Abstract
Context
Most methods for assessing dietary intake have considerable measurement error. Dietary biomarkers are objective tools for dietary assessment. Dietary biomarkers of dietary patterns have not been well described, despite modern dietary guidelines endorsing dietary patterns.
Objective
This systematic review sought to describe the dietary biomarkers commonly used to assess dietary patterns, and the novel biomarkers of dietary patterns identified by exploratory studies.
Data Sources
MEDLINE, Embase, Cochrane Central, PreMEDLINE, and CINAHL databases were searched.
Data Extraction
Data extraction and bias assessment were undertaken in duplicate.
Data Analysis
A qualitative approach was applied, without statistical analysis.
Conclusion
In controlled settings, dietary biomarkers of single nutrients or of individual foods or food groups are commonly used to assess compliance with dietary patterns. However, currently, there are no dietary biomarkers or biomarker profiles that are able to identify the specific dietary pattern that has been consumed by an individual. Future work should seek to validate novel dietary biomarkers and biomarker profiles that are indicative of specific dietary patterns and their characteristics. A dietary biomarker panel consisting of multiple biomarkers is almost certainly necessary to capture the complexity of dietary patterns.
Systematic Review Registration
PROSPERO registration no. CRD42019129839.
Keywords: biological markers, biomarker, dietary assessment, dietary pattern
INTRODUCTION
A “single-nutrient approach” that focuses on individual nutrients, foods, and food groups has traditionally been the dominant modality for nutrition research and policy. This approach, however, may not fully capture the complexities of dietary intake, given nutrient–nutrient interactions, intercorrelations, and food matrix characteristics.1 An alternative approach is to view nutrition through the prism of dietary patterns, which capture the overall combination of the dietary components, including the quality, quantity, and frequency of consumption.1 This approach acknowledges the synergistic and antagonistic effects of nutrients and foods, and, therefore, better aligns with the complexity of real-world dietary intake.2
The application of both approaches is impaired by the relatively low accuracy of current methods for assessing habitual dietary intake. Food frequency questionnaires are commonly used in large-scale clinical trials and cohort studies, despite the inaccuracy inherent in recall of habitual intake over an extended period of time. Multiple short-term dietary recalls using multipass methods are increasingly seen as the most accurate method of assessing usual dietary intake,3 although this method is not exempt from random or systematic measurement error due to the subjective nature of self-report, estimation of portion size, etc. Also, a high number of recalls is required to capture infrequently consumed nutrients or foods, especially at an individual level.4 More-objective dietary assessment would enhance nutritional research and improve the evidence base for the development of dietary guidelines.
Dietary biomarkers are an attractive alternative or additional measure for assessing dietary intake. Defined as measurable and quantifiable biological indicators of dietary intake or nutritional status,5 dietary biomarkers have not only been used in validating dietary assessment tools,6 but have demonstrated the ability to predict dietary intake independent of traditional dietary assessment tools.7 Dietary biomarkers can be categorized as either direct biomarkers of dietary exposure (ie, measures of consumed nutrients) or as biomarkers of nutritional status (ie, indicators of dietary intake affected by metabolism and nutrient–nutrient interactions).8 Both types of dietary biomarkers are commonly used for assessing consumption of specific nutrients, or of individual foods or food groups.9–11 The widespread adoption of metabolomics has shifted the field further during the past decade.12 Metabolomics is the study of the different molecules synthesized by an organism and can provide a broad profile of the metabolites that are present in a biospecimen,13 including many associated with dietary intake. One strength of metabolomics, in comparison with the use of traditional nutritional biomarkers, is that it is often applied in an unbiased manner as part of exploratory research; however, this approach requires subsequent validation. A key weakness of metabolomics is that these metabolites may be associated with a variety of foods, and as indicators they may lack specificity; however, they have great potential for development and application within biomarker profiles of dietary patterns.
One previous systematic review investigated urinary biomarkers of dietary intake, including dietary patterns. That review included 191 articles on urinary biomarkers, but only 4 of the articles described their relationship to dietary patterns14; the review reported on the Mediterranean diet, the vegetarian diet, diets based on dietary guidelines, and dietary clusters identified by cluster analysis. However, the use of urinary biomarkers as dietary pattern indicators was not a focus and was not discussed in depth. The present comprehensive review of biomarkers for dietary patterns sought to identify the nature of those currently being used within clinical trials, and those for which there is strong evidence for their value as biomarkers of dietary patterns.
Accordingly, a systematic review of randomized controlled trials (RCTs) was undertaken to identify dietary biomarkers currently used to assess compliance with dietary patterns, as well as those biomarkers and metabolites affected by intake of a distinct dietary pattern in exploratory metabolomics studies.
METHODS
Protocol and registration
The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, Registration no. CRD42019129839), and conducted as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.15 The PRISMA checklist is included in Appendix S1 in the Supporting Information online.
Selection criteria
The inclusion criteria were (1) RCT study design; (2) healthy adult participants, including some participants who have a chronic disease; (3) food-based intervention focusing on a dietary pattern, without nutritional supplementation; (4) having an appropriate comparator, ie, a different dietary pattern; (5) dietary biomarkers or metabolomic profiles were reported based on different dietary patterns. The exclusion criteria were (1) studies with all participants selected on the basis of a current diagnosis of metabolic syndrome, diabetes, cardiovascular diseases, cancer, or other diseases; (2) study participants selected on the basis of pregnancy or lactation; (3) the dietary intervention focused on one component of the diet such as a single nutrient, or individual foods or food groups; (4) the intervention involved supplementation or energy restriction; (5) the control group(s) differed in less than 2 components (foods/food groups) of the intervention diet; (6) article not written in English language; (7) research only published in the form of a scientific abstract. The PICOS (participants, interventions, comparators, outcomes, and study design) criteria are shown in Table 1.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Criteria |
|---|---|
| Participants | Healthy adult participants; however, including studies in which some of the participants have a chronic disease |
| Interventions | Food-based intervention focusing on a dietary pattern, without nutritional supplementation |
| Comparators | A different dietary pattern |
| Outcomes | Dietary biomarkers, including metabolomic profiles |
| Study design | Randomized controlled trials |
Information source
The information was sourced from databases, including MEDLINE, Embase, Cochrane Central, PreMEDLINE, and CINAHL until November 16, 2020. Reference lists from included publications and from reviews on dietary pattern analysis were also reviewed to supplement the electronic database search.
Search strategy
The search strategy was developed in MEDLINE and adapted for Embase, Cochrane Central, PreMEDLINE, and CINAHL. The search terms used in MEDLINE were “Biomarkers,” “Metabolome” and “Metabolomics,” which were then combined with “Diet, Carbohydrate Loading,” “Diet, Atherogenic,” “exp Diet, Carbohydrate-Restricted,” “Diet, Fat-Restricted,” “Diet, Gluten-Free,” “Diet, High-Fat,” “exp Diet, High-Protein,” “Diet, Mediterranean,” “Diet, Paleolithic,” “Diet, Protein-Restricted,” “Diet, Sodium-Restricted,” “exp Diet, Vegetarian,” “Diet, Western,” “Dietary Approaches To Stop Hypertension,” and “Healthy Diet.” A line of key word searching was also included as “([Biomarker* or biochemical marker* or biological marker* or metabolomic* or metabolite* or metabolome*] adj5 [nutrition* or food* or diet* or DASH or eating]).tw.” The search was then limited to RCTs in human adults published in the English language. The study type and animal filters used were derived from the Scottish Intercollegiate Guidelines Network (SIGN) systematic review filters.16 The full electronic search strategy for MEDLINE is presented in Appendix S2 in the Supporting Information online.
Study selection
First-round screening was undertaken by reviewer S.L. A random sample of 12% of all abstracts was checked by reviewer F.M.O’L. and a 100% agreement was reached. The full text of each article was then assessed in duplicate by 3 reviewers (S.L., R.F.N., and C.A.T.) independently, and any discrepancies were resolved by consultation with reviewers (F.M.O’L., M.R.S., and K.S.B.-A.). The screening process was completed on reference management software EndNote (version 9, Clarivate Analytics, Philadelphia, PA, USA).
Data collection process
Data extraction was completed with the use of Research Electronic Data Capture (REDCap), a secure online application for data collection and management.17,18 Data were extracted from each of the identified articles by 2 reviewers (S.L. and R.F.N.) independently, and verified by reviewers F.M.O’L. and M.R.S.
Data extracted include study title, first author, publication year, country, study design, aim, selection criteria, study time frame, population size, participant characteristics, dietary pattern details, biomarkers, biological compartment, assay technique, statistical method, conclusions, limitations, and funding sources.
Risk of bias
The Cochrane risk-of-bias tool version 219 was used by 2 reviewers (S.L. and R.F.N.) independently to assess the risk of bias of each publication. A total of 5 domains were assessed: (1) bias arising from the randomization process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in measurement of the outcome, and (5) bias in selection of the reported result. An overall risk of bias judgment was made as “Low risk of bias” (if judged as having “low risk of bias” for all domains), “Some concerns” (if judged as having “some concerns” for at least 1 domain and not judged as having “high risk of bias” for any domain), and “High risk of bias” (if judged as having “high risk of bias” for at least 1 domain, or “some concerns” for multiple domains in a way that substantially increased the risk of bias).
RESULTS
Study selection
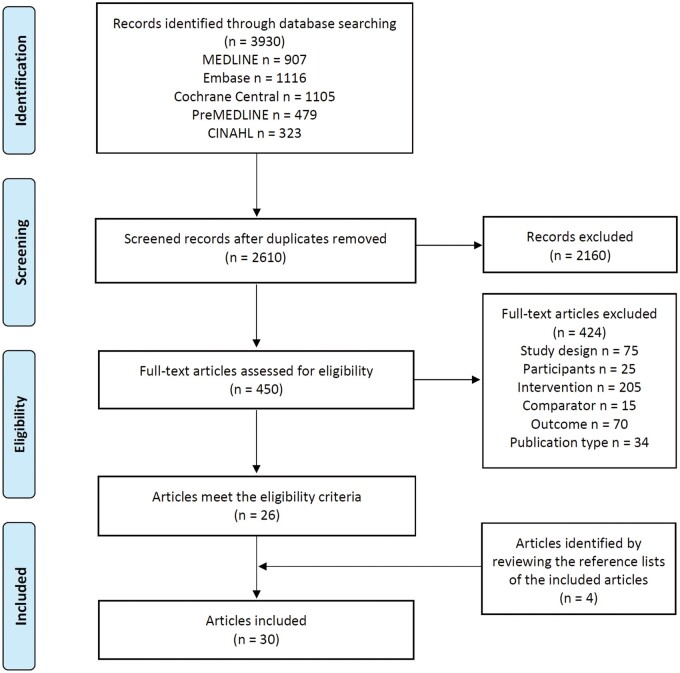
The complete study selection procedure is summarized in Figure 1. Briefly, the literature search retrieved 3930 records; a total of 2610 records were screened for titles and abstracts, of which 2160 records were excluded. From the remaining 450 full texts screened, a total of 30 publications met the inclusion criteria. A number of publications provided data on different biomarkers from the same RCTs,20–32 using various biospecimen types, or measurement approaches; thus, results were included from 22 RCTs.
Figure 1.
PRISMA flowchart of the study selection procedure.
Study characteristics
The main characteristics of the included studies are shown in Table 2.20–49 Of the 30 publications identified, 13 utilized prospectively identified dietary biomarkers.23,25–27,29,33–35,37–39,42,45 These dietary biomarkers have all been previously proposed as indicators of a dietary characteristic (eg, nutrient, food, or food group). The other 17 publications applied a metabolomics approach to identify novel biomarkers of dietary patterns.20–22,24,28,30–32,36,40,41,43,44,46–49 Publications were from the United States (n = 9),22,25–28,30,35,43,49 Denmark (n = 5),20,21,24,29,31 and Spain (n = 3),23,32,39 2 each from Australia,33,42 Germany,34,38 and the UK,36,45 and 1 each from Canada,48 Italy,40 Korea,46 the Netherlands41 and Sweden.44 Two publications were based on multinational trials from European37 and Nordic countries47 (n = 1 each). The 30 publications reported findings from 22 trials, with 5 papers from a Danish trial,20,21,24,29,31 4 from a US trial,25–27,30 and 2 each from Spanish23,32 and US trials.22,28 Sixteen publications reported trials with sample size of <100 participants,22,28,32,34–36,38–44,46,48,49 10 with a sample size of 100–200 participants,20,21,24,26,27,29,31,33,45,47 and 4 with a sample size of >300 participants23,25,30,37 (maximum 1127 subjects37). The participants’ mean age ranged from 22 to 71 years, and between 31% to 100% of participants were female.
Table 2.
Characteristics of included randomized controlled trials
| Reference | Country | Trial name (if available) | Study design | Participants (sample size, mean age, % female (F), mean BMI) | Intervention | Control | Biomarkers | Statistical method, Technique |
|---|---|---|---|---|---|---|---|---|
| Mediterranean diet | ||||||||
| Davis et al (2017)33 | Australia | Parallel | n = 152, 71 y, 56% F, 27.0 kg/m2 |
|
|
|
|
|
| Diekmann et al (2019)34 | Germany | Crossover | n = 26, 70 y, 31% F, 30.3 kg/m2 |
|
|
|
|
|
| Djuric et al (2009)35 | USA | Parallel | n = 69, 44 y, 100% F, 24 kg/m2 |
|
|
|
|
|
| Fitó et al (2014)23 | Spain | PREDIMED | Parallel |
|
|
Low-fat diet (reduce all fat and American Heart Association guidelines) |
|
|
| Jennings et al (2020)37 | Europe (France, Italy, Netherlands, Poland, UK) | New Dietary Strategies Addressing the Specific Needs of the Elderly Population for Healthy Aging in Europe (NU-AGE) | Parallel | n = 1142, 71 y, 55% F, 26.6 kg/m2 |
|
|
|
|
| Marin et al (2011)39 | Spain | Crossover |
|
|
|
|
|
|
| Meslier et al (2020)40 | Italy | Parallel | n = 82, 43 y, 52% F, 31.1 kg/m2 |
|
Habitual (Western) diet (n = 39) |
|
|
|
| Michielsen et al (2019)41 | Netherlands | FoodBALL | Parallel | n = 47, 56 y, 57% F, 27.4 kg/m2 |
|
|
|
|
| Park et al (2019)43 | USA | Crossover | n = 18, 31 y, 35% F, 22.6 kg/m2 |
|
High-fat diet (Atkins) Very-low-fat diet (Ornish) |
|
|
|
| Vázquez-Fresno et al (2015)32 | Spain | PREDIMED (nondiabetic) | Parallel |
|
|
Low-fat diet n = 30 (reduce all fat and American Heart Association guidelines) |
|
|
| Zhu et al (2020)49 | USA | Crossover | n = 10, 22 y, 50% F, 24.4 kg/m2 |
|
|
|
|
|
| Dietary approaches to stop hypertension | ||||||||
| McClure et al (2019)25 | USA | DASH Trial | Parallel | n = 397, 45 y, 48% F, 28.2 kg/m2 |
|
|
|
ANOVA followed by pairwise comparisons with Tukey’s honest significant difference test only if ANOVA P < 0.05 |
| Miller et al (2005)27 | USA | Parallel |
|
|
TAD, n = 52 |
|
|
|
| Miller et al (1998)26 | USA | Ancillary study within the DASH trial | Parallel | n = 123, 48.5 y, 47% F, 27.5 kg/m2 |
|
|
|
t-test (ANCOVA) with Bonferroni adjustment HPLC |
| Nowson et al (2009)42 | Australia | Parallel |
|
|
|
|
|
|
| Rebholz et al (2018)30 | USA | DASH Trial subset | Parallel |
|
|
|
|
|
| Healthy Nordic diet | ||||||||
| Acar et al (2019)20 | Denmark | SHOPUS | Parallel |
|
|
Average Danish diet (Higher in imported and processed foods, refined grains, meat, dairy, sugary products, convenience foods, low fiber vegetables, imported fruit) |
|
|
| Anderson et al (2014)21 | Denmark | SHOPUS | Parallel |
|
|
Average Danish diet |
|
|
| Khakimov et al (2016)24 | Denmark | SHOPUS | Parallel | n = 145, age range 18–65 y, 69% F, BMI not reported Centrally obese |
Run-in: 1 wk Intervention: 26 wks New Nordic diet |
Average Danish diet |
|
|
| Poulsen et al (2014)29 | Denmark | SHOPUS | Parallel | n = 181, 42 y, 71% F, 30.2 kg/m2 Centrally obese | Run-in: 1 wk Intervention: 26 wks New Nordic diet |
Average Danish diet |
|
Student’s t-test |
| Trimigno et al (2020)31 | Denmark | OPUS | Parallel |
|
New Nordic diet | Average Danish diet |
|
|
| Tuomainen et al (2019)47 | Nordic countries (Finland, Sweden, Denmark, Iceland) | SYSDIET | Parallel | n = 164, 55 y, 66% F, 31.7 kg/m2 |
|
|
|
|
| Diet based on dietary guidelines | ||||||||
| Garcia-Perez et al (2017)36 | UK | Crossover | n = 19, 55.8 y, 47% F, 25.6 kg/m2 |
|
Diet 4: least concordant with WHO healthy eating guidelines. |
|
|
|
| Reidlinger et al (2015)45 | UK | CRESSIDA | Parallel | n = 162, 52 y, 60% F, 26.2 kg/m2 Nonsmoking healthy individuals |
|
|
|
|
| Low-glycemic-load diet | ||||||||
| Barton et al (2015)22 | USA | Carbohydrates And Related Biomarkers (CARB) study | Crossover | n = 19, 31.6 y, 53% F, BMI not reported 58% overweight/obese |
|
|
|
|
| Navarro et al (2019)28 | USA | Carbohydrates And Related Biomarkers (CARB) study | Crossover | n = 80, 30 y, 50% F, BMI not reported |
|
|
|
|
| Vegetarian diet | ||||||||
| Lederer et al (2019)38 | Germany | Parallel | n = 53, 32 y, 62% F, 23.1 kg/m2 |
|
|
|
|
|
| Raådjursöga et al (2018)44 | Sweden | Crossover | n = 32, 29 y, 50% F, 22.1 kg/m2 |
|
Omnivore diet |
|
|
|
| Prudent diet | ||||||||
| Wellington et al (2019)48 | Canada | Subset of Diet and Gene Intervention (DIGEST) pilot study | Parallel | n = 42, 47 y, 64% F, 27 kg/m2 |
|
|
|
|
| Korean diet | ||||||||
| Shin et al (2019)46 | Korea | Crossover | n = 54, 41 y, 48% F, 27.5 kg/m2 |
|
|
Urinary:
|
|
|
Values are means ± SDs; +, higher in intervention group; –, lower in intervention group; =, no difference between groups, unless otherwise indicated. Use of established dietary biomarkers of specific foods or nutrients.
Use of metabolomics to study biomarkers and biomarker profiles; targeted metabolomics focused on defined subsets of metabolites within specific metabolic pathways, and untargeted metabolomics quantified all metabolites globally (including known and unknown) in a biospecimen.
Metabolomics type (targeted or untargeted) was ascertained from the reported methodology as this information was not specified in the article.Abbreviations: 1H-NMR, hydrogen-1 proton nuclear magnetic resonance; 3-HB, 3-hydroxybutyrate; ANCOVA, analysis of covariance; ANOVA, analysis of variance; BMI, body mass index; BP, blood pressure; CHO, carbohydrate; CVD, cardiovascular disease; DASH, dietary approaches to stop hypertension; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; EVOO, extra virgin olive oil; FAs, fatty acids; FFA, free fatty acid; GC-MS, Gas Chromatography–Mass Spectrometry; GL, glycemic load; HPLC, high-performance liquid chromatography; HUFA, highly unsaturated fatty acid; LC-MS, liquid chromatography–mass spectrometry; LFD, low-fat diet; MUFAs, monounsaturated fatty acids; n–3, omega 3; n–6, omega 6; N-AGN, n-acetylglutamine; NHANES, National Health and Nutrition Examination Survey; NS, not significant; PAGN, phenylacetylglutamine; PLS-DA, partial least-squares discriminant analysis; PUFAs, polyunsaturated fatty acids; RAD, recommended American diet; SFAs, saturated fatty acids; TAD, typical American diet; TFAs, trans fatty acids; TMAO, trimethylamine N-oxide; VOO, virgin olive oil.
The main dietary patterns studied were the Mediterranean diet (n = 11),23,32–35,37,39–41,43,49 the healthy Nordic diet (n = 6),20,21,24,29,31,47 the DASH diet (n = 5),25–27,30,42 a low-glycemic-load (GL) diet (n = 2),22,28 a vegetarian diet (n = 2),38,44 diets based on national and international guidelines (n = 2) (ie, UK and World Health Organization [WHO] Eating Guidelines),36,45 Korean diet [n = 1],46 and a prudent diet (n = 1).48 The Mediterranean diet was compared with a Western habitual diet (n = 8),33–35,37,39–41,49 a low-fat diet (n = 4),23,32,39,43 a low-carbohydrate, high-fat, high-protein (Atkins) diet (n = 1),43 and a monounsaturated fatty acid (MUFA)-rich diet but otherwise similar to a Western habitual diet (n = 1).41 The healthy Nordic diet was compared with a typical Nordic47 or Danish diet20,21,24,29,31 (n = 6), while the DASH diet was compared with a typical American diet (n = 4),25–27,30 a diet high in fruit and vegetables (n = 3),25,26,30 and a healthy diet based on the Australian dietary guidelines (n = 1).42
Two studies detailed dietary biomarkers within the acute postprandial stage (3 and 4.5 hours)34,44; the intervention period of the other studies ranged from 3 days to 5 years. Most of the RCTs used a parallel design (n = 14)20,21,23–27,29–33,35,37,38,40–42,45,47,48 and the rest used a crossover design (n = 8).22,28,34,36,39,43,44,46,49 The majority of the trials provided intervention foods (n = 19)20,22,23,26,30,33,34,36–39,41,42,44–49; most provided all foods (n = 13),20,22,26,30,34,36,38,39,41,44,46,48,49 but some trials provided only key food items (n = 6).23,33,37,42,45,47 Meals were monitored20,22,26,30,34,36,44,46,49 or assessed by food records or questionnaires23,33,37–39,41,42,45,47,48 in trials that provided foods. Food was not provided in 3 trials, but food records were collected to confirm dietary compliance.35,40,43
Risk of bias within studies
Thirteen publications included in the review were rated as having low risk of bias, 16 publications were rated as having some concerns, and 1 publication was rated as having high risk of bias, mostly due to issues with the randomization process. The full results are shown in Table 3.20–49
Table 3.
Risk of bias for individual studies
| Reference | Randomization process | Deviations from the intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall |
|---|---|---|---|---|---|---|
| Acar et al (2019)20 | Low | Low | Low | Low | Low | Low |
| Anderson et al (2014)21 | Low | Low | Low | Low | Low | Low |
| Barton et al (2015)22 | Low | Low | Low | Low | Low | Low |
| Davis et al (2017)33 | Low | Low | Low | Low | Low | Low |
| Diekmann et al (2019)34 | Some concerns | Low | Low | Low | Low | Some concerns |
| Djuric et al (2009)35 | Some concerns | Low | Low | Low | Low | Some concerns |
| Fitó et al (2014)23 | High | Low | High | Low | Some concerns | High |
| Garcia-Perez et al (2017)36 | Low | Low | Low | Low | Low | Low |
| Jennings et al (2020)37 | Some concerns | Low | Low | Low | Low | Some concerns |
| Khakimov et al (2016)24 | Low | Low | Low | Low | Low | Low |
| Lederer et al (2019)38 | Low | Low | Low | Low | Low | Low |
| Marin et al (2011)39 | Some concerns | Low | Low | Low | Low | Some concerns |
| McClure et al (2019)25 | Some concerns | Low | Low | Low | Low | Some concerns |
| Meslier et al (2020)40 | Some concerns | Low | Low | Low | Low | Some concerns |
| Michielsen et al (2019)41 | Some concerns | Low | Low | Low | Low | Some concerns |
| Miller et al (2005)27 | Some concerns | Low | Low | Low | Low | Some concerns |
| Miller et al (1998)26 | Some concerns | Low | Low | Low | Low | Some concerns |
| Navarro et al (2019)28 | Low | Low | Low | Low | Low | Low |
| Nowson et al (2009)42 | Some concerns | Low | Low | Low | Low | Some concerns |
| Park et al (2019)43 | Some concerns | Low | Low | Low | Low | Some concerns |
| Poulsen et al (2014)29 | Low | Low | Low | Low | Low | Low |
| Rådjursöga et al (2018)44 | Some concerns | Low | Low | Low | Low | Some concerns |
| Rebholz et al (2018)30 | Low | Low | Low | Low | Low | Low |
| Reidlinger et al (2015)45 | Low | Low | Low | Low | Low | Low |
| Shin et al (2019)46 | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Trimigno et al (2020)31 | Low | Low | Low | Low | Low | Low |
| Tuomainen et al (2019)47 | Some concerns | Low | Low | Low | Low | Some concerns |
| Vázquez-Fresno et al (2015)32 | Some concerns | Low | Low | Low | Low | Some concerns |
| Wellington et al (2019)48 | Low | Low | Low | Low | Low | Low |
| Zhu et al (2020)49 | Some concerns | Low | Low | Low | Low | Some concerns |
Mediterranean diet
Eleven publications generated from 10 studies reported dietary biomarkers of the Mediterranean diet. Three were conducted in the United States, 2 in Spain, and 1 each in Australia, Germany, Italy, the Netherlands, and Europe.
A study from Australia measured serum carotenoids (β-cryptoxanthin, lycopene, α-carotene, β-carotene, and lutein:zeaxanthin ratio), erythrocyte fatty acids (saturated fatty acids, trans fatty acids [TFAs], MUFAs, omega-3 [n–3], omega-6 [n–6], docosahexenoic acid [DHA], n–6:n–3, and n–3 index), and 24-h urinary electrolytes (sodium, potassium, calcium and magnesium) in 152 participants to assess their compliance with a Mediterranean diet intervention in comparison with that of a habitual diet group.33 The serum α-carotene, and erythrocyte MUFA %, DHA %, and n–3 index were higher, and the percentage of saturated fatty acids and TFA % were lower at 6 months compared with baseline in the Mediterranean diet group. No within- or between-group differences were identified in the other biomarkers tested.
A study conducted in the United States analyzed plasma lipids and carotenoids in 69 females following a Mediterranean diet or their habitual diet for 6 months.35 Oleic acid, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, total carotenoids, and γ-tocopherol were higher in the Mediterranean diet group compared with the control group, whereas linoleic acid was lower.
A multinational trial from Europe assessed serum selenium, ferritin, iron, and soluble transferrin receptor in 1142 participants who followed a Mediterranean-style diet or a habitual Western diet for 1 year.37 No differences were observed between the groups in these nutritional biomarkers.
Plasma α-tocopherol, retinol, β-carotene, and vitamin C were assessed 4.5 hours after the consumption of a meal during two study visits in a study in Germany, with the 26 study participants consuming a Mediterranean-type diet meal and a Western-diet high-fat meal in a random order.34 Vitamin C, α-tocopherol, and β-carotene were higher, but retinol did not differ following the Mediterranean-type diet meal compared with the Western-diet high-fat meal.
Plasma β-carotene and α-tocopherol levels were also determined in a 3-arm crossover trial conducted in Spain in which 20 participants followed the Mediterranean diet, a high-saturated fat diet, and a low-fat/high-carbohydrate diet.39 β-carotene was higher following a 4-week Mediterranean diet compared with either a high-saturated fat diet or a low-fat/high-carbohydrate diet. No variation was found in the plasma α-tocopherol level between groups.
The other study from Spain, the PREDIMED study, is one of the largest dietary RCTs with a parallel design and it compared the Mediterranean diet with virgin olive oil (VOO) or mixed nuts with a low-fat diet. That study measured urinary tyrosol and hydroxytyrosol, which are the main phenolic components in olive oil, and plasma α-linolenic acid in a random sample of 450 participants.23 Tyrosol increased in the Mediterranean diet + VOO group when compared with the low-fat group. Hydroxytyrosol increased in the Mediterranean diet + VOO group when compared with the low-fat, and compared with the Mediterranean diet + nuts group. For α-linolenic acid, the only intragroup difference identified was an increase in participants on the Mediterranean diet + nuts group at 1 year.
Urinary metabolomics was determined in a subset of 98 participants without diabetes in the PREDIMED study.32 Statistical analysis by partial least-squares discriminant analysis and analysis of covariance identified a number of Mediterranean diet–associated metabolites, including ketone bodies, amino acids and their derived metabolites, gut microbiota cometabolites, and fatty acids. Metabolites associated with consumption of a low-fat diet were hippurate, trimethylamine N-oxide (TMAO), histidine and its derived metabolites, as well as carnosine, proline-betaine, and xanthosine.
The other metabolomics studies were from the United States (n = 2) and the Netherlands and Italy (n = 1 each). Zhu et al from the United States assigned 10 participants to a Mediterranean diet and a fast food diet, each for 4 days in a random order,49 and found 3 indole derivatives that are related to tryptophan metabolism increased after the Mediterranean diet and decreased after the fast food diet, while 2 other indole derivatives changed in opposite direction. TMAO and choline were stable with both diets. In the other metabolomics study from the United States, Park et al assessed intra- and intergroup differences in plasma metabolites in 14 subjects after a 4-week Mediterranean diet, a high-fat (Atkins) diet, or a very-low-fat (Ornish) diet.43 No differences were identified except for higher TMAO, leucine, and valine in the Atkins diet when compared with the Ornish diet. Michielsen et al in the Netherlands analyzed serum metabolome after an 8-week Mediterranean-type diet, a Western diet high in saturated fat, or a Western-type diet in which part of the saturated fatty acid was replaced by MUFA.41 Five metabolites were identified by sparse partial least-squares discriminant analysis as having the most important differences between the diets; all of these 5 metabolites were associated with fatty acid metabolism, with 2 reflecting fish consumption, 2 reflecting butter consumption, and 1 reflecting olive oil consumption. In a trial in Italy, Meslier et al collected blood, urine, and fecal samples from 82 participants who followed a Mediterranean diet or a habitual Western diet for 8 weeks.40 Metabolites that were lower in the Mediterranean diet group were generally associated with meat and animal-based foods, while those that were higher were associated with vegetables/berries, whole grains, legumes, fish, and nuts.
Taken together, the most important biomarkers for measurement of a Mediterranean-style diet are those relating to fruit and vegetables, fish, olive oil, and nut intake. These biomarkers include but are not limited to carotenoids, vitamin C, DHA, n–3 index, MUFA %, tyrosol, hydroxytyrosol, α-linolenic acid, and γ-tocopherol.
DASH diet
The 5 publications that examined the DASH and DASH-based diets were derived from 3 studies: 2 conducted in the United States and 1 in Australia.
A study from Baltimore in the United States compared a DASH diet, a fruit and vegetable diet, and a typical American diet, using an 8-week 3-arm parallel design. Three publications based on this study were included in this review. One analyzed established serum biomarkers in 34 participants: β-carotene, cryptoxanthin, and zeaxanthin were increased in the DASH and fruit and vegetable diet groups, when compared with the typical American diet (P < 0.05).26 Lutein was increased in the DASH diet group when compared with the typical American diet (P < 0.05). Lycopene, retinol, and α-tocopherol levels did not differ among or between groups. McClure et al reported higher 24 h-urinary phosphorus in the DASH diet group when compared with the other 2 groups in 397 subjects.25 The third publication (with 329 subjects) identified 44 serum metabolites that were significantly different in participants consuming the DASH diet compared with those consuming either the fruit and vegetable diet or the typical American diet.30 The majority of the metabolites identified were lipids (n = 41); the others included the amino acid trans-4-hydroxyproline and 2 vitamin A metabolites (isomers of carotene diol). Two panels of the 10 most useful metabolites in distinguishing between consumers of the 3 diets are listed in Table 2; 1 distinguished the DASH diet from the fruit and vegetable diet; the other distinguished consumers of the DASH from consumers of the typical American diet.
The other US study, from the same group, compared a 3-month DASH diet with a typical American diet, and measured serum carotenoids, retinol, and tocopherols in 103 participants.27 Lutein, cryptoxanthin, zeaxanthin, and β-carotene were higher in the DASH diet group (P < 0.001), whereas lycopene, α-carotene, and γ-tocopherol were lower (P < 0.001). There were no significant differences for retinol or α-tocopherol.
A study from Australia with 95 participants looked at a DASH-type diet (with a low dietary acid load) compared with a healthy diet based on dietary guidelines (with a high acid load).42 The group measured 24-h urinary metabolites to assess compliance with the 14-week interventions. They reported lower sodium, calcium, chloride, and phosphate excretion, and higher excretion of potassium in the DASH-type diet group compared with the control group, although the results for phosphate and potassium were not significant. No differences were reported for magnesium and urea.
Overall, these results indicate that sodium, potassium, and carotenoids are the primary biomarkers in assessing compliance with a DASH diet. Emerging evidence indicates that metabolites relating to lipid and protein metabolism may also be useful.
Healthy Nordic diet
Six publications from 2 trials investigating the healthy Nordic diet were eligible for inclusion in this systematic review.
A trial from Copenhagen, Denmark, randomly allocated participants to a 26-week intervention of either a New Nordic diet or an average Danish diet. The New Nordic diet was based on the Nordic Nutrition Recommendations50 but with a slightly higher protein content. Plasma and urinary samples underwent untargeted metabolomics determination, as described in 4 publications (2 each for plasma and urinary metabolomics). The plasma samples were analyzed by gas chromatography–mass spectrometry (n = 145)24 and again by an ultra-performance liquid chromatography (UPLC) system coupled to quadruple time-of-flight mass spectrometry (n = 146).20 The analysis using gas chromatography–mass spectrometry identified 9 metabolites associated with the New Nordic diet and 8 metabolites associated with the average Danish diet (Table 2). The UPLC-quadruple time-of-flight mass spectrometry analysis reported complementary information. Eight metabolites were identified as being associated with the New Nordic diet; of these, 4 have been suggested to relate to fish intake, and 1 each to whole grains and fat intake; 2 do not have known dietary associations. Thirteen metabolites were identified as being associated with the average Danish diet: 5 that relate to lipid metabolism, 3 to protein metabolism, 1 each to intake of chocolate, citrus fruits, and fish, 1 to food heating, and 1 with unknown food origin. The urinary samples were analyzed by UPLC (n = 107)21 and hydrogen-1 proton nuclear magnetic resonance (n = 142).31 Analysis using UPLC identified 5 metabolites associated with the New Nordic diet: 1 relating to fish intake and the others with no clear food origin. Fifteen metabolites were associated with the average Danish diet: 5 limonene metabolites relating to orange juice, wine gums, and soft drink intakes, 1 citrus metabolite relating to citrus fruit intake, 5 relating to chocolate intake, 1 metabolite relating to heat-treated food that has undergone Maillard reactions,51 and 3 of unknown food origin. Using hydrogen-1 proton nuclear magnetic resonance and partial least-squares discriminant analysis, 15 metabolites were deemed to be higher in the New Nordic diet group and 10 lower. Suggested food origins are detailed in Table 2. The use of established biomarkers within the same trial was reported in Poulsen et al.29 No significant differences between the 2 diets were identified in terms of 24-h urinary nitrogen or sodium levels in consumers (n = 143). Whole-blood lipids were also assessed (n = 145). The percentages of n–3 fatty acids, the n–3 index, the n–6/n–3 ratio, and the proportion of total highly unsaturated fatty acids as n–3 highly unsaturated fatty acids differed significantly between the groups, whereas percentages of saturated fatty acids, MUFAs, polyunsaturated fatty acids, and n–6 fatty acids in whole blood did not differ.
Similarly, a Nordic multinational trial47 randomly assigned 164 participants to a healthy Nordic diet based on the Nordic Nutrition Recommendations,50 or a typical Nordic diet for 18 or 24 weeks. Plasma metabolomics were measured, and the only difference in metabolites found between the 2 groups was that pipecolic acid betaine (homostachydrine, which is a substance found in coffee beans and citrus fruits) was higher in the healthy Nordic diet group.
These results suggest that biomarkers related to fish, fruit and vegetables, whole grains, and lipids are the top candidates for healthy Nordic diet measurement.
Diets based on dietary guidelines
Two trials from the United Kingdom investigated diets based on government healthy eating guidelines. The CRESSIDA trial45 compared the UK dietary guidelines with a traditional British diet in 162 participants. In order to verify participants’ compliance, biomarkers measured included 24-h urinary electrolytes, erythrocyte lipids, plasma alkylresorcinol, and serum vitamin D, folate, ferritin, and homocysteine. Potassium, n–3 index, alkylresorcinol, and vitamin D were reported to be higher in the intervention group compared with the control group, whereas sodium, folate, sucrose, and fructose were lower. No differences were reported for ferritin or homocysteine.
The other study was from London (UK) in which 19 participants consumed 4 diets that varied in their concordance with the WHO healthy eating guidelines.36 Twenty-four-hour urinary samples were collected during each 72-h intervention period. A total of 28 metabolites had significant changes, with 19 of them being present in higher concentrations, and 9 metabolites having lower concentrations, after the consumption of the diet with the highest concordance with WHO healthy eating guidelines, compared with the diet with the lowest concordance (Table 2). Most of the metabolites had well-known dietary associations with individual foods, including 7 associated with fruit and/or vegetable intake, 7 with fish or meat intake, and 6 with single nutrients. Food and nutrient origins were unknown for the remaining 8 metabolites. The metabolomic model developed in this study was further validated against DASH scores in 225 subjects from a UK cohort of the INTERMAP study, as well as an additional external Danish cohort of 66 subjects. Significant associations between urinary metabolic levels and DASH scores were observed. Additionally, 4 metabolites related to healthy eating were quantified individually in the INTERMAP study samples. All 4 metabolites increased in participants with higher, compared with lower, DASH scores (Table 2).
Due to the limited evidence, no definitive summary can be provided. However, possible candidates include biomarkers of fruit and vegetables, fish, whole grains, salt, and sugar intakes.
Low-glycemic-load diet
One trial from Seattle, United States, investigated the plasma metabolome response to GL. Subjects followed a low-GL or a high-GL diet for 4 weeks each in a random crossover design. Targeted metabolomics were reported in 2 articles derived from this 1 trial, with 1922 and 8028 participants, respectively. One article reported the levels of 14 metabolites as differing significantly between the low-GL and high-GL intervention diets.22 Nine metabolites were found to be higher in concentration in the low-GL diet, while 5 were found to be lower. The other article reported on 20 metabolites, of which 8 were found to be higher and 12 were found to be lower in the low-GL diet when compared with the high-GL diet.28 Five metabolites were found to differ in both of the metabolomics determinations: cystamine, acetylcholine, hydroxyproline, and creatine were consistently lower in the low-GL diet; however, carnitine was higher in the low-GL diet in 1 article but lower in the other (Table 2).
Vegetarian diet
A Swedish study from the University of Gothenburg examined the postprandial metabolic responses in 32 participants consuming a lacto-ovo vegetarian diet, compared with a vegan diet or an omnivore diet, using a crossover design.44 Serum 3-hydroxyisobutyrate, proline, propylene glycol, and tyrosine, in addition to variables that included overlapping metabolites (carnitine & acetoacetate, N-acetylcysteine & proline & glutamate, proline & glutamate & unknown), were consistently higher post lacto-ovo vegetarian diet consumption compared with the vegan or omnivore diets. The metabolites that increased in participants consuming the vegan or omnivore diets are listed in Table 2.
Lederer et al randomly assigned 53 participants to a 4-week strict vegan diet or a meat-rich diet38 and measured the levels of a group of nutritional biomarkers. Serum vitamin B12, holotranscobalamin, and arachidonic acid were lower in consumers of the vegan diet compared with consumers of the meat-rich diet, whereas plasma nitrite and nitrate concentrations were higher. No significant differences were detected in serum methylmalonic acid, homocysteine, DHA, eicosanoic acid, eicosenoic acid, linoleic acid, linolenic acid, oleic acid, 25-OH-vitamin D2/D3, or urinary creatinine.
These results indicate that biomarkers related to vitamin B12 metabolism, as well as nitrites and nitrates are potentially important markers of this diet. However, more studies are required.
Prudent diet
A group from Hamilton, Canada, contrasted a prudent diet with a typical Western diet and reported metabolomics in 42 participants following a 2-week intervention.48 Fourteen metabolites in plasma and 9 in urine samples were selected as the top-ranked metabolites distinguishing the contrasting diets (Table 2). The limited evidence supported the fatty acid profile as being a potential marker of a prudent diet.
Korean diet
A trial conducted at the Seoul National University compared a typical Korean diet based on the Korean food guide, with a recommended American diet and a typical American diet using a crossover design.46 Fifty-four participants followed each intervention for 4 weeks. At the end of each dietary phase, serum and urinary metabolites that changed significantly compared with baseline were identified and are listed in Table 2. While limited, the current evidence showed inverse associations between the Korean diet and some serum essential amino acid (isoleucine, leucine, and valine) concentrations.
DISCUSSION
This is the first systematic review to describe the use of dietary biomarkers for assessing dietary patterns. A number of biomarkers used in clinical trials were identified, and information gained from metabolomic studies on the metabolites that characterize dietary pattern intakes was synthesized. These biomarkers/metabolites and their related dietary patterns are summarized in Table 4. Currently, the most commonly used biomarkers for assessing dietary patterns are those related to specific nutrients and foods characteristic of the patterns, including the n–3 index, 24-h urinary electrolytes, and carotenoids. The metabolites most frequently identified in exploratory studies were those broadly associated with fish, protein, and lipid intakes.
Table 4.
Summary of biomarkers/metabolites and their related dietary patterns
| Biomarker | Related dietary pattern(s) |
|---|---|
| 25-hydroxyvitamin D | Diets based on dietary guidelines |
| α-linolenic acid | Mediterranean diet |
| γ-tocopherol | Mediterranean diet |
| DASH dieta | |
| n–3 index | Mediterranean diet |
| Healthy Nordic diet | |
| Diets based on dietary guidelines | |
| Alkylresorcinol | Diets based on dietary guidelines |
| Calcium | DASH dieta |
| Carotenoids | Mediterranean diet |
| Chloride | DASH dieta |
| Docosahexaenoic acid (DHA) | Mediterranean diet |
| Folate | Diets based on dietary guidelinesa |
| Linoleic acid | Mediterranean dieta |
| MUFA | Mediterranean diet |
| MUFA-rich Western dietb | |
| Nitrites and nitrates | Vegetarian diet |
| Nitrogen | Healthy Nordic diet |
| Oleic acid | Mediterranean diet |
| Phosphorus | DASH diet |
| Potassium | DASH diet |
| Diets based on dietary guidelines | |
| Saturated fatty acids (SFAs) | Mediterranean dieta |
| Sodium | DASH dieta |
| Diets based on dietary guidelinesa | |
| Sucrose, fructose | Diets based on dietary guidelinesa |
| Total fatty acids | Mediterranean dieta |
| Trimethylamine N-oxide | Healthy Nordic diet |
| Diets based on dietary guidelines | |
| Low-GL diet | |
| Atkins dieta, b | |
| Low-fat dietb | |
| Tyrosol, hydroxytyrosol | Mediterranean diet |
| Vitamin B12, holotranscobalamin | Vegetarian dieta |
| Vitamin C | Mediterranean diet |
| Essential amino acids, eg, isoleucine, leucine, valinec | Mediterranean diet |
| Healthy Nordic diet | |
| Vegetarian diet | |
| Korean diet | |
| Atkins dietb | |
| Animal-based foods/meat-related metabolitesc | Mediterranean diet |
| Healthy Nordic diet | |
| Diets based on dietary guidelines | |
| Vegetarian diet | |
| Prudent diet | |
| Western dietb | |
| Fish intake–related metabolitesc | Healthy Nordic diet |
| Diets based on dietary guidelines | |
| Average Danish dietb | |
| Fruit and vegetable intake–related metabolitesc | Mediterranean diet |
| Healthy Nordic diet | |
| Diets based on dietary guidelines | |
| Prudent diet | |
| Fast-food dietb | |
| Lipid intake–related metabolitesc | DASH diet |
| Healthy Nordic diet | |
| Low-GL diet | |
| Vegetarian diet | |
| Prudent diet | |
| Western dietb | |
| Average Danish dietb | |
| Protein intake–related metabolitesc | DASH diet |
| Healthy Nordic diet | |
| Low-GL diet | |
| Vegetarian diet | |
| Prudent diet | |
| Low-fat dietb | |
| Average Danish dietb | |
| Whole grains intake–related metabolitesc | Mediterranean diet |
| Healthy Nordic diet |
Dietary biomarker was inversely associated with the dietary pattern.
Dietary pattern investigated as a control group.
For essential amino acids and intake-related metabolites, there are both direct and inverse associations (see Table 2 for details).
Abbreviations: DASH, Dietary Approaches to Stop Hypertension; GL, glycemic load; MUFA, monounsaturated fatty acid.
The common dietary biomarkers in use for assessing dietary patterns relate to specific foods or nutritional aspects of a diet, but appear to lack specificity for a given dietary pattern. For example, the n–3 index is the combined proportion of eicosapentaenoic acid and DHA as a percentage of total fatty acids in erythrocytes or whole blood. The n–3 index is frequently included as a biomarker of oily fish intake to assess adherence to fish-rich dietary patterns, including the Mediterranean diet,33 the New Nordic diet,29 and a diet based on the UK dietary guidelines.45 While this index may be useful under certain controlled conditions, it not only reflects fish consumption, but also a shift in fatty acid equilibrium, as the index is by definition inversely proportional to the levels of all other fatty acids combined and therefore may lack sensitivity.52
Twenty-four-hour urinary sodium, potassium, and nitrogen are established biomarkers for sodium, potassium, and protein intake, respectively. They are not affected by metabolism, and thus there is a direct relationship with absolute intake, making them robust dietary intake biomarkers.53,54 Urinary sodium and potassium were included to assess compliance with the Mediterranean diet,33 DASH diet,42 New Nordic diet,29 and the diet based on the UK dietary guidelines,45 to inform on salt (sodium), and fruit and vegetables (potassium) intake. Twenty-four-hour urinary nitrogen was used in the study comparing the New Nordic diet with the average Danish diet.29 These biomarkers only differentiate between dietary patterns where the difference in the corresponding dietary characteristics (eg, salt, fruit and vegetables, and protein) differ as individual components or characteristics of the dietary patterns tested. Therefore, the choice of such biomarkers needs to be carefully considered based on the knowledge of the specific characteristics of the dietary pattern being studied.
Serum or plasma carotenoids are established biomarkers for fruit and vegetable intake. These biomarkers were measured in 4 studies that investigated the Mediterranean diet33,34,39 or the DASH diet.26,27 No consistent results were seen across the studies. Although it was not the intention to assess dietary compliance but to study oxidative stress in 3 of the studies,27,34,39 these studies were included in this review as they are about established dietary biomarkers and inform the research question. The lack of consistency seen in these results, may be explained by the broad spectrum of carotenoids that are present in various fruit and vegetables, the analysis methods used, or that the comparator dietary patterns had similar levels of carotenoid-rich fruit and vegetables. Future research may seek to identify whether a panel of specific carotenoids, or indeed a panel of dietary biomarkers, may better reflect a specific dietary pattern.
The other dietary biomarkers identified were 24-h urinary sucrose and fructose, which are biomarkers commonly used for assessing sugar intake. These biomarkers successfully differentiated between a diet based on the UK dietary guidelines and a typical American diet, where added sugar was discouraged in the former and no restriction was placed in the latter.45 It should be noted that urinary sucrose and fructose cannot distinguish between the dietary intakes of naturally occurring or added sources and so is a marker of total sugar intake55; in this study, however, it was interpreted as reflecting added sugar intake.45 Plasma alkylresorcinol, serum 25-hydroxyvitamin D, and folate were also measured in the aforementioned study to capture intakes of whole grains, oily fish, and folic-acid–fortified breakfast cereals, respectively.45 All 3 of these biomarkers performed well in differentiating between the 2 dietary patterns. In another study using serum 25-hydroxyvitamin D as a biomarker, no difference was seen when an unsupplemented vegan diet and a meat-rich diet were compared.38 This is not surprising, as the meat-rich diet did not emphasize oily fish intake, while other protein sources in the diet were not necessarily good sources of vitamin D.
Serum or plasma α-tocopherol concentrations differed between dietary patterns in only 134 of 5 included studies,26,27,34,35,39 suggesting poor specificity. There is evidence that α-tocopherol is not a reliable biomarker of intake, given its plasma concentration is highly regulated and affected by factors other than dietary intake, such as genetic differences in absorption and metabolism.56 Two studies assessed γ-tocopherol and both reported a significant difference between dietary patterns. One compared the Mediterranean diet and a habitual American diet,35 the other compared the DASH diet and a typical American diet.27 In contrast to α-tocopherol, γ-tocopherol is more closely associated with dietary intake56 and therefore may be superior as a biomarker of vitamin E intake in dietary patterns.
Biomarkers of nutritional status that are indicators of not only dietary intake, but also nutrient metabolism8 and that reflect longer-term intakes,57 are possible contenders for dietary pattern biomarkers. Some examples of these include retinols, B vitamin, and iron status indicators. Serum or plasma retinol, ferritin, and homocysteine were measured in multiple trials for vitamin A, iron, and B vitamin status26,27,34,37,38,45; serum selenium, iron, soluble transferrin receptor,37 methylmalonic acid, and urinary creatinine38 were measured in only 1 trial. No differences across dietary patterns were reported for any of these biomarkers. These biomarkers reflect dietary intake but are also affected by nutrient metabolism.58 The only biomarkers of nutritional status that differed in the context of a dietary pattern were serum vitamin B12 and holotranscobalamin38; however, these biomarkers were only assessed in 1 study. The usefulness of these and other nutritional status biomarkers as a means by which to discriminate between different dietary patterns should be further investigated.
Established biomarkers of specific foods or nutrients lack specificity for a given dietary pattern, and there is considerable overlap between biomarkers used for various dietary patterns. For instance, 24-hour urinary sodium and potassium were determined in all dietary pattern studies included in this systematic review. Healthy dietary patterns share many aspects in common, including increased intake of fruit and vegetables, fish, whole grains, nuts, and healthy fats, and decreased intake of sodium and added sugar. Accordingly, these established biomarkers are currently unable to individually quantify dietary pattern intakes in observational situations. Taken together, these results suggest that a biomarker profile consisting of multiple biomarkers that reflect relevant foods, nutrients, and/or changes in metabolic status is likely required to accurately identify and discriminate between individual dietary patterns.
Exploratory studies have sought to identify dietary biomarker profiles that reflect different dietary patterns in an unbiased way. As such, inclusion of these exploratory studies within this systematic review complements the findings for established dietary biomarkers by providing unbiased information on the associations of a broad set of metabolic markers with the consumption of distinct dietary patterns. Exploratory studies have predominantly leveraged metabolomic methodologies, although these metabolomic profiles have generally not yet been validated in subsequent independent trials. Only 1 trial validated36 their metabolomic profile model developed from a diet based on WHO healthy eating guidelines in independent cohorts. However, the association that was confirmed was between the metabolomic profiles and the DASH diet, rather than the original dietary pattern that the model was developed from (ie, the diet based on WHO healthy eating guidelines). This result reinforces the similarities shared by healthy dietary patterns.
Of the individual metabolites identified as having a dietary source, the most frequently identified were associated with fish (n = 29), proteins (n = 21), and lipids (n = 18), followed by meat (n = 17), vegetables (n = 9), fruit (n = 8), dairy (n = 6), chocolate (n = 6), vitamins (n = 6), whole grains (n = 4), legumes (n = 4), nuts (n = 2), sugar (n = 2), and wine/grape (n = 1). Most of these dietary components overlap across many dietary patterns, again suggesting a lack of specificity for a given dietary pattern if used in isolation. For example, TMAO has been shown to be a biomarker for both red meat and deep-sea fish consumption.59 These foods can be consumed in distinct dietary patterns with distinctly different associations with mortality and heart disease.60,61 Biomarker profile is also dependent on the nature of the sample and the error in the analytic method. Urinary samples from one study were analyzed by UPLC–quadruple time-of-flight mass spectrometry21 and hydrogen-1 proton nuclear magnetic resonance.31 Only 1 overlapping metabolite (TMAO) was identified.59 In another study, plasma metabolomic profiles were assessed twice using the same analysis technique LC-MS/MS in 1922 and 8028 participants. Fourteen and 20 metabolites were identified, respectively, with only 5 of them being identified on both occasions. Accordingly, there remain analytic challenges with handling complex data from metabolomics, which adds to the difficulty of developing objective dietary pattern assessment tools.
Currently, the metabolic profiles for identifying particular dietary patterns are not sufficiently developed; they require external validation and determination of sensitivity and specificity. This systematic review and summary of the current evidence base provides a clear direction for future research seeking to identify dietary biomarkers profiles. Biomarkers related to intakes of fish, proteins, and lipids, are likely to be important for objective assessment of dietary patterns. Future metabolomic studies should be hypothesis-driven, investigating a priori metabolic profiles, but should also test for study-specific exploratory associations.
One of the strengths of this review is the comprehensive search that was conducted. A total of 5 databases were searched without restricting biological specimen type, intervention duration, or publication year. To the authors’ knowledge, this is the first systematic review on dietary biomarkers of dietary patterns. A limitation is that this review is based on studies conducted in generally healthy populations, and the findings may not be generalizable to those with noncommunicable diseases. Furthermore, assessing the dietary biomarkers of dietary patterns was not the primary aim of some of these studies; however, they nonetheless provided relevant data that was suitable for inclusion in this systematic review.
CONCLUSION
Using dietary biomarkers of single nutrients or of individual foods or food groups can be useful for assessing dietary compliance in controlled settings. However, identifying an individual’s specific or broad dietary pattern on the basis of their biomarker profile remains an area for future research. This is particularly challenging given the large degree of variation within dietary patterns. The application of established biomarkers is limited due to their poor specificity. A framework that incorporates a panel of dietary biomarkers is likely necessary in order to accurately capture the full complexity of dietary patterns.
Supplementary Material
Acknowledgment
The literature search has been greatly assisted by Monica Cooper, Academic Liaison Librarian from the library of the University of Sydney.
Author contributions. The authors’ responsibilities were as follows: S.L., K.S.B.-A., F.M.O’L., and M.R.S. conceived the idea and designed the study; S.L. performed the systematic search and drafted the systematic review protocol and manuscript; S.L., R.F.N., and C.A.T. performed the screening; S.L. and R.F.N. performed the data extraction and bias assessment; M.R.S., F.M.O’L., and K.S.B.-A. critically reviewed the manuscript for meaningful intellectual insight; and all authors read and approved the final manuscript.
Funding. The authors report that no funding was received for this study.
Declaration of interest. M.R.S. is employed by the University of Sydney as the Maurice Blackmore Principal Research Fellow in Integrative Medicine. This position was established through a gift from the Blackmores Institute. M.R.S. receives no research funding or in-kind support from Blackmores Limited. The authors report no other conflicts of interest.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1 PRISMA 27-item checklist
Appendix S2 MEDLINE search
Contributor Information
Shuang Liang, The Boden Initiative, Charles Perkins Centre, The University of Sydney, Sydney, New South Wales, Australia; Sydney Medical School, The University of Sydney, Sydney, New South Wales, Australia.
Reeja F Nasir, The Boden Initiative, Charles Perkins Centre, The University of Sydney, Sydney, New South Wales, Australia; Sydney Medical School, The University of Sydney, Sydney, New South Wales, Australia.
Kim S Bell-Anderson, School of Life and Environmental Sciences, The University of Sydney, Sydney, New South Wales, Australia.
Clémence A Toniutti, The Boden Initiative, Charles Perkins Centre, The University of Sydney, Sydney, New South Wales, Australia.
Fiona M O’Leary, Susan Wakil School of Nursing and Midwifery, The University of Sydney, Sydney, New South Wales, Australia.
Michael R Skilton, The Boden Initiative, Charles Perkins Centre, The University of Sydney, Sydney, New South Wales, Australia; Sydney Medical School, The University of Sydney, Sydney, New South Wales, Australia; Sydney Institute for Women, Children and their Families, Sydney Local Health District, Sydney, New South Wales, Australia.
References
- 1. Hu BF. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 2. Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr. 2001;73:1–2. [DOI] [PubMed] [Google Scholar]
- 3. Moshfegh AJ, Rhodes DG, Staples RC, et al. The US Department of Agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–332. [DOI] [PubMed] [Google Scholar]
- 4. Basiotis PP, Welsh SO, Cronin FJ, et al. Number of days of food intake records required to estimate individual and group nutrient intakes with defined confidence. J Nutr. 1987;117:1638–1641. [DOI] [PubMed] [Google Scholar]
- 5. Potischman N, Freudenheim JL. Biomarkers of nutritional exposure and nutritional status: an overview. J Nutr. 2003;133:873S–874S. [DOI] [PubMed] [Google Scholar]
- 6. Freedman LS, Kipnis V, Schatzkin A, et al. Can we use biomarkers in combination with self-reports to strengthen the analysis of nutritional epidemiologic studies? Epidemiol Perspect Innov. 2010;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gibbons H, Michielsen CJR, Rundle M, et al. Demonstration of the utility of biomarkers for dietary intake assessment; proline betaine as an example. Mol Nutr Food Res. 2017;61:1700037. [DOI] [PubMed] [Google Scholar]
- 8.Cambridge Biomedical Research Centre. Nutritional biomarkers. Available at: https://dapa-toolkit.mrc.ac.uk/diet/objective-methods/biomarkers. Accessed September 23, 2021.
- 9. Poppitt SD, Kilmartin P, Butler P, et al. Assessment of erythrocyte phospholipid fatty acid composition as a biomarker for dietary MUFA, PUFA or saturated fatty acid intake in a controlled cross-over intervention trial. Lipids Health Dis. 2005;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heinzmann SS, Holmes E, Kochhar S, et al. 2-Furoylglycine as a candidate biomarker of coffee consumption. J Agric Food Chem. 2015;63:8615–8621. [DOI] [PubMed] [Google Scholar]
- 11. McGrath A, Hamill L, Cardwell C, et al. Combining vitamin C and carotenoid biomarkers better predicts fruit and vegetable intake than individual biomarkers in dietary intervention studies. Eur J Nutr. 2016;55:1377–1388. [DOI] [PubMed] [Google Scholar]
- 12. Gibbons H, Brennan L. Metabolomics as a tool in the identification of dietary biomarkers. Proc Nutr Soc. 2017;76:42–53. [DOI] [PubMed] [Google Scholar]
- 13. Fiehn O. Metabolomics – the link between genotypes and phenotypes. Plant Mol Biol. 2002;48:155–171. [PubMed] [Google Scholar]
- 14. Clarke ED, Rollo ME, Pezdirc K, et al. Urinary biomarkers of dietary intake: a review. Nutr Rev. 2020;78:364–381. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:E1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scottish Intercollegiate Guideline Network. Healthcare Improvement Scotland. Search filters. Available at: https://www.sign.ac.uk/what-we-do/methodology/search-filters/. Accessed November 16, 2020.
- 17. Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:L4898. [DOI] [PubMed] [Google Scholar]
- 20. Acar E, Gurdeniz G, Khakimov B, et al. Biomarkers of individual foods, and separation of diets using untargeted LC-MS–based plasma metabolomics in a randomized controlled trial. Mol Nutr Food Res. 2019;63:e1800215. [DOI] [PubMed] [Google Scholar]
- 21. Andersen MBS, Rinnan A, Manach C, et al. Untargeted metabolomics as a screening tool for estimating compliance to a dietary pattern. J Proteome Res. 2014;13:1405–1418. [DOI] [PubMed] [Google Scholar]
- 22. Barton S, Navarro SL, Buas MF, et al. Targeted plasma metabolome response to variations in dietary glycemic load in a randomized, controlled, crossover feeding trial in healthy adults. Food Funct. 2015;6:2949–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fitó M, Estruch R, Salas-Salvadó J, et al. ; PREDIMED Study Investigators. Effect of the Mediterranean diet on heart failure biomarkers: a randomized sample from the PREDIMED trial. Eur J Heart Fail. 2014;16:543–550. [DOI] [PubMed] [Google Scholar]
- 24. Khakimov B, Poulsen SK, Savorani F, et al. New Nordic diet versus average Danish diet: a randomized controlled trial revealed healthy long-term effects of the new Nordic diet by GC-MS blood plasma metabolomics. J Proteome Res. 2016;15:1939–1954. [DOI] [PubMed] [Google Scholar]
- 25. McClure ST, Rebholz CM, Phillips KM, et al. The percentage of dietary phosphorus excreted in the urine varies by dietary pattern in a randomized feeding study in adults. J Nutr. 2019;149:816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller ER, Appel LJ, Risby TH. Effect of dietary patterns on measures of lipid peroxidation: results from a randomized clinical trial. Circulation. 1998;98:2390–2395. [DOI] [PubMed] [Google Scholar]
- 27. Miller ER, Erlinger TP, Sacks FM, et al. A dietary pattern that lowers oxidative stress increases antibodies to oxidized LDL: results from a randomized controlled feeding study. Atherosclerosis. 2005;183:175–182. [DOI] [PubMed] [Google Scholar]
- 28. Navarro SL, Tarkhan A, Shojaie A, et al. Plasma metabolomics profiles suggest beneficial effects of a low–glycemic load dietary pattern on inflammation and energy metabolism. Am J Clin Nutr. 2019;110:984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poulsen SK, Due A, Jordy AB, et al. Health effect of the New Nordic Diet in adults with increased waist circumference: a 6-mo randomized controlled trial. Am J Clin Nutr. 2014;99:35–45. [DOI] [PubMed] [Google Scholar]
- 30. Rebholz CM, Lichtenstein AH, Zheng Z, et al. Serum untargeted metabolomic profile of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am J Clin Nutr. 2018;108:243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trimigno A, Khakimov B, Savorani F, et al. Human urine 1H NMR metabolomics reveals alterations of the protein and carbohydrate metabolism when comparing habitual average Danish diet vs. healthy new Nordic diet. Nutrition. 2020;79–80:110867. [DOI] [PubMed] [Google Scholar]
- 32. Vazquez-Fresno R, Llorach R, Urpi-Sarda M, et al. Metabolomic pattern analysis after Mediterranean diet intervention in a nondiabetic population: a 1- and 3-year follow-up in the PREDIMED study. J Proteome Res. 2015;14:531–540. [DOI] [PubMed] [Google Scholar]
- 33. Davis CR, Bryan J, Hodgson JM, et al. A Mediterranean diet reduces F2-isoprostanes and triglycerides among older Australian men and women after 6 months. J Nutr. 2017;147:1348–1355. [DOI] [PubMed] [Google Scholar]
- 34. Diekmann C, Huber H, Preuß M, et al. Moderate postmeal walking has no beneficial effects over resting on postprandial lipemia, glycemia, insulinemia, and selected oxidative and inflammatory parameters in older adults with a cardiovascular disease risk phenotype: a randomized crossover trial. J Nutr. 2019;149:1930–1941. [DOI] [PubMed] [Google Scholar]
- 35. Djuric Z, Ren J, Blythe J, et al. A Mediterranean dietary intervention in healthy American women changes plasma carotenoids and fatty acids in distinct clusters. Nutr Res. 2009;29:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia-Perez I, Posma JM, Gibson R, et al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017;5:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jennings A, Tang J, Gillings R, et al. Changing from a Western to a Mediterranean-style diet does not affect iron or selenium status: results of the New Dietary Strategies Addressing the Specific Needs of the Elderly Population for Healthy Aging in Europe (NU-AGE) 1-year randomized clinical trial in elderly Europeans. Am J Clin Nutr. 2020;111:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lederer A-K, Hannibal L, Hettich M, et al. Vitamin B12 status upon short-term intervention with a vegan diet—a randomized controlled trial in healthy participants. Nutrients. 2019;11:2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marin C, Ramirez R, Delgado-Lista J, et al. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am J Clin Nutr. 2011;93:267–274. [DOI] [PubMed] [Google Scholar]
- 40. Meslier V, Laiola M, Roager HM, et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69:1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michielsen CCJR, Hangelbroek RWJ, Feskens EJM, et al. Disentangling the effects of monounsaturated fatty acids from other components of a Mediterranean diet on serum metabolite profiles: a randomized fully controlled dietary intervention in healthy subjects at risk of the metabolic syndrome. Mol Nutr Food Res. 2019;63:E1801095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nowson CA, Wattanapenpaiboon N, Pachett A. Low-sodium dietary approaches to stop hypertension-type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res. 2009;29:8–18. [DOI] [PubMed] [Google Scholar]
- 43. Park JE, Miller M, Rhyne J, et al. Differential effect of short-term popular diets on TMAO and other cardio-metabolic risk markers. Nutr Metab Cardiovasc Dis. 2019;29:513–517. [DOI] [PubMed] [Google Scholar]
- 44. Radjursoga M, Lindqvist HM, Pedersen A, et al. Nutritional metabolomics: postprandial response of meals relating to vegan, lacto-ovo vegetarian, and omnivore diets. Nutrients. 2018;10:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reidlinger DP, Darzi J, Hall WL, et al. How effective are current dietary guidelines for cardiovascular disease prevention in healthy middle-aged and older men and women? A randomized controlled trial. Am J Clin Nutr. 2015;101:922–930. [DOI] [PubMed] [Google Scholar]
- 46. Shin J-H, Jung S, Kim S-A, et al. Differential effects of typical Korean versus American-style diets on gut microbial composition and metabolic profile in healthy overweight Koreans: a randomized crossover trial. Nutrients. 2019;11:2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tuomainen M, Karkkainen O, Leppanen J, et al. Quantitative assessment of betainized compounds and associations with dietary and metabolic biomarkers in the randomized study of the healthy Nordic diet (SYSDIET). Am J Clin Nutr. 2019;110:1108–1118. [DOI] [PubMed] [Google Scholar]
- 48. Wellington N, Shanmuganathan M, de Souza RJ, et al. Metabolic trajectories following contrasting prudent and Western diets from food provisions: identifying robust biomarkers of short-term changes in habitual diet. Nutrients. 2019;11:2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu C, Sawrey-Kubicek L, Beals E, et al. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and Mediterranean diet in 4 days: a pilot study. Nutr Res. 2020;77:62–72. [DOI] [PubMed] [Google Scholar]
- 50. Becker W, Lyhne N, Pedersen AN, et al. Nordic Nutrition Recommendations 2004 – integrating nutrition and physical activity. Scand J Food Nutr. 2004;48:178–187. [Google Scholar]
- 51. Henle T. Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids. 2005;29:313–322. [DOI] [PubMed] [Google Scholar]
- 52. Baylin A, Kim MK, Donovan-Palmer A, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162:373–381. [DOI] [PubMed] [Google Scholar]
- 53. Bingham S. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr. 2003;133:921S–924S. [DOI] [PubMed] [Google Scholar]
- 54.National Cancer Institute. Dietary assessment primer. Learn more about biomarkers. Available at: https://dietassessmentprimer.cancer.gov/learn/biomarkers.html. Accessed September 23, 2020.
- 55. Ramne S, Gray N, Hellstrand S, et al. Comparing self-reported sugar intake with the sucrose and fructose biomarker from overnight urine samples in relation to cardiometabolic risk factors. Front Nutr. 2020;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. El-Sohemy A, Baylin A, Ascherio A, et al. Population-based study of α- and γ-tocopherol in plasma and adipose tissue as biomarkers of intake in Costa Rican adults. Am J Clin Nutr. 2001;74:356–363. [DOI] [PubMed] [Google Scholar]
- 57. Jenab M, Slimani N, Bictash M, et al. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet. 2009;125:507–525. [DOI] [PubMed] [Google Scholar]
- 58. Picó C, Serra F, Rodríguez AM, et al. Biomarkers of nutrition and health: new tools for new approaches. Nutrients. 2019;11:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Abbasi J. TMAO and heart disease: the new red meat risk? JAMA. 2019;321:2149–2151. [DOI] [PubMed] [Google Scholar]
- 60. Koay YC, Chen YC, Wali JA, et al. Plasma levels of trimethylamine-N-oxide can be increased with ‘healthy’ and ‘unhealthy’ diets and do not correlate with the extent of atherosclerosis but with plaque instability. Cardiovasc Res. 2021;117:435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nowiński A, Ufnal M. Trimethylamine N-oxide: a harmful, protective or diagnostic marker in lifestyle diseases? Nutrition. 2018;46:7–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.