Abstract
Hypothesis
Both clinical outcomes and early rates of failure will not be associated with glenoid retroversion.
Methods
All patients who underwent an anatomic total shoulder arthroplasty with minimal, noncorrective reaming between 2006 and 2016 with minimum 2-year follow-up were reviewed. Measurements for retroversion, inclination, and posterior subluxation were obtained from magnetic resonance imaging or computerized tomography. A regression analysis was performed to assess the association between retroversion, inclination and subluxation, and their effect on patient reported outcomes (PROs). Clinical failures and complications were reported.
Results
One hundred fifty-one anatomic total shoulder arthroplasties (90% follow-up) with a mean follow-up of 4.6 years (range, 2-12 years) were assessed. The mean preoperative retroversion was 15.6° (range, 0.2-42.1), the mean posterior subluxation was 15.1% (range, −3.6 to 44.1%), and the mean glenoid inclination was 13.9° (range, −11.3 to 44.3). All median outcome scores improved significantly from pre- to post-operatively (P < .001). The median satisfaction was 10/10 (1st quartile = 7 and 3rd quartile = 10). Linear regression analysis found no significant association between retroversion and any postoperative PRO. A total of 5 (3.3%) failures occurred due to glenoid implant loosening (3 patients) and Cutibacterium acnes infection (2 patients) with no association between failure causation and increased retroversion or inclination. No correlation could be found between the Walch classification and postoperative PROs.
Conclusion
Anatomic total shoulder replacement with minimal and noncorrective glenoid reaming demonstrates reliable increases in patient satisfaction and clinical outcomes at a mean of 4.6-year follow-up in patients with up to 40° of native retroversion. Higher values of retroversion were not associated with early deterioration of clinical outcomes, revisions, or failures. Long-term studies are needed to see if survivorship and outcomes hold up over time.
Keywords: Glenoid retroversion, Total shoulder arthroplasty, Patient-reported outcomes, Implant survivorship
Total shoulder arthroplasty (TSA) has proven to be a reliable treatment option, demonstrating improvement in both pain and function for patients presenting with primary osteoarthritis of the glenohumeral joint.10,32,45,49 As osteoarthritis of the shoulder naturally progresses, increases in glenoid retroversion, glenoid bone loss, and subluxation of the humeral head can pose technical difficulties that warrant extensive preoperative planning to optimize the patient’s outcome.31,32,46 As the total number of arthroplasties have drastically increased over the past decade, so too have the need for revision surgeries.4 Revision shoulder arthroplasty is performed in the setting of implant loosening, fracture, infection, stiffness, instability, and rotator cuff tears.24,47
The most frequent cause for revision TSA is early loosening and failure of the glenoid component.22,26,53 Factors that have shown to increase the rates of loosening include eccentric loading of the humeral head, excessive retroversion, and patients demonstrating severe posterior glenoid bone loss.9,19,21,23,51 Sustained eccentric loading of the glenoid implant can lead to the rocking horse phenomenon and early glenoid implant loosening.19,20,22,26,50 Centering forces can be native soft tissue structures, such as the rotator cuff, but different surgical steps can contribute to postoperative balanced glenohumeral articulation.35 Various strategies have been used when approaching posterior glenoid wear, including high side eccentric reaming,14,52 posterior glenoid bone grafting,17,30,41 augmented glenoid implants40,42,48,54 and in extreme retroversion cases, reverse TSA (rTSA).29 These steps are taken to correct retroversion to ≤15° with the theoretical hope of decreasing the risks of eccentric implant loading and early TSA failure. Excessive reaming, however, can lead to removal of strong and structural subchondral bone, which can compromise glenoid component fixation and lead to early glenoid implant migration and failure. Published series using bone graft augmentation in conjunction with polyethylene glenoid implants have shown suboptimal results with graft resorption. The optimal glenoid management in cases of increased posterior bone loss remains elusive.37
Because of conflicting outcomes, the ideal method for treating patients with increased retroversion in the setting of anatomic TSA remains unknown. The purpose of this study was to introduce a technique of subchondral bone sparing, noncorrective reaming in anatomic TSA, and to review outcomes, complications, and survivorship while specifically assessing the impact of preoperative retroversion on both clinical outcomes and failure. The hypothesis was that both clinical outcomes and early rates of failure would not be associated with glenoid retroversion.
Methods
Study cohort
In this institutional review board approved study 2019-17, a retrospective collection of prospectively collected clinical outcomes was performed in consecutive patients undergoing anatomic TSA treated by a single surgeon between 2006 and 2016. Exclusion criteria were (1) patients who had prior arthroplasty, (2) patients who were not 2 years out from surgery at the time of follow-up, (3) patients who refused to participate or had died before the initiation of the current study, or (4) patients who did not have preoperative imaging available such as computerized tomography (CT) or magnetic resonance imaging (MRI).
Radiographic evaluation
Radiographic parameters, measured by 3 independent reviewers (T.J.D., W.J.G., and B.T.G.), included degrees of glenoid retroversion, inclination, and percent of humeral head subluxation. Interclass and intraclass correlation were performed by 2 evaluators rerecording all data points on 21 randomly assigned (12.5%) patients >4 weeks apart from the initial data collection time point.
Retroversion
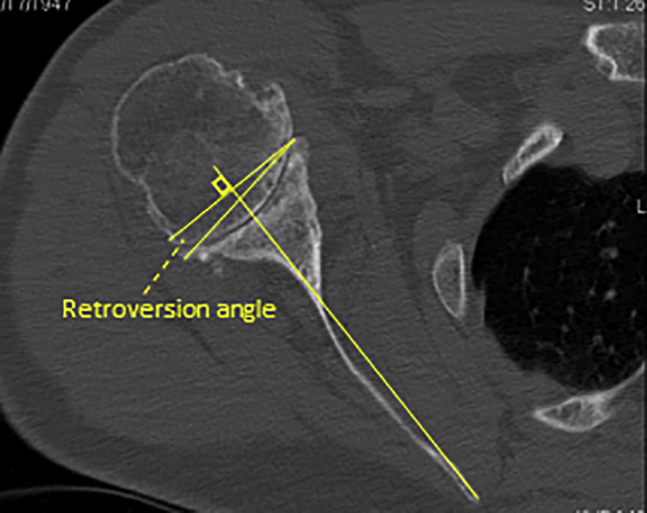
Glenoid retroversion was measured using methods consistent with prior studies.5,11 Using advanced 3D imaging of CT or MRI, a line between the tip of the medial border of the scapula—with the entire scapula visualized—and central aspect of the midglenoid on axial scans was first identified to give the scapula axis (Friedman’s line).11 The version was recorded by taking the angle between 2 points (the anterior and posterior glenoid border) and the scapula axis with the use of an angle measuring tool on a picture archiving and communication system (Fig. 1).
Figure 1.
Retroversion angle measured between the longitudinal axis of the scapula body and the line parallel to the face of the glenoid.
Inclination
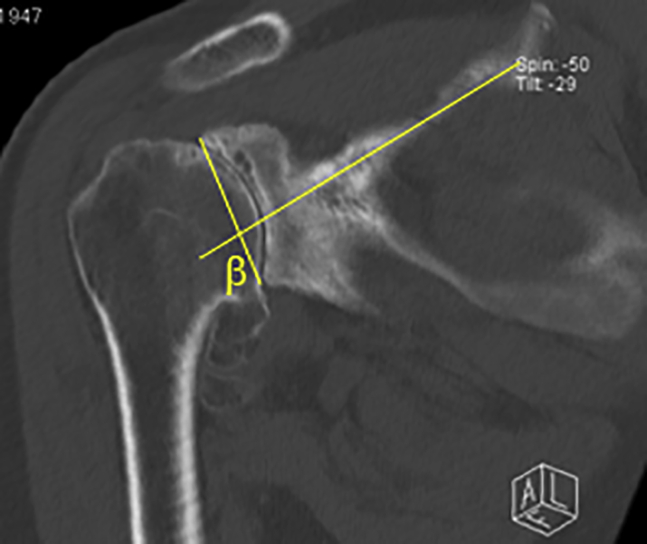
Glenoid inclination was recorded in a similar fashion to that described by Walch and Maurer et al using the picture archiving and communication system system.3,27 Coronal sections of advanced imaging modalities (CT or MRI) were obtained at the deepest point of the supraspinatus fossa. The inclination angle was defined using a Cobb angle tool with a line paralleling the sclerotic line of the supraspinatus fossa and a line connecting the most lateral points on superior and inferior glenoid rim (Fig. 2). The angle was then subtracted by 90°, which shows positive numbers reflecting superior glenoid inclination and negative numbers reflecting inferior inclination.
Figure 2.
A line is drawn paralleling the sclerotic line of the supraspinatus fossa and the glenoid fossa line to determine the inclination (β-angle).
Subluxation
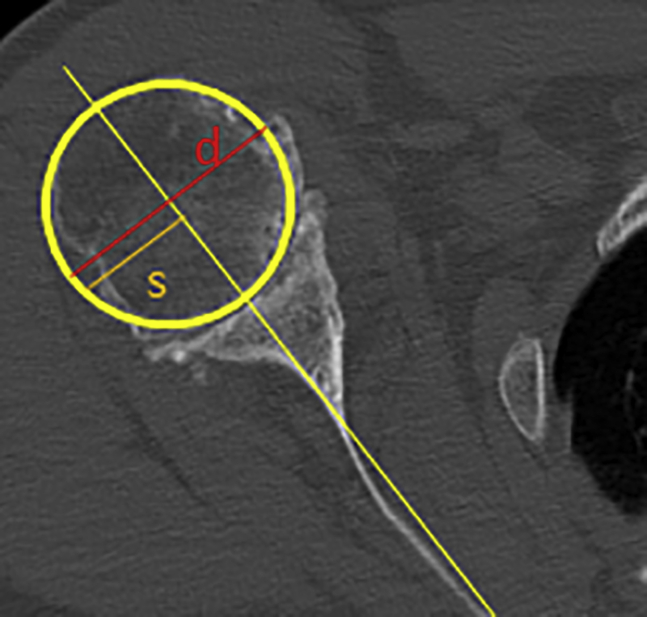
Using the method as defined by Walch et al,24 subluxation was defined by the percentage of the humeral head lying posterior to the scapula axis. Using a mid-glenoid axial image on either CT or MRI, a line is drawn through the scapular body axis into the humeral head. The anteroposterior intersection of this line in relation to the diameter of the humeral head at this axial slice is used to calculate the total percent of posterior subluxation and serve as a surrogate for total posterior wear (Fig. 3).50
Figure 3.
Subluxation is determined by comparing the diameter of the humeral head to a line perpendicular through the central aspect of the humeral head to that of the scapular axis line to give a total percent of posterior subluxation (s/d × 100).
Surgical technique
All surgeries were performed by the senior surgeon (P.J.M.) using a standard anatomic TSA implant (Apex or Univers 2, Arthrex, Inc., Naples, FL, USA) with an all-polyethylene pegged cemented glenoid. Following a standard deltopectoral approach, the subscapularis tendon was released via a lesser tuberosity osteotomy.38 The humeral head was then exposed and osteotomized to mimic native humeral version. Next, the glenoid was prepared for component placement, and minimal noncorrective reaming was performed. Osteophytes were removed with a rongeur.
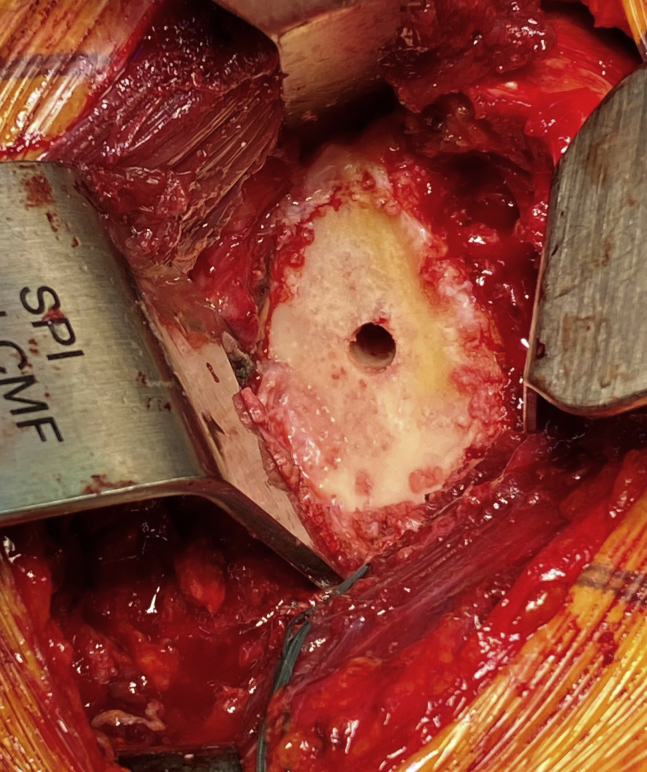
Minimal noncorrective reaming was defined as reaming until there was concentricity of the reamer and the native glenoid and thus a stable fit of the glenoid implant on the bone. The goal of reaming was to decorticate the glenoid changing the version and inclination only until concentricity of the reamed bone and glenoid component was achieved (Fig. 4). No attempts were made to correct glenoid version to neutral. Posterior coverage of the glenoid component was maximized, and before proceeding to final implantation, the glenoid implant trial had to be stable without cement.
Figure 4.
View of the glenoid post minimal, noncorrective reaming.
Cement was pressurized in the glenoid with a Toomey syringe and a mechanical pressurizing instrument. Cement was placed on the backside of the glenoid component around the pegs before its insertion. The pegged, all-polyethylene glenoid component was then inserted and impacted into the glenoid followed by removal of any excess cement.
After implantation of the glenoid component, the humerus was reamed and broached to an appropriate size, and the stemmed humeral implant was inserted. Because of the span of time and number of procedures performed, both metaphyseal and diaphyseal stabilizing implants were used. Finally, after trialing heads to ensure adequate soft tissue balance, stability, and range of motion, an anatomic humeral head component was placed. The lesser tuberosity osteotomy was repaired using 3 sutures passed through the lesser tuberosity bone fragment, passed around the humeral component shaft before final seating of the humeral implant.36
Postoperative rehabilitation
Postoperative rehabilitation included immediate passive range of motion without restriction in all planes with the exception of external rotation, which was limited to 30° for the first 3 weeks postoperatively. At 3 weeks, full passive and active ranges of motion were permitted.28 Strengthening commenced at 5-6 weeks postoperatively. After full range of motion and strength were obtained (typically 4-5 months postoperatively), full activities were permitted. No restrictions were placed on patients’ recreational, work, or sporting activities after 4-6 months.28
Outcome scores
The following clinical outcomes were collected prospectively at baseline and at regular postoperative follow-up: American Shoulder and Elbow Surgeons (ASES) score, Single Assessment Numerical Evaluation (SANE) score, Quick Disabilities of the Arm, Shoulder and Hand (QuickDASH) score, the physical component summary (PCS) of the 12-item Short Form (SF-12) questionnaire, and patient satisfaction (integer scale 1-10 with 10 representing highest satisfaction). Complications and further surgical interventions were assessed.
At a minimum follow-up of 2 years postoperatively, questionnaires with the aforementioned scores were sent to the patients electronically. If patients did not return their questionnaires, they were contacted via telephone or email and were asked to complete the shoulder survey. No questions regarding the patient reported outcomes (PROs) were asked via telephone to avoid response bias.
Statistical analysis
The primary analysis method to assess the relationship between glenoid retroversion and each patient-reported outcome scale was simple ordinary least squares (OLS) regression. As a sensitivity analysis, multivariable ordinal logistic regression models with multiple imputation and restricted cubic spline relationships were constructed to adjust for age, sex, glenoid inclination, percent subluxation, Walch classification, and baseline PRO score. In addition to providing adjusted effects for the primary hypothesis, the latter more complex analysis was performed to better handle the skewed response variable distributions that can lead to suboptimal OLS model fits. Our a priori intention was to report the more straightforward analysis as the primary results and then secondarily report the sensitivity analysis models where clinically relevant differences in interpretation were identified.
Comparison between preoperative and postoperative outcome scores was assessed using the Wilcoxon signed-rank test. Interrater and intrarater measurement reliability were assessed with the absolute agreement, single-measures intraclass correlation coefficient (ICC) was assessed using a subset of 20 randomly assigned patients. Bivariate nonlinear (LOESS) correlations were run for data that were not normally distributed. The ICC values were graded using the scale described by Fleiss et al (excellent reliability (0.75> ICC ≤ 1.00), fair to good reliability (0.40 = ICC ≤ 0.75), and poor reliability (0.00 = ICC ≤ 0.40).8 ICC values were calculated using SPSS, version 11.0 (SPSS Inc, IBM, Armonk, NY, USA). All other graphs and analyses were completed with the statistical package R Version 3.5.2 (R Development Core Team, Vienna, Austria with additional package rms; access date June 10, 2019).15,39
Results
Study population
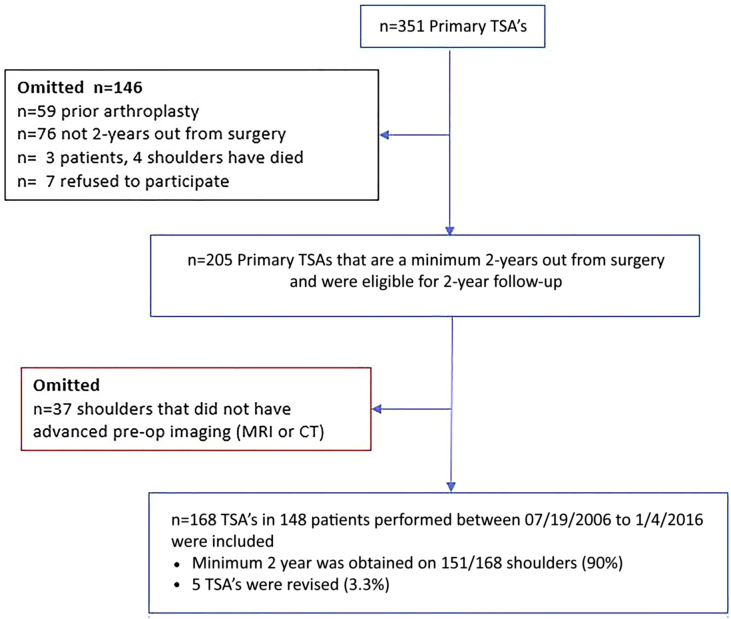
A total of 168 consecutive TSA surgeries in 148 patients were identified between 2006 and 2016. There was a total of 148 patients (43 females and 105 males) with average age of 63.9 years (range 20.6-82.7 years). Walch classification of glenoid morphology of the study population included A1 = 51, A2 = 10, B1 = 32, B2 = 51, B3 = 4, and C = 20. There were 160 TSAs for primary osteoarthritis, and 8 for secondary or post-traumatic osteoarthritis. Complete follow-up (minimum 2-years) was available for 151/168 anatomic TSAs (90% follow-up), with a mean follow-up of 4.6 years (range 2-12 years; Fig. 5).
Figure 5.
Flow diagram of patients included in this study. TSA, total shoulder arthroplasty.
The mean retroversion and inclination determined retrospectively on preoperative advanced imaging of MRI or CT for all included shoulders was 15.6° (range, 0.2-42.1) and 13.9° (range, −11.3 to 44.3), respectively. The mean posterior subluxation was 15.1 % (range, −3.6 to 44.1%).
All median [1st quartile, 3rd quartile] outcome scores improved significantly from pre- to postoperatively: ASES (48 [37, 62] to 92 [71, 98]; P < .001), SANE (50 [30, 69] to 89 [59, 97]; P < .001), QuickDASH (41 [30, 50] to 9 [5, 32]; P < .001), and SF-12 PCS (37 [33, 45] to 53 [42, 57]; P < .001). At final follow-up, the median satisfaction was 10/10 (1st quartile = 7, 3rd quartile = 10).
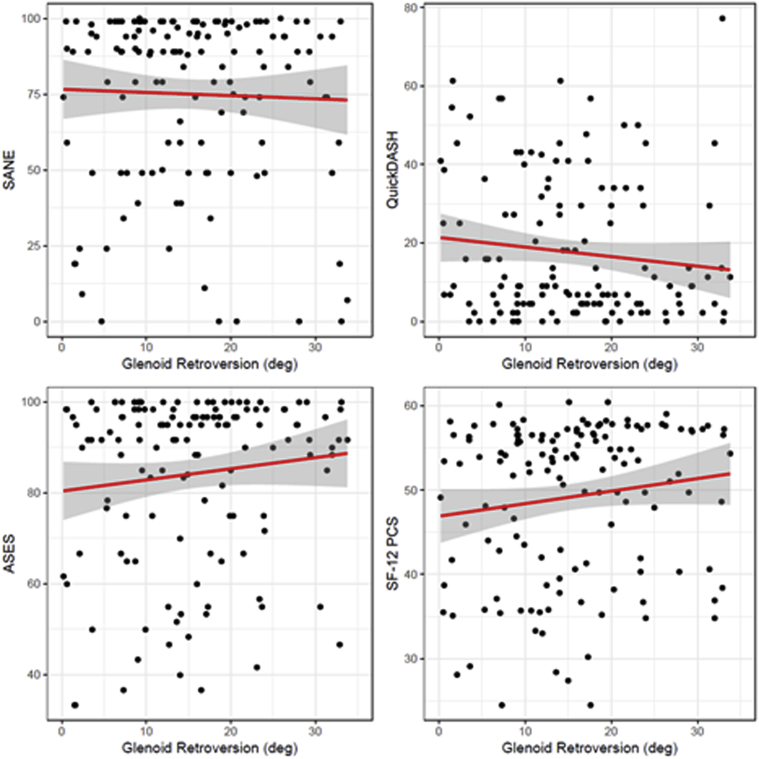
Glenoid retroversion was not significantly associated with any postoperative PRO scale; ASES (beta = 0.27, standard error [SE] = 0.18, P = .141), QuickDASH (beta = −0.27, SE = 0.17, P = .122), SANE (beta = −0.10, SE = 0.28, P = .712), and SF-12 PCS (beta = 0.16, SE = 0.09, P = .082). Scatter plots and linear regression fits are presented in Figure 6. Multiple ordinal logistic regression, performed as a sensitivity analysis, did not identify clinically meaningful differences in the interpretation of the relationship between retroversion and each PRO.
Figure 6.
Scatterplots and linear regression associations between glenoid retroversion and 4 postoperative patient-reported outcome scales.  represent individual patients, whereas
represent individual patients, whereas  represent estimated regression relationships, and the gray shaded areas represent 95% confidence regions for each regression.
represent estimated regression relationships, and the gray shaded areas represent 95% confidence regions for each regression.
Furthermore, simple OLS regression found no significant associations between inclination or subluxation and any of the postoperative PROs. Finally, 5 total failures occurred, which required revision or conversion to rTSA 3.3% (5/151) (Table I).
Table I.
Retroversion, inclination, and subluxation of TSA failures.
| Patient | Age | Sex | Glenoid type | Inclination | Subluxation (%) | Causation of failure | Months from primary surgery |
|---|---|---|---|---|---|---|---|
| 1 | 60.5 | F | B2 | 35.4 | 61.5 | Glenoid Implant loosening | 26.4 |
| 2 | 55.8 | M | B1 | 12.4 | 88.1 | C. Acnes infection | 36.3 |
| 3 | 53.4 | M | B1 | 14.9 | 50.2 | C. Acnes infection | 39.2 |
| 4 | 57.8 | M | C | 23.7 | 62.7 | Glenoid implant loosening | 27.6 |
| 5 | 58.7 | F | A1 | 28.1 | 57.2 | Glenoid implant loosening | 49 |
F, female; M, male; TSA, total shoulder arthroplasty.
Finally, bivariate analysis found no statistically significant difference in postoperative SANE, QuickDASH, ASES, SF-12 PCS, or patient satisfaction depending on the Walch classification. The results are further confirmed by multiple regression models where the Walch classification was not a statistically significant independent predictor of any PROM when adjusting for age, sex, baseline PRO, inclination, subluxation, and retroversion.
Discussion
The most important finding in this study was that in this large single surgeon series at a mean of 4.6 years and minimum 2-year follow-up, increased retroversion was not associated with increased risk of failure nor any difference in clinical outcome scores. Although many previous studies empirically recommended correction to a glenoid retroversion (5°-15°) with the intention of avoiding abnormal positioning, a possible rocking horse effect, and potentially early glenoid polyethylene loosening, this study finds that minimal corrective reaming had no bearing on clinical outcome scores and, furthermore, demonstrated no clinical symptoms of early loosening or failure.19,44 The data from this study suggest that bone preservation with minimal corrective reaming in the setting of anatomic total shoulder replacement can produce reliable and durable results with improvement in all PRO domains at 4.6-year follow-up. The data suggest that the quality of the fixation of the glenoid component in the bone may be an important factor and that preserving bone quality by avoiding excessive reaming might enhance outcomes.
Because of past biomechanical and clinical series, there are concerns of higher complication rates and risks of revision in cases with severe retroversion when performing anatomic TSA. In complex shoulders with increased posterior bone loss and retroversion, there are several strategies to address and correct the bone deficiency and retroversion: high side corrective reaming, posterior glenoid bone grafting, augmented glenoid implants, or even rTSA in extreme cases.14,17,29,30,40, 41, 42,48,52,54 Previous dogma recommended placement of polyethylene glenoids in <15° of retroversion to avoid early loosening and possible failure.2,7,13,19,44 However, several recent clinical series have shown reliable outcomes with bone preserving reaming techniques that are noncorrective.6,34,43 Our findings are consistent with Service et al, who found equivalent outcomes at 2-year follow-up when glenoid implants were placed with minimal corrective high-sided reaming and no attempts of changing native version.43 Furthermore, these studies showed excellent and durable outcomes with revision rates <6% regardless of preoperative retroversion 6,43 The findings from our cohort demonstrate no association between retroversion and failure rates and a low risk of failure (3.3%) regardless of native preoperative version when bone preserving and noncorrective reaming strategy for glenoid preparation is used.
Similar to retroversion, increased glenoid inclination (β angle) is associated with more severe and degenerative osteoarthritis of the shoulder.16 Increases in glenoid inclination and scapular tilt have been implicated in scapular notching and impingement with rTSA.12 Biomechanical studies have been performed evaluating the importance of residual coronal plane deformity in the setting of anatomic TSA. These have demonstrated increased risk of superior migration and subluxation if superior inclination is not corrected.33 There remains a paucity of clinical outcome studies focusing on coronal plane deformities in anatomic TSA and its long-term implications both on implant survivorship and patient outcomes. Unlike the implications associated with rTSA, at mid-term follow-up, glenoid inclination did not adversely affect survivorship or PROs when evaluated as an independent variable. The implications of these findings are important, as surgeons should note preoperative inclination for planning purposes, but surgical correction back to a normal glenoid inclination1 is not necessary with bone preserving techniques.
Even when using augmented implants to neutralize prosthetic version, preoperative posterior humeral head subluxation has been associated with lower postoperative outcome scores and satisfaction.18 However, subluxation has not been associated with increased risk of loosening or signs of early implant failure with modern implants.25 In our series, when controlling for possible confounding variables, subluxation did not have an effect on the need for revision arthroplasty or inferior clinical outcomes. Although native mechanics may not be reestablished when using a minimal reaming technique, we surmise that improvements seen in function and satisfaction are because of the placement of the prosthesis in a position that the soft tissues had conformed to over years of degenerative wear and use.
The strengths of this study include the evaluation of 3 independent radiographic variables (retroversion, subluxation, and inclination) and their association to the clinical variables of outcome scores, failure rates, and patient satisfaction at mid-term follow-up. Furthermore, the technique was performed by a single surgeon, which acts to limit the variability of technique, controls for implants, and standardizes the postoperative protocol. Finally, with 90% follow-up, to our knowledge, this is the largest series to date.
Limitations
Advanced imaging was not routinely obtained postoperatively on all patients to critically evaluate the degree of correction from each individual patient. Ideally, patients would undergo advanced CT imaging at minimum 6-month follow-up to evaluate final version achieved; however, in such a large cohort that is asymptomatic, this was not possible nor feasible to do, as it would subject patients to unnecessary radiation. Furthermore, routine radiographs are taken up to 6 months postoperatively unless patients return with symptoms at a later date, which would necessitate further radiographic workup beyond this time point; as a result, this study cannot specifically comment on early loosening but instead focused on patient outcomes, satisfaction, and need for revision surgery. Although no decline in scores was seen when controlling for retroversion, inclination, and subluxation, the findings of this study are limited to only these pathologies. In more severe cases (subluxation >44%, retroversion >40°, and inclination >30°) or over longer durations of follow-up, the results of this study may not hold true. Further investigations are necessary with longer follow-up to carefully evaluate long-term outcomes and risk of loosening/failure in patients who undergo anatomic TSA in the setting of increased retroversion, subluxation, and inclination.
Conclusion
Anatomic total shoulder replacement with minimal and noncorrective glenoid reaming demonstrates reliable increases in patient satisfaction and clinical outcomes at a mean of 4.6-year follow-up in patients with up to 40° of native retroversion. Higher values of retroversion were not associated with early deterioration of clinical outcomes, revisions, or failures. Long-term studies are needed to see if survivorship and outcomes hold up over time.
Disclaimers
Funding: No funding was disclosed by the authors.
Conflicts of interest: Peter J. Millett, MD, MSc: Reports receiving royalties and consultant payments from Arthrex and owns stock in VuMedi. He received royalties from MedBridge and Springer Publishing. Reports being a part owner of ProofPoint Biologics. He has an affiliation with and receives research support from the Steadman Philippon Research Institute, a 501(c)(3) nonprofit institution supported financially by private donations and corporate support from the following entities: Arthrex, Ossur, Siemens, Smith & Nephew, DoD, and DJO.
Travis Dekker, MD: Travis’ position and this research were supported by the Steadman Philippon Research Institute, which is a 501(c)(3) nonprofit institution supported financially by private donations and corporate support from the following entities: Arthrex, Ossur, Siemens, Smith & Nephew, DoD, and DJO.
W. Jeffery Grantham, MD: Dr Grantham reports education support from Smith & Nephew Inc. outside of the submitted work. Dr Grantham’s position and this research supported by the Steadman Philippon Research Institute, which is a 501(c)(3) nonprofit institution supported financially by private donations and corporate support from the following entities: Arthrex, Ossur, Siemens, Smith & Nephew, DoD, and DJO.
Lucca Lacheta, MD: Dr Lacheta reports grants from AGA outside of the submitted work. This research supported by the Steadman Philippon Research Institute, which is a 501(c)(3) nonprofit institution supported financially by private donations and corporate support from the following entities: Arthrex, Ossur, Siemens, Smith & Nephew, DoD, and DJO.
Brandon Goldenberg, MD: Dr. Goldenberg’s position and this research supported by the Steadman Philippon Research Institute, which is a 501(c)(3) nonprofit institution supported financially by private donations and corporate support from the following entities: Arthrex, Ossur, Siemens, Smith & Nephew, DoD, and DJO.
Rony-Orijit Dey Hazra, MD: Dr. Rony-Orijit Dey Hazra reports grants from AGA outside of the submitted work sponsored by Arthrex Inc. This research supported by the Steadman Philippon Research Institute, which is a 501(c)(3) nonprofit institution supported financially by private donations and corporate support from the following entities: Arthrex, Ossur, Siemens, Smith & Nephew, DoD, and DJO.
Dylan Rakowski, BS: Dylan’s position and this research supported by the Steadman Philippon Research Institute, which is a 501(c)(3) nonprofit institution supported financially by private donations and corporate support from the following entities: Arthrex, Ossur, Siemens, Smith & Nephew, DoD, and DJO.
Marilee Horan, MPH: This research supported by the Steadman Philippon Research Institute, which is a 501(c)(3) nonprofit institution supported financially by private donations and corporate support from the following entities: Arthrex, Ossur, Siemens, Smith & Nephew, DoD, and DJO.
Grant Dornan, MS: This research supported by the Steadman Philippon Research Institute, which is a 501(c)(3) nonprofit institution supported financially by private donations and corporate support from the following entities: Arthrex, Ossur, Siemens, Smith & Nephew, DoD, and DJO.
Footnotes
Research performed at the Steadman Philippon Research Institute, Vail, CO.
This study was approved by Vail Health Hospital Institutional Review Board under study number 2019-17.
References
- 1.Churchill R.S., Brems J.J., Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001;10:327–332. doi: 10.1067/mse.2001.115269. [DOI] [PubMed] [Google Scholar]
- 2.Clavert P., Millett P.J., Warner J.J. Glenoid resurfacing: what are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16:843–848. doi: 10.1016/j.jse.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Daggett M., Werner B., Gauci M.O., Chaoui J., Walch G. Comparison of glenoid inclination angle using different clinical imaging modalities. J Shoulder Elbow Surg. 2016;25:180–185. doi: 10.1016/j.jse.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Day J.S., Lau E., Ong K.L., Williams G.R., Ramsey M.L., Kurtz S.M. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19:1115–1120. doi: 10.1016/j.jse.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Denard P.J., Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22:1589–1598. doi: 10.1016/j.jse.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 6.DeVito P., Agyeman K.D., Judd H., Moor M., Berglund D., Malarkey A., et al. Outcomes of anatomic total shoulder arthroplasty in patients with excessive glenoid retroversion: a case-control study. J Shoulder Elbow Surg. 2019;28:1948–1955. doi: 10.1016/j.jse.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Farron A., Terrier A., Buchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg. 2006;15:521–526. doi: 10.1016/j.jse.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Fleiss J. John Wiley Sons; New York: 1986. The design and analysis of clinical experiments. [Google Scholar]
- 9.Fox T.J., Cil A., Sperling J.W., Sanchez-Sotelo J., Schleck C.D., Cofield R.H. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:859–863. doi: 10.1016/j.jse.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Fox T.J., Foruria A.M., Klika B.J., Sperling J.W., Schleck C.D., Cofield R.H. Radiographic survival in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:1221–1227. doi: 10.1016/j.jse.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Friedman R.J., Hawthorne K.B., Genez B.M. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74:1032–1037. [PubMed] [Google Scholar]
- 12.Gerber C., Pennington S.D., Nyffeler R.W. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17:284–295. doi: 10.5435/00124635-200905000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Gillespie R., Lyons R., Lazarus M. Eccentric reaming in total shoulder arthroplasty: a cadaveric study. Orthopedics. 2009;32:21. doi: 10.3928/01477447-20090101-07. [DOI] [PubMed] [Google Scholar]
- 14.Habermeyer P., Magosch P., Lichtenberg S. Recentering the humeral head for glenoid deficiency in total shoulder arthroplasty. Clin Orthop Relat Res. 2007;457:124–132. doi: 10.1097/BLO.0b013e31802ff03c. [DOI] [PubMed] [Google Scholar]
- 15.Harrell F., Jr. rms: regression Modeling Strategies. R package version 5.1-3. 2019. https://CRAN.R-project.org/package=rms Accessed June 10, 2019.
- 16.Hawi N., Magosch P., Tauber M., Lichtenberg S., Martetschlager F., Habermeyer P. Glenoid deformity in the coronal plane correlates with humeral head changes in osteoarthritis: a radiographic analysis. J Shoulder Elbow Surg. 2017;26:253–257. doi: 10.1016/j.jse.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Hill J.M., Norris T.R. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83:877–883. [PubMed] [Google Scholar]
- 18.Ho J.C., Amini M.H., Entezari V., Jun B.J., Alolabi B., Ricchetti E.T., et al. Clinical and radiographic outcomes of a posteriorly augmented glenoid component in anatomic total shoulder arthroplasty for primary osteoarthritis with posterior glenoid bone loss. J Bone Joint Surg Am. 2018;100:1934–1948. doi: 10.2106/JBJS.17.01282. [DOI] [PubMed] [Google Scholar]
- 19.Ho J.C., Sabesan V.J., Iannotti J.P. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95:e82. doi: 10.2106/JBJS.L.00336. [DOI] [PubMed] [Google Scholar]
- 20.Hsu J.E., Ricchetti E.T., Huffman G.R., Iannotti J.P., Glaser D.L. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:1298–1308. doi: 10.1016/j.jse.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Iannotti J.P., Norris T.R. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85:251–258. doi: 10.2106/00004623-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Karelse A., Van Tongel A., Verstraeten T., Poncet D., De Wilde L.F. Rocking-horse phenomenon of the glenoid component: the importance of inclination. J Shoulder Elbow Surg. 2015;24:1142–1148. doi: 10.1016/j.jse.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Keller J., Bak S., Bigliani L.U., Levine W.N. Glenoid replacement in total shoulder arthroplasty. Orthopedics. 2006;29:221–226. doi: 10.3928/01477447-20060301-05. [DOI] [PubMed] [Google Scholar]
- 24.Kidder J.F., Rouleau D.M., Pons-Villanueva J., Dynamidis S., DeFranco M.J., Walch G. Humeral head posterior subluxation on CT scan: validation and comparison of 2 methods of measurement. Tech Shoulder Elbow Surg. 2010;11:72–76. doi: 10.1097/BTE.0b013e3181e5d742. [DOI] [Google Scholar]
- 25.Levy J.C., Berglund D., Vakharia R., Tahal D.S., Mijc D., DeVito P., et al. Midterm results of anatomic total shoulder arthroplasty with a third-generation implant. J Shoulder Elbow Surg. 2019;28:698–705. doi: 10.1016/j.jse.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 26.Matsen F.A., III, Clinton J., Lynch J., Bertelsen A., Richardson M.L. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90:885–896. doi: 10.2106/JBJS.G.01263. [DOI] [PubMed] [Google Scholar]
- 27.Maurer A., Fucentese S.F., Pfirrmann C.W., Wirth S.H., Djahangiri A., Jost B., et al. Assessment of glenoid inclination on routine clinical radiographs and computed tomography examinations of the shoulder. J Shoulder Elbow Surg. 2012;21:1096–1103. doi: 10.1016/j.jse.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Millett P.J., Gobezie R., Boykin R.E. Shoulder osteoarthritis: diagnosis and management. Am Fam Physician. 2008;78:605–611. [PubMed] [Google Scholar]
- 29.Mizuno N., Denard P.J., Raiss P., Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95:1297–1304. doi: 10.2106/JBJS.L.00820. [DOI] [PubMed] [Google Scholar]
- 30.Neer C.S., II, Morrison D.S. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70:1154–1162. [PubMed] [Google Scholar]
- 31.Neer C.S., II, Watson K.C., Stanton F.J. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64:319–337. [PubMed] [Google Scholar]
- 32.Norris T.R., Iannotti J.P. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11:130–135. doi: 10.1067/mse.2002.121146. [DOI] [PubMed] [Google Scholar]
- 33.Oosterom R., Rozing P.M., Bersee H.E. Effect of glenoid component inclination on its fixation and humeral head subluxation in total shoulder arthroplasty. Clin Biomech (Bristol, Avon) 2004;19:1000–1008. doi: 10.1016/j.clinbiomech.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Orvets N.D., Chamberlain A.M., Patterson B.M., Chalmers P.N., Gosselin M., Salazar D., et al. Total shoulder arthroplasty in patients with a B2 glenoid addressed with corrective reaming. J Shoulder Elbow Surg. 2018;27(6S):S58–S64. doi: 10.1016/j.jse.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Patel R.J., Choi D., Wright T., Gao Y. Nonconforming glenoid increases posterior glenohumeral translation after a total shoulder replacement. J Shoulder Elbow Surg. 2014;23:1831–1837. doi: 10.1016/j.jse.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Petri M., Euler S.A., Dornan G.J., Greenspoon J.A., Horan M.P., Katthagen J.C., et al. Predictors for satisfaction after anatomic total shoulder arthroplasty for idiopathic glenohumeral osteoarthritis. Arch Orthop Trauma Surg. 2016;136:755–762. doi: 10.1007/s00402-016-2452-6. [DOI] [PubMed] [Google Scholar]
- 37.Phipatanakul W.P., Norris T.R. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15:84–87. doi: 10.1016/j.jse.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Ponce B.A., Ahluwalia R.S., Mazzocca A.D., Gobezie R.G., Warner J.J., Millett P.J. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(Suppl 2):1–8. doi: 10.2106/JBJS.E.00441. [DOI] [PubMed] [Google Scholar]
- 39.R Core Team . R Foundation for Statistical Computing V; Austria: 2018. R: A language and environment for statistical computing. [Google Scholar]
- 40.Rice R.S., Sperling J.W., Miletti J., Schleck C., Cofield R.H. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466:579–583. doi: 10.1007/s11999-007-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabesan V., Callanan M., Ho J., Iannotti J.P. Clinical and radiographic outcomes of total shoulder arthroplasty with bone graft for osteoarthritis with severe glenoid bone loss. J Bone Joint Surg Am. 2013;95:1290–1296. doi: 10.2106/JBJS.L.00097. [DOI] [PubMed] [Google Scholar]
- 42.Sabesan V., Callanan M., Sharma V., Iannotti J.P. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23:964–973. doi: 10.1016/j.jse.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Service B.C., Hsu J.E., Somerson J.S., Russ S.M., Matsen F.A., III Does postoperative glenoid retroversion affect the 2-year clinical and radiographic outcomes for total shoulder arthroplasty? Clin Orthop Relat Res. 2017;475:2726–2739. doi: 10.1097/CORR.0000000000000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapiro T.A., McGarry M.H., Gupta R., Lee Y.S., Lee T.Q. Biomechanical effects of glenoid retroversion in total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 Suppl):S90–S95. doi: 10.1016/j.jse.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Simovitch R.W., Friedman R.J., Cheung E.V., Flurin P.H., Wright T., Zuckerman J.D., et al. Rate of improvement in clinical outcomes with anatomic and reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2017;99:1801–1811. doi: 10.2106/JBJS.16.01387. [DOI] [PubMed] [Google Scholar]
- 46.Singh J.A., Sperling J.W., Cofield R.H. Revision surgery following total shoulder arthroplasty: analysis of 2588 shoulders over three decades (1976 to 2008) J Bone Joint Surg Br. 2011;93:1513–1517. doi: 10.1302/0301-620X.93B11.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sperling J.W., Hawkins R.J., Walch G., Mahoney A.P., Zuckerman J.D. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135–141. [PubMed] [Google Scholar]
- 48.Stephens S.P., Spencer E.E., Wirth M.A. Radiographic results of augmented all-polyethylene glenoids in the presence of posterior glenoid bone loss during total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:798–803. doi: 10.1016/j.jse.2016.09.053. [DOI] [PubMed] [Google Scholar]
- 49.Torchia M.E., Cofield R.H., Settergren C.R. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6:495–505. doi: 10.1016/s1058-2746(97)90081-1. [DOI] [PubMed] [Google Scholar]
- 50.Walch G., Ascani C., Boulahia A., Nove-Josserand L., Edwards T.B. Static posterior subluxation of the humeral head: an unrecognized entity responsible for glenohumeral osteoarthritis in the young adult. J Shoulder Elbow Surg. 2002;11:309–314. doi: 10.1067/mse.2002.124547. [DOI] [PubMed] [Google Scholar]
- 51.Walch G., Moraga C., Young A., Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21:1526–1533. doi: 10.1016/j.jse.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 52.Wang T., Abrams G.D., Behn A.W., Lindsey D., Giori N., Cheung E.V. Posterior glenoid wear in total shoulder arthroplasty: eccentric anterior reaming is superior to posterior augment. Clin Orthop Relat Res. 2015;473:3928–3936. doi: 10.1007/s11999-015-4482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wirth M.A., Rockwood C.A., Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78:603–616. doi: 10.2106/00004623-199604000-00018. [DOI] [PubMed] [Google Scholar]
- 54.Youderian A.R., Napolitano L.A.J., Davidson I.U., Iannotti J.P. Management of glenoid bone loss with the use of a New augmented all-polyethylene glenoid component. Tech Shoulder Elbow Surg. 2012;13:163–169. doi: 10.1097/BTE.0b013e318265354d. [DOI] [Google Scholar]