This randomized clinical trial evaluates the efficacy and safety of theophylline added to saline nasal irrigation compared with placebo for COVID-19–related olfactory dysfunction.
Key Points
Question
Is the use of theophylline added to saline nasal irrigation efficacious and safe for treatment of COVID-19–related olfactory dysfunction?
Findings
In this phase 2 randomized clinical trial of 51 adults with chronic COVID-19–related olfactory dysfunction, the number of participants who self-reported improvement in their olfactory function after using theophylline nasal irrigation was 16% higher than those receiving placebo (59% vs 43%). The theophylline arm had 24% more participants with a clinically meaningful change in their objective smell identification test score.
Meaning
The use of theophylline added to saline nasal irrigations may result in clinically beneficial improvements in smell function compared with saline irrigation alone.
Abstract
Importance
Recent studies suggest that theophylline added to saline nasal irrigation (SNI) can be an effective treatment for postviral olfactory dysfunction (OD), a growing public health concern during the COVID-19 pandemic.
Objective
To evaluate the efficacy and safety of theophylline added to SNI compared with placebo for COVID-19–related OD.
Design, Setting, and Participants
This triple-blinded, placebo-controlled, phase 2 randomized clinical trial was conducted virtually between March 15 and August 31, 2021. Adults residing in Missouri or Illinois were recruited during this time period if they had OD persisting for 3 to 12 months following suspected COVID-19 infection. Data analysis was conducted from October to December 2021.
Interventions
Saline sinus rinse kits and bottles of identical-appearing capsules with either 400 mg of theophylline (treatment) or 500 mg of lactose powder (control) were mailed to consenting study participants. Participants were instructed to dissolve the capsule contents into the saline rinse and use the solution to irrigate their nasal cavities in the morning and at night for 6 weeks.
Main Outcomes and Measures
The primary outcome was the difference in the rate of responders between the treatment and the control arms, defined as a response of at least slightly better improvement in the Clinical Global Impression-Improvement scale posttreatment. Secondary outcome measures included changes in the University of Pennsylvania Smell Identification Test (UPSIT), the Questionnaire for Olfactory Disorders, the 36-Item Short Form Health Survey on general health, and COVID-19–related questions.
Results
A total of 51 participants were enrolled in the study; the mean (SD) age was 46.0 (13.1) years, and 36 (71%) participants were women. Participants were randomized to SNI with theophylline (n = 26) or to SNI with placebo (n = 25). Forty-five participants completed the study. At the end of treatment, 13 (59%) participants in the theophylline arm reported at least slight improvement in the Clinical Global Impression-Improvement scale (responders) compared with 10 (43%) in the placebo arm (absolute difference, 15.6%; 95% CI, −13.2% to 44.5%). The median difference for the UPSIT change between baseline and 6 weeks was 3.0 (95% CI, −1.0 to 7.0) for participants in the theophylline arm and 0.0 (95% CI, −2.0 to 6.0) for participants in the placebo arm. Mixed-model analysis revealed that the change in UPSIT scores through study assessments was not statistically significantly different between the 2 study arms. Eleven (50%) participants in the theophylline arm and 6 (26%) in the placebo arm had a change of 4 or more points in UPSIT scores from baseline to 6 weeks. The difference in the rate of responders as measured by the UPSIT was 24% (95% CI, −4% to 52%) in favor of theophylline.
Conclusions and Relevance
This randomized clinical trial suggests that the clinical benefit of theophylline nasal irrigations on olfaction in participants with COVID-19–related OD is inconclusive, though suggested by subjective assessments. Larger studies are warranted to investigate the efficacy of this treatment more fully.
Trial Registration
ClinicalTrials.gov Identifier: NCT04789499
Introduction
The ongoing COVID-19 pandemic has resulted in an unprecedented rise in the incidence of postviral olfactory dysfunction (OD), a growing public health concern affecting up to 1.6 million individuals in the US alone.1 As of 2016, about 13 million Americans were diagnosed with anosmia or hyposmia, with postviral OD being the leading cause.2 With the rising burden of chronic OD and emerging evidence on its effects on quality of life (QOL) and safety, the need for a viable treatment is imperative.3
Chronic OD can considerably affect QOL. In one observational study, only adults who experienced loss of smell and/or taste as part of their COVID-19 infection experienced increased depression and anxiety.4 Other studies reported considerable alterations in the daily lives of individuals with chronic OD, including inability to eat and prepare food, substantial weight fluctuations, nutritional insufficiency, decreased emotional well-being, disruptions in intimacy and social bonding, and an altered relationship with self.5 Chronic OD has also been known to cause loss of cortical gray matter and contribute to the anorexia of aging in older individuals.6,7,8,9,10,11
The novel SARS-CoV-2 virus has been shown to cause anosmia, dysgeusia, parosmia, and phantosmia.12 There is no consensus on the mechanisms of postviral anosmia, parosmia, and phantosmia; however, angiotensin-converting enzyme 2 receptors, which act as a key site of viral entry, are expressed throughout the nervous system and may explain the SARS-CoV-2 neurological manifestations.6,13,14 Evidence exists to suggest that infection may cause changes within the central nervous system,15 to the olfactory neural complex,16,17 and to nasal epithelial cells.18
Theophylline, a phosphodiesterase inhibitor, has been theorized to promote neural olfactory signaling and sensory axonal regeneration by preventing the breakdown of important secondary messengers cyclic adenosine monophosphate and cyclic guanosine monophosphate.19,20,21,22,23,24,25,26,27 Although systemic theophylline has a narrow therapeutic index, 2 pilot studies of participants who had postviral OD refractory to multiple treatments reported statistically significant improvement in subjective rating of smell with intranasal theophylline.28,29,30 A previous phase 2 double-blinded, placebo-controlled randomized clinical trial (RCT) using a theophylline dose of 12 mg dissolved in 240 mL of saline nasal irrigation (SNI) in 22 patients with postviral OD confirmed the safety of intranasal theophylline and suggested improved olfaction-related QOL.31 The failure to observe differences in the patient-reported and psychophysical measures of olfaction between the theophylline and placebo SNI arms, as well as the imprecise estimates for all of the outcome measures, however, suggested the need for a future study with a larger sample size and increased theophylline dose. After a single-arm dose escalation trial32 established the safety of increased theophylline dosing delivered via SNI, the aim of this trial was to evaluate the efficacy and safety of 400 mg of theophylline added to 240 mL of SNI twice daily compared with placebo SNI for participants with post–COVID-19 chronic OD.
Methods
Study Design and Participants
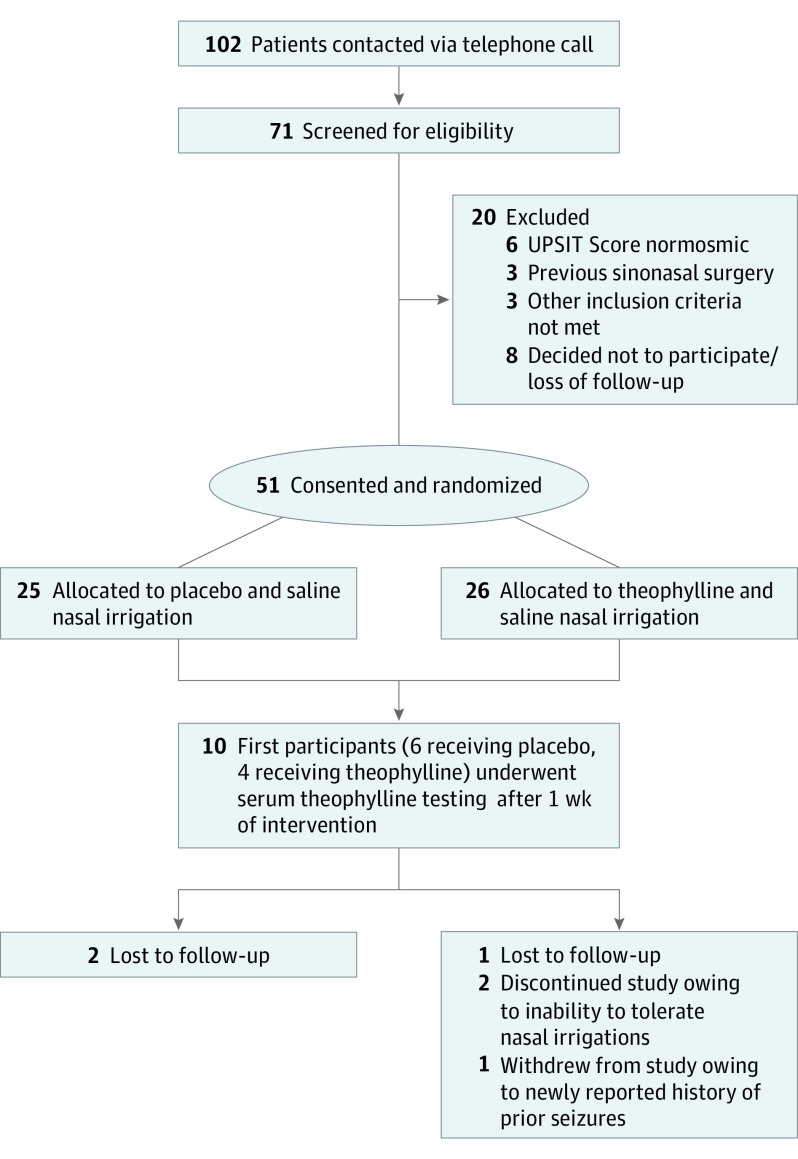
This study was a triple-blinded, phase 2, placebo-controlled RCT of individuals with post–COVID-19 chronic OD. Participants were recruited virtually from March 15 to August 31, 2021, with the support of the ResearchMatch database, physicians at the Otolaryngology–Head & Neck Surgery Clinic at Washington University in St Louis, and local news media outlets. The flow diagram of study participation is shown in Figure 1. Adults residing in Missouri or Illinois were recruited if they had OD persisting for 3 to 12 months following suspected COVID-19 infection. Documentation of COVID-19 infection with results from a positive test was not required. Given the high incidence of COVID-19 infection in the community and the relationship between OD and presumed COVID-19 infection, we felt confident that all enrolled participants were experiencing COVID-19–associated anosmia and not from other causes. Presence of OD at baseline was defined as a score of 33 or lower for men and 34 or lower for women on the validated University of Pennsylvania Smell Identification Test (UPSIT).33 Participants were excluded if they reported a history of OD prior to infection with COVID-19, a previous diagnosis of nasal polyps, prior sinonasal or anterior skull base surgery, neurodegenerative disease, or prior seizures or any arrhythmia. Pregnant or breastfeeding individuals were excluded. Individuals were also excluded if they were concomitantly using other therapies for OD, were dependent on theophylline or another methylxanthine for any comorbid conditions such as asthma, had an allergic reaction to theophylline or a methylxanthine, or had other contraindications to the use of theophylline.
Figure 1. CONSORT Diagram.
UPSIT indicates the University of Pennsylvania Smell Identification Test.
The study protocol (Supplement 1) was approved by the institutional review board, human protection research office, and COVID-19 clinical research committee at Washington University in St Louis. Interested adults were screened via telephone call. Informed consent was required and obtained by participants who passed screening and were invited to participate in the study. All responses to surveys were obtained online via REDCap, version 9.0 (Vanderbilt University). This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines for RCTs.
Interventions
Participants underwent simple randomization to study arms following a computer-generated randomization list created and stored by an independent study pharmacist. The participants and all members of the study team except the study pharmacist were blinded to the randomization assignment. Enrolled participants received an 8-oz Sinus Rinse Regular Bottle Kit (NeilMed Pharmaceuticals) and a 6-week supply of United States Pharmacopeia–grade sodium chloride and sodium bicarbonate mixture via mail. Participants received 84 capsules of theophylline (400 mg/capsule) or an identical appearing and tasting 84 capsules of lactose powder (500 mg/capsule). Participants were instructed to dissolve a capsule of the study drug and saline mixture into distilled water or tap water boiled for 5 minutes within the rinse bottle, then to irrigate the left and right nasal cavity equally with the solution twice daily for 6 weeks. All participants received verbal, written, and video instructions on how to conduct the irrigation properly.
To determine the serum theophylline concentration level after twice daily SNI with 400 mg of theophylline, the first 10 participants enrolled into the trial had their blood drawn 1 week after treatment initiation. If any participant had a serum theophylline level greater than 20 µg/mL,27 the study would be discontinued. If serum theophylline levels were undetectable or lower than 5 µg/mL, the study would continue as planned. If any participant had serum theophylline levels 5 to 20 µg/mL, then 10 more participants would undergo serum testing.
Assessments
At baseline, participants completed the UPSIT, the 36-Item Short Form Health Survey (SF-36), and questions related to infection with COVID-19. The UPSIT is a clinically validated 40-question, forced-choice odor identification test where microencapsulated odorants on a strip are released by scratching.33 Out of a total of 40 points, normosmia is defined as 34 or more points for males and 35 or more points for females, and an increase in 4 or more points was deemed to be a clinically significant improvement in symptoms. The SF-36 was used to determine general health status in 8 different domains for each participant, with the range of scores for each domain from 0 to 100, with 0 indicating death and 100 indicating perfect health.34
At baseline, participants also completed the Clinical Global Impression-Severity (CGI-S) scale for perceived severity of smell dysfunction35,36 and the Questionnaire for Olfactory Disorders (QOD).37 The CGI-S poses the question, “Overall, what is your current ability to smell?” with 6 response options (absent, poor, fair, good, very good, or excellent) and was adapted from a validated patient-reported outcome measure commonly used in psychometric studies.35 The QOD measures smell-related QOL, including topics such as eating, mental health, social interactions, and fear of dysfunction. The survey is scored based on changes in QOL overall, negative changes to QOL only, the parosmia experience (important owing to its association with COVID-19–related OD37), and a sincerity score assessing a patient’s tendency to give socially desired answers.
At 3 and 6 weeks postintervention, the participants completed the Clinical Global Impression-Improvement (CGI-I) scale. The CGI-I for smell dysfunction poses the question, “Compared to your sense of smell before you started the nasal irrigations, how would you rate your sense of smell now?” with 7 response options (much better, somewhat better, slightly better, neither better nor worse, slightly worse, somewhat worse, and much worse). The CGI-I for distortion poses the question, “How intrusive is parosmia or phantosmia in your life now after [3,6] weeks of treatment?” with the same 7 response options. The primary outcome measure for this study was the rate of responders to intervention defined as the number of participants who self-reported at least a “slightly better” improvement in their sense of smell in the CGI-I scale, divided by the total number of participants in each arm. We are aware of the frequently observed discrepancy between a patient’s assessment of their own ability to smell and objective testing and selected the CGI-I as the primary outcome measure, rather than UPSIT, because we believe a global, single-item, patient-reported outcome measure most accurately reflects the patient’s response to treatment.
At 3 and 6 weeks postintervention, participants repeated the UPSIT, CGI-S, and QOD. Participants also completed an adverse effects questionnaire at 3 and 6 weeks postintervention, which allowed for reporting of any unusual adverse effects experienced during the study. Lastly, at the end of the study, participants completed a survey, which included a compliance question based on the final pill count and a question that asked participants what intervention they believed they received.
Statistical Analysis
Because, to our knowledge, this was the first study using 400 mg of theophylline in SNI, no data were available for effect size or variance estimation. Therefore, a sample size of 50 participants for this pilot study was proposed based on feasibility considering the daily rate of new cases of COVID-19 in Missouri (about 3500 cases) at the time of protocol development, as well as an estimated rate of chronic OD of 10%. Given an anticipated 20% dropout rate, the sample size of 50 participants would provide us with 40 participants with complete data. Assuming the rate of responders in the placebo arm is 30%,31 the total sample size of 40 participants (20 participants in each arm) would provide a 95% CI of 5% to 55% around the predefined minimally clinically important difference (MCID) of 25% in the rate of responders between arms.
Data were analyzed following principles of intention-to-treat analysis. Means (SDs) were reported for normally distributed continuous-level variables. Medians and ranges were reported for nonnormally distributed variables based on the Shapiro-Wilk test. Frequency and percentages were used for reporting categorical data. Fisher exact test was used to compare the responders’ rates based on CGI-I scores between the 2 arms at 6 weeks.
Independent samples t test or Mann-Whitney U test were used to compare the change in UPSIT scores and other continuous-level outcome measures between the 2 arms. A mixed-model analysis with participants as a random factor, and group and time as fixed factors, was used to compare the change in outcomes through different study assessments between the study groups. Effect sizes and 95% CIs were reported for all analyses. All statistical analyses were conducted in SPSS Statistics, version 27 (IBM Corp).
Results
Participants
A total of 71 individuals were screened, and 51 participants were enrolled and randomized to SNI with theophylline (n = 26) or to SNI with lactose as placebo (n = 25) (Figure 1). The baseline characteristics of the participants are summarized in Table 1. The mean (SD) age of participants was 46.0 (13.1) years, and the majority were women (n = 36 [71%]). The scores of the SF-36 subscales were not different between the 2 study arms. Participants in the theophylline arm had lower UPSIT scores at baseline than participants in the placebo arm (median difference, 3.0; 95% CI, −1.0 to 7.0). The majority of participants (n = 45 [88%]) reported issues with loss of taste during their acute COVID-19 infection. Further information on COVID-19 presentation can be found in eTable 1 in Supplement 2. Of the 51 enrolled participants, 17 reported attempting previous treatments for OD.
Table 1. Summary of Baseline Characteristics for the 51 Participants Enrolled and Randomized in the Trial.
| Characteristic | Median (range) | |
|---|---|---|
| Theophylline arm (n = 26) | Placebo arm (n = 25) | |
| Age, mean (SD), y | 42.4 (11.6) | 47.1 (13.9) |
| Sex, No. (%) | ||
| Female | 19 (73) | 17 (68) |
| Male | 7 (27) | 8 (32) |
| SF-36 Domain | ||
| Physical | 100 (65-100) | 100 (40-100) |
| Physical limitations | 100 (0-100) | 100 (0-100) |
| Emotional limitations | 100 (0-100) | 100 (0-100) |
| Fatigue | 63 (25-90) | 60 (15-90) |
| Emotional | 80 (36-100) | 80 (28-96) |
| Social | 100 (37-100) | 88 (25-100) |
| Pain | 100 (45-100) | 100 (58-100) |
| General health | 75 (40-100) | 80 (50-100) |
| Baseline UPSIT score | 20 (7-34) | 23 (11-32) |
| Time from COVID-19 infection to start of intervention, moa | 5.7 (4.1-13.2) | 7.2 (4.1-13.0) |
Abbreviation: SF-36, 36-Item Short Form Health Survey; UPSIT, University of Pennsylvania Smell Identification Test.
Maximum time from COVID-19 infection to start of intervention may exceed 12 months owing to delay in enrollment and receipt of treatment.
The CGI-I and CGI-S scores for each arm at each study assessment are summarized in Table 2. At study completion, 13 (59%) participants in the theophylline arm and 10 (43%) participants in the placebo arm were responders (difference, 15.6%; 95% CI −13.2% to 44.5%). The upper bound of the 95% CI for the rate difference is much larger than the predetermined MCID of 25%.
Table 2. Self-reported Assessment of Smell and Changes in Ability to Smell at Baseline, 3 Weeks, and 6 Weeksa.
| Assessment | Categories | Theophylline arm, No. (%) (n = 26) | Placebo arm, No. (%) (n = 25) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 3 Weeks | 6 Weeks | Baseline | 3 Weeks | 6 Weeks | ||
| Change in ability to smell from baseline (CGI-I) | Much better | NA | 0 | 0 | NA | 0 | 1 (4) |
| Somewhat better | NA | 0 | 4 (18) | NA | 2 (9) | 4 (17) | |
| Slightly better | NA | 8 (35) | 9 (41) | NA | 7 (30) | 5 (22) | |
| Neither better nor worse | NA | 15 (58) | 5 (23) | NA | 13 (57) | 12 (52) | |
| Slightly worse | NA | 0 | 1 (4) | NA | 1 (4) | 0 | |
| Somewhat worse | NA | 0 | 1 (4) | NA | 0 | 0 | |
| Much worse | NA | 0 | 2 (9) | NA | 0 | 1 (4) | |
| Overall ability to smell (CGI-S) | Very good | 0 | 0 | 0 | 0 | 0 | 1 (4) |
| Good | 0 | 1 (4) | 1 (4) | 1 (4) | 1 (4) | 2 (9) | |
| Fair | 1 (4) | 3 (13) | 6 (27) | 3 (12) | 6 (26) | 6 (26) | |
| Poor | 17 (71) | 16 (70) | 10 (46) | 16 (64) | 12 (52) | 11 (48) | |
| Absent | 6 (25) | 3 (13) | 5 (23) | 5 (20) | 4 (18) | 3 (13) | |
Abbreviations: CGI-I, Clinical Global Impression-Improvement; CGI-S, Clinical Global Impression-Severity; NA, not applicable.
Four participants in the theophylline arm and 2 participants in the placebo arm were either lost to follow-up, discontinued the study for inability to tolerate nasal irrigations, or were withdrawn for medical reasons.
Efficacy
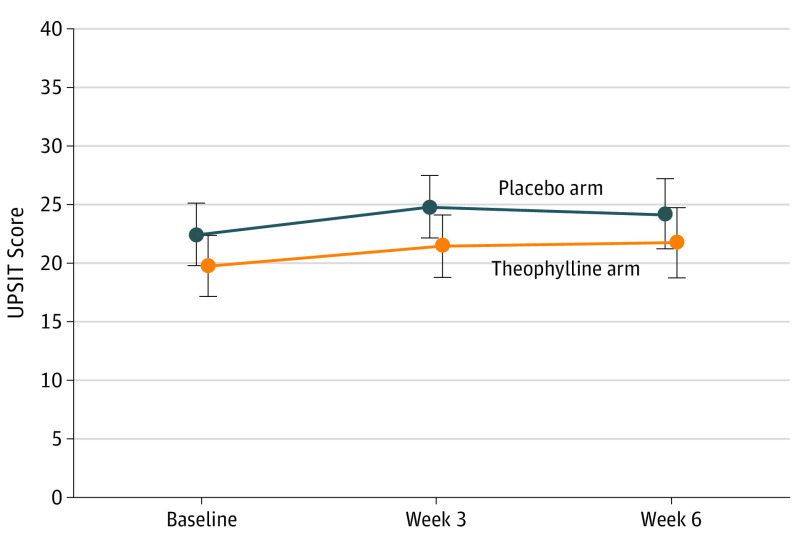
The median preintervention to postintervention difference for the UPSIT between baseline and 6 weeks was 3.0 (95% CI, −1.0 to 7.0) for participants in the theophylline arm and 0.0 (95% CI, −2.0 to 6.0) for participants in the placebo arm. Eleven of 22 (50%) participants in the theophylline arm and 6 of 23 (26%) participants in the placebo arm had a change of 4 or more points from baseline to week 6. The difference in the rate of responders as measured by the UPSIT was 24% (95% CI, −4% to 52%). Mixed-model analysis revealed that the change in UPSIT scores was not statistically significantly different between the 2 study groups (Figure 2).
Figure 2. Comparisons of UPSIT Scores Between the 2 Study Arms at Different Time Points.
Error bars represent 95% CIs. UPSIT indicates the University of Pennsylvania Smell Identification Test.
Scores on the QOD related to smell loss assessments are summarized in Table 3. Mixed-model analysis revealed that the change in score on each of the 4 QOL assessments through study visits was not different between the study arms.
Table 3. Quality-of-Life Metrics Related to Smell Loss by Study Arm and Study Assessment Using the Questionnaire for Olfactory Disorders.
| Assessmenta | Assessment time point | Theophylline arm (n = 26) | Placebo arm (n = 25) | ||
|---|---|---|---|---|---|
| No. | Median score (range) | No. | Median score (range) | ||
| Quality of life | Baseline | 24 | 17.39 (6.27 to 26.22) | 25 | 15.96 (5.13 to 30.21) |
| 3 weeks | 23 | 15.39 (3.99 to 25.65) | 23 | 12.54 (3.99 to 27.93) | |
| 6 weeks | 22 | 15.39 (4.56 to 25.65) | 23 | 14.25 (2.28 to 26.79) | |
| Baseline to 6 weeks’ change, median difference (95% CI) | 22 | 0.86 (−0.57 to 2.85) | 23 | 1.43 (−0.29 to 3.14) | |
| Quality of life-negative statements | Baseline | 24 | 26.50 (10.00 to 42.00) | 25 | 22.00 (8.00 to 48.00) |
| 3 weeks | 23 | 25.00 (6.00 to 40.00) | 23 | 20.00 (5.00 to 43.00) | |
| 6 weeks | 22 | 25.00 (6.00 to 40.00) | 23 | 22.00 (2.00 to 43.00) | |
| Baseline to 6 weeks’ change, median difference (95% CI) | 22 | 1.50 (−1.00 to 4.50) | 23 | 1.80 (−1.00 to 5.00) | |
| Parosmia | Baseline | 24 | 1.08 (0.36 to 1.44) | 25 | 1.32 (0.00 to 1.44) |
| 3 weeks | 23 | 0.96 (0.00 to 1.44) | 23 | 0.96 (0.00 to 1.44) | |
| 6 weeks | 22 | 1.20 (0.24 to 1.44) | 23 | 1.08 (0.24 to 1.44) | |
| Baseline to 6 weeks’ change, median difference (95% CI) | 22 | −0.06 (−0.18 to 0.06) | 23 | 0.06 (−0.60 to 0.24) | |
| Sincerity | Baseline | 24 | 1.44 (0.36 to 2.70) | 25 | 1.08 (0.18 to 2.52) |
| 3 weeks | 23 | 1.26 (0.18 to 2.70) | 23 | 1.44 (0.00 to 3.24) | |
| 6 weeks | 22 | 1.44 (0.54 to 2.34) | 23 | 1.26 (0.36 to 2.70) | |
| Baseline to 6 weeks’ change, median difference (95% CI) | 22 | 0.00 (−0.09 to 0.18) | 23 | −0.14 (−0.36 to 0.00) | |
Higher scores for quality of life, quality of life-negative statements, and parosmia indicate a higher degree of impairment. Lower scores for sincerity suggest a greater tendency to give socially desirable answers.
The study was completed by 35 of 39 participants who reported experiencing parosmia or phantosmia at baseline, with 14 (40%) participants reporting a change in the intrusiveness of the parosmia as “much better,” “somewhat better,” or “slightly better.” Eight (44%) participants in the theophylline arm and 6 (35%) participants in the placebo arm reported improvement in parosmia or phantosmia (proportion difference, 9.1%; 95% CI, −24.0% to 42.2%).
The final pill count showed that 20 (44%) participants had 0 theophylline or placebo capsules left, while only 3 (7%) participants had between 12 and 25 (15%-30%) pills left. All participants were asked to guess which treatment they believed they were receiving, and 19 of 23 (83%; 95% CI, 61%-95%) participants in the placebo arm guessed placebo while 10 of 22 (45%; 95% CI, 24%-68%) participants in the theophylline arm correctly guessed their allocation.
Safety
All 10 participants who underwent serum theophylline concentration testing after 1 week of intervention had results of less than 5 µg/mL, reflecting no clinically meaningful systemic absorption. The adverse effects that were reported by participants as possibly related to the study are summarized in eTable 2 in Supplement 2, showing similar results at week 6 for both groups. At week 3, the theophylline arm had 2 participants reporting insomnia, and the placebo arm had 3 participants reporting tachycardia. There were no severe adverse effects. Two participants assigned to the theophylline arm reported experiencing considerable parosmia and foul taste with the intervention, resulting in 1 withdrawal and 1 with poor compliance (>30% of pills remaining at 6 weeks).
Discussion
In this triple-blinded, placebo-controlled RCT, we found that the use of 400 mg of theophylline in SNI twice daily may have the potential to improve OD in adults with COVID-19–related anosmia. The upper bound of the 95% CI for the absolute difference in the percentage of responders (44.5%), as defined by the CGI-I, was much larger than the predefined MCID (25%); thus, these results, while not definitive, do include a clinically meaningful effect. Based on the UPSIT, a much greater percentage of participants who received theophylline achieved a clinically meaningful change. Given the small sample size and the imprecision of the estimates, it is difficult to make definitive conclusions about the efficacy of theophylline. Furthermore, many participants randomized to the placebo arm correctly guessed that they had received placebo, suggesting possible inadequate blinding. Participants in a placebo-controlled randomized trial who did not experience benefit might be more likely than participants who did receive benefit to judge their intervention as a placebo.38 In this trial, the participants in the placebo group demonstrated a better improvement in their olfactory function than expected, and so the explanation that participants in the placebo group were more likely to correctly guess placebo owing to the failure to experience improvement in olfactory function is hard to explain. The theophylline irrigations were well tolerated overall, and no serious adverse effects related to intervention were reported. Two participants in the theophylline group experienced foul taste, which likely limited their use of theophylline. Improvement was observed in several of the outcome measures. While the results of this study are encouraging, future studies with larger sample sizes are needed to assess the efficacy of theophylline SNI postviral OD.
Currently, there is no evidence-based treatment for chronic COVID-19–related OD.39 Intranasal and oral corticosteroids and olfactory training are currently used to treat postviral OD; however, these treatments demonstrate limited efficacy, suggesting that there is no current gold standard of care.40,41,42 Research shows that SNIs can improve smell function that was altered owing to minor obstructions present in the nasal cavity.31,43 An added benefit of large-volume, low-pressure nasal irrigation over a nasal spray is the comprehensive distribution of the therapeutic compound within the sinonasal cavities. The nasal saline method is also more targeted to olfactory tissue than oral administration of therapeutics, which is particularly beneficial for compounds with a narrow therapeutic index, such as theophylline.27 In the present study, the serum theophylline concentrations were well below the 10 to 15 µg/mL range thought to be required to achieve the majority of the drug’s potential benefit while minimizing adverse effects for asthma and chronic obstructive pulmonary disease.
In comparison with the RCT of 12 mg of theophylline SNI twice daily in 22 patients with postviral OD,31 this study of 400 mg of theophylline twice daily in 51 participants with COVID-19–related OD demonstrated a greater effect size for all outcomes measured. This suggests that the higher dose of theophylline is likely more efficacious while maintaining a robust safety profile. Theoretically, adding theophylline to these SNIs helps target inflammation, relaxes smooth muscle, and promotes neural recovery. While this study was conducted for a feasible period of 6 weeks, neural recovery can often take months to years, depending on the mechanism of injury.44 Therefore, the effects of long-term theophylline SNI therapy should be studied to determine whether theophylline can affect olfactory recovery in adults with postviral OD.
While viral-mediated OD is a well-known entity, the long-term prognosis of COVID-19–related OD is still not fully understood. Previous studies have noted that 49% to 89% of patients with OD will have complete remission within 4 weeks of infection.45 A systematic review found that patients with persistent OD do not have substantial spontaneous improvement after 8 weeks.46 For that reason, patients who had been diagnosed with COVID-19 infection within 3 months of enrollment were excluded to reduce the rate of spontaneous olfactory recovery. Interestingly, participants in both arms of this study reported fluctuations in their ability to smell for many months after their COVID-19 infection, including during their participation in this trial.
Limitations
There are several limitations of this study. Because the study was conducted virtually, we were unable to perform a physical examination of the nasal cavity of the participants, thereby only relying on participant accounts of the presence of nasal polyps, septal deviation, or other anatomic or structural abnormalities as a contributing factor to their anosmia. No participant reported an inability to use the nasal irrigation. Most participants were female, and this gender imbalance has been noted in other studies of OD. Another limitation of the study was that we did not collect data on vaccination status of the participants. Anecdotally, several participants reported changes in their OD severity related to vaccination. As previously alluded, the 6-week intervention may not have been enough time to observe neural recovery. This was a pilot study and the effect size observed can help inform sample size for a more definitive phase 3 trial. The need to study effective treatments for chronic COVID-19–related OD was demonstrated by the number of interested individuals who reached out to the study research office, as well as the accounts of decreased QOL and anxiety shared by these individuals. While the results of this study are interesting, true clinical efficacy of theophylline has not been demonstrated. Therefore, the use of theophylline SNI as an outpatient treatment for COVID-19–related OD should not be considered as standard practice. Instead, we believe further study is warranted.
Conclusions
This RCT demonstrated that the use of theophylline SNI is well tolerated, yet the evidence of efficacy is inconclusive. Clinically meaningful benefits in olfactory function for patients with chronic COVID-19–related OD are suggested by subjective assessments but are not definitively demonstrated. These findings can be used to inform the design of future, larger RCTs.
Trial Protocol
eTable 1. Report of COVID-19 Infection Symptoms and Severity of Disease for the 51 Enrolled Patients With Chronic Olfactory Dysfunction
eTable 2. Side Effects as Reported by the Patients Who Completed the Study
Data Sharing Statement
References
- 1.Khan AM, Kallogjeri D, Piccirillo JF. Growing public health concern of COVID-19 chronic olfactory dysfunction. JAMA Otolaryngol Head Neck Surg. 2022;148(1):81-82. doi: 10.1001/jamaoto.2021.3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman HJ, Rawal S, Li CM, Duffy VB. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 2016;17(2):221-240. doi: 10.1007/s11154-016-9364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitcroft KL, Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020;323(24):2512-2514. doi: 10.1001/jama.2020.8391 [DOI] [PubMed] [Google Scholar]
- 4.Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Mood, anxiety and olfactory dysfunction in COVID-19: evidence of central nervous system involvement? Laryngoscope. 2020;130(11):2520-2525. doi: 10.1002/lary.28964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burges Watson DL, Campbell M, Hopkins C, Smith B, Kelly C, Deary V. Altered smell and taste: anosmia, parosmia and the impact of long Covid-19. PLoS One. 2021;16(9):e0256998. doi: 10.1371/journal.pone.0256998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffman SS, Warwick ZS. Flavor enhancement of foods for the elderly can reverse anorexia. Neurobiol Aging. 1988;9(1):24-26. doi: 10.1016/S0197-4580(88)80009-5 [DOI] [PubMed] [Google Scholar]
- 7.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307-2312. doi: 10.1001/jama.288.18.2307 [DOI] [PubMed] [Google Scholar]
- 8.Bitter T, Gudziol H, Burmeister HP, Mentzel HJ, Guntinas-Lichius O, Gaser C. Anosmia leads to a loss of gray matter in cortical brain areas. Chem Senses. 2010;35(5):407-415. doi: 10.1093/chemse/bjq028 [DOI] [PubMed] [Google Scholar]
- 9.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 2014;9(10):e107541. doi: 10.1371/journal.pone.0107541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Luo Z, Pinto JM, et al. Relationship between poor olfaction and mortality among community-dwelling older adults: a cohort study. Ann Intern Med. 2019;170(10):673-681. doi: 10.7326/M18-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Regemorter V, Hummel T, Rosenzweig F, Mouraux A, Rombaux P, Huart C. Mechanisms linking olfactory impairment and risk of mortality. Front Neurosci. 2020;14:140. doi: 10.3389/fnins.2020.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schambeck SE, Crowell CS, Wagner KI, et al. Phantosmia, parosmia, and dysgeusia are prolonged and late-onset symptoms of COVID-19. J Clin Med. 2021;10(22):5266. doi: 10.3390/jcm10225266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998. doi: 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 14.Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. 2020;41(5):102581. doi: 10.1016/j.amjoto.2020.102581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19 and anosmia. JAMA Neurol. 2020;77(8):1028-1029. doi: 10.1001/jamaneurol.2020.2125 [DOI] [PubMed] [Google Scholar]
- 16.Morbini P, Benazzo M, Verga L, et al. Ultrastructural evidence of direct viral damage to the olfactory complex in SARS-CoV-2-positive patients. JAMA Otolaryngol Head Neck Surg. 2020;146(10):972-973. doi: 10.1001/jamaoto.2020.2366 [DOI] [PubMed] [Google Scholar]
- 17.Hawkes C. Parosmia: treatment, mechanism, and types. BMJ. 2020;371:m4739. doi: 10.1136/bmj.m4739 [DOI] [PubMed] [Google Scholar]
- 18.Brann DT, Tsukahara T, Weinreb C, Logan DW, Datta SR. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. bioRxiv. Preprint posted online March 28, 2020. doi: 10.1101/2020.03.25.009084 [DOI]
- 19.Pace U, Hanski E, Salomon Y, Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985;316(6025):255-258. doi: 10.1038/316255a0 [DOI] [PubMed] [Google Scholar]
- 20.Anholt RR. Molecular neurobiology of olfaction. Crit Rev Neurobiol. 1993;7(1):1-22. [PubMed] [Google Scholar]
- 21.Levy LM, Henkin RI, Lin CS, Hutter A, Schellinger D. Increased brain activation in response to odors in patients with hyposmia after theophylline treatment demonstrated by fMRI. J Comput Assist Tomogr. 1998;22(5):760-770. doi: 10.1097/00004728-199809000-00019 [DOI] [PubMed] [Google Scholar]
- 22.Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34(6):885-893. doi: 10.1016/S0896-6273(02)00702-X [DOI] [PubMed] [Google Scholar]
- 23.Moon C, Simpson PJ, Tu Y, Cho H, Ronnett GV. Regulation of intracellular cyclic GMP levels in olfactory sensory neurons. J Neurochem. 2005;95(1):200-209. doi: 10.1111/j.1471-4159.2005.03356.x [DOI] [PubMed] [Google Scholar]
- 24.Henkin RI, Velicu I. cAMP and cGMP in nasal mucus: relationships to taste and smell dysfunction, gender and age. Clin Invest Med. 2008;31(2):E71-E77. doi: 10.25011/cim.v31i2.3366 [DOI] [PubMed] [Google Scholar]
- 25.Henkin RI, Velicu I. cAMP and cGMP in nasal mucus related to severity of smell loss in patients with smell dysfunction. Clin Invest Med. 2008;31(2):E78-E84. doi: 10.25011/cim.v31i2.3367 [DOI] [PubMed] [Google Scholar]
- 26.Henkin RI, Velicu I, Schmidt L. An open-label controlled trial of theophylline for treatment of patients with hyposmia. Am J Med Sci. 2009;337(6):396-406. doi: 10.1097/MAJ.0b013e3181914a97 [DOI] [PubMed] [Google Scholar]
- 27.Barnes PJ. Theophylline. Pharmaceuticals (Basel). 2010;3(3):725-747. doi: 10.3390/ph3030725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henkin RI, Schultz M, Minnick-Poppe L. Intranasal theophylline treatment of hyposmia and hypogeusia: a pilot study. Arch Otolaryngol Head Neck Surg. 2012;138(11):1064-1070. doi: 10.1001/2013.jamaoto.342 [DOI] [PubMed] [Google Scholar]
- 29.Goldstein MF, Hilditch GJ, Frankel I, Chambers L, Dvorin DJ, Belecanech G. Intra-nasal theophylline for the treatment of chronic anosmia and hyposmia. J Allergy Clin Immunol. 2017;139(2):AB252. doi: 10.1016/j.jaci.2016.12.810 [DOI] [Google Scholar]
- 30.Nigwekar SU, Weiser JM, Kalim S, et al. Characterization and correction of olfactory deficits in kidney disease. J Am Soc Nephrol. 2017;28(11):3395-3403. doi: 10.1681/ASN.2016121308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JJ, Peterson AM, Kallogjeri D, et al. Smell Changes and Efficacy of Nasal Theophylline (SCENT) irrigation: a randomized controlled trial for treatment of post-viral olfactory dysfunction. Am J Otolaryngol. 2022;43(2):103299. doi: 10.1016/j.amjoto.2021.103299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JJ, Gupta S, Kallogjeri D, Piccirillo JF. Safety of high-dose nasal theophylline irrigation in the treatment of postviral olfactory dysfunction: a dose-escalation study. JAMA Otolaryngol Head Neck Surg. Published online July 7, 2022. doi: 10.1001/jamaoto.2022.1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489-502. doi: 10.1016/0031-9384(84)90269-5 [DOI] [PubMed] [Google Scholar]
- 34.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 35.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28-37. [PMC free article] [PubMed] [Google Scholar]
- 36.Dunlop BW, Gray J, Rapaport MH. Transdiagnostic clinical global impression scoring for routine clinical settings. Behav Sci (Basel). 2017;7(3):E40. doi: 10.3390/bs7030040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattos JL, Schlosser RJ, Mace JC, Smith TL, Soler ZM. Establishing the minimal clinically important difference for the Questionnaire of Olfactory Disorders. Int Forum Allergy Rhinol. 2018;8(9):1041-1046. doi: 10.1002/alr.22135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moscucci M, Byrne L, Weintraub M, Cox C. Blinding, unblinding, and the placebo effect: an analysis of patients’ guesses of treatment assignment in a double-blind clinical trial. Clin Pharmacol Ther. 1987;41(3):259-265. doi: 10.1038/clpt.1987.26 [DOI] [PubMed] [Google Scholar]
- 39.O’Byrne L, Webster KE, MacKeith S, Philpott C, Hopkins C, Burton MJ. Interventions for the treatment of persistent post-COVID-19 olfactory dysfunction. Cochrane Database Syst Rev. 2021;7:CD013876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorokowska A, Drechsler E, Karwowski M, Hummel T. Effects of olfactory training: a meta-analysis. Rhinology. 2017;55(1):17-26. doi: 10.4193/Rhino16.195 [DOI] [PubMed] [Google Scholar]
- 41.Whitcroft KL, Hummel T. Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Head Neck Surg. 2019;145(9):846-853. doi: 10.1001/jamaoto.2019.1728 [DOI] [PubMed] [Google Scholar]
- 42.Nguyen TP, Patel ZM. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int Forum Allergy Rhinol. 2018;8(9):977-981. doi: 10.1002/alr.22140 [DOI] [PubMed] [Google Scholar]
- 43.Tait S, Kallogjeri D, Suko J, Kukuljan S, Schneider J, Piccirillo JF. Effect of budesonide added to large-volume, low-pressure saline sinus irrigation for chronic rhinosinusitis: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2018;144(7):605-612. doi: 10.1001/jamaoto.2018.0667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menorca RM, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29(3):317-330. doi: 10.1016/j.hcl.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei G, Gu J, Gu Z, et al. Olfactory dysfunction in patients with coronavirus disease 2019: a review. Front Neurol. 2022;12:783249. doi: 10.3389/fneur.2021.783249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jafar A, Lasso A, Shorr R, Hutton B, Kilty S. Olfactory recovery following infection with COVID-19: a systematic review. PLoS One. 2021;16(11):e0259321. doi: 10.1371/journal.pone.0259321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Report of COVID-19 Infection Symptoms and Severity of Disease for the 51 Enrolled Patients With Chronic Olfactory Dysfunction
eTable 2. Side Effects as Reported by the Patients Who Completed the Study
Data Sharing Statement