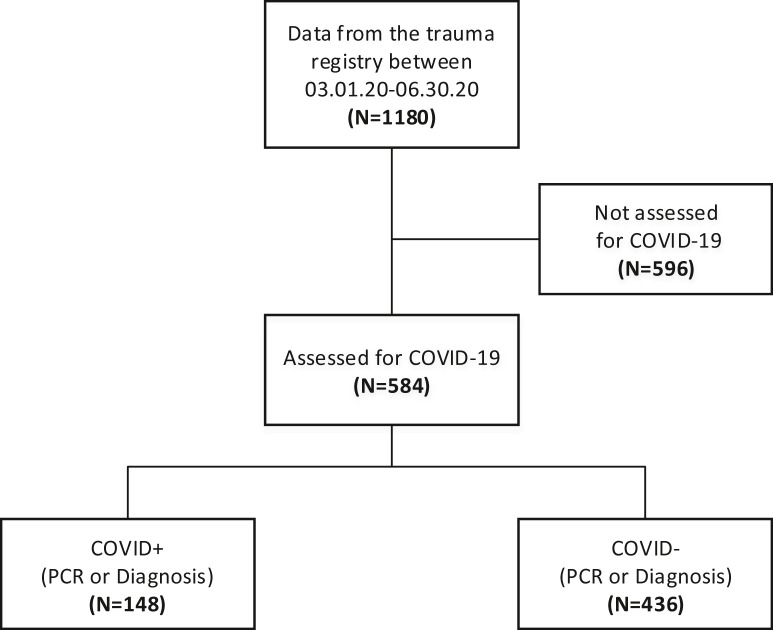
Figure 1 summarizes patient selection for the study. There were 1180 trauma patients admitted during the study period from March 1, 2020, to June 30, 2020. Of these, 596 were not tested for COVID-19 and were excluded from the study. The remaining 584 patients were divided into two arms: COVID-19+ (n = 148) and COVID-19− (n = 436).
Fig. 1.
Data flow showing how many patients were included and excluded from the database. Patients not assessed for COVID-19 were excluded.
To reduce errors, we compared trauma patients during the pandemic to patients before the pandemic, looking to identify any difference in injury severity score (ISS), mortality, and morbidities. When patients from 2020 were compared with those from the same period in 2019, patients in 2020 were older, with an average age of 76.8 y compared with 73.0 y (P < 0.001). Trauma patients in 2020 also had significantly more comorbidities: obesity, hypertension, diabetes, heart failure, renal failure, cardiac arrhythmias, coagulopathy, neurological disorders, and pulmonary circulation disorders.
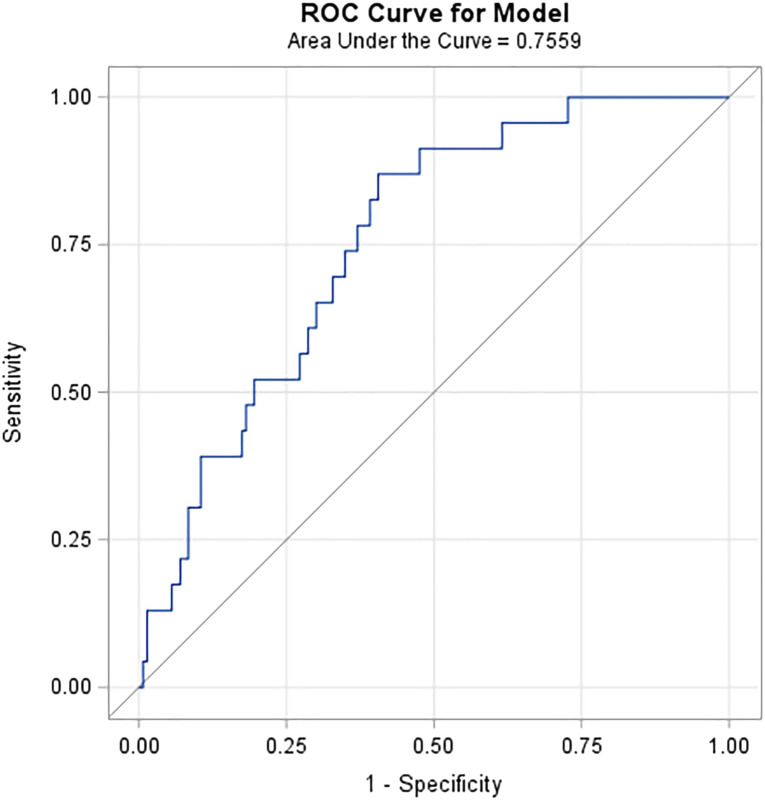
Table 1 compares demographics and clinical characteristics between the 2020 cohorts. COVID-19+ patients were significantly older (79.6 versus 74.7 y; P < 0.001) and presented with a lower ISS (4.0 versus 5.0; P < 0.001). COVID-19+ patients were also more likely to be admitted to the urban hospital setting than the suburban hospital. Notably, a significantly higher percentage of COVID-19+ patients had the following risk factors: obesity, hypertension, diabetes, heart failure, chronic pulmonary disease, renal failure, cardiac arrhythmias, coagulopathy, neurological disorders, and weighted Elixhauser comorbidity score. D-dimer, ferritin, CRP, and lactate dehydrogenase (LDH) were compared with significantly higher levels of CRP identified in COVID-19+ patients. In our sample, CRP significantly (P < 0.001) predicted death in trauma patients with moderate accuracy and area under the receiver operating characteristic curve of 0.76 (Fig. 2 ).
Table 1.
Demographics and clinical characteristics.
| Variable | COVID+ (N = 148) | COVID− (N = 436) | Overall (N = 584) | P value∗ | n |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (y) | 79.64 (67.7-87.0) | 74.7 (55.8-85.4) | 76.83 (58.6-86.3) | <0.001 | 584 |
| ISS | 4.0 (1.0-9.0) | 5.0 (4.0-10.0) | 5.0 (2.0-9.0) | <0.001 | 501 |
| Female gender | 74 (50.0%) | 232 (53.2%) | 306 (52.4%) | 0.499 | 584 |
| Race/ethnicity | 0.697 | 584 | |||
| White | 101 (68.2%) | 277 (63.5%) | 378 (64.7%) | ||
| Black | 11 (7.4%) | 37 (8.5%) | 48 (8.2%) | ||
| Hispanic | 17 (11.5%) | 51 (11.7%) | 68 (11.6%) | ||
| Asian/Pacific Islander | 9 (6.1%) | 25 (5.7%) | 34 (5.8%) | ||
| Other/Unknown | 10 (6.8%) | 46 (10.6%) | 56 (9.6%) | ||
| Current/former smoker | 53 (42.4%) | 156 (43.7%) | 209 (43.4%) | 0.801 | 482 |
| Current/former vaping | 7 (6.0%) | 19 (5.5%) | 26 (5.6%) | 0.847 | 462 |
| Urban versus suburban hospital | 75 (50.7%) | 160 (36.7%) | 235 (40.2%) | 0.003 | 584 |
| Comorbidities | |||||
| Alcohol use | 16 (10.9%) | 64 (14.7%) | 80 (13.7%) | 0.248 | 583 |
| Obese | 65 (44.2%) | 106 (24.3%) | 171 (29.3%) | <0.001 | 583 |
| HTN with complication | 72 (49.0%) | 119 (27.3%) | 191 (32.8%) | <0.001 | 583 |
| DM with complication | 42 (28.6%) | 70 (16.1%) | 112 (19.2%) | <0.001 | 583 |
| CHF | 52 (35.4%) | 92 (21.1%) | 144 (24.7%) | <0.001 | 583 |
| Chronic pulmonary disease | 49 (33.3%) | 94 (21.6%) | 143 (24.5%) | 0.004 | 583 |
| Renal failure | 57 (38.8%) | 97 (22.2%) | 154 (26.4%) | <0.001 | 583 |
| Liver disease | 19 (12.9%) | 44 (10.1%) | 63 (10.8%) | 0.339 | 583 |
| Blood loss anemia | 10 (6.8%) | 13 (3.0%) | 23 (3.9%) | 0.04 | 583 |
| Cardiac arrhythmias | 81 (55.1%) | 164 (37.6%) | 245 (42.0%) | <0.001 | 583 |
| Coagulopathy | 44 (29.9%) | 64 (14.7%) | 108 (18.5%) | <0.001 | 583 |
| Deficiency anemia | 38 (25.9%) | 42 (9.6%) | 80 (13.7%) | <0.001 | 583 |
| Drug abuse | 8 (5.4%) | 28 (6.4%) | 36 (6.2%) | 0.67 | 583 |
| Fluid and electrolyte disorders | 122 (83.0%) | 216 (49.5%) | 338 (58.0%) | <0.001 | 583 |
| Other neurological disorders | 55 (37.4%) | 105 (24.1%) | 160 (27.4%) | 0.002 | 583 |
| Paralysis | 6 (4.1%) | 28 (6.4%) | 34 (5.8%) | 0.295 | 583 |
| Pulmonary circulation disorders | 31 (21.1%) | 50 (11.5%) | 81 (13.9%) | 0.004 | 583 |
| Psychoses | 10 (6.8%) | 10 (2.3%) | 20 (3.4%) | 0.009 | 583 |
| Peripheral vascular disorders | 41 (27.9%) | 77 (17.7%) | 118 (20.2%) | 0.008 | 583 |
| Valvular disease | 33 (22.4%) | 92 (21.1%) | 125 (21.4%) | 0.731 | 583 |
| Weighted Elixhauser score (AHRQ) | 20.00 (10.0-31.0) | 10.0 (0.0-22.0) | 12.0 (1.0-25.0) | <0.001 | 583 |
| Biomarkers† | |||||
| D-dimer (ng/mL DDU) | 575 (352-1668) | 632 (264-1840) | 620 (299-1767) | 0.770 | 149 |
| Ferritin (ng/mL) | 291 (130-648) | 212 (96-382) | 269 (115-512) | 0.068 | 173 |
| CRP (mg/L) | 66 (17-107) | 25 (9-84) | 54 (11-104) | 0.028 | 166 |
| LDH (U/L) | 259 (212-359) | 248 (205-308) | 252 (207-230) | 0.146 | 261 |
CHF = congestive heart failure; CRP = C-reactive protein; DM = diabetes mellitus; HTN = hypertension; ISS = Injury Severity Score; LDH = lactated dehydrogenase.
P values are from Wilcoxon rank-sum test for continuous variables and chi-square or Fisher's exact test for categorical variables.
Presented values are area under the curve computed using all available measurements for each patient.
Fig. 2.
Predictive ability of CRP for mortality.
The primary endpoint was time from admission to in-hospital death, assuming hospital discharge or end of follow-up as censored. Table 2 shows that of 148 COVID-19+ trauma patients, only 37 of them were discharged within 40 d.
Table 2.
Death censored time to discharge Kaplan–Meier failure curves.
| Summary of the number of censored and uncensored values | |||||
|---|---|---|---|---|---|
| Stratum | Group | Total | Failed | Censored | Percent censored |
| 1 | COVID | 148 | 111 | 37 | 25.00 |
| 2 | Non-COVID | 436 | 406 | 30 | 6.88 |
| Total | 584 | 517 | 67 | 11.47 | |
Censored: (46 death + 20 were not discharged before June 25, 2020).
Multivariable analysis using Cox proportional hazard regression revealed a significantly higher risk of death in COVID-19+ trauma patients compared with those who were COVID-19− (hazard ratio [95% confidence interval] = 2.7 [1.5-5.2]; P = 0.002; Table 3 ). In addition to COVID-19 status, age, coagulopathy, and other neurological disorders were independently associated with higher risk of mortality. Similarly, multivariable analysis demonstrated that the COVID-19+ patients are less likely to get discharged (hazard ratio [95% confidence interval] = 0.63 [0.49-0.80]; P < 0.001), that is, they spend longer time in the hospital (Table 4 ). The significant factors influencing longer discharge time in COVID-19+ trauma patients were congestive heart failure, fluid/electrolyte disorders, neurological disorders, and pulmonary circulation disorders (Table 5 ).
Table 3.
Unadjusted and adjusted hazard ratios for “time to death” estimated via Cox proportional hazard regression models.
| Variable | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI)† | P value |
|---|---|---|---|---|
| COVID positive versus negative∗ | 3.61 (1.91-6.83) | <0.0001 | 2.74 (1.45-5.17) | 0.002 |
| Age | 1.03 (1.01-1.05) | 0.007 | 1.04 (1.01-1.06) | 0.004 |
| Urban versus suburban hospital | 1.35 (0.76-2.4) | 0.306 | --- | --- |
| ISS | 0.97 (0.92-1.02) | 0.226 | --- | --- |
| Obesity | 1.85 (1.04-3.0) | 0.036 | --- | --- |
| Hypertension w/ complication | 1.87 (1.03-3.38) | 0.039 | --- | --- |
| Diabetes w/ complication | 1.22 (0.67-2.25) | 0.514 | --- | --- |
| Congestive heart failure | 2.07 (1.16-3.68) | 0.013 | --- | --- |
| Renal failure | 1.27 (0.71-2.27) | 0.43 | --- | --- |
| Blood loss anemia | 0.73 (0.18-3.03) | 0.667 | --- | --- |
| Cardiac arrhythmias | 1.86 (1.01-3.45) | 0.048 | --- | --- |
| Coagulopathy | 2.70 (1.51-4.8) | <0.001 | 2.40 (1.33-4.32) | 0.004 |
| Deficiency anemia | 1.49 (0.77-2.88) | 0.234 | --- | --- |
| Depression | 0.91 (0.49-1.67) | 0.754 | --- | --- |
| Fluid and electrolyte disorders | 3.1 (1.10-8.72) | 0.032 | --- | --- |
| Other neurological disorders | 2.85 (1.57-5.18) | <0.001 | 2.67 (1.45-4.92) | 0.002 |
| Pulmonary circulation disorders | 2.0 (1.10-3.62) | 0.023 | --- | --- |
| Psychoses | 1.99 (0.71-5.55) | 0.191 | --- | --- |
| Peripheral vascular disorders | 1.49 (0.82-2.70) | 0.194 | --- | --- |
| Weight loss | 1.10 (0.60-2.03) | 0.758 | --- | --- |
CI = confidence interval; HR = hazard ratio.
Patients were assumed COVID+ based on a positive PCR test or COVID diagnosis.
Please note our main purpose was to evaluate if the COVID status significantly affects death in trauma patients. So, only characteristics with P values <0.25 comparing COVID+ and COVID− groups (Table 1) were considered for the multivariable model. Factors with P value ≥0.25 were deemed irrelevant in this context.
Table 4.
Unadjusted and adjusted hazard ratios for “time to discharge” estimated via Cox proportional hazard regression models.
| Variable | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI)∗ | P value |
|---|---|---|---|---|
| COVID positive versus negative | 0.60 (0.48-0.74) | <0.0001 | 0.63 (0.49-0.80) | <0.001 |
| Age | 0.99 (0.988-0.996) | <0.001 | --- | --- |
| Urban versus suburban hospital | 1.06 (0.89-1.27) | 0.491 | --- | --- |
| ISS | 0.98 (0.96-0.990) | 0.002 | 0.97 (0.95-0.98) | <0.0001 |
| Obesity | 0.88 (0.73-1.07) | 0.187 | --- | --- |
| Hypertension w/ complication | 0.67 (0.56-0.81) | <0.0001 | --- | --- |
| Diabetes w/ complication | 0.73 (0.58-0.92) | 0.007 | --- | --- |
| Congestive heart failure | 0.64 (0.52-0.79) | <0.0001 | 0.72 (0.57-0.92) | 0.008 |
| Renal failure | 0.72 (0.59-0.88) | 0.001 | --- | --- |
| Blood loss anemia | 0.86 (0.55-1.33) | 0.491 | --- | --- |
| Cardiac arrhythmias | 0.71 (0.60-0.85) | <0.001 | --- | --- |
| Coagulopathy | 0.64 (0.50-0.81) | <0.001 | --- | --- |
| Deficiency anemia | 0.80 (0.62-1.04) | 0.096 | --- | --- |
| Depression | 0.81 (0.66-0.99) | 0.037 | --- | --- |
| Fluid and electrolyte disorders | 0.51 (0.43-0.61) | <0.0001 | 0.58 (0.48-0.71) | <0.0001 |
| Other neurological disorders | 0.64 (0.52-0.78) | <0.0001 | 0.69 (0.55-0.87) | 0.002 |
| Pulmonary circulation disorders | 0.55 (0.42-0.72) | <0.0001 | 0.69 (0.51-0.95) | 0.022 |
| Psychoses | 0.79 (0.48-1.30) | 0.36 | --- | --- |
| Peripheral vascular disorders | 0.69 (0.55-0.85) | <0.001 | --- | --- |
| Weight loss | 0.62 (0.49-0.78) | <0.0001 | 0.73 (0.56-0.95) | 0.019 |
Please note our main purpose was to evaluate if the COVID status significantly affects death in trauma patients. So, only characteristics with P values <0.25 comparing COVID+ and COVID− groups (Table 1) were considered for the multivariable model. Factors with P value ≥0.25 were deemed irrelevant in this context.
Table 5.
Demographics and clinical characteristics between 2019 and 2020 patients.
| Variable | Year 2019 (n = 759) | Year 2020 (n = 584) | P value∗ |
|---|---|---|---|
| Demographics | |||
| Age (y) | 73.0 (50.8-84.8) | 76.8 (58.6-86.3) | 0.001 |
| Female gender | 392 (51.6%) | 306 (52.4%) | 0.785 |
| Race/ethnicity | <0.0001 | ||
| White | 553 (72.9%) | 378 (64.7%) | |
| Black | 56 (7.4%) | 48 (8.2%) | |
| Hispanic | † | 68 (11.6%) | |
| Asian/Pacific Islander | 43 (5.7%) | 34 (5.8%) | |
| Other/unknown | 107 (14.1%) | 56 (9.6%) | |
| Comorbidities | |||
| Alcohol use | 69 (9.2%) | 80 (13.7%) | 0.01 |
| Obese | 137 (18.3%) | 171 (29.3%) | <0.001 |
| HTN with complication | 163 (21.8%) | 191 (32.8%) | <0.001 |
| DM with complication | 106 (14.2%) | 112 (19.2%) | 0.014 |
| CHF | 128 (17.1%) | 144 (24.7%) | <0.001 |
| Chronic pulmonary disease | 138 (18.4%) | 143 (24.5%) | 0.007 |
| Renal failure | 117 (15.6%) | 154 (26.4%) | <0.001 |
| Liver disease | 44 (5.9%) | 63 (10.8%) | 0.001 |
| Blood loss anemia | 16 (2.1%) | 23 (3.9%) | 0.053 |
| Cardiac arrhythmias | 244 (32.6%) | 245 (42.0%) | <0.001 |
| Coagulopathy | 62 (8.3%) | 108 (18.5%) | <0.001 |
| Deficiency anemia | 67 (9.0%) | 80 (13.7%) | 0.006 |
| Depression | 146 (19.5%) | 146 (25.0%) | 0.016 |
| Drug abuse | 44 (5.9%) | 36 (6.2%) | 0.824 |
| Fluid and electrolyte disorders | 292 (39.0%) | 338 (58.0%) | <0.001 |
| Other neurological disorders | 108 (14.4%) | 160 (27.4%) | <0.001 |
| Paralysis | 29 (3.9%) | 34 (5.8%) | 0.096 |
| Pulmonary circulation disorders | 57 (7.6%) | 81 (13.9%) | <0.001 |
| Psychoses | 8 (1.1%) | 20 (3.4%) | 0.003 |
| Peripheral vascular disorders | 107 (14.3%) | 118 (20.2%) | 0.004 |
| Weighted Elixhauser score (AHRQ) | 5.0 (0.0-15.0) | 12.0 (1.0-25.0) | <0.001 |
CHF = congestive heart failure; DM = diabetes mellitus; HTN = hypertension.
P values are from Wilcoxon rank-sum test for continuous variables, and chi-square or Fisher's exact test for categorical variables.
Information on race/ethnicity category Hispanic not available in 2019 data.
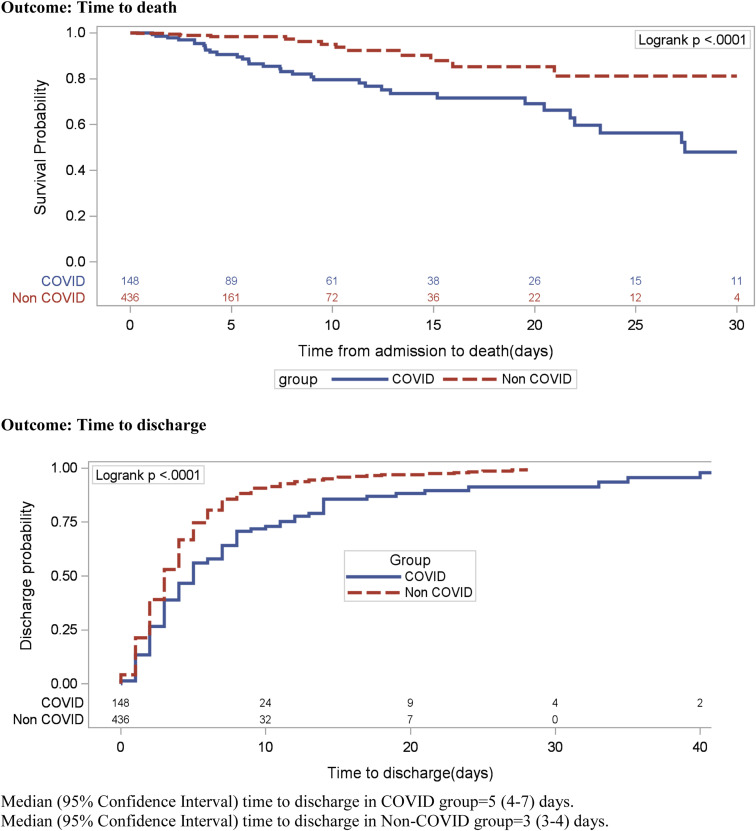
COVID-19+ patients had a lower overall survival probability than COVID-19− patients (Fig. 3 ). Similarly, COVID-19+ patients had longer discharge probability with a longer time to discharge (median [IQR] = 5 [4-7] versus 3 [3-4] d) compared with COVID-19− patients (Fig. 3).
Fig. 3.
Comparing Kaplan–Meier survival curves for time to death and time to discharge between groups.
Discussion
In this study, we were able to demonstrate increased mortality in COVID-19+ patients, our primary endpoint, despite a lower ISS compared with the COVID−cohort. We also reported that trauma patients with a diagnosis of COVID-19 requiring a hospital admission could have longer hospital LOS. One major contributing factor was the need for negative COVID-19 polymerase chain reaction (PCR) results for discharge of our trauma patients to a rehabilitation center, with many of the recovering trauma patients showing no symptoms of COVID-19 continuing to test positive, prolonging their hospital stay.
After the first reported case of COVID-19 in the United States, new protocols were developed by the health care system to diagnose patients presenting with COVID-19 symptoms. Diagnosis included the PCR, radiographic imaging such as chest computed tomography imaging, and a high clinical suspicion of COVID-19 after exclusion of other causes.13 In New York City, a rapid increase in patients was attributed to factors such as dense population and social practices. The health care system was unprepared for the vast number of patients presenting with COVID-19 respiratory symptoms. Because of limited available personnel, facilities, disposable equipment, and finite severe acute respiratory syndrome coronavirus-2 testing technology, only selective COVID-19 PCR testing was implemented early in the pandemic. Therefore, only 580 of 1180 patients were tested for COVID-19 in our study (Fig. 1).14 On admission, we used high clinical suspicion coupled with findings on routine trauma chest x-rays and chest computed tomography scans (if obtained) to selectively test and diagnose for COVID-19. This practice was consistent across campuses. As tests became more available however, we routinely tested every trauma patient admitted to the institution.
Older adult patients with COVID-19 are at a higher risk of rapid progressive deterioration in comparison to younger patients.15 In our study, worse outcome of the trauma patient was independent of age and correlated with the presence of COVID-19 disease. When we controlled for age and ISS, COVID-19+ patients had worse outcomes.16, 17 Surprisingly, in our study, patients who were COVID-19− had a higher ISS but still had significantly better outcomes. These associations could be explained by the systemic inflammatory response in COVID-19+ patients, leading to multiorgan dysfunction, potentially responsible for worse outcomes.
Currently, the Centers for Disease Control and Prevention report a nearly similar positive COVID-19 caseload between different ethnicities in the United States.18 In our study, one-third of each ethnicity tested for COVID-19 returned positive, echoing similar results to that published by the Centers for Disease Control and Prevention. The ratio of COVID-19− patients was higher in the suburban trauma centers, perhaps a reflection of the lower population density, which may allow for decreased contact spread.
Systemic coagulopathy is now a recognized manifestation of COVID-19 illness.19 When combined with the coagulopathy of trauma, systemic coagulopathy may complicate the management of those patients. Patients with contraindications to chemoprophylaxis, such as patients with traumatic brain injuries, patients undergoing major procedures that require holding anticoagulation, or patients with active bleeding are at increased risk.20 At the time of our data collection, there were no standardized guidelines for prophylactic anticoagulant dosing in COVID-19+ trauma patients. At our institution, we implemented a process that relied on the level of D-dimer in those patients. However, elevation of D-dimer levels is a known sequelae of trauma, and it is unclear what the impact of the presence of COVID-19 had on these values in the setting of trauma.21, 22, 23
Inflammatory markers were recognized early in the pandemic as methods by which COVID-19+ patients could be managed. Biomarkers such as LDH, CRP, ferritin, and D-dimer were routinely measured in that population. Our data demonstrate an association with CRP and COVID-19+ trauma patients and outcomes. Similarly, D-dimer levels have been used as indicators for initiation of anticoagulation. D-dimer is a useful marker for inflammation, clot breakdown, and blood vessel damage.24 D-dimer levels are routinely checked in COVID-19+ patients to assess their coagulopathy and disease severity, aiding in their management. However, D-dimer could also be elevated due to traumatic injury, minimizing its usefulness in assessing COVID-19 severity and coagulopathy in trauma patients.25, 26 Ferritin and LDH are other inflammatory markers that are both elevated due to trauma and COVID-19. In our population, COVID-19+ patients had significantly elevated CRP levels compared with patients who were COVID-19−. This finding persisted in analysis, suggesting that COVID-19+ injured patients could have their outcomes predicted by following the CRP trend. Understanding how this could influence care requires assessing a larger population in a controlled setting. Although CRP is elevated in traumatic injury, our COVID-19+ patients’ CRP levels were significantly elevated in comparison to COVID-19− patients, suggesting utility as a prognostic tool.27
This is a prospectively collected retrospective review of a patient population with inherent limitations. In this model, the outcome is subject to confounding bias unlike a prospective randomized study. Because the data were also reviewed retrospectively, some data were missed or unmeasured, further compounding bias. Another limitation is the restricted COVID-19 testing at the start of the pandemic due to limited testing availability and different protocols that stated only patients with symptoms were to be tested. It is possible that some patients may have been positive with the virus while remaining asymptomatic. It is therefore impossible to know if this led to an underestimation of COVID-19+ patients, thereby magnifying the impact of the virus on outcomes. Based on subsequent experience, we do not believe this to be likely however.
Conclusions
COVID-19+ trauma patients admitted to our two level 1 trauma centers had increased morbidity and mortality compared with admitted COVID-trauma patients despite age and lower ISS. CRP may play a role in monitoring COVID-19 activity in trauma patients. A better understanding of the physiologic impact of COVID-19 on injured patients warrants further investigation.
Author Contributions
D.K.J., P.P., and G.B. contributed to design. H.H. and N.G. contributed to data acquisition. S.I. and J.D. contributed to statistical analysis. H.H., A.S., A.H.S., R.J., A.S., and L.V. drafted the article. D.K.J., S.I., and P.P. contributed to supervision. H.H., S.I., P.P., A.S., G.B., A.H.S., N.G., R.J., A.S., L.V., J.D., and D.K.J. contributed to final approval of the article.
Disclosure
None declared.
Funding
None.
Meeting Presentation
This work was presented as a Poster at the 80th Annual Meeting of the American Association for the Surgery of Trauma. Atlanta, GA. September 29 to October 2, 2021.
References
- 1.Cdc COVID data tracker weekly review. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html Available at:
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A., Haider Y., Passey J., Khan R., Gaba S., Kumar M. Mortality predictors in covid-19 positive patients with fractures: a systematic review. Bull Emerg Trauma. 2021;9:51–59. doi: 10.30476/BEAT.2021.87742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Farii H., Al Rawahi S., Samaila E., Lavini F., Magnan B., Al Maskari S. Thirty-day mortality in COVID-19 positive patients with hip fractures: a case-series and literature review. Geriatr Orthop Surg Rehabil. 2020;11 doi: 10.1177/2151459320972681. 2151459320972681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patralekh M.K., Jain V.K., Iyengar K.P., Upadhyaya G.K., Vaishya R. Mortality escalates in patients of proximal femoral fractures with COVID-19: a systematic review and meta-analysis of 35 studies on 4255 patients. J Clin Orthop Trauma. 2021;18:80–93. doi: 10.1016/j.jcot.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman E.J., Ong A.W., Cipolle M.D., et al. The impact of COVID-19 infection on outcomes after injury in a state trauma system. J Trauma Acute Care Surg. 2021;91:559–565. doi: 10.1097/TA.0000000000003310. [DOI] [PubMed] [Google Scholar]
- 7.Sheets N.W., Fawibe O.S., Mahmoud A., Chawla-Kondal B., Ayutyanont N., Plurad D.S. Impact of the COVID-19 pandemic on trauma encounters. Am Surg. 2021;4 doi: 10.1177/00031348211029858. 31348211029858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.COVID Surg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Quan H., Sundararajan V., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 11.Lin D.Y., Wei L.J., Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 12.Wei L.J. Testing goodness of fit for proportional hazards model with censored observations. J Am Stat Assoc. 1984;79:649–652. [Google Scholar]
- 13.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadi N., Khelfaoui M. Population density, a factor in the spread of COVID-19 in Algeria: statistic study. Bull Natl Res Cent. 2020;44:138. doi: 10.1186/s42269-020-00393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrotta F., Corbi G., Mazzeo G., et al. COVID-19 and the elderly: insights into pathogenesis and clinical decision-making. Aging Clin Exp Res. 2020;32:1599–1608. doi: 10.1007/s40520-020-01631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulger E.M., Arneson M.A., Mock C.N., Jurkovich G.J. Rib fractures in the elderly. J Trauma. 2000;48:1040–1047. doi: 10.1097/00005373-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Witt C.E., Bulger E.M. Comprehensive approach to the management of the patient with multiple rib fractures: a review and introduction of a bundled rib fracture management protocol. Trauma Surg Acute Care Open. 2017;2:e000064. doi: 10.1136/tsaco-2016-000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COVID-19 hospitalization and death by race/ethnicity. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html Available at:
- 19.Biffl W.L., Harrington D.T., Majercik S.D., Starring J., Cioffi W.G. The evolution of trauma care at a level I trauma center. J Am Coll Surg. 2005;200:922–929. doi: 10.1016/j.jamcollsurg.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Maegele M., Schöchl H., Cohen M.J. An update on the coagulopathy of trauma. Shock. 2014;41(Suppl 1):21–25. doi: 10.1097/SHK.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 21.Becker R.C. COVID-19 update: covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosse C., Grosse A., Salzer H.J.F., Dünser M.W., Motz R., Langer R. Analysis of cardiopulmonary findings in COVID-19 fatalities: high incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. 2020;49:107263. doi: 10.1016/j.carpath.2020.107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Paassen J., Vos J.S., Hoekstra E.M., Neumann K.M.I., Boot P.C., Arbous S.M. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24:696. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchhof M.G., Lee A.Y., Dutz J.P. D-dimer levels as a marker of cutaneous disease activity: case reports of cutaneous polyarteritis nodosa and atypical recurrent urticaria. JAMA Dermatol. 2014;150:880–884. doi: 10.1001/jamadermatol.2013.9944. [DOI] [PubMed] [Google Scholar]
- 26.Johna S., Cemaj S., O'Callaghan T., Catalano R. Effect of tissue injury on D-Dimer levels: a prospective study in trauma patients. Med Sci Monit. 2002;8:CR5–CR8. [PubMed] [Google Scholar]
- 27.Zhang L., Yan X., Fan Q., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]