Abstract
The finding of Trichinella in the Arctic was foreseen because captive polar bears and arctic foxes had been found infected during the first decades of the 20th century. Human trichinellosis outbreaks were reported to have taken place in 1944 in Franz Josef Archipelago and 1947 in Greenland, and previous outbreaks in Greenland also appeared to have been trichinellosis. Now, it is known that Trichinella parasites thrive in the Arctic and subarctic and pose a risk for public health. We collated the available information, which show that infection prevalences are high in many animal host species, and that outbreaks of human trichinellosis have been described also recently. The species diversity of Trichinella in the Arctic and subarctic is relatively high, and the circulation is in non-domestic cycles with transmission by predation, scavenging and cannibalism. There are also sporadic reports on the synanthropic species Trichinella spiralis in arctic wild mammals with little known or assumed contact to potential synanthropic cycles. In this paper, we summarize the knowledge on epidemiology of Trichinella parasites in the circumpolar Arctic and subarctic regions, and discuss the challenges and solutions for their control.
Keywords: Arctic, Subarctic, Epidemiology, Trichinella, Zoonosis
Graphical abstract
Highlights
-
•
Trichinella infection is common in wild animals in the Arctic and subarctic regions.
-
•
The high prevalence of Trichinella infection in some arctic marine mammal species suggests a marine cycle.
-
•
Outbreaks of human trichinellosis have been described, and public health importance still remains obvious.
-
•
In this review, we had access to the large amount of Trichinella literature published in the Russian language.
1. Introduction
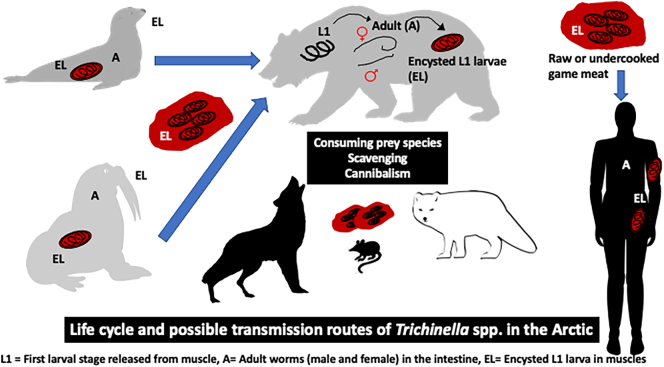
The parasitic genus Trichinella (Nematoda: Trichinellidae) includes 10 species and 3 nameless but recognized and numbered genotypes in carnivores and omnivores, especially carrion feeders (Zarlenga et al., 2020; Sharma et al., 2020). The adults are short-lived in the small intestine of the host, while the majority of the Trichinella biomass and individuals consists of muscle larvae (L1). After ingestion, the larvae are released from the muscle tissue and develop into adult worms in the gastric mucosa within 48 h. The newborn larvae are released by the adult female and migrate into the striated musculature of the host, where they penetrate the muscle cell, encapsulate and wait for the muscle to be ingested by the next host, after which the larvae mature. The life cycle and the possible transmission routes in the Arctic are summarized in Fig. 1. In addition, there are species whose L1 do not encapsulate. Currently, there are seven encapsulating species recognized (Trichinella spiralis, Trichinella nativa, Trichinella britovi, Trichinella murrelli, Trichinella nelsoni, Trichinella patagoniensis and Trichinella chanchalensis), and three numbered genotypes with no name (Trichinella T6, T8, T9). Also, three non-encapsulating species are known (Trichinella pseudospiralis, Trichinella papuae and Trichinella zimbabwensis), and they are, in contrast to the encapsulating species, infective also to non-mammalian vertebrates (birds and reptiles). Current Trichinella taxonomy groups the encapsulating species and genotypes in one clade, and the non-encapsulating species in another (Zarlenga et al., 2020).
Fig. 1.
Infectivity of species of Trichinella vary with the type of host species. Possibly, they are all zoonotic and infective to human beings. In the global ranking of importance of food-borne parasites (FAO/WHO, 2014), Trichinella spiralis was ranked seventh, while Trichinella spp. (all other species but T. spiralis) were ranked sixteenth.
1.1. The Arctic, the subarctic and the boreal taiga forest zone
The Arctic got its name from the Great Bear, Ursa Major Constellation, which by the ancient Greeks was called Arctos. It is situated in the North sky and thus the land below it was later named the Arctic. The ancient Greeks referred to it as Thule (or ultima Thule), meaning the farthest location in the north. The Arctic has many definitions; the region beyond the Arctic Circle (about 66°33′ N); the region beyond the July 10 °C isotherm, or above the tree line. The Arctic Council lists Canada, The Kingdom of Denmark, Finland, Iceland, Norway, The Russian Federation, Sweden, and The United States of America as Arctic States (Arctic Council, 2021). The subarctic is not very clearly defined either, being the region located immediately south of the Arctic, it overlaps with the boreal (northern, from Greek Boreas, the god of the north wind) taiga forest zone. Within the European Union, the boreal region includes most of the subarctic Sweden and Finland, and the Baltic States, Estonia, Latvia and Lithuania (European Commission, 2021). With the ongoing climate change, the definitions of the Arctic and subarctic may change, and we considered it justified to include the boreal region in this review.
Previous reviews by other authors have elucidated Trichinella infections in the Arctic (e.g. Connell, 1949; Abs and Schmidt, 1954; Thorshaug and Rosted, 1956; Leikina, 1961; Lukashenko et al., 1971; Kapel, 1997; Pozio, 2016), but new research has continuously revealed previously unknown aspects, including new Trichinella species circulating in the region. In this review, we also cover Russian Trichinella literature as this is important information on the circumpolar knowledge on the zoonotic parasite genus. Moreover, we briefly touch relevant aspects from comparable environments from Antarctic and Alpines. Finally, we discuss the challenges and solutions for control of Trichinella in the Arctic and subarctic.
1.2. History of trichinellosis in the Arctic
The freeze resistant Trichinella nativa may “always” have been present in Arctic areas (Korhonen et al., 2016). Apparently the first accurately reported human Trichinella infection in the Arctic was initially a military secret. In 1943, during World War II, a German undercover weather station was established on Alexandra Land, Franz Josef Archipelago, Arkhangelsk Oblast, Russia. In late May 1944, the staff shot a polar bear (Ursus maritimus) and ate it raw (steak tartare). About 15 men became ill and were evacuated by plane in July, and the station was abandoned (Connell, 1949; Barr, 1995). The infection caused no fatalities, but several patients required long periods for convalescence. However, almost half a century earlier, in July 1897, the Swedish three-man Andrée expedition set out from Spitsbergen to reach the North Pole by balloon. They did not reach the North Pole (Tryde, 1952; Akkuratov, 1964). After 65 h of flight, the balloon, gathering ice, could not carry the three men further and they landed on an ice pack. For the next three months, the men wandered across the ice to the island Kvitøya and wrote in their diaries that in late September, they again shot a polar bear. Andrée noted that the men liked the taste of raw polar bear meat. The camp and skeletons of the three men were found 30 years later. The Danish doctor Ernst Adam Tryde wrote in 1952 that he and his colleagues had detected Trichinella in a few pieces of dried muscle tissue from remains of a polar bear brought back from the campsite of the Andrée expedition (Tryde, 1952). Since then, it has been a popular hypothesis that the men died from acute trichinellosis, but although the hypothesis about Trichinella as the cause of death is intriguing, it has not been proven.
Other evidence suggests that trichinellosis has been prevalent for centuries in the Arctic. Muscle tissue from a child mummy from the 16th century from Pissisarfik in West Greenland near the capital Nuuk showed infection with Trichinella larvae (Lynnerup, 2015).
1.3. Particular aspects of human trichinellosis in the highly endemic Arctic
As symptoms of trichinellosis are nonspecific, many cases in endemic areas may still go undetected. Correspondingly, serologic studies indicate that Trichinella exposure is much more prevalent in Arctic populations than identified through outbreaks of clinical cases only (e.g. Berezantsev, 1974; Goyette et al., 2014). There is some evidence that symptoms associated with trichinellosis in populations repeatedly exposed to Trichinella may differ from the classical symptoms, which include enteral phase symptoms – abdominal pain, nausea, vomiting and diarrhea – and parenteral/muscle symptoms – edema, fever, myalgia and rash (Bruschi and Murrell, 2002; Dupouy-Camet et al., 2002; Gottstein et al., 2009). In the 1980s, in Inuit from northern Canada, two syndromes were identified; one representing the classical myopathic form as described above with edema, fever, myalgia and rash; while the second was a persistent diarrheal illness with little edema, myalgia or fever (Viallet et al., 1986; MacLean et al., 1989; MacLean et al., 1992). The authors hypothesized that the first syndrome represented a primary infection with T. nativa, while the second represented a secondary infection in previously sensitized individuals who had acquired immunity against the enteral and parenteral stages of the parasite. Likewise, clinical manifestations of Trichinella infection in persons from 51 villages in Papua New Guinea, of which 10% were seropositive, were never severe, which could suggest that seropositive persons were reinfected relative often, but with few larvae (Owen et al., 2005).
2. Epidemiology
2.1. Geographical distribution – Prevalence rates and reported outbreaks
2.1.1. Nearctic
The Arctic and subarctic areas of the Nearctic region include Alaska, northern Canada and Greenland. Multiple species of Trichinella have been documented in animals and humans in this region. Range of Trichinella prevalence (based on the presence of muscle larvae) in arctic wildlife of Nearctic is summarized in Table 1, Table 2. Most surveys in wildlife used muscle digestion or trichinoscopy to detect Trichinella infection, and studies using serological approaches have contributed to the knowledge on exposure to the parasite.
Table 1.
Prevalence of Trichinella in animal species reported as hosts in the northern Canada and Alaska.
| Host | Prevalence range (%) | Method(s) | Reference |
|---|---|---|---|
| Family Canidae | |||
| Domestic dog (Canis familiaris) | 6–93 | MD | (Rausch et al., 1956; Schiller, 1952) |
| Arctic fox (Vulpes lagopus) | 2–21 | NR*, MD | (Forbes, 2000*; Owsiacki et al., 2020; Roth and Madsen, 1953*) |
| Red fox (Vulpes vulpes) | 6–41 | MD | (Rausch et al., 1956; Smith and Snowdon, 1988) |
| Wolf (Canis lupus) | 13–52 | MD | (Larter et al., 2011; Smith and Snowdon, 1988) |
| Coyote (Canis latrans) | 13 | MD | (Rausch et al., 1956) |
| Family Felidae | |||
| Lynx (Lynx canadensis) | 7–24 | MD | (Gajadhar and Forbes, 2010; Rausch et al., 1956) |
| Family Mustelidae | |||
| Wolverine (Gulo gulo) | 50–88 | MD | (Rausch et al., 1956; Reichard et al., 2008) |
| Ermine (Mustela erminea) | 9–43 | MD | (Rausch et al., 1956) |
| Least Weasel (Mustela nivalis) | 2 | MD | (Rausch et al., 1956) |
| Marten (Martes americana) | 1–3 | MD,TR* | (Gajadhar and Forbes, 2010; Poole et al., 1983*) |
| Family Odobenidae | |||
| Walrus (Odobenus rosmarus) | 1–41 | NR*, MD | (Kuitunen-Ekbaum (1954)*; Gajadhar and Forbes, 2010) |
| Family Phocidae | |||
| Various seals | <1 | (Rausch et al., 1956) | |
| Family Ursidae | |||
| Black Bear (Ursus americanus) | 1–24 | NR*,MD | (Frechette and Rau, 1977*; Rausch et al., 1956) |
| Grizzly Bear (Ursus arctos) | 6–88 | MD | (Choquette et al., 1969; Harms et al., 2021; Rausch et al., 1956) |
| Polar Bear (Ursus maritimus) | 53–66 | NR*, MD | (Rausch et al., 1956*; Gajadhar and Forbes, 2010) |
| Family Monodontidae | |||
| Beluga Whale (Delphinapterus leucas) | 2 | MD | (Rausch et al., 1956) |
| Family Leporidae | |||
| Snowshoe Hare (Lepus americanus) | 5 | MD | (Rausch et al., 1956) |
| Family Cricetidae | |||
| Brown Lemming (Lemmus trimucronatus) | 5 | MD | (Rausch et al., 1956) |
| Muskrat (Ondatra zibethicus) | <1 | MD | (Rausch et al., 1956) |
| Narrow-skulled Vole (Microtus gregalis) | 2 | MD | (Rausch et al., 1956) |
| Red-backed Vole (Myodes/Clethrionomys rutilus) | 4 | MD | (Rausch et al., 1956) |
| Family Sciuridae | |||
| Red squirrel (Tamiasciurus hudsonicus) | 4 | MD | (Rausch et al., 1956) |
| Ground squirrel (Citellus undulatus) | <1 | MD | (Rausch et al., 1956) |
| Family Muridae | |||
| Brown rat (Rattus norvegicus) | 12 | MD | (Schiller, 1952) |
| Family Castoridae | |||
| Beaver (Castor canadensis) | 3 | MD | (Rausch et al., 1956) |
Table 2.
Prevalence of Trichinella in animals reported as hosts in Greenland.
| Host | Prevalence [overall %, range % (n)] | Method(s) | Reference |
|---|---|---|---|
| Family Canidae | |||
| Arctic fox (Alopex lagopus) | 0 (6) 1.4, 0–13.3 (1743) 6, 0–35 (266) |
MD, TR TR MD |
(Masterton and Lewis, 1955) (Madsen, 1961) (Kapel et al., 1996) |
| Dog (Canis familiaris) | 77.8 (9) 11.3, 2.5–66.7 (133) 71, 0–94 (807) |
MD, TR MD TR |
(Masterton and Lewis, 1955) (Møller, 2006) (Madsen, 1961) |
| Wolf (Canis lupus) | 50 (4) | NR | (Roth and Madsen, 1953) |
| Family Ursidae | |||
| Polar bear (Ursus maritimus) | 32 (38) 24.2, 11–67 (231) |
MD TR |
(Born and Henriksen, 1990) (Madsen, 1961) |
| Family Odobenidae | |||
| Walrus (Odobenus rosmarus) | 1.6 (126) 1, 0–9.1 (489) 4.2 (24) |
MD, TR TR MD, TR |
(Born et al., 1982) (Madsen, 1961) (Thing et al., 1976) |
| Family Phocidae | |||
| Bearded seal (Erignathus barbatus) | 0/0 (7/42)⁎ 0.8, 0–12.5 (245) |
MD/SE TR |
(Møller, 2006) (Madsen, 1961; Roth and Madsen, 1953) |
| Harp seal (Pagophilus groenlandicus) | 0/2.1 (284/435)⁎ 0 (2405) |
MD/SE | (Møller, 2006) (Madsen, 1961) |
| Hooded seal (Cystophora cristata) | 2.3/0.33 (215/304)⁎ 0 (203) |
MD/SE TR |
(Møller, 2006) (Madsen, 1961) |
| Ringed seal (Phoca hispida) | 0.16/1.5 (627/1377)⁎ 0.06 (1775) |
MD/SE TR |
(Møller, 2006) (Madsen, 1961) |
| Seal (not specified) | 0.06 (1657) | TR | (Madsen, 1961) |
n: number of samples tested.
MD: Muscle Digestion, NR: Not Recorded, SE: Serology, TR: Trichinoscopy.
MD/SE.
2.1.1.1. Canada
Northern Canada comprises three territories, viz. Yukon, Northwest Territories, and Nunavut and the regions north of the southern limit of discontinuous permafrost in the following provinces: Alberta, British Columbia, Labrador, Manitoba, Ontario, Québec (Nunavik and the James Bay region) and Saskatchewan (Jenkins et al., 2013). A wide range of prevalence of Trichinella infection (1–88%) has been reported from wild carnivores in northern Canada (Reichard et al., 2008; Gajadhar and Forbes, 2010; Jenkins et al., 2013). Prevalence documented ranged from 11 to 88% in the Yukon, 3–77% in the Northwest Territories and 3–88% in Nunavut (Reichard et al., 2008; Gajadhar and Forbes, 2010; Jenkins et al., 2013; Sharma et al., 2021). Similarly, varied prevalence has been reported in Québec (1–66%, Frechette and Rau, 1977; Gajadhar and Forbes, 2010), Newfoundland and Labrador (4–59%, Thorshaug and Rosted, 1956; Smith and Snowdon, 1988) as well as in Alberta, British Columbia, Manitoba and Saskatchewan (1–33%, Gajadhar and Forbes, 2010; Jenkins et al., 2013).
Among terrestrial wild carnivores, high prevalence rates (>50%) were reported in wolverines (Gulo gulo, 88% in Nunavut and 78% in Yukon, Reichard et al., 2008; Sharma et al., 2021), grizzly bears (Ursus arctos horribilis, 88% and 71% in Yukon; 73% in Northwest Territories, Choquette et al., 1969; Larter et al., 2011; Harms et al., 2021), polar bears (66% in Nunavut and Quebec; Gajadhar and Forbes, 2010) and wolves (Canis lupus, 52% in Northwest Territories; Larter et al., 2011). Recently, 66% seroprevalence for Trichinella was described in 409 polar bears from western Hudson Bay, Canada (Pilfold et al., 2021). However, varied prevalence in the same animal host species has also been reported in regions of northern Canada, for example, 29% in grizzly bears from Nunavut and British Columbia and 43% in wolves from Nunavut, British Columbia, and the Yukon (Gajadhar and Forbes, 2010). In contrast, lower prevalence (1–11%) has been documented in other terrestrial carnivores, including arctic (Vulpes lagopus) and red fox (Vulpes vulpes), black bear (Ursus americanus), lynx (Lynx canadensis), marten (Martes americana), and skunk (Mephitis mephitis) as reviewed in Jenkins et al. (2013). The variation in the prevalence rate in wildlife species could be due to differences in sampling method (most often convenience sampling), sample size, type of muscle sampled, detection method, geographical or dietary variation (between different species of animals as well as due to regional variation within an animal host species). Within a province/territory, variation in prevalence was also reported; for example, prevalence of Trichinella infection was higher in wolverines from southeast than northeast region of the Yukon (Sharma et al., 2021). This could be due to the variation in the wolverine diet. Other reasons may involve environmental factors (temperature, humidity, altitude etc.) affecting the biology, including population density, of various host animal species. Moreover, environmental factors affect survival of Trichinella larvae in dead animals.
Among marine mammals, walrus (Odobenus rosmarus) was the only species found positive for Trichinella in surveys conducted in the Canadian north; prevalence varied between 4 and 41% (Kuitunen-Ekbaum, 1954; Gajadhar and Forbes, 2010). Highest prevalence was reported in walrus from the Nunavut and northern Quebec (Gajadhar and Forbes, 2010). Previously, walrus meat has been associated with human cases and outbreaks in the Nearctic regions including Canada. Larvae of Trichinella were not detected in surveys in ringed seals (Pusa hispida, n = 244), bearded seals (Erignathus barbatus, n = 29) (Forbes, 2000) and grey seals (Halichoerus grypus, n = 52) from northern Canada (Sauvé et al., 2020). The absence of observations of Trichinella infection in the Canadian seals indicates low risk via consumption of seal meat; however, species of Trichinella have been reported both in experimentally and in naturally infected seals elsewhere (Kapel et al., 2003; Seymour et al., 2014).
Of 13 species and genotypes of Trichinella, five have been documented in the wildlife of northern Canada: Trichinella T6, T. nativa, T. chanchalensis, T. spiralis, and T. pseudospiralis. Species identification of Trichinella was not performed in all the surveys; moreover, only a proportion of isolates recovered were identified to species in most of the studies. Trichinella T6 and T. nativa are the ones most commonly reported in northern Canada. Trichinella T6 has been documented in wildlife of Yukon, Northwest Territories, Nunavut and British Columbia (Reichard et al., 2008; Gajadhar and Forbes, 2010; Larter et al., 2011; Owsiacki et al., 2020; Harms et al., 2021; Sharma et al., 2021). Trichinella nativa has been reported in the wildlife of the Yukon, Northwest Territories, Nunavut, British Columbia, Saskatchewan and Quebec (Reichard et al., 2008; Gajadhar and Forbes, 2010; Jenkins et al., 2013; Owsiacki et al., 2020; Harms et al., 2021; Sharma et al., 2021). Trichinella chanchalensis, a recently discovered species, has been documented only in wolverines from two territories of northern Canada, viz. the Yukon and Northwest Territories (Sharma et al., 2020). Trichinella T6, T. chanchalensis, and T. nativa occur in sympatry geographically (Yukon) and in the same individual host(s) (wolverine) (Sharma et al., 2021). Similarly, sympatry between T. nativa and Trichinella T6 has been previously documented in wolverine in Nunavut (Reichard et al., 2008). Other species of Trichinella (T. spiralis and T. pseudospiralis) have been reported sporadically in wild animals in Canada; for example, T. spiralis has been reported in red foxes and coyotes (Canis latrans) originating from Prince Edward Island (Appleyard et al., 1998). Recently, T. spiralis and T. pseudospiralis were reported in wolverine from the Yukon, the first cases documented in northern Canada (Sharma et al., 2019, Sharma et al., 2021). Trichinella spiralis has been eradicated from the market pigs in Canada (Appleyard and Gajadhar, 2000; Gajadhar and Forbes, 2010), and only sporadic reports have been documented in the wild animals (Appleyard et al., 1998; Sharma et al., 2019, Sharma et al., 2021). Game meat (where T. nativa and Trichinella T6 were mostly reported) could pose the highest public health risk for acquiring Trichinella spp. infection in Canada. Also T. pseudospiralis has been reported sporadically, and the comparison of the nucleotide sequence analysis with other local and global isolates of T. pseudospiralis raised the possibility of transmission from Eurasia or the Neotropical regions to northern Canada via migratory birds.
Several serological surveys to detect exposure to zoonotic pathogens including Trichinella have been conducted in northern Canada. One of the largest surveys was conducted in 36 Inuit communities in Inuvialuit Settlement Region (ISR), Nunavut, and Nunatsiavut, and 19% of the tested humans (n = 2212) were seropositive for antibodies to Trichinella. Seroprevalence was higher in Nunavut (24%) than ISR (1%) and Nunatsiavut (1%) (Goyette et al., 2014). Seroprevalence was 1% of 251 people in two Cree communities (Eastmain and Wemindji) (Campagna et al., 2011) and among 267 people examined, none were found seropositive in 2 other Cree communities of Nunavik (Chisasibi and Waskaganish) (Sampasa-Kanyinga et al., 2012). The variation in the seroprevalence in the regions of northern Canada could be attributed to the differences in the local hunting and culinary practices as well as study design and detection method.
Black bear, polar bear, grizzly bear, and walrus are considered the major source(s) for human infections and outbreaks of trichinellosis in northern Canada (reviewed in Jenkins et al., 2013). One outbreak involved 10 persons who consumed dried meat (jerky) from a black bear hunted in Northern Ontario (Dalcin et al., 2017). Larvae of Trichinella recovered from the frozen bear meat were identified as T. nativa (Dalcin et al., 2017). An outbreak occurred in a crew of five members from France, who during their stopover at Cambridge Bay (Iqaluktuuttiaq), Victoria Island, Nunavut consumed meat of grizzly bear after pan-frying or barbequing (Houzé et al., 2009). Interestingly, the rest of the grizzly bear meat was consumed locally in Cambridge Bay after proper cooking, and no cases of Trichinella infection were reported (Houzé et al., 2009). One of the largest outbreaks of trichinellosis in Canada occurred in two communities (Black Lake and Stony Rapids) of northern Saskatchewan; 78 residents consumed meat of black bear, and 31 were confirmed positive for Trichinella infection (Schellenberg et al., 2003). Higher number and proportion of patients with confirmed Trichinella infection had consumed dried as well as boiled meat than only boiled meat of bear (Schellenberg et al., 2003).
2.1.1.2. Alaska
>6 decades back, reported prevalence based on detection of larvae of Trichinella in the wildlife of Alaska ranged from <1% (in seals) to around 50% in wolverine and polar bear (Rausch et al., 1956). In addition, high (93%) prevalence of Trichinella infection was documented in 41 sledge dogs (Canis familiaris) in a village of Barrow region of Alaska (Rausch et al., 1956). Moderate prevalence (>20%) has been documented in polar bear, black bear, grizzly bear, wolf, wolverine, red fox, and ermine (Mustela erminea). Seroprevalence was 28% in black bear (Chomel et al., 1998), 49% in grizzly bear (Zarnke et al., 1997) and 46–100% in polar bear (Chomel et al., 1998; Rah et al., 2005). However, antibodies to Trichinella were not detected in 155 brown bears (Ursus arctos, Ramey et al., 2019), and authors from the study (Ramey et al., 2019) emphasized the need of testing and validating of serological assays for Trichinella spp., along with use of detection methods that analyse tissues for the presence of larvae.
In a study on Pacific walrus, none of 137 tested were positive (Seymour et al., 2014), although cases of positive walruses were reported in an earlier study in Alaska (Fay, 1960); in any case, prevalence in Pacific walrus appears low as compared to the reported prevalence in Atlantic walrus of Canada (41%, Gajadhar and Forbes, 2010). Ringed seals are known to be consumed by the Pacific walruses, however higher prevalence of Trichinella spp., in walruses could not be attributed to consumption of ringed seals as none were found positive in the aforementioned studies. Therefore, further studies are required to assess the components of diet of walrus (how much contribution ringed and other seals make) to determine if relationship exists between Trichinella spp. infection and their diet (e.g. seal eating) (Seymour et al., 2014). Trichinella T6 and T. nativa were detected in sympatry in wolves from Alaska, and T. nativa was the predominant species (La Rosa et al., 2003). In Alaska, T. nativa has also been reported in arctic fox, polar bear, and ringed seal (Seymour et al., 2014).
In 2016–17, two outbreaks (each involving five patients) were reported from the Norton Sound region of Alaska, both linked to the ingestion of raw or undercooked walrus meat (Springer et al., 2017). Meat was not available for testing of larvae of Trichinella in first outbreak, however, larvae recovered from the walrus meat in second outbreak were identified as T. nativa. It was reported that there was a sharp decline of about 12.6 times (from 6.3 cases per year during 1975–1992 in comparison to 0.5 cases per year during 1993–2017) of cases of walrus associated trichinellosis in Alaska (reported in Springer et al., 2017). Only one case associated with walrus meat in Alaska was recorded during the 23 years preceding these events (reported in Springer et al., 2017). Of 241 cases of trichinellosis in Alaska reported from 1975 to 2012, 94% were linked to wild game (black bear, polar bear, grizzly bear, walrus and seal); 41% were associated with walrus meat, and 10% with seal or walrus meat (Springer et al., 2017). Such outbreaks emphasize the importance of the integration of traditional knowledge about the culinary and hunting practices with risk communication to prevent foodborne infections in the consumers.
2.1.1.3. Greenland
The first known official report of a human outbreak likely to be trichinellosis in Greenland occurred in 1933 in the Upernavik district in North Greenland and was caused by ingestion of walrus meat (Thorborg et al., 1948). Subsequently, many outbreaks have been described, mostly on the west coast of Greenland, with the largest outbreak in the mine settlement of Qullissat in 1947 with 131 cases and 11 deaths. From 1944 to 1983, approximately 680 cases of trichinellosis from west Greenland were identified, including 95 deaths. Outbreaks in Greenland have been reviewed in literature (Madsen, 1961; Møller, 2007), and most outbreaks were associated with ingestion of walrus meat, and a few with polar bear or seal meat.
Outbreaks of trichinellosis in humans have also been reported in more recent years, and not only in residents, but also in tourists eating polar bear (Nozais et al., 1996). The most recent known outbreak occurred in 2016 in South Greenland during a conference, where polar bear meat had been barbecued in a modern way on spears with capsicums (Rosa, 2016). Outbreaks during the 1940s and 1950s were larger and more frequent than in later years, most likely because of changes in lifestyle from being reliant on hunting to a more western lifestyle.
Following the series of human trichinellosis outbreaks in Greenland in 1947 (Thorborg et al., 1948), the study of Trichinella in animals in Greenland was commenced to investigate the potential sources of human infection (Roth, 1949). During the late 1940s and the 1950s, animal samples were tested for Trichinella larvae (Thorborg et al., 1948; Roth, 1949; Roth and Madsen, 1953; Masterton and Lewis, 1955). The prevalence estimates in different animal host species reported by Roth and colleagues were summarized in Roth and Madsen (1953). The work continued throughout the 1950s with a large-scale survey of Trichinella in wild animals and sled dogs (Madsen, 1961). Later, studies have been conducted within selected carnivore and seal species (Thing et al., 1976; Born et al., 1982; Born and Henriksen, 1990; Kapel et al., 1996; Møller, 2006).
All Trichinella parasites recovered from animals in Greenland and identified by molecular methods are reported as T. nativa (La Rosa et al., 1990; Kapel et al., 1999; Møller, 2006). Prevalence estimates of reported hosts for Trichinella are presented in Table 2. Trichinella has not been detected in stoats (ermines), rodents, multiple herbivores, whale species, and a few fish that have been investigated (Madsen, 1961). Highest prevalence has been reported in dogs (overall prevalence of 11% in 2006) and polar bears (overall prevalence >20%). The prevalence in dogs reported in 2006 (Møller, 2006) is lower than reported in 1961 (71%, Madsen, 1961), even within the same locations. It has been suggested that this apparent decrease in prevalence may be related to a change in hunting practices in Greenland with a decrease in fulltime hunters (Møller, 2006).
Generally, a large geographic variation in Trichinella prevalence is apparent within all the studied host species. For example, no Trichinella larvae were detected in 56 arctic foxes from Kangerlussuaq, West Greenland, while a prevalence of 35% (n = 20) was reported in arctic foxes from Ittoqqortoormiit (cited as Scoresbysund) in East Greenland (Kapel et al., 1996). It has been hypothesized that the highest prevalence is found in areas where polar bears are traditionally hunted (Kapel et al., 1996), leaving a higher number of carcasses available for scavenging and, thereby, allowing for transmission to other hosts. Further, in communities where polar bear and walrus are commonly hunted, the meat will often be used as dog feed, leading to high Trichinella prevalence in the dog population (Møller, 2006). Age composition of the dog population has also been suggested as a reason for local differences in prevalence (Madsen, 1961).
2.1.2. Palearctic
Out of the currently recognized ten encapsulated taxa, the distribution areas of T. nativa and T. britovi overlap partially or entirely in boreal regions of the Palearctic (Pozio, 2016). The northern geographic boundary of T. britovi is assumed to be around the isotherms −6 to −5 °C in January (Shaikenov, 1992; Pozio et al., 1998; Pozio, 2000, Pozio, 2016). However, the southern distribution boundary of T. nativa, the northern species of Trichinella, is considered between the isotherms −5 to −4 °C in January (Shaikenov, 1992; Pozio, 2016; Deksne et al., 2020).
2.1.2.1. Estonia
In Estonia, the January isotherms between −4 to −6 °C were close together (Pozio et al., 1998). However, the isotherms move to the north around 15 km per year, thus probably changing the distribution area of host species and sylvatic Trichinella species (Beniston, 2014, Pozio, 2016). Trichinella spp. infections are endemic in sylvatic animals (Table 3) and were reported also in fur animal farms and three pig farms during the 1980s and 1990s (Pozio et al., 1998; Järvis et al., 2001). Recent study in the most important reservoir animal species in Estonia, raccoon dogs (Nyctereutes procyonoides) and red foxes, confirmed that the prevalence of Trichinella was one of the highest in Europe (57.5% and 69.0%, respectively) and biomass of sylvatic Trichinella species carried by reservoir animals had increased during the last decades (Kärssin et al., 2017). The commonest Trichinella species was T. nativa in raccoon dogs and T. britovi in red foxes, and mixed infection was confirmed in 13% of raccoon dogs and 8% of red foxes (Kärssin et al., 2017).
Table 3.
Prevalence of Trichinella in animals reported as hosts in Estonia.
| Host | Sampling period | Prevalence [overall %, range % (n/N)] | Method(s) | References |
|---|---|---|---|---|
| Order Artiodactyla | ||||
| Domestic pig (Sus scrofa) | 1992–1996⁎ 1992–1999⁎ 2012 |
0.5 (5/1001) 0.6 (6/1002) 0.0 (0/374) |
MD MD SE |
(Pozio et al., 1998) (Järvis et al., 2001) (Kärssin et al., 2016) |
| Wild boar (Sus scrofa) | 1992–1996(1999) 2000–2002 2007–2014 2012–2013 |
0.7–1.0 (5/667–7/695) 0.3 (2/710) 0.9 (0.4–1.6) (281/30,566) 42.1⁎⁎ (198/470) |
MD MD MD SE |
(Pozio et al., 1998) (Järvis et al., 2001) (Malakauskas et al., 2007) (Kärssin et al., 2021) (Kärssin et al., 2016) |
| Order Carnivora | ||||
| Wolf (Canis lupus) | 1992–1996(1999) 2000–2002 |
75.0–79.4 (18/24–27/34) 63.2 (12/19) |
MD MD |
(Pozio et al., 1998) (Järvis et al., 2001) (Malakauskas et al., 2007) |
| Brown bear (Ursus arctos) | 1992–1996(1999) 2007–2014 |
29.4–38.5 (5/13–5/17) 14.7 (7.9–35.7) (63/429) |
MD MD |
(Pozio et al., 1998) (Järvis et al., 2001) (Kärssin et al., 2021) |
| Eurasian lynx (Lynx lynx) | 1992–1996(1999) 1999–2001 2000–2002 2007–2014 |
38.5–47.4 (5/13–9/19) 29.6 (8/27) 50.0 (15/30) 65.6 (50.0–90.9) (59/90) |
MD TR MD MD |
(Pozio et al., 1998) (Järvis et al., 2001) (Valdmann et al., 2004) (Malakauskas et al., 2007) (Kärssin et al., 2021) |
| European badger (Meles meles) | 1992–1999 2007–2014 |
1/2 3/5 |
MD MD |
(Järvis et al., 2001) (Kärssin et al., 2021) |
| Raccoon dog (Nyctereutes procyonoides) | 1992–1996(1999) 2000–2002 2011–2012 |
50.0–52.6 (10/19–11/22) 42.0 (66/157) 57.5 (65/113) |
MD MD MD |
(Pozio et al., 1998) (Järvis et al., 2001) (Malakauskas et al., 2007) (Kärssin et al., 2017) |
| Red fox (Vulpes vulpes) | 1992–1996(1999) 2000–2002 2011–2012 |
42.1–44.4 (8/18–19) 40.6 (181/446) 69.0 (60/87) |
MD MD MD |
(Pozio et al., 1998) (Järvis et al., 2001) (Malakauskas et al., 2007) (Kärssin et al., 2017) |
| Pine marten (Martes martes) | 1992–1996(1999) 2000–2002 |
1/4–1/6 30.4 (17/56) |
MD MD |
(Pozio et al., 1998) (Järvis et al., 2001) (Malakauskas et al., 2007) |
| Arctic fox (Vulpes lagopus)⁎⁎⁎ | 1992–1996(1999) 1992–2000 |
24.5 (13/53) 30.6 (15/103) |
MD MD |
(Pozio et al., 1998) (Järvis et al., 2001) (Miller et al., 2006) |
| Silver fox (Vulpes vulpes fulva)⁎⁎⁎ | 1992–1996(1999) | 1.4–3.2 (1/32–1/70) | MD | (Pozio et al., 1998) (Järvis et al., 2001) (Miller et al., 2006) |
| Mink (Neogale vison or Mustela lutreola)⁎⁎⁎ | 1992–2000 | 14.3–17.9 (5/28–5/35) | MD | (Järvis et al., 2001) (Miller et al., 2006) |
| Domestic cat (Felis catus) | 1992–1996(1999) | 1/1–1/2 | MD | (Pozio et al., 1998) (Järvis et al., 2001) |
| Order Rodentia | ||||
| Brown rat (Rattus norvegicus) | 1992–2000 | 11.1 (2/18) | MD | (Pozio et al., 1998) (Järvis et al., 2001) (Miller et al., 2006) |
| Order Pinnipedia | ||||
| Grey seal (Halichoerus grypus) | 1/3 | NR | (Turovski et al., 1995) | |
MD: Muscle Digestion, NR: Not Recorded, TR: Trichinoscopy, SE: Serology (indirect enzyme linked immunosorbent assay).
overlapping data, sampling was first connected with case-studies.
apparent seroprevalence, extrapolated from dataset of confirmed seroprevalence was 17.4%.
sampled from fur-bearing animal farms.
In wild boars, the overall prevalence was 0.9% in 2007–2014, but varied by counties and by year, being the highest (1.6%) in 2013 (Kärssin et al., 2021). The higher prevalence of T. britovi and overall Trichinella spp. were detected in the western part of Estonia (Kärssin et al., 2021). Infections with T. nativa, including mixed infections with T. nativa and T. britovi, were detected in 0.13% of wild boars in the mainland of the country (Kärssin et al., 2021). In addition, focal distribution of T. spiralis as single or mixed infections with T. britovi was found in wild boars and Eurasian lynxes (Lynx lynx). A few wild boars hunted in the western part of the country were infected with T. pseudospiralis (Kärssin et al., 2021). The overall prevalence for lynxes was highest in western part of the country (85%) (Kärssin et al., 2021). Three Trichinella species have been found in lynxes: T. britovi, T. nativa, and T. spiralis as mono- or mixed infection (T. britovi and T. nativa, or T. britovi and T. spiralis), and T. britovi was confirmed in 84% of larval detections (Kärssin et al., 2021). During the last decades, the first findings of T. britovi have been detected in brown bears: in 2007–2014, 54% of larval detections were confirmed as T. britovi infections and 5% were concurrently infected with T. nativa. A recent study on badgers confirmed the presence of T. britovi, in addition to previously found T. nativa infections (Järvis et al., 2001; Kärssin et al., 2021). Recent studies have shown the presence of T. nativa only in the mainland of the country (Kärssin et al., 2017; Kärssin et al., 2021), however, in previous studies, T. nativa was also reported in western islands (Saaremaa and Hiiumaa) (Pozio et al., 1998; Malakauskas et al., 2007).
Trichinellosis is rare in humans in Estonia. The most common meat in the local diet is pork; game meat is an important food source mainly for hunters' families but not for the general population (Kana, 2017). During 1969–2020, 108 human cases were reported in Estonia (Health Board, 2021). Of the 71 cases when the source was known, 93% occurred after the consumption of wild boar (Sus scrofa) meat, 4% from pork, and 3% from badger (Meles meles) meat (Health Board, 2021). The latest small outbreak was confirmed in two persons from Tartumaa in 2015, the source was uninspected wild boar meat (Health Board, 2021). In a recent study in humans, the crude seroprevalence of Trichinella spp. detected by ELISA was 3.1% in the general population and 4.9% in hunters, after confirmatory testing with Western blot, estimates of 2.7% and 3.5%, were yielded, respectively (Lassen et al., 2016).
2.1.2.2. Fennoscandia
Fennoscandian countries are Norway (mainland), Sweden and Finland. There was a Trichinella outbreak with 50 known human cases in Sweden in 1937 (Hallen, 1938), soon followed by outbreaks involving about 700 people, most of them were German soldiers, in Norway in 1940. The “revival” of trichinellosis was suspected to be associated with fur animal farms, regarded as a Trichinella reservoir (Thorshaug, 1940). Therefore, farmed fur animals, wild foxes, and other potential hosts, including human cadavers, were tested in southeastern Norway (Aaser, 1941). The observed prevalence in 92 wild red foxes examined was 22%, while in 18,567 farmed foxes it was 6.2% and in 2063 farmed American minks (Neogale vison), 11.1%. One of the 286 human corpses was positive. In Finland, meat inspection revealed 10 infections in swine in 1954–1956, and also they appeared associated with fur animal farming. Wild red foxes (n = 38) and farmed fur animals (89 foxes and 42 minks) were tested revealing rather similar prevalence rates (16%, 10% and 7%, respectively) as in Norway (Rislakki, 1956).
Further, there was a large outbreak originating from pork sausage in Blekinge, southern Sweden in 1961, involving 338 human patients (Ringertz et al., 1961) and another one of 15 patients in Vadstena, also in southern Sweden (Odelram, 1973), but apart from them, human infections in Fennoscandia have been rare and sporadic. In 1967, a zoo employee in Helsinki was diagnosed with trichinellosis obviously derived from a wild boar raised in the zoo (Mäkelä, 1970), and in 1977 three hunters got infected by eating bear meat in Lapland (Salmi, 1978). In Sweden, in 2007, 2013, 2014, and 2015, single, and in 2016, two imported cases were reported (Folkhälsomyndigheten, 2021). The latest autochthonous human infection in Norway was reported in 1980 (Folkehelseinstituttet, 2021).
The latest infected swine both in Sweden and in Norway were found in 1994 (Folkhälsomyndigheten, 2021; Folkehelseinstituttet, 2021), while in Finland, a single infected swine was detected in 2010, and another, an outdoor reared Mangalica pig infected with T. nativa, in 2021 (Ruokavirasto, 2021). There was a period of swine Trichinella endemicity, or a long-lasting outbreak, between 1980 and 2005, (Hirvelä-Koski et al., 1985; Oivanen and Oksanen, 2009), ending probably due to changes in swine production after Finland joined the European Union in 1995, which discontinued most of the small and bio-insecure pig farms due to unprofitability.
Norwegian red foxes have been studied at times. In the western part of the country, a low prevalence of 2% (1/50) was found (Alne and Rossebø, 1987). Trichinella nativa was first isolated in Fennoscandia when 20 farmed arctic foxes fed with, among other fodder, frozen fox carcasses were found to have a high prevalence (95%) of infection in Finnmark, northern Norway (Handeland et al., 1995b). In studies conducted in 1994–1995 and 2002–2005, a proportion of wild red foxes were found positive (18 of 222 examined, 8.1%) in south-eastern part of Norway (Østlandet, “Eastern Norway”), while no positives were discovered among 101 animals from central and northern Norway (Davidson et al., 2006). Trichinella nativa was the most frequently identified species, while T. britovi was found in one fox.
In Sweden, 4639 badgers were meat inspected in 1942–1943, and Trichinella infection was found in 2% (Ekstam, 1964). In 476 brown rats from different parts of Sweden, no Trichinella infection was detected (Hülphers and Henricson, 1943).
Trichinella was prevalent (average 19.6% of 1151 animals examined) in red foxes almost all over Sweden, apart from the north and the rather highland Jämtland where the prevalence was lower, and from the island of Gotland, where no Trichinella was found (Roneus and Christensson, 1979). Also, Trichinella was detected in free-living badgers, polecats, ermines, martens, lynxes, minks (apparently American) and brown rats, as well as in farmed foxes, minks, domestic dogs and cats, but not in any of 20 brown bears examined.
Subsequently, from 1985 to 2003, Trichinella prevalence in Swedish wildlife decreased considerably: 4.5% of 1800 red foxes and 5.0% of 200 lynxes were found positive (Pozio et al., 2004). Muscle larvae isolated from 14 animals (seven red foxes, three wild boars, two domestic pigs, one lynx, and one wolf) were identified to species and all the four Trichinella species known from Europe (T. spiralis, T. nativa, T. britovi and T. pseudospiralis) were identified, all at least in two animals (Pozio et al., 2004).
In 1994–2011, cryopreserved muscle tissues from 59 carnivorous birds were tested for the presence of Trichinella; two tawny owls (Strix aluco) were found infected with T. pseudospiralis (Hurníková et al., 2014).
In Finland, during 1955–1956, 16% of 38 wild red foxes were Trichinella positive (Rislakki, 1956), while after some years, 4% of 105 foxes were found infected (Freeman, 1964). After the (re)emergence of Trichinella infection in Finnish slaughter swine around 1980, the National Veterinary Institute (currently Finnish Food Authority) has intermittently surveyed wildlife for presence of Trichinella. In these surveys, Trichinella prevalence in red foxes has been high: 33% of 15 foxes, (Hirvelä-Koski et al., 1985), 37% of 158 foxes (Oivanen et al., 2002), 19% of 1010 foxes (Airas et al., 2010), and 25% of 454 foxes (Oksanen et al., 2018). The somewhat lower prevalence reported in the latter two publications was explained by the high number of red foxes examined from northern Lapland, where the prevalence is much lower than in the south, as has been reported also from Sweden and Norway (Roneus and Christensson, 1979; Davidson et al., 2006). The lower Trichinella prevalence in the north obviously follows the same pattern in all the three countries but in Finland it is accentuated by the commonness of infection in southern Finland. This in turn has been attributed to the presence of the raccoon dog, an invasive alien species introduced into European parts of the previous Soviet Union and spread into Finland, yet apart from northern Lapland so far. Raccoon dog has been found to be the pivotal Trichinella reservoir due to high population density with high prevalence and high intensity of infection. The median number of larvae per gram of muscle examined was 40 in 186 infected raccoon dogs, 9.5 in 189 foxes, 6.8 in 7 pine martens, 3.6 in 40 wolves, 1.0 in 186 lynxes, 0.4 in 4 badgers and 0.2 in 7 brown bears. The Finnish raccoon dogs were infected with all the four species of Trichinella reported in Europe (Airas et al., 2010). Experimentally, Trichinella infection in raccoon dogs did not cause severe disease, and the raccoon dog could be confirmed to be an ideal propagation host and more than just a reservoir, an incubator species for Trichinella spp. in Finnish fauna (Näreaho et al., 2000).
The emergence of Trichinella infection detected in Finnish swine happened simultaneously with the rapid increase in raccoon dog population in southern Finland (Kauhala and Helle, 1995). The raccoon dog is an omnivorous carnivore species having scavenging as an important source of nutrition (Kauhala et al., 1998). To elucidate whether raccoon dogs and other carnivores might get infected by eating sylvatic rodents, 1761 bank voles (Myodes glareolus) and 138 field voles (Microtus agrestis) trapped on 30 transect trapping locations, together with 60 shrews (Sorex spp.) found accidentally succumbed in the traps, were tested by muscle digestion. No Trichinella infection was detected and, thus, no evidence was found of small mammals being an important part of sylvatic Trichinella life cycle in Finland (Välimaa et al., 2010).
One of 102 Finnish domestic dogs (1%) examined at necropsy was found Trichinella positive by digestion technique. Of another 727 dogs examined serologically by ELISA, 7.3% were classified as seropositive (Oivanen et al., 2005).
Based on testing 801 bears, 312 wolves and 1958 lynxes, it was concluded (Kojola et al., 2016) that males of all the three host species were more frequently infected than females, and that age increased prevalence. Of all bears, 4% were Trichinella positive, and even of bears older than 4 years, only 10% were infected, which is low compared with Alaskan, Canadian and Russian Far East bears (20–90%, e.g. Rausch et al., 1956; Seryodkin et al., 2020; Harms et al., 2021), and even compared with Estonian bears, whereof 15% were positive (Kärssin et al., 2021), but higher than the zero prevalence reported in 20 (Roneus and Christensson, 1979) and 41 Swedish bears (Mörner et al., 2005). The clear increase in prevalence in Finnish bears with increasing age indicates that the low prevalence is probably caused by low exposure and not by some undefined unsuitability of Fennoscandian bears to hosting Trichinella.
In a study of 327 Finnish lynx, with a 40% overall prevalence, it was found that the highest prevalence, ~60%, was in 4–5-year-old animals, and after that age, the prevalence decreased somewhat. Moreover, the generally low infection density (median 1.04 larvae/g of muscle, range 0.02–40.76 lpg) decreased slightly with age. These reductions were by the authors interpreted to have been caused by the population becoming saturated due to high incidence and indicate death of muscle larvae (Oksanen et al., 1998).
There is no indication about any significant change in Trichinella prevalence in Finnish wildlife during the last three decades, but the species distribution has changed drastically, and the winner has, quite surprisingly, been T. nativa. While the ongoing climate change might have been expected to hamper the well-being of the arctic species, there have been other drivers, too. The eradication of the synanthropic T. spiralis from pig farms in Finland around 2000–2005 has obviously cut the spillover to the sylvatic cycle (Oksanen et al., 2018). Of Trichinella isolates from four carnivore species (lynx, raccoon dog, red fox, wolf), T. spiralis constituted 15.0% in 1993–7 (Oivanen et al., 2002), 14.5% in 1999–2005 (Airas et al., 2010) and only 1.0% in 2011–2013 (Oksanen et al., 2018). Another loser was T. pseudospiralis, with 10, 2.2, and 0.4%. The reasons for this collapse are unknown. Corresponding percentages for T. nativa were 62.5, 74.7, and 90.2, respectively.
Antibodies against Trichinella were detected in 11% of 99 and 2% of 197 farmed Finnish wild boars that had been tested negative for muscle larvae in meat inspection (Sukura et al., 2001; Jokelainen et al., 2012). The seropositive wild boars had possibly been exposed to T. nativa, which has low infectivity to swine, including wild boar (Kapel, 2001).
In 1994 and 2000, a relatively high (19%) prevalence of Trichinella was found in 767 brown rats trapped from Finnish waste disposal pits. The only species identified from 28 rats was T. spiralis (Mikkonen et al., 2005).
The range expanding golden jackal (Canis aureus) has been observed in Finland a couple of times since 2018. In March 2022, an adult male jackal was trapped in Sodankylä, Lapland. It was found to harbour 9 lpg of T. nativa (Ruokavirasto, unpublished, 2022).
Grey (n = 171) and ringed seals (Phoca (=Pusa) hispida botnica) (n = 56) from the Finnish coastal waters of the Baltic Sea were tested and T. nativa was found in one grey seal (0.6%). This was interpreted as caused by spillover from the highly endemic terrestrial ecosystem (Isomursu and Kunnasranta, 2011). Fennoscandian Trichinella prevalences are summarized in Table 4.
Table 4.
Prevalence of Trichinella in animal species reported as hosts in Fennoscandia.
| Host | Prevalence [overall %, (n)] | Method(s) | Reference |
|---|---|---|---|
| ORDER ARTIODACTYLA | |||
| Family Suidae | |||
| Domestic pig (Sus domesticus) | 0.00004 (66 × 106) 0.00000–0.0016 |
MD MD |
(Pozio et al., 2004) (Oivanen and Oksanen, 2009) |
| Wild boar (Sus scrofa) | 0.05 (8000) 1.5 (1084) |
MD MD |
(Pozio et al., 2004) (Ruokavirasto, 2021) |
| ORDER CARNIVORA | |||
| Family Canidae | |||
| Dog (Canis familiaris) | 1.0 (102) 7.3 (727) |
MD SE |
(Oivanen et al., 2005) (Oivanen et al., 2005) |
| Wolf (Canis lupus) | 14.3 (7) 0 (9) 33 (18) 39.2 (102) 34.1 (85) 32.9 (319) |
MD MD MD MD MD MD |
(Pozio et al., 2004) (Mörner et al., 2005) (Oivanen et al., 2002) (Airas et al., 2010) (Oksanen et al., 2018) (Kojola et al., 2016) |
| Arctic fox (Vulpes lagopus) | 16.7 (6) | MD | (Pozio et al., 2004) |
| Red fox (Vulpes vulpes) Raccoon dog (Nyctereutes procyonoides) |
22 (92) 2 (50) 5.6 (323) 19.6 (1151) 4.5 (1800) 16 (38) 4(105) 33 (15) 37 (158) 19 (1010) 25 (454) 38 (199) 28 (662) 33 (952) |
TR NR MD TR, MD MD TR TR MD MD MD MD MD MD MD |
(Aaser, 1941) (Alne and Rossebø, 1987) (Davidson et al., 2006) (Roneus and Christensson, 1979) (Pozio et al., 2004) (Rislakki, 1956) (Freeman, 1964) (Hirvelä-Koski et al.,1985) (Oivanen et al., 2002) (Airas et al., 2010) (Oksanen et al., 2018) (Oivanen et al., 2002) (Airas et al., 2010) (Oksanen et al., 2018) |
| Family Felidae | |||
| Lynx (Lynx lynx) | 5 (200) 40 (327) 53 (96) 45 (402) 48 (1245) 47 (1958) |
MD MD MD MD MD MD |
(Pozio et al., 2004) (Oksanen et al., 1998) (Oivanen et al., 2002) (Airas et al., 2010) (Oksanen et al., 2018) (Kojola et al., 2016) |
| Family Mustelidae | |||
| Wolverine (Gulo gulo) | 50 (4) | MD | (Oksanen et al., 2018) |
| Common otter (Lutra lutra) | 3 (31) | MD | (Airas et al., 2010) |
|
Pine marten (Martes martes) |
2 (49) 9 (75) 12 (69) |
MD MD MD |
(Oksanen et al., 2018) (Airas et al., 2010) (Oksanen et al., 2018) |
| Badger (Meles meles) | 2 (4639) 33 (6) 7.5 (53) 7.5 (40) |
NR MD MD MD |
(Ekstam, 1964) (Oivanen et al., 2002) (Airas, 2010) (Oksanen et al., 2018) |
| American mink (Neogale vision) | 0 (23) 0 (37) |
MD MD |
(Airas et al., 2010) (Oksanen et al., 2018) |
| Family Phocidae | |||
| Ringed seal (Phoca hispida botnica) | 0 (56) | MD | (Isomursu and Kunnasranta, 2011) |
| Grey seal (Halichoerus grypus) | 0.6 (171) | MD | (Isomursu and Kunnasranta, 2011) |
| Family Ursidae | |||
| Brown bear (Ursus arctos) | 0 (20) 0 (41) 9 (150) 5.6 (125) 4.4 (801) 6.1 (162) |
MD, TR MD, TR MD MD MD MD |
(Ronéus and Christensson, 1979) (Mörner et al., 2005) (Oivanen et al., 2002) (Airas et al., 2010) (Kojola et al., 2016) (Oksanen et al., 2018) |
| ORDER RODENTA | |||
| Family Cricetidae | |||
| Bank vole (Myodes glareolus) Field vole (Microtus agrestis) |
0 (1761) 0 (138) |
MD MD |
(Välimaa et al., 2010) (Välimaa et al., 2010) |
| Family Muridae | |||
| Brown rat (Rattus norvegicus) | 0 (476) 19 (767) |
NR MD |
(Hülphers and Henricson, 1943) (Mikkonen et al., 2005) |
MD: Muscle Digestion, NR: Not Recorded, TR: Trichinoscopy, SE: Serology.
2.1.2.3. Arctic ocean, Norwegian sealing grounds
Polar bears and other potential hosts in the Svalbard Archipelago, Greenland Sea and Barents Sea of the Arctic Ocean have been studied for Trichinella since the late 1940s. As reviewed (Connell, 1949), evidence of arctic Trichinella existence had accumulated already during the early decades of the 20th century. The fate of the German military weather station in Franz Josef Land abandoned in 1944 due to trichinellosis acquired by eating raw polar bear meat (Connell, 1949) was probably not totally unknown in occupied Norway.
In 1948, 7 out of 9 polar bear skins (scraps of muscle adhered to them) from Spitsbergen (Svalbard) were reported infected (Eieland, 1948). In 1949–1953, a large survey was conducted on marine mammals in the Norwegian sealing regions in arctic waters (Western, Northern and Eastern Ice, and Newfoundland grounds); 58.6% of 278 polar bears and 9.5% of 74 walruses were infected, but none of 2455 seals (bearded, ringed, harp (Pagophilus groenlandicus), and hooded (Cystophora cristata) seals), 224 whales (beluga, fin (Balaenoptera physalus) and sperm whales (Physeter macrocephalus)), or 66 Greenland sharks (Somniosus microcephalus) were positive (Thorshaug and Rosted, 1956). In 1992, 1175 harp and 175 hooded seals were examined, with no Trichinella found (Handeland et al., 1995a).
Between 1950 and 1980, 376 polar bears, 77 arctic foxes and 336 seals (ringed and bearded), were examined and 32.7% of polar bears but no seals were found infected. Arctic foxes sampled in 1954–1955 (n = 15) had a prevalence of 67%, while those from 1979 (n = 62) were much more seldomly infected (3%) (Larsen and Kjos-Hanssen, 1983). Freeze-resistance was demonstrated in muscle larvae of polar bears (Kjos-Hanssen, 1983).
Polar bears are considered a vulnerable species, and Svalbard polar bear hunt was prohibited in 1973. After that, effective Trichinella surveillance could no more be based on muscle samples. In 1991–2000 and 2006–2008, live-caught polar bears (n = 441 and 226, respectively) were blood sampled for various studies, including testing for antibodies against Trichinella by ELISA. In cubs-of-the-year (n = 54), the prevalence was low, 1.9%, but in ≥1-year-olds, antibodies were found in 78% in Svalbard and 51% in Barents Sea pack ice population. The difference could be possibly due to variation in diet, scavenging on dead polar bears being more difficult on the pack ice where carcasses are easily swallowed by sea. In Svalbard, on the other hand, there is a chance that a dead animal lies on firm ground. An association was found between anti-Trichinella and anti-Toxoplasma gondii antibodies, which may be caused by similar transmission routes, both being food-borne infections the prevalence of which increase with age (Åsbakk et al., 2010).
In 1983–88, 8.5% of 697 arctic foxes were demonstrated infected by trichinoscopy; the prevalence increasing with age (juveniles, 4% and > 6-year-olds, 36%) (Prestrud et al., 1993). Antibodies against Trichinella were tested in 429 Svalbard arctic foxes from 1996 to 2004 using ELISA. The prevalence (overall 11.7%) increased with age and was significantly higher in males (50%) than females (20%) of age ≥ 4 years (Åsbakk et al., 2015).
In Iceland, despite the presence of arctic foxes and the introduced American mink, no autochthonous Trichinella infection has been reported, even though these animals have been examined. The reason may be that the infection has not yet been introduced. However, two polar bears wandering into Iceland from the Greenland Sea have been found infected, the latter infection was species identified as T. nativa. There are reports of over 500 polar bears stranded in Iceland during centuries, many of which have been killed by humans. Moreover, infected pinnipeds might also have introduced infection and, if dead, eaten by foxes (Skírnisson et al., 2010).
2.1.2.4. Russia
In a large Russia-wide review on Trichinella covering 1950–2019, wolves were reported having highest prevalence (regionally up to 97%), followed by red foxes (up to 80%), and wild boars (up to 21%) (Andreyanov et al., 2019). Yet, at the coastal Arctic, marine mammals may play a more important role. Twelve out of 20 polar bears (60%) from Franz Josef Land and Novaya Zemlya tested with an ELISA kit in 2011 turned out to be positive for antibodies against T. spiralis (Naidenko et al., 2013). In the Republic of Sakha (Yakutia; Russia) the brown bear is considered the most relevant host (Odnokurtsev, 2015). In Chukotka Peninsula, Trichinella infection is maintained by combination of sylvatic and synanthropic cores involving both marine and terrestrial animals (Bukina, 2015).
From 1996 to 2014, 88 trichinellosis patients were registered in the Yamalo-Nenets Autonomous Okrug (YNAO) (population around 500,000), including 31 children. The trend was diminishing, with no cases reported in Revitch, 2009–2014. Since the 1990s, seven outbreaks have been reported in the YNAO, with totally >100 people infected. In the Khanty-Mansiysk Autonomous Okrug (KMAO) (population around 1,500,000), from 1996 to 2014, 85 cases of trichinellosis were officially registered, of which 15 cases were among children under 17 years old. (Peklo and Stepanova, 2020). In the Republic of Sakha (Yakutia) (population around 1,000,000) in Revitch, 2009, 16 cases were reported (3 in children) (Odnokurtsev, 2015). So called “family” cases are typical for Russia when hunters offer their prey to family, friends, and neighbors (Peklo and Stepanova, 2020).
2.2. Associated risk factors
2.2.1. Animals
Circulation of Trichinella among arctic animals has been considered to occur through predation, cannibalism, and carrion feeding (Naidenko et al., 2013; Ivanov et al., 2020).
In several wildlife surveys, age was a significant risk factor for infection with/exposure to Trichinella, which could be attributed to cumulative exposure over a lifetime (Zarnke et al., 1997; Kojola et al., 2016; Owsiacki et al., 2020; Pilfold et al., 2021; Sharma et al., 2021). A higher prevalence of Trichinella in male than female animals was also observed (Kojola et al., 2016; Pilfold et al., 2021; Sharma et al., 2021); the possible reasons for this male bias could be larger home ranges for males and thus, more dispersal and risks for exposure, as well as a wider range of prey species. Higher exposure to Trichinella spp. and higher frequency of cannibalism in older male polar bears advocates cannibalism as a route of transmission in them (Taylor et al., 1985). Cannibalizing their own species is frequent in polar bears from western Hudson Bay, Canada (Russell, 1975; Derocher et al., 1993; Gormezano and Rockwell, 2013).
High prevalence reported in wolverines, wolves, polar bears, grizzly bears, and arctic foxes could be attributed to their high mobility, ability to disperse rapidly over great distances, as well as the proportion of other carnivores and diversity of prey species in their diet (Jenkins et al., 2013). Moreover, some wild animals, for example, wolverines and foxes, are both scavengers and predators, and could act as sentinel species for Trichinella in the Arctic. In historical times, dogs in the Nearctic scavenged meat from harvested carnivores and had high prevalence of infection with Trichinella (Rausch et al., 1956); however, the current status of Trichinella infection in dogs is unknown. Knowledge would help to understand the transmission pattern and epidemiology of this and other foodborne parasites. Results from Greenland suggest continuously high prevalence of Trichinella in sled dogs from regions where polar bears and walrus are commonly hunted (Møller, 2006).
Transmission of Trichinella in marine mammals is enigmatic. Low prevalence in ringed seals (primary food for polar bears) seems incompatible with the high prevalence reported in polar bears. However, the diet of polar bears also includes walrus, and scavenging and cannibalism could also maintain the life cycle in polar bears (Forbes, 2000; Pilfold et al., 2021). Equally enigmatic is the rather high prevalence in walrus, which primarily feed on benthic invertebrates. It is hypothesized that walruses are exposed through scavenging carcasses of terrestrial carnivores, or possibly through consumption of muscle larvae released from decomposing carcasses and concentrated by filter feeding benthic invertebrates. Further surveys are required using large sample size and wide geographical distribution, and perhaps also experimental studies on filter feeders, to investigate these hypotheses and to determine the dietary contribution of prey animals (e.g. seal, walrus, beluga whale etc.) to the predators (e.g. polar bears).
Moreover, human activities could also help in persistence of the parasite in the environment, for example leaving carcasses of trapped wild carnivores available to scavengers, using meat as a bait, feeding meat to the sled/domestic dogs and improper disposal of dog carcasses (Kapel, 1997; Pozio et al., 2001; Peklo and Stepanova, 2020).
2.2.2. Humans
In a serosurvey, age (50+ years), level of education (primary only), and consumption of products from marine mammals were associated with seropositivity for Trichinella (Goyette et al., 2014). In another study, age (40+ years), occupation as fisherman or hunter, and a high intake of polar bear meat were risk factors associated with Trichinella seropositivity (Møller, 2007). Many members of communities in the Arctic are subsistence hunters; game meat plays a pivotal role in their cultural and traditional values and is essential for their health and well-being. Country foods include meat from marine and terrestrial animals as well as plants (Rochette and Blanchet, 2007). Communities commonly consume country food either raw or frozen, boiled or dried; however, if meat is not cooked properly, consumption may lead to Trichinella infection (MacLean et al., 1989). Freezing does not inactivate larvae of arctic adapted species of Trichinella. The effect of drying, fermenting, and smoking on the infectivity of larvae remains unknown, and cannot be trusted on. Cooking meat thoroughly (not microwaving), however, does inactivate larvae, though, this is not always culturally acceptable in the Canadian North, neither among all other indigenous peoples in the Arctic. Therefore, it becomes important to incorporate risk communication and local food inspection programs. One of such programs, “Nunavik Trichinellosis Prevention Program” was implemented in Nunavik, after 11 outbreaks occurred there in 1982–1999, and this program resulted in considerable decrease in outbreaks in Nunavik (Proulx et al., 2002). This program has had marked success in Nunavik (Larrat et al., 2012), and could be expanded geographically and to other wildlife species harvested for food (bear, lynx) across the Arctic. Such local food safety programs are needed to address food insecurity and facilitate food sharing practices common in the North.
Perhaps due to common knowledge that Trichinella is transmitted through raw or undercooked meat, infection reports in Russia include only 2% of cases involving raw ground meat and “kotlety” (pan-fried minced meat croquettes / cutlet-shaped patties), 7% – corned meat, and 18% – “shashlyk” (a dish of skewered and grilled cubes of meat), whereas boiled or stewed meat was the source in 73% (Chuelov and Rossina, 2019).According to the authors larvae can survive, for instance, inside an 8 cm thick piece of meat during its boiling for 2–2.5 h (Chuelov and Rossina, 2019), which would indicate that “boiling” meat as cooking method does not necessarily everywhere mean the same. In Alaska, a trichinellosis outbreak-implicated meal consisted of frozen walrus meat boiled for approximately 1 h, after which the exterior was fully cooked, but the interior remained undercooked, as preferred by the community (Springer et al., 2017). In 2000 and 2019, International Commission on Trichinellosis (ICT) recommended all parts of pork muscle to be heated to 62.2 °C for instant inactivation of Trichinella larvae, or, alternatively, 1 min (at 60 °C) to 21 h (at 49 °C, Gamble et al., 2000; Noeckler et al., 2019).
Another threat is poorly controlled private roadside eateries and raw meat selling along the highways where no meat inspection is practiced, and dog meat can be offered as pork or beef, keeping in mind that dogs can be highly infected – 12% of 527 human infection cases in Russia were derived from dog meat (Berezantsev, 1974). Commitment of the Indigenous Peoples of the North to traditional dishes such as Kopalhen / Igunaq (meat and fat of marine mammals caught in the summer is buried in the ground as steaks, which then ferment over autumn and freeze over winter, ready for consumption the next year) significantly contributes to the epidemiology of Trichinella (Emelyanova and Yuzhaninova, 2002). Traditionally, Orochi People prefer raw bear and boar meat, saving time during labor season, and for prevention of scurvy. Evenky honor bear very much, which involves a complicated hunting ritual. Once hunted, bears must be presented to other tribes and eaten by as many people as possible. Also, Evenky and some other Peoples believe that they must not break bones, therefore they boil meat (attached to the bones) in big pieces (so that inside the chunk the temperature could remain rather low). Nivkhi, once having hunted the first animal in the season, have to share the prey with all neighbors. Nivkhi also believe that they would lose (good) luck if they do not share a killed dog with neighbors. There is also a tradition in different Peoples to leave a piece of prey they hunted at the place of hunting or to return it to the forest or seawater (Emelyanova and Yuzhaninova, 2002). Thus, serological examination by enzyme-linked immunosorbent assay (ELISA) revealed seropositivity to Trichinella antigen in 24% of 642 indigenous inhabitants of Chukotka examined in 1969 (Berezantsev, 1974) and 29% of 159 in 2015 (Bukina, 2016). The Arctic and subarctic territories of Russia are home to about 160,000 people belonging to the Indigenous Peoples of the North. The life expectancy of the Indigenous Peoples of the North is much lower than that of the general population of Russia and the Indigenous population of the non-Russian North (Revitch Revitch, 2009). Trichinella muscle larvae have been found to survive in decaying human corpse tissue and survive freezing (Chuelov and Rossina, 2019). If a human corpse is somehow available to carrion feeders, it may become a source of infection to them.
2.3. Host range
Trichinella Prevalence reports in Russian arctic mammals are listed in Table 5. It should be noted that before the PCR and DNA sequencing era, helminthologists relied on morphology of Trichinella larvae. Therefore, other similar-looking nematode larvae were probably mistakenly counted, such as Physocephalus sexalatus whose larvae have similar dimensions (Berezantsev, 1974). Of course, there is no reason why this would not be the case also today if proper identification is not performed.
Table 5.
Prevalence of Trichinella in animal species reported as hosts in Russia.
| Host | Prevalence [overall %, (n)] | Method(s) | Reference |
|---|---|---|---|
| ORDER ARTIODACTYLA | |||
| Family Suidae | |||
| Domestic pig (Sus domesticus) | 0.33 (?) 0.02 (?) 0.62 (?) |
NR NR NR |
(Merkushev, 1954)* (Bessonov, 1970)* (Konovalov, 2003)* |
| Wild boar (Sus scrofa) | 1.3 (2815) 11.5 (?) |
NR NR |
(Bessonov, 1970)* (Konovalov, 2003)* |
| ORDER CARNIVORA | |||
| Family Canidae | |||
| Dog (Canis familiaris) | 1.6 (?) 72 (?) 58 (?) 2.67 (23611) 26 (23) 17 (?) 29.4 (?) |
NR NR NR NR NR NR NR |
(Merkushev, 1954)* (Kozlov, 1963)** (Ovsyukova, 1966)** (Bessonov, 1970)* (Sorochenkova, 1971)** (Oshevskaya et al., 2001)* (Konovalov, 2003)* |
| Wolf (Canis lupus) | 100 (?) 51 (1616) 16 (?) 65.4 (26) 15 (?) 14.8 (?) 86.7 (68) 15.2 (59) |
NR NR NR NR NR NR MD, TR NR |
(Merkushev, 1954)* (Ovsyukova, 1966)** (Bessonov, 1970)* (Sorochenkova, 1971)** (Oshevskaya et al., 2001)* (Romashov et al., 2006)* (Bukina, 2015) (Odnokurtsev, 2015) |
| Arctic fox (Vulpes lagopus) | 13 (270) 10 (?) 15.26 (1134) 13.3 (45) 75.6 (995) |
NR NR NR NR MD, TR |
(Lukashenko & Brzhesky, 1963)** (Ovsyukova, 1966)** (Bessonov, 1970)* (Sorochenkova, 1971)** (Bukina, 2015) |
| Red fox (Vulpes vulpes) | 66.6 (?) 18 (?) 10 (?) 18.4 (8135) 8.1 (74) 27.8 (?) 27.6 (?) 20.3 (?) 66.7 (21) 3.3 (90) |
NR NR NR NR NR NR NR NR MD, TR NR |
(Merkushev, 1954)* (Kozlov, 1963)** (Ovsyukova, 1966)** (Bessonov, 1970)* (Sorochenkova, 1971)** (Konovalov, 2003)* (Romashov et al., 2006)* (Andreyanov, 2012)* (Bukina, 2015) (Odnokurtsev, 2015) |
| Family Felidae | |||
| Cat (Felix catus) | 11.8 (?) 7.67 (8958) 17 (?) 30.8 (?) 10.5 (?) 90.9 (22) |
NR NR NR NR NR MD, TR |
(Merkushev, 1954)* (Bessonov, 1970)* (Oshevskaya et al., 2001)* (Konovalov, 2003)* (Romashov et al., 2006)* (Bukina, 2015) |
| Lynx (Felis lynx) | 26.8 (112) 16.7 (6) |
NR NR |
(Bessonov, 1970)* (Odnokurtsev, 2015) |
| Family Mustelidae | |||
| Wolverine (Gulo gulo) | 100 (?) 33.3 (10) 20 (15) |
NR NR NR |
(Ovsyukova, 1966)** (Bessonov, 1970)* (Odnokurtsev, 2015) |
| Common otter (Lutralutra) | 1.9 (106) | NR | (Bessonov, 1970)* |
| Pine marten (Martes martes) | 13 (1100) 36.8 (?) 3.4 (?) |
NR NR NR |
(Bessonov, 1970)* (Romashov et al., 2006)* (Andreyanov, 2012)* |
| Sable (Martes zibellina) | 0.64 (2804) | NR | (Bessonov, 1970)* |
| Badger (Meles meles) | 50 (?) 6 (1118) 58.7 (?) 33.3 (?) 100 (?) |
NR NR NR NR NR |
(Merkushev, 1954)* (Bessonov, 1970)* (Konovalov, 2003)* (Romashov et al., 2006)* (Andreyanov, 2012)* |
| Stoat or short-tailed weasel (Mustela erminea) | 6 (150) 5.2 (846) |
NR NR |
(Lukashenko & Brzhesky, 1963)** (Bessonov, 1970)* |
| European mink (Mustela lutreola) | 0.84 (239) 20 (?) |
NR NR |
(Bessonov, 1970)* (Vagin, 2011)* |
| Least weasel (Mustela nivalis) | 2.48 (161) 14.2 (?) |
NR NR |
(Bessonov, 1970)* (Vagin, 2011)* |
| American mink (Neogale vision) | 32.8 (?) | NR | (Vagin, 2011)* |
| Family Odobenidae | |||
| Walrus (Odobenus rosmarus) | 2 (50) 0.8 (254) 1.5 (598) |
NR NR MD, TR |
(Kozlov& Berezantsev, 1968)** (Bessonov, 1970)* (Bukina, 2015) |
| Family Phocidae | |||
| Bearded seal (Erignathus barbatus nauticus) | 4.3 (675) | MD, TR | (Bukina, 2015) |
| Harp seal (Pagophilus groenlandicus) | 2.3 (210) 1.48 (473) |
NR NR |
(Britov, 1962)** (Bessonov, 1970)* |
| Ringed seal (Phoca hispida) | 1.6 (1131) | MD, TR | (Bukina, 2015) |
| Spotted seal (Phoca largha) | 0.9 (425) | MD, TR | (Bukina, 2015) |
| Family Ursidae | |||
| Brown bear (Ursus arctos) | 58 (?) 18 (280) 40 (5) 80 (15) 19.7 (71) |
NR NR NR MD, TR NR |
(Ovsyukova, 1966)** (Bessonov, 1970)* (Sorochenkova, 1971)** (Bukina, 2015) (Odnokurtsev, 2015) |
| Polar bear (Ursus maritimus) | 20 (20) ? (3) ? (1) 100 (3) |
NR NR NR NR |
(Bessonov, 1970)* (Pereverseva et al., 1971)** (Sorochenkova, 1971)** (Odnokurtsev, 2006)*** |
| ORDER EULIPOTYPHLA | |||
| Family Erinaceidae | |||
| Common hedgehog (Erinaceus europaeus) | 6.88 (218) 14.3 (?) |
NR NR |
(Bessonov, 1970)* (Romashov et al., 2006)* |
| Family Soricidae | |||
| Common shrew (Sorex araneus) | 0.39 (760) | NR | (Bessonov, 1970)* |
| Laxmann's shrew (Sorex caecutiens) | 0.4 (251) | NR | (Bessonov, 1970)* |
| ORDER RODENTA | |||
| Family Cricetidae | |||
| Arctic lemming (Dicrostonyx torquatus) | 0.38 (266) 0.37 (?) |
NR NR |
(Bessonov, 1970)* (Sorochenkova, 1971)** |
| Tundra vole (Microtus oeconomus) | 0.03 (3135) | NR | (Bessonov, 1970)* |
| Bank vole (Myodes glareolus) | 0.18 (2175) 19.5 (?) |
NR NR |
(Bessonov, 1970)* (Oshevskaya et al., 2001)* |
| Muskrat (Ondatra zibethica) | 0.04 (2546) | NR | (Bessonov, 1970)* |
| Family Muridae | |||
| Wood mouse (Apodemus sylvaticus) | 0.53 (4143) | NR | (Bessonov, 1970)* |
| House mouse (Mus musculus) | 0.8 (?) 0.34 (19223) |
NR NR |
(Merkushev, 1954)* (Bessonov, 1970)* |
| Brown rat (Rattus norvegicus) | 2.5 (?) 2.12 (15262) 3.2 (?) 31.1 (?) 3.6 (?) 20 (45) |
NR NR NR NR NR MD, TR |
(Merkushev, 1954)* (Bessonov, 1970)* (Oshevskaya et al., 2001)* (Konovalov, 2003)* (Vagin, 2011)* (Bukina, 2015) |
| Black rat (Rattus rattus) | 0.96 (1141) 4.6 (?) |
NR NR |
(Bessonov, 1970)* (Oshevskaya et al., 2001)* |
| Family Sciuridae | |||
| Red squirrel (Sciurus vulgaris) | 8.3 (24) 0.3 (648) |
NR NR |
(Ovsyukova, 1966)** (Bessonov, 1970)* |
| Long-tailed ground squirrel (Urocitellus undulatus) | 0.34 (866) | NR | (Bessonov, 1970)* |
n: number of samples tested.
MD: Muscle Digestion, NR: Not Recorded, TR: Trichinoscopy.
After: (*) Andreyanov et al., 2019; (**) Berezantsev, 1974; Bukina, 2015; (***) Odnokurtsev, 2015
There are some arctic marine mammal species reported as hosts of Trichinella in the Nearctic, but studies in the Palearctic have not demonstrated infection in them. We refer to beluga whale – 8 animals were studied in Russia and 175 in Norway; grey whale (Eschrichtius robustus) – 119 from Russia, and also one bowhead whale (Balaena mysticetus) in Russia, and Trichinella was not detected in any of them (Berezantsev, 1974; Forbes, 2000; Bukina, 2015).
There are also animal species, candidates-to-be on the host list, involved in Trichinella life cycles. Among them are orcas (Orcinus orca), top predators hunting different pinnipeds. Yet no data on orcas infected with Trichinella is available yet (Forbes, 2000). Another one is reindeer (Rangifer tarandus). Though ruminants, reindeer diversify the diet as much as needed, because they often struggle to obtain food. Therefore, they eat not only grass, herbs and reindeer lichen, but also mushrooms, seaweed, brackish soil, discarded antlers of different cervids, bird eggs, chicks and excrements, as well as lemmings – the Sami people even call the latter “koont saplyg” meaning “reindeer mice” (Oksanen et al., 2000; Loginova and Belova, 2019). Experiments also support the possibility for reindeer to be infected, especially with T. spiralis (Oksanen et al., 2000). It has in 2017 even been claimed by authorities, without much direct evidence presented, that Trichinella infection is widespread among reindeer (Prosto Gazeta, 2017). Unofficial report of Trichinella larvae found in reindeer meat during food examination in Murmansk region appeared in 2018. Nevertheless, until further evidence shown, the reliability of such statements is questionable.
Trichinella pseudospiralis reported in the Palearctic was capable to infect birds of >13 species (Berezantsev, 1974; La Rosa et al., 2001; Pozio, 2005). Nineteen European herring gulls (Larus argentatus) and 31 glaucous gulls (Larus hyperboreus) in Chukotka Peninsula were experimentally exposed to T. nativa, which resulted in finding Trichinella larvae in bird faeces, and mice becoming infected after ingesting those larvae (Odoevskaya et al., 2013; Bukina, 2015). Given that Trichinella larvae, even after being decapsulated, can survive in fresh and sea water for days and months (depending on its temperature), raptorial and carrion birds should, according to the authors, be considered as least potential transport hosts of encapsulated Trichinella species (Odoevskaya et al., 2013; Bukina, 2015).
In Arkhangelsk Oblast and Chukotka Peninsula, Trichinella larvae were species identified from tissues of eleven animals (polar bear, wolverine, arctic fox, ringed seal, brown bear, domestic cat (Felis catus), sled dog, domestic pig (Sus domesticus) and northern sea lion (Eumetopias jubatus)). Trichinella nativa was identified in 8 of these animals. Trichinella spiralis/T. pseudospiralis mixed infection was found in a domestic pig and T. spiralis/T. nativa in two arctic foxes and a northern sea lion (Goździk et al., 2017).
Experimentally, it was found that at least 11 insect species can mechanically transmit infective Trichinella larvae (Bukina, 2015; Andreyanov et al., 2019). This list contains terrestrial necrophages (that can be eaten by terrestrial insectivores), aquatic beetles and blood-sucking insects. Also, small crustaceans were experimentally found to function as transport hosts for Trichinella (Odoevskaya et al., 2013; Bukina, 2015).
Mollusks also were proved to ingest Trichinella larvae and excrete them in faeces (Bukina, 2015). Moreover, larvae seemed to be more protected in faecal pellets of snails and therefore could survive longer. But “alive” is not equal to “infective”, and faecal spreading was not considered having epidemiological significance (Berezantsev, 1974). However, some authors disagree; invertebrates (or fish feeding on them and excreting Trichinella larvae with faeces) were considered a missing link of the food chain leading to walruses, since the latter are often not considered predators. Thus, carcasses of infected carnivores, once on the Arctic coast, are skeletonized by seawater invertebrates, which may then be eaten by walruses (Bukina, 2015). However, there are also solid reports on carnivorous walruses (Fay, 1960; Lowry and Fay, 1984).
3. Comparison with findings from the Antarctic and alpines
In addition to the Arctic at high northern latitudes, Trichinella, as other living organisms, meet rather similar challenges of cold climate also in the Antarctic at high southern latitudes, and in Alpine regions at high altitudes, generally higher than 3.000 m above sea level.
There are no native terrestrial mammals in the Antarctic, and it has been stated that Trichinella spp. are present on all continents apart from Antarctic (Pozio and Zarlenga, 2013). However, there are many species of marine mammals in the Antarctic seas. A total of 101 seals of 3 species (Ross (Ommatophoca rossii), Weddell (Leptonychotes weddellii), and leopard seal (Hydrurga leptonyx) were tested by trichinoscopy. All were negative (Thorshaug and Rosted, 1956). A survey on the health of seals belonging to four different species (Weddell, Ross, crabeater (Lobodon carcinophaga) and Antarctic fur seals (Arctocephalus gazella) did not reveal antibodies against Trichinella in any of the 137 animals tested (Tryland et al., 2012).
On many subantarctic islands and archipelagos, such as Kerguelen and South Georgia, there was an intentional or accidental introduction of alien mammal species, such as reindeer, sheep (Ovis aries), rabbits (Oryctolagus cuniculus), mice (Mus musculus), brown and black rats (Rattus rattus) and cats. We are not aware of any Trichinella studies on these mammalian populations, but it does not appear totally impossible that they could sustain Trichinella life cycles. Anyhow, it must be noted that T. spiralis is the only species known with high affinity to rats (Pozio and Murrell, 2006).
Skuas (Stercorarius spp.) and giant petrels (Macronectes spp.) are opportunistic carrion feeder and predatory bird genera from the Antarctic and the Southern Ocean. We do not know that these birds would have been studied for Trichinella, but their feeding habits might indicate potential to act as suitable hosts for the non-encapsulating T. pseudospiralis which they might encounter during yearly winter migration.
While there is no evidence on Trichinella life cycles in the Antarctic, including subantarctic islands, it cannot be excluded that the lack of evidence may be based on lack of research. However, at least so far, Trichinella infection is still known to be present virtually world-wide, apart from the Antarctic region (Pozio and Zarlenga, 2013).
Trichinella patagoniensis (T12) was described from mountain lions or cougars (Puma concolor) from Argentina (Krivokapich et al., 2012). Muscle larvae tolerate freezing at −5 °C for three months, which might be a favorable adaptation to the cold in alpine regions of South America.
In North America, using Maxent modeling and based on the environmental factors, minimum January temperature, maximum July temperature, elevation, and land cover, Masuoka et al. (2009), found a predicted range of the freeze-resistant Trichinella T6 extending down the mountainous hot continental eco-region, and even in the Sierra Madre Mountains of Mexico. However, in addition to the environment factors, also suitable host animal populations are of course needed for the life cycle to establish. So far, there are obviously no reports of Trichinella species in Sierra Madre.
In the Alps, the snow cover has been found essential for the survival of T. britovi larvae in experimental fox (and raccoon dog) carcasses during the winter by reducing temperature variation in the carcass (Rossi et al., 2019). Climate change is predicted to reduce the presence of snow cover everywhere, exposing carcasses to more extreme temperature fluctuation. This might mean shorter survival of muscle larvae in carcasses. On the other hand, the lack of snow cover might make carcasses easier available to scavengers.
The observations concerning epidemiology of Trichinella in Arctic and subarctic have similarities to those in Antarctic and Alpines. In the changing world, sharing information across these areas with similar expected changes will be relevant.
4. Challenges and solutions to control Trichinella in the Arctic and subarctic
The statement from over 70 years ago - “For the future, it is difficult to see how trichinosis can ever be less than a major public health problem of the Arctic so long as man depends for food upon the spoils of the chase” (Connell, 1949) - remains valid. Trichinella still pose a public health issue in the Arctic.
Trichinella infection prevalences in some animal host species used for human consumption are high (Table 1, Table 2, Table 3, Table 4, Table 5). Further monitoring the situation over time and space is needed to understand the circulation of the parasites, and to identify areas with high parasite prevalence and intensity. Epidemiological research focusing on Trichinella in the Arctic has been relatively limited and scattered, and reviews like this current one are hopefully helpful for obtaining an overview. Identified knowledge gaps should be filled, and research results made available to other researchers and risk assessors.
Trichinella parasites thrive in the Arctic and subarctic, and the genetic diversity within the genus in the region is noteworthy, with T. nativa (T2) and T6 as the main genotypes, but including also T. britovi (T3), T. pseudospiralis (T4) and T. spiralis (T1). In addition to synanthropic animals, T. spiralis has been found e.g. in wolverine in Yukon (Sharma et al., 2021), in red fox in Lapland (Airas et al., 2010) and in northern sea lion in Chukotka (Goździk et al., 2017). Trichinella chanchalensis has so far only been found in wolverines in Yukon and Northwest Territories, but it is a cryptic species which cannot be differentiated from T. nativa by multiplex PCR (Sharma et al., 2021). Therefore, its true distribution is yet unknown.
The circulation of Trichinella in its animal hosts is driven by carnivorism and scavenging, and human actions may facilitate these. Biosecurity practices of farms are important. Carcass and offal disposal practices could be improved to limit easy access. Hunters' practice to leave animal carcasses in the field after skinning has been associated with high Trichinella infection prevalence among wildlife (Pozio et al., 2001; Peklo and Stepanova, 2020).
In many parts of the Arctic, testing the meat before consumption using artificial digestion is not a practical possibility. Routine testing would yield relevant data on the epidemiological situation, and the larvae collected can be identified to species level. Overall, various methods have been utilized to test animals for Trichinella. For instance, Roth and Madsen (Roth and Madsen, 1953; Madsen, 1961) used the compression method with 56 cuts, corresponding to about 1 g of muscle examined, while Rausch in Alaska already in 1949 utilized the digestion method with 50 g samples (Brandly and Rausch, 1950). Serology, often by ELISA (see Barlow et al., 2021), has become a common method especially when testing protected wildlife species (e.g. Rah et al., 2005; Åsbakk et al., 2010).
Sharing data and knowledge are also important. One of the strengths of the current review is its geographical coverage: while the data available are not easily comparable, together they provide an overview of the situation, and help to identify knowledge gaps. Harmonized methods would enable obtaining comparable data, and international collaboration and sharing results internationally are encouraged.
Post-harvest control by proper heat-treatment of meat before consumption is regarded as an efficient way to prevent human infections (Gamble et al., 2000; Noeckler et al., 2019). Education about the importance of sufficient temperature should target relevant audiences, and it needs to take into account traditional food habits, which are culturally sensitive. Information about risks and their avoidance is essential. Heat-treating meat that is given to dogs and cats should also be advocated.
Outbreaks of human trichinellosis occur and should be better prevented. The preparedness to detect and manage outbreaks needs to be supported, and diagnostic capacity should be increased. Source attribution approaches could inform targeted interventions by detecting the foods with highest risk. The relative importance of different sources likely varies by region and may change, and thus local and updated information are needed. A number of outbreaks have originated from eating polar bear meat, but even though infection prevalence in them is high, because Indigenous Peoples traditionally consume polar bear meat well-cooked and trophy hunters are not allowed to kill this protected species, polar bear meat is no longer considered a very important source of human infections (Dupouy-Camet et al., 2017).
Understanding and controlling Trichinella requires a One Health approach that is adapted to the situation. Expected changes in the environment, animal populations and human actions may affect the epidemiology of Trichinella in the Arctic and subarctic, and preparedness and control approaches need to adapt as well. To control Trichinella and to prevent human Trichinella infections, awareness is the first step. Trichinella parasites thrive in the Arctic.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
We thank colleagues who helped to find local literature on the topic.
References
- Aaser C.S. Trichinelliasis in fur-bearing animals. Norsk Veterinaertidsskrift. 1941;53(6):198–226. (In Norwegian) [Google Scholar]
- Abs O., Schmidt H.W. Die arktische Trichinose und ihr Verbreitungsweg. Norsk Polarinstitutt. Skrifter Nr. 1954;105:34. (In German) [Google Scholar]
- Airas N., Saari S., Mikkonen T., Virtala A.M., Pellikka J., Oksanen A., Isomursu M., Kilpelä S.S., Lim. C.W., Sukura A. Sylvatic Trichinella spp. infection in Finland. J. Parasitol. 2010;96(1):67–76. doi: 10.1645/GE-2202.1. [DOI] [PubMed] [Google Scholar]
- Akkuratov V. Dolgoye puteshestviye v nikuda (O puteshestvii S. Andrée na vozdushnom share k Severnomu polyusu, 1897); A long journey to nowhere (About S. Andrée’s journey in a balloon to the North Pole, 1897) Vokrug sveta. 1964;1:46. (In Russian) [Google Scholar]
- Alne J.I., Rossebø L. Forekomst av trikiner i vill-rev på Haugalandet. Nor. Vet. Tidsskr. 1987;99:227–228. (In Norwegian) [Google Scholar]
- Andreyanov O.N., Uspensky A.V., Skvortsova F.K. LLC Scientific and Production Association “Sel’skokhozyaystvennyye tekhnologii”; Moscow: 2019. Trikhinellez v prirodnom biotsenoze: -biologiya vozbuditelya, diagnostika i profilaktika (Trichinellosis in a natural biocenosis: biology of the pathogen, diagnosis and prevention) [In Russian] [Google Scholar]
- Appleyard G., Conboy G., Gajadhar A. Trichinella spiralis in sylvatic hosts from Prince Edward Island. J. Wildl. Dis. 1998;34:158–160. doi: 10.7589/0090-3558-34.1.158. [DOI] [PubMed] [Google Scholar]
- Appleyard G.D., Gajadhar A.A. A review of trichinellosis in people and wildlife in Canada. Can. J. Public Health. 2000;91(4):293–297. doi: 10.1007/BF03404292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arctic Council 2021. https://arctic-council.org/about/states/
- Åsbakk K., Aars J., Derocher A.E., Wiig Ø., Oksanen A., Born E.W., Dietz R., Sonne C., Gofroid J., Kapel C.M. Serosurvey for Trichinella in polar bears (Ursus maritimus) from Svalbard and the Barents Sea. Vet. Parasitol. 2010;172:256–263. doi: 10.1016/j.vetpar.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Åsbakk K., Mørk T., Fuglei E. A serosurvey for Trichinella in Arctic foxes (Vulpes lagopus) in Svalbard. Polar Biol. 2015;38:755–762. [Google Scholar]
- Barlow A., Roy K., Hawkins K., Ankarah A.A., Rosenthal B. A review of testing and assurance methods for Trichinella surveillance programs. Food Waterborne Parasitol. 2021;24 doi: 10.1016/j.fawpar.2021.e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr S. In: Franz Josef Land. Barr s., editor. Norsk Polarinstitutt; Oslo: 1995. The history of western activity in Franz Josef Land; pp. 59–106. [Google Scholar]
- Beniston M. European isotherms move northwards by up to 15 km year: using climate analogues for awareness-raising: speed of climate change in Europe. Int. J. Climatol. 2014;34:1838–1844. doi: 10.1002/joc.3804. [DOI] [Google Scholar]
- Berezantsev Yu.A. “Meditsyna” Leningradskoe Otdelenie, Leningrad. 1974. Trichinellosis; p. 160. [Google Scholar]
- Born E.W., Henriksen S.A. Prevalence of Trichinella sp. in polar bears (Ursus maritimus) from northeastern Greenland. Polar Res. 1990;8:313–315. doi: 10.1111/j.1751-8369.1990.tb00396.x. [DOI] [Google Scholar]
- Born E.W., Clausen B., Henriksen S.A. Trichinella spiralis in walruses from the Thule District, North Greenland, and possible routes of transmission. Zeitschrift Fur Saugetierkunde-Mamm Biol. 1982;47:246–251. [Google Scholar]
- Bruschi F., Murrell K.D. New aspects of human trichinellosis: the impact of new Trichinella species. Postgrad. Med. J. 2002;78:15–22. doi: 10.1136/PMJ.78.915.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukina L.A. Vyatka State Agricultural Academy; Kirov: 2015. Trikhinellez V Pribrezhnykh Rayonakh Chukotskogo Poluostrova, Rasprostraneniye, Mery Profilaktiki (Trichinellosis in the Coastal Regions of the Chukotka Peninsula, Spreading, Preventive Measures) (In Russian) [Google Scholar]
- Bukina L.A. Traditsionnaya pishcha – istochnik zarazheniya trikhinellezom korennogo naseleniya Chukotki (traditional food is a source of Trichinellosis infection of the indigenous population of Chukotka) Aktual’nyye voprosy veterinarnoy biologii. 2016;1:45–50. (In Russian) [Google Scholar]
- Campagna S., Lévesque B., Anassour-Laouan-Sidi E., Côté S., Serhir B., Ward B., Libman M., Drebot M., Makowski K., Andonova M., Ndao M., Dewailly E. Seroprevalence of 10 zoonotic infections in 2 Canadian Cree communities. Diagn. Microbiol. Infect. Dis. 2011;70:191–199. doi: 10.1016/J.DIAGMICROBIO.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Chomel B., Kasten R., Chappuis G., Soulier M., Kikuchi Y. Serological survey of selected canine viral pathogens and zoonoses in grizzly bears (Ursus arctos horribilis) and black bears (Ursus americanus) from Alaska. Rev. Sci. Tech. 1998;17:756–766. doi: 10.20506/RST.17.3.1134. [DOI] [PubMed] [Google Scholar]
- Choquette L.P., Gibson G.G., Pearson A.M. Helminths of the grizzly bear, Ursus arctos L., in northern Canada. Can. J. Zool. 1969;47:167–170. doi: 10.1139/Z69-038. [DOI] [PubMed] [Google Scholar]
- Chuelov S.B., Rossina A.L. Trikhinellez – aktual’naya problema zdravookhraneniya (Trichinellosis is a topical health issue) Detskie Infektsii. 2019;18:30–35. doi: 10.22627/2072-8107-2019-18-2-30-35. [DOI] [Google Scholar]
- Connell F.H. Trichinosis in the Arctic: A review. Arctic. 1949;2:98–107. http://www.jstor.org/stable/40506354 [Google Scholar]
- Dalcin D., Zarlenga D., Larter N., Hoberg E., Boucher D., Merrifield S., Lau R., Ralevski F., Cheema K., Schwartz K., Boggild A. Trichinella nativa outbreak with rare thrombotic complications associated with meat from a black bear hunted in northern Ontario. Clin. Infect. Dis. 2017;64:1367–1373. doi: 10.1093/CID/CIX165. [DOI] [PubMed] [Google Scholar]
- Davidson R.K., Gjerde B., Vikøren T., Lillehaug A., Handeland K. Prevalence of Trichinella larvae and extra-intestinal nematodes in Norwegian red foxes (Vulpes vulpes) vet. Parasitol. 2006;136:307–316. doi: 10.1016/j.vetpar.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Deksne G., Davidson R.K., Buchmann K., Kärssin A., Kirjušina M., Gavarāne I., Miller A.L., Pálsdóttir G.R., Robertson L.J., Mørk T., Oksanen A., Palinauskas V., Jokelainen P. Parasites in the changing world - ten timely examples from the Nordic-Baltic region. Parasite epidemiology and control. 2020;10 doi: 10.1016/j.parepi.2020.e00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derocher A.E., Andriashek D., Stirling I. Terrestrial foraging by polar bears during the ice-free period in western Hudson Bay. Arctic. 1993;46(3):251–254. [Google Scholar]
- Dupouy-Camet J., Kociecka W., Bruschi F., Bolas-Fernandez F., Pozio E. Opinion on the diagnosis and treatment of human trichinellosis. Expert. Opin. Pharmacother. 2002;3(8):1117–1130. doi: 10.1517/14656566.3.8.1117. [DOI] [PubMed] [Google Scholar]
- Dupouy-Camet J., Bourée P., Yera H. Trichinella and polar bears: a limited risk for humans. J. Helminthol. 2017;91:440–446. doi: 10.1017/S0022149X17000219. [DOI] [PubMed] [Google Scholar]
- Eieland E. Norsk Veterinaertidsskrift. Vol. 60. 1948. Trichinosis in polar bears; pp. 414–415. (cited by Thorshaug & Rosted, 1956) [Google Scholar]
- Ekstam M. Svensk Veterinärtidning. Vol. 16. 1964. Trikinos; pp. 11–49. (cited by Roneus and Christensson, 1979) [Google Scholar]
- Emelyanova T.N., Yuzhaninova M.V. Severnye Prostory; Moscow: 2002. Narody Krainego Severa i Dal’nego Vostoka Rossii v trudakh issledovatelei. XX vek (Peoples of the Far North and Far East of Russia in the works of researchers. XX century) [In Russian] [Google Scholar]
- European Commission 2021. https://ec.europa.eu/environment/nature/natura2000/biogeog_regions/boreal/index_en.htm
- Fay F.H. Carnivorous walrus and some Arctic Zoonoses. Arctic. 1960;13:111–122. doi: 10.14430/ARCTIC3691. [DOI] [Google Scholar]
- Folkehelseinstituttet 2021. https://www.fhi.no/nettpub/smittevernveilederen/sykdommer-a-a/trikinose/
- Folkhälsomyndigheten 2021. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/smittsamma-sjukdomar/trikinos-/
- Forbes L.B. The occurrence and ecology of Trichinella in marine mammals. Vet. Parasitol. 2000;93:321–334. doi: 10.1016/S0304-4017(00)00349-6. [DOI] [PubMed] [Google Scholar]
- Frechette J., Rau M. Helminths of the black bear in Quebec. J. Wildl. Dis. 1977;13:432–434. doi: 10.7589/0090-3558-13.4.432. [DOI] [PubMed] [Google Scholar]
- Freeman R.S. Leveä heisimato ja trikiini luonnonvaraisissa ketuissa Suomessa. (broad fish tape-worm and trichina worm in wild red foxes in Finland) Suomen eläinlääkärilehti. 1964;70:279–282. (In Finnish) [Google Scholar]
- Gajadhar A.A., Forbes L.B. A 10-year wildlife survey of 15 species of Canadian carnivores identifies new hosts or geographic locations for Trichinella genotypes T2, T4, T5, and T6. Vet. Parasitol. 2010;168:78–83. doi: 10.1016/J.VETPAR.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Gamble H.R., Bessonov A.S., Cuperlovic K., Gajadhar A.A., Van Knapen F., Noeckler K., Schenone H., Zhu X. International commission on Trichinellosis: recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet. Parasitol. 2000;93(3–4):393–408. doi: 10.1016/s0304-4017(00)00354-x. [DOI] [PubMed] [Google Scholar]
- Gazeta Prosto. Общественно-информационная газета «Просто газета». Public information newspaper “Prosto Gazeta”. 2017. http://просто-газета.рф/articles/media/2017/12/5/na-zametku-ohotnikam/
- Gormezano L.J., Rockwell R.F. What to eat now? Shifts in polar bear diet during the ice-free season in western Hudson Bay. Ecol. Evol. 2013;3(10):3509–3523. doi: 10.1002/ece3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottstein B., Pozio E., Nöckler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009;22:127–145. doi: 10.1128/CMR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyette S., Cao Z., Libman M., Ndao M., Ward B.J. Seroprevalence of parasitic zoonoses and their relationship with social factors among the Canadian Inuit in Arctic regions. Diagn. Microbiol. Infect. Dis. 2014;78:404–410. doi: 10.1016/j.diagmicrobio.2013.08.026. [DOI] [PubMed] [Google Scholar]
- Goździk K., Odoevskaya I.M., Movsesyan S.O., Cabaj W. Molecular identification of Trichinella isolates from wildlife animals of the Russian Arctic territories. Helminthologia. 2017;54(1):11–16. [Google Scholar]
- Hallen L.G. Beobachtungen ueber eine Trichinoseepidemie in Lindesberg (Schweden). Bericht ueber 40 Fälle. Acta Medica Scandinavica. 1938;94:355–365. (In German) [Google Scholar]
- Handeland K., Skjerve E., Stuen S., Moustgaard A. Trichinella examinations of commercially hunted harp and hooded seals. Bull. Scand. Soc. Parasitol. 1995;5(3):15–16. http://sbsp.eu/images/PDF/Bulletin_SBSP/Old/SSP_Bulletin_1995_Vol_5_No_3.pdf [Google Scholar]
- Handeland K., Slettbakk T., Helle O. Freeze-resistant Trichinella (Trichinella nativa) established on the Scandinavian peninsula. Acta Vet. Scand. 1995;36:149–151. doi: 10.1186/BF03547712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms N.J., Larivee M., Scandrett B., Russell D. High prevalence and intensity of Trichinella infection in Yukon American black (Ursus americanus) and grizzly (Ursus arctos) bears. J. Wildl. Dis. 2021;57:429–433. doi: 10.7589/JWD-D-20-00135. [DOI] [PubMed] [Google Scholar]
- Health Board 2021. https://www.terviseamet.ee/et/nakkushaigused-menuu/tervishoiutootajale/nakkushaigustesse-haigestumine accessed 19.12.2021.
- Hirvelä-Koski V., Aho M., Asplund K., Hatakka M., Hirn J. Trichinella spiralis in wild animals, cats, mice, rats and farmed fur animals in Finland. Nordisk veterinaermedicin. 1985;37:234–242. [PubMed] [Google Scholar]
- Houzé S., Ancelle T., Matra R., Boceno C., Carlier Y., Gajadhar A.A., Dupouy-Camet J. Trichinellosis acquired in Nunavut, Canada in September 2009: meat from grizzly bear suspected. Eurosurveillance. 2009;14:19383. doi: 10.2807/ESE.14.44.19383-EN. [DOI] [PubMed] [Google Scholar]
- Hülphers G., Henricson T. Svensk Veterinär Tidning. Vol. 48. 1943. Undersökningar på förekomst av infektionsämnen och trikiner hos råttor. (Studies on the occurrence of microbes and Trichinella in rats) pp. 245–254. (cited by Roneus and Christensson, 1979) [Google Scholar]
- Hurníková Z., Hrčková G., Ågren E., Komorová P., Forsman J., Chovancová B., Molnár L., Letková V. First finding of Trichinella pseudospiralis in two tawny owls (Strix aluco) from Sweden. Helminthologia. 2014;51:190–197. doi: 10.2478/s11687-014-0228-5. [DOI] [Google Scholar]
- Isomursu M., Kunnasranta M. Trichinella nativa in grey seal Halichoerus grypus: spill-over from a highly endemic terrestrial ecosystem. J. Parasitol. 2011;97:735–736. doi: 10.1645/GE-2717.1. [DOI] [PubMed] [Google Scholar]
- Ivanov E.A., Mizin I.A., Kirilov A.G., Platonov N.G., Mordvintsev I.N., Naidenko S.V., et al. Observations of intraspecific killing, cannibalism, and aggressive behavior among polar bears (Ursus maritimus) in the eastern Barents Sea and the Kara Sea. Polar Biol. 2020;43:2121–2127. doi: 10.1007/s00300-020-02771-7. [DOI] [Google Scholar]
- Järvis T., Miller I., Pozio E. Epidemiological studies on animal and human trichinellosis in Estonia. Parasite. 2001;8:S86–S87. doi: 10.1051/parasite/200108s2086. [DOI] [PubMed] [Google Scholar]
- Jenkins E., Castrodale L., de Rosemond S., Dixon B., Elmore S., Gesy K., EP H., Polley L., Schurer J., Simard M., Thompson R. Tradition and transition: parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Adv. Parasitol. 2013;82:33–204. doi: 10.1016/B978-0-12-407706-5.00002-2. [DOI] [PubMed] [Google Scholar]
- Jokelainen P., Näreaho A., Hälli O., Heinonen M., Sukura A. Farmed wild boars exposed to toxoplasma gondii and Trichinella spp. Vet. Parasitol. 2012;187(1–2):323–327. doi: 10.1016/j.vetpar.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Kana S. CONSUME mapping study for Estonia. Estonian Fund for Nature (ELF) 2017. http://media.voog.com/0000/0037/1265/files/Consumemappingstudy_silja_final_updated_version_22_03_2018.pdf Available at. Accessed 19.12.2021.
- Kapel C., Measures L., Møller L., Forbes L., Gajadhar A. Experimental Trichinella infection in seals. Int. J. Parasitol. 2003;33:1463–1470. doi: 10.1016/S0020-7519(03)00202-9. [DOI] [PubMed] [Google Scholar]
- Kapel C.M. Trichinella in arctic, subarctic and temperate regions: Greenland, the Scandinavian countries and the Baltic States. Southeast Asian J. Trop. Med. Public Health. 1997;28:14–19. (PMID: 9656341) [PubMed] [Google Scholar]
- Kapel C.M. Sylvatic and domestic Trichinella spp. in wild boars; infectivity, muscle larvae distribution, and antibody response. J. Parasitol. 2001;87 doi: 10.1645/0022-3395(2001)087[0309:SADTSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kapel C.M.O., Henriksen S.A., Berg T.B., Nansen P. Epidemiologic and zoogeographic studies on Trichinella nativa in arctic fox, Alopex lagopus, in Greenland. Proc. Helminthol. Soc. Wash. 1996;63(2):226–232. [Google Scholar]
- Kapel C.M.O., Pozio E., Sacchi L., Prestrud P. Freeze tolerance, morphology, and RAPD-PCR identification of Trichinella nativa in naturally infected arctic foxes. J. Parasitol. 1999;85:144–147. doi: 10.2307/3285722. [DOI] [PubMed] [Google Scholar]
- Kärssin A., Velström K., Gómez-Morales M.A., Saar T., Jokelainen P., Lassen B. Cross-sectional study of anti-Trichinella antibody prevalence in domestic pigs and hunted wild boars in Estonia. Vector Borne Zoonotic Dis. 2016;16:604–610. doi: 10.1089/vbz.2016.1943. [DOI] [PubMed] [Google Scholar]
- Kärssin A., Häkkinen L., Niin E., Peik K., Vilem A., Jokelainen P., Lassen B. Trichinella spp. biomass has increased in raccoon dogs (Nyctereutes procyonoides) and red foxes (Vulpes vulpes) in Estonia. Parasit. Vectors. 2017;10:609. doi: 10.1186/s13071-017-2571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärssin A., Häkkinen L., Vilem A., Jokelainen P., Lassen B. Trichinella spp. in wild boars (Sus scrofa), brown bears (Ursus arctos), Eurasian lynxes (Lynx lynx) and badgers (Meles meles) in Estonia, 2007–2014. Animals. 2021;11:183. doi: 10.3390/ani11010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauhala K., Helle E. Population ecology of the raccoon dog in Finland - a synthesis. Wildl. Biol. 1995;1:3–9. doi: 10.2981/wlb.1995.004. [DOI] [Google Scholar]
- Kauhala K., Laukkanen P., Von Rége I. Summer food composition and food niche overlap of the raccoon dog, red fox and badger in Finland. Ecography. 1998;21:457–463. doi: 10.1111/j.1600-0587.1998.tb00436.x. [DOI] [Google Scholar]
- Kjos-Hanssen B. Freeze-resistance of Trichinella cysts in polar bears from the high-arctic region of Norway (Svalbard) Acta Vet. Scand. 1983;24:244. doi: 10.1186/BF03546752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojola I., Holmala K., Huhta E., Oksanen A., Kokko S. Prevalence of Trichinella infection in three sympatric large carnivores: effects of the host’s sex and age. J. Zool. 2016;301:69–74. doi: 10.1111/jzo.12394. [DOI] [Google Scholar]
- Korhonen P., Pozio E., La Rosa G., et al. Phylogenomic and biogeographic reconstruction of the Trichinella complex. Nat. Commun. 2016;7:10513. doi: 10.1038/ncomms10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivokapich S.J., Pozio E., Gatti G.M., Prous C.L., Ribicich M., Marucci G., et al. Trichinella patagoniensis n. sp. (Nematoda), a new encapsulated species infecting carnivorous mammals in South America. Int. J. Parasitol. 2012;42:903–910. doi: 10.1016/j.ijpara.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Kuitunen-Ekbaum E. Walrus meat as a source of trichinosis in Eskimos. Can. J. Public Heal. 1954;45:30. [Google Scholar]
- La Rosa G., Pozio E., Henriksen S.A. Biochemical characterization of Trichinella in Greenland. Acta Vet. Scand. 1990;31(3):381–383. doi: 10.1186/BF03547552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Marucci G., Zarlenga D.S., Pozio E. Trichinella pseudospiralis populations of the Palearctic region and their relationships with populations of the Nearctic and Australian regions. Int. J. Parasitol. 2001;31(3):297–305. doi: 10.1016/S0020-7519(01)00110-2. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Marucci G., Zarlenga D., Casulli A., Zarnke R., Pozio E. Molecular identification of natural hybrids between Trichinella nativa and Trichinella T6 provides evidence of gene flow and ongoing genetic divergence. Int. J. Parasitol. 2003;33:209–216. doi: 10.1016/S0020-7519(02)00258-8. [DOI] [PubMed] [Google Scholar]
- Larrat S., Simard M., Lair S., Bélanger D., Proulx J.F. From science to action and from action to science: the Nunavik Trichinellosis prevention program. Int. J Circumpolar Health. 2012;71:18595. doi: 10.3402/ijch.v71i0.18595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen T., Kjos-Hanssen B. Trichinella sp. in polar bears from Svalbard, in relation to hide length and age. Polar Res. 1983;1(1):89–96. [Google Scholar]
- Larter N.C., Forbes L.B., Elkin B.T., Allaire D.G. Prevalence of Trichinella spp. in black bears, grizzly bears, and wolves in the Dehcho region, northwest territories, Canada, including the first report of T. nativa in a grizzly bear from Canada. J. Wildl. Dis. 2011;47:745–749. doi: 10.7589/0090-3558-47.3.745. [DOI] [PubMed] [Google Scholar]
- Lassen B., Janson M., Viltrop A., Neare K., Hütt P., Golovljova I., Tummeleht L., Jokelainen P. Serological evidence of exposure to globally relevant zoonotic parasites in the Estonian population. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0164142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E.S. Natural foci of infection of some helminthiases. (translated from Russian) Public Health Rep. 1961;76:263–272. (PMID: 13760561) [PMC free article] [PubMed] [Google Scholar]
- Loginova O.A., Belova L.M. Teoriya i praktika bor'by s parazitarnymi boleznyami, Proceedings of the 20th International Conference. Nauka; Moscow: 2019. Food habits of reindeer contributing to their infestation with helminths; pp. 318–322.10.31016/978-5-9902340-8-6.2019.20.318-322 [In Russian] [Google Scholar]
- Lowry L.F., Fay F.H. Seal eating by walruses in the Bering and Chukchi seas. Polar Biol. 1984;3:11–18. [Google Scholar]
- Lukashenko N.P., Vol’fson A.G., Istomin V.A., Chernov V.Y. Trichinellosis of animals in Chukotka, USSR: a general review. Int. J. Parasitol. 1971;1(3–4):287–296. doi: 10.1016/0020-7519(71)90031-2. [DOI] [PubMed] [Google Scholar]
- Lynnerup N. The Thule inuit mummies from Greenland. Anat. Rec. 2015;298(6):1001–1006. doi: 10.1002/AR.23131. [DOI] [PubMed] [Google Scholar]
- MacLean J.D., Viallet J., Law C., Staudt M. Trichinosis in the Canadian Arctic: report of five outbreaks and a new clinical syndrome. J. Infect. Dis. 1989;160(3):513–520. doi: 10.1093/INFDIS/160.3.513. [DOI] [PubMed] [Google Scholar]
- MacLean J.D., Poirier L., Gyorkos T.W., et al. Epidemiologic and serologic definition of primary and secondary trichinosis in the Arctic. J. Infect. Dis. 1992;165(5):908–912. doi: 10.1093/INFDIS/165.5.908. [DOI] [PubMed] [Google Scholar]
- Madsen H. The distribution of Trichinella spiralis in sledge dogs and wild mammals in Greenland. Medd. Grønland. 1961;159(7):1–124. [Google Scholar]
- Mäkelä T. Trichinosis in a zoo employee. Scand. J. Infect. Dis. 1970;2:75–76. doi: 10.3109/inf.1970.2.issue-1.13. [DOI] [PubMed] [Google Scholar]
- Malakauskas A., Paulauskas V., Järvis T., Keidans P., Eddi C., Kapel C.M.O. Molecular epidemiology of Trichinella spp. in three Baltic countries: Lithuania, Latvia, and Estonia. Parasitol. Res. 2007;100(4):687–693. doi: 10.1007/s00436-006-0320-y. [DOI] [PubMed] [Google Scholar]
- Masterton J.P., Lewis H.E. Trichinosis in greenland. Lancet. 1955;269(6890):591. doi: 10.1016/s0140-6736(55)92587-3. [DOI] [PubMed] [Google Scholar]
- Masuoka P.M., Burke R., Colaccico M., Razuri H., Hill D., Murrell K.D. Predicted geographic ranges for north American sylvatic Trichinella species. J. Parasitol. 2009;95(4):829–837. doi: 10.1645/GE-1952.1. [DOI] [PubMed] [Google Scholar]
- Mikkonen T., Valkama J., Wihlman H., Sukura A. Spatial variation of Trichinella prevalence in rats in Finnish waste disposal sites. J. Parasitol. 2005;91(1):210–213. doi: 10.1645/GE-3230RN. [DOI] [PubMed] [Google Scholar]
- Miller I., Järvis T., Pozio E. Epidemiological investigations on Trichinella infections in farmed fur animals of Estonia. Vet. Parasitol. 2006;139(1–3):140–144. doi: 10.1016/j.vetpar.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Møller N.L. The Royal Veterinary and Agricultural University; Denmark: 2006. Epidemiology of Trichinella in Greenland – Occurrence in Animals and Man & Observations on Anisakidae Infections in Humans. Ph.D. thesis. [Google Scholar]
- Møller N.L. Epidemiology of Trichinella in Greenland – occurrence in animals and man. Int. J. Circumpolar Health. 2007;66(1):77–79. doi: 10.3402/ijch.v66i1.18230. [DOI] [PubMed] [Google Scholar]
- Mörner T., Eriksson H., Bröjer C., Nilsson K., Uhlhorn H., Ågren E., Hård af Segerstad C., Jansson D.S., Gavier-Widén D. Diseases and mortality in free-ranging brown bear (Ursus arctos), gray wolf (Canis lupus), and wolverine (Gulo gulo) in Sweden. J. Wildl. Dis. 2005;41(2):298–303. doi: 10.7589/0090-3558-41.2.298. [DOI] [PubMed] [Google Scholar]
- Naidenko S.V., Ivanov E.A., Mordvintsev I.N., Platonov N.G., Ershov R.V., Rozhnov V.V. Seropositivity for different pathogens in polar bears (Ursus maritimus) from Barents Sea Islands. Biol. Bull. 2013;40(9):779–782. doi: 10.1134/S1062359013090082. [DOI] [Google Scholar]
- Näreaho A., Sankari S., Mikkonen T., Oivanen L., Sukura A. Clinical features of experimental trichinellosis in the raccoon dog (Nyctereutes procyonoides) Vet. Parasitol. 2000;91(1–2):79–91. doi: 10.1016/s0304-4017(00)00261-2. [DOI] [PubMed] [Google Scholar]
- Noeckler K., Pozio E., van der Giessen J., Hill D.E., Gamble H.R. International commission on Trichinellosis: recommendations on post-harvest control of Trichinella in food animals. Food Waterborne Parasitol. 2019;14 doi: 10.1016/j.fawpar.2019.e00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozais J.P., Mannevy V., Danis M. Deux cas de trichinose après ingestion de viande d’ours blanc (Thalarctos maritimus) au Groenland (two cases of trichinosis from polar bear (Thalarctos maritimus) meat) Med. Mal. Infect. 1996;26(6–7):732–733. doi: 10.1016/S0399-077X(96)80107-7. [DOI] [Google Scholar]
- Odnokurtsev V.A. SB RAS; Novosibirsk: 2015. Parasitic Fauna of Vertebrates in Yakutia. (In Russian) [Google Scholar]
- Odoevskaya I., Uspensky A., Voronin M., Movsesyan S., Bukina L., Panayotova-Pencheva M., Malczewski A., Cabaj W., Shuikina E. Role of birds and invertebrates in epidemiology and epizootology of trichinellosis at Chukotka seashores. Acta zoologica bulgarica. 2013;65:531–536. [Google Scholar]
- Oivanen L., Oksanen A. Synanthropic Trichinella infection in Finland. Vet. Parasitol. 2009;159(3–4):281–284. doi: 10.1016/j.vetpar.2008.10.057. [DOI] [PubMed] [Google Scholar]
- Oivanen L., Kapel C.M., Pozio E., La Rosa G., Mikkonen T., Sukura A. Associations between Trichinella species and host species in Finland. J. Parasitol. 2002;88(1):84–88. doi: 10.1645/0022-3395(2002)088[0084:ABTSAH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Oivanen L., Näreaho A., Jokela S., Rikula U., Gamble R., Sukura A. The prevalence of Trichinella infection in domestic dogs in Finland. Vet. Parasitol. 2005;132(1–2):125–129. doi: 10.1016/j.vetpar.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Oksanen A., Lindgren E., Tunkkari P. Epidemiology of trichinellosis in lynx in Finland. J. Helminthol. 1998;72(1):47–53. doi: 10.1017/s0022149x00000973. [DOI] [PubMed] [Google Scholar]
- Oksanen A., Oivanen L., Eloranta T., Tirkkonen T., Åsbakk K. Experimental trichinellosis in reindeer. J. Parasitol. 2000;86(4):763–767. doi: 10.1645/0022-3395(2000)086[0763:ETIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Oksanen A., Interisano M., Isomursu M., Heikkinen P., Tonanzi D., Oivanen L., Pozio E. Trichinella spiralis prevalence among wildlife of a boreal region rapidly reduced in the absence of spillover from the domestic cycle. Vet. Parasitol. 2018;262:1–5. doi: 10.1016/j.vetpar.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Owen I.L., Gomez Morales M.A., Pezzotti P., Pozio E. Trichinella infection in a hunting population of Papua New Guinea suggests an ancient relationship between Trichinella and human beings. Trans. R. Soc. Trop. Med. Hyg. 2005;99(8):618–624. doi: 10.1016/J.TRSTMH.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Owsiacki R., Buhler K.J., Sharma R., Branigan M., Fenton H., Tomaselli M., Kafle P., Lobanov V.A., Bouchard É., Jenkins E. Trichinella nativa and Trichinella T6 in arctic foxes (Vulpes lagopus) from northern Canada. Int. J. Parasitol. Parasites Wildl. 2020;13:269–274. doi: 10.1016/j.ijppaw.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peklo G.N., Stepanova T.F. Zabolevayemost’ trikhinellezom v Ural’skom federal’nom okruge (Trichinellosis incidence in the Ural Federal District) Zdorov’ye naseleniya i sreda obitaniya. 2020;6(327):55–62. 10.35627/2219-5238/2020-327-6-55-62 [in Russian] [Google Scholar]
- Pilfold N.W., Richardson E.S., Ellis J., Jenkins E., Scandrett W.B., Hernández-Ortiz A., Buhler K., McGeachy D., Al-Adhami B., Konecsni K., Lobanov V.A., Owen M.A., Rideout B., Lunn N.J. Long-term increases in pathogen seroprevalence in polar bears (Ursus maritimus) influenced by climate change. Glob. Chang. Biol. 2021;27:4481–4497. doi: 10.1111/gcb.15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B.C., Chadee K., Dick T.A. Helminth parasites of pine marten, Martes americana (Turton), from Manitoba, Canada. J. Wildl. Dis. 1983;19:10–13. doi: 10.7589/0090-3558-19.1.10. [DOI] [PubMed] [Google Scholar]
- Pozio E. Factors affecting the flow among domestic, synanthropic and sylvatic cycles of Trichinella. Vet. Parasitol. 2000;93(3–4):241–262. doi: 10.1016/S0304-4017(00)00344-7. [DOI] [PubMed] [Google Scholar]
- Pozio E. The broad spectrum of Trichinella hosts: from cold- to warm-blooded animals. Vet. Parasitol. 2005;132:3–11. doi: 10.1016/j.vetpar.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Pozio E. Adaptation of Trichinella spp. for survival in cold climates. Food Waterborne Parasitol. 2016;4:4–12. doi: 10.1016/j.fawpar.2016.07.001. [DOI] [Google Scholar]
- Pozio E., Murrell K.D. Systematics and epidemiology of Trichinella. Adv. Parasitol. 2006;63:367–439. doi: 10.1016/S0065-308X(06)63005-4. [DOI] [PubMed] [Google Scholar]
- Pozio E., Zarlenga D.S. New pieces of the Trichinella puzzle. Int. J. Parasitol. 2013;43(12−13):983–997. doi: 10.1016/j.ijpara.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Pozio E., Miller I., Jarvis T., Kapel C.M.O., Rosa G.L. Distribution of sylvatic species of Trichinella in Estonia according to climate zones. J. Parasitol. 1998;84(1):193. doi: 10.2307/3284561. [DOI] [PubMed] [Google Scholar]
- Pozio E., Casulli A., Bologov V.V., Marucci G., La Rosa G. Hunting practices increase the prevalence of Trichinella infection in wolves from European Russia. J. Parasitol. 2001;87(6):1498–1501. doi: 10.1645/0022-3395(2001)087[1498:HPITPO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pozio E., Christensson D., Stéen M., Marucci G., La Rosa G., Bröjer C., Mörner T., Uhlhorn H., Ågren E., Hall M. Trichinella pseudospiralis foci in Sweden. Vet. Parasitol. 2004;125(3–4):335–342. doi: 10.1016/j.vetpar.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Prestrud P., Stuve G., Holt G. The prevalence of Trichinella sp. in arctic foxes (Alopex lagopus) in Svalbard. J. Wildl. Dis. 1993;29(2):337–340. doi: 10.7589/0090-3558-29.2.337. [DOI] [PubMed] [Google Scholar]
- Proulx J.F., MacLean J.D., Gyorkos T.W., Leclair D., Richter A.K., Serhir B., Forbes L., Gajadhar A.A. Novel prevention program for trichinellosis in Inuit communities. Clin. Infect. Dis. 2002;34:1508–1514. doi: 10.1086/340342. [DOI] [PubMed] [Google Scholar]
- Rah H., Chomel B.B., Follmann E.H., Kasten R.W., Hew C.H., Farver T.B., Garner G.W., Amstrup S.C. Serosurvey of selected zoonotic agents in polar bears (Ursus maritimus) Vet. Rec. 2005;156:7–13. doi: 10.1136/vr.156.1.7. [DOI] [PubMed] [Google Scholar]
- Ramey A.M., Cleveland C.A., Hilderbrand G.V., Joly K., Gustine D.D., Mangipane B., Leacock W.B., Crupi A.P., Hill D.E., Dubey J.P., Yabsley M.J. Exposure of Alaska brown bears (Ursus arctos) to bacterial, viral, and parasitic agents varies spatiotemporally and may be influenced by age. J. Wildl. Dis. 2019;55:576–588. doi: 10.7589/2018-07-173. [DOI] [PubMed] [Google Scholar]
- Rausch R., Babero B., Rausch R.V., Schiller E. Studies on the helminth fauna of Alaska. XXVII. The occurrence of larvae of Trichinella spiralis in Alaskan mammals. J. Parasitol. 1956;42:259–271. doi: 10.2307/3274850. [DOI] [PubMed] [Google Scholar]
- Reichard M.V., Torretti L., Snider T.A., Garvon J.M., Marucci G., Pozio E. Trichinella T6 and Trichinella nativa in wolverines (Gulo gulo) from Nunavut, Canada. Parasitol. Res. 2008;103:657–661. doi: 10.1007/s00436-008-1028-y. [DOI] [PubMed] [Google Scholar]
- Revitch B.A. Klimaticheskiye izmeneniya kak novyy faktor riska dlya zdorov’ya naseleniya rossiyskogo Severa (climatic changes as new risk factor for population health in Russian north) Ekologiya cheloveka. 2009;06:11–16. (In Russian) [Google Scholar]
- Ringertz O., Lundbäck H., Zetterberg B., Hederstedt B., Kallings L.O., Damgaard K., Löfdahl A. Trichinosis in Sweden in 1961. Acta Path Microbiol Scand. 1961;54:351–352. [Google Scholar]
- Rislakki V. Trikineistä ja niitten esiintymisestä Suomessa. [On the incidence of trichinae in Finland] Suom. Eläinlääkäril. 1956;62:382–395. (In Finnish) [Google Scholar]
- Rochette L., Blanchet C. Qanuippitaa ? How are we? Methodological report. Inst. Natl. santé publique du Québec. 2007:325. [Google Scholar]
- Ronéus O., Christensson D. Presence of Trichinella spiralis in free-living red foxes (Vulpes vulpes) in Sweden related to Trichinella infection in swine and man. Acta Vet. Scand. 1979;20(4):583–594. doi: 10.1186/BF03546586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa A. Toppolitiker alvorligt syg af trikiner. 2016, December 02. https://sermitsiaq.ag/node/192297 Sermitsiaq.AG.
- Rossi L., Interisano M., Deksne G., Pozio E. The subnivium, a haven for Trichinella larvae in host carcasses. Int. J. Parasitol. Parasites Wildl. 2019;8:229–233. doi: 10.1016/j.ijppaw.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth H. Trichinosis in arctic animals. Nature. 1949;163(4151):805–806. doi: 10.1038/163805b0. [DOI] [PubMed] [Google Scholar]
- Roth H., Madsen H. Die Trichinose in Grönland, abschliessender Bericht der Jahre 1948-1953 (trichinosis in Greenland, final report for the years 1948-1953) Proc. XIV Int. Congr. Zool. 1953:340–341. (in German) [Google Scholar]
- Ruokavirasto 2021. https://www.ruokavirasto.fi/teemat/zoonoosikeskus/zoonoosit/loisten-aiheuttamat-taudit/trikinelloosi/
- Russell R.H. The food habits of polar bears of James Bay and Southwest Hudson Bay in summer and autumn. Arctic. 1975;28:117–129. [Google Scholar]
- Sampasa-Kanyinga H., Lévesque B., Anassour-Laouan-Sidi E., Côté S., Serhir B., Ward B., Libman M., Drebot M., Ndao M., Dewailly E. Zoonotic infections in native communities of James Bay, Canada. Vector Borne Zoonotic Dis. 2012;12:473–481. doi: 10.1089/VBZ.2011.0739. [DOI] [PubMed] [Google Scholar]
- Sauvé C.C., Hernández-Ortiz A., Jenkins E., Mavrot F., Schneider A., Kutz S., Saliki J.T., Daoust P.Y. Exposure of the gulf of St. Lawrence grey seal Halichoerus grypus population to potentially zoonotic infectious agents. Dis. Aquat. Org. 2020;142:105–118. doi: 10.3354/DAO03536. [DOI] [PubMed] [Google Scholar]
- Schellenberg R., Tan B., Irvine J., Stockdale D., Gajadhar A., Serhir B., Botha J., Armstrong C., Woods S., Blondeau J., McNab T. An outbreak of trichinellosis due to consumption of bear meat infected with Trichinella nativa, in 2 northern Saskatchewan communities. J. Infect. Dis. 2003;188:835–843. doi: 10.1086/378094. [DOI] [PubMed] [Google Scholar]
- Schiller E.L. Studies on the helminth fauna of Alaska. V. Notes on Adak rats (Rattus norvegicus Berkenhout) with special reference to helminth parasites. J. Mammal. 1952;33:38–49. doi: 10.2307/1375639. [DOI] [Google Scholar]
- Seryodkin I.V., Odoyevskaya I.М., Konyaev S.V., Spiridonov S.E. Trichinella infection of wild carnivorans in Primorsky Krai, Russian Far East. Nature Conservation Research. Заповедная наука. 2020;5(S2):31–40. [Google Scholar]
- Seymour J., Horstmann-Dehn L., Rosa C., Lopez J. Occurrence and genotypic analysis of Trichinella species in Alaska marine-associated mammals of the Bering and Chukchi seas. Vet. Parasitol. 2014;200:153–164. doi: 10.1016/J.VETPAR.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Shaikenov B.S. Ecological border of distribution of Trichinella nativa Britov et Boev, 1972 and T. nelsoni Britov et Boev, 1972. Wiad. Parazytol. 1992;38(3–4):85–91. [PubMed] [Google Scholar]
- Sharma R., Thompson P., Elkin B., Mulders R., Branigan M., Pongracz J., Wagner B., Scandrett B., Hoberg E., Rosenthal B., Jenkins E. Trichinella pseudospiralis in a wolverine (Gulo gulo) from the Canadian north. Int. J. Parasitol. Parasites Wildl. 2019;9:274–280. doi: 10.1016/J.IJPPAW.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Thompson P.C., Hoberg E.P., Scandrett B.W., Konecsni K., Harms N.J., Kukka P.M., Jung T.S., Elkin B., Mulders R., Larter N.C., Branigan M., Pongracz J., Wagner B., Kafle P., Lobanov V.A., Rosenthal B.M., Jenkins E.J. Hiding in plain sight: discovery and phylogeography of a cryptic species of Trichinella (Nematoda: Trichinellidae) in wolverine (Gulo gulo) Int. J. Parasitol. 2020;50:277–287. doi: 10.1016/j.ijpara.2020.01.003. [DOI] [PubMed] [Google Scholar]
- Sharma R., Harms N.J., Kukka P.M., Jung T.S., Parker S.E., Ross S., Thompson P., Rosenthal B., Hoberg E.P., Jenkins E.J. High prevalence, intensity, and genetic diversity of Trichinella spp. in wolverine (Gulo gulo) from Yukon. Canada. Parasites and Vectors. 2021;14:1–9. doi: 10.1186/s13071-021-04636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skírnisson K., Marucci G., Pozio E. Trichinella nativa in Iceland: an example of Trichinella dispersion in a frigid zone. J. Helminthol. 2010;84(2):182–185. doi: 10.1017/S0022149X09990514. [DOI] [PubMed] [Google Scholar]
- Smith H., Snowdon K. Sylvatic trichinosis in Canada. Can. J. Vet. Res. 1988;52:488–489. [PMC free article] [PubMed] [Google Scholar]
- Springer Y.P., Casillas S., Helfrich K., Mocan D., Smith M., Arriaga G., Mixson L., Castrodale L., McLaughlin J. Two outbreaks of trichinellosis linked to consumption of walrus meat — Alaska, 2016–2017. MMWR Morb. Mortal. Wkly Rep. 2017;66:692. doi: 10.15585/MMWR.MM6626A3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukura A., Näreaho A., Veijalainen P., Oivanen L. Trichinellosis in farmed wild boar: meat inspection findings and seroprevalence. Parasite. 2001;8:S243–S245. doi: 10.1051/parasite/200108s2243. [DOI] [PubMed] [Google Scholar]
- Taylor M., Larsen T., Schweinsburg R.E. Observations of intraspecific aggression and cannibalism in polar bears (Ursus maritimus) Arctic. 1985;38(4):303–309. [Google Scholar]
- Thing H., Clausen B., Henriksen S.A. Finding of Trichinella spiralis in a walrus (Odobenus rosmarus L) in Thule District. Northwest Greenland. Nordisk Veterinaer Medicin. 1976;28(1):59. [PubMed] [Google Scholar]
- Thorborg N.B., Tulinius S., Roth H. Trichinosis in Greenland. Acta Pathologica et Microbiologica Scandinavica. 1948;25(6):778–794. [Google Scholar]
- Thorshaug, K., 1940. Trikinose i Norge. Norsk Veterinaertidsskrift, 475 (In Norwegian).
- Thorshaug K., Rosted A.F. 1956. Researches into the Prevalence of Trichinosis in Animals in Arctic and Antarctic waters; p. 19. [Google Scholar]
- Tryde E.A. Bonnier; Stockholm: 1952. De döda på Vitön: sanningen om Andrée; p. 172. (In Swedish) [Google Scholar]
- Tryland M., Nymo I.H., Nielsen O., Nordøy E.S., Kovacs K.M., Krafft B.A., et al. Serum chemistry and antibodies against pathogens in antarctic fur seals, Weddell seals, crabeater seals, and Ross seals. J. Wildl. Dis. 2012;48(3):632–645. doi: 10.7589/0090-3558-48.3.632. [DOI] [PubMed] [Google Scholar]
- Turovski A., Jussi I., Jussi M. The parasitofauna of seals in Estonian waters. Bull. Scand. Soc. Parasitol. 1995;5(2):55. http://sbsp.eu/images/PDF/Bulletin_SBSP/Old/SSP_Bulletin_1995_Vol_5_No_2.pdf [Google Scholar]
- Valdmann H., Moks E., Talvik H. Helminth fauna of Eurasian lynx (Lynx lynx) in Estonia. J. Wildl. Dis. 2004;40(2):356–360. doi: 10.7589/0090-3558-40.2.356. [DOI] [PubMed] [Google Scholar]
- Välimaa H., Niemimaa J., Oksanen A., Henttonen H. Sylvatic Trichinella reservoir not found among voles in Finland. Acta Vet. Scand. 2010;52(Suppl 1):S15. [Google Scholar]
- Viallet J., MacLean J., Goresky C., Staudt M., Routhier M., Law C. Arctic trichinosis presenting as prolonged diarrhea. Gastroenterology. 1986;91(4):938–946. doi: 10.1016/0016-5085(86)90698-0. [DOI] [PubMed] [Google Scholar]
- Zarlenga D., Thompson P., Pozio E. Trichinella species and genotypes. Res. Vet. Sci. 2020;133:289–296. doi: 10.1016/j.rvsc.2020.08.012. [DOI] [PubMed] [Google Scholar]
- Zarnke R.L., Gamble R., Heckert R.A., Hoef J. Ver. Serologic survey for Trichinella spp. in grizzly bears from Alaska. J. Wildl. Dis. 1997 doi: 10.7589/0090-3558-33.3.474. [DOI] [PubMed] [Google Scholar]