Abstract
Background
Granulomatous cardiomyopathy (GCM) is relatively uncommon in patients presenting with ventricular tachycardia (VT). Sarcoidosis and tuberculosis are the most common causes of GCM with VT. The aim of study was to evaluate their clinical characteristics and the long-term outcomes.
Methods
We retrospectively analyzed patients from March 2004 to January 2020, presenting with VT and subsequently diagnosed to have GCM. Patients were divided into three groups (sarcoid, tuberculosis and indeterminate) based on serologic tests, imaging and histopathology. The response to anti-arrhythmic and disease specific therapy on long-term follow-up were analyzed.
Results
There were 52 patients, comprising 27 males and 25 females, age 40 ± 10 years. The follow-up period was 5.9 ± 3.9 years. Sarcoidosis was diagnosed in 20 (38%); tuberculosis (TB) in 15(29%) and 17(33%) patients were indeterminate. Left ventricular ejection fraction (LVEF) of the entire cohort was 0.45 ± 0.14. Erythrocyte Sedimentation Rate(ESR) was found to be significantly higher in TB(43.6 ± 18.4) patients vs sarcoid(18.9 ± 6.7)p < 0.0001, but not the indeterminate group (36.2 ± 21.1), p = 0.3. Implantable Cardioverter Defibrillator (ICD) implantation was performed in 12/20(60%) patients in the sarcoid group, in 4/15(27%) patients in the TB group and in 10/17(59%) patients in the indeterminate group. At a mean follow-up of six years, VT recurrences were noted in 6, 2, and 7 patients in the sarcoid, TB and indeterminate groups respectively.
Conclusion
Despite the advances in diagnostic modalities for tuberculosis and sarcoidosis, in real-world practice, almost one-third of the patients with VT and GCM have uncertain etiology. Long term outcomes of patients presenting with GCM and VT with mild left ventricle dysfunction treated appropriately seems favorable.
Keywords: Sarcoidosis, Tuberculosis, Ventricular tachycardia, Cardiac imaging
Abbreviations and acronyms
- 1. GCM
Granulomatous cardiomyopathy
- 2. CS
Cardiac Sarcoidosis
- 3. MRI
Magnetic Resonance Imaging
- 4. CT
Computed Tomography
- 5. TB
Tuberculosis
- 6. VT
Ventricular Tachycardia
- 7. 18FDG PET-CT
18- Fluoro-deoxyglucose Positron Emission Tomography with Computed Tomogram
- 8. DNA PCR
Deoxyribonucleic Acid Polymerase Chain Reaction
- 9. ACE
Angiotensin Convertase Enzyme
- 10. ICD
Implantable Cardioverter Defibrillator
- 11. ESR
Erythrocyte Sedimentation Rate
- 12. CRP
C Reactive protein
- 13. ZN
Ziehl-Neelsen
- 14. LV
Left Ventricle
- 15. RFA
Radio Frequency Ablation
- 16. AADs
Anti Arrhythmic Drugs
1. Background
Granulomatous cardiomyopathy is a relatively uncommon cardiac disease and occurs either in isolation or along with systemic manifestations. Granulomatous infiltration of the myocardium can be due to sarcoidosis, tuberculosis, fungal infections, Wegener's granulomatosis, eosinophilic granulomatosis or idiopathic giant cell myocarditis [1]. The spectrum of cardiac involvement in GCM includes myocarditis, heart failure, conduction blocks, atrial arrhythmias, ventricular arrhythmias and sudden cardiac arrest [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]].
Sarcoidosis and TB are the most common causes of GCM. Clinical manifestations of cardiac sarcoidosis (CS) and TB are dependent on the location, extent, and activity of the disease [2,3]. Sarcoidosis and TB share several common clinical presentations and imaging features, making accurate diagnosis difficult [2,10,[12], [13], [14], [15]].
Our study analyzed patients presenting with VT and previously not known to have granulomatous disease. We assessed the clinical presentation, diagnostic modalities, and response to various therapies on outcomes at long-term follow up.
1.1. Methods
We retrospectively included 52 patients from March 2004 to January 2020, who presented with unexplained VT and were subsequently diagnosed to have GCM. This study was done after institutional ethics committee approval. The patients were pooled from the data of six hospitals and three clinics in three cities in India (Mumbai, Vadodara and Ahmedabad). Patients were investigated for granulomatous infiltration, after ruling out congenital or other structural heart disease by transthoracic echocardiography, and if one or both of the following were seen: i) VT morphology did not match any known idiopathic variety; ii) there were pleomorphic VTs (defined as two or more morphologies of monomorphic VT). Patients with typical idiopathic VT, ischemic heart disease, ion channelopathies or reversible causes of VT were excluded from the study. A cardiac magnetic resonance (CMR) or computed tomography (CT) of the chest or a positron emission tomography (PET) scan was performed to identify infiltration. Histopathologic diagnosis of granuloma was done by lymph node biopsy.
Imaging: All patients who were included in the study underwent transthoracic echocardiography, CMR with delayed gadolinium enhancement and contrast-enhanced CT scan (64-slice) with or without PET scan. Transthoracic echocardiography was done to document the LV ejection fraction and rule out other structural or congenital heart disease. Delayed enhancement on MRI was looked for in the myocardium, to see whether subendocardial, epicardial, mid-myocardial or transmural scar was present (Fig. 1A and B, 2A-D). CT chest was done to look for pulmonary involvement and lymphadenopathy (and if present, to demarcate the location and size of lymph nodes amenable to biopsy). We followed a standard special diet preparation before the PET CT scan, to suppress the physiological myocardial uptake of FDG and to facilitate pathological FDG uptake in the inflammatory cells. This included a low carbohydrate, high fat diet followed by prolonged fasting (at least 18 h) before the FDG PET CT scan. This helps to increase the provision of free fatty acids [FFAs] to the myocardium and in turn reduces the physiological myocardial glucose uptake [16]. Additionally, the patients were given intravenous unfractionated heparin [50 units/kg] 15 min prior to 18F-FDG injection, which also helped to increase FFAs, inhibit GLUT-4 expression in cardiac muscle, and eventually help to reduce physiological myocardial glucose uptake, reducing the possibility of a false positive study. Subsequent to this, cardiac image acquisition was done after 90 min. Additionally, PET CT of neck, chest, abdomen/pelvis was also acquired to look for any metabolically active adenopathy as well as any other metabolically active lesion in other organs. The presence of ‘focal’/‘patchy’ or ‘focal on diffuse’ uptake of FDG is pathological and consistent with cardiac inflammation. High 18 FDG uptake was interpreted as inflammation; matched perfusion and metabolism defects were interpreted as scar (Fig. 3).
Fig. 1.
A. Cardiac magnetic resonance imaging showing mid-myocardial late gadolinium enhancement in the mid and apical interventricular septum (arrows). B. Cardiac magnetic resonance imaging showing subepicardial late gadolinium enhancement (arrows).
Fig. 2.
(A–D): Tuberculous granulomatous cardiomyopathy. The short axis STIR (arrow in A) image shows edema along with the short axis T2 map (arrow in B), derived from T2 images with abnormal late gadolinium enhancement suggestive of fibrosis (arrows) in the late gadolinium inversion recovery short axis (C) and four-chamber (D) images. The four-chamber image also shows an enlarged necrotic subcarinal node (labeled), which was biopsied under CT scan guidance and proven to be tuberculosis on histology and on microbiology.
Fig. 3.
A&B: Cardiac Sarcoidosis: 40 year old man, under evaluation for suspected cardiac sarcoidosis. The scan is positive for cardiac sarcoidosis. Abnormal increased metabolic activity is seen in the myocardium. Arrows in A and B (axial views for PET CT fusion and PET respectively) show abnormal increased metabolic activity (in red colour in A, and in black in B) in myocardium which is distinctly more than the normal physiological metabolic activity in cardiac blood pool. C&D: Normal Scan: 43 year old woman under evaluation for suspected cardiac sarcoidosis. However, the scan is negative for cardiac sarcoidosis. No abnormal metabolic activity is seen in the myocardium. Arrows in C and D (axial views for PET CT fusion and PET respectively) show absence of any metabolic activity in myocardium with normal physiological metabolic activity in cardiac blood pool.
Biopsy: Image-guided biopsy of the mediastinal nodes, or extra-mediastinal lymph nodal excision (more than 1 cm size) was performed in all patients. Histopathologic examination determined the type of involvement. Sarcoidosis was characterized by the presence of closely packed well-formed non-necrotizing granulomas, while central small or large areas of caseation necrosis was often found in tuberculous granulomas (Fig. 4A and B). The histopathological features were also correlated with modified Ziehl-Neelsen (ZN) staining on the paraffin sections, culture and/or polymerase chain reaction (PCR) for Mycobacterium tuberculosis. The diagnosis of sarcoidosis was made in the presence of non-caseating granulomas with negative results of ZN stains, culture and TB-PCR. Caseating granulomas with or without demonstration of acid-fast bacilli along with positive Mantoux/culture/TB-PCR was considered as TB. The patient was labeled to have indeterminate GCM if the histopathology showed ill-formed granulomatous inflammation or predominant sclerosis (Fig. 4C) with no further confirmation from culture or TB-PCR.
Fig. 4.
A. The normal lymph node architecture is replaced by closely packed compact non-necrotizing granulomas composed of epithelioid cells and Langerhans giant cells, characteristically seen in sarcoidosis; B. Tuberculous lymphadenitis with a large focus of caseation necrosis CN is bordered by a granulomatous reaction with few giant cells (arrows); C. The lymph node shows foci of hyaline sclerosis with a giant cell in the centre and small ill-formed clusters (arrows) of epithelioid cells and macrophages (H and E x 250). This is an example of indeterminate histology, where clear-cut features of tuberculosis and sarcoidosis are not seen.
Additional etiologic evaluation: All patients were evaluated with Mantoux test, erythrocyte sedimentation rate (ESR) and serum angiotensin converting enzyme (ACE) levels. The tuberculin skin test was performed with 5 tuberculin units of purified protein derivative. Horizontal/transverse induration of more than 10 mm at 48 h was considered positive.
Arrhythmia treatment: All patients were initiated on appropriate anti-arrhythmic drug therapy for VT. Radiofrequency ablation was offered in patients having drug-refractory or incessant monomorphic VT. This was performed at least two months after initiation of disease-specific therapy. Implantable cardioverter-defibrillator (ICD) was recommended to patients with resistant VT and a cardiac scar on imaging. Those who had VTs controlled with drugs but showed a myocardial scar were also offered ICD. Bilateral cardiac sympathetic denervation (T1-T4 ganglia) via video-assisted thoracoscopy was performed in patients with VT storm or frequent VTs (despite multiple anti-arrhythmic drugs and ICD shocks).
Disease specific therapy: Disease specific therapy for tuberculosis, sarcoidosis or both was instituted. Treatment duration was individualized as per the disease subset, ranging from 6 months to 1 year for tuberculosis; for sarcoidosis and the indeterminate group, the dose and duration of immunosuppressants was left to the individual discretion of treating physician. This decision was based on inflammatory markers, continuing ventricular arrhythmias and, increased uptake in the myocardium among patients undergoing repeat PET imaging. Prednisolone was the primary drug. Methotrexate was often used along with prednisolone or added subsequently if necessary. Azathioprine and cyclophosphamide were added if there was no response or if there were adverse effects to previous immunosuppressant drugs. A 3-month period was considered appropriate for disease specific therapy to take effect.
Follow-up: The response to treatment was judged at 3 months using clinical recurrences of VT or inflammatory markers as a surrogate endpoint or by repeating CT/MRI/PET-CT scan. Additionally, the patients were evaluated during symptomatic recurrence. Once in remission, they were followed every 6 months. Each visit included ECG, echocardiography, interrogation of ICD and evaluation of disease markers. The follow up data for disease markers at follow up was not available for all patients.
1.2. Statistical analysis
All analyses were performed using SPSS (version 20.0, IBM Corp., Armonk, NY, USA). Categorical variables were expressed as percentages, whereas continuous variables were presented as mean ± standard deviation, and these continuous variables were approximated to the form of the normal distribution and compared by ANOVA test.for statistically significant findings by ANOVA where the p value < 0.05, t-test was used to confirm if the comparison between each of the groups also showed statistical significance. χ2 test and Fisher's exact test were used for the categorical variables between the three groups. All hypothesis tests of significance were two-tailed and significance was defined as p < 0.05.
2. Results
From March 2004 to January 2020, 52 patients were included in the study. The male/female distribution was 27/25 and the mean age was 40 ± 10 years (range 16–63 years). Their response to treatment were analyzed on follow-up, which was 5.9 ± 3.9 years (range 1–15 years). 51 out of 52 patients (98%) were available for follow up, except one patient who was lost to follow up at 3 months.
Clinical Characteristics: The demographic profile and clinical presentation of the study population are shown in Table 1. Based on histopathology and culture reports, CS was diagnosed in 20 (38%) patients; TB in 15 (29%) patients and the remaining 17 (33%) patients were classified as indeterminate. Clinical characteristics, investigative profile, treatment details and follow up of the three subgroups are shown in Table 2.
Table 1.
Clinical characteristics and presentation of patients having granulomatous cardiomyopathy
TB:- Tuberculosis, VT:- Ventricular Tachycardia, LVEF:- Left ventricular Ejection Fraction.
| Parameters | Sarcoid (n = 20) |
TB (n = 15) |
Indeterminate (n = 17) |
P value |
|---|---|---|---|---|
| n(%) or mean ± SD | n(%) or mean ± SD | n(%) or mean ± SD | ||
| Age (years) | 39.4 ± 9.7 | 38.4 ± 11.6 | 42.2 ± 8.9 | 0.54 |
| Male/Female | 9/11 | 9/6 | 9/8 | 0.68 |
| Arrhythmia | ||||
| Monomorphic VT | 8 (40) | 9 (73.3) | 5 (29.4) | 0.21 |
| Pleomorphic VT | 7 (35) | 3 (20) | 10 (58.8) | |
| Polymorphic VT | 5 (25) | 3 (20) | 2 (11.7) | |
| VT storm | 5 (25) | 3 (20) | 1 (5.9) | 0.29 |
| LVEF | 0.44 | 0.42 | 0.49 | 0.41 |
| >0.55 | 7 (35) | 4 (26.7) | 7 (41.2) | |
| 0.35–0.55 | 9 (45) | 7 (46.7) | 8 (47.1) | 0.84 |
| <0.35 | 4 (20) | 4 (26.7) | 2 (11.7) | |
Table 2.
Investigations, treatment and follow-up of patients with granulomatous cardiomyopathy
TB:- Tuberculosis, AFB:- Acid Fast Bacilli, PCR:- Polymerase chain reaction, MRI:- Magnetic Resonance imaging, FDG PET:- Fluoro-deoxyglucose Positron Emission Tomography, AICD:- Automatic Implantable cardiac defibrillator, Cyclo:- Cyclophosphamide, ACE:- Angiotensin Converting Enzyme, ESR:-Erythrocyte Sedimentation Rate, LVEF:- Left ventricular Ejection Fraction.
| Parameters | Sarcoid (n = 20) |
TB (n = 15) |
Indeterminate (n = 17) |
P value |
|---|---|---|---|---|
| n(%) or mean ± SD | n(%) or mean ± SD | n(%) or mean ± SD | ||
| Imaging | ||||
| Cardiac MRI scarring | 19 (95) | 15 (100) | 16 (94.1) | 0.9780 |
| FDG PET inflammation | 8/8 (100) | 8/8 (100) | 5/5 (100) | 0.9384 |
| Histopathology lymph node: | ||||
| caseating granulomas | 0 (0) | 15 (100) | 0 (0) | <0.001 |
| Non caseating granulomas | 20 (100) | 0 (0) | 17 (100) | |
| Etiological analysis: | ||||
| Mantoux positive | 0 (0) | 15 (100) | 17 (100) | <0.001 |
| AFB culture positive | 0 (0) | 3 (20.0). | 0 (0) | 0.0205 |
| TB PCR positive | 0 (0)) | 5 (33.3) | 0 (0) | 0.0012 |
| ACE level (U/L) | 64.8 ± 21.8 | 39.2 ± 11.2 | 52.8 ± 15.4 | 0.0003 (<0.0001 for Sarcoid vs TB; 0.059 for Sarcoid vs Indeterminate; 0.0076 for TB vs Indetermi-nate) |
| ESR level (mm) | 18.9 ± 6.7 | 43.6 ± 18.4 | 36.2 ± 21.1 | 0.0001 (<0.0001 for Sarcoid vs TB; 0.004 for Sarcoid vs Indeterminate; 0.2959 for TB vs Indeterminate) |
| Treatment: | ||||
| Antiarrhythmic therapy: | ||||
| Amiodarone/Sotalol/Mexiletine | 15 (75) | 13 (86.7) | 15 (88.2) | 0.5072 |
| Beta blockers | 18 (90) | 15 (100) | 16 (94.1) | 0.7692 |
| sympathetic denervation | 1 (5)) | 0 (0) | 1 (5.8) | 1.000 |
| Radiofrequency ablation | 6 (30) | 3 (20.0) | 8 (47.1) | |
| AICD | 12 (60 | 4 (26.7)) | 10 (58.8) | 0.1004 |
| Disease specific therapy | ||||
| steroids | 20 (100) | 2 (13.3) | 16 (94.1) | <0.0001 |
| Cyclo/Aza/Metho | 9 (45) | 0 (0) | 4 (23.5) | 0.0072 |
| ATT | 1 (5) | 15 (100) | 14 (82.4) | <0.0001 |
| Follow up | ||||
| Arrhythmia recurrence | 6 (30) | 2 (13.3) | 7 (41.2) | 0.2197 |
| LVEF (%) | 50.2 ± 12.4 | 52.7 ± 8.0 | 53.7 ± 7.4 | |
| Death | 0 (0) | 1 (6.7) | 0 (0) | |
Ventricular tachycardia: Twenty-two (42%) of the 52 patients presented with monomorphic VT; multiple morphologies of monomorphic VT (pleomorphic) were seen in 20/52 (38%) and 10 (19%) presented with polymorphic VT. Nine patients presented with VT storm (two or more hemodynamically unstable VTs requiring electric cardioversion/defibrillation). In all, 16 patients had 249 VT recurrences during 307 patient years of follow-up, including 15 VT storms in 9 patients. Three patients had been labeled as idiopathic VT before coming to us, two of whom had already undergone an attempt at ablation.
Trans-thoracic 2 D echocardiography: The mean LVEF of the entire cohort at presentation was 0.45 ± 0.14. Left ventricular systolic dysfunction (EF < 0.55) was present in 34 (65%) patients. Severe LV dysfunction (EF < 0.35) was noted in 10 (19%) patients.
Cardiac MRI: This was done in all 52 patients. Myocardial scarring in form of LGE was seen in 95% of cardiac sarcoidosis, 100% of tuberculosis, and 94% of indeterminate patients (Fig. 2). Both Sarcoidosis and Tuberculosis had predominantly mid myocardial and subepicardial scarring, and had predilection for abnormalities involving the basal, and both lateral and septal segments of left ventricle.
CT chest: Contrast enhanced CT and high-resolution CT was done for delineation of hilar and mediastinal lymphadenopathy, and to assess for parenchymal and extra-parenchymal involvement. Lung involvement was seen in 7/52 (13.5%) patients. Ten percent of the sarcoidosis group, 20% of the tuberculosis group and 12% of the indeterminate group showed lung involvement. Mediastinal lymphadenopathy was noted in all patients while extra-mediastinal lymphadenopathy was seen in 11 patients (20%, 33% and 12% of sarcoidosis, tuberculosis, and indeterminate patients respectively). Data about change of lymph node size with treatment was not available.
PET scan: FDG PET scans were done in 21 patients and all showed evidence of focal inflammation. FDG avid mediastinal nodes were also present in all.
2.1. Etiologic correlations with investigational findings
Sarcoidosis group: 20 (38%) patients were diagnosed to have sarcoidosis. The ESR was 18.9 ± 6.7 mm. Serum ACE level was high in only 9 (45%) patients, with a level of 64.8 ± 21.8 U/L. Follow up data for inflammatory markers were not available for analysis. Extra-mediastinal lymphadenopathy was seen only in 4 (20%) patient and asymptomatic extra-parenchymal lung involvement on CT scan was seen in 2 (10%) patients.
The VT was monomorphic in 8/20 (40%), pleomorphic in 7/20 (35%) and polymorphic in 5/20 (25%). Five patients in this group had VT storm.
The LVEF was 0.44 , with 9 patients having mild to moderate LV dysfunction (LVEF 0.35 to < 0.55) and 4 patients with severe LV dysfunction (LVEF <0.35).
TB group: 15 (29%) patients were diagnosed to have TB. Mantoux test was positive in all patients in the TB group with three patients showing an exuberant, necrotic local reaction to the purified tubercular protein derivative. Culture for AFB was positive in 3 and the TB PCR was positive in 5 patients. The ESR was 43 ± 18.4 mm, and the ACE level was 39.2 ± 11.2 U/L. Follow up data for inflammatory markers were not available for analysis. Extra-mediastinal lymphadenopathy was noted in 5 (33%) patients, asymptomatic parenchymal lung involvement on CT scan was seen in 3(20%) patients and two had pleural effusion.
The VT was predominantly monomorphic VT (9/15, 73%); pleomorphic and polymorphic VT was noted in 3/15 (20%) each. Three patients presented with VT storm.
The LVEF was 0.42 , with 11 patients having mild to moderate LV dysfunction (LVEF 0.35 to < 0.55) and 4 patients with severe LV dysfunction (LVEF <0.35).
Indeterminate group: The lymph node biopsy in 17 (33%) patients showed un-definable granuloma and no evidence of TB on AFB stain, culture and TB-PCR test. The serum ACE level was high in only 3 (18%) patients. The ESR and ACE levels were 36.2 ± 21.1 mm and 52.8 ± 15.4 U/L respectively. Follow up data for inflammatory markers were not available for analysis.
Extra-mediastinal lymphadenopathy and asymptomatic extra-parenchymal lung involvement on CT scan was seen in 2 (12%) patients each. Pleural effusion was seen in one patient.
The VT morphology was monomorphic in 5/17 patients (29%), pleomorphic VTs in 10/17 (59%) patients and polymorphic VT in 2/17 (11.7%) patients. One patient had VT storm.
The LVEF was 0.49 , with 15 patients having mild to moderate LV dysfunction (LVEF 0.35 to < 0.55) and 2 patients had LVEF <0.35.
The serum ACE levels were significantly higher in the CS group compared to the TB group 64.8 ± 21.8 vs 39.2 ± 11.2, (p < 0.0001). The ESR was significantly higher in the TB group compared to the sarcoid group (43.6 ± 18.4 vs 18.9 ± 6.7, p < 0.0001).
2.2. Response to treatment (Table 2)
Antiarrhythmic drugs were used in all the patients. Beta-blockers were used in 49/52 (94%) patients. Amiodarone, sotalol or mexiletine in isolation or combination were given in 43/52 (83%) of patients.
In spite of the above anti-arrhythmic drugs, VT was not controlled in 19/52 (36.5%) of patients.
Radiofrequency ablation (RFA) was performed in 17 patients for drug resistant, recurrent and incessant, monomorphic VT. The RFA was performed in 6, 3 and 8 patients in the CS, TB and the indeterminate groups respectively. Sustained monomorphic VT was inducible in 13 of these 17 patients, and in 4 only non-sustained VT was inducible. Pleomorphic VTs were inducible in 11 out of 13 patients. Induced VTs were consistent with a macro re-entrant mechanism in 4 and focal micro-reentry in 6; the mechanism could not be determined in 3 cases. Low voltage areas (bipolar voltage <1.5 mV) on substrate map and sites of RFA corresponded to the sites of scar in delayed enhancement CMR or to the site of inflammation in 18 FDG PET-CT. No patient underwent epicardial mapping. VT recurred in 8 of 17 patients (53%) patients who underwent RFA.
Bilateral cardiac sympathetic denervation (T1-T4 ganglia) was performed via thoracoscopy in 2 patients who continued to have frequent VTs and presented with VT storm, despite the above anti-arrhythmic measures. Both the patients responded well to sympathectomy.
Disease specific therapy: All patients in the tuberculosis group were started with anti-tubercular treatment (ATT). In this group, 2 patients empirically received steroids during a VT storm; these were subsequently tapered off over the next 3 months.
Patients in the CS group received a period of high-dose prednisolone (40–60 mg daily) for 4–8 weeks, followed by a taper to <10 mg of prednisone daily, typically 5 mg daily. In this group, 9/20 (45%) patients required second line immunotherapy (cyclophosphamide, methotrexate or azathioprine) due either to no response or steroid intolerance. Patients in the indeterminate group were initiated on both steroids and ATT in 14/17 (82%) of cases, while the remaining three were given steroids only based on biopsy characteristics and absence of any evidence of TB. Only 4/17 (24%) patients in this group required second line immunotherapy.
The duration from presentation of patients to an etiological diagnosis was 16 ± 3 days. Before the initiation of disease-specific therapy, a total of 23 patients had recurrence of VT. Once the disease specific therapy was started, in the first month, two more patients had recurrence of VT. Among the 15 patients who experienced VT recurrence while receiving initial therapy, disease-specific therapy over 3 months could successfully control VT in 10 patients (4 patients had no further VT and 6 patients had a significant decrease in frequency of VT). At 3 month follow up in the CS group, 6/20 (30%) patients had recurrence of VT; the indeterminate group had 7/17 (41%) patients with VT recurrence and in the TB group 2/15 (13%) patients had recurrent VT. None of the patients had VT storm after disease-specific therapy. Overall, disease-specific therapy helped to reduce the VT burden (in addition to the antiarrhythmic medications, radiofrequency ablation and sympathectomy). Reduction in VT burden correlated with resolution of myocardial inflammation on post-treatment 18 FDG PET-CTs. This response was observed among all disease-specific therapy protocols (Fig. 5). Remarkably, only 5/52 (9.6%) patients presenting with ventricular arrhythmia had persistent VT after 3 months of therapy, which was appropriately treated by ICD or up-titration of immunosuppressive therapy.
Fig. 5.
Line diagram depicting response of VT over time with initiation of disease specific therapy.
Implantable cardioverter-defibrillators (ICD) were implanted in 26 patients once the acute phase was controlled well with antiarrhythmic drugs and disease specific medications. Fifteen patients with recurrent, drug resistant VT were implanted with ICD, whereas 11 patients with controlled arrhythmia, but scarring documented on cardiac MRI underwent ICD implantation. Nearly 60% (22/37) of patients in the CS and the indeterminate groups were implanted with ICD compared to only 26% (4/15) patients in the TB group. Appropriate ICD therapies occurred in 20 of 26 ICD recipients (80%) on follow up. Appropriate shocks were noted in 13 out of the 15 ICD patients with recurrent, drug resistant VT, as compared to 7 of the 11 scar only ICD patients. The other 26 patients did not undergo an ICD implantation either due to no VT recurrences, good clinical response to therapy, financial constraints, or non-acceptance of the procedure. We unfortunately do not have data regarding number of recurrences of VT in the group of patients who did not receive and ICD and were managed medically.
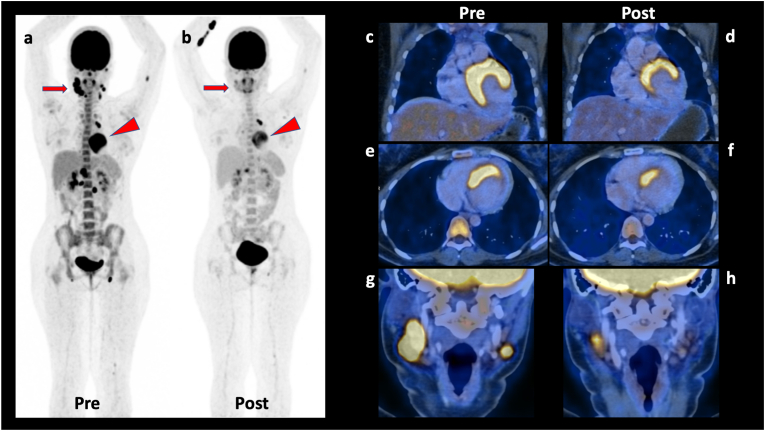
PET scan: Follow up PET scans were done in 13 patients at 5.2 ± 2.3 months duration. There was resolution of inflammation in all the 13 patients (Fig. 6).
Fig. 6.
Cardiac Sarcoidosis. 21 years old young woman, diagnosed with sarcoidosis with involvement of supra- and infradiaphragmatic lymph nodes as well as myocardium. The baseline PET/CT scan (images: a: whole body MIP image; c: coronal PET/CT fusion view of myocardium, e: axial PET/CT fusion view of myocardium; g: coronal PET/CT fusion view of neck showing metabolically active and enlarged nodes) shows highly metabolically active bilateral neck, mediastinal, upper abdominal, retroperitoneal, and bilateral inguinal nodes, along with few metabolically active intermuscular nodules in left arm and left thigh, and also the active myocardial involvement. The PET/CT scan was acquired after special preparation of high fat meal, prolonged fasting, and pre-scan IV heparin injection (cardiac sarcoidosis protocol). Subsequently she underwent biopsy of one of the active neck nodes, which showed sarcoidosis. This was the single sarcoidosis case who received steroids, methotrexate and anti-tubercular treatment as part of therapy. The follow-up PET/CT scan was performed after around two months of the above treatment, which shows partial metabolic response with variable reduction in the size and metabolic activity of the nodes, intermuscular nodules, as well as myocardial metabolic activity (images b, d, f, h – corresponding sections from the post-therapy scan images).
Improvement in the LVEF by more than 10% absolute value was observed in 18 patients after therapy; 5/20 (25%) from CS group, 7/15 (47%) from TB group and 6/17 (35%) from the indeterminate group. There was 1 death during the follow up due to accidental burns. Only one patient in the TB group was lost to follow-up after 3 months.
3. Discussion
Our study is a cohort of 52 patients presenting with VT and identified to have granulomatous infiltration of the heart based on imaging and histopathologic examination of lymph nodes. When patients present with atypical (for idiopathic VT) and/or pleomorphic VT without any well-defined structural heart disease or extra-cardiac features of granulomatous disease, it poses a diagnostic challenge. A suspicion that the VT is due to infiltrative myopathy, based on morphologic characteristics, is the first step in reaching the correct diagnosis. Non-invasive imaging techniques including CMR, CT scan and FDG-PET scan, help to establish myocardial infiltration and inflammation. Histopathologic examination helps in establishing the diagnosis and in planning appropriate treatment. Endomyocardial biopsy is limited by the patchy involvement of the myocardium, expertise in performing the biopsy and potentially lethal complications with left ventricular biopsy. We therefore considered LN biopsy, which is relatively easy to access with a high yield, as a surrogate for myocardial involvement.
Cardiac sarcoidosis was diagnosed in 38% of patients, TB in 29%, while in 33% precise diagnosis was not reached and this constituted the indeterminate group. Infiltration in CMR and abnormal FDG PET CT was noted in 50/52 patients and 21/21 patients respectively. Lymph node biopsy showed granuloma in all the patients, non-caseating in the CS group, caseating in TB group and ill-defined in the indeterminate group. This highlights the practical difficulty in accurate diagnosis despite the availability of advanced cardiac imaging techniques and histopathologic examination.
The Mantoux test in isolation has a limited diagnostic role in India where tuberculous infection is so common. Interestingly in our study, all patients in the TB and the indeterminate group had Mantoux test positive and none in the CS group. Acid-fast bacilli culture and TB PCR test was positive in only 20% and 33% respectively in the TB group. Serum ACE levels were elevated in less than half of the CS group. Serum ACE levels were significantly higher in the CS group as compared to the TB group, while the difference between the CS and indeterminate groups did not reach statistical significance. The ESR, a non-specific marker of inflammation, was significantly higher in the TB group as compared to the CS group, while the difference between the TB and indeterminate groups did not reach statistical significance. up. Thus, elevated serum ACE levels supports the diagnosis of sarcoidosis and raised ESR supports TB. The mild elevation of both these markers in the indeterminate group continues to remain non-diagnostic.
The clinical features are not very dissimilar in the three groups. The mean age of presentation was in fourth decade of life with similar gender distribution. Monomorphic VT at presentation was seen in 70% patients in the TB group as opposed to 40% and 30% respectively in the sarcoid and the indeterminate groups. Pleomorphic VTs were more in the indeterminate group (59%). Polymorphic VT was noted less commonly in all the groups. Ventricular arrhythmia storm occurred in 9 patients (17%) and seemed to be more frequent in the sarcoid group. The morphologic characteristics of VT were not statistically different between the groups, primarily due to small numbers. Review of literature suggests that almost 20–30% of patients with monomorphic VTs have granulomatous cardiomyopathy [17,18]. The LVEF was only mildly reduced in the entire cohort and this seems predominantly due to selection bias of including patients who presented with VT. Compared to idiopathic VT, GCM with VT may have slightly lower LVEF (as almost 65% of patients had LVEF less than 0.55). However, a preserved LVEF does not rule out GCM.
Antiarrhythmic drug (AAD) therapy, constituting of beta-blockers, amiodarone, sotalol and mexiletine in various combinations were used to control the VT. Disease specific therapy with steroids, cyclophosphamide, azathioprine, methotrexate and anti-tubercular treatment were started along with AAD. Assessment of VT control was done on both therapeutic lines. Radio-frequency ablation was necessary in one-third of patients due to VT recurrences. The indeterminate group more often (47%; 8/17 patients) required to undergo ablation as opposed to 30% (6/20) in the CS group and only 20% (3/15) in the TB group. Koplan et al. in their study underscored the difficulties in ablating these arrhythmias [19]. Presence of myocardial scar and or ineffective suppression of VT despite AAD, disease specific therapy and RF ablation, was noted in almost 60% of patients in the CS and indeterminate group, requiring ICD implantation. Suppression of arrhythmia was more in the TB group. Only 4 patients (26%) in the TB group underwent ICD implantation. Bilateral cardiac sympathetic denervation for recurrent VTs and VT storm was infrequently required, for one patient each in the CS and the indeterminate group.
Cardiac involvement in sarcoidosis occurs in 20–27% of patients and some series report incidence as high as 58%. Despite a high incidence, only 5% of patients with sarcoidosis have clinical manifestations of cardiac disease [13]. The manifestation of CS may be high grade AV block, sustained atrial arrhythmias, or malignant ventricular arrhythmias (including sudden death). The substrate for arrhythmia may be active granuloma formation in the myocardium or myocardial fibrosis. In our CS group the presentation was more with monomorphic and pleomorphic VT and almost 25% patients had a VT storm. Control of VT in this group required a more aggressive approach with almost 60% patients undergoing ICD implantation and one patient underwent cardiac sympathetic denervation. Steroids were the main stay of immunosuppressive therapy. However, 9 patients (45%) required additional immunosuppressive therapy with cyclophosphamide, azathioprine or methotrexate. In one patient ATT was started because there were features to suggest both sarcoid and TB. Almost 30% patients had VT recurrence on follow-up despite anti-arrhythmic therapy and immunosuppressants. There was a marginal improvement in LVEF in this group and this is likely because of a diligent follow-up and guideline directed medical therapy.
Myocardial TB was the likely cause of VT in 29% of patients in our series. Although cardiac tuberculosis most commonly affects the pericardium, the myocardium may also be involved. Three patterns of involvement have been described-granulomatous mass lesions (tuberculomas), diffuse myocardial infiltration and rarely miliary infiltration. A tuberculoma is usually iso-intense or hypo-intense to myocardium on T1and T2-weighted CMR images, and heterogeneous late gadolinium enhancement pattern in non-enhancing areas represent necrosis. We stress on the importance of histopathological diagnosis, as it very frequently serves as a tie breaker between TB and sarcoidosis. In our TB group, the patients responded well to anti-mycobacterial chemotherapy with only 2 patients having arrhythmia recurrence while on treatment and only one patient had recurrence of VT after completion of the course. There was an improvement in LVEF from baseline of 0.41–0.52 on follow-up. There was one death in this group due to accidental burns, unrelated to cardiac disease. Adjunct steroids for three months in myocardial TB with VT was reported in a cohort of 11 patients with myocardial TB presenting with VT previously [20].
The indeterminate group constituted one-third of our study population. We encourage further definition and validation of the term sarco-tuberculosis when there are overlapping features of both sarcoid (non-caseating granulomas) and TB (positive Mantoux). The classification of the indeterminate group is important in India, because TB is common and many patients without active disease may have a positive Mantoux test. Majority of the patients in this group received both ATT and immunosuppressants. Almost 23% patients required additional immunosuppressants beyond steroids. The follow-up showed improvement in LVEF and no fatality. However, almost 40% patients had VT recurrences and nearly 60% patients underwent an ICD implantation.
Recognition of disease activity in the form of raised inflammatory markers, continuing ventricular arrhythmias, increased uptake in the myocardium or increasing size of lymph nodes, helps to guide therapy. Aggressive treatment with disease specific therapy, immunosuppressive drugs (including second line immunosuppressive therapy) may help suppress arrhythmias and possibly prevent myocardial damage and preserve the LVEF.
Overall, our study shows that patients with VT at presentation due to granulomatous infiltration and diagnosed before the onset of severe left ventricle dysfunction, when meticulously evaluated and appropriately treated, have a good long-term prognosis and survival. Cardiac sarcoidosis and the indeterminate groups require more aggressive anti-arrhythmia therapy and often underwent ICD implantation. It appears that systemic diseases like sarcoid or TB when presenting only with VT and no other cardiac or extra-cardiac diseases, respond well to a combined approach of anti-arrhythmic strategies and disease specific therapy and their LV function remains preserved. However, there was no significant difference in long term prognosis, and arrhythmia recurrence, when the groups were compared.
4. Conclusion
Granulomatous cardiomyopathy patients presenting with VT had extra-cardiac lymph node enlargement, which could be uniformly biopsied. Although many patients could be definitively diagnosed as CS or TB, the diagnosis in one-third patients remained uncertain. The ESR was significantly elevated in the TB group as compared to the CS or the indeterminate group. The overall long-term outcome is favorable with the LVEF being preserved. Optimal management with a combination of antiarrhythmic drugs, disease-specific therapy, ablation and ICD is often necessary. It is likely that it is the combination of these two factors - diagnosis at the stage of mild/no left ventricle dysfunction and institution of disease specific therapy early in the course of disease that leads to good outcomes.
5. Limitations
Ours is a retrospective analysis, from six different centers. The diagnostic and treatment approaches were at the discretion of the individual clinicians. We excluded classical idiopathic VT cases, so we could have missed GCM cases which mimicked these ECG patterns. However, three patients had been labeled as idiopathic VT before coming to us, two of whom had already undergone attempt at ablation. PET-CT was not done in all patients. This was due to a variety of reasons - logistic, financial and when it was presumed that the management would not be affected. Demonstrating granulomas in the heart by endomyocardial biopsy would have unequivocally established the etiology of the VT. However, we relied on an easier and safer approach of lymph node biopsies. The clinical follow-up assessment was considered as a practical surrogate in the control of disease. The study was also limited by the fact, that we did not have data regarding VT recurrences in non- ICD patients, which challenges our capacity to recommend indications for ICD implantation.
Consent
Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
Funding
Nil.
CRediT authorship contribution statement
Shomu Bohora: Conceptualization, Methodology, Writing – review & editing. Zeeshan Mumtaz: Methodology, Writing – original draft, Writing – review & editing, Software. Milind Phadke: Conceptualization, Methodology, Writing – review & editing. Vishnu Bhute: Methodology, Investigation, Writing – original draft. Varun Bhatia: Investigation. Amit Vora: Supervision, Validation, Writing – review & editing. Ajay Naik: Supervision, Validation. Ashish Nabar: Supervision, Validation. Bhavin Jankharia: Writing – review & editing. Pradeep Vaideeswar: Validation, Writing – review & editing. Gopi Panicker: statistical analysis, Formal analysis. Ujwal Bhure: Writing – review & editing. Yash Lokhandwala: Conceptualization, Methodology, Writing – review & editing.
Declaration of competing interest
The authors report no relationships that could be construed as a conflict of interest.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Ferrans V., René Rodríguez E., McAllister H. Granulomatous inflammation of the heart. Heart Ves. 1985;1:262–270. doi: 10.1007/bf02072406. [DOI] [PubMed] [Google Scholar]
- 2.Ayyala U., Nair A., Padilla M. Cardiac sarcoidosis. Clin Chest Med. 2008;29:493–508. doi: 10.1016/j.ccm.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Dubrey S., Falk R. Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis. 2010;52:336–346. doi: 10.1016/j.pcad.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Chapelon-Abric C., de Zuttere D., Duhaut P., Veyssier P., Wechsler B., Huong D., de Gennes C., Papo T., Blétry O., Godeau P., Piette J. Cardiac sarcoidosis. Medicine. 2004;83:315–334. doi: 10.1097/01.md.0000145367.17934.75. [DOI] [PubMed] [Google Scholar]
- 5.Fleming H., McMahon J., McCarthy C., Kelehan P. Sarcoid heart disease. Heart. 1983;50:498. doi: 10.1136/hrt.50.5.498. 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsui Y., Iwai K., Tachibana T., Fruie T., Shigematsu N., Izumi T., Homma A., Mikami R., Hongo O., Hiraga Y., Yamamoto M. Clinicopathological study on fatal myocardial sarcoidosis. Ann NY Acad Sci. 1976;278:455–469. doi: 10.1111/j.1749-6632.1976.tb47058.x. [DOI] [PubMed] [Google Scholar]
- 7.Roberts W., McAllister H., Ferrans V. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group I) and review of 78 previously described necropsy patients (group II) Am J Med. 1977;63:A81. doi: 10.1016/0002-9343(77)90145-0. [DOI] [PubMed] [Google Scholar]
- 8.Yazaki Y., Isobe M., Hiroe M., Morimoto S., Hiramitsu S., Nakano T., Izumi T., Sekiguchi M. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 9.Uusimaa P., Ylitalo K., Anttonen O., Kerola T., Virtanen V., Paakko E., Raatikainen P. Ventricular tachyarrhythmia as a primary presentation of sarcoidosis. Europace. 2008;10:760–766. doi: 10.1093/europace/eun110. [DOI] [PubMed] [Google Scholar]
- 10.Dubrey S., Falk R. Diagnosis and management of cardiac sarcoidosis. Progress Cardiovasc Dis. 2010;52:336–346. doi: 10.1016/j.pcad.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Birnie D., Sauer W., Bogun F., Cooper J., Culver D., Duvernoy C., Judson M., Kron J., Mehta D., Cosedis Nielsen J., Patel A., Ohe T., Raatikainen P., Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1304–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Khurana R., Shalhoub J., Verma A., Assomull R., Prasad S., Kooner J., Sethi A. Tubercular myocarditis presenting with ventricular tachycardia. Nat Clin Pract Cardiovasc Med. 2008;5:169–174. doi: 10.1038/ncpcardio1111. [DOI] [PubMed] [Google Scholar]
- 13.Thachil A., Christopher J., Sastry B., Reddy K., Tourani V., Hassan A., Raju B., Narasimhan C. Monomorphic ventricular tachycardiaand mediastinal adenopathy due to granulomatous infiltration in patients with preserved ventricular function. J Am Coll Cardiol. 2011;58:48–55. doi: 10.1016/j.jacc.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Hoey E., Gulati G., Ganeshan A., Watkin R., Simpson H., Sharma S. Cardiovascular MRI for assessment of infectious and inflammatory conditions of the heart. AJR Am J Roentgenol. 2011;197:103–112. doi: 10.2214/ajr.10.5666. [DOI] [PubMed] [Google Scholar]
- 15.Kim R., Shah D., Judd R. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson. 2003;5:505–514. doi: 10.1081/jcmr-120022267. [DOI] [PubMed] [Google Scholar]
- 16.Slart R., Glaudemans A., Lancellotti P., Hyafil F., Blankstein R., Schwartz R.G., et al. A joint procedural position statement on imaging in cardiac sarcoidosis: from the cardiovascular and inflammation & infection committees of the European association of nuclear medicine, the European association of cardiovascular imaging, and the American Society of Nuclear Cardiology. J Nucl Cardiol. 2018;25(1):298–319. doi: 10.1007/s12350-017-1043-4. [DOI] [PubMed] [Google Scholar]
- 17.Nery P., Mc Ardle B., Redpath C., Leung E., Lemery R., Dekemp R., et al. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2013;37(3):364–374. doi: 10.1111/pace.12277. [DOI] [PubMed] [Google Scholar]
- 18.Nery P., Beanlands R., Nair G., Green M., Yang J., Mcardle B., et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25(8):875–881. doi: 10.1111/jce.12401. [DOI] [PubMed] [Google Scholar]
- 19.Koplan B., Soejima K., Baughman K., Epstein L., Stevenson W. Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006;3:924–929. doi: 10.1016/j.hrthm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Mohan A., Thachil A., Sundar G., Sastry B.K.S., Hasan A., Sridevi C., Narasimhan C. Ventricular tachycardia and tuberculous lymphadenopathy: sign of myocardial tuberculosis? J Am Coll Cardiol. 2015;65:218–220. doi: 10.1016/j.jacc.2014.09.087. [DOI] [PubMed] [Google Scholar]