Abstract
Breast cancer is globally the most common invasive cancer in women and remains one of the leading causes of cancer-related deaths. Surgery, radiotherapy, chemotherapy, immunotherapy, and endocrine therapy are currently the main treatments for this cancer type. However, some breast cancer patients are prone to drug resistance related to chemotherapy or immunotherapy, resulting in limited treatment efficacy. Consequently, traditional Chinese medicinal materials (TCMMs) as natural products have become an attractive source of novel drugs. In this review, we summarized the current knowledge on the active components of animal-derived TCMMs, including Ophiocordyceps sinensis-derived cordycepin, the aqueous and ethanolic extracts of O. sinensis, norcantharidin (NCTD), Chansu, bee venom, deer antlers, Ostrea gigas, and scorpion venom, with reference to marked anti-breast cancer effects due to regulating cell cycle arrest, proliferation, apoptosis, metastasis, and drug resistance. In future studies, the underlying mechanisms for the antitumor effects of these components need to be further investigated by utilizing multi-omics technologies. Furthermore, large-scale clinical trials are necessary to validate the efficacy of bioactive constituents alone or in combination with chemotherapeutic drugs for breast cancer treatment.
Keywords: Breast cancer, Traditional Chinese medicinal material (TCMM), Bioactive constituent, Anticancer effect
1. Introduction
As the most common female malignancy worldwide, breast cancer accounts for 32% and 12.8% of female malignancies in incidence and mortality, respectively (DeSantis et al., 2019; Lei et al., 2021; Sung et al., 2021). The optimal therapy for breast cancer depends on the tumor subtype, the cancer stage, and the patient preferences (Waks and Winer, 2019). Besides surgery, radiotherapy, endocrine therapy, chemotherapy, and immunotherapy play vital roles in the management of breast cancer (Hassan et al., 2010; Bhushan et al., 2021). Chemotherapy can be used as a treatment option in almost all stages of breast cancer, either alone or in combination with other therapies to inhibit tumor progression and thus extend patient survival (Waks and Winer, 2019). In comparison, immunotherapy has proven more effective for cases of triple-negative breast cancer (TNBC) (Emens, 2018; Mediratta et al., 2020), an aggressive and lethal subtype of breast cancer, due to the high heterogeneity and the lack of treatment options (Zhu et al., 2021). However, some breast cancer patients are prone to drug-resistance to chemotherapy or immunotherapy, resulting in limited efficacy (Nedeljković and Damjanović, 2019).
Natural products comprise a valuable pool of drug sources for chemotherapy and immunotherapy (Dutta et al., 2019; Deng et al., 2020). For example, more than 60% of the approximately 200 drugs approved by the Food and Drug Administration (FDA) to treat different types of malignant tumors, such as taxol, doxorubicin, and vincasar, are derived from natural ingredients (Demain and Vaishnav, 2011; Cragg and Pezzuto, 2016). Therefore, refining, processing, and standardizing active constituents in natural products is an established approach for the development of novel chemotherapies or immunotherapy drugs.
Traditional Chinese medicinal materials (TCMMs) as natural products are an attractive source of novel drugs. The Shen Nong Ben Cao Jing alone, which is the earliest pharmacological monograph of Chinese medicine written between about 200 and 250 CE, has a record of 365 kinds of Chinese medicinal materials. Among them, numerous active ingredients in Chinese herbal medicines have potential utility for the development of new drugs, with the examples of artemisinin, which exhibits anti-malarial (Adekunle et al., 2016; Guo, 2016) and anti-systemic lupus erythematosus effects (An et al., 2017; Mu and Wang, 2018), and thapsigargin A, which exerts anti-neuroinflammatory effects by acting directly on the inosine monophosphate dehydrogenase type II (IMPDH2) protein (Liao et al., 2017). More recently, Chinese herbal medicines have played an irreplaceable role in the fight against coronavirus disease 2019 (COVID-19) in China (Ni et al., 2020; Xiao et al., 2020; Zhao et al., 2021). These successes suggest that looking into the bioactive components derived from TCMMs may lead to the discovery of novel effective chemotherapeutic or immunotherapeutic agents.
Previous reviews have systematically analyzed and evaluated the anticancer effects of Chinese herbal medicines and their active ingredients (Zhang Y et al., 2020; Wang KL et al., 2021; Wang S et al., 2021). Animal-derived TCMMs are central components of traditional Chinese medicine, and many of them are key agents for well-known compound prescriptions presenting satisfactory efficacy. The active ingredients of animal-derived TCMMs are mainly allogeneic proteins or peptides and vitamins, which showed marked efficacy in treating rheumatoid arthritis, ankylosing spondylitis, cancers, and other diseases (Carpena et al., 2020; Li et al., 2021). However, according to our literature search, no systematic summary of the anti-breast cancer effects or clinical applications has been performed for animal-derived TCMMs.
Therefore, the objective of this review was to systematically summarize and analyze the relevant animal-derived TCMMs along with their active components and anti-breast cancer effects, and to provide inspiration for the development of novel anti-breast cancer chemotherapeutic or immunotherapeutic drugs.
2. Active ingredients of animal-derived TCMMs for breast cancer
2.1. Components of the aqueous and ethanolic extracts of Ophiocordyceps sinensis
Ophiocordyceps sinensis (or Chinese caterpillar fungus), generally called "Dong Chong Xia Cao," is a well-known and valued natural TCMM, which has been used to treat a wide range of diseases for hundreds of years in China and other Asian regions (Chen et al., 2013). O. sinensis is made into a dried composite consisting of the stroma of the fungus (O. sinensis (Berk) Sacc.) parasitized on the larva of some species of insects and dead caterpillars (Chen et al., 2013). O. sinensis is a potential source of novel drugs and is known to have therapeutic effects, including immunomodulatory (Lee et al., 2020), anti-inflammatory (Qi et al., 2020), anti-tumor (Qi et al., 2020), antioxidant (Li et al., 2001), and anti-aging effects (Li XT et al., 2010).
Cordycepin (Kato et al., 2021), found in the aqueous and ethanolic extracts of O. sinensis (Yong et al., 2016; Lin et al., 2021), and O. sinensis polysaccharides (Yang et al., 2021) have been demonstrated to exert the main therapeutic effects of O. sinensis. It is a nucleoside analog (3'-deoxyadenosine), which is considered as the most important bioactive constituent of O. sinensis with significant therapeutic potential (Paterson, 2008; Tuli et al., 2013). Lee et al. (2019) recently found that cordycepin significantly inhibited the cell viability of MCF-7 breast cancer cells, and its half-maximal inhibitory concentration (IC50) value was found to be 9.58 μmol/L. Furthermore, cordycepin's potential antitumor targets and pathways were associated with Sonic hedgehog (Shh) signaling, apoptosis, p53, and estrogen pathways based on network pharmacological analysis (Lee et al., 2019). Some of the available evidence on the anti-breast cancer effects of cordycepin is discussed below. First, it has been demonstrated that cordycepin can cause apoptosis or persistent cell cycle arrest by inhibiting RNA synthesis and DNA double-strand breaks in breast cancer cell lines (Lee et al., 2012). Next, a pro-apoptosis effect from cordycepin was observed in MCF-7 breast cancer cells, which indicated that the apoptotic cell death induced by cordycepin treatment was related to caspase-dependent pathways (Song et al., 2016). In addition, a study demonstrated that cordycepin treatment induced apoptotic cell death in MDA-MB 231 cells, triggered by the release of cytochrome C from mitochondria to the cytosol, leading to caspase activation and poly-(ADP-ribose) polymerase (PARP) cleavage (Kim et al., 2011). Finally, cordycepin caused autophagic cell death in MCF-7 cells irrespective of the estrogen receptor (ER) response, indicating that cordycepin could be used to treat ER-independent human breast cancers (Choi et al., 2011). However, the apoptosis pathway was only confirmed in vitro, and thus cordycepin's MCF-7 cell inhibitory effects that depend on estrogen signaling pathways require further confirmation in vivo.
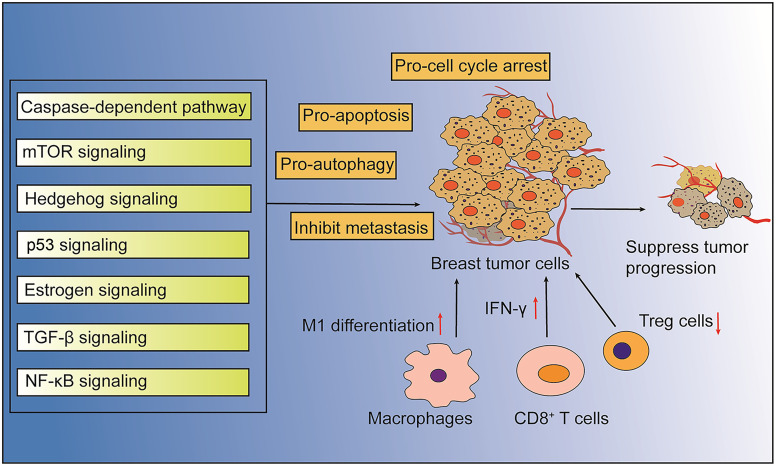
In addition to the pro-apoptosis and pro-autophagic effects of cordycepin on human breast cancers, O. sinensis also possesses immunoregulatory functions. It was reported that the water extracts of O. sinensis significantly inhibited 4T1 cell viability and reduced lung metastasis without affecting body weight in a mouse model of breast cancer metastasis (Cai et al., 2018). Furthermore, metastasis-related cytokines including C-C motif chemokine ligand 17 (CCL17), matrix metalloprotein-9 (MMP-9), osteopontin (OPN), and interleukin-33 (IL-33) were reduced, indicating that the aqueous extracts of O. sinensis could inhibit breast cancer cell metastasis via downregulating metastasis-related cytokines (Cai et al., 2018). However, the exact mechanisms remained elusive. Add‑itionally, the ethanolic extracts of O. sinensis could induce immunogenic and apoptotic cell death in breast cancer (Quan et al., 2020). It is widely accepted that M1 macrophages with pro-inflammatory activity can exert antitumor effects and that M2-polarized macrophages contribute to malignant tumor growth, angiogenesis, and migration (Liu et al., 2021; Mehta et al., 2021). Thus, increasing the proportion of M1 macrophages and inhibiting the M2 phenotypic differentiation of macrophages in the tumor microenvironment could be a promising approach for cancer treatment (Tariq et al., 2017; DeNardo and Ruffell, 2019). Recently, the aqueous extracts of O. sinensis were shown to inhibit TNBC progression by promoting the M1 phenotypic differentiation of macrophages and producing inflammatory cytokines via activating the nuclear factor-κB (NF-κB) signaling pathway in the tumor microenvironment (Li J et al., 2020). However, the aqueous and ethanolic extracts of O. sinensis may contain various active components; therefore, the specific mechanisms of action of individual compon‑ents in these extracts need to be further investigated.
Recent studies have demonstrated that O. sinensis polysaccharides can also inhibit cancer cell growth via inducing apoptosis and cell cycle arrest (Xu J et al., 2021), prompting autophagy flux blockage via the mammalian target of rapamycin (mTOR) signaling (Qi et al., 2020). Jeong et al. (2013) found that cordycepin-enriched Cordyceps militaris effectively reduced the regulatory T cell (Treg) population and increased the number and percentage of interferon-γ (IFN-γ)-expressing cluster of differentiation 8-positive (CD8+) T cells in a breast cancer mice model, which implied that C. militaris could enhance IFN-γ production and effector T cell function to exert antitumor effects. Another mechanistic hypothesis is related to epithelial-mesenchymal transition (EMT), that is, the biological changes of polarized epithelial cells to a mesenchymal cell phenotype, which plays a significant role in cancer metastasis and chemoresistance (Mittal, 2018). In particular, transforming growth factor-β (TGF-β) signaling plays a crucial role in mediating this process (Hao et al., 2019). Lin et al. (2016) found that MHP-1, an isolated polysaccharide from Mortierella hepiali (anamorph of O. sinensis), significantly inhibited breast cancer metastasis in the MDA-MB-231 xenograft mice model and restored sensitivity in topotecan-resistant MCF-7 cells via downregulating TGF-β signaling and EMT. Yang et al. (2015) purified a novel antitumor protein named C. militaris immunoregulatory protein (CMIP) from the fruiting body of C. militaris (Yang et al., 2015). They found that despite showing less ability to kill breast tumor cells, CMIP promoted macrophage proliferation, which indicated that it could activate the immune system. In addition, CMIP inhibited the lung metastasis of 4T1 breast cancer cells and extended the life span of tumor-bearing mice (Yang et al., 2015).
Due to the vast market demand and limited natural resources, the price of natural Chinese Cordyceps has increased exponentially in the last decades in China. Thus, the artificial fermentation product of O. sinensis, namely Hirsutella sinensis fungus (anamorph of C. sinensis), has become a widely used substitute for C. sinensis (Dong et al., 2015). A recent study demonstrated that H. sinensis fungus could inhibit tumor growth in mice with spontaneous polyomavirus middle T antigen (PyMT) breast cancer and reduce orthotopic lung metastasis by promoting the transcription factor Eomes to increase the production of IFN-γ and granzyme B, leading to enhanced cytotoxic capacity among effector T cells. In addition, H. sinensis fungus reduced programmed death 1 (PD-1) expression in T cells and decreased exhausted CD8+ T (Tex) cell formation via inhibiting the transcription factor T-bet (Jin et al., 2021).
In summary, the existing evidence suggests that the bioactive ingredients of O. sinensis exert anti-breast cancer effects via multiple approaches, including apoptosis, autophagy, metastasis, inflammation, and cell cycle signaling (Fig. 1). Therefore, O. sinensis may hold great promise for anti-breast cancer drug discovery. However, further scientific methods such as genomics, transcriptomics, proteomics, and metabonomics should be applied to identify more active constituents associated with the anti-breast cancer effects of this fungus. The wild resources of O. sinensis are decreasing due to global warming and ecological changes in its original area, the Tibetan Plateau, driving up the cost of natural O. sinensis medicinal materials and limiting the performance of related clinical trials. Therefore, manufactured products from O. sinensis are gradually becoming an alternative option due to the increasing demand in the pharmaceutical market (Dong et al., 2015). Thus, the validation of action mechanisms and safety and quality control of the manufactured products from O. sinensis warrant further attention. With the aid of the systems biology research method and multi-omics technology platform, the anti-breast cancer mechanisms of O. sinensis can be studied further, and substances related to the formation and efficacy of O. sinensis may open a path for the artificial cultivation of the species. Additionally, clinical trials investigating the effects of artificial O. sinensis anamorphs of C. sinensis and supplementation therapy involving these anamorphs for breast cancer should also be established.
Fig. 1. Action pathways involved in the anti-tumor effects of bioactive components of Ophiocordyceps sinensis. mTOR: mammalian target of rapamycin; TGF-β: transforming growth factor-β; NF-κB: nuclear factor-κB; IFN-γ: interferon-γ; Treg: regulatory T cell; CD8+: cluster of differentiation 8-positive.
2.2. Chansu
Chansu, also known as toad venom, is a popular but expensive TCMM extracted from the skin or parotid venom glands of Bufo gargarizans Cantor (Chen et al., 2018). It is a Class II wild TCMM under national protection and is one of the 28 species of poisonous herbs that require unique management promulgated by the State Council of China (Zhang and Li, 2004). The chemical components of Chansu are divided into small- and large-molecule compounds (Cao et al., 2019). Thus far, more attention has been focused on the small-molecule compounds, including toad lactones, indole alkaloids, and sterols (Li et al., 2021). However, the qualitative analysis of Chansu has been mainly limited to bufadienolides (a group of the toad lactones, including bufalin, cinobufagin, and resibufogenin), which are cardiotonic steroids isolated from Chansu (Zhang et al., 2005; Qi et al., 2011). Bufadienolides are Na+/K+ ATPase inhibitors that can regulate renal sodium transport and have cardiotonic, anesthetic, and blood pressure stimulation effects (Garcia et al., 2019).
Accumulating evidence suggests that Chansu can inhibit the proliferation and induce the apoptosis of various cancer cells (Qi et al., 2011; Lee et al., 2014; Lan et al., 2019). Among the components isolated from Chansu, bufalin and cinobufagin are the primary agents responsible for its antitumor effects (Zhang JH et al., 2020). One hypothesis regarding its antitumor mechanism is related to the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a critical member of the TNF superfamily that triggers apoptosis upon attachment to death receptors (DRs) on the cell surface via the extrinsic caspase activation cascade (Rowinsky, 2005). TRAIL selectively kills cancer cells without causing damage to normal cells. Thus, it is considered an attractive novel anticancer agent (Yuan et al., 2018; Wong et al., 2019). In terms of breast cancer, Yan et al. (2014b) found that bufalin can enhance TRAIL-induced apoptosis in different breast cancer cells via redistributing DRs in lipid rafts. They further demonstrated that bufalin prompted enhanced TRAIL-induced apoptosis via the upregulation of DR4 and DR5, the activation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38-mitogen-activated protein kinase (MAPK) signaling pathways, and the downregulation of Casitas B-lineage lymphoma-b (Cbl-b) (Yan et al., 2012). Consistently, another study also reported that the inhibition of signal transducer and activator of transcription 3 (STAT3)/myeloid cell leukemia-1 (Mcl-1) pathway contributed to enhanced TRAIL-induced apoptosis in MCF-7 and MDA-MB-231 breast cancer cells by bufalin (Dong et al., 2011). Another hypothesis was proposed by Chen et al. (2020), who demonstrated that bufalin inhibited cell proliferation by inducing G2/M cell cycle arrest, triggering apoptosis in the TNBC cell lines MDA-MB-231 and HCC-1937, and reducing the stemness of TNBC stem cells via suppressing the SRY-box transcription factor 2 (SOX2)/octamer-binding transcription factor 4 (OCT4) signaling pathway (Chen et al., 2020). Furthermore, bufalin can inhibit breast cancer tumorigenesis by inducing cell necroptosis via targeting the reactive oxygen species (ROS)-mediated receptor-interacting protein kinase-1 (RIP1)/RIP3/PARP-1 and RIP1/RIP3/phosphoglycerate mutase family member 5 (PGAM5) pathways (Li YL et al., 2018, 2019). Another hypothesis was related to the steroid receptor coactivator-3 (SRC-3), which is overexpressed in multiple solid tumors. The overexpression of SRC-3 correlates with high levels of epidermal growth factor receptor (EGFR), ER, and human epidermal growth factor receptor 2 (HER2), and thus enhances tamoxifen resistance in human breast cancer (Osborne et al., 2003). Several studies have concluded that bufalin acts as a potent molecule inhibitor of SRC-3 to reduce tumor growth in breast cancer cells and animal models (Wang Y et al., 2014; Song et al., 2015). It has been demonstrated that bufalin can downregulate the activity of the protein kinase B (Akt) pathway to prompt the apoptosis of cancer cells (Yan et al., 2014a). In addition, the combinatorial regimen of bufalin and the histone deacetylase (HDAC) inhibitor suberanilohydroxamic acid inhibited Akt and reduced B-cell lymphoma-2 (Bcl-2) levels, indicating that the combination of HDAC and SRC-3 inhibition might be a novel and promising therapeutic approach for breast cancer (Zou et al., 2016).
Combination treatment with bufalin and chemotherapeutic drugs can synergistically induce the apoptosis of breast cancer cells. Yan et al. (2016b) found that bufalin and cisplatin could synergistically suppress the proliferation and apoptosis of MCF-7 breast cancer cells by downregulating the cisplatin-induced activation of EMT factor and SRC in a dose-dependent manner. Treatment with bufalin at 10 nmol/L plus paclitaxel at 40 nmol/L also increased the apoptotic fraction of cancer cells from 12.5% (paclitaxel alone) to 23.7%, indicating that the combination of bufalin and paclitaxel synergistically suppresses proliferation and induces apoptosis (Yan et al., 2016a). Further analysis showed that bufalin and paclitaxel treatment downregulated the activity of Akt and upregulated the activity of p38, thus inhibiting tumor growth (Yan et al., 2016a).
Cinobufagin is another water-soluble component of B. gargarizans Cantor with various biological effects, such as antineoplastic (Zhang C et al., 2019) and immunomodulatory roles (Xie et al., 2016). Cinobufagin has apparent cytotoxicity in cancer cells, including those of colorectal cancer (Li XW et al., 2019), liver cancer (Jin et al., 2020), and prostate cancer (Yu et al., 2008). Zhu et al. (2018) demonstrated that cinobufagin could induce cell apoptosis and cause G1 phase arrest in MCF-7 breast cancer cells in a dose-dependent manner based on an increase in the Bcl-2-associated X (Bax)/Bcl-2 ratio, indicating that cinobufagin exerts an anti-breast tumor effect via the mitochondrial apoptotic pathway. Similarly, Ma et al. (2012) showed that cinobufagin induced apoptosis and cytotoxicity in MDA-MB-231 cells.
These findings imply that a wide range of signaling pathways correlated with cell proliferation, apoptosis, metastasis, and drug resistance participate in the anti-breast cancer effects of the active components of Chansu. It should be noted that Chansu has a complex chemical composition. However, the current research on its anti-breast cancer effects is mainly performed on bufadienolides; the content ratios of each component in Chansu and their contribution to the overall efficacy are still unknown. In addition, Chansu and bufadienolides as its active ingredients are highly toxic and can cause heart rate disturbance, tissue ischemia, and even hypoxia (Li M et al., 2020). The cardiotoxicity induced by bufadienolides was associated with membrane depolarization, binding to the retinoic acid receptor and γ-aminobutyric acid (GABA) receptor, the positive regulation of nuclear division, the negative regulation of genome replication, and cell cycle changes (Zhang et al., 2018). Therefore, it is necessary to explore the dose threshold for the efficacy and toxicity of this ingredient in depth, and to develop a more rigorous and practical extraction process as well as a quantitative determination method. With the continuous advancement of molecular biology, medicinal chemistry, and drug metabolism research, the active ingredients and toxicity mechanisms of Chansu can be more scientifically and accurately elucidated. Stable and quality-controlled Chansu-derived drugs with low toxicity and high efficiency should be developed to promote more widespread, safer, and feasible applications in clinical practice. In addition, due to the dwindling resources of wild Chansu, its supply cannot meet the rising demand. Meanwhile, it is challenging to discover satisfactory alternatives to Chansu because of its high efficacy and potent bioactive ingredients.
2.3. Norcantharidin
The utility of the Chinese blister beetle and the method of concoction were first recorded in the Shen Nong Ben Cao Jing as early as a thousand years ago. Cantharidin (CTD) is the main active ingredient and toxic component of the Chinese blister beetle. It has been confirmed that CTD is an inhibitor of protein phosphatase 2A (PP2A) (Li W et al., 2010), enabling the effective regulation of cell proliferation and protein transcription (Seshacharyulu et al., 2013). CTD exerts significant inhibitory effects in various cancer types, including gastric cancer (Song et al., 2020), breast cancer (Pan et al., 2019), prostate cancer (Nazim et al., 2020), and lung cancer (Hsia et al., 2014). However, the clinical application of CTD is greatly limited due to various adverse effects, including nausea, vomiting, intense burning sensation in the digestive tract, as well as urinary toxicity such as urinary pain, hematuria, proteinuria, and even renal failure (Liu and Chen, 2009).
Norcantharidin (NCTD, 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic anhydride) can be extracted from CTD or synthesized from furan and maleic anhydride to alleviate the side effects of CTD (Tu et al., 2014). It is a small-molecule synthetic compound with low cytotoxicity and few side effects after the 1,2-methyl groups on the chemical structure of CTD are removed (Dorn et al., 2009; Jiang et al., 2017). In addition, NCTD is an efficacious anticancer drug that has been applied for cancer therapy in China for many years. The underlying mechanisms of NCTD in various cancers have already been discussed in a previous review (Pan et al., 2020). Here, we mainly focus on its effects on breast cancer.
Accumulating evidence indicates that NCTD can inhibit the proliferation and induce the apoptosis of breast cancer cells. Liu et al. (2012) found that NCTD induced dose-dependent DNA damage and reduced the G1 peak in BCaP-37 breast cancer cells, accompanied by increased ROS levels and decreased mitochondrial membrane potential. In addition, NCTD can trigger cell senescence and cell cycle arrest by simultaneously downregulating the Akt and ERK signaling pathways in TNBC (He et al., 2019). Similarly, NCTD at concentrations of 6, 30, and 60 μmol/L inhibited the Akt and NF-κB signaling pathways and reduced the Bcl-2/Bax ratio in a dose-dependent manner to induce cell apoptosis and cell cycle arrest, thus blocking tumor growth (Huang et al., 2009). The inhibition of Wnt/β-catenin (Yin et al., 2019), STAT3, STAT5, ERK1/2, JNK, and p38 (MAPK) by NCTD also contributed to NCTD-induced breast cancer cell apoptosis (Shou et al., 2013). One hypothesis to explain this phenomenon is that both CTD and NCTD can inhibit the ability of MCF-7 breast cancer cells to adhere to platelets through the downregulation of α2 integrin, an adhesion molecule on the surface of cancer cells involved in the interaction between cancer cells and platelets, thus repressing the metastatic potential (Shou et al., 2013). Furthermore, the repression of α2 integrin was observed through the downregulation of the protein kinase C (PKC) pathway (Shou et al., 2013). Huang et al. (2010) also found that NCTD suppressed the cell migration and invasion ability via inhibiting PKC signaling in several human breast cancer lines, including MCF-7, SKBR3, and MDA-MB231. These findings were consistent with a previous observation in pancreatic cancer (Xie et al., 2015). In addition, a growing body of evidence suggests that NCTD can enhance the antitumor effects or alleviate the resistance to chemotherapeutic drugs. Zhang XM et al. (2019) demonstrated that NCTD inhibited cell proliferation and tumor growth by regulating ERα signaling and tamoxifen resistance in breast cancer cells, wherein the microRNA-873 (miR-873)/cyclin-dependent kinase 3 (CDK3) axis mediated the increased tamoxifen mechanism sensitivity and the inhibition of cell proliferation.
Second mitochondria-derived activator of caspase (SMAC) mimetics have emerged as novel anticancer agents due to their potential for inducing cell apoptosis in various types of cancer (Morrish et al., 2020; Zhao et al., 2020). However, resistance to SMAC mimetics was found to be widespread among cancer patients, which limits their more comprehensive clinical applications (Fulda, 2015; Noonan et al., 2016). Recently, it was found that the combination of SMAC mimetics with NCTD could enhance SMAC mimetic Birinapant-mediated apoptosis in breast cancer cells via the downregulation of cellular Fas-associated protein with death domain (FADD)-like IL-1β-converting enzyme (FLICE)-like inhibitory proteins (c-FLIP), leading to the enhancement of caspase-8 activation and cell death (Zhao et al., 2017). In addition, NCTD treatment can reverse the resistance to doxorubicin and vinorelbine through inhibiting Shh and the downstream multidrug resistance 1 (MDR-1)/P-glycoprotein (P-gp) expression in human breast cancer cells (Chen et al., 2012).
In summary, the anti-breast cancer effects of CTD and NCTD can be achieved via multiple targets and pathways, including the inhibition of proliferation, induction of apoptosis and cell cycle arrest, increase of cell sensitivity to chemotherapeutic drugs, and repression of metastasis (Fig. 2). However, other possible anti-breast cancer mechanisms of NCTD, such as anti-angiogenesis and immune regulation, have been less studied. The present evidence indicates that NCTD, a demethylation derivative of CTD, may be a complementary anticancer drug. Therefore, well-designed and large-scale clinical trials investigating the therapeutic effects of NCTD are necessary.
Fig. 2. Action pathways associated with the anti-breast cancer effects of NCTD. NCTD: norcantharidin; STAT: signal transducer and activator of transcription; MAPK: mitogen-activated protein kinase; JNK: c-Jun N-terminal kinase; ERK: extracellular signal-regulated kinase; NF-κB: nuclear factor-κB; Akt: protein kinase B; Bcl-2: B-cell lymphoma-2; Bax: Bcl-2-associated X; ERα: estrogen receptor α; PKC: protein kinase C; Shh: sonic hedgehog; MDR-1: multidrug resistance 1; P-gp: P-glycoprotein; miR-873: microRNA-873; CDK3: cyclin-dependent kinase 3.
2.4. Bee venom
Bee venom is a complex mixture of compounds in an aromatic yellowish transparent venom secreted by the venom glands of worker bees, which has various pharmacological and biological activity (van Vaerenbergh et al., 2013). There is a long history of using bee venom in Chinese medicine to treat rheumat‑ism, rheumatoid arthritis, and other diseases (Son et al., 2007). In recent years, the composition and chemical structure of bee venom have been gradually verified thanks to the advancement of separation and purification technologies. Function and action mechanism studies have revealed that bee venom exhibits potent antitumor effects (Oršolić, 2012). Bee venom contains several biologically active peptides, including melittin, apamin, adolapin, mast cell degranulating peptide, and enzymes (phospholipase A2 and hyaluronidase) as well as non-peptide components such as histamine, dopamine, and norepinephrine (Wehbe et al., 2019).
Melittin is the major peptide component, accounting for nearly 40%‒50% of the total dry weight of bee venom (Ceremuga et al., 2020). A previous review summarized the evidence on the possible mechanisms of bee venom and melittin in various types of cancers (Rady et al., 2017). The anticancer effects were associated with the regulation of angiogenesis and necrosis, cell proliferation, motility, migration, metastasis, invasion, and cell apoptosis in many cancers, including ovarian, prostate, lung, and breast cancers as well as hepatocellular carcinoma, melanoma, leukemia (Rady et al., 2017). Moghaddam et al. (2020) reported that melittin exerted cytotoxic effects on 4T1 breast cancer cells involved in upregulated dynamin-related protein 1 (Drp1) and mitofusin-1 (Mfn1) expression. Another study reported that melittin inhibited tumor motility and invasion in vitro and in vivo by suppressing the phosphatidylinositol-3-kinase (PI3K)/Akt/mTOR signaling pathway and reducing focal adhesion kinase phosphorylation levels via the inhibition of mTOR/p70 ribosomal protein S6 kinase (p70S6K)/eukaryotic initiation factor 4E-binding protein-1 (4E-BP1) pathway (Jeong et al., 2014). Cho et al. (2010) found that melittin can inhibit MMP-9 expression in MCF-7 cells by suppressing NF-κB via the p38 MAPK and JNK signaling pathways, and thus exerts anti-metastatic and anti-invasive effects. Furthermore, melittin can also shape the tumor microenvironment. It has been demonstrated that melittin treatment suppresses hypoxia-inducible factor-1α (HIF-1α) involved in tumor microenvironment formation via the downregulation of NF-κB, leading to the decreased expression of vascular endothelial growth factor A (VEGFA) and lactate dehydrogenase A (LDHA), which participate in angiogenesis and anaerobic respir‑ation, leading to the inhibition of tumor growth. Furthermore, melittin activates the extrinsic and intrinsic apoptotic pathways by upregulating TNF-α and Bax expression to exert pro-apoptosis effects (Mir Hassani et al., 2021). Another possible mechanism is related to IL-2, which holds great potential for tumor immunotherapy. High and low doses of IL-2 lead to apparent systemic adverse effects and less satisfactory antitumor efficacy, respectively (Boyman and Arenas-Ramirez, 2019). Liu et al. (2016) recently reported that the melittin-mutant recombinant IL-2 fusion protein could induce the more vital cytolytic activity of T cells and natural killer (NK) cells, promote the production of IFN-γ, and inhibit lung metastasis in breast cancer. In addition, it was found that bee venom and melittin induced potent cell death in HER2- and EGFR-overexpressing breast cancer cells through interfering with growth factor-dependent receptor tyrosine kinase (RTK) interactions, critical for receptor phosphorylation and the activation of PI3K/Akt signaling (Duffy et al., 2020). The sensitization of breast cancer cells to docetaxel treatment in vivo by melittin involved the downregulation of microtubule-associated protein tau (MAPT) and β-tubulin class III (TUBB3) genes, consistent with previous findings (Wang RP et al., 2014). The potential underlying mechanisms for bee venom and melittin to alleviate cancer cells' resistance to chemotherapeutic agents warrant further investigations.
Bee venom, which exhibits diverse pharmaceutical properties, is an attractive therapeutic agent in China for treating pain, rheumatoid arthritis, atherosclerosis, etc. (Zhang et al., 2018). In addition, its effects on tumors and neurodegenerative diseases have been highlighted (Carpena et al., 2020). However, even though the effects of the main components of bee venom, such as melittin, have been extensively elucidated, the mechanisms of action and metabolic pathways of its other ingredients, including phospholipase A2, mast cell-degranulating peptide, and apamin, are barely known. Therefore, there is still a long way to go before bee venom can be applied for breast cancer therapy. Furthermore, clinical studies investigating the incidence of adverse events, doses, or administration forms have not yet been well established or critically assessed.
2.5. Deer antler
The deer antler, as a periodically regenerable mammalian organ, is composed of cartilage, bone, blood vessels, nerves, and skin tissue (Li, 2012). The process of deer antler regeneration is based on the proliferation and differentiation of stem cells. Deer antlers grow extremely quickly without canceration, and therefore can be used as a biomedical model for regeneration science (Wang et al., 2019). Antler polypeptides and polysaccharides are the main active components of deer antler, which possess the characteristics of neuroprotection, the promotion of reproductive function, the protection of cartilage tissue, the enhancement of immunity, the protection of cardiomyocytes and vascular endothelial cells, the improvement of liver and kidney damage, and anti-inflammation (Huo et al., 2014; Sui et al., 2014). Recently, it was dis‑covered that antler polypeptides and polysaccharides also exhibited antitumor effects. It was reported that antler velvet extract reduced proliferation, inhibited migration, and promoted apoptosis in glioblastoma cell lines, indicating that it may serve as a new therapeutic strategy for glioblastoma treatment (Chonco et al., 2021). Using liquid chromatography-mass spectrometry (LC-MS), Zheng et al. (2020) identified several bioactive polypeptides with potential tumor-suppressing properties from the water-soluble extract of pilose antler, including extracellular matrix repair proteins, intercellular adhesion proteins, and angiogenesis inhibitors. Furthermore, it was demonstrated that pilose antler water-soluble polypeptides inhibited the growth and metastasis of 4T1 breast cancers mainly by activating the cleaved caspase-3, downregulating the expression of platelet-endothelial cell adhesion molecule, suppressing angiogenesis and EMT, inhibiting MMP-2 and MMP-9, and increasing the ratio of cadherin-1/cadherin-2 (Zheng et al., 2020). Similarly, Xu GG et al. (2021) reported that antler extract could inhibit the invasion and EMT of human breast cancer cells in a dose-dependent manner, and this antitumor effect was mediated by the inhibition of NF-κB signaling. Hu et al. (2015) also found that polypeptides from sika antler inhibited rat breast cancer cell proliferation and telomerase activity.
To date, no in-depth studies have been conducted on the effects and mechanisms of action of deer antler or its active components in breast cancer. In China, the clinical use of deer antlers has seen a gradual decline due to the strict animal ethics policy and increased price. The chemical components and standards for the quality control, identification, or pharmacodynamics of deer antler have not been validated, thus limiting its use for cancer therapy. In future work, the identification and screening of the active components of deer antler for their antitumor effects based on fingerprint analysis combined with a quantitative analysis of multi-components and multi-omics may inspire the development of novel antitumor chemotherapeutic agents.
2.6. Others
In addition to the materials mentioned above, a number of other animal-derived TCMMs have been attributed antitumor effects. For instance, oyster is an edible shellfish commonly found in coastal countries worldwide, which is rich in glycogen, taurine, amino acids, B vitamins, polysaccharides, low-molecular active peptides, Fe, Zn, Se, and other trace elements. In China, the use of oysters to treat breast lumps was recorded as early as a millennium ago. Chintalapati et al. (2009) reported that ceramide methylamino‑ethylphosphonate (CMAEPn), isolated from eastern oyster, exerted antitumor effects via anti-angiogenesis, inducing autophagic cell death and inhibiting cell migration as well as the invasion in MCF-7 and MDA-MB-435s breast cancer cell lines. However, no in-depth research has focused on the effects of oyster extract or its active ingredients on breast cancer. In another example, scorpions have been used in China to treat epilepsy, stroke, and physical mobility disorders for a long time. Scorpion venom, extracted from the scorpion, is the main component exhibiting a multitude of biological effects. The scorpion sting can cause pain and may lead to various complications such as hypotension, cardiac arrhythmia, and respiratory distress (Ahmadi et al., 2020), which results in the inhibition of the Na+/K+-ATPase pump, thus paralyzing the sympathetic and parasympathetic nervous systems (Díaz-García and Varela, 2020). On the other hand, despite its neurotoxicity, scorpion venom has beneficial components that may inspire drug discovery (Ortiz et al., 2015). It has been demonstrated that scorpion venom has the potential for inhibiting several cancers, including leukemia (Song et al., 2012), breast cancer (Kampo et al., 2019), and colorectal cancer (Al-Asmari et al., 2018). A previous mini-review discussed potential anticancer peptides from scorpion venom and the action mechanisms of scorpion venom in treating breast cancer (Sarfo-Poku et al., 2016). It was suggested that scorpion venom exhibited anti-breast cancer functions mainly via increasing nitric oxide production, promoting apoptosis, and inhibiting DNA synthesis. A recent study reported that Buthus martensii Karsch antitumor-analgesic peptide, a scorpion venom peptide, can inhibit cancer cell stemness, EMT, migration, and invasion by downregulating pentraxin 3 (PTX3) through the NF-κB and Wnt/β-catenin signaling pathways in breast cancer cells and a xenograft tumor mouse model (Kampo et al., 2019). Additionally, scorpion venom induced cell apoptosis, reduced cell invasion and IL-6 cytokine levels, and downregulated several proteins, including Ras homolog gene family member C (RhoC), ERK1/2, and STAT3, associated with cancer survival in MDA-MB-231 cell lines (Al-Asmari et al., 2018). Although the anti-breast cancer effects of these animal-derived active ingredients have been illustrated, their pharmacological mechanisms remain undiscovered. Moreover, their hepatic or renal toxicity has not been well established.
3. Future perspectives and approaches
As highlighted above, despite the growing volume of scientific evidence suggesting that the bioactive components of these animal-derived TCMMs may possess attractive anti-breast cancer effects both in vivo and in vitro, several issues remain in this area that require urgent consideration. Firstly, studies investigating the mechanisms of anti-breast cancer effects of active ingredients have mainly focused on a single signaling pathway associated with apoptosis, proliferation, metastasis, cell cycle, or drug resistance, without considering associations with other biological processes, which may undermine their conclusions to an extent. The genes, proteins, metabolites, and signaling pathways involved in inhibiting tumor progression should be studied in depth using state-of-the-art bioinformatics analysis and multi-omics technologies, including genomics, transcriptomics, proteomics, and metabolomics, which may broaden the understanding of the mechanisms of action of these components. Secondly, these TCMMs show various levels of toxicity, and the complexity of their composition makes their identification, quantification, and standardization more difficult. Therefore, analytical methods have become indispensable for identifying marker components. In this regard, the fingerprint analysis and quantitative analysis of components using high-performance liquid chromatography (HPLC), mass spectrometry (MS) coupled to electrospray ionization (ESI), or matrix-assisted laser desorption/ionization (MALDI) techniques will increase the current knowledge of these animal-derived TCMMs and promote drug discovery for breast cancer. Besides, combinations with developed chemotherapeutic drugs to reduce drug resistance or produce synergistic antitumor effects may also be considered. Thirdly, drug delivery and transportation systems technologies, such as microspheres (Huang et al., 2019), nanoparticles (Garbayo et al., 2020), liposomes (Vahed et al., 2017), and microemulsions (Callender et al., 2017), also hold great potential for new formulations involving these components. Drug delivery and transportation systems can enhance drug stability, prolong drug action, reduce drug dosage and administration frequency, and promote sustained and slow drug release rate while reducing the side effects (Singh et al., 2017). For example, Tian et al. (2014) designed a co-delivery system comprising bufalin-loaded biotinylated chitosan nanoparticles, which exhibited the sustained and slow release of bufalin, as well as superior intracellular uptake. This drug vehicle system significantly enhanced the cytotoxicity on breast cancer cells compared to native bufalin, with no or few apparent side effects. In a further example, melittin delivered by citric acid-functionalized Fe3O4 magnetic nanoparticles (Hematyar et al., 2018), carbon nanoparticles (Daniluk et al., 2019) and perfluorocarbon nanoparticles (Soman et al., 2009), or an estrone-appended polyion complex micelle exhibited substantially greater antitumor efficacy against several breast cancer cells (Raveendran et al., 2020). Furthermore, an NCTD-loaded RGD (Arg-Gly-Asp)-decorated lipid-polymer hybrid nanoparticle inhibited TNBC tumor growth and metastasis via attenuating the Wnt/β-catenin pathway more effectively than free NCTD (Li YF et al., 2019). These findings highlight that drug delivery and transportation systems possess great potential for the clinical application of these bioactive components derived from TCMMs.
In the future, methods for the screening, identification, and standardization of active compounds with higher efficiency and lower toxicity should be further developed. It should also be noted that the optimization of drug carrier materials to improve the encapsulation and drug delivery rates requires a multidisciplinary collaboration among the areas of Chinese medicine, oncology, pharmacology, toxicology, and material science.
4. Conclusions
In summary, the active components of animal-derived TCMMs, including O. sinensis, NCTD, Chansu, bee venom, deer antler, O. gigas, and scorpion venom, exert marked anti-breast cancer effects that include the regulation of cell cycle arrest, proliferation, apoptosis, metastasis, cell cycle, and drug resistance. In the future, the underlying mechanisms for the antitumor effects of these constituents need to be further investigated by utilizing multi-omics technologies. In addition, the development of appropriate drug delivery and transportation systems to increase the stability of these drugs in vivo, to prolong the duration of drug action, to reduce drug dosage, and to simultaneously alleviate toxic side effects, is also an important direction for further research. Furthermore, large-scale clinical trials are required to validate the efficacy of bioactive constituents alone or in combination with chemotherapeutic drugs in breast cancer treatment.
Acknowledgments
This work was supported by the Integrated Chinese and Western Medicine Key Research Project of the Health Commission of Hubei Province (No. 6, 2017) and the Key Research & Development Project of the Department of Science and Technology of Hubei (No. 2020BCB006), China.
Author contributions
Chaochao YU conceived the main ideas and wrote this paper. Yi LI and Guopeng CHEN helped search the literature. Chaoyan WU and Xiuping WANG illustrated figures. Yingwen ZHANG revised the manuscript. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Chaochao YU, Yi LI, Guopeng CHEN, Chaoyan WU, Xiuping WANG, and Yingwen ZHANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Adekunle AI, Cromer D, Davenport MP, 2016. Artemisinin-based treatments in pregnant women with malaria. N Engl J Med, 375(3): 283-284. 10.1056/NEJMc1604709 [DOI] [PubMed] [Google Scholar]
- Ahmadi S, Knerr JM, Argemi L, et al. , 2020. Scorpion venom: detriments and benefits. Biomedicines, 8(5): 118. 10.3390/biomedicines8050118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Asmari AK, Riyasdeen A, Islam M, 2018. Scorpion venom causes upregulation of p53 and downregulation of Bcl-xL and BID protein expression by modulating signaling proteins Erk1/2 and STAT3, and DNA damage in breast and colorectal cancer cell lines. Integr Cancer Ther, 17(2): 271-281. 10.1177/1534735417704949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Minie M, Sasaki T, et al. , 2017. Antimalarial drugs as immune modulators: new mechanisms for old drugs. Annu Rev Med, 68: 317-330. 10.1146/annurev-med-043015-123453 [DOI] [PubMed] [Google Scholar]
- Bhushan A, Gonsalves A, Menon JU, 2021. Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics, 13(5): 723. 10.3390/pharmaceutics13050723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O, Arenas-Ramirez N, 2019. Development of a novel class of interleukin-2 immunotherapies for metastatic cancer. Swiss Med Wkly, 149: w14697. 10.4414/smw.2019.14697 [DOI] [PubMed] [Google Scholar]
- Cai HW, Li J, Gu BH, et al. , 2018. Extracts of Cordyceps sinensis inhibit breast cancer cell metastasis via down-regulation of metastasis-related cytokines expression. J Ethnopharmacol, 214: 106-112. 10.1016/j.jep.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Callender SP, Mathews JA, Kobernyk K, et al. , 2017. Microemulsion utility in pharmaceuticals: implications for multi-drug delivery. Int J Pharm, 526(1-2): 425-442. 10.1016/j.ijpharm.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Cao YT, Wu JH, Pan HY, et al. , 2019. Chemical profile and multicomponent quantitative analysis for the quality evaluation of toad venom from different origins. Molecules, 24(19): 3595. 10.3390/molecules24193595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpena M, Nuñez-Estevez B, Soria-Lopez A, et al. , 2020. Bee venom: an updating review of its bioactive molecules and its health applications. Nutrients, 12(11): 3360. 10.3390/nu12113360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceremuga M, Stela M, Janik E, et al. , 2020. Melittin—a natural peptide from bee venom which induces apoptosis in human leukaemia cells. Biomolecules, 10(2): 247. 10.3390/biom10020247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Zhu L, Hu JY, et al. , 2020. Bufalin attenuates triple-negative breast cancer cell stemness by inhibiting the expression of SOX2/OCT4. Oncol Lett, 20(5): 171. 10.3892/ol.2020.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PX, Wang SN, Nie SP, et al. , 2013. Properties of Cordyceps sinensis: a review. J Funct Foods, 5(2): 550-569. 10.1016/j.jff.2013.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Kuo CD, Chen SH, et al. , 2012. Small-molecule synthetic compound norcantharidin reverses multi-drug resistance by regulating Sonic hedgehog signaling in human breast cancer cells. PLoS ONE, 7(5): e37006. 10.1371/journal.pone.0037006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Bian XL, Guo FJ, et al. , 2018. Two new 19-norbufadienolides with cardiotonic activity isolated from the venom of Bufo bufo gargarizans . Fitoterapia, 131: 215-220. 10.1016/j.fitote.2018.10.023 [DOI] [PubMed] [Google Scholar]
- Chintalapati M, Truax R, Stout R, et al. , 2009. In vitro and in vivo anti-angiogenic activities and inhibition of hormone-dependent and -independent breast cancer cells by ceramide methylaminoethylphosphonate. J Agric Food Chem, 57(12): 5201-5210. 10.1021/jf803818y [DOI] [PubMed] [Google Scholar]
- Cho HJ, Jeong YJ, Park KK, et al. , 2010. Bee venom suppresses PMA-mediated MMP-9 gene activation via JNK/p38 and NF-κB-dependent mechanisms. J Ethnopharmacol, 127(3): 662-668. 10.1016/j.jep.2009.12.007 [DOI] [PubMed] [Google Scholar]
- Choi S, Lim MH, Kim KM, et al. , 2011. Cordycepin-induced apoptosis and autophagy in breast cancer cells are independent of the estrogen receptor. Toxicol Appl Pharmacol, 257(2): 165-173. 10.1016/j.taap.2011.08.030 [DOI] [PubMed] [Google Scholar]
- Chonco L, Landete-Castillejos T, Serrano-Heras G, et al. , 2021. Anti-tumour activity of deer growing antlers and its potential applications in the treatment of malignant gliomas. Sci Rep, 11: 42. 10.1038/s41598-020-79779-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg GM, Pezzuto JM, 2016. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract, 25(Suppl 2): 41-59. 10.1159/000443404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniluk K, Kutwin M, Grodzik M, et al. , 2019. Use of selected carbon nanoparticles as melittin carriers for MCF-7 and MDA-MB-231 human breast cancer cells. Materials (Basel), 13(1): 90. 10.3390/ma13010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain AL, Vaishnav P, 2011. Natural products for cancer chemotherapy. Microb Biotechnol, 4(6): 687-699. 10.1111/j.1751-7915.2010.00221.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Ruffell B, 2019. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol, 19(6): 369-382. 10.1038/s41577-019-0127-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng LJ, Qi M, Li N, et al. , 2020. Natural products and their derivatives: promising modulators of tumor immunotherapy. J Leukoc Biol, 108(2): 493-508. 10.1002/JLB.3MR0320-444R [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis CE, Ma JM, Gaudet MM, et al. , 2019. Breast cancer statistics, 2019. CA Cancer J Clin, 69(6): 438-451. 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- Díaz-García A, Varela D, 2020. Voltage-gated K+/Na+ channels and scorpion venom toxins in cancer. Front Pharmacol, 11: 913. 10.3389/fphar.2020.00913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CH, Guo SP, Wang WF, et al. , 2015. Cordyceps industry in China. Mycology, 6(2): 121-129. 10.1080/21501203.2015.1043967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YH, Yin ST, Li JH, et al. , 2011. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis, 16(4): 394-403. 10.1007/s10495-011-0573-5 [DOI] [PubMed] [Google Scholar]
- Dorn DC, Kou CA, Png KJ, et al. , 2009. The effect of cantharidins on leukemic stem cells. Int J Cancer, 124(9): 2186-2199. 10.1002/ijc.24157 [DOI] [PubMed] [Google Scholar]
- Duffy C, Sorolla A, Wang E, et al. , 2020. Honeybee venom and melittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. NPJ Precis Oncol, 4: 24. 10.1038/s41698-020-00129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Mahalanobish S, Saha S, et al. , 2019. Natural products: an upcoming therapeutic approach to cancer. Food Chem Toxicol, 128: 240-255. 10.1016/j.fct.2019.04.012 [DOI] [PubMed] [Google Scholar]
- Emens LA, 2018. Breast cancer immunotherapy: facts and hopes. Clin Cancer Res, 24(3): 511-520. 10.1158/1078-0432.Ccr-16-3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, 2015. Promises and challenges of Smac mimetics as cancer therapeutics. Clin Cancer Res, 21(22): 5030-5036. 10.1158/1078-0432.ccr-15-0365 [DOI] [PubMed] [Google Scholar]
- Garbayo E, Pascual-Gil S, Rodríguez-Nogales C, et al. , 2020. Nanomedicine and drug delivery systems in cancer and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 12(5): e1637. 10.1002/wnan.1637 [DOI] [PubMed] [Google Scholar]
- Garcia IJP, de Oliveira GC, de Moura Valadares JM, et al. , 2019. New bufadienolides extracted from Rhinella marina inhibit Na, K-ATPase and induce apoptosis by activating caspases 3 and 9 in human breast and ovarian cancer cells. Steroids, 152: 108490. 10.1016/j.steroids.2019.108490 [DOI] [PubMed] [Google Scholar]
- Guo ZR, 2016. Artemisinin anti-malarial drugs in China. Acta Pharm Sin B, 6(2): 115-124. 10.1016/j.apsb.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Baker D, Ten Dijke P, 2019. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci, 20(11): 2767. 10.3390/ijms20112767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MSU, Ansari J, Spooner D, et al. , 2010. Chemotherapy for breast cancer (Review). Oncol Rep, 24(5): 1121-1131. 10.3892/or_00000963 [DOI] [PubMed] [Google Scholar]
- He Q, Xue SY, Tan YQ, et al. , 2019. Dual inhibition of Akt and ERK signaling induces cell senescence in triple-negative breast cancer. Cancer Lett, 448: 94-104. 10.1016/j.canlet.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Hematyar M, Soleimani M, Es-Haghi A, et al. , 2018. Synergistic co-delivery of doxorubicin and melittin using functionalized magnetic nanoparticles for cancer treatment: loading and in vitro release study by LC-MS/MS. Artif Cells Nanomed Biotechnol, 46(sup3): S1226-S1235. 10.1080/21691401.2018.1536063 [DOI] [PubMed] [Google Scholar]
- Hsia TC, Yu CC, Hsu SC, et al. , 2014. Cantharidin induces apoptosis of H460 human lung cancer cells through mitochondria-dependent pathways. Int J Oncol, 45(1): 245-254. 10.3892/ijo.2014.2428 [DOI] [PubMed] [Google Scholar]
- Hu W, Qi L, Tian YH, et al. , 2015. Studies on the purification of polypeptide from sika antler plate and activities of antitumor. BMC Complement Altern Med, 15: 328. 10.1186/s12906-015-0845-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PS, Wang XL, Liang XY, et al. , 2019. Nano-, micro-, and macroscale drug delivery systems for cancer immunotherapy. Acta Biomater, 85: 1-26. 10.1016/j.actbio.2018.12.028 [DOI] [PubMed] [Google Scholar]
- Huang SY, Yao YD, Chen LL, et al. , 2010. Anti-invasive and anti-metastasis effect of norcantharidin on high-metastatic human breast cancer cell lines. J Trop Med, 10(9): 1034-1038 (in Chinese). [Google Scholar]
- Huang Y, Liu Q, Liu K, et al. , 2009. Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology, 59(3): 201-208. 10.1007/s10616-009-9210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo YS, Huo H, Zhang J, 2014. The contribution of deer velvet antler research to the modern biological medicine. Chin J Integr Med, 20(10): 723-728. 10.1007/s11655-014-1827-1 [DOI] [PubMed] [Google Scholar]
- Jeong MH, Lee CM, Lee SW, et al. , 2013. Cordycepin-enriched Cordyceps militaris induces immunomodulation and tumor growth delay in mouse-derived breast cancer. Oncol Rep, 30(4): 1996-2002. 10.3892/or.2013.2660 [DOI] [PubMed] [Google Scholar]
- Jeong YJ, Choi Y, Shin JM, et al. , 2014. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem Toxicol, 68: 218-225. 10.1016/j.fct.2014.03.022 [DOI] [PubMed] [Google Scholar]
- Jiang ZW, Chi JH, Han BQ, et al. , 2017. Preparation and pharmacological evaluation of norcantharidin-conjugated carboxymethyl chitosan in mice bearing hepatocellular carcinoma. Carbohydr Polym, 174: 282-290. 10.1016/j.carbpol.2017.06.072 [DOI] [PubMed] [Google Scholar]
- Jin L, Jin LS, Wu RJ, et al. , 2021. Hirsutella sinensis fungus regulates CD8+ T cell exhaustion through involvement of T-bet/Eomes in the tumor microenvironment. Front Pharmacol, 11: 612620. 10.3389/fphar.2020.612620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin XH, Wang JB, Zou S, et al. , 2020. Cinobufagin triggers defects in spindle formation and cap-dependent translation in liver cancer cells by inhibiting the AURKA-mTOR-eIF4E axis. Am J Chin Med, 48(3): 651-678. 10.1142/s0192415x20500330 [DOI] [PubMed] [Google Scholar]
- Kampo S, Ahmmed B, Zhou TT, et al. , 2019. Scorpion venom analgesic peptide, BmK AGAP inhibits stemness, and epithelial-mesenchymal transition by down-regulating PTX3 in breast cancer. Front Oncol, 9: 21. 10.3389/fonc.2019.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Nishimura K, Suparmin A, et al. , 2021. Effects of cordycepin in Cordyceps militaris during its infection to silkworm larvae. Microorganisms, 9(4): 681. 10.3390/microorganisms9040681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Seo HS, Choi HS, et al. , 2011. Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells. Arch Pharm Res, 34(8): 1363-1372. 10.1007/s12272-011-0817-5 [DOI] [PubMed] [Google Scholar]
- Lan YL, Lou JC, Jiang XW, et al. , 2019. A research update on the anticancer effects of bufalin and its derivatives (Review). Oncol Lett, 17(4): 3635-3640. 10.3892/ol.2019.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Huang KS, Shaw JF, et al. , 2020. Trends in the immunomodulatory effects of Cordyceps militaris: total extracts, polysaccharides and cordycepin. Front Pharmacol, 11: 575704. 10.3389/fphar.2020.575704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Lee WY, Jung K, et al. , 2019. The inhibitory effect of cordycepin on the proliferation of MCF-7 breast cancer cells, and its mechanism: an investigation using network pharmacology-based analysis. Biomolecules, 9(9): 414. 10.3390/biom9090414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Burger P, Vogel M, et al. , 2012. The nucleoside antagonist cordycepin causes DNA double strand breaks in breast cancer cells. Invest New Drugs, 30(5): 1917-1925. 10.1007/s10637-012-9859-x [DOI] [PubMed] [Google Scholar]
- Lee S, Lee Y, Choi YJ, et al. , 2014. Cyto-/genotoxic effects of the ethanol extract of Chan Su, a traditional Chinese medicine, in human cancer cell lines. J Ethnopharmacol, 152(2): 372-376. 10.1016/j.jep.2014.01.023 [DOI] [PubMed] [Google Scholar]
- Lei SY, Zheng RS, Zhang SW, et al. , 2021. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond), 41(11): 1183-1194. 10.1002/cac2.12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, 2012. Deer antler regeneration: a stem cell-based epimorphic process. Birth Defects Res C Embryo Today, 96(1): 51-62. 10.1002/bdrc.21000 [DOI] [PubMed] [Google Scholar]
- Li FJ, Hu JH, Ren X, et al. , 2021. Toad venom: a comprehensive review of chemical constituents, anticancer activities, and mechanisms. Arch Pharm (Weinheim), 354(7): 2100060. 10.1002/ardp.202100060 [DOI] [PubMed] [Google Scholar]
- Li J, Cai HW, Sun HH, et al. , 2020. Extracts of Cordyceps sinensis inhibit breast cancer growth through promoting M1 macrophage polarization via NF-κB pathway activation. J Ethnopharmacol, 260: 112969. 10.1016/j.jep.2020.112969 [DOI] [PubMed] [Google Scholar]
- Li M, Wang XJ, Zhao Q, et al. , 2020. Bufalin-induced cardiotoxicity: new findings into mechanisms. Chin J Nat Med, 18(7): 550-560. 10.1016/s1875-5364(20)30065-0 [DOI] [PubMed] [Google Scholar]
- Li SP, Li P, Dong TTX, et al. , 2001. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia. Phytomedicine, 8(3): 207-212. 10.1078/0944-7113-00030 [DOI] [PubMed] [Google Scholar]
- Li W, Xie L, Chen Z, et al. , 2010. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Sci, 101(5): 1226-1233. 10.1111/j.1349-7006.2010.01523.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XT, Li HC, Li CB, et al. , 2010. Protective effects on mitochondria and anti-aging activity of polysaccharides from cultivated fruiting bodies of Cordyceps militaris . Am J Chin Med, 38(6): 1093-1106. 10.1142/s0192415x10008494 [DOI] [PubMed] [Google Scholar]
- Li XW, Chen CH, Dai Y, et al. , 2019. Cinobufagin suppresses colorectal cancer angiogenesis by disrupting the endothelial mammalian target of rapamycin/hypoxia-inducible factor 1α axis. Cancer Sci, 110(5): 1724-1734. 10.1111/cas.13988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Xiao YJ, Lin HP, et al. , 2019. In vivo β-catenin attenuation by the integrin α5-targeting nano-delivery strategy suppresses triple negative breast cancer stemness and metastasis. Biomaterials, 188: 160-172. 10.1016/j.biomaterials.2018.10.019 [DOI] [PubMed] [Google Scholar]
- Li YL, Tian X, Liu XD, et al. , 2018. Bufalin inhibits human breast cancer tumorigenesis by inducing cell death through the ROS-mediated RIP1/RIP3/PARP-1 pathways. Carcinogenesis, 39(5): 700-707. 10.1093/carcin/bgy039 [DOI] [PubMed] [Google Scholar]
- Li YL, Gong PC, Kong CC, et al. , 2019. Bufalin engages in RIP1-dependent and ROS-dependent programmed necroptosis in breast cancer cells by targeting the RIP1/RIP3/PGAM5 pathway. Anticancer Drugs, 30(7): 706-713. 10.1097/cad.0000000000000770 [DOI] [PubMed] [Google Scholar]
- Liao LX, Song XM, Wang LC, et al. , 2017. Highly selective inhibition of IMPDH2 provides the basis of antineuroinflammation therapy. Proc Natl Acad Sci USA, 114(29): E5986-E5994. 10.1073/pnas.1706778114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SS, Lyu XD, Yu J, et al. , 2016. MHP-1 inhibits cancer metastasis and restores topotecan sensitivity via regulating epithelial-mesenchymal transition and TGF-β signaling in human breast cancer cells. Phytomedicine, 23(10): 1053-1063. 10.1016/j.phymed.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Lin YE, Chen YC, Lu KH, et al. , 2021. Antidepressant-like effects of water extract of Cordyceps militaris (Linn. ) Link by modulation of ROCK2/PTEN/Akt signaling in an unpredictable chronic mild stress-induced animal model. J Ethnopharmacol, 276: 114194. 10.1016/j.jep.2021.114194 [DOI] [PubMed] [Google Scholar]
- Liu DW, Chen ZW, 2009. The effects of cantharidin and cantharidin derivates on tumour cells. Anticancer Agents Med Chem, 9(4): 392-396. 10.2174/1871520610909040392 [DOI] [PubMed] [Google Scholar]
- Liu DW, Shi PG, Yin X, et al. , 2012. Effect of norcantharidin on the human breast cancer Bcap-37 cells. Connect Tissue Res, 53(6): 508-512. 10.3109/03008207.2012.694928 [DOI] [PubMed] [Google Scholar]
- Liu JY, Geng XF, Hou JX, et al. , 2021. New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int, 21: 389. 10.1186/s12935-021-02089-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MJ, Wang HT, Liu LJ, et al. , 2016. Melittin-MIL-2 fusion protein as a candidate for cancer immunotherapy. J Transl Med, 14: 155. 10.1186/s12967-016-0910-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LN, Song B, Jin H, et al. , 2012. Cinobufacini induced MDA-MB-231 cell apoptosis-associated cell cycle arrest and cytoskeleton function. Bioorg Med Chem Lett, 22(3): 1459-1463. 10.1016/j.bmcl.2011.11.095 [DOI] [PubMed] [Google Scholar]
- Mediratta K, El-Sahli S, D'Costa V, et al. , 2020. Current progresses and challenges of immunotherapy in triple-negative breast cancer. Cancers (Basel), 12(12): 3529. 10.3390/cancers12123529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AK, Kadel S, Townsend MG, et al. , 2021. Macrophage biology and mechanisms of immune suppression in breast cancer. Front Immunol, 12: 643771. 10.3389/fimmu.2021.643771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir Hassani Z, Nabiuni M, Parivar K, et al. , 2021. Melittin inhibits the expression of key genes involved in tumor microenvironment formation by suppressing HIF-1α signaling in breast cancer cells. Med Oncol, 38(7): 77. 10.1007/s12032-021-01526-6 [DOI] [PubMed] [Google Scholar]
- Mittal V, 2018. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol, 13: 395-412. 10.1146/annurev-pathol-020117-043854 [DOI] [PubMed] [Google Scholar]
- Moghaddam FD, Mortazavi P, Hamedi S, et al. , 2020. Apoptotic effects of melittin on 4T1 breast cancer cell line is associated with up regulation of Mfn1 and Drp1 mRNA expression. Anticancer Agents Med Chem, 20(7): 790-799. 10.2174/1871520620666200211091451 [DOI] [PubMed] [Google Scholar]
- Morrish E, Brumatti G, Silke J, 2020. Future therapeutic directions for Smac-mimetics. Cells, 9(2): 406. 10.3390/cells9020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu XZ, Wang CC, 2018. Artemisinins—a promising new treatment for systemic lupus erythematosus: a descriptive review. Curr Rheumatol Rep, 20(9): 55. 10.1007/s11926-018-0764-y [DOI] [PubMed] [Google Scholar]
- Nazim UM, Yin HH, Park SY, 2020. Downregulation of c-FLIP and upregulation of DR-5 by cantharidin sensitizes TRAIL‑mediated apoptosis in prostate cancer cells via autophagy flux. Int J Mol Med, 46(1): 280-288. 10.3892/ijmm.2020.4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeljković M, Damjanović A, 2019. Mechanisms of chemotherapy resistance in triple-negative breast cancer—how we can rise to the challenge. Cells, 8(9): 957. 10.3390/cells8090957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni LQ, Chen LL, Huang X, et al. , 2020. Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm Sin B, 10(7): 1149-1162. 10.1016/j.apsb.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan AM, Bunch KP, Chen JQ, et al. , 2016. Pharmacodynamic markers and clinical results from the phase 2 study of the SMAC mimetic birinapant in women with relapsed platinum-resistant or -refractory epithelial ovarian cancer. Cancer, 122(4): 588-597. 10.1002/cncr.29783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oršolić N, 2012. Bee venom in cancer therapy. Cancer Metastasis Rev, 31(1-2): 173-194. 10.1007/s10555-011-9339-3 [DOI] [PubMed] [Google Scholar]
- Ortiz E, Gurrola GB, Schwartz EF, et al. , 2015. Scorpion venom components as potential candidates for drug development. Toxicon, 93: 125-135. 10.1016/j.toxicon.2014.11.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, et al. , 2003. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst, 95(5): 353-361. 10.1093/jnci/95.5.353 [DOI] [PubMed] [Google Scholar]
- Pan MS, Cao J, Fan YZ, 2020. Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. Chin Med, 15: 55. 10.1186/s13020-020-00338-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YH, Zheng Q, Ni WT, et al. , 2019. Breaking glucose transporter 1/pyruvate kinase M2 glycolytic loop is required for cantharidin inhibition of metastasis in highly metastatic breast cancer. Front Pharmacol, 10: 590. 10.3389/fphar.2019.00590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson RRM, 2008. Cordyceps—a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry, 69(7): 1469-1495. 10.1016/j.phytochem.2008.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi FH, Li AY, Inagaki Y, et al. , 2011. Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int Immunopharmacol, 11(3): 342-349. 10.1016/j.intimp.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Qi WC, Zhou XT, Wang JQ, et al. , 2020. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr Polym, 237: 116113. 10.1016/j.carbpol.2020.116113 [DOI] [PubMed] [Google Scholar]
- Quan XG, Kwak BS, Lee JY, et al. , 2020. Cordyceps militaris induces immunogenic cell death and enhances antitumor immunogenic response in breast cancer. Evid Based Complement Alternat Med, 2020: 9053274. 10.1155/2020/9053274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rady I, Siddiqui IA, Rady M, et al. , 2017. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett, 402: 16-31. 10.1016/j.canlet.2017.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveendran R, Chen F, Kent B, et al. , 2020. Estrone-decorated polyion complex micelles for targeted melittin delivery to hormone-responsive breast cancer cells. Biomacromolecules, 21(3): 1222-1233. 10.1021/acs.biomac.9b01681 [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, 2005. Targeted induction of apoptosis in cancer management: the emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J Clin Oncol, 23(36): 9394-9407. 10.1200/jco.2005.02.2889 [DOI] [PubMed] [Google Scholar]
- Sarfo-Poku C, Eshun O, Lee KH, 2016. Medical application of scorpion venom to breast cancer: a mini-review. Toxicon, 122: 109-112. 10.1016/j.toxicon.2016.09.005 [DOI] [PubMed] [Google Scholar]
- Seshacharyulu P, Pandey P, Datta K, et al. , 2013. Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett, 335(1): 9-18. 10.1016/j.canlet.2013.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou LM, Zhang QY, Li W, et al. , 2013. Cantharidin and norcantharidin inhibit the ability of MCF-7 cells to adhere to platelets via protein kinase C pathway-dependent downregulation of α2 integrin. Oncol Rep, 30(3): 1059-1066. 10.3892/or.2013.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Singh S, Lillard JW, et al. , 2017. Drug delivery approaches for breast cancer. Int J Nanomedicine, 12: 6205-6218. 10.2147/IJN.S140325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soman NR, Baldwin SL, Hu G, et al. , 2009. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest, 119(9): 2830-2842. 10.1172/JCI38842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son DJ, Lee JW, Lee YH, et al. , 2007. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther, 115(2): 246-270. 10.1016/j.pharmthera.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Song JJ, Wang YW, Teng MY, et al. , 2016. Cordyceps militaris induces tumor cell death via the caspase-dependent mitochondrial pathway in HepG2 and MCF-7 cells. Mol Med Rep, 13(6): 5132-5140. 10.3892/mmr.2016.5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MY, Wang XF, Luo YJ, et al. , 2020. Cantharidin suppresses gastric cancer cell migration/invasion by inhibiting the PI3K/Akt signaling pathway via CCAT1. Chem Biol Interact, 317: 108939. 10.1016/j.cbi.2020.108939 [DOI] [PubMed] [Google Scholar]
- Song XF, Zhang GJ, Sun AP, et al. , 2012. Scorpion venom component III inhibits cell proliferation by modulating NF-κB activation in human leukemia cells. Exp Ther Med, 4(1): 146-150. 10.3892/etm.2012.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XZ, Zhang CW, Zhao MK, et al. , 2015. Steroid receptor coactivator-3 (SRC-3/AIB1) as a novel therapeutic target in triple negative breast cancer and its inhibition with a phospho-bufalin prodrug. PLoS ONE, 10(10): e0140011. 10.1371/journal.pone.0140011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui ZG, Zhang LH, Huo YS, et al. , 2014. Bioactive components of velvet antlers and their pharmacological properties. J Pharm Biomed Anal, 87: 229-240. 10.1016/j.jpba.2013.07.044 [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. , 2021. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 71(3): 209-249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Tariq M, Zhang JQ, Liang GK, et al. , 2017. Macrophage polarization: anti-cancer strategies to target tumor-associated macrophage in breast cancer. J Cell Biochem, 118(9): 2484-2501. 10.1002/jcb.25895 [DOI] [PubMed] [Google Scholar]
- Tian X, Yin HZ, Zhang SC, et al. , 2014. Bufalin loaded biotinylated chitosan nanoparticles: an efficient drug delivery system for targeted chemotherapy against breast carcinoma. Eur J Pharm Biopharm, 87(3): 445-453. 10.1016/j.ejpb.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Tu GG, Zhan JF, Lv QL, et al. , 2014. Synthesis and antiproliferative assay of norcantharidin derivatives in cancer cells. Med Chem, 10(4): 376-381. 10.2174/15734064113099990037 [DOI] [PubMed] [Google Scholar]
- Tuli HS, Sharma AK, Sandhu SS, et al. , 2013. Cordycepin: a bioactive metabolite with therapeutic potential. Life Sci, 93(23): 863-869. 10.1016/j.lfs.2013.09.030 [DOI] [PubMed] [Google Scholar]
- Vahed SZ, Salehi R, Davaran S, et al. , 2017. Liposome-based drug co-delivery systems in cancer cells. Mater Sci Eng C Mater Biol Appl, 71: 1327-1341. 10.1016/j.msec.2016.11.073 [DOI] [PubMed] [Google Scholar]
- van Vaerenbergh M, Cardoen D, Formesyn EM, et al. , 2013. Extending the honey bee venome with the antimicrobial peptide apidaecin and a protein resembling wasp antigen 5. Insect Mol Biol, 22(2): 199-210. 10.1111/imb.12013 [DOI] [PubMed] [Google Scholar]
- Waks AG, Winer EP, 2019. Breast cancer treatment: a review. JAMA, 321(3): 288-300. 10.1001/jama.2018.19323 [DOI] [PubMed] [Google Scholar]
- Wang DT, Berg D, Ba HX, et al. , 2019. Deer antler stem cells are a novel type of cells that sustain full regeneration of a mammalian organ—deer antler. Cell Death Dis, 10(6): 443. 10.1038/s41419-019-1686-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KL, Chen Q, Shao YY, et al. , 2021. Anticancer activ [Google Scholar]
- ities of TCM and their active components against tumor metastasis. Biomed Pharmacother, 133: 111044. 10.1016/j.biopha.2020.111044 [DOI] [PubMed] [Google Scholar]
- Wang RP, Huang SR, Zhou JY, et al. , 2014. Synergistic interaction between melittin and chemotherapeutic agents and their possible mechanisms: an experimental research. Chin J Integr Tradit Western Med, 34(2): 224-229 (in Chinese). 10.7661/CJIM.2014.02.0224 [DOI] [PubMed] [Google Scholar]
- Wang S, Fu JL, Hao HF, et al. , 2021. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol Res, 170: 105728. 10.1016/j.phrs.2021.105728 [DOI] [PubMed] [Google Scholar]
- Wang Y, Lonard DM, Yu Y, et al. , 2014. Bufalin is a potent small-molecule inhibitor of the steroid receptor coactivators SRC-3 and SRC-1. Cancer Res, 74(5): 1506-1517. 10.1158/0008-5472.CAN-13-2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehbe R, Frangieh J, Rima M, et al. , 2019. Bee venom: overview of main compounds and bioactivities for therapeutic interests. Molecules, 24(16): 2997. 10.3390/molecules24162997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SHM, Kong WY, Fang CM, et al. , 2019. The TRAIL to cancer therapy: hindrances and potential solutions. Crit Rev Oncol Hematol, 143: 81-94. 10.1016/j.critrevonc.2019.08.008 [DOI] [PubMed] [Google Scholar]
- Xiao MZ, Tian JX, Zhou YN, et al. , 2020. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res, 161: 105126. 10.1016/j.phrs.2020.105126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie SS, Spelmink L, Codemo M, et al. , 2016. Cinobufagin modulates human innate immune responses and triggers antibacterial activity. PLoS ONE, 11(8): e0160734. 10.1371/journal.pone.0160734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wu MY, Shou LM, et al. , 2015. Tamoxifen enhances the anticancer effect of cantharidin and norcantharidin in pancreatic cancer cell lines through inhibition of the protein kinase C signaling pathway. Oncol Lett, 9(2): 837-844. 10.3892/ol.2014.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GG, Zhao HP, Xu JD, et al. , 2021. Hard antler extract inhibits invasion and epithelial-mesenchymal transition of triple-negative and Her-2+ breast cancer cells by attenuating nuclear factor-κB signaling. J Ethnopharmacol, 269: 113705. 10.1016/j.jep.2020.113705 [DOI] [PubMed] [Google Scholar]
- Xu J, Tan ZC, Shen ZY, et al. , 2021. Cordyceps cicadae polysaccharides inhibit human cervical cancer hela cells proliferation via apoptosis and cell cycle arrest. Food Chem Toxicol, 148: 111971. 10.1016/j.fct.2021.111971 [DOI] [PubMed] [Google Scholar]
- Yan SC, Qu XJ, Xu CA, et al. , 2012. Down-regulation of Cbl-b by bufalin results in up-regulation of DR4/DR5 and sensitization of TRAIL-induced apoptosis in breast cancer cells. J Cancer Res Clin Oncol, 138(8): 1279-1289. 10.1007/s00432-012-1204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SC, Liu YP, Qu XJ, et al. , 2014a. Bufalin induced apoptosis of MCF-7 breast cancer cells by down-regulating the activity of AKT. J China Med Univ, 43(7): 598-601, 611 (in Chinese). 10.3969/j.issn.0258-4646.2014.07.006 [DOI] [Google Scholar]
- Yan SC, Qu XJ, Xu L, et al. , 2014b. Bufalin enhances TRAIL-induced apoptosis by redistributing death receptors in lipid rafts in breast cancer cells. Anticancer Drugs, 25(6): 683-689. 10.1097/cad.0000000000000095 [DOI] [PubMed] [Google Scholar]
- Yan SC, Jiao X, Li K, et al. , 2016a. The mechanism of bufalin and paclitaxel synergistically suppress the proliferation of breast cancer cells. J Mod Oncol, 24(20): 3173-3176 (in Chinese). 10.3969/j.issn.1672-4992.2016.20.001 [DOI] [Google Scholar]
- Yan SC, Jiao X, Li K, et al. , 2016b. The mechanism of bufalin and cisplatin synergistically suppress the proliferation of breast cancer MCF-7 cells. Chin Clin Oncol, 21(12): 1057-1062 (in Chinese). [Google Scholar]
- Yang Q, Yin YL, Yu GJ, et al. , 2015. A novel protein with anti-metastasis activity on 4T1 carcinoma from medicinal fungus Cordyceps militaris . Int J Biol Macromol, 80: 385-391. 10.1016/j.ijbiomac.2015.06.050 [DOI] [PubMed] [Google Scholar]
- Yang XQ, Lin P, Wang J, et al. , 2021. Purification, characterization and anti-atherosclerotic effects of the polysaccharides from the fruiting body of Cordyceps militaris . Int J Biol Macromol, 181: 890-904. 10.1016/j.ijbiomac.2021.04.083 [DOI] [PubMed] [Google Scholar]
- Yin SL, Zhang H, Jia LN, et al. , 2019. Apoptosis in breast cancer cells MCF-7 induced by norcantharidin through the Wnt/β-catenin signaling pathway. J Shenyang Med Coll, 21(6): 500-504 (in Chinese). 10.16753/j.cnki.1008-2344.2019.06.003 [DOI] [Google Scholar]
- Yong TQ, Zhang ML, Chen DL, et al. , 2016. Actions of water extract from Cordyceps militaris in hyperuricemic mice induced by potassium oxonate combined with hypoxanthine. J Ethnopharmacol, 194: 403-411. 10.1016/j.jep.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Yu CH, Kan SF, Pu HF, et al. , 2008. Apoptotic signaling in bufalin- and cinobufagin-treated androgen-dependent and -independent human prostate cancer cells. Cancer Sci, 99(12): 2467-2476. 10.1111/j.1349-7006.2008.00966.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Gajan A, Chu Q, et al. , 2018. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev, 37(4): 733-748. 10.1007/s10555-018-9728-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ma K, Li WY, 2019. Cinobufagin suppresses the characteristics of osteosarcoma cancer cells by inhibiting the IL-6-OPN-STAT3 pathway. Drug Des Devel Ther, 13: 4075-4090. 10.2147/dddt.s224312 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang ED, Li B, 2004. Resources of Chinese Materia Medica and Conservation of Endangered Wild Animals and Plants. Shanghai Pujiang Education Press, Shanghai, China: (in Chinese). [Google Scholar]
- Zhang JH, Hong YJ, Xie PS, et al. , 2020. Spatial lipidomics reveals anticancer mechanisms of bufalin in combination with cinobufagin in tumor-bearing mice. Front Pharmacol, 11: 593815. 10.3389/fphar.2020.593815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Cui Z, Liu YS, et al. , 2005. Quality evaluation of traditional Chinese drug toad venom from different origins through a simultaneous determination of bufogenins and indole alkaloids by HPLC. Chem Pharm Bull (Tokyo), 53(12): 1582-1586. 10.1248/cpb.53.1582 [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Ye Y, et al. , 2018. Bee venom therapy: potential mechanisms and therapeutic applications. Toxicon, 148: 64-73. 10.1016/j.toxicon.2018.04.012 [DOI] [PubMed] [Google Scholar]
- Zhang XM, Zhang BF, Zhang PH, et al. , 2019. Norcantharidin regulates ERα signaling and tamoxifen resistance via targeting miR-873/CDK3 in breast cancer cells. PLoS ONE, 14(5): e0217181. 10.1371/journal.pone.0217181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lou YN, Wang JB, et al. , 2020. Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Front Immunol, 11: 609705. 10.3389/fimmu.2020.609705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Yang GS, Bai H, et al. , 2017. NCTD promotes Birinapant-mediated anticancer activity in breast cancer cells by downregulation of c-FLIP. Oncotarget, 8(16): 26886-26895. 10.18632/oncotarget.15848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XY, Wang XY, Wei QY, et al. , 2020. Potency and selectivity of SMAC/DIABLO mimetics in solid tumor therapy. Cells, 9(4): 1012. 10.3390/cells9041012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZY, Li YD, Zhou LY, et al. , 2021. Prevention and treatment of COVID-19 using traditional Chinese medicine: a review. Phytomedicine, 85: 153308. 10.1016/j.phymed.2020.153308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng KX, Li QL, Lin DD, et al. , 2020. Peptidomic analysis of pilose antler and its inhibitory effect on triple-negative breast cancer at multiple sites. Food Funct, 11(9): 7481-7494. 10.1039/D0FO01531H [DOI] [PubMed] [Google Scholar]
- Zhu L, Chen YX, Wei C, et al. , 2018. Anti-proliferative and pro-apoptotic effects of cinobufagin on human breast cancer MCF-7 cells and its molecular mechanism. Nat Prod Res, 32(4): 493-497. 10.1080/14786419.2017.1315575 [DOI] [PubMed] [Google Scholar]
- Zhu YX, Zhu XD, Tang CJ, et al. , 2021. Progress and challenges of immunotherapy in triple-negative breast cancer. Biochim Biophys Acta Rev Cancer, 1876(2): 188593. 10.1016/j.bbcan.2021.188593 [DOI] [PubMed] [Google Scholar]
- Zou ZZ, Luo XY, Nie PP, et al. , 2016. Inhibition of SRC-3 enhances sensitivity of human cancer cells to histone deacetylase inhibitors. Biochem Biophys Res Commun, 478(1): 227-233. 10.1016/j.bbrc.2016.07.063 [DOI] [PubMed] [Google Scholar]