Abstract
Osimertinib is active against T790M-positive epidermal growth factor receptor mutant non-small cell lung cancer. We enrolled 122 sensitive epidermal growth factor receptor mutant non-small cell lung cancer patients who were planned to receive or were receiving first-/second-generation epidermal growth factor receptor tyrosine kinase inhibitors without disease progression and monitored plasma T790M every 1–2 months using the cobas® EGFR Mutation Test v2. We previously reported the concordance between T790M status in plasma and tissue. This is the final report on the sensitivity of plasma T790M and the efficacy of sequential osimertinib. The sensitivity was 21.1% (95% confidence interval: 6.1–45.6%). The best overall response was 25.0% (95% confidence interval: 9.8–46.7) in the plasma T790M-positive group and 28.6% (95% confidence interval: 8.4–58.1) in the plasma T790M-negative but tissue T790M-positive group. Median progression-free survival was 7.9 months (95% confidence interval: 4.7–17.5) for the former and 4.4 months (95% confidence interval: 3.0–N.E.) for the latter, with no statistically significant difference (P = 0.74).
Keywords: lung neoplasm, epidermal growth factor, mutation, liquid biopsy
In our study, with regular monitoring of plasma circulating tumor DNA (ctDNA) T790 in daily practice, there was no statistically significant difference in progression-free survival between the plasma ctDNA-positive and ctDNA-negative groups.
Introduction
Treatment with first- or second-generation epidermal growth factor (EGFR)-tyrosine kinase inhibitors (TKIs) is effective for patients with non-small cell lung cancer (NSCLC) who harbor a sensitizing EGFR mutation. However, acquired resistance is inevitable after 9–14 months (1–3), and the most common mechanism of resistance is the EGFR T790M mutation, which accounts for approximately 60% of cases (4). Osimertinib, a third-generation EGFR-TKI, is reported to be highly active against T790M-positive NSCLC (5). To detect the T790M mutation, tumor re-biopsy is necessary. However, re-biopsies are often infeasible during standard care of NSCLC patients. Circulating tumor DNA (ctDNA) detected in plasma is recognized as a noninvasive biomarker for the molecular analysis of NSCLC. The cobas® EGFR Mutation Test (Roche Diagnostics K.K., Switzerland) is a companion diagnostic test for the detection of EGFR mutations in plasma specimens and has been approved to identify such patients with NSCLC (6). We conducted an observational study of plasma ctDNA in NSCLC patients with EGFR mutations receiving EGFR-TKIs and reported the monitoring results from baseline to the initiation of osimertinib (7). In this article, we present the final report of this study, including treatment results with osimertinib.
Patients and methods
Study population
Key eligibility criteria were as follows: (i) histologically and/or cytologically confirmed advanced or post-operative recurrent NSCLC harboring sensitizing EGFR mutations, with receipt or planned receipt of first-line EGFR-TKIs (gefitinib, erlotinib or afatinib); (ii) EGFR mutations with Exon 19 deletion, Exon 21 L858R or another sensitive minor mutation (i.e. Exon 18 G719X); (iii) Eastern Cooperative Oncology Group performance status of 0 to 2. Patients were excluded if they had undergone prior EGFR-TKI treatment with disease progression (PD).
Plasma ctDNA analysis
Plasma ctDNA was collected at baseline and every 1–2 months. The following events were specifically noted: (i) radiological PD, (ii) clinical PD, (iii) re-biopsy at PD and (iv) treatment change. Plasma ctDNA was analyzed at SRL Laboratory (Tokyo, Japan) using the cobas® EGFR Mutation Test version 2 (v2) to detect sensitizing EGFR mutations and the T790M mutation.
Re-biopsy and EGFR mutation analysis
When the disease had progressed, tissue re-biopsy was recommended. The EGFR genotypes of re-biopsied materials were analyzed at each hospital using the peptide nucleic acid-locked nucleic acid clamp method or the cobas® EGFR Mutation Test v2.
Clinical data collection
The case report form (CRF) included clinical information about radiological PD (date, site of PD), clinical PD (date, pattern of PD), survival (date last verified), death (date) and cause of death. Radiological PD was assessed according to the Response Evaluation Criteria in Solid Tumors v1.1 at each institution. Adverse events were assessed according to the Common Terminology Criteria for Adverse Events v4.0.
Statistical analysis
This study is an observational study to estimate the usefulness of plasma ctDNA monitoring in patients with EGFR mutant NSCLC who received first-line EGFR-TKIs. The primary endpoint was the plasma ctDNA T790M-positivity rate using the cobas® EGFR Mutation Test v2, and secondary endpoints were the best response rate and progression-free survival (PFS) with osimertinib. We defined PFS in this study as from the date of osimertinib initiation to the date of RECIST PD or death. This study used descriptive statistics and the target sample size was set to 120 cases in consideration of the feasibility of the research.
Ethical considerations
This study protocol was approved by the institutional review board at each participating institution. Declaration of Helsinki ethical standards and local and national regulations were followed. All patients provided written informed consent before participation.
Results
From September 2016 to March 2017, 122 patients were enrolled in this study. One patient was excluded because he was primarily refractory to first-line EGFR-TKI therapy. EGFR-TKIs were used continuously in 103 patients before enrollment and in 18 patients after enrollment; the first-line EGFR-TKI regimen was gefitinib in 50 patients, erlotinib in 40 patients and afatinib in 31 patients. For more information, please refer to our previous report (7). At the data cut-off, the median follow-up duration was 33.0 months (26.6–33.8).
The final result for the concordance rate of T790M identification between the re-biopsied tissue (N = 19) and plasma ctDNA was 21.1% [95% confidence interval (CI): 6.1–45.6] (Table 1).
Table 1.
Final results of T790M concordance between re-biopsied tissue and plasma
| T790M in plasma | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| T790M in tissue | Positive | 4 (21.1%) | 15 (79.0%) | 19 |
| Negative | 5 (16.7%) | 25 (83.3%) | 30 | |
| Unknown | 16 (22.2%) | 56 (77.8%) | 72 | |
| Total | 25 (20.1%) | 96 (79.3%) | 121 | |
The best response treated with osimertinib
The best response in all 46 patients treated with osimertinib after a first- or second-generation EGFR-TKIs was 30.4% (95% CI: 17.7–45.8). In the plasma ctDNA T790M-positive group (Liquid-positive group; LP), the response rate was 25.0% (95% CI: 9.8–46.7). There were 14 cases with plasma ctDNA T790M negative/tissue T790M positive (Liquid-negative group; LN), and here, the response rate was 28.6% (95% CI: 8.4–58.1). There was no statistically significant difference between the two groups (P = 0.29). There were eight patients in whom both plasma ctDNA and tissue T790M were negative or unknown, and four responded to osimertinib. Three patients in this group discontinued prior EGFR-TKIs due to adverse events without PD and were switched to osimertinib without re-biopsy; two responded (Table 2).
Table 2.
The best overall response to osimertinib by ctDNA status
| ctDNA T790M | Tissue T790M | N | RR (%, 95% CI) | DCR (%, 95% CI) |
|---|---|---|---|---|
| + | +/− | 24*1 | 25.0 (9.8–46.7) | 66.7 (44.7–84.4) |
| − | + | 14 | 28.6 (8.4–58.1) | 71.4 (41.2–91.6) |
| −/ukn | −/ukn | 8*2 | 50.0 (15.7–84.3) | 87.5 (47.4–99.7) |
| All | All | 46 | 30.4 (17.7–45.8) | 71.7 (56.5–84.0) |
*1: One of the 25 patients with positive plasma ctDNA T790M was excluded from the analysis because osimertinib was not used.
*2: This group included three patients who discontinued first- or second-generation EGFR-TKIs due to adverse events and were switched to osimertinib without T790M re-biopsy.
ctDNA, circulating tumor DNA; RR, response rate; DCR, disease control rate; ukn, unknown.
PFS treated with osimertinib
PFS with osimertinib was estimated by the Kaplan–Meier method. The median PFS was 7.9 months (4.7–17.5) in the LP group and 4.4 months (3.0–N.E.) in the LN group, without a statistically significant difference (P = 0.74) (Fig. 1).
Figure 1.
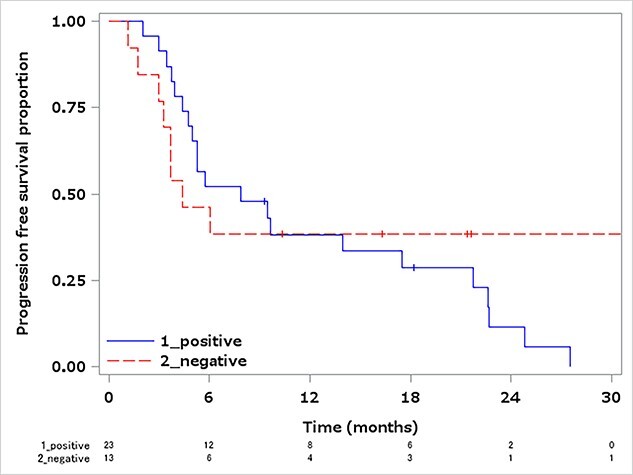
PFS in the ctDNA T790M-positive group and the ctDNA-negative/tissue T790M-positive group, treated with osimertinib. Median PFS in the ctDNA T790M-positive group: 7.9 months (95% CI: 4.7–17.5). Median PFS in the ctDNA-negative/tissue T790M-positive group: 4.4 months (95% CI: 3.0–N.E.). Log-rank test P = 0.74. PFS, progression-free survival; ctDNA, circulating tumor DNA; CI, confidential interval; N.E., could not evaluable.
Discussion
In the AURA3 study, Papadimitrakopoulou et al. reported that the T790M mutant concordance rate between tissue and liquid biopsy was 51% (110 of 215 samples) using the cobas® EGFR Mutation Test v2 (8). In our study, it was lower, at 21.1% (95% CI: 6.1–45.6%). As heterogeneity is known to occur in NSCLC, it is possible that T790M was not detected in the small re-biopsied samples and appeared at other sites. Papadimitrakopoulou et al. also reported that the detection rate in plasma was related to the baseline size of the tumor target lesion, with a median diameter of 56 mm in cases with detection and 39 mm in those without; the detection rate was also higher in cases with extrathoracic metastatic disease. There are some reports that the frequency of plasma T790M positivity varies depending on the presence of extrathoracic lesions or the site of exacerbation (9–10). Thus, when trying to detect plasma T790M in a single-point test, it is more frequently detected when the tumor target size is larger or there is extrathoracic metastasis. On the other hand, in our study, we monitored plasma T790Mregularly. As a result, plasma T790M might be detected with smaller tumor size and better control of extrathoracic lesions than AURA3, and osimertinib might have been initiated earlier than AURA3.
The best overall response was 25.0% in the LP group and 28.6% in the LN group. In a pooled analysis of the AURA extension and AURA2 trials, the overall response in the tissue T790M-positive group was 66% and in the plasma T790M-negative/tissue-positive group was 70%. The overall response rate was 71% in the AURA3 trial (11). Compared to these reports, the response rate in this study is low. As mentioned above, in our study, plasma ctDNA was measured regularly, which may have led to the initiation of osimertinib while the tumor was still small. Moreover, the timing of the imaging was not consistent due to the clinical setting.
In AURA3, the median PFS of osimertinib for patients who were T790M positive in tissue was 10.1 months (95% CI: 8.3–12.3) and for patients who were also T790M positive in plasma was 8.2 months (95% CI: 6.8–9.7). In our study, the median PFS was 7.9 months, which was similar to the results of the plasma T790M-positive group in AURA3. In our study, there was no statistical difference in survival between the LP group and LN group, despite AURA3 reporting a tendency for plasma T790M-positive patients to have inferior PFS to plasma T790M-negative patients. Detecting resistance at a relatively early timing of tumor progression by repeated tests of plasma T790M, and the initiation of osimertinib could have had a similar effect to that of the plasma T790M-negative group, although its impact on the prognosis is unknown.
This study has several limitations: (i) the small number of cases, particularly the small number where there was a matching T790M mutation detected in both tissue and plasma. Tissue biopsy could, however, not be mandated in daily practice; (ii) this was an observational study and the timing of imaging tests was not well defined, and no radiologic central review was made. These may have affected the response rate and PFS; (iii) PFS also includes a guarantee-time bias/lead-time bias and thus cannot simply be compared with the results of AURA3 or other studies of EGFR-TKIs; (iv) currently, osimertinib is recommended as first-line therapy (12), but other treatment strategies such as ramucirumab plus erlotinib (13) and afatinib followed by sequential osimertinib (14) are being actively studied. In those studies, osimertinib is considered as second-line therapy. In this study, osimertinib was also used as second-line therapy or later. However, in actual practice, osimertinib is often used as first-line therapy, so the clinical relevance of this study might be limited.
Conclusions
In this study, we regularly monitored plasma T790M. Although the concordance between tissue and plasma ctDNA for T790M mutations was low, the PFS of the plasma ctDNA-positive group was similar to that of the plasma ctDNA-negative tissue T790M-positive group, suggesting its role in clinical decision-making. Relatively smaller tumor volume at detection of plasma ctDNA T790M during monitoring might affect treatment outcomes in a real-world setting.
Acknowledgements
We thank all patients, their families, caregivers and staff at all institutions.
Sites participating in this study were as follows:
• NTT Medical Center Tokyo
• Kasukabe Medical Center
• Japanese Red Cross Medical Center
• Kyorin University Hospital
• Iwate Prefectural Central Hospital
• National Center for Global Health and Medicine
• Showa University Hospital
• Fujisawa City Hospital
• Toranomon Hospital
• Kansai Electric Power Hospital
• National Hospital Organization Shibukawa Medical Center
• Mitsui Memorial Hospital
• Showa University Fujigaoka Hospital
• Gunma Prefectural Cancer Center
• Saitama Red Cross Hospital.
We would like to acknowledge the late Dr Yasuo Ohashi, a former Professor at Chuo University, for his valuable advice during this study and also for his contribution to the study conception and design, data acquisition, analysis and interpretation, as well as drafting and revising the article.
Conflict of interest statement
Dr Naka has received honoraria from AstraZeneca, Chugai Pharmaceutical and Ono Pharmaceutical. Dr Kishi has received honoraria from AstraZeneca. Dr Kunitoh has received honoraria from AstraZeneca, Johnson & Johnson, Taiho Pharmaceutical, Boehringer Ingelheim and Daiichi Sankyo. All remaining authors have declared no conflicts of interest.
Funding
This work (Clinical Trial Number: UMIN 000023248) was supported by AstraZeneca. The study funding was provided to the Comprehensive Support Project for Oncology Research (CSPOR) with support from an investigator-sponsored study program of AstraZeneca.
References
- 1. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- 2. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicenter, open-label, randomized phase 3 trial. Lancet Oncol 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- 3. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised phase 3 trials. Lancet Oncol 2015;16:141–51. [DOI] [PubMed] [Google Scholar]
- 4. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD 9291. Lung Cancer 2015;90:509–15. [DOI] [PubMed] [Google Scholar]
- 7. Usui K, Yokoyama T, Naka G, et al. Plasma ctDNA monitoring during epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor treatment in patients with EGFR-mutant non-small cell lung cancer (JP-CLEAR trial). Jpn J Clin Oncol 2019;49:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papadimitrakopoulou VA, Han JY, Ahn MJ, et al. Epidermal growth factor receptor mutation analysis in tissue and plasma from the AURA3 trial: osimertinib versus platinum-pemetrexed for T790M mutation-positive advanced non-small cell lung cancer. Cancer 2020;126:373–80. [DOI] [PubMed] [Google Scholar]
- 9. Minari R, Mazzaschi G, Bordi P, et al. Detection of EGFR-activating and T790M mutations using liquid biopsy in patients with EGFR-mutated non-small-cell lung cancer whose disease has progressed during treatment with first- and second-generation tyrosine kinase inhibitors: a multicenter real-life retrospective study. Clin Lung Cancer 2020;21:e464–73. [DOI] [PubMed] [Google Scholar]
- 10. Zhang S, Zhu L, Xia B, et al. Epidermal growth factor receptor (EGFR) T790M mutation identified in plasma indicates failure sites and predicts clinical prognosis in non-small cell lung cancer progression during first-generation tyrosine kinase inhibitor therapy: a prospective observational study. Cancer Commun 2018;38:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M mutant lung cancer. N Engl J Med 2017;376:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- 13. Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:1655–69. [DOI] [PubMed] [Google Scholar]
- 14. Hochmair MJ, Morabito A, Hao D, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: updated analysis of the observational GioTag study. Future Oncol 2019;15:2905–014. [DOI] [PubMed] [Google Scholar]


