Abstract
When grown on xanthan as a carbon source, the bacterium Bacillus sp. strain GL1 produces extracellular xanthan lyase (75 kDa), catalyzing the first step of xanthan depolymerization (H. Nankai, W. Hashimoto, H. Miki, S. Kawai, and K. Murata, Appl. Environ. Microbiol. 65:2520–2526, 1999). A gene for the lyase was cloned, and its nucleotide sequence was determined. The gene contained an open reading frame consisting of 2,793 bp coding for a polypeptide with a molecular weight of 99,308. The polypeptide had a signal peptide (2 kDa) consisting of 25 amino acid residues preceding the N-terminal amino acid sequence of the enzyme and exhibited significant homology with hyaluronidase of Streptomyces griseus (identity score, 37.7%). Escherichia coli transformed with the gene without the signal peptide sequence showed a xanthan lyase activity and produced intracellularly a large amount of the enzyme (400 mg/liter of culture) with a molecular mass of 97 kDa. During storage at 4°C, the purified enzyme (97 kDa) from E. coli was converted to a low-molecular-mass (75-kDa) enzyme with properties closely similar to those of the enzyme (75 kDa) from Bacillus sp. strain GL1, specifically in optimum pH and temperature for activity, substrate specificity, and mode of action. Logarithmically growing cells of Bacillus sp. strain GL1 on the medium with xanthan were also found to secrete not only xanthan lyase (75 kDa) but also a 97-kDa protein with the same N-terminal amino acid sequence as that of xanthan lyase (75 kDa). These results suggest that, in Bacillus sp. strain GL1, xanthan lyase is first synthesized as a preproform (99 kDa), secreted as a precursor (97 kDa) by a signal peptide-dependent mechanism, and then processed into a mature form (75 kDa) through excision of a C-terminal protein fragment with a molecular mass of 22 kDa.
Xanthan is an extracellular polysaccharide produced by a plant-pathogenic bacterium, Xanthomonas campestris, and has a cellulosic chain as a backbone and a linear trisaccharide as a side chain consisting of a mannosyl-glucuronyl-mannose sequence attached at the C-3 position on the alternate glucosyl residue of the main chain (22, 32). The internal and terminal mannosyl residues of the side chain are frequently modified with an O-acetyl group at the C-6 position and with pyruvate ketal at the C-4 to C-6 positions, although the extent of modification varies depending on both the growth conditions and bacterial strains (37). Because xanthan shows superior rheological properties, such as pseudoplasticity, high viscosity at low concentration, and tolerance toward a wide range of pHs and temperatures (21, 23), the polymer has been widely used as a gelling and stabilizing agent in the food, pharmaceutical, and oil industries (36).
However, application of the polymer is fairly restricted due to its high viscosity, and modified xanthans with novel physicochemical and physiological functions are therefore sought to exploit new application fields. Chemical modification of xanthan is thought to be difficult because of the complex structure of the polymer. Though Xanthomonas mutants created by genetic engineering produce variant xanthans (4, 16), their production levels are far from what is required for practical use (45). Therefore, because of the molecular design of xanthan, the use of relevant enzymes seems to be the most suitable and promising way to prepare modified xanthans.
Although some bacteria and microbial mixed cultures have been reported to assimilate xanthan (2, 6, 7, 8, 19, 40, 41), we first elucidated an enzymatic route for complete depolymerization of xanthan in Bacillus sp. strain GL1 (12, 29). Xanthan is, at first, attacked by extracellular xanthan lyase (75 kDa) to remove the pyruvylated mannose from xanthan side chains and then depolymerized to tetrasaccharides by extracellular β-d-glucanase (350 kDa). The tetrasaccharide is incorporated into cells and further degraded to the constituent monosaccharides by successive reactions catalyzed by β-d-glucosidase (51 kDa), unsaturated glucuronyl hydrolase (42 kDa), and α-d-mannosidase (330 kDa). Among these xanthan-depolymerizing enzymes, xanthan lyase is thought to be a useful biochemical agent for modification of xanthan, since the modified xanthan with disaccharide as a side chain has been experimentally confirmed to show excellent food-technological properties hitherto unexplored, especially in its interaction with other edible biopolymers (28a; unpublished data).
An analysis of the structure-function relationship of xanthan lyase in combination with other polysaccharide lyases (alginate lyases [A1-I, A1-II, and A1-III] [48], oligoalginate lyase [13], and gellan lyase [15]) gives rise to fundamental and essential insight into the nature of polysaccharide lyases, since polysaccharide lyases, when they act on polysaccharides, strictly recognize uronic acid residues in the molecules and are therefore hypothesized to contain a common structural feature in their catalytic sites. The crystal structures of a few polysaccharide lyases acting endolytically have recently been determined (11, 20, 25, 28, 31, 46, 47, 49). The xanthan lyase presented in this article will be suitable as a model for a structural analysis of the exolytic enzyme.
A few xanthan lyases from bacteria or mixed culture fluids have been characterized (1, 12, 33, 42), but the molecular cloning of their genes and overproduction of the enzymes have not yet been achieved. As the first step to prepare modified xanthans and analyze the structure-function relationship of the enzyme, in this study we cloned a gene encoding the xanthan lyase of Bacillus sp. strain GL1 and analyzed its nucleotide sequence and product.
MATERIALS AND METHODS
Materials.
Pyruvylated xanthan (average molecular mass, 2 × 106 Da; pyruvylation of terminal mannosyl residue in the side chain, 50%) was a gift from Kohjin Co., Tokyo, Japan. Silica gel 60-Kieselguhr F254 thin-layer chromatography (TLC) plates were obtained from E. Merck, Darmstadt, Germany. DEAE-Toyopearl 650M and Butyl-Toyopearl 650M were purchased from Tosoh Co., Tokyo, Japan. Sephacryl S-200HR and QAE-Sephadex A-25 were from Pharmacia Biotech, Uppsala, Sweden. A polyvinylidene difluoride (PVDF) membrane (Immobilon PSQ) was from Millipore Co., Bedford, Mass. Ponceau S, sodium hyaluronate, and chondroitin A were from Nacalai Tesque Co., Kyoto, Japan. A cloning vector of Charomid 9-36 and an expression vector of pET17b were from Nippon Gene Co., Tokyo, Japan, and Novagen, Inc., Madison, Wis., respectively. Restriction endonucleases and DNA-modifying enzymes were purchased from Takara Shuzo Co., Kyoto, and Toyobo Co., Tokyo, Japan.
Microorganisms and culture conditions.
For the purification of xanthan lyase, cells of Bacillus sp. strain GL1 were aerobically cultured at 30°C for 24 h in a xanthan medium consisting of 0.1% (NH4)2SO4, 0.1% KH2PO4, 0.1% Na2HPO4, 0.01% MgSO4 · 7H2O, 0.01% yeast extract, and 0.5% xanthan (pH 7.2). Six kinds of E. coli strains [BL21(DE3), BL21(DE3)pLysE, BL21(DE3)pLysS, HMS174(DE3), HMS174(DE3)pLysE, and HMS174(DE3)pLysS], purchased from Novagen, Inc., were used as hosts for expression of xanthan lyase. For the purification of xanthan lyase expressed in E. coli, the cells were aerobically precultured in Luria-Bertani (LB) medium (35) at 28°C. When the turbidity reached 0.5 at 600 nm, the culture received 0.1 mM isopropyl β-d-thiogalactopyranoside and was incubated further at 16°C for 38 h.
Assays for enzyme and protein.
Xanthan lyase was assayed as described previously (12). Briefly, the enzyme was incubated in a 1-ml reaction mixture containing 0.05% xanthan and 50 mM sodium acetate buffer, pH 5.5, and the activity was determined by monitoring the increase in absorbance at 235 nm. One unit of enzyme activity was defined as the amount of enzyme required to produce an increase in the absorbance at 235 nm of 1.0 per min. In order to investigate the substrate specificity of the enzyme, various polysaccharides such as hyaluronate, chondroitin, gellan, heparin, and pectin were used as substrates in the place of xanthan. Protein content was determined by the method of Lowry et al. (26) with bovine serum albumin as a standard or by measuring absorbance at 280 nm, by assuming that an E280 of 1.0 corresponds to 1 mg/ml.
Purification of xanthan lyase from Bacillus sp. strain GL1.
Unless otherwise specified, all procedures were carried out at 0 to 4°C. After cultivation of cells of Bacillus sp. strain GL1 at 30°C for 24 h in 10 liters of xanthan medium (1 liter/flask), the culture fluid was obtained by centrifugation at 13,000 × g and 4°C for 10 min and applied to a DEAE-Toyopearl 650M column (4.1 by 30 cm) equilibrated with 20 mM potassium phosphate buffer (KPB), pH 7.0. The enzyme was eluted with a linear gradient of NaCl (0 to 1.0 M) in 20 mM KPB, pH 7.0 (2 liters), and 17-ml fractions were collected every 9 min. The active fractions, which were eluted with 0.5 M NaCl, were saturated with ammonium sulfate (30%), and then the enzyme solution was applied to a Butyl-Toyopearl 650M column (2.7 by 17 cm) equilibrated with 20 mM KPB, pH 7.0, and saturated with ammonium sulfate (30%). The enzyme was eluted with a linear gradient of ammonium sulfate (30 to 0%) in 20 mM KPB, pH 7.0 (500 ml), and 4-ml fractions were collected every 4 min. The active fractions that eluted at 9% saturation with ammonium sulfate in 20 mM KPB, pH 7.0, were combined, concentrated by ultrafiltration with a molecular weight cutoff of 10,000 (model 8200; Amicon Co., Beverly, Mass.) to about 3 ml, and then applied to a Sephacryl S-200HR column (2.7 by 64 cm) equilibrated with 20 mM KPB, pH 7.0, containing 0.15 M NaCl. The enzyme was eluted with the same buffer, and 3-ml fractions were collected every 6 min. The enzyme was eluted between fractions 60 and 70, and each of the fractions contained two proteins with molecular masses of 97 and 75 kDa. The fractions were combined and dialyzed against 20 mM KPB, pH 7.0, overnight. The dialysate was immediately applied to a QAE-Sephadex A-25 column (0.8 by 2.8 cm) equilibrated with 20 mM KPB, pH 7.0. The enzyme was eluted with a linear gradient of NaCl (0 to 0.3 M) in 20 mM KPB, pH 7.0 (100 ml), and 1-ml fractions were collected every 0.5 min. The active fractions, which were eluted with 0.2 M NaCl, were used as the purified xanthan lyase (75 kDa) from Bacillus sp. strain GL1.
Electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and native gradient PAGE were done as described previously (10, 24).
Transfer of proteins on polyacrylamide gel to PVDF membrane.
Separated proteins on SDS-polyacrylamide gel were transferred to a PVDF membrane by electroblotting as described previously (27). The proteins on the membrane were visualized by staining with Ponceau S and subjected to analysis of their N-terminal amino acid sequences.
Preparation of internal peptides of xanthan lyase.
After denaturation at 37°C for 2 h in the presence of 8 M urea, the purified xanthan lyase (75 kDa, 600 pmol) from Bacillus sp. strain GL1 was hydrolyzed at 37°C for 6 h with trypsin (10 pmol) in 100 mM Tris-HCl buffer, pH 8.0. The resultant peptides were subjected to a capillary high-performance liquid chromatography system composed of a model 140B pump, a model 785A UV monitor (Applied Biosystems Division of Perkin-Elmer, Foster City, Calif.), a Micro Flow processor, a UZ flow cell, a Micro Injector (LC Packings, Amsterdam, The Netherlands), and a Probot micro fractionator (bai, Lautern, Germany). Peptides were eluted for 60 min with a linear gradient of acetonitrile (5 to 60%) in 0.1% trifluoroacetc acid (TFA) through a reversed-phase column (FUS-15-03-C18, 0.3 mm by 15 cm; LC Packings) and detected by measuring the absorbance at 220 nm.
N-terminal amino acid sequence.
N-terminal amino acid sequences of xanthan lyase and internal peptides derived from the enzyme were determined by Edman degradation using the Procise 492 protein sequencing system (Applied Biosystems Division of Perkin-Elmer).
Molecular cloning of the xanthan lyase gene.
A genomic DNA library (15) of Bacillus sp. strain GL1 previously constructed in E. coli DH5α using the Charomid 9-36 cloning vector was screened by the colony hybridization method (3) with a 32P-labeled probe (TAYGCNCARGAYCAYGCSGT; 20-mer, 128 mixtures) corresponding to the N-terminal amino acid sequence of an internal peptide prepared as described above. Several positive clones were obtained and cultivated in LB medium supplemented with ampicillin at 100 μg/ml. A plasmid vector was extracted from one of them and subjected to subcloning and DNA sequencing.
DNA sequence and DNA manipulations.
The nucleotide sequence of the xanthan lyase gene was determined by the dideoxy-chain termination method using an automated DNA sequencer (model 377; Applied Biosystems Division of Perkin-Elmer) (38). Subcloning, transformation, gel electrophoresis, and Southern hybridization were performed as described previously (35).
Construction of an expression plasmid.
To subclone the xanthan lyase gene into the expression vector of pET17b, PCR was performed by using KOD polymerase (Toyobo, Co.) with high-fidelity, genomic DNA from Bacillus sp. strain GL1 as a template and two synthetic oligonucleotides (5′-GGCATATGTCGGATGAATTCGACGCGCTTCGA-3′ and 5′-CCGAGCTCCTAGCCGACGGCCACGAACTT-3′) with NdeI and SacI sites added at their termini as primers. The PCR conditions recommended by the manufacturer (Toyobo, Co.) were used. The amplified gene, which encoded truncated xanthan lyase (26Ser to 930Gly) without the signal peptide, was digested with NdeI and SacI and ligated with the NdeI- and SacI-double-digested expression vector (pET-17b). The resultant plasmid containing the xanthan lyase gene was designated pET17b-XL4.
Purification of xanthan lyase from E. coli.
Cells of E. coli harboring the plasmid pET17b-XL4 were grown in 6 liters of LB medium (1.5 liters/flask), collected by centrifugation at 13,000 × g and 4°C for 5 min, washed with 20 mM KPB, pH 7.0, and then resuspended in the same buffer. The cells were ultrasonically disrupted (Insonator model 201M; Kubota, Tokyo, Japan) at 0°C and 9 kHz for 20 min, and the clear solution obtained after centrifugation at 15,000 × g and 4°C for 20 min was used as a cell extract. The cell extract after supplementation with 1 mM phenylmethylsulfonyl fluoride and 0.1 μM pepstatin A was applied to a DEAE-Toyopearl 650M column (2.6 by 30 cm) equilibrated with 20 mM KPB, pH 7.0. The enzyme was eluted with a linear gradient of NaCl (0 to 0.7 M) in 20 mM KPB, pH 7.0 (400 ml), and 4-ml fractions were collected every 3 min. Active fractions, which were eluted with 20 mM KPB, pH 7.0, containing around 0.5 M NaCl were combined and dialyzed against 20 mM Tris-HCl buffer, pH 7.2, and the dialysate was used as the purified enzyme from E. coli.
TLC.
Xanthan degradation products by xanthan lyase were analyzed by TLC with a solvent system of 1-butanol-acetic acid-water (3:2:2, vol/vol/vol). The products were visualized by heating a TLC plate at 110°C for 5 min after spraying it with 10% (vol/vol) sulfuric acid in ethanol.
Identification of the product by xanthan lyase.
The products released from xanthan by the enzyme were hydrolyzed with TFA and subjected to TLC analysis and pyruvate assay (12).
Nucleotide sequence accession number.
The nucleotide sequence for the xanthan lyase gene reported in this paper has been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB037178.
RESULTS
Biosynthesis of xanthan lyase in Bacillus sp. strain GL1.
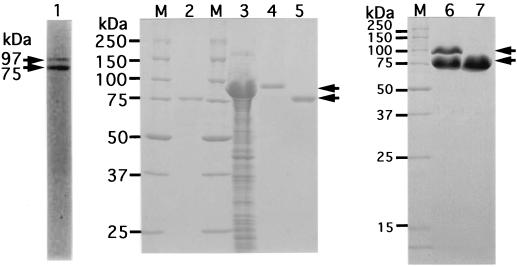
Although the cells of Bacillus sp. strain GL1 in stationary phase on a medium with xanthan (48-h culture) produce extracellular xanthan lyase of 75 kDa (12), an additional protein with a molecular mass of 97 kDa other than xanthan lyase (75 kDa) was copurified from mid-logarithmic cultures (24-h culture) (Fig. 1, lane 1). The N-terminal amino acid sequence of the 97-kDa protein that electroblotted on a PVDF membrane was determined to be NH2-SDEFDALRIK, which completely matched that of a previously purified xanthan lyase with a molecular mass of 75 kDa from the bacterial cells (12). However, the 97-kDa protein found in the Sephacryl S-200HR column chromatography step was absent in a final enzyme preparation, and only a 75-kDa enzyme was purified about 50-fold from the culture fluid, with an activity yield of 1% (Fig. 1, lane 2).
FIG. 1.
Electrophoretic profile of xanthan lyases. The preparations were subjected to SDS-PAGE. Lane M, synthetic polypeptides with molecular masses of 250, 150, 100, 75, 50, 37, 25, and 15 kDa; lane 1, partially purified xanthan lyase (after Sephacryl S-200HR column chromatography) from Bacillus sp. strain GL1; lane 2, purified xanthan lyase (after QAE-Sephadex A-25 column chromatography) from Bacillus sp. strain GL1; lane 3, cell extract of E. coli transformed with xanthan lyase gene; lane 4, purified xanthan lyase (97 kDa) from E. coli; lanes 5 and 7, processed xanthan lyase (75 kDa) from E. coli; lane 6, conversion of 97-kDa enzyme from E. coli to 75-kDa enzyme. Arrows indicate the positions of xanthan lyases.
N-terminal amino acid sequence of the internal peptide from xanthan lyase.
The N-terminal amino acid sequence (NH2-SDEFDALRIK) of xanthan lyase (75 kDa) was not suitable to prepare a hybridization probe for cloning of the enzyme gene. Therefore, the internal amino acid sequence of the enzyme was determined to prepare appropriate probes. Several peptides were generated from the purified xanthan lyase (75 kDa) after treatment with trypsin, isolated by high-performance liquid chromatography, and subjected to an analysis of its N-terminal amino acid sequence. The sequences of three kinds of peptides (P1, P2, and P3) were NH2-XXVDDPXIAP, NH2-XYAQDHAVGH, and NH2-LAQFAPAPHA (with X being an unidentified amino acid), respectively, and the amino acid sequence of the P2 peptide was used for the preparation of the oligonucleotide probe, since the length of the probe was sufficient and degeneracy was low (20-mer, 128 mixtures).
Molecular cloning and sequence analysis of the xanthan lyase gene.
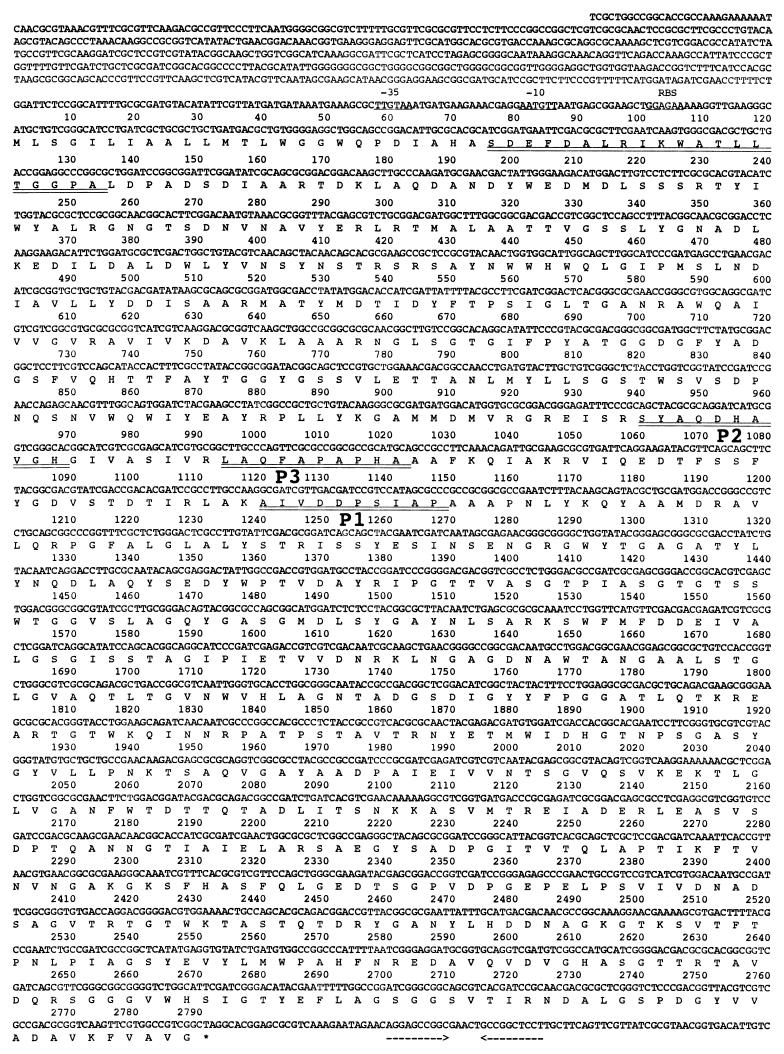
The gene for xanthan lyase was screened in a genomic DNA library of Bacillus sp. strain GL1, which was constructed in E. coli DH5α, which has no xanthan lyase activity. Several positive clones that hybridized with the probe corresponding to the amino acid sequence of the P2 peptide were obtained. One of them harbored a plasmid (designated pXL1) having a 6-kb fragment of genomic DNA in the Charomid 9-36 cloning vector and showed apparent xanthan lyase activity. A nucleotide sequence of a part (about 4 kb) of the genomic fragment contained in pXL1 was determined (Fig. 2). The fragment was found to contain a gene consisting of 2,793 bp. The gene encoded a polypeptide composed of 930 amino acid residues with a molecular weight of 99,308 and coded for the N-terminal amino acid sequence SDEFDALRIK (26Ser to 35Lys) of the purified xanthan lyase (75 kDa) from Bacillus sp. strain GL1 and amino acid sequences of the internal peptides (P1, P2, and P3) (Fig. 2), thus confirming that the predicted amino acid sequence represents the primary structure of the enzyme. Hereinafter, the xanthan lyase gene will be designated xly. Judging from the N-terminal amino acid sequence of xanthan lyase purified from the culture fluid of Bacillus sp. strain GL1, a signal sequence consisting of 25 amino acid residues was positioned preceding the N terminus of the enzyme, thus supporting the localization of the enzyme in the external medium. A predicted ribosome-binding site (Shine-Dalgarno sequence) (39) existed just before the start codon (ATG) of the gene, and an apparent promoter with homology to the E. coli consensus promoter (17) was found in 5′ regions of the gene (Fig. 2). A hairpin structure containing a stem composed of 20 nucleotides was observed downstream the stop codon (TAG) (Fig. 2), and the structure had a free energy of −28.2 kcal. The endogenous promoter and terminator of Bacillus sp. strain GL1 may function in E. coli cells transformed with pXL1.
FIG. 2.
Nucleotide sequence of the xanthan lyase gene and its deduced amino acid sequence. Putative promoters (−35 and −10) and the ribosome-binding site (RBS) are underlined. The N-terminal amino acids of xanthan lyase and internal peptides (P1, P2, and P3) determined by protein sequencing are double underlined. An inverted repeat (possible terminator) is indicated by facing arrows.
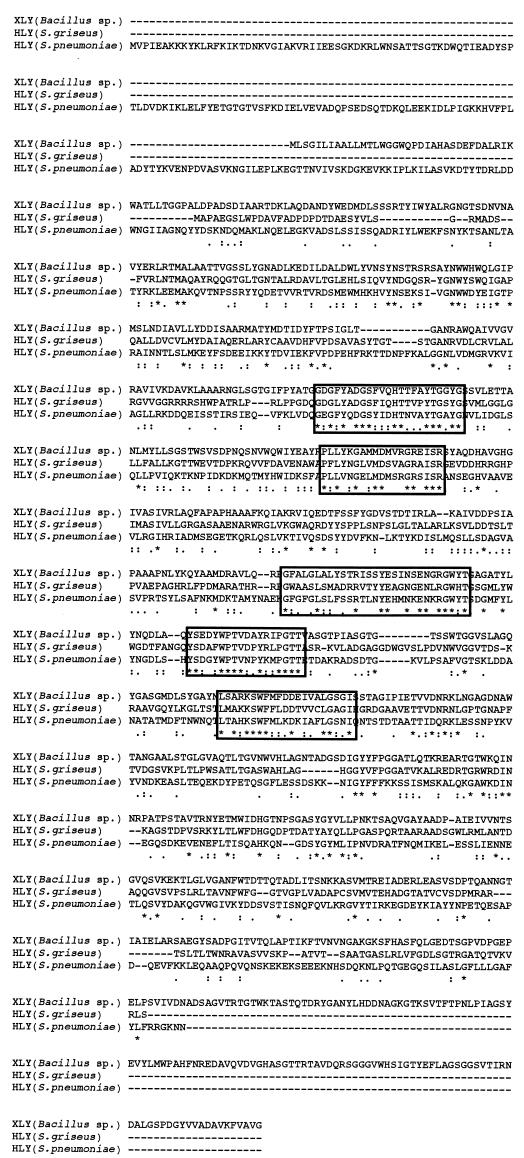
The polypeptide with a molecular mass of 99 kDa from Bacillus sp. strain GL1 was used to search for similarity with sequences in protein databases (PIR, Swiss Prot, and DAD) with the FASTA program (30). The polypeptide displayed the highest identity score with hyaluronidase of Streptomyces griseus (37.7% identity in a 697-amino-acid overlap; accession no. AB028210), followed by that of Streptococcus pneumoniae (27.1% identity in a 724-amino-acid overlap; accession no. L20670) (5). From an alignment of these proteins, five well-conserved regions among xanthan lyase and hyaluronidases were observed (Fig. 3). Since hyaluronidase is a polysaccharide lyase that catalyzes the β-elimination reaction by using hyaluronate as a substrate (43), these regions are thought to play an important role in the β-elimination reaction of polysaccharide lyases. Xanthan lyase also shows homology with chondroitin AC lyase (one of the polysaccharide lyases) of Pedobacter heparinus (25.9% identity in a 649-amino-acid overlap; accession no. U27583) (44), though xanthan lyase from Bacillus sp. strain GL1 showed no activity against hyaluronate and chondroitin A under our assay conditions (data not shown).
FIG. 3.
Amino acid sequence alignment of xanthan lyase (XLY) of Bacillus sp. strain GL1 (AB037178) and hyaluronidases (HLY) of Streptomyces griseus (AB028210) and S. pneumoniae (L20670). Five well-conserved regions are boxed. Identical and similar amino acid residues among the three enzymes are marked with asterisks and dots, respectively.
Southern blot analysis.
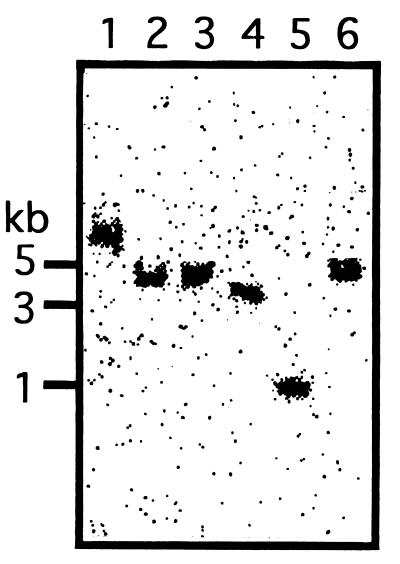
In order to identify xanthan lyase with a molecular mass of 75 kDa as a mature form derived from a 99-kDa preproform, the copy number of the xanthan lyase gene in genomic DNA of Bacillus sp. strain GL1 was investigated by Southern blot analysis using a probe (5′-TCGGATGAATTCGACGCGCTTCGAA-3′) corresponding to the N-terminal amino acid sequence of the mature enzyme. A single band was detected in the genomic DNA digested with various restriction enzymes (Fig. 4), indicating that the xanthan lyase gene is unique in the genomic DNA of Bacillus sp. strain GL1. Therefore, it was concluded that the 75-kDa xanthan lyase was derived from a 99-kDa preproform.
FIG. 4.
Southern blot analysis. Genomic DNA from Bacillus sp. strain GL1 was digested with various restriction enzymes and subjected to Southern hybridization using the oligonucleotide coding for the N-terminal amino acids of xanthan lyase (75 kDa) as a probe. Lane 1, BamHI; lane 2, HindIII; lane 3, PstI; lane 4, SmaI; lane 5, SphI; lane 6, KpnI.
Overexpression, purification, and characterization of xanthan lyase in E. coli.
For overproduction of xanthan lyase, the expression plasmid (pET17b-XL4) with the truncated enzyme gene (xly) under the T7 promoter was constructed and introduced into six kinds of E. coli strains [BL21(DE3), BL21(DE3)pLysE, BL21(DE3)pLysS, HMS174(DE3), HMS174(DE3)pLysE, and HMS174(DE3)pLysS]. The transformant of BL21(DE3)pLysS with the plasmid showed the highest xanthan lyase activity (448 kU [400 mg]/liter of culture). The expression level of the lyase in the transformant was over 1,000-fold higher than that (0.226 kU/liter of culture) in Bacillus sp. strain GL1 (12). In fact, xanthan lyase expressed in cell extract of E. coli was estimated to occupy over 80% of total proteins (Fig. 1, lane 3).
Xanthan lyase was purified 1.15-fold from E. coli, with a recovery of 96.4% (Table 1). The purified enzyme was confirmed to be almost homogeneous by SDS-PAGE (Fig. 1, lane 4). Properties of the enzyme from E. coli are as follows.
TABLE 1.
Purification of xanthan lyase from E. coli
| Step | Total protein (mg) | Total activity (kU) | Sp act (U/mg) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Cell extraction | 2,952 | 2,690 | 911 | 100 | 1.00 |
| DEAE-Toyopearl 650M column chromatography | 2,479 | 2,592 | 1,047 | 96.4 | 1.15 |
(i) Molecular mass.
The molecular mass of the enzyme was determined to be 97 kDa by SDS-PAGE (Fig. 1, lane 4). This value was comparable with the theoretical value (96,778 Da) calculated from the predicted amino acid sequence of xanthan lyase without the signal sequence. The enzyme formed a band at a molecular mass of 97 kDa on the native gradient polyacrylamide gel after being stained with Coomasie brilliant blue R-250 (data not shown), indicating that the enzyme was monomeric. However, during storage of the enzyme for several days at 4°C in 20 mM KPB, pH 7.0, or 20 mM Tris-HCl buffer, pH 7.2, the 97-kDa enzyme was gradually converted to a protein (75 kDa) that shows the same mobility (Fig. 1, lane 5) as that of the enzyme from Bacillus sp. strain GL1 (Fig. 1, lane 2) on SDS-polyacrylamide gel. The excision of the 22-kDa fragment had no appreciable effect on the activity of enzyme, since the specific activity (1.00 kU/mg) of the 75-kDa enzyme was almost equal to that (1.05 kU/mg) of the 97-kDa enzyme. The N-terminal amino acid sequence of both the 97- and 75-kDa proteins was determined to be NH2-SDEFD, indicating that the 97-kDa enzyme expressed in E. coli was converted to the 75-kDa protein by removal of the C-terminal region corresponding to the 22-kDa fragment.
(ii) pH and temperature.
The enzyme with a molecular mass of 75 kDa was most active at pH 5.2 (sodium acetate buffer) and 50°C and was stable below 40°C. These properites of the enzyme (75 kDa) from E. coli were comparable with those of the enzyme from Bacillus sp. strain GL1 (12).
(iii) Substrate specificity.
The 75-kDa enzyme was highly specific for xanthan, especially pyruvylated xanthan. Although xanthan lyase shows homology with microbial polysaccharide lyases for hyaluronate and chondroitin, hyaluronate and chondroitin A were inert as substrates. Other than on these polysaccharides, the enzyme was inactive on gellan, heparin, and pectin.
(iv) Mode of action.
The enzyme (75 kDa) from Bacillus sp. strain GL1 is shown to produce pyruvylated mannose from xanthan (12). The reaction products from xanthan by the enzyme (75 kDa) expressed in E. coli were highly viscous, and the property made TLC analysis difficult. So, the products were separated into low- and high-molecular-weight products by using ultrafiltration with a molecular weight cutoff of 10,000. The low-molecular-weight products were hydrolyzed in the presence of TFA and then subjected to TLC analysis and pyruvate assay. The hydrolysates of the products with TFA showed the same mobility as that of mannose on a TLC plate (Fig. 5, lanes 4 and 5) and contained pyruvate (data not shown). Therefore, as seen with the enzyme from Bacillus sp. strain GL1, the 75-kDa enzyme from E. coli was found to release pyruvylated mannose. On the other hand, the high-molecular-weight products revealed high viscosity and had absorbency at 235 nm, suggesting that the products are the modified xanthan with unsaturated glucuronyl mannose as the side chains. Judging from the identification of xanthan degradation products, the enzyme was confirmed to act exolytically on side chains of xanthan and release the pyruvylated mannose specifically.
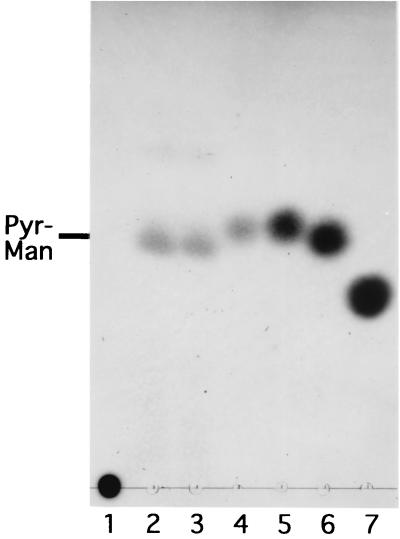
FIG. 5.
Release of pyruvylated mannose from xanthan by xanthan lyases. Xanthan (0.25%) was incubated for 3 h with 20 U of xanthan lyases from Bacillus sp. strain GL1 and E. coli per μl. Products were analyzed by TLC. Lane 2, product produced by the enzyme from Bacillus sp. strain GL1; lane 3, product produced by the enzyme from E. coli; lane 4, hydrolysate of the product produced by the enzyme from E. coli. Authentic samples: lane 1, xanthan (25 μg); lane 5, d-mannose (45 μg); lane 6, d-glucose (45 μg); lane 7, d-glucuronic acid (45 μg). Pyr-Man indicates pyruvylated mannose.
DISCUSSION
For the first time, we have cloned the xanthan lyase gene from Bacillus sp. strain GL1 and obtained a substantial amount of enzyme. As a result, the maturation route of the enzyme in cells of Bacillus sp. strain GL1 was supposed and a large amount of the modified xanthan, which may be applicable to food technology (28a), became readily obtainable. Xanthan lyase is first synthesized as a preproform (99 kDa), secreted as a precursor form (97 kDa) by a general signal sequence-dependent mechanism, and finally converted to a mature form (75 kDa) through the removal of a C-terminal 22-kDa fragment. Specifically, xanthan lyase becomes a mature form through two steps of posttranslational processing: release of the signal peptide (2 kDa) and excision of the C-terminal protein fragment (22 kDa). The intrinsic function of the C-terminal protein fragment is obscure, since the protein with a molecular mass of 22 kDa showed no appreciable effects on the enzyme activity and little homology with other proteins, including hyaluronidases (Fig. 3) and proteases. Judging from the N-terminal amino acid sequences and molecular mass of 75-kDa enzymes of Bacillus sp. strain GL1 and E. coli containing the xly gene, the probable processing site is in the vicinity of valinyl residue 719. The disappearance of the 22-kDa fragment on SDS-polyacrylamide gels (Fig. 2, lanes 5 to 7) is possibly due to immediate degradation of the released fragment by aminopeptidase or to depolymerization of the 97-kDa protein by carboxypeptidase contaminated in the preparation of xanthan lyase. However, the possibility that the xanthan lyase is autoprocessed by the protease activity inherent in the enzyme is not excluded, since the posttranslational processing of xanthan lyase is observed with lyases expressed in both Bacillus sp. strain GL1 and E. coli containing the xly gene. A more detailed analysis of this posttranslational modification process is apparently required.
After submission of this article, results on the xanthan lyase gene (xalA) of Paenibacillus alginolyticus strain XL-1 were reported by Ruijssenaars et al. (34). The xalA gene codes for a polypeptide consisting of 936 amino acid residues with a molecular weight of 100,823, including a signal sequence of 36 amino acid residues. Bacillus sp. strain GL1 is classified into Paenibacillus species by 16S rRNA analysis (29), and xanthan lyase of Bacillus sp. strain GL1 shows significant homology (56.3% identity in a 933-amino-acid overlap) with that of P. alginolyticus strain XL-1 (accession no. AF242413). However, the maturation system of the enzyme in Bacillus sp. strain GL1 is quite different from that in P. alginolyticus strain XL-1, since the posttranslational processing observed in Bacillus sp. strain GL1 has not occurred in P. alginolyticus strain XL-1 (34).
The properties of microbial glycosyl hydrolases acting on poly- and oligosaccharides have been well documented, and the three-dimensional structures of a large number of polysaccharide hydrolases such as amylases, chitinases, and cellulases have been reviewed (9, 18). On the other hand, a structural study of polysaccharide lyases has largely been restricted and, to the best of our knowledge, the structures of only four polysaccharide lyases (lyases for pectate, alginate, chondroitin, and hyaluronate) have been determined (11, 20, 25, 28, 31, 46, 47, 49). Although all polysaccharide lyases recognize uronic acid residues in polysaccharides and catalyze the β-elimination reaction, no information on the structure-function relationship that specifies the recognition sites and reaction types of the lyases has been accumulated. To elucidate the molecular basis underlying the polysaccharide lyase reaction, we have focused our attention on the bacterial heteropolysaccharide lyases (lyases for alginate, oligoalginate, gellan, and xanthan) with different types of reactions—endo- or exo-type reactions and backbone- or side chain-type reactions (13, 14)—and have already determined the crystal structure of the alginate lyase (endo- and backbone type from Sphingomonas sp. strain A1) responsible for the depolymerization of alginate produced by bacteria (49). The xanthan lyase in this article will be suitable for structural analysis of polysaccharide lyase acting exolytically on side chains of polysaccharide.
ACKNOWLEDGMENTS
We thank Toyofumi Miya, Kohjin Co., for his kind gift of xanthan and Yukari Ohyama, Kyoto University, for her excellent technical assistance.
REFERENCES
- 1.Ahlgren J A. Purification and characterization of a pyruvated-mannose-specific xanthan lyase from heat-stable, salt-tolerant bacteria. Appl Environ Microbiol. 1991;57:2523–2528. doi: 10.1128/aem.57.9.2523-2528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahlgren J A. Purification and properties of a xanthan depolymerase from a heat-stable salt-tolerant bacterial consortium. J Ind Microbiol. 1993;12:87–92. [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1987. [Google Scholar]
- 4.Bellach M R, Capage M A, Doherty D H, Hassler R A, Henderson N M, Vanderslice R W, Marellia J D, Ward M B. Genetically engineered polymers: manipulation of xanthan biosynthesis. In: Yalpani M, editor. Industrial polysaccharides: genetic engineering, structure/property relations and applications. Amsterdam, The Netherlands: Elsevier; 1987. pp. 35–80. [Google Scholar]
- 5.Berry A M, Lock R A, Thomas S M, Rajan D P, Hansman D, Paton J C. Cloning and nucleotide sequence of the Streptococcus pneumoniae hyaluronidase gene and purification of the enzyme from recombinant Escherichia coli. Infect Immun. 1994;62:1101–1108. doi: 10.1128/iai.62.3.1101-1108.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadmus M C, Jackson L K, Burton K A, Plattner R D, Slodki M E. Biodegradation of xanthan gum by Bacillus sp. Appl Environ Microbiol. 1982;44:5–11. doi: 10.1128/aem.44.1.5-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadmus M C, Slodki M E, Nicholson J J. High-temperature, salt-tolerant xanthanase. J Ind Microbiol. 1989;4:127–133. [Google Scholar]
- 8.Cheetham N W H, Mashimba E N M. Characterisation of some enzymic hydrolysis products of xanthan. Carbohydr Polym. 1991;15:195–206. [Google Scholar]
- 9.Davies G, Henrissat B. Structures and mechanisms of glycosyl hydrolases. Structure. 1995;3:853–859. doi: 10.1016/S0969-2126(01)00220-9. [DOI] [PubMed] [Google Scholar]
- 10.Davis B J. Disc electrophoresis, II. Method and application to human serum proteins. Ann NY Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 11.Fethiere J, Eggimann B, Cygler M. Crystal structure of chondroitin AC lyase, a representative of a family of glycosaminoglycan degrading enzymes. J Mol Biol. 1999;288:635–647. doi: 10.1006/jmbi.1999.2698. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto W, Miki H, Tsuchiya N, Nankai H, Murata K. Xanthan lyase of Bacillus sp. strain GL1 that liberates pyruvylated mannose from xanthan side chains. Appl Environ Microbiol. 1998;64:3765–3768. doi: 10.1128/aem.64.10.3765-3768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto W, Miyake O, Momma K, Kawai S, Murata K. Molecular identification of oligoalginate lyase of Sphingomonas sp. strain A1 as one of the enzymes required for complete depolymerization of alginate. J Bacteriol. 2000;182:4572–4577. doi: 10.1128/jb.182.16.4572-4577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto W, Momma K, Miki H, Mishima Y, Kobayashi E, Miyake O, Kawai S, Nankai H, Mikami B, Murata K. Enzymatic and genetic basis on assimilation, depolymerization, and transport of heteropolysaccharides in bacteria. J Biosci Bioeng. 1999;87:123–136. doi: 10.1016/s1389-1723(99)89001-x. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto W, Sato N, Kimura S, Murata K. Polysaccharide lyase: molecular cloning of gellan lyase gene and formation of the lyase from a huge precursor protein in Bacillus sp. GL1. Arch Biochem Biophys. 1998;354:31–39. doi: 10.1006/abbi.1998.0674. [DOI] [PubMed] [Google Scholar]
- 16.Hassler R A, Doherty D H. Genetic engineering of polysaccharide structure: production of variants of xanthan gum in Xanthomonas campestris. Biotechnol Prog. 1990;6:182–187. doi: 10.1021/bp00003a003. [DOI] [PubMed] [Google Scholar]
- 17.Hawley D K, McClure W R. Comparison and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolase. Curr Opin Struct Biol. 1997;7:637–644. doi: 10.1016/s0959-440x(97)80072-3. [DOI] [PubMed] [Google Scholar]
- 19.Hou C T, Barnabe N, Greaney K. Purification and properties of a novel xanthan depolymerase from a salt-tolerant bacterial culture, HD1. Appl Environ Microbiol. 1986;52:37–44. doi: 10.1128/aem.52.1.37-44.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang B, Matte A, Li Y, Kim Y S, Linhardt R J, Su H, Cygler M. Crystal structure of chondroitinase B from Flavobacterium heparinum and its complex with a disaccharide product at 1.7 Å resolution J. Mol Biol. 1999;294:1257–1269. doi: 10.1006/jmbi.1999.3292. [DOI] [PubMed] [Google Scholar]
- 21.Janes A, Pittsley J E, Senti F R. Polysaccharide B-1459: a new hydrocolloid polyelectrolyte produced from glucose by bacterial fermentation. J Appl Polym Sci. 1961;5:519–526. [Google Scholar]
- 22.Jansson P-E, Kenne L, Lindberg B. Structure of the extracellular polysaccharide from Xanthomonas campestris. Carbohydr Res. 1975;45:275–282. doi: 10.1016/s0008-6215(00)85885-1. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy J F, Bradshaw I J. Production, properties and applications of xanthan. In: Bushell M E, editor. Progress in industrial microbiology. Vol. 19. Amsterdam, The Netherlands: Elsevier/North-Holland Publishing Co.; 1984. pp. 319–371. [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Kelly S J, Lamani E, Ferraroni M, Jedrzejas M J. Structural basis of hyaluronan degradation by Streptococcus pneumoniae hyaluronate lyase. EMBO J. 2000;19:1228–1240. doi: 10.1093/emboj/19.6.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 28.Mayans O, Scott M, Connerton I, Gravesen T, Benen J, Visser J, Pickersgill R, Jenkins J. Two crystal structures of pectin lyase A from Aspergillus reveal a pH driven conformational change and striking divergence in the substrate-binding clefts of pectin and pectate lyases. Structure. 1997;5:677–689. doi: 10.1016/s0969-2126(97)00222-0. [DOI] [PubMed] [Google Scholar]
- 28a.Miki H. Masters thesis. Kyoto, Japan: Kyoto University; 1999. [Google Scholar]
- 29.Nankai H, Hashimoto W, Miki H, Kawai S, Murata K. Microbial system for polysaccharide depolymerization: enzymatic route for xanthan depolymerization by Bacillus sp. strain GL1. Appl Environ Microbiol. 1999;65:2520–2526. doi: 10.1128/aem.65.6.2520-2526.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickersgill R, Jenkins J, Harris G, Nasser W, Robert-Baudouy J. The structure of Bacillus subtilis pectate lyase in complex with calcium. Nat Struct Biol. 1994;1:717–723. doi: 10.1038/nsb1094-717. [DOI] [PubMed] [Google Scholar]
- 32.Rogovin S P, Anderson R F, Cadmus M C. Production of polysaccharide with Xanthomonas campestris. J Biochem Microbiol Technol Eng. 1961;3:51–63. [Google Scholar]
- 33.Ruijssenaars H J, Bont J A M D, Hartmans S. A pyruvated mannose-specific xanthan lyase involved in xanthan degradation by Paenibacillus alginolyticus XL-1. Appl Environ Microbiol. 1999;65:2446–2452. doi: 10.1128/aem.65.6.2446-2452.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruijssenaars H J, Hartmans S, Verdoes J C. A novel gene encoding xanthan lyase of Paenibacillus alginolyticus strain XL-1. Appl Environ Microbiol. 2000;66:3945–3950. doi: 10.1128/aem.66.9.3945-3950.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Sandford P A, Baird J. Industrial utilization of polysaccharides. In: Aspinall G O, editor. The polysaccharides. Vol. 2. New York, N.Y: Academic Press, Inc.; 1983. pp. 411–490. [Google Scholar]
- 37.Sandford P A, Pittsley J E, Knutson C A, Watson P R, Cadmus M C, Janes A. Variation in Xanthomonas campestris NRRL B-1459: characterization of xanthan products of differing pyruvic acid content. Am Chem Soc Symp Ser. 1977;45:192–210. [Google Scholar]
- 38.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shine J, Dalgarno L. The 3′ terminal sequence of E. coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosomal binding sites. Proc Natl Acad Sci USA. 1974;71:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutherland I W. An enzyme system hydrolyzing the polysaccharides of Xanthomonas species. J Appl Microbiol. 1982;53:385–393. [Google Scholar]
- 41.Sutherland I W. Hydrolysis of unordered xanthan in solution by fungal cellulases. Carbohydr Res. 1984;131:93–104. [Google Scholar]
- 42.Sutherland I W. Xanthan lyases—novel enzymes found in various bacterial species. J Gen Microbiol. 1987;133:3129–3134. doi: 10.1099/00221287-133-11-3129. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland I W. Polysaccharide lyases. FEMS Microbiol Rev. 1995;16:323–347. doi: 10.1111/j.1574-6976.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 44.Tkalee A L, Fink D, Blain F, Zhang-Sun G, Laliberte M, Bennett D C, Gu K, Zimmermann J J F, Su H. Isolation and expression in Escherichia coli and cslA and cslB, genes coding for the chondroitin sulfate-degrading enzymes chondroitinase AC and chondroitinase B, respectively, from Flavobacterium heparinum. Appl Environ Micobiol. 2000;66:29–35. doi: 10.1128/aem.66.1.29-35.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderslice R W, Doherty D H, Capage M A, Betlach M R, Hassler R A, Henderson N M, Ryan-Graniero J, Tecklenburg M. Genetic engineering of polysaccharide structure in Xanthomonas campestris. In: Crescenzi V, Dea I C M, Paoletti S, Stivala S S, Sutherland I W, editors. Biochemical and biotechnological advances in industrial polysaccharides. New York, N.Y: Gordon and Breach; 1989. pp. 145–156. [Google Scholar]
- 46.Vitali J, Schick B, Kester H C, Visser J, Jurnak F. The three-dimensional structure of Aspergillus niger pectin lyase B at 1.7 Å resolution. Plant Physiol. 1998;116:69–80. doi: 10.1104/pp.116.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoder M D, Keen N T, Jurnak F. New domain motif: the structure of pectate lyase C, a secreted plant virulence factor. Science. 1993;260:1503–1507. doi: 10.1126/science.8502994. [DOI] [PubMed] [Google Scholar]
- 48.Yoon H-J, Hashimoto W, Miyake O, Okamoto M, Mikami B, Murata K. Overexpression in Escherichia coli, purification, and characterization of Sphingomonas sp. A1 alginate lyases. Protein Expr Purif. 2000;19:84–90. doi: 10.1006/prep.2000.1226. [DOI] [PubMed] [Google Scholar]
- 49.Yoon H-J, Mikami B, Hashimoto W, Murata K. Crystal structure of alginate lyase A1-III from Sphingomonas species A1 at 1.78 Å resolution. J Mol Biol. 1999;290:505–514. doi: 10.1006/jmbi.1999.2883. [DOI] [PubMed] [Google Scholar]