Abstract
Microplastics (MPs) are environmental pollutants of growing concern, and awareness of MPs pollution in marine and freshwater environments has increased in recent years. However, knowledge of MPs contamination in riverine sediments in Ireland is limited. To address this, we collected and analysed sediment samples from 16 selected sites along the River Barrow. Microplastics were extracted through a density separation method, after which their size, colour, and shape were analysed under a stereo microscope (Optica SZM-2). Attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy was used to identify polymer types. A total of 690 MPs were recovered from the 16 sites, with fibres as the dominant MP type. The highest concentration of MPs was 155 MP fibres kg−1 wet sediment found in samples collected from Graiguenamanagh, Co. Kilkenny (GK). The majority of the recovered MPs were polyethylene (PE), polypropylene (PP), nylon, and cellulose acetate (CA) fibres. Overall, this study highlighted the presence of MPs in Irish river sediments and provided a baseline for future studies on MPs pollution. Further research is needed to better understand sources, distribution, and effects of MPs in freshwater ecosystems.
Keywords: River sediments, MPs, Density separation, Quantification, Characterisation, ATR-FTIR
Graphical abstract
River sediments; MPs; Density separation; Quantification; Characterisation; ATR-FTIR.
1. Introduction
The global production of plastics has rapidly increased over the past 70 years reaching almost 370 million tons in 2019, compared to 1.5 million tons in 1950 (Plastics Europe, 2020). Due to the high disposability and limited recovery of plastic materials, plastics have been accumulating in aquatic environments, including rivers, lakes, and marine environments, leading to increased concern about their environmental effects (Rillig, 2012; Rocha-santos and Duarte, 2015). Plastics have been widely used in daily life in a multitude of products such as packaging (39.6%), building and construction (20.4%), automotive (9.6%), electrical and electronic (6.2%), household, leisure, and sports (4.1%), agriculture (3.4%), and others including appliances, mechanical engineering, furniture, and medical (Andrady and Neal, 2009; Bouwman et al., 2018; Plastics Europe, 2020). The most common plastic materials used are high-density polyethylene (HDPE), low density polyethylene (LDPE), polypropylene (PP), polyvinylchloride (PVC), polystyrene (PS), and polyethylene terephthalate (PET) or polyester (Boyle and Örmeci, 2020; Ivar Do Sul et al., 2014; Magalhães et al., 2020; Ziajahromi et al., 2016).
Microplastics (MPs) are defined as plastic fragments less than 5 mm in diameter and are of concern in part due to their potential to accumulate organic contaminants (Masura et al., 2015; Murphy and Quinn, 2018; Wagner et al., 2014). Microplastics can be classified into two categories based on their origin: primary and secondary MPs. Primary MPs are intentionally manufactured in small size and used as industrial pellets for further processing, to be added directly in cosmetic products such as facial scrubs or other personal care products and toothpaste, or to be used in abrasive blasting and medical vector applications. In contrast, secondary MPs result from the breakdown of larger plastics, such as MP fibres produced by mechanical stress to synthetic textiles and the products of in situ litter decomposition (Horton et al., 2017; Magalhães et al., 2020; Rocha-santos & Duarte, 2015). Plastics degradation may occur once exposed to aquatic environments due to the effects of sunlight, pH, temperature, biological, physical, and chemical conditions (Law and Thompson, 2014; Li et al., 2020a). Plastics often include a variety of chemical additives such as plasticizers, flame retardants, cross-linking additives, antioxidants, and other stabilizers, which may leach out to the environment or within organisms following ingestion (Sartain et al., 2018). Microplastics have been shown to adsorb persistent organic pollutants (POPs) such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), dichlorodiphenyltrichloroethanes (DDTs), and nonylphenols due to their hydrophobicity (Menéndez-Pedriza and Jaumot, 2020). Because of their small size, MPs have a high bioavailability when ingested. Once MPs enter organisms, they can release chemicals added during production as well as absorbed contaminants from their surface, which can potentially cause toxic effects (Gabriel et al., 2019). Therefore, MPs may pose threats to organisms and to human health as they may be ingested deliberately or accidentally by fish and other species, thus entering the food chain (Gabriel et al., 2019; Gall and Thompson, 2015; Li et al., 2020b; Zhang et al., 2019). Some of the chemicals used to produce plastics such as bisphenol A (BPA), phthalates, and flame retardants used in household and food packaging, have been confirmed to affect human health if ingested or inhaled (Campanale et al., 2020a, 2020b; Li et al., 2020a, 2020b, 2020c). Household products that may contain BPA include canned foods, glass jars lids, food packaging products such as baby bottles, drinking containers, and snack packaging (Chen et al., 2017). Polyvinyl chloride (PVC) floors, PVC leather, food packaging, toys, polishing products, and skincare items have all been shown to contain phthalates (Zhang et al., 2020). Brominated flame retardants are used in household objects such as furniture and textiles (Niu et al., 2019). A recent study suggested that bottle fed infants are consuming 1 million MP particles daily (Li et al., 2020a, 2020b, 2020c). In the United States, estimations of MPs consumed per person annually via the food chain and inhalation have ranged from 74000 to 121000 (Cox et al., 2019) and MPs have also been detected in human stool (Li et al., 2020b). However, the health impacts of MPs in humans are yet to be established (Li et al., 2018; Wright and Kelly, 2017).
Microplastics have been observed in a variety of shapes, such as fragments, fibres, microbeads, films, and pellets. Microfibres and microbeads are mostly discussed because of their prevalence in everyday life (Boyle and Örmeci, 2020; Lenaker et al., 2021). Microfibres account for 59% of MPs released into the environment, due to fragmentation during household washing of synthetic fabrics and wastewater discharges from textile industries, which significantly contribute to river and ocean pollution (Kole et al., 2017; Reineccius et al., 2020). Discharges from domestic wastewater treatment plants, landfill leachate, and surface run off from urban areas have been identified as important sources of MPs in freshwater environments (He et al., 2019; Li et al., 2020a).
Microplastics in aquatic sediments can be ingested by a range of benthic organisms (Li et al., 2020c; Tan et al., 2019) and are recognized as an emerging issue in both marine, freshwater, and terrestrial environments. Most studies have focused on MPs in marine ecosystems (Li et al., 2020a). However, a review of literature between 2012 and 2020 revealed a total of only 158 publications that have referred to freshwater compared to 2864 for marine ecosystems (Cera et al., 2020; He et al., 2020). Over the last decade, MPs have been documented in freshwater ecosystems globally, as the number of studies has rapidly increased (Eerkes-Medrano and Thompson, 2018). The detection of MPs in Irish freshwater ecosystems is limited. A preliminary study on MPs in Irish freshwaters by Cedro and Cleary (2015) showed the presence of MPs in river, lake, and wastewater (Cedro and Cleary, 2015). The current study is aimed at assessing the presence of MPs in the River Barrow. Microplastics were extracted from sediment samples through the density separation method using various high-density brine solutions (Nakajima et al., 2019). Density separation using concentrated salt solutions have been shown to be effective for recovery of MPs from marine and freshwater sediments. Attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) was used to identify different polymers. Infrared spectroscopy has been used as the most common method for MP polymers identification (Zobkov and Esiukova, 2018). Attenuated total reflection Fourier transform infrared (ATR-FTIR) was used in this study since it has been considered as one of the useful techniques for obtaining spectra from small particles and examining surface interactions.
2. Materials and methods
2.1. Sampling sites
The River Barrow is Ireland’s second longest river. It is roughly 196 km long from source to sea, having a catchment area of 3067 km2. Before it joins the River Nore, the River Barrow has an average flow rate of 37.40 m3/s. Counties Laois, Kildare, Kilkenny, Carlow, Wexford, and Waterford are all bordered by the River Barrow. It flows north and east past Mountmellick and Portarlington to Monasterevin, rising on the northern slopes of the Slieve Bloom Mountains in Co. Laois. The River Barrow flows from Monasterevin to Saint Mullin's, passing through the towns of Athy, Carlow, Leighlinbridge, Bagenalstown, Goresbridge, Borris, and Graiguenamanagh (Figure 1). Waterford Harbour is formed when the River Barrow meets the River Nore upstream of New Ross, Co. Wexford, and merges with the River Suir. The River Barrow is influenced by the tides from Waterford Harbour to St. Mullins, Co. Carlow. The Barrow has been designated as a valuable resource for waterside and waterborne recreation (Delanty et al., 2017).
Figure 1.
Sampling locations along the River Barrow. Blue circles illustrate the sediment sampling sites. Red markers indicate the location of major urban wastewater discharge points. Insert shows the location of the Barrow catchment on the island of Ireland.
The River Barrow is designated as a special area of conservation (SAC) under the EU Habitats Directive for the conservation of flora, fauna, and habitats of European importance. Under the EU Water Framework Directive (WFD) rivers are classified as either high, good, moderate, poor, or bad Ecological Status depending on whether they meet certain biological, chemical, and hydromorphological criteria (EPA, 2017). The sites in the lower half of the River Barrow catchment were assigned good or high Ecological Status while sites in the upper catchment were mainly classified as good or moderate Ecological Status. In the most recent ecological assessment of the River Barrow and its tributaries (2015–2018), just over 70% of surveyed sites on the main channel were below good status (EPA, 2021).
Waste water discharges have been identified as the most significant pressure on the Barrow by the Irish Environmental Protection Agency (EPA), putting the environment and public health at risk (EPA, 2019, 2018). Wastewater inputs to the Barrow come from locations including Laois – Portarlington (moderate status), Kildare – Athy (moderate status), Carlow – Tullow, Carlow – Borris (poor status), Carlow – Bagenalstown (poor), Kilkenny – Goresbridge (moderate status). These discharges are likely to be major sources of MPs entering the river. Although the levels of MPs are not among the chemical and physical parameters considered in official water quality assessments, they have been shown to affect biological parameters including algae and fish (Okubo et al., 2018; Pannetier et al., 2020; Savinelli et al., 2020), which are part of the WFD assesment criteria. Therefore it is necessary to assess the abundance and chemical nature of MPs in freshwater ecosystems in order to consider their potential to cause harm to riverine ecology.
In August 2018 and July 2019, 5 to 10 kg river sediment samples were collected in triplicate from sixteen locations along the River Barrow from a 10 cm depth using a Dutch auger (Figure 1; Table 2). The collected samples were stored at 4 °C (to minimize biological activity which could affect the subsequent separation/analysis) using pre-labelled 5–10 L high-density polyethylene (HDPE) containers (Baptista et al., 2019; Sartain et al., 2018).
Table 2.
Abundance and sizes of microplastics found in samples from Irish river sediment samples (August 2017–2018).
| Sampling locations' coordinates | Sampling sites and codes | Size range (mm) | Total number of MPs recovered | ||
|---|---|---|---|---|---|
| 1 | 53°05′31.4″N7°31′54.5″W | Slieve Blooms – Laois | SBL | <2 | 24 |
| 2 | 53°08′58.0″N 7°28′53.9″W | Clarahill – Laois | CHL | <4 | 21 |
| 3 | 53°10′55.3″N 7°25′26.4″W | Clonduff – Laois | CDL | <3 | 13 |
| 4 | 53°09′49.8″N 7°11′21.1″W | Portarlington – Laois | PL | <3 | 16 |
| 5 | 53°08′46.6″N 7°04′12.2″W | Monasterevin – Kildare | MK | <3 | 13 |
| 6 | 52°59′46.3″N 6°59′08.5″W | Athy – Kildare | AK | <4 | 54 |
| 7 | 52°49′58.5″N 6°55′30.0″W | Carlow town – Carlow | CTC | <2 | 7 |
| 8 | 52°48′49.5″N 6°57′11.5″W | Mortarstown – Carlow | MUC | <3 | 33 |
| 9 | 52°48′49.5″N 6°57′11.5″W | Dolmen Hotel – Carlow | DHC | <6 | 31 |
| 10 | 52°47′04.7″N 6°57′60.0″W | Milford – Carlow | MFC | <4 | 15 |
| 11 | 52°42′28.0″N 6°57′16.1″W | Bagenalstown – Carlow | BTC | <3 | 6 |
| 12 | 52°29′26.9″N 6°56′05.2″W | St. Mullin's – Carlow | SMC | <6 | 91 |
| 13 | 52°37′51.7″N 6°59′26.1″W | Goresbridge – Kilkenny | GBK | <4 | 9 |
| 14 | 52°32′26.4″N 6°56′53.7″W | Graiguenamanagh – Kilkenny | GK | <3 | 155 |
| 15 | 52°18′48.9″N 6°59′56.6″W | Fisherstown – Wexford | FTW | <6 | 124 |
| 16 | 52°16′58.9″N 6°59′52.3″W | Great Island – Wexford | GIW | <4 | 78 |
| Total | 690 | ||||
2.2. Contamination control
To mitigate sample contamination from synthetic clothing and equipment used during processing, a strict procedure was followed to avoid airborne contamination during the sediment sampling and transport in the laboratory. Prior to sediment sampling, the equipment was cleaned with Virkon™ (LANXESS Rely + On) high level surface disinfectant and with deionised water. To prevent contamination, the lids of the containers were checked for any loose material. The containers were then rinsed with deionised water which was then filtered through Whatman Nº 4 filter paper. The filter paper was dried and examined under a microscope for fibres and evidence of MP contamination. This procedure did not indicate any sources of potential contamination. The laboratory windows remained closed during the experiments. The laboratory bench tops were cleaned four times with 70 % ethanol before the sediment analysis. Hair was tied back, and 100 % cotton lab coat and gloves were worn at all times. All apparatus was washed with Virkon, and materials were examined under a stereomicroscope, which was also cleaned during the samples analysis to ensure that it was free of contamination.
2.3. Preparation of brine solution for MP extraction from sediments
The density separation method described by Coppock et al. (2017), Imhof et al. (2012), and Vermeiren et al. (2020) was used in this study to extract MPs from sediment samples using brine solutions of various densities. In contrast to most of the selected methods, the sediment samples in this study were not dried because high-organic-matter-content sediments dry into compacted agglomerations which can impact the state of MPs within the samples (Vermeiren et al., 2020). Sodium iodide (NaI), zinc chloride (ZnCl2), potassium hydroxide (KOH), and sodium chloride (NaCl) (Table 1) were used for the recovery of MPs from River Barrow sediment samples.
Table 1.
Brine solutions of different densities used to recover microplastics from sediment samples.
| Salt | Mass (g) | Water volume (mL) | Density (g mL−1) | MPs Recovery (%) |
|---|---|---|---|---|
| Sodium iodide (NaI) | 140 | 200 | 1.5 | 99.8 |
| Zinc chloride (ZnCl2) | 300 | 200 | 1.7 | 100 |
| Potassium hydroxide (KOH) | 70 | 200 | 1.2 | 93.5 |
| Sodium chloride (NaCl) | 70 | 220 | 1.15 | 92 |
2.4. Validation of density separation
The density separation technique has been applied in 65 % of the studies for the recovery of MPs from sediments (Nakajima et al., 2019; Quinn et al., 2017). NaCl has been the most commonly used in brine solution for MPs density separation; it has been recommended for use by the Marine Strategy Framework Directive (MSFD) Technical Subgroup on Marine Litter, as NaCl is cheap, abundant, and environmentally benign. However, due to the lower density of NaCl solutions the resulting recovery of denser polymers has been found to be limited (European Commission, 2013). In this study a number of brine solutions were trialled to identify which would give the highest recovery of MPs. NaCl was prepared by dissolving 70 g in 200 mL deionised water (1.15 g cm−3). A hydrometer was used to measure the density of brine solutions. 50 g of sediment was spiked with 1.2001 g of MP particles and placed in a 250 mL glass beaker. A volume of 200 mL of the NaCl brine solution was added to the beaker. The mixture was stirred using a metal spatula for 2 min and left to settle for 10 min to allow MPs flotation while sediment particles sank. The suspension was filtered through Whatman Nº 4 filter paper by vacuum filtration. Filter paper was placed on a watch glass, dried at room temperature overnight and dried in an oven at 60 °C for 5 min. Once dried, an analytical balance was used to weigh the MPs and percentage recovery was calculated. The amount of 1.1081g (92 %) of MPs was recovered. This procedure was repeated with the other salts (Table 1). ZnCl2 was selected and used throughout this work as it gave the highest recovery (100 %) (Table 1). Microplastics percentage recovery was calculated using the equation:
2.5. Extraction and analysis of MPs from sediment samples
400 mL of ZnCl2 (1.7 g mL−1) was added to 160 g of wet sediment and stirred for 3 min using a metal spatula. The beaker containing the ZnCl2 solution was covered loosely with aluminum foil and left to settle for 2 h at room temperature until the solution was clarified. The solution was filtered through Whatman Nº 4 filter paper by vacuum filtration to reduce the risk of potential contamination from any microfibres from the laboratory air. To avoid any impact from the heat on MPs (Vermeiren et al., 2020), the filter paper was dried at room temperature overnight and then dried in an oven at 60 °C for 5 min. The density separation using ZnCl2 solution was repeated twice for each sample to ensure full recovery of MP particles. ZnCl2 solution was freshly prepared for each sample prior to MPs extraction. The density of the solution was checked after the first use. Forceps were used to transfer the recovered particles to a clean Petri dish which was sealed with Parafilm. A stereomicroscope (Optica SZM-2) was used to examine the filters with the recovered MP particles at total magnifications ranging from 7X (minimum) to 45X (maximum) to identify, quantify, and measure MPs using a ruler. Microplastic concentrations were expressed as number of particles kg−1 wet sediment. Furthermore, MP particles were characterised using ATR-FTIR spectroscopy. Spectra were collected from 4000 cm−1 to 500 cm−1 using a Perkin Elmer Spectrum 65 FT-IR Spectrometer with a LiTa detector and a UATR ZnSe crystal with a 3–4 mm aperture. A background scan was performed between each sample after cleaning the ATR diamond crystal with 70 % 2-propanol. As recommended by Perkin Elmer, each sample was compressed against the crystal to ensure good contact. Each spectrum was the average of 8 scans at 4 cm−1 resolution. Absorption bands were recorded and compared to reference spectra of polymers obtained from Cospheric (Cospheric, http://www.cospheric.com/); absorption bands have been reported in the literature, such as by Hummel (2002), Jung et al. (2018), and Tiwari et al. (2019). Each sampling site was analysed in triplicate (160 g sediment/replicate).
A single factor one-way ANOVA analysis of the data showed significant variation among the 16 sampling locations. The statistical analysis was conducted using Microsoft Office Excel. Microplastics abundance in River Barrow sediment samples were significantly different between sites (Figure 3), one-way ANOVA (F = 3.670, P = 0.000299), with mean abundance ranging from 1.5 particles kg−1 (BTC samples) to 38.75 particles kg−1 (GK samples).
Figure 3.
Abundance and colours of MP fibres found in River Barrow sediment samples. Colours on figure represent the actual colour categories of recovered MPs. Sampling sites: Slieve Blooms (SBL); Clarahill (CHL); Clonduff (CDL); Portarlington (PL); Monasterevin (MK), Athy – Kildare (AK); Carlow town – Carlow (CTC); Mortarstown – Carlow (MC); Dolmen Hotel – Carlow (DHC); Milford – Carlow (MFC); Bagenalstown – Carlow (BTC), St. Mullin's – Carlow (SMC); Goresbridge – Kilkenny (GBK); Graiguenamanagh – Kilkenny (GK); Fisherstown – Wexford (FTW); and Greatisland – Wexford (GIW).
3. Results
3.1. Abundance of MPs in River Barrow sediments
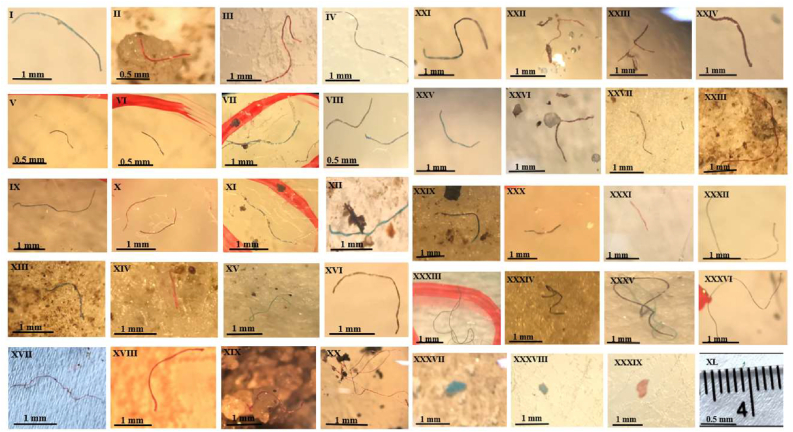
Results showed the ubiquitous presence of MPs along the entire channel of the River Barrow as they were recovered from sediment samples at all 16 sites (Figure 3). A total number of 690 MP fibres were detected (see Table 2). Plastic fibres were the most common type of MPs recovered followed by a small number of fragments detected from Graiguenamanagh – Kilkenny (GK), and Milford – Carlow (MFC) (Figure 2). Microplastic fibre abundance in the sediments ranged from 6 to 155 pieces per 160 g wet sediment samples. Microplastic fibres were mostly red, blue, white, and black in colour. The blue fibres were found in a higher number across all the samples, followed by the red and white fibres. Black fibres were observed in lower number among all the sampling locations (Table 3).
Figure 2.
Microplastic particles recovered from the River Barrow sediment, ranging from 100 μm to 5 mm in size.
Table 3.
Colours and types of microplastic fibres and fragments recovered from sediment samples.
| Number of MP found | Polymer colour | Polymer type | Number of MP identified by ATR-FTIR |
|---|---|---|---|
| 198 | Red | Polyethylene | 7 |
| 244 | Blue | Polypropylene | 14 |
| 181 | White | Cellulose acetate | 28 |
| 67 | Black | Polyethylene terephthalate | 4 |
| Nylon | 5 |
Sediment samples from GK (155 MP fibres kg−1) and Fisherstown – Wexford (FTW) (124 MP fibres kg−1) presented the highest abundance of MP fibres, followed by those from St. Mullin's – Carlow (SMC) (91 MP fibres kg−1). The lowest number of MPs was observed in samples from Bagenalstown – Carlow (BTC) (6 MP fibres kg−1) and Carlow Town – Carlow (CTC) (7 MP fibres kg−1) (Figure 3; Table 2). The average extraction rate was 172.5 ± 116.0 MPs per Kg of sediment from the 16 locations. Despite the significant variation in MPs quantity and size from different sites, particles less than 3 mm in size predominated in GK samples, and particles less than 6 mm were most dominant in FTW (Table 2).
4. Discussion
To our knowledge this is the first study to evaluate the presence and abundance of MPs in sediments from the River Barrow. The majority of similar studies in relation to Irish freshwater have been focused on the toxicity of MPs to freshwater organisms (Mateos-Cárdenas et al., 2021; O'Connor et al., 2020).
All plastics found in the present study were classified as secondary MPs, with no particle greater than 5mm in size. Most of the recovered MPs from river sediment samples were fibres. The higher occurrence of plastic fibres is consistent with other studies showing MPs composition in freshwater and marine environments to be dominated by fibres at 52%, followed by fragments at 29% (Ballent et al., 2016; Burns and Boxall, 2018; Dodson et al., 2020; Nakajima et al., 2019; Shruti et al., 2019; Turner et al., 2019).
Out of a total of 690 observed particles, ATR-FTIR was used to identify 58 that were visible to the naked eye and transferable from the filter paper under the microscope to a Petri dish for further analysis. This represents a limitation on MP characterisation due to their size. Of the identified MPs, seven were classified as polyethylene, 14 as polypropylene, 28 as cellulose acetate, four as polyethylene terephthalate, and five as nylon (Table 4; Figure 4a– – j). Absorption bands were used to identify polymers, as shown in Figure 4, and detailed in Table 5. Figure 4 (a) shows the spectrum of a recovered fibre with absorption bands that match polyethylene (PE) with additional bands (1, 4, and 6, see Table 5). The broad absorption band for hydroxyl (OH) at 3442 cm−1 (peak 1) could suggest water absorption, C=C stretch at 1619 cm−1 (peak 4) and C–O stretch at 1057 cm−1 (peak 6) could potentially indicate PE degradation due to oxidation induced by ultraviolet light after exposure to various environment mechanisms. Similar findings on PE degradation have been observed in recent studies (Castelvetro et al., 2021; Chamas et al., 2020). Various additives can also contribute absorption peaks to polymer spectra which are not explained by the polymer structure (Maléchaux et al., 2021).
Table 4.
Identification and characterization of microplastics obtained from river sediment samples.
| Location | Sediment description | Identified particle number | Microplastics type |
|---|---|---|---|
| Clarahill – Laois | Gravel | 1 | Polyethylene |
| 1 | Cellulose acetate | ||
| Clonduff – Laois | Gravel | 2 | Cellulose acetate |
| Portarlington – Laois | Gravel | 13 | Cellulose acetate |
| Athy – Kildare | Sandy | 3 | Cellulose acetate |
| 2 | Polyethylene | ||
| 4 | Polypropylene | ||
| 1 | Polyethene terephthalate | ||
| 1 | Nylon | ||
| Carlow town – Carlow | Gravel | 1 | Polyethene terephthalate |
| Dolmen Hotel – Carlow | Sandy/Gravel | 1 | Nylon |
| Milford – Carlow | Gravel | 4 | Cellulose acetate |
| 1 | Polyethylene | ||
| Bagenalstown – Carlow | Gravel | 1 | Cellulose acetate |
| St. Mullin’s – Carlow | Sandy | 3 | Polyethylene |
| 1 | Polyethene terephthalate | ||
| 2 | Polypropylene | ||
| Graiguenamanagh – Kilkenny | Silt | 1 | Nylon |
| 4 | Polypropylene | ||
| 2 | Cellulose acetate | ||
| Fisherstown – Wexford | Sandy | 1 | Polypropylene |
| 1 | Polyethene terephthalate | ||
| 1 | Nylon | ||
| 1 | Cellulose acetate | ||
| Great Island – Wexford | Muddy | 3 | Polypropylene |
| 1 | Cellulose acetate | ||
| 1 | Nylon | ||
| Total | 58 |
Figure 4.
ATR-FTRI spectra of recovered MP fibre recovered from (a) Milford – Carlow corresponding to polyethylene (PE); (b) Athy – Kildare corresponding to PE; (c) St. Mullin's – Carlow corresponding to polypropylene (PP); (d) Graiguenamanagh – Kilkenny Carlow corresponding to PP; (e) Graiguenamanagh – Kilkenny corresponding to cellulose acetate (CA); (f) Great Island – Wexford corresponding to nylon; (g) Greatisland – Wexford corresponding to a mixture of PP and CA; (h) St. Mullin’s – Carlow corresponding to PE; (i) Fisherstown – Wexford corresponding to PP and (j) Athy – Kildare corresponding to PET. Each polymer's distinctive absorption bands (cm−1) are represented by numbers. See Table 5 for an assignment of the peaks.
Table 5.
ATR-FTIR peak data and assignments of the infrared absorption bands for identified polymers (Figure 4), based on Jung et al. (2018), Sathish et al. (2019) and Tiwari et al. (2019).
| Microplastic type and chemical structure | Figures | Absorption bands (cm−1) | Assignment | |
|---|---|---|---|---|
1. Polyethylene (PE)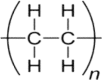
|
1a. MFC | 1. | 3442 | O–H stretch |
| 2. | 2917 | C–H stretch | ||
| 3. | 2848 | C–H stretch | ||
| 4. | 1619 | C=C stretch | ||
| 5. | 1467 | CH2 bend | ||
| 6. | 1057 | C–O stretch | ||
| 7. | 717 | CH2 rock | ||
| 1b. AK | 1. | 3381 | O–H stretch | |
| 2. | 2917 | C–H stretch | ||
| 3. | 2849 | C–H stretch | ||
| 4. | 1628 | C=C stretch | ||
| 5. | 1476 | C=C stretch | ||
| 6. | 1467 | CH2 bend | ||
| 7. | 1032 | CH2 rock | ||
| 8. | 718 | CH2 rock | ||
2. Polypropylene (PP)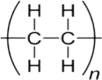
|
2a. SMC | 1. | 2950 | C–H stretch |
| 2. | 2917 | C–H stretch | ||
| 3. | 2838 | C–H stretch | ||
| 4. | 1455 | CH2 bend | ||
| 5. | 1376 | CH3 bend | ||
| 6. | 1164 | CH3 bend, C–C stretch | ||
| 7. | 973 | CH3 rock, C–C stretch | ||
| 8. | 876 | C–H bend | ||
| 9. | 713 | CH2 rock | ||
| 2b. GK | 1. | 2950 | C–H stretch | |
| 2. | 2917 | C–H stretch | ||
| 3. | 2838 | C–H stretch | ||
| 4. | 1455 | CH2 bend | ||
| 5. | 1376 | CH3 bend | ||
| 6. | 1166 | CH3 bend | ||
| 7. | 1033 | CH2 rock | ||
| 8. | 997 | CH3 rock, CH2 rock | ||
| 9. | 972 | CH3 rock, C–C stretch | ||
| 10. | 808 | C–C stretch | ||
3. Cellulose acetate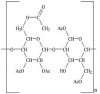
|
3a. GK | 1. | 3417 | O–H stretch |
| 2. | 2931 | O–H stretch | ||
| 3. | 1718 | C=O stretch | ||
| 4. | 1390 | CH3 bend | ||
| 5. | 1257 | C–O stretch | ||
| 6. | 1075 | C–C stretch | ||
| 7. | 1065 | C–C stretch | ||
4. Nylon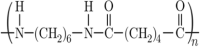
|
3b. GIW | 1. | 3285 | N–H stretch |
| 2. | 2931 | C–H stretch | ||
| 3. | 2859 | C–H stretch | ||
| 4. | 1629 | C=O stretch | ||
| 5. | 1538 | N–H bend, C–N stretch | ||
| 6. | 1462 | O–H bend | ||
| 7. | 1256 | C–O stretch | ||
This study confirmed the ubiquitous presence of MP fibres in all River Barrow sediment samples, with fibres making up the majority of MPs recovered. Similar studies have made similar observations based on sediment analysis. Baptista et al. (2019); Dodson et al. (2020) and Gerolin et al. (2020) have also found MP fibres to be the most common type of MPs in sediment samples. Microplastic particles were found in all sampling sites in the present study. These fibres could originate from the breakdown of larger plastic items such as plastic rope, fishing materials, or clothing, hence considered as secondary MPs. Microfibres from synthetic clothing have been proven to be one of the major sources of MPs in the environment, released in discharges from municipal wastewater treatment plants (Borges et al., 2019; Napper and Thompson, 2016). Recent studies have also found synthetic clothing and fishing nets to be the main source of MP fibres in the marine and freshwater environments (De Falco et al., 2019; Yang et al., 2021).
In our study we found five polymers represented in MPs recovered from river sediment, including PE, PET, PP, CA, and nylon. Cellulose acetate fibres were the most abundant in all sediment samples, followed by PP, PE nylon, and PET, all of which are widely used in the plastics sector (de Haan et al., 2019). Other studies have reported polyethylene and polypropylene as the main types of MPs in their sediment samples (Claessens et al., 2011; Gimiliani et al., 2020; Pohl et al., 2020). Polypropylene polymers constituted 19.4 % of plastic by resin type in 2019 and are widely used in food packaging, snack and sweet wrappers, pipes, hinged caps, etc. However, low and high-density polyethylene represents 29.8 % of plastic by resin type and is used in food packaging film, reusable bags, trays, and agricultural films, toys, milk bottles, pipes etc. Polyethylene terephthalate accounts for 7.9 % and is used in bottles for soft drinks, juices, cleaners etc. (Plastics Europe, 2020). Cellulose acetate is used in the manufacture of clothing and cigarettes. Nylon is commonly found in musical strings, rugs, textiles and rope (Zhu et al., 2019). A study conducted by Corcoran et al. (2020) in the River Thames (Canada), investigated MP abundance in freshwater river sediment. They found MPs abundance ranging from 6 to 2444 particles per Kg of dry weight sediment with fibres as the predominant MP morphology, and PE as the most common polymer identified (Corcoran et al., 2020), which is higher compared to our finding for the River Barrow.
Transport and deposition of MPs is affected by a number of variables. These include geographical position, wind, currents, and streamflow rate (Bellasi et al., 2020). Tibbetts et al. (2018) found that in the River Tame (Birmingham, UK), variation in MP abundance was primarily driven by flow velocities, with increased particle concentrations reported in areas of decreased flow. The same study reported that MP concentrations in freshwater sources were generally elevated in urban locations relative to rural settings (Tibbetts et al., 2018). Rummel et al. (2017) have pointed out that biofilm formation on plastic debris has substantial and varying implications for its transport/deposition potential. The accumulation of fouling organisms such as diverse communities of bacteria, algae, protozoans, and fungi may lead to an increase in the density of MP particles and a decrease in their buoyancy, leading to deposition. Conversely, the buoyancy of particles that originally had a higher density than water may increase as a result of biofouling, rendering such MPs more susceptible to transport. Buoyancy can be increased if less dense biofilms form on a dense material (since the average density of the particle is decreased). Furthermore, biofilm formation increases the adhesiveness of MP particles, promoting the formation of heteroaggregates including MPs, microbial communities, organic detritus, and inorganic solids (Rummel et al., 2017). Quantity and characteristics of suspended organic and inorganic solids can also affect the transport and deposition of MPs by providing greater or lesser opportunity for the formation of assemblages, with potential density effects similar to those of biofilm formation. Temporal variation in river flow rate and in local flow velocity, and turbulence patterns in the immediate vicinity of sampling locations, represent additional complicating factors when assessing the origins and implications of MP abundance at a given location. Hence, we limit our discussion to the following observations and suggestions regarding the reasons for the different MP abundances found in this study and shown in Figure 3.
There was an overall trend towards higher abundance of MPs in sampling sites in the lower and tidal-influenced reaches of the River Barrow, with the four highest MP numbers occurring within the five sampling locations in the lower catchment areas. The exception among these five locations, with much lower MP numbers, was the GBK site. At this location the flow was relatively rapid with gravelly sediment, both factors which are likely to favour transport rather than deposition of MPs. The SMC and GK sites are popular amenity areas with recreational activities including bathing, boating, kayaking, and fishing being likely local contributors to the high abundances of MPs in these locations. The GK sampling location, which had the highest MP numbers, consisted of fine sandy sediment, indicating a general tendency towards deposition at this location as well as providing a benthic environment suitable to trap and act as a sink for deposited MPs. The two sampling sites lowest in the catchment had high abundance of MPs. Fisherstown – Wexford (FTW) has a strong tidal influence and very fine silty/muddy sediment, which again appears to represent a sink for MPs, with the second highest number of MPs recovered from this location. Greatisland – Wexford (GIW) is an estuarine location with likely marine influence on its high MP abundance from sources such as fisheries, shipping, buoy mooring ropes etc. in addition to inputs from the River Barrow itself.
The five uppermost sampling locations had relatively low MP abundances, although the uppermost two locations, SBL and CHL, despite being in upland areas with low population densities, had MP numbers higher than the next three sampling locations. These include urban locations such as PL (Portarlington, population approx. 6000) and MK (Mountmellick, population approx. 4000). In contrast, AK (Athy, population approx. 8200) had the highest MP numbers outside of the lower reaches of the river. Possible contributors to this high abundance include relatively slow flow, turbid conditions, recreational activities, and general plastic litter. Athy’s wastewater treatment plant discharges to the River Barrow significantly downstream of the sampling location. Additionally, possible sources of MPs in upper catchment may include agricultural materials such as fibres from baler twine (polypropylene), fragments from agricultural film/sheeting – polyethylene), storage bags and containers, plumbing components (PE, PP, PVC), as well as atmospheric deposition of artificial textiles, and fragments from general litter (Tian et al., 2022).
Moving downstream, the next sampling location was CTC (Carlow Town, population approx. 20,600), which had one of the lowest MP numbers with just 7 MPs recovered. This sampling location is again, upstream of the town’s wastewater treatment discharge. The following two locations, MUC and DHC, are downstream of Carlow Town’s wastewater discharge point and relatively high MP numbers of MPs (33 and 31 respectively) were recovered from these locations. The wastewater discharge represents a plausible source of MPs in this stretch of the river, however recreational activities such as rowing and fishing, as well as general plastic litter, are also possible contributors. Wastewater discharges are well established as sources of MPs (Tibbetts et al., 2018), and the abundance of fibre morphology across all sampling locations, as represented in Figure 2, suggests that secondary MPs from clothing wear via laundry water discharges are likely to be significant contributors to the overall MP abundance in many locations. Tibbetts et al. (2018) point out that there is no simple relationship between MP numbers and population density or proximity to wastewater treatment sites (Tibbetts et al., 2018). In general, we observed that sampling locations with fine, silty, or muddy sediments had higher abundances than locations with coarser sand or gravelly sediments, and we suggest that these variations, as well as local flow rates and flow patterns, are likely to be stronger determinants of MP abundance at a given sampling site than the proximity to inputs.
Due to their invisibility to the naked eye, the majority of MP particles smaller than 0.5 mm in size were challenging for further analysis due to difficulty in separating them from the filter paper under the microscope, and also due to limitations on the methods available, which for the current study do not include microspectroscopy. Studies by Pakhomova et al. (2020) and Vermeiren et al. (2020) also encountered a similar issue with samples containing MPs less than 1 mm in size. Further research needs to be done on the recovery of MPs less than 1 mm for further identification by infrared spectroscopy. Microplastics with a size of 1μm in environmental samples can be detected, but due to methodological limitations, few studies have identified particles less than 50 μm (Campanale et al., 2020b). Primpke et al. (2020) identified some limitations that can occur during sample analysis, where MP particles less than 1 mm are not easily visible to the naked eye. For this reason, only particles with sizes of 1–5 mm were identified.
Our findings highlight the ubiquitous presence of MPs along the river channel, including in areas of low population density. There is a clear knowledge gap on the sources and fate of MPs entering the River Barrow. Microplastics provide surfaces for microorganism colonisation, including pathogens, in both marine and freshwater environments (Murphy et al., 2020; Yang et al., 2020) and they may act as a ‘trap’ for the accumulation of harmful organic chemicals such as pesticides, detergents, and pharmaceuticals. Microplastics occurrence in River Barrow may have impacts on aquatic organisms and potentially pose risk in humans via the food chain (as the estuary into which the Barrow flows has a significant shellfish industry). Therefore, limiting the use of non-biodegradable plastics is important as little can be done to remediate MPs once they are released into the environment.
5. Conclusions
Density separation using high density brine solutions such as NaI and ZnCl2 were found to be successful with a higher recovery of MP fibres from sediment samples compared to KOH, and NaCl. ZnCl2 (1.7 g cm−3) gave the highest recovery of MPs and was chosen for the analysis of all samples. This study provided evidence of the presence of MPs in Irish freshwater sediments. By analysing sediment samples from 16 sites along the River Barrow, the results revealed the ubiquitous nature of the MPs, highlighting the extensive level of MP contamination in River Barrow. In general, MPs were more abundant in the lower reaches and tidal-influenced section of the River Barrow. Higher abundances were also typically associated with locations in which sediments were fine, silty or muddy rather than coarser sand or gravel, and these conditions appeared to be more reliable predictors of abundance than was proximity to urban centres or wastewater treatment discharges.
This study corroborates other international studies in terms of the predominance of fibres as the main type of MPs in river sediments. Improving wastewater treatment systems by (for example) adding a filtration step prior to discharge, to filter out MP fibres beforehand, may significantly reduce the quantity of MP entering rivers (Schell et al., 2021). The findings of this study will inform future research and serve as a platform for management efforts to better understand MP distribution and fate in the River Barrow. Further studies are required to establish the impact of MPs on the river ecosystem, and to better understand the sources of MPs in Irish freshwater ecosystems in general. This study contributes to the limited work on MPs characterisation and quantification in Irish freshwater environments.
Declarations
Author contribution statement
Loriane Murphy: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Kieran Germaine, Thomais Kakouli-Duarte & John Cleary: Conceived and designed the experiments; Wrote the paper.
Funding statement
Mrs Loriane Murphy was supported by Irish Research Council [GOIPG/2019/2786].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Loriane Murphy, Email: loriane.murphy@itcarlow.ie.
John Cleary, Email: john.cleary@itcarlow.ie.
References
- Andrady A.L., Neal M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:1977–1984. doi: 10.1098/rstb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballent A., Corcoran P.L., Madden O., Helm P.A., Longstaffe F.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016;110:383–395. doi: 10.1016/j.marpolbul.2016.06.037. [DOI] [PubMed] [Google Scholar]
- Baptista J.A., Gaylarde C., Beech I., Bastos A.C., da Silva Quaresma V., de Carvalho D.G. Microplastics and attached microorganisms in sediments of the Vitória bay estuarine system in SE Brazil. Ocean Coast Manag. 2019;169:247–253. [Google Scholar]
- Bellasi A., Binda G., Pozzi A., Galafassi S., Volta P., Bettinetti R. Microplastic contamination in freshwater environments: a review, focusing on interactions with sediments and benthic organisms. Environ. - MDPI. 2020;7 [Google Scholar]
- Borges M.M., Dzul Caamal R., Rendón von Osten J. Occurrence and seasonal distribution of microplastics and phthalates in sediments from the urban channel of the Ria and coast of Campeche, Mexico. Sci. Total Environ. 2019;672:97–105. doi: 10.1016/j.scitotenv.2019.03.472. [DOI] [PubMed] [Google Scholar]
- Bouwman H., Minnaar K., Bezuidenhout C., Verster C. Water Research Comission; 2018. Microplastic in Freshwater Environments A Scoping Study Report to the Water Research Commission. [Google Scholar]
- Boyle K., Örmeci B. Microplastics and nanoplastics in the freshwater and terrestrial environment: a review. Water (Switzerland) 2020;12 [Google Scholar]
- Burns E.E., Boxall A.B.A. Microplastics in the aquatic environment: evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018;37:2776–2796. doi: 10.1002/etc.4268. [DOI] [PubMed] [Google Scholar]
- Campanale C., Massarelli C., Savino I., Locaputo V., Uricchio V.F. 2020. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanale C., Savino I., Pojar I., Uricchio V.F. A practical overview of methodologies for sampling and analysis of microplastics in riverine environments. Sustain. Times. 2020;12 [Google Scholar]
- Castelvetro V., Corti A., Biale G., Ceccarini A., Degano I., La Nasa J., Lomonaco T., Manariti A., Manco E., Modugno F., Vinciguerra V. New methodologies for the detection, identification, and quantification of microplastics and their environmental degradation by-products. Environ. Sci. Pollut. Res. 2021;28:46764–46780. doi: 10.1007/s11356-021-12466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedro A., Cleary J. 2015. Microplastics in Irish freshwaters : a preliminary study; pp. 1–4. (Proc. 14th Int. Conf. Environ. Sci. Technol.). [Google Scholar]
- Cera A., Cesarini G., Scalici M. Microplastics in freshwater: what is the news from the world? Diversity. 2020;12 [Google Scholar]
- Chamas A., Moon H., Zheng J., Qiu Y., Tabassum T., Jang J.H., Abu-Omar M., Scott S.L., Suh S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020;8:3494–3511. [Google Scholar]
- Chen S., Chang Q., Yin K., He Q., Deng Y., Chen B., Liu C., Wang Y., Wang L. Rapid analysis of bisphenol A and its analogues in food packaging products by paper spray ionization mass spectrometry. J. Agric. Food Chem. 2017;65:4859–4865. doi: 10.1021/acs.jafc.7b02061. [DOI] [PubMed] [Google Scholar]
- Claessens M., Meester S., De Landuyt L., Van Clerck K. De, Janssen C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011;62:2199–2204. doi: 10.1016/j.marpolbul.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Coppock R.L., Cole M., Lindeque P.K., Queirós A.M., Galloway T.S. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut. 2017;230:829–837. doi: 10.1016/j.envpol.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Corcoran P.L., Belontz S.L., Ryan K., Walzak M.J. Factors controlling the distribution of microplastic particles in benthic sediment of the Thames River, Canada. Environ. Sci. Technol. 2020;54:818–825. doi: 10.1021/acs.est.9b04896. [DOI] [PubMed] [Google Scholar]
- Cox K.D., Covernton G.A., Davies H.L., Dower J.F., Juanes F., Dudas S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019;53:7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- De Falco F., Di Pace E., Cocca M., Avella M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-43023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan W.P., Sanchez-Vidal A., Canals M. Floating microplastics and aggregate formation in the western mediterranean sea. Mar. Pollut. Bull. 2019;140:523–535. doi: 10.1016/j.marpolbul.2019.01.053. [DOI] [PubMed] [Google Scholar]
- Delanty Kelly, McLoone Matson, Briain Gordon, Connor Corcoran, Coyne Feeney, Morrissey Cierpal. Fish stock assessment of the river barrow catchment 2015. Inl. Fish. Irel. 2017:1–132. [Google Scholar]
- Dodson G.Z., Shotorban A.K., Hatcher P.G., Waggoner D.C., Ghosal S., Noffke N. Microplastic fragment and fiber contamination of beach sediments from selected sites in Virginia and North Carolina, USA. Mar. Pollut. Bull. 2020;151 doi: 10.1016/j.marpolbul.2019.110869. [DOI] [PubMed] [Google Scholar]
- Eerkes-Medrano D., Thompson R. Elsevier Inc.; 2018. Occurrence, fate, and effect of microplastics in freshwater systems; pp. 95–132. (Microplastic Contamination in Aquatic Environments: An Emerging Matter of Environmental Urgency). [Google Scholar]
- EPA . 2021. 3 Rd Cycle Draft Barrow Catchment Report. [Google Scholar]
- EPA . Official Journal of the European Union; 2019. Urban Waste Water Treatment in 2019. [Google Scholar]
- EPA . 2018. Barrow Catchment Assessment 2015. [Google Scholar]
- EPA . 2017. Code of Practice Code of Practice. (Quality). [Google Scholar]
- European Commission . 2013. MSDF Guidance on Monitoring Marine Litter. [Google Scholar]
- Gabriel L., Barboza A., Lopes C., Oliveira P., Bessa F., Otero V. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci.Total Environ. 2019 doi: 10.1016/j.scitotenv.2019.134625. [DOI] [PubMed] [Google Scholar]
- Gall S.C., Thompson R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015;92:170–179. doi: 10.1016/j.marpolbul.2014.12.041. [DOI] [PubMed] [Google Scholar]
- Gerolin C.R., Pupim F.N., Sawakuchi A.O., Grohmann C.H., Labuto G., Semensatto D. Microplastics in sediments from Amazon rivers, Brazil. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141604. [DOI] [PubMed] [Google Scholar]
- Gimiliani G.T., Fornari M., Redígolo M.M., Willian Vega Bustillos J.O., Moledo de Souza Abessa D., Faustino Pires M.A. Simple and cost-effective method for microplastic quantification in estuarine sediment: a case study of the Santos and São Vicente Estuarine System. Case Stud. Chem. Environ. Eng. 2020;2 [Google Scholar]
- He D., Bristow K., Filipović V., Lv J., He H. Microplastics in terrestrial ecosystems: a scientometric analysis. Sustainability. 2020;12:1–15. [Google Scholar]
- He P., Chen L., Shao L., Zhang H., Lü F. Municipal solid waste (MSW)landfill: a source of microplastics?-evidence of microplastics in landfill leachate. Water Res. 2019;159:38–45. doi: 10.1016/j.watres.2019.04.060. [DOI] [PubMed] [Google Scholar]
- Horton A.A., Walton A., Spurgeon D.J., Lahive E., Svendsen C. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017 doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- Hummel . Springer Berlin/Heidelberg; Germany: 2002. Atlas of Plastics Additives: Analysis by Spectrometric Nethods. [Google Scholar]
- Imhof H.K., Schmid J., Niessner R., Ivleva N.P., Laforsch C. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol Oceanogr. Methods. 2012;10:524–537. [Google Scholar]
- Ivar Do Sul J.A., Costa M.F., Fillmann G. Microplastics in the pelagic environment around oceanic islands of the western Tropical Atlantic Ocean. Water, Air, Soil Pollut. 2014;225 [Google Scholar]
- Jung M.R., Horgen F.D., Orski S.V., Rodriguez C.,V., Beers K.L., Balazs G.H., Jones T.T., Work T.M., Brignac K.C., Royer S.J., Hyrenbach K.D., Jensen B.A., Lynch J.M. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018;127:704–716. doi: 10.1016/j.marpolbul.2017.12.061. [DOI] [PubMed] [Google Scholar]
- Kole P.J., Löhr A.J., Van Belleghem F.G.A.J., Ragas A.M.J. Wear and tear of tyres: a stealthy source of microplastics in the environment. Int. J. Environ. Res. Publ. Health. 2017 doi: 10.3390/ijerph14101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law K.L., Thompson R.C. 2014. Microplastics in the Sea. [Google Scholar]
- Lenaker P.L., Corsi S.R., Mason S.A. Spatial distribution of microplastics in surficial benthic sediment of Lake Michigan and Lake Erie. Environ. Sci. Technol. 2021;55:373–384. doi: 10.1021/acs.est.0c06087. [DOI] [PubMed] [Google Scholar]
- Li C., Busquets R., Campos L.C. Assessment of microplastics in freshwater systems: a review. Sci. Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.135578. [DOI] [PubMed] [Google Scholar]
- Li Green, C., Reynolds A., Shi H., Rotchell J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018;241:35–44. doi: 10.1016/j.envpol.2018.05.038. [DOI] [PubMed] [Google Scholar]
- Li Shi, Y., Yang L., Xiao L., Kehoe D.K., Gun’ko Y.K., Boland J.J., Wang J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food. 2020;1:746–754. doi: 10.1038/s43016-020-00171-y. [DOI] [PubMed] [Google Scholar]
- Li Z., Hongyuan Wang, J., Chen C. Microplastics in surface water and sediments of chongming island in the Yangtze estuary, China. Environ. Sci. Eur. 2020;32 [Google Scholar]
- Magalhães S., Alves L., Medronho B., Romano A., da Graça Rasteiro M. Microplastics in ecosystems: from current trends to bio-based removal strategies. Molecules. 2020;25:1–19. doi: 10.3390/molecules25173954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maléchaux A., Colombani J., Amat S., Marque S.R.A., Dupuy N. Influence of gamma irradiation on electric cables models: study of additive effects by mid-infrared spectroscopy. Polymers (Basel) 2021;13 doi: 10.3390/polym13091451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masura J., Baker J., Foster G., Arthur C. Laboratory methods for the analysis of microplastics in the marine environment. NOAA Mar. Debris Progr. Natl. 2015:1–39. [Google Scholar]
- Mateos-Cárdenas A., O’Halloran J., van Pelt F.N.A.M., Jansen M.A.K. Beyond plastic microbeads – short-term feeding of cellulose and polyester microfibers to the freshwater amphipod Gammarus duebeni. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141859. [DOI] [PubMed] [Google Scholar]
- Menéndez-Pedriza A., Jaumot J. Interaction of environmental pollutants with microplastics: a critical review of sorption factors, bioaccumulation and ecotoxicological effects. Toxics. 2020;8 doi: 10.3390/toxics8020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F., Quinn B. The effects of microplastic on freshwater Hydra attenuata feeding, morphology & reproduction. Environ. Pollut. 2018;234:487–494. doi: 10.1016/j.envpol.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Murphy L., Germaine K., Dowling D.N., Kakouli-Duarte T., Cleary J. 2020. Association of Potential Human Pathogens with Microplastics in Freshwater Systems 112–120. [Google Scholar]
- Nakajima R., Tsuchiya M., Lindsay D.J., Kitahashi T., Fujikura K., Fukushima T. A new small device nade of glass for separating microplastics from marine and freshwater sediments. PeerJ. 2019:2019. doi: 10.7717/peerj.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper I.E., Thompson R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016;112:39–45. doi: 10.1016/j.marpolbul.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Niu D., Qiu Y., Du X., Li L., Zhou Y., Yin D., Lin Z., Chen L., Zhu Z., Zhao J., Bergman Å. Novel brominated flame retardants in house dust from Shanghai, China: levels, temporal variation, and human exposure. Environ. Sci. Eur. 2019;31 [Google Scholar]
- O’Connor J.D., Murphy S., Lally H.T., O’Connor I., Nash R., O’Sullivan J., Bruen M., Heerey L., Koelmans A.A., Cullagh A., Cullagh D., Mahon A.M. Microplastics in brown trout (Salmo trutta Linnaeus, 1758) from an Irish riverine system. Environ. Pollut. 2020;267 doi: 10.1016/j.envpol.2020.115572. [DOI] [PubMed] [Google Scholar]
- Okubo N., Takahashi S., Nakano Y. Microplastics disturb the anthozoan-algae symbiotic relationship. Mar. Pollut. Bull. 2018;135:83–89. doi: 10.1016/j.marpolbul.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Pakhomova S., Zhdanov I., van Bavel B. Polymer type identification of marine plastic litter using a miniature near-infrared spectrometer (Micronir) Appl. Sci. 2020;10:1–14. [Google Scholar]
- Pannetier P., Morin B., Le Bihanic F., Dubreil L., Clérandeau C., Chouvellon F., Van Arkel K., Danion M., Cachot J. Environmental samples of microplastics induce significant toxic effects in fish larvae. Environ. Int. 2020;134 doi: 10.1016/j.envint.2019.105047. [DOI] [PubMed] [Google Scholar]
- Plastics Europe Plastics – the facts 2020. Plast Eur. 2020;1–64 [Google Scholar]
- Pohl F., Eggenhuisen J.T., Kane I.A., Clare M.A. Transport and burial of microplastics in deep-marine sediments by turbidity currents. Environ. Sci. Technol. 2020;54:4180–4189. doi: 10.1021/acs.est.9b07527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primpke S., Christiansen S.H., Cowger W., De Frond H., Deshpande A., Fischer M., Holland E.B., Meyns M., O’Donnell B.A., Ossmann B.E., Pittroff M., Sarau G., Scholz-Böttcher B.M., Wiggin K.J. Applied Spectroscopy; 2020. Critical Assessment of Analytical Methods for the Harmonized and Cost-Efficient Analysis of Microplastics. [DOI] [PubMed] [Google Scholar]
- Quinn B., Murphy F., Ewins C. Validation of density separation for the rapid recovery of microplastics from sediment. Anal. Methods. 2017;9:1491–1498. [Google Scholar]
- Reineccius J., Appelt J.S., Hinrichs T., Kaiser D., Stern J., Prien R.D., Waniek J.J. Abundance and characteristics of microfibers detected in sediment trap material from the deep subtropical North Atlantic Ocean. Sci. Total Environ. 2020;738 doi: 10.1016/j.scitotenv.2020.140354. [DOI] [PubMed] [Google Scholar]
- Rillig M.C. 2012. Microplastics in Terrestrial Ecosystems and the soil? pp. 6453–6454. [DOI] [PubMed] [Google Scholar]
- Rocha-santos T., Duarte A.C. Trends in analytical chemistry: a critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Anal. Chem. 2015;65:47–53. [Google Scholar]
- Rummel C.D., Jahnke A., Gorokhova E., Kühnel D., Schmitt-Jansen M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017;4:258–267. [Google Scholar]
- Sartain M., Wessel C., Sparks E. Mississippi State Univ. MP Proj; 2018. pp. 14–20. (Microplastics Sampling and Processing Guidebook). [Google Scholar]
- Sathish N., Jeyasanta K.I., Patterson J. Abundance, characteristics and surface degradation features of microplastics in beach sediments of five coastal areas in Tamil Nadu, India. Mar. Pollut. Bull. 2019;142:112–118. doi: 10.1016/j.marpolbul.2019.03.037. [DOI] [PubMed] [Google Scholar]
- Savinelli B., Vega Fernández T., Galasso N.M., D’Anna G., Pipitone C., Prada F., Zenone A., Badalamenti F., Musco L. Microplastics impair the feeding performance of a Mediterranean habitat-forming coral. Mar. Environ. Res. 2020;155 doi: 10.1016/j.marenvres.2020.104887. [DOI] [PubMed] [Google Scholar]
- Schell T., Hurley R., Nizzetto L., Rico A., Vighi M. Spatio-temporal distribution of microplastics in a Mediterranean river catchment: the importance of wastewater as an environmental pathway. J. Hazard Mater. 2021;420 doi: 10.1016/j.jhazmat.2021.126481. [DOI] [PubMed] [Google Scholar]
- Shruti V.C., Jonathan M.P., Rodriguez-Espinosa P.F., Rodríguez-González F. Microplastics in freshwater sediments of atoyac river basin, Puebla city, Mexico. Sci. Total Environ. 2019;654:154–163. doi: 10.1016/j.scitotenv.2018.11.054. [DOI] [PubMed] [Google Scholar]
- Tan X., Yu X., Cai L., Wang J., Peng J. Chemosphere microplastics and associated PAHs in surface water from the feilaixia reservoir in the beijiang river, China. Chemosphere. 2019;221:834–840. doi: 10.1016/j.chemosphere.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Tian L., Jinjin C., Ji R., Ma Y., Yu X. Microplastics in agricultural soils: sources, effects, and their fate. Curr. Opin. Environ. Sci. Health. 2022;25 [Google Scholar]
- Tibbetts J., Krause S., Lynch I., Smith G.H.S. Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water (Switzerland) 2018;10 [Google Scholar]
- Tiwari M., Rathod T.D., Ajmal P.Y., Bhangare R.C., Sahu S.K. Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar. Pollut. Bull. 2019;140:262–273. doi: 10.1016/j.marpolbul.2019.01.055. [DOI] [PubMed] [Google Scholar]
- Turner S., Horton A.A., Rose N.L., Hall C. A temporal sediment record of microplastics in an urban lake, London, UK. J. Paleolimnol. 2019;61:449–462. [Google Scholar]
- Vermeiren P., Muñoz C., Ikejima K. Microplastic identification and quantification from organic rich sediments: a validated laboratory protocol. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114298. [DOI] [PubMed] [Google Scholar]
- Wagner M., Scherer C., Alvarez-Muñoz D., Brennholt N., Bourrain X., Buchinger S., Fries E., Grosbois C., Klasmeier J., Marti T., Rodriguez-Mozaz S., Urbatzka R., Vethaak A.D., Winther-Nielsen M., Reifferscheid G. Microplastics in freshwater ecosystems: what we know and what we need to know. Environ. Sci. Eur. 2014;26:1–9. doi: 10.1186/s12302-014-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.L., Kelly F.J. Plastic and human health: a micro issue? Environ. Sci. Technol. 2017;51:6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- Yang L., Zhang Y., Kang S., Wang Z., Wu C. Microplastics in freshwater sediment: a review on methods, occurrence, and sources. Sci. Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.141948. [DOI] [PubMed] [Google Scholar]
- Yang Y., Liu W., Zhang Z., Grossart H.P., Gadd G.M. Microplastics provide new microbial niches in aquatic environments. Appl. Microbiol. Biotechnol. 2020;104:6501–6511. doi: 10.1007/s00253-020-10704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhou H., Cui Y., Wang C., Li Y., Zhang D. Microplastics in offshore sediment in the Yellow Sea and East China Sea, China. Environ. Pollut. 2019;244:827–833. doi: 10.1016/j.envpol.2018.10.102. [DOI] [PubMed] [Google Scholar]
- Zhang Qinghao, Sun Y., Zhang Qingnan, Hou J., Wang P., Kong X., Sundell J. Phthalate exposure in Chinese homes and its association with household consumer products. Sci. Total Environ. 2020;719 doi: 10.1016/j.scitotenv.2020.136965. [DOI] [PubMed] [Google Scholar]
- Zhu X., Nguyen B., You J.B., Karakolis E., Sinton D., Rochman C. Identification of microfibers in the environment using multiple lines of evidence. Environ. Sci. Technol. 2019;53:11877–11887. doi: 10.1021/acs.est.9b05262. [DOI] [PubMed] [Google Scholar]
- Ziajahromi S., Neale P.A., Leusch F.D.L. Wastewater treatment plant effluent as a source of microplastics: review of the fate, chemical interactions and potential risks to aquatic organisms. Water Sci. Technol. 2016;74:2253–2269. doi: 10.2166/wst.2016.414. [DOI] [PubMed] [Google Scholar]
- Zobkov, Esiukova E.E. Microplastics in a marine environment: review of methods for sampling, processing, and analyzing microplastics in water, bottom sediments, and coastal deposits. Oceanology. 2018;58:137–143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.