Abstract
Objectives
To identify and map all trials in maternal health conducted in low and middle-income countries (LMIC) over the 10-year period from 2010 to 2019, to identify geographical and thematic trends, as well as comparing to global causes of maternal death and preidentified priority areas.
Design
Systematic scoping review.
Primary and secondary outcome measures
Extracted data included location, study characteristics and whether trials corresponded to causes of mortality and identified research priority topics.
Results
We searched the Cochrane Central Register of Controlled Trials database, a combined registry of trials from multiple sources. Our search identified 7269 articles, 874 of which were included for analysis. Between 2010 and 2019, maternal health trials conducted in LMICs more than doubled (50–114). Trials were conducted in 61 countries—231 trials (26.4%) were conducted in Iran. Only 225 trials (25.7%) were aligned with a cause of maternal mortality. Within these trials, pre-existing medical conditions, embolism, obstructed labour and sepsis were all under-represented when compared with number of maternal deaths globally. Large numbers of studies were conducted on priority topics such as labour and delivery, obstetric haemorrhage and antenatal care. Hypertensive disorders of pregnancy, diabetes and health systems and policy—despite being high-priority topics—had relatively few trials.
Conclusion
Despite trials conducted in LMICs increasing from 2010 to 2019, there were significant gaps in geographical distribution, alignment with causes of maternal mortality and known research priority topics. The research gaps identified provide guidance and insight for future research conduct in low-resource settings.
Trial registration number
10.17605/OSF.IO/QUJP5.
Keywords: obstetrics, maternal medicine, public health
Strengths and limitations of this study.
We undertook a broad, extensive search to identify as many trials as possible, using a trial-specific database that draws from a wide range of other databases.
This resulted in a large number of trials to analyse, ensuring as much as possible that overall trends found in the data were instructive and informative.
All data were double extracted by two independent reviewers, ensuring consistency and accuracy of the individual findings.
We acknowledge that as a review of trials only, not all research pertaining to maternal health is captured, and that other study designs are important to the overall body of work done in any given field.
We also acknowledge that the nature of a scoping review means that no quality assessment of trials is undertaken, and so we cannot comment on the quality of research conducted.
Background
In 2017, an estimated 295 000 women died worldwide during pregnancy, childbirth or the immediate postpartum period, equivalent to 211 deaths per 100 000 live births.1 While this represents a near 38% reduction from the 2000 estimates, acceleration is required to meet the global Sustainable Development Goals (SDG) target of 70 deaths per 100 000 live births by 2030.1 2 Based on a 2014 systematic analysis, the leading causes of maternal death include indirect causes (27.5%), obstetric haemorrhage (27.1%), hypertensive disorders (14.0%) and sepsis (10.7%).3 Maternal mortality data have consistently shown that a majority of maternal deaths occur in low and middle-income countries (LMICs), with countries in sub-Saharan Africa and Southern Asia accounting for 86% of all maternal deaths.1 4 The disparity in maternal mortality between higher and lower income countries is a stark example of how profound inequities in the quality of healthcare services between higher and lower resourced settings have tragic consequences for women, families and communities.5
Robust and reliable research is a critical component of the global effort to address the global burden of maternal death and disability, the majority of which is preventable.6 Recent global research prioritisation exercises have been conducted to identify the most impactful research areas to drive improvements in global maternal and perinatal health outcomes.7 8 For example, the WHO-led prioritisation exercise by Souza et al in 2014 identified and prioritised 190 research questions for improving global maternal and perinatal health in the period 2015–2025—suggesting eight broad topics of maternal health of importance (box 1).7 A separate prioritisation exercise by Chapman et al in 2014 on reducing maternal mortality in LMICs identified 100 high-priority research questions—categorised into seven key topics (box 1).8
Box 1. Priority maternal health topics from global prioritisation exercises.
Souza et al—‘Maternal and perinatal health research priorities beyond 2015: an international survey and prioritization exercise’7
Questions identified by a reference group of experts and refined by a technical working group were given a score based on five criteria. Questions were given a normalised research priority score (NRPS) to determine the highest priority topics, which were as follows:
Labour and delivery.
Obstetric haemorrhage.
Neonatal care.
Hypertensive disorders of pregnancy.
Antenatal care.
Abortion.
Health systems.
Other.
Chapman et al—‘A survey study identified global research priorities for decreasing maternal mortality’8
An initial list of questions derived from 178 Cochrane systematic reviews was prioritised and refined into a list of 100 questions. Thematic analysis of these questions was used to determine rank of priority by weighting within the set, with the following list of topics:
Health systems and policy.
Diabetes and other causes*.
Abortion and unplanned pregnancy.
Postpartum haemorrhage.
Hypertensive disorders.
Labour and caesarean.
Say et al—‘Global causes of maternal death: a WHO systematic analysis’3
A WHO working group analysed specialised and general bibliographic databases, as well as the WHO mortality database for vital registration data, to identify and report estimated causes of maternal death between 2003 and 2012. Their work found that in the ‘developing regions’, the leading causes of maternal death were:
Obstetric haemorrhage (27.1%).
Pre-existing medical conditions (14.8%).
Hypertensive disorders (14.0%).
Other (11.2%).
Sepsis (10.7%).
Abortion (7.9%).
HIV related (5.5%).
Embolism (3.1%).
Obstructed labour (2.9%).
Complications of delivery (2.8%).
*Including HIV, malaria, anaemia and violence.
Randomised controlled trials are the preferred study design for assessing effectiveness of interventions such as medicines.9 They can also be used to evaluate the effectiveness of more complex interventions, such as changes in health system arrangements.10 A 2016 scoping review conducted by Chersich et al—which searched for maternal health intervention research conducted in LMICs on five key conditions—observed a marked rise in the number of trials published on maternal health topics between 2000 and 2012.11 However, it is not known whether these trials are aligned with the major causes of maternal deaths, or aligned with the priority topics identified in global research prioritisation exercises. To our knowledge, no such review has been undertaken across all aspects of maternal health. As such, we sought to identify and assess all published maternal health trials conducted in LMICs in the past 10 years to identify the overall trends, and to what degree this research addresses established maternal mortality burden and research priorities.
Methods
We elected to use a scoping review design as it is the preferred methodology for examining the scope, content and knowledge gaps in a body of literature.12 This was conducted in accordance with a prespecified scoping review protocol registered via the Open Science Framework website.13 Findings have been reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews.14
Patient and public involvement
No patients or members of the public were involved in the design, conduct or dissemination of results for this paper.
Eligibility criteria
We considered any trial conducted in or across any one or more LMICs to be eligible for this scoping review. LMICs were defined according to the World Bank classification of 2019, which identifies 139 countries as LMICs.15 Trials were eligible if they included women who were pregnant, in labour, giving birth or in the postpartum period (up to 42 days post partum) and if they used any intervention primarily aimed at improving maternal or fetal health or preventing morbidity or mortality (ie, the primary outcome/s of the study was related to maternal or fetal health or well-being). Trials published between 1 January 2010 and 31 December 2019 (inclusive) in any language were eligible. We included trials that were aimed at the maternal health system level if the primary outcome remained relevant to our population of interest. Classification of a study as a trial by the reviewers was based on Cochrane Handbook guidance.16 Studies were excluded if they used quasirandomised or non-randomised designs; had a primary outcome related to a different population (eg, neonates or infants); were conducted in both high-income countries and LMICs and presented only combined results (if trial results from LMICs were reported separately for LMICs and high-income countries the trial was included); or pertained to management of infertility, early pregnancy loss or abortion.
Literature searching and assessment of eligibility
With support from an information specialist, a search strategy was devised to capture eligible studies (online supplemental table 1). Search terms for maternal and perinatal health were derived from search strategies used by Cochrane Pregnancy and Childbirth to maintain and update their specialised register.17 We consulted the search filters developed by Cochrane EPOC to identify search terms relating to LMICs.18 The search strategy was applied to the Cochrane Central Register of Controlled Trials (CENTRAL), which retrieves records from PubMed/MEDLINE, Embase, CINAHL, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP), KoreaMed, Cochrane Review Group’s Specialised Registers and hand-searched biomedical sources.19 Searching CENTRAL directly had the benefit of restricting search results to trials only, keeping the volume of citations to screen to a manageable level. Trial register records from ClinicalTrials.gov and WHO ICTRP were not included in the records retrieved from CENTRAL. The search was conducted on 1 May 2020.
bmjopen-2021-059473supp001.pdf (78.9KB, pdf)
Citation management, identification of duplicates and screening articles for eligibility were conducted using EndNote20 and Covidence.21 Two reviewers independently screened titles and abstracts of all retrieved citations to identify those that were potentially eligible. Full texts for these articles were accessed and assessed by two independent reviewers according to the eligibility criteria. At both steps, any disagreements were resolved through discussion or consulting a third author.
Data collection and analysis
For each included trial we extracted information on title, author, year of publication, location where trial was conducted (country and SDG region22), unit of randomisation (individual or cluster), category of intervention, intervention level (public health, community, primary care, hospital and health system) and category of primary outcome(s). The intervention and outcome categories were adapted from Cochrane’s list of ‘higher-level categories for interventions and outcomes’.23 For trials with more than one primary outcome, we identified a single, most appropriate outcome category through discussion and consensus among review authors. The level of intervention was determined based on the level of the healthcare system that the trial was primarily targeting—for example, trials recruiting women at an antenatal clinic were classified as primary care level. Public health and preventative care were defined as interventions for those in the community who were well, while home; and community care was defined as interventions for those in the community who were unwell. Based on the trial’s primary objective, we tagged each trial to one of 35 maternal health topics, as well as classifying them by relevance to a cause of maternal death identified by Say et al in their global systematic analysis (box 1).3
Included trials were additionally categorised into global research priority topics identified by Souza et al and Chapman et al.7 8 The research priorities identified by Souza et al were ranked based on the distribution of maternal health themes across the 190 priority research questions—that is, the theme with the most research questions was considered the highest ranked priority topic. This mirrored the process used by Chapman et al where research topics with the greatest representation within the 100 research questions, based on percentage, were given the highest rank. For each trial identified in our review, we used the variables extracted to classify it according to priority topics identified in Souza et al or Chapman et al where possible (box 1). All data were extracted by two independent reviewers, with results compared to ensure consistency and any disputes resolved through discussion or consultation with a third author. As this was a scoping review, we did not perform quality assessment on individual trials.
We conducted descriptive analyses using Excel to determine frequencies of extracted variables and used line graphs to explore trends. We assessed trends over time using proportions of each variable within studies available for a given year. While we initially planned to look at trends in individual countries and interventions, many had few or no data points.
Results
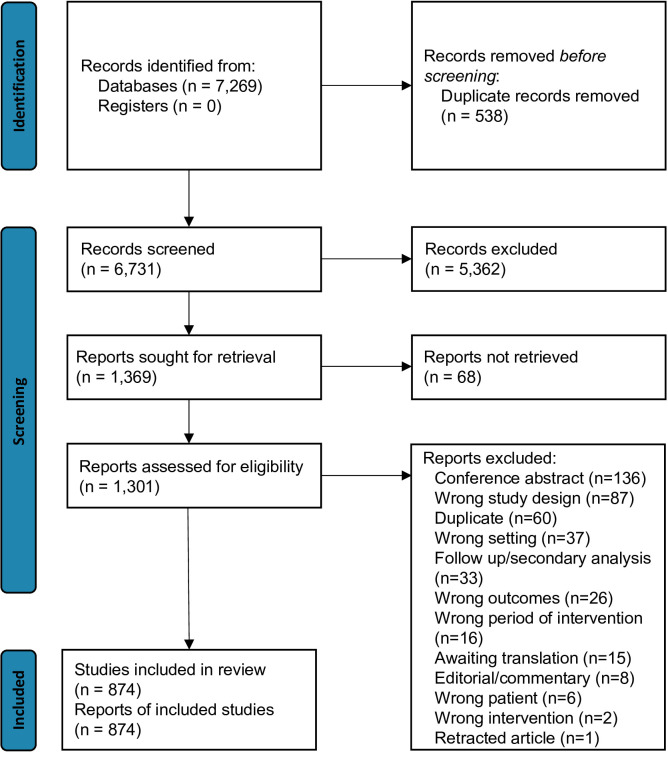
A total of 7269 articles were identified in the search, from which 538 duplicates were removed, and 6731 studies underwent title and abstract screening. This resulted in 1369 articles sought for retrieval, of which 68 were not located, leaving 1301 for assessment of eligibility. After reviewing these full texts, 874 studies were included (figure 1). The most common reasons for exclusion were conference abstracts (136 studies) and ineligible study design (87 studies).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of screening process.
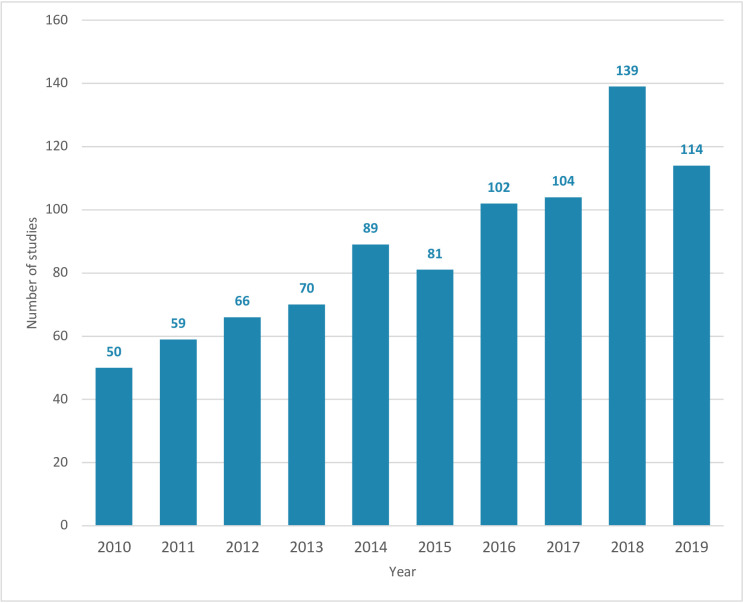
A total of 874 trials were included. The number of published trials conducted in LMICs steadily increased over the 10-year period—from 50 in 2010 to 114 in 2019 (figure 2). Across all years, 2018 had the highest number of trials published (139 trials). In total, 786 (89.9%) were individually randomised trials and 88 (10.1%) were cluster-randomised trials. Trials addressed a range of health topics, the most frequent being caesarean section (81 trials, 9.3%), obstetric haemorrhage (80 trials, 9.2%), health system, resources, and infrastructure (57 trials, 6.5%), induction of labour (55 trials, 6.3%) and hypertensive disorders of pregnancy (53 trials, 6.1%). These proportions were relatively consistent over time, apart from some slight variation in trials of caesarean section (8.0% of trials in 2010, 17.1% in 2013, 9.6% in 2019) and nutrition during pregnancy (4.0% of trials in 2010, 12.4% in 2014, 4.4% in 2019).
Figure 2.
Number of maternal health trials in low and middle-income countries by year of publication (2010–2019).
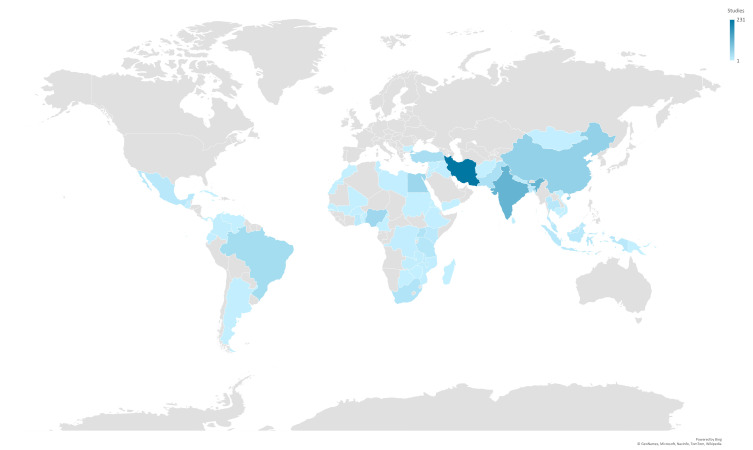
Trials were conducted in 61 LMICs—no trials were identified from the remaining 78 LMICs (figure 3). Iran had the highest number of trials (231 trials, 26.4%), followed by India (113 trials, 12.9%), China (58 trials, 6.6%), Egypt (47 trials, 5.4%) and Nigeria (44 trials, 5.0%). Forty countries had five or fewer trials, and 20 countries had only one trial. The SDG region with the highest number of trials was Central and Southern Asia (399 trials), accounting for nearly half of all identified trials (45.7%) (table 1). The next highest region was sub-Saharan Africa with 185 trials (21.2%), followed by Eastern and South-Eastern Asia with 110 trials (12.6%). Most SDG regions saw increases in the number of trials over time. For example, Eastern and South-Eastern Asia increased from three trials published in 2010 to 22 in 2019, while sub-Saharan Africa increased from nine trials published in 2010 to 33 in 2019.
Figure 3.
Number of identified maternal health trials per low and middle-income country.
Table 1.
Number and proportions of identified trials by Sustainable Development Goals (SDG) region, 2010–2019
| Sustainable Development Goals region* | Total number of trials | % of trials |
| All | 874 | 100 |
| Sub-Saharan Africa | 185 | 21.2 |
| Northern Africa and Western Asia | 95 | 10.9 |
| Central and Southern Asia | 399 | 45.7 |
| Eastern and South-Eastern Asia | 110 | 12.6 |
| Latin America and the Caribbean | 70 | 8.0 |
| Oceania | 1 | 0.1 |
| Europe and Northern America† | 2 | 0.2 |
| Multiregion‡ | 12 | 1.4 |
Pharmacological interventions were the most frequent intervention studied, accounting for 33.8% of all trials (295 trials). Trials of complementary interventions (129 trials, 14.8%) were also common, which included interventions such as aromatherapy, acupuncture and massage therapy. This was followed by educational interventions (90 trials, 10.3%), and nutritional and supplementary interventions (77 trials, 8.8%). Some intervention categories had few trials, hence change over time is not detectable. However, complementary interventions decreased from 18.0% of all trials published in 2010 (9/50) to 10.5% of all trials published in 2019 (12/114). Nutritional and supplementary interventions decreased from 16.0% of trials published in 2010 (8/50) to 6.1% of trials in 2019 (7/114). Conversely, educational interventions increased from 4.0% of trials published in 2010 (2/50) to 15.8% in 2019 (18/114), and resources and infrastructure interventions increased from 4.0% of trials published in 2010 (2/50) to 14.9% in 2019 (17/114).
Half of all trials within the data set pertained to care in a health facility (448 trials, 51.3%). A further 342 trials (39.1%) were in primary care settings. The remaining trials were at health system level (60 trials, 6.9%), public health and preventative care (14 trials, 1.6%) and home and community care (10 trials, 1.1%). The proportion of trials of facility-based care decreased from 60.0% of all trials published in 2010 (30/50) to 41.7% of all in 2019 (48/114), while trials at the health system level rose from 4.0% in 2010 (2/50) to 14.8% in 2019 (17/114).
In assessing the primary outcomes of identified trials—using the predefined Cochrane list of ‘higher-level categories for interventions and outcomes’—development of complications (124 trials, 14.2%), pain-related outcomes (92 trials, 10.5%), outcomes related to women’s knowledge, skills or attitudes (66 trials, 7.6%) and infection-related outcomes (50 trials, 5.7%) were the most common. A large number of trials reported non-descript physiological or clinical outcomes (394 trials, 45.1%) which were categorised into the Cochrane category of ‘other physiological or clinical’. These proportions were largely consistent over time; however, outcomes related to coverage of care increased from 2.0% of trials published in 2010 (1/50) to 13.2% of those in 2019 (15/114). Outcomes on woman’s knowledge, skills and attitudes increased from 0.0% of trials published in 2010 (0/50) to 14.4% in 2018 (16/114), whereas development of complications decreased from 22.0% of trials published in 2010 (11/50) to 10.5% in 2019 (12/114).
Comparison to causes of maternal mortality
Of the 874 trials published between 2010 and 2019, a total of 225 (25.7%) were aimed at preventing or managing one of the causes of maternal mortality. Of these 225 trials, 81 (36.0%) pertained to obstetric haemorrhage, 55 (24.4%) to hypertensive disorders, 38 (16.9%) to HIV, 23 (10.2%) to sepsis, 15 (6.7%) to complications of delivery, 10 (4.4%) to pre-existing medical conditions and 3 (1.3%) to obstructed labour. Table 2 describes each of these causes of death, comparing their percentage contribution to global maternal mortality against the percentage of these 225 trials. The largest discrepancy is in the pre-existing medical conditions category, causing 14.8% of maternal deaths but accounting for only 4.4% of trials. Haemorrhage, hypertensive disorders, complications of delivery and HIV-related causes all had higher proportions of research relative to their contribution to global maternal mortality. Despite accounting for 3.4% of maternal deaths globally, no trials on embolism were identified in our search.
Table 2.
Relationship between contribution of a cause of mortality to maternal deaths in the ‘developing regions’, and research output within maternal health trials in low and middle-income countries, 2010–2019
| Causes of maternal mortality | Contribution to mortality in ‘developing regions’* (%) | Number of trials (% of all trials) | Percentage of trials addressing a cause of mortality (n=225) |
| Abortion† | 7.9 | N/A | N/A |
| Embolism | 3.1 | 0 (0.0) | 0.0 |
| Haemorrhage | 27.1 | 81 (9.3) | 36.0 |
| Hypertensive disorders | 14.0 | 55 (6.3) | 24.4 |
| Sepsis | 10.7 | 23 (2.6) | 10.2 |
| Complications of delivery | 2.8 | 15 (1.7) | 6.7 |
| Obstructed labour | 2.9 | 3 (0.3) | 1.3 |
| HIV related | 5.5 | 38 (4.3) | 16.9 |
| Pre-existing medical conditions | 14.8 | 10 (1.1) | 4.4 |
| Other | 11.2 | 649 (74.3) | N/A |
| Total | 100.0 | 874 (100.0) | 100.0 |
*Mortality figures were taken from the 2014 Say et al report.3
†Abortion was excluded from this review, and hence no results are reported.
N/A, not applicable.
Comparison to research priority topics
The WHO global maternal and perinatal health research prioritisation by Souza et al identified eight priority topics (box 1).7 Among the trials included in this review, the most frequent were trials of antenatal care interventions (333 trials, 38.1%), labour and delivery interventions (292 trials, 33.4%) and trials of interventions for obstetric haemorrhage (80 trials, 9.2%), health systems (65 trials, 7.4%), hypertensive disorders of pregnancy (54 trials, 6.2%) and other (50 trials, 5.7%) (table 3). The greatest differences between the priority topics identified in Souza et al and trials in this review were seen in antenatal care, ranked fourth priority by Souza et al but contributing the highest proportion of research output. The remaining priorities were approximately aligned with the research output identified in this review.
Table 3.
Maternal health trials from low and middle-income countries (2010–2019), compared with Souza et al’s maternal health research priority topics7
| Research priority topics, as ranked by Souza et al | Number of trials (% of all trials) | Rank (based on number of trials) |
| 1. Labour/delivery | 292 (33.4) | 2 |
| 2. Obstetric haemorrhage | 80 (9.2) | 3 |
| 3. Neonatal care* | N/A | N/A |
| 4. Hypertensive disorders of pregnancy | 54 (6.2) | 5 |
| 5. Antenatal care | 333 (38.1) | 1 |
| 6. Abortion* | N/A | N/A |
| 7. Health systems | 65 (7.4) | 4 |
| 8. Other | 50 (5.7) | 6 |
| Total | 874 (100.0) |
*Categories were excluded from this review and hence no results are reported.
N/A, not applicable.
A similar analysis was performed for the research priority topics identified by Chapman et al (box 1).8 In total, 245 trials (28.0%) were not related to one of the categories described by Chapman et al. Aside from these, the most frequent category was labour and caesarean section (292 trials, 33.4%), followed by diabetes and other causes (140 trials, 16.0%), postpartum haemorrhage (80 trials, 9.2%), health policy and systems (63 trials, 7.2%) and hypertensive disorders (54 trials, 6.2%) (table 4). The volume of trial research was almost completely inverted against priority research topics identified by Chapman et al. For example, the lowest ranked Chapman et al priority topic (labour and delivery) accounted for the highest proportion of research output. Relatively few trials were available for some categories.
Table 4.
Maternal health trials from low and middle-income countries (2010–2019), compared with Chapman et al’s maternal health research priority topics8
| Theme, as ranked by Chapman et al | Number of trials (% of all trials) | Rank (based on number of trials) |
| 1. Health policy and system | 63 (7.2) | 5 |
| 2. Diabetes and other causes* | 140 (16.0) | 3 |
| 3. Abortion and unplanned pregnancy† | N/A | N/A |
| 4. Postpartum haemorrhage | 80 (9.2) | 4 |
| 5. Hypertensive disorders | 54 (6.2) | 6 |
| 6. Labour and caesarean | 292 (33.4) | 1 |
| Other‡ | 245 (28.0) | 2 |
| Total | 874 (100.0) |
*Other causes include HIV, malaria, anaemia and violence.
†Category was excluded from this review and hence no results are reported.
‡Other was not a reported result from the Chapman et al’s paper, it has been used to capture any studies that did not fit one of the above categories.
N/A, not applicable.
Discussion
Summary of main findings
A total of 874 trials in maternal health were conducted in LMICs between 2010 and 2019, with a steady increase in trials each year until 2018. Pharmacological interventions accounted for a third of all trials. Nearly half (45.7%) of trials were conducted in Central and Southern Asian countries, and, importantly, of the 139 countries classified as LMIC,15 only 61 had at least one maternal health trial over this 10-year period. Most trials were conducted at facility or primary care levels (51.3% and 39.1%, respectively). Only a quarter of trials explicitly targeted one of the major causes of maternal mortality. Within these studies, trials of pre-existing medical conditions (such as cardiac or endocrine diseases3) and embolism were under-represented relative to their contribution to the global maternal mortality burden. On comparison of our findings to two global research prioritisation exercises by Souza et al and Chapman et al, gaps were identified for research priority topics such as health systems, hypertensive disorders of pregnancy and obstetric haemorrhage. Comparatively, a substantial number of trials addressed antenatal care and labour/delivery topics. These findings suggest that trials conducted in LMICs are not well aligned with either the burden of mortality or identified research priority topics.
Interpretation
To our knowledge, this is the first systematic scoping review to describe the characteristics of maternal health trials conducted in LMICs during 2010–2019. In 2016, Chersich et al published a broad review of the publication of studies (of any design) from LMICs between 2000 and 2012 on five health conditions—haemorrhage, hypertension, malaria, HIV and other sexually transmitted infections—as well as health systems strengthening.24 They reported that the number of articles published per year more than doubled over this time period, from an average of 92 studies between 2000 and 2003 to 237 studies between 2008 and 2012. In line with this, the number of trials increased from 66 in the 2000–2003 period to 119 in the 2008–2012 period. However, Chersich et al reported that the proportion of studies that were trials declined due to the more rapid increase in systematic reviews, qualitative studies and mixed-methods studies. This is broadly similar to our findings, where the number of trials had more than doubled by 2018. The apparent decrease to 114 trials in 2019 might reflect a time lag between publication and inclusion in bibliographic databases, though this is not certain. The rate of increase in published trials is similar to that described by Bornmann and Mutz in their 2015 analysis of research studies published across all scientific fields—they reported that in recent decades the number of cited references approximately doubles every 9 years.25
Iran, an upper middle-income country of nearly 83 million people, was the largest country in terms of maternal health trial output, contributing over 26% of all trials. This was considerably higher than the second largest country, India, with 13% of trials. For the period 2010–2019, Iran’s trial output increased from eight trials a year to a peak of 51 trials in 2018. The global trend of increasing number of trials annually was similar even when excluding trials from Iran. Interestingly, the rapid increase in Iran’s output is in contrast to the Chersich et al’s review, which assessed studies from 2000 to 2012 and did not identify Iran within the top five countries in terms of publications.24 A 2019 report by Stanford University, however, identified that across all scientific fields, publication output from Iran increased dramatically from approximately 1000 studies in 1997 to over 50 000 studies in 2018.26 The authors hypothesised that an increase in graduate student numbers, combined with government policies regarding publication requirements for graduation and promotion, has driven this rapid increase.
Consistent with scoping review methodology, we did not conduct quality assessment of individual trials and are unable to determine whether there are differences in study quality across countries. However, we note that concerns regarding quality of randomised trials are increasingly frequent across a range of health areas. For example, a 2019 analysis of 1082 retracted publications estimated that 2.5 retractions occur for every 10 000 papers globally, though this rate was highest for studies from Iran (15.52 per 10 000), Egypt (11.75 per 10 000) and China (8.26 per 10 000 papers).27 A separate 2019 study of retracted articles from open-access journals found that Iran was one of the top four contributors globally, alongside China, India and the USA.28 In a future analysis of this database, we intend to appraise the quality of identified trials to explore possible differences.
Over 90% of trials were conducted at either a facility or primary care level, a finding consistent with Chersich et al in which only 5% of studies involved a community service component.24 This is perhaps not surprising considering that trials of health system or community-wide interventions can be larger scale and complex endeavours, and hence more challenging and resource intensive to conduct. Conversely, our findings may reflect that the relative scarcity of community-level intervention trials is a missed opportunity, and that greater investment in such trials is warranted. Strengthening community-based approaches is particularly important in resource-limited settings where maternity care facilities and services are scarce. The increase in trials of health system-level interventions from two studies in 2010 to 17 studies in 2019 is already suggestive of greater effort in evaluating more complex interventions to improve maternal health outcomes.
Overall, there is a substantial mismatch between the areas being addressed in trials, leading causes of maternal mortality and priority research topics. Our finding that only a quarter of trials in LMICs are addressing a cause of maternal mortality, despite the maternal death burden, indicates that greater investment and research focused on leading causes of maternal death is required, particularly on underevaluated topics such as pre-existing medical conditions, obstructed labour and embolism. Additionally, our finding that available trials are not closely aligned with identified priority topics suggests that more effort is needed to ensure that research activities would benefit from being better targeted to agreed global priorities.
Strengths and limitations
We undertook a broad, inclusive search with screening in duplicate for eligible studies conducted according to a prespecified review protocol. While it is possible that some trials were not identified, we used the Cochrane CENTRAL database of randomised trials, and hence consider the risk of missing trials to be low. While we focused this analysis on published randomised trials, we acknowledge that further insights could be gleaned from analyses of registered trial protocols on platforms such as ClinicalTrials.gov or the WHO ICTRP. While exploring registered trial protocols was beyond the scope of this analysis, we intend to update and expand this database in the future. We acknowledge that, after extensive efforts, we were unable to locate the full text for 68 of the trials initially identified. We observed that a majority of these were from journals not currently indexed in PubMed.
We opted to focus on randomised trials only, considering their importance in evidence-based practice and evaluating the effects of interventions. However, we acknowledge that this review is limited in that other types of study designs—non-randomised interventional studies, qualitative studies and mixed-methods studies—are also integral to clinical research and improving maternal health outcomes globally. As such, the trends on trial publication reported here may not be applicable to trends in other types of research output. Another limitation was the exclusion of important reproductive health topics such as contraception, preconception health, fertility treatment and abortion, as well as care of newborns in the postnatal period. While these are important health areas, we opted to focus on antenatal, intrapartum and postpartum care of the woman to keep this review to a manageable size and scope. A similar, future analysis of trials from LMICs on these health topics would be important in identifying whether similar trends exist.
Implications for practice, policy and research
Substantial global targets have been set for improving maternal health and well-being by 2030.29 Conducting more and better trials to drive improvements in clinical care is a critical part of efforts to achieve those goals.30 Our findings can guide maternal health researchers and research funding organisations to identify and address overlooked priority topics. This includes LMICs where no maternal health trials were identified, or maternal health conditions (such as pre-existing conditions) where too few trials have been conducted. Where significant numbers of trials are underway, such as individual countries or maternal health topics, reflection on the benefit and necessity of new research may provide impetus for realignment to areas of greater need. This database of randomised trials will be used to conduct further analyses of the maternal health trial literature, such as exploring variations in study quality between countries and over time, trial protocol registration and trial funding practices, and bibliometric analyses to identify the most impactful individuals, institutions and collaborations.
Conclusion
While the volume of maternal health trials in LMICs has steadily increased over the 10-year period from 2010 to 2019, there remains a deficit of trials addressing important causes of maternal mortality. Topics such as pre-existing medical conditions and embolism, as well as the previously identified priority topics of haemorrhage, hypertensive disorders of pregnancy and diabetes in pregnancy, remain relatively under-represented. On a geographical level, the majority of trial output is from a small number of countries, with nearly 40% of studies emanating from only two of the 139 LMICs. These findings suggest that a different approach to selecting topics for trials of maternal health interventions in LMICs may be required—one where trial research is more focused on high-burden conditions and high-priority health issues. Findings can also aid researchers and funding agencies to identify current research gaps for further investment and improve allocation of resources for research.
Supplementary Material
Footnotes
Twitter: @josh_vogel
Contributors: AJE, JPV and TT developed the review protocol and data extraction tools. AJE and SM developed the search strategy. AJE and AR conducted the title/abstract and full-text screening, while AJE, AR, EF, WCT, JW and ASH conducted the data extraction. AJE prepared the first draft of the analysis, which was reviewed by all the authors and revised following their input. AJE is responsible for the overall content as guarantor for this review. All named authors contributed to the writing of this manuscript.
Funding: JPV is supported by a National Health and Medical Research Council Investigator Grant (GNT1194248).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Extra data can be accessed via the Dryad data repository at http://datadryad.org/ with the doi: 10.5061/dryad.hhmgqnkj8.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
As a systematic review of publicly available data, ethical approval was not required.
References
- 1.World Health Organization . Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the. Geneva: United Nations Population Division, 2019. [Google Scholar]
- 2.United Nations . Sustainable development goals report. New York, NY: United Nations, 2019. [Google Scholar]
- 3.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323–33. 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 4.Alkema L, Chou D, Hogan D, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN maternal mortality estimation inter-agency group. Lancet 2016;387:462–74. 10.1016/S0140-6736(15)00838-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham W, Woodd S, Byass P, et al. Diversity and divergence: the dynamic burden of poor maternal health. Lancet 2016;388:2164–75. 10.1016/S0140-6736(16)31533-1 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . Strategies toward ending preventable maternal mortality (EPMM). Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 7.Souza JP, Widmer M, Gülmezoglu AM, et al. Maternal and perinatal health research priorities beyond 2015: an international survey and prioritization exercise. Reprod Health 2014;11:61. 10.1186/1742-4755-11-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman E, Reveiz L, Sangalang S, et al. A survey study identified global research priorities for decreasing maternal mortality. J Clin Epidemiol 2014;67:314–24. 10.1016/j.jclinepi.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 9.CEBM . Centre for evidence based medicine. Available: https://www.cebm.net/ [Accessed 1 May 2020].
- 10.Eccles M, Grimshaw J, Campbell M, et al. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care 2003;12:47–52. 10.1136/qhc.12.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chersich M, Blaauw D, Dumbaugh M, et al. Mapping of research on maternal health interventions in low- and middle-income countries: a review of 2292 publications between 2000 and 2012. Global Health 2016;12:52. 10.1186/s12992-016-0189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munn Z, Peters MDJ, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018;18:143. 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggleston AV, Turner T. Randomised trials in maternal and perinatal health in low- and middle-income countries from 2010-2019: a systematic scoping review 2020. Available: osf.io/wcrph [Accessed 2 Oct 2021]. [DOI] [PMC free article] [PubMed]
- 14.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 15.World Bank Group . Low & middle income, 2019. Available: https://data.worldbank.org/income-level/low-and-middle-income [Accessed 25 Mar 2020].
- 16.Higgins JTJ, Chandler J, Cumpston M, et al. Cochrane Handbook for systematic reviews of interventions: cochrane, 2019. [Google Scholar]
- 17.Cochrane Pregnancy and Childbirth . Cochrane pregnancy and chilbirth’s trials register: the cochrane collaboration, 2020. Available: https://pregnancy.cochrane.org/pregnancy-and-childbirth-groups-trials-register [Accessed Mar 2020].
- 18.Cochrane Effective Practice and Organisation of Care . LMIC Filters: The Cochrane Collaboration; 2020 [cited 2021 November 8]. Available from:. Available: https://epoc.cochrane.org/lmic-filters [Accessed March 2020].
- 19.Cochrane Library . Cochrane central register of controlled trials (central) United Kingdom: cochrane, 2020. Available: https://www.cochrane.org/contact [Accessed 14 Oct 2020].
- 20.Web of Science Group . Endnote USA: clarivate, 2020. Available: https://endnote.com/ [Accessed 14 Oct 2020].
- 21.Covidence . About covidence Australia, 2020. Available: https://www.covidence.org/ [Accessed 14 Oct 2020].
- 22.United Nations Statistics Division . SDG indicators: regional groupings used in report and statistical Annex New York, USA: United nations, 2020. Available: https://unstats.un.org/sdgs/indicators/regional-groups [Accessed 15 Jun 2020].
- 23.Cochrane Linked Data . Metadata and vocabularies London, UK: the Cochrane collaboration, 2020. Available: https://linkeddata.cochrane.org/linked-data-project/metadata-and-vocabularies [Accessed 15 Jun 2020].
- 24.Centre for Health Policy . Report on systematic review of health system, health promotion and clinical interventions for improving maternal health in low- and middle-income countries: MASCOT 2013.
- 25.Bornmann L, Mutz R. Growth rates of modern science: a bibliometric analysis based on the number of publications and cited references. J Assoc Inf Sci Technol 2015;66:2215–22. 10.1002/asi.23329 [DOI] [Google Scholar]
- 26.Sadeh S, Mirramezani M, Mesgaran MB. The scientific output of Iran: quantity, quality, and corruption 2019.
- 27.Campos-Varela I, Ruano-Raviña A. Misconduct as the main cause for retraction. A descriptive study of retracted publications and their authors. Gac Sanit 2019;33:356–60. 10.1016/j.gaceta.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Xing Q-R, Wang H, et al. Retracted publications in the biomedical literature from open access journals. Sci Eng Ethics 2019;25:855–68. 10.1007/s11948-018-0040-6 [DOI] [PubMed] [Google Scholar]
- 29.United Nations . Goal 3: ensure healthy lives and promote well-being for all at all ages Geneva, Switzerland: United nations, 2015. Available: https://www.un.org/sustainabledevelopment/health/ [Accessed 29 Apr 2020].
- 30.World Health Organization, Światowa Organizacja Zdrowia . Research for universal health coverage. World Health Organization, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059473supp001.pdf (78.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Extra data can be accessed via the Dryad data repository at http://datadryad.org/ with the doi: 10.5061/dryad.hhmgqnkj8.