Abstract
Cryopreservation was recommended to ensure continuity in allogeneic hematopoietic progenitor cells (HPC) transplantation during the COVID-19 pandemic. Several groups have shown no impact on clinical outcomes for patients who underwent HPC transplantation with cryopreserved products during the first months of this pandemic. However, concerns about quality control attributes after cryopreservation have been raised. We investigated, in 155 allogeneic peripheral blood cryopreserved HPC, leukocytapheresis characteristics influencing viable CD34+ and CD3+ cells, and CFU-GM recoveries after thawing. Collection characteristics such as volume, nucleated cells (NC)/mL and hematocrit correlated with viable CD34+ and CD3+ cells recoveries after thawing in univariate analysis but only CD3+ cells remained statistically significant in multivariate analysis (r2 = 0.376; P = < 0.001). Additionally, transit time also showed correlation with viable CD34+ (r2 = 0.186), CD3+ (r2 = 0.376) and CFU-GM recoveries (r2 = 0.212) in multivariate analysis. Thus, diluting leukocytapheresis below 200 × 106 NC/mL, avoiding red cells contamination above 2%, cryopreserving below 250 × 106 NC/mL and minimizing transit time below 36 h, prevented poor viable CD34+ and CD3+ cells, and CFU-GM recoveries. In summary, optimizing leukocytapheresis practices and minimizing transportation time may better preserve the quality attributes of HPC when cryopreservation is indicated.
Subject terms: Haematopoietic stem cells, Bone marrow transplantation
Introduction
Allogeneic hematopoietic progenitor cells (HPC) are usually transported as fresh products to be transfused without the need for a cryopreservation step [1]. However, cryopreservation has undoubted advantages, such as securing graft integrity before starting the conditioning regimen and the ease of logistics for transplant procurement. On the other hand, there are some concerns about cryopreservation such as products not being used, adverse reactions to DMSO and cell damage [2–4].
In 2020, because of the Coronavirus Disease 2019 (COVID-19) pandemic, widespread cryopreservation of allogeneic HPC was recommended by national authorities and international organizations to guarantee the receipt of a product before starting conditioning [5–7]. COVID-19 is still highly active in many countries, and in Europe, there has been an ongoing 6th wave during the winter of 2021–2022. Although the severity of the disease has decreased due to vaccinations and the omicron variant, the social and medical disease burden is still significant. The emergence of new variants has forced different countries to continually re-appraise travel restrictions and consider the need for HPC cryopreservation [6]. Several groups and registries have shown no differences in engraftment, acute graft versus host disease (GVHD), infections and early post-transplant survival during the first months of the COVID-19 pandemic when comparing cryopreserved versus fresh allogeneic hematopoietic stem cell transplantations (HSCT) [3, 8–11]. Despite these results, concerns about product quality after cryopreservation have been raised [10, 12]. In the setting of cord blood and autologous hematopoietic stem cell transplantation (HSCT), post thaw graft quality (viability and colony forming units (CFU)) has been demonstrated to influence recipient engraftment [13–15], but the factors influencing quality control attributes are known and mostly under control [16]. However, limited data are available about the effect of pre-cryopreservation characteristics on post thaw quality control of frozen allogeneic peripheral blood (PB) HPC.
The aim of the present study was to retrospectively investigate collection and transport factors that might influence the quality control attributes after cryopreservation (like cell viability and clonogenicity) of allogeneic PB HPC for transplantation. This assessment may harmonize collection center practices and transportation conditions policies when cryopreservation is recommended.
Materials and methods
Eligibility criteria
This is a collaborative study between Banc de Sang i Teixits (BST), Barcelona, Spain, and Anthony Nolan (AN), Nottingham, UK. Allogeneic related and unrelated, unmanipulated PB HPC that were cryopreserved from November 2019 to November 2021 were retrospectively included in the study. All donors and recipients provided written informed consent prior to collection and transplantation, respectively. The Vall d’Hebron Hospital’s Ethics Committee reviewed and approved the study [PR(BS)529/2021].
Apheresis collection
Leukocytapheresis was performed with either citrate dextrose solution A (ACDA) anticoagulant or ACDA + heparin according to collection center protocols and end time of collection was recorded. Products were hand-carried in validated boxes for delivery to processing facilities and kept refrigerated (2–8 °C) for overnight storage. Fresh graft characterization was performed in the associated transplant center (TC) cellular laboratory on leukocytapheresis material with: an automated complete blood count (CBC) using an hematology analyzer (XN550, Sysmex, Kobe, Japan; XE2000, Sysmex UK, Milton Keynes, UK) analysis of nucleated cells (NC) concentration, percentage of mononuclear cells (MNC), hematocrit and platelet concentration; phenotyping by flow cytometry (Navios, Beckman Coulter, Brea, EEUU; BD FACS Canto II, BD UK, Oxfordshire) assessing CD45+, CD34+ and CD3+ cells expression and viability assessment using 7-aminoactinomycin D (7-AAD) staining according to the single-platform International Society for Hematotherapy and Graft Engineering (ISHAGE) protocol; potency was analyzed with CFU granulocyte-macrophage (GM) assay using Methocult 4434 (Stem Cell Technologies, (Vancouver, Canada) supplemented with IMDM (Iscove’s Modified Dulbecco’s Medium, BIOWEST, Nuaillé, France). Frozen bone marrow proficiency testing program (stem cell technologies) was performed for quality control of CFU counts.
Cryopreservation
Cryopreservation was performed in two cellular processing laboratories (BST, cell therapy department, and AN Cell Therapy Centre, Nottingham, UK) and was destined for 4 TCs (BST) and 8 TCs (AN).
Prior to cryopreservation, HPC were plasma reduced. The cryopreservation solution was added volume: volume over 10 min at increasing speed, to a final concentration of 10% dimethyl sulfoxide (DMSO) and human serum albumin in Plasma-Lyte solution into a cryobag (50/250/750 dependent upon aliquot volume; Milteny). Final NC concentration and start time of cryopreservation was recorded. A maximum of 30 min was permitted between initial additions of cryoprotectant solution to initiation of cryopreservation. A controlled-rate/programmable freezer was used to produce a freezing rate of −1 °C/minute until −40 °C, then continuing with a rate of −5 °C/min until −150 °C; storage was performed at less than −150 °C in vapor phase or liquid nitrogen tanks.
Quality control of cryovial reference sample
Three 0.5 mL cryovials of the cells preparation were frozen in parallel with the cryobag, and remained for a minimum of 24 h in the same conditions as the cryobag. Then one cryovial was thawed using a water bath at 37 °C. Red cells lysis and dilution was performed prior to flow cytometry.
The same quality control tests were performed on the cryovial reference sample as in fresh leukacytopharesis, but adjusting flow cytometry gating for thawed cells and following a similar gating strategy as in the ISHAGE protocol [17].
Definitions
Transit time was considered as the time between the end of the collection procedure to the start of cryopreservation.
Recoveries were calculated as absolute number of NC, viable CD45+, CD34+, CD3+ cells and CFU-GM in the post thaw cryovial divided by absolute number of NC, viable CD45+, CD34+ and CD3+ cells, and CFU-GM in the initial fresh material sample and multiplied by 100.
Poor post thaw viability and clonogenicity were defined as less than 50% of viable CD34+ and CD3+ cells, and CFU-GM recoveries in a cryovial after thawing.
Study endpoints
We investigated factors, regarding graft characteristics and transit time, influencing the post thaw quality control attributes (viable CD34+ and CD3+ cells recoveries, and CFU-GM recovery) of allogeneic cryopreserved PB HPC, potentially resulting in reduced graft performance. Additionally, we evaluated the thresholds of those variables that predict poor viability and clonogenicity after cryopreservation.
Statistical analysis
Patient- (age, sex, weight and diagnosis), donor- (age, sex, weight, and type of donor (related or unrelated)), graft- (transit time, volume, NC/mL, % MNC, hematocrit, platelet/mL and CD45+, CD34+ and CD3+ cells viability), cell processing- (NC dose/kg, viable CD34+ and CD3+ cells dose/kg, CFU-GM/kg and NC/mL at cryopreservation) and post thaw quality control attributes (NC dose/kg, viable CD34+ and CD3+ cells dose/kg and CFU-GM/kg) were assessed. Median and range or frequency and percentage were used for quantitative and qualitative variables, respectively. The endpoints considered were viable CD34+ and CD3+ cells and CFU-GM recovery, in a cryopreserved reference sample of HPC. Mann-Whitney U test and Chi-Square test were performed for continuous and categorical variables, respectively.
A multivariate model was added, considering as explanatory variables all those with a p-value less than 0.1 in the bivariate analysis, and excluding those that presented collinearity problems. The final model was obtained using the Least Square Method to find the estimated multiple linear regression.
Statistical analysis was performed with the software R Studio (Version 4.1.2). The significance level was set at 0.05.
Results
Baseline characteristics of patients, donors and grafts
Our series comprises a total of 155 cryovial reference samples of allogeneic PB HPC that were cryopreserved from November 2019 to November 2021 (Table 1). Eighteen (12%) leukocytapheresis were performed for pediatric patients. Regarding donors, the median age was 34 years old, mostly men (67%) and 45% of them were unrelated donors. Patient’s and donors’ characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of patients and donors, and pre and post cryopreservation characteristics of HPC.
| Characteristics | |
|---|---|
| N = 155 cryiovial reference samples | |
| Patients | |
| Age (years), median (range) | 53 (2–70) |
| Sex, n (%) | |
| Male | 95 (61.3) |
| Female | 60 (38.7) |
| Weight (kg), median (range) | 70 (15–110) |
| Diagnosis, n (%) | |
| Acute leukaemia | 86 (55.5) |
| Lymphoma | 23 (14.8) |
| MDS/MPN | 22 (14.2) |
| Othersa | 24 (15.5) |
| Donors | |
| Age (years), median (range) | 34 (17–66) |
| Sex, n (%) | |
| Male | 104 (67.1) |
| Female | 51 (32.9) |
| Weight (kg), median (range) | 77 (51–141) |
| Type of donor, n (%) | |
| Related | 85 (54.8) |
| Unrelated | 70 (45.2) |
| Collected grafts | |
| Transit time (hours), median (range) | 23.3 (2.6–57) |
| Overseas, n (%) | 2 1 |
| Overnight prior cryopreservation, n (%) | 96 62 |
| Volume (ml), median (range) | 318.5 (84.0–782.9) |
| NC/mL (x106), median (range) | 176.2 (94.2–559.4) |
| MNC (%), median (range) | 84.5 (51.6–98.0) |
| Hematocrit, median (range) | 1.6 (0.2–6.1) |
| Platelet/mL (x106), median (range) | 1385.0 (491.0–3473.0) |
| Pre-freeze aliquots | |
| NC x 106/mL median (range) | 225.1 (73.5–469.1) |
| NC x 108 cell dose/kg, median (range) | 5.7 (1.6–18.3) |
| CD34+ x 106 cell dose/kg, median (range) | 6.0 (0.3–22.1) |
| CD3+ x 108 cell dose/kg, median (range) | 1.8 (0.4–6.1) |
| CFU-GM x 105/kg, median (range) | 9.3 (0.0–67.0) |
| Cryovial sample | |
| NC x 108/kg, median (range) | 5.6 (1.6–19.9) |
| Viable CD34+ x 106/kg, median (range) | 4.4 (0.3–19.7) |
| Viable CD3+ x 108/kg, median (range) | 1.0 (0.1–3.9) |
| CFU-GM x 105/kg, median (range) | 7.9 (0.4–38.0) |
| Viability, median (range) | |
| CD45+/7-AAD negative (%) | 70.3 (26.5–89.4) |
| CD34+/7-AAD negative (%) | 89.2 (7.9–100.0) |
| CD3+/7-AAD negative (%) | 61.5 (10.1–96.8) |
HPC hematopoietic progenitor cells, MDS/MPN myelodysplastic syndrome/myeloproliferative neoplasms, NC nucleated cells, MNC mononucleated cells, CFU-GM colony-forming units granulocyte/macrophage, 7-AAD 7-Aminoactinomicine D.
aEleven patients with chronic leukaemia, 6 with myeloma/plasma cell disorders, 4 with secondary acute leukaemia, 2 with inherited disorders, 1 with haemoglobinopathy and 1 with bone marrow failure.
We analyzed the graft’s characteristics (Table 1). The median time between ending collection and starting cryopreservation was 23.3 h. The median CD45+, CD34+ and CD3+ cells viability at cell processing reception was 99%, 100% and 100%, respectively.
Baseline characteristics of cryopreservation and quality control after thawing
One hundred and thirty-three (86%) and twenty-two (14%) products were processed in BST and AN laboratories, respectively. The median (range) of cells concentration at cryopreservation was 225 (74–469) NC/mL. Only, 16 (10%) leukocytapheresis were frozen with a final NC/mL higher than 250 × 106. The median (range) of CD34+ cells dose/kg, CD3+ cells dose/kg and CFU-GM/kg in frozen aliquots were 6.0 (0.3–22.1) x 106, 1.8 (0.4–6.1) x 108 and 9.3 (0.0–67) x 105, respectively.
A cryovial reference sample was thawed for each frozen cryobag to assess cell content, viability and clonogenicity (Table 1). The median post thaw cryovial viability of CD45+ 7-AAD-, CD34+ 7-AAD- and CD3+ 7-AAD- was 70%, 89% and 62%, respectively. The loss of viable CD34+, CD3+ cells and CFU-GM after cryopreservation was 19%, 43% and 14%, respectively (Table 1S. Supplementary data). Comparison between the two facilities is showed in supplementary data (Table 2S). Non relevant differences between laboratories were found regarding viable CD3 + cells and CFU-GM recoveries.
Variables influencing viable CD34+ and CD3+ cells recoveries after thawing
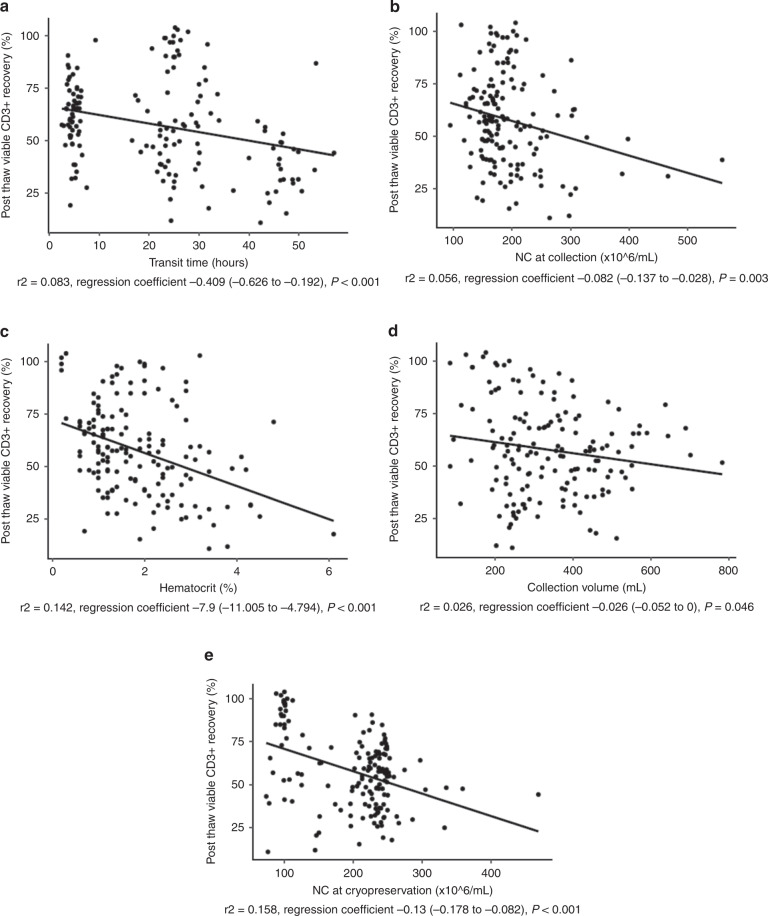
We investigated the effects of fresh cell product characteristics and transit time on the post thaw attributes of cryovial reference samples from cryopreserved allogeneic PB HPC. On univariate linear regression analysis, a weak correlation was observed between viable CD34+ cells recovery after cryopreservation and graft attributes such as NC/mL, MNC (%), CD45+ 7-AAD- (%) and CD3+ 7-AAD- (%), hematocrit and transit time. Interestingly, a higher correlation was observed between the above parameters for viable CD3+ cells recovery in cryovials than for viable CD34+ cells (Table 2). In multivariate analysis, only transit time and the percentage of CD45+ 7-AAD- at reception remained statistically significant with viable CD34+ cells after thawing. In contrast, regarding viable CD3+ cells recovery, more graft attributes (transit time, collection volume, NC/mL at collection and hematocrit) remained statistically significant (Table 2). Correlation of those variables and viable CD34+ and CD3+ cells recoveries are shown in Figs. 1 and 2, respectively.
Table 2.
Univariate and multivariate linear regression analysis of viable CD34+ and CD3+ cells, and CFU-GM recoveries on post thaw cryovial of cryopreserved HPC.
| Variable | Viable CD34+ cells recovery | Viable CD3+ cells recovery | CFU-GM recovery | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||||||
| r2 | RC (95% CI) | P | r2 | RC (95% CI) | P | r2 | RC (95% CI) | P | r2 | RC (95% CI) | P | R2 | RC (95% CI) | P | R2 | RC (95% CI) | P | |
| Female donor | 0.009 | −3.64 (−9.57 to 2.29) | 0.228 | 0.034 | 8.60 (.33 to 15.87) | 0.021 | 0.000 | −1.93 (−20.20 to 16.33) | 0.835 | |||||||||
| Unrelated donor | 0.022 | −5.45 (−11.23 to 0.32) | 0.064 | 0.025 | −6.90 (−13.80 to −0.00) | 0.05 | 0.072 | −29.30 (−46.45 to −12.14) | 0.001 | |||||||||
| Collection volume | 0.019 | −0.02 (−0.03 to 0.00) | 0.088 | 0.026 | −0.03 (−0.05 to 0.00) | 0.046 | 0.376 | −0.04 (−0.07 to −0.01) | 0.007 | 0.012 | 0.05 (−0.02 to 0.11) | 0.177 | ||||||
| Transit time | 0.085 | −0.35 (−0.53 to −0.17) | <0.001 | 0.186 | −0.29 (−0.48 to −0.10) | 0.003 | 0.083 | −0.41 (−0.63 to −0.19) | <0.001 | 0.376 | −0.32 (−0.54 to −0.11) | 0.004 | 0.073 | −0.94 (−1.49 to −0.39) | 0.001 | 0.212 | −2.43 (−3.38 to −0.49) | 0.019 |
| NC/mL collection | 0.027 | −0.05 (−0.09 to −0.00) | 0.041 | 0.056 | −0.08 (−0.14 to −0.03) | 0.003 | 0.376 | −0.06 (−0.12 to −0.01) | 0.032 | 0.010 | −0.09 −0.23 to 0.05) | 0.214 | ||||||
| % MNC collection | 0.033 | 0.39 (0.05 to 0.69) | 0.025 | 0.026 | 0.39 (0.05 to 0.73) | 0.026 | 0.004 | 0.37 (−0.62 to 1.53) | 0.462 | |||||||||
| CD45+ 7-AAD neg collection | 0.094 | 11.85 (5.96 to 17.73) | <0.001 | 0.186 | 7.76 (1.61 to 13.92) | 0.014 | 0.088 | 13.73 (6.66 to 20.80) | <0.001 | 0.025 | 18.36 (−0.28 to 37.00) | 0.053 | ||||||
| CD3+ 7-AAD neg collection | 0.044 | 6.74 (1.72 to 11.77) | 0.009 | 0.070 | 10.23 (4.30 to 16.17) | 0.001 | 0.018 | 12.65 (−2.78 to 28.08) | 0.107 | |||||||||
| Hto collection | 0.062 | −4.35 (−7.06 to −1.64) | 0.002 | 0.142 | −7.90 (−11.00 to −4.79) | < 0.001 | 0.376 | −5.64 (−9.32 to −1.96) | 0.003 | 0.035 | −9.87 (−18.34 to −1.40) | 0.023 | ||||||
| Platelet/mL collection | 0.002 | −0.00 (−0.01 to −0.00) | 0.604 | 0.112 | −0.00 (−0.01 to 0.00) | 0.174 | 0.026 | −0.01 (−0.03 to 0) | 0.05 | |||||||||
| NC/mL cryo | 0.047 | −0.06 (−0.10 to −0.02) | 0.007 | 0.186 | −0.07 (−0.11 to −0.03) | 0.001 | 0.158 | −0.13 (−0.18 to −0.08) | <0.001 | 0.376 | −0.10 (−0.16 to −0.05) | <0.001 | 0.011 | 0.09 (−0.05 to 0.22) | 0.204 | |||
CFU-GM colony-forming units granulocyte/macrophage, HPC hematopoietic progenitor cells, RC regression coefficient, CI confidence interval, 7-AAD 7-Aminoactinomicine D, NC nucleated cells, MNC mononucleated cells, Hto hematocrit.
Bold values indicate statistical significance.
Fig. 1. Variables affecting viable CD34+ cells recovery in the criovial.
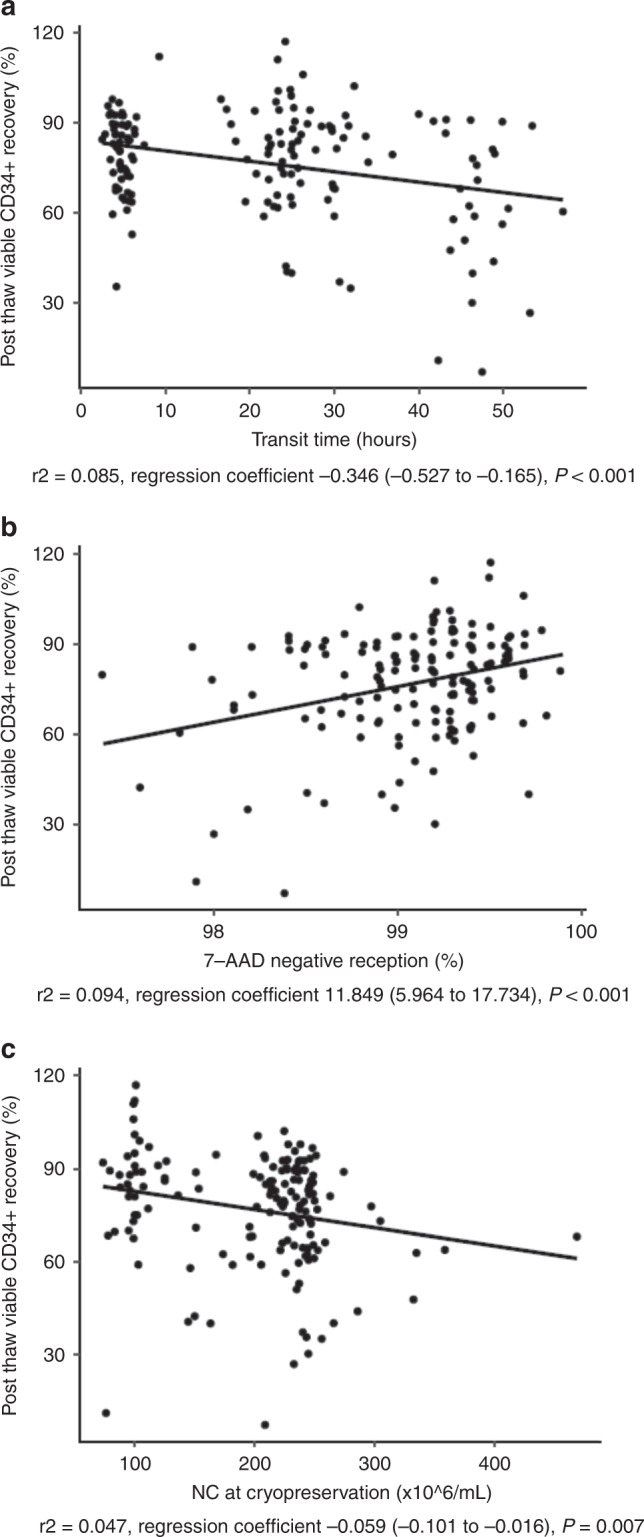
Viable CD34+ cells recovery in the cryovial is plotted against transit time (hours) (a), CD45+ cells 7-AAD- (%) at reception (b) and NC/mL (x106) at cryopreservation (c).
Fig. 2. Variables affecting viable CD3+ cells recovery in the criovial.
Viable CD3+ cells recovery in the cryovial is plotted against transit time (hours) (a), NC/mL (x106) collection (b), hematocrit (%) (c), collection volume (ml) (d) and NC/mL (x106) at cryopreservation (e).
Regarding donor characteristics, female and unrelated donors correlated weakly with viable CD3+ cells recovery after thawing in univariate but not multivariate analysis (Table 2).
HPC plasma reduction, considered as NC/mL at cryopreservation, weakly correlated with both viable CD34+ and CD3+ cells recoveries after thawing on univariate analysis but only with CD3+ cells recoveries in multivariate analysis (Table 2 and Fig. 2).
Variables influencing CFU-GM recovery after thawing
Donor characteristics (unrelated donor), graft attributes (transit time, hematocrit and platelet/mL) showed a weak correlation on univariate analysis (Table 2). However, only transit time remained statistically significant in multivariate analysis (Table 2). Weak correlation with transit time and CFU-GM recovery after thawing is shown in Fig. 3.
Fig. 3. 2. Variables affecting CFU-GM recovery in the criovial.
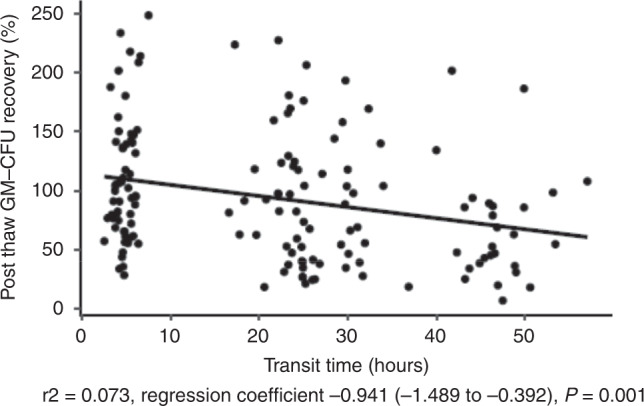
CFU-GM recovery (%) after thawing is plotted against transit time (h).
Viable CD34+ cells recovery has a weak correlation albeit significant with CFU-GM recovery (r2 = 0.033; P = 0.002)). However, even in cases with poor CD34+ cells recovery, CFU-GM recovery was observed (Fig. 1S. Supplementary data).
Dichotomy variables influencing poor viability and poor potency after thawing
Those variables that influenced the viable CD34+ and CD3+ cells, and CFU-GM recoveries after thawing in multivariate analysis were selected and converted into dichotomy variables.
Exploratory analysis between threshold dichotomy variables and significant poor viability (<50% viable CD34+ and CD3+ cells recoveries) and poor clonogenicity (<50% CFU-GM recovery) was performed (Table 3). Seven out of twenty-eight (25%) allogeneic PB HSC with more than 36 h from collection until starting cryopreservation had poor viable CD34+ cells recovery (P = 0.001). Only 36 h was statistically associated in univariate analysis with poor viability and clonogenicity (Table 3). Forty-eight (31%) of leukocytapheresis were collected with a higher concentration than 200 × 106 NC/mL and associated with poor viable CD3+ cells recoveries (P = 0.020). Fifty-eight (37%) grafts had a higher hematocrit than 2% and were associated with poor viable CD3+ cells recoveries (P = <0.001). Finally, plasma reduced HPC with a higher NC concentration than 250 × 106/mL were associated with poor viable CD34+ and CD3+ cells recoveries in univariate analysis (Table 3).
Table 3.
Dichotomy variables resulting in <50% viable CD34+ and CD3+ cells, and CFU-GM recoveries on postthaw cryovial of cryopreserved HPC.
| Variable | <50% viable CD34+ cell recovery | <50% viable CD3+ cell recovery | <50% CFU-GM recovery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | P | No | Yes | P | NO | Yes | P | |
| Transit time, n | 0.002 | <0.001 | 0.005 | ||||||
| ≤ 36 h | 121 | 6 | 90 | 37 | 98 | 23 | |||
| > 36 h | 21 | 7 | 5 | 23 | 15 | 13 | |||
| NC/mL at reception, n | 0.035 | 0.021 | 0.034 | ||||||
| ≤200 × 106 | 101 | 5 | 72 | 34 | 83 | 19 | |||
| >200 × 106 | 41 | 8 | 23 | 26 | 30 | 17 | |||
| Hto at reception, n | 0.005 | <0.001 | 0.142 | ||||||
| ≤2% | 95 | 3 | 71 | 27 | 74 | 18 | |||
| >2% | 47 | 10 | 24 | 33 | 39 | 18 | |||
| NC/mL at cryopreservation, n | 0.040 | 0.017 | 0.695 | ||||||
| ≤250 × 106 | 130 | 9 | 89 | 50 | 102 | 31 | |||
| >250 × 106 | 12 | 4 | 6 | 10 | 11 | 5 | |||
CFU-GM colony-forming units granulocyte/macrophage, HPC hematopoietic progenitor cells, NC nucleated cells, hto hematocrit.
Four samples showed a very poor viable CD34 + cells recovery (<30%) after thawing. All of them were cryopreserved at more than 42 h old from end of collection. Three samples were frozen as leftover aliquots and they have not been transfused to date. The only patient that underwent HSCT with a with very poor viable CD34 + cells recovery showed neutrophil and platelet engraftment at 17 and 87 days after transplantation, respectively. It reflects a delay in engraftment-mainly in platelets.
Discussion
We retrospectively analyzed pre-freeze variables affecting viability and clonogenicity after thawing of 155 samples of allogeneic cryopreserved PB HPC. In our study, transit time longer than 36 h influenced poor viable CD34+ and CD3+ cells recoveries and also poor CFU-GM recovery. Interestingly, viable CD3+ cells recoveries were affected more by graft attributes such as NC/mL >200 × 106, collection volume and hematocrit >2%. Finally, cryopreservation with an NC concentration higher than 250 × 106/mL was associated with poor viability but without affecting clonogenicity.
Several other observations in our study are worth mentioning. First, our data are consistent with recent scientific literature in terms of viable CD34+ cells recovery after thawing; Purtill D and colleagues reported a similar median of 74% CD34+ cells recoveries [10]. Other groups found >90% CD34+ cells recoveries [18, 19]. However, a central cryopreservation hub published a 42% viable CD34+cells recovery but 94% viability after thaw [20]. It is possible that this may be attributed to the fact that the assessment of cryopreserved material is more difficult even using a standardized ISHAGE protocol. This means that alternative approaches for viable CD34+ cells quantification after thawing may help in defining a more reproducible method [17]. Second, CD3+ cells recoveries were more sensitive to cryopreservation (lower than 60%), as others groups reported [18, 19]. Interestingly we observed new parameters associated with this impairment such as transit time, collection volume, NC/mL during transport, hematocrit and also with cells concentration at freezing (NC/mL at cryopreservation). Some differences between BST and AN were observed regarding viable CD3 + cells without affecting overall results. In our series, BST samples were cryopreserved with longer transit time and higher NC/mL cryopreservation than AN samples, leading to a lower viable CD3 + cells recovery.
Third, only transit time was associated with CFU-GM recovery, showing that clonogenicity of stem cells are well protected even when low viability is reported.
Leukocytapheresis is a well-defined and automated process. However, operators can potentially negatively affect product quality attributes by modulating collection parameters such as red blood cells contamination and NC/mL. Our study suggests that long collections, defined as high graft volumes, could result in poor CD3+ cells viability after thawing. It may be explained by cell damage due to prolonged centrifugation in the collection device. A higher hematocrit than 2% was also associated with poor CD3+ cells viability. Red blood cells contamination may also adversely affect T cells expansion in culture for advanced cell therapies [21]. Our group supports diluting products to a maximum of 200 × 106NC/mL after collection to preserve viability after thawing, a lower threshold than other groups [12]. In regards to cell processing after collection, plasma reduction of products to a higher than 250 × 106/mL was associated to a poor viability.
Despite different graft attributes factors influencing cells recovery after thawing, only transit time was associated in multivariate analysis with low viability and clonogenicity. In our study, allogeneic HPC cryopreserved more than 36 h after collection (estimated threshold for overseas products) were associated with poor viability as reported by Purtill D and colleagues [10], and with poor CFU-GM recovery. The creation of a central European cryopreservation site for Australian TC’s using DKMS registry donors has guaranteed a lower transit time and therefore, better quality control of HPC attributes [20]. Viable CD34+/kg and CFU-GM/kg pre and post cryopreservation impacts on engraftment of autologous HSCT and CBU transplantation [14, 22, 23]. However, limited data are available regarding impact of viable CD34+/kg and CFU-GM after thawing of cryopreserved allogeneic PB HPC. The COVID-19 pandemic made it necessary to cryopreserve allogeneic HPC, and mostly groups have shown no differences regarding engraftment and clinical outcomes versus fresh allogeneic HSCT [3, 8–11]. Probably, despite cell losses after cryopreservation, enough viable CD34+ cells dose and CFU-GM are usually transfused because a good mobilization is achieved from healthy donors, and therefore no clinical impact has been shown. However, even in those healthy donors, approximately 2% of them are at risk of poor mobilization [24] and the difference in weight between recipient and donor exists in allogeneic HSCT. So, cryopreservation of allogeneic HPC could potentially result in reduced graft performance in those situations. Therefore, good control of collection variables (transit time, NC/mL at transportation, collection volume and hematocrit) and transit time could better preserve the viability and clonogenicity after cryopreservation.
Nevertheless, our study has some limitations, first of all because of its retrospective design. Second, we focus our analysis on the CD34+ and CD3+ cells populations. Third, 2 different cell processing laboratories were involved but with the same cryopreservation and quality control methodology. Finally, CFU assays require training and experience to reduce variability. In spite of both laboratories are running external proficiency testing, CFU assessment is a temperamental assay and due to technician variation in identifying and counting colonies, pre and post processing inconsistencies and consequently interlaboratory performance variations can be expected as our study showed [14]. However, this issue is not affecting the general results nor our conclusions.
In summary, to the best of our knowledge, this is the first study to assess the impact of pre-freeze characteristics not only on CD34+ cells viability after thawing, but also on T cells lymphocytes and clonogenicity in allogeneic PB HPC. In our study, transit time longer than 36 h was associated with poor viability and clonogenicity after thawing. Diluting leukacytapheresis below 200 × 106NC/mL, avoiding red blood cells contamination higher than 2% hematocrit and cryopreserving with lower than 250 × 106 NC/mL may increase viability especially for CD3+ cells. These results may help to harmonize collection centers and transit time policies when cryopreservation is recommended.
Supplementary information
Acknowledgements
Authors want to thank Jesus Maroto and Silvia Tuset for their administrative support. Luciano Rodriguez for his manuscript review. We also want to acknowledge BST Cell Therapy Service technical team (Sonia Maroto, Maria Ruz, Yolanda Chacon, Sergio Arteaga, Marina Hortola, and Camila Cardona) and cellular laboratory (Silvia Torrents, Javier Algar, Begoña Amill, Margarita Blanco, Ruth Forner, Isabel Tarrago, Mireia Lloret, Mariam Jallow and Fatima Shettiyarand) for their effort. We would like to thank the scientific team at Anthony Nolan for their efforts in processing these donations (Pam Sami, Farzana Shah, Liam Wynn, Monika Jurgielewicz, Marnie Wilson, Zara Chiverton, Whitney Holden, Mimi Nolan and Jordan Wright) as well as those who helped with the signoff and issue (Compliance and Release team, Quality Team and Medical team) Also, we thank donors, patients, couriers, Spanish and other registries, and everyone who contributed to make HSCT possible.
Author contributions
Conception and design of the study: J.F., R.H., S.Q. Acquisition of data: J.F., A.G., Analysis and interpretation of data: J.F., R.H., S.Q. Drafting or revising the manuscript: J.F., R.H., J.C., C.A., A.G., E.V., L.M, N.R., M.C., G.A., J.M., A.M., R.M., C.D., C.F., D.V., M.L., A.A., E.G., N.G., L.M., E.C., J.V., M.L., D.G., S.G. All authors have approved the final article.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-022-01750-2.
References
- 1.Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the “cold shoulder”? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transpl. 2006;38:399–405. doi: 10.1038/sj.bmt.1705462. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt AH, Buk D, Platz A, van den Brink MRM. Cryopreservation for All Is No Option in Unrelated Stem Cell Transplantation. Comment on Dholaria B, et al. Securing the Graft During Pandemic: Are We Ready for Cryopreservation for All? Biol Blood Marrow Transpl. 2020;26:e145–6. doi: 10.1016/j.bbmt.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Sojo J, Azqueta C, Valdivia E, Martorell L, Medina-Boronat L, Martinez-Llonch N, et al. Cryopreservation of unrelated donor hematopoietic stem cells: the right answer for transplantations during the COVID-19 pandemic? Bone Marrow Transpl. 2021;56:2489–96. doi: 10.1038/s41409-021-01367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DH, Jamal N, Saragosa R, Loach D, Wright J, Gupta V, et al. Similar outcomes of cryopreserved allogeneic peripheral stem cell transplants (PBSCT) compared to fresh allografts. Biol Blood Marrow Transpl. 2007;13:1233–43. doi: 10.1016/j.bbmt.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Update of October 5th, 2020, of the guidelines of the Spanish National Transplant Organization (Spain Ministry of Health, Consumer Affairs and Social Welfare) about the impact of SARS-CoV-2 and COVID-19 on donation and transplantation, reference BV-ES-20200122-11. http://www.ont.es/infesp/Paginas/COVID-19.aspx.
- 6.Recommendations of the European Society for Blood and Marrow Transplantation (EBMT) regarding the situation generated by the COVID-19 pandemic. 2020 https://www.ebmt.org/sites/default/files/2020-03/EBMT%20COVID19%20guidelines%20v.4.3%20%282020-03-23%29.pdf
- 7.Recommendations of the World Marrow Donor Association (WMDA) regarding donor medical suitability in the situation generated by the COVID-19 pandemic 2021. https://share.wmda.info/display/DMSR/Coronavirus+-+COVID-19#/
- 8.Maurer K, Kim HT, Kuczmarski TM, Garrity HM, Weber A, Reynolds CG, et al. Impact of Cryopreservation and Transit Times of Allogeneic Grafts on Hematopoietic and Immune Reconstitution. Blood Adv. 2021;5:5140–9. doi: 10.1182/bloodadvances.2021005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abstracts from the 2021 ASH Annual Meeting and Exposition. Devine S. 2846;2021.
- 10.Purtill D, Hutchins C, Kennedy G, McClean A, Fraser C, Shaw PJ, et al. Good Engraftment but Quality and Donor Concerns for Cryopreserved Hemopoietic Progenitor Cell Products Collected During the COVID-19 Pandemic. Transpl Cell Ther. 2021;27:1022.e1–e6. doi: 10.1016/j.jtct.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valentini CG, Chiusolo P, Bianchi M, Metafuni E, Orlando N, Giammarco S, et al. Coronavirus disease 2019 pandemic and allogeneic hematopoietic stem cell transplantation: a single center reappraisal. Cytotherapy. 2021;23:635–40. doi: 10.1016/j.jcyt.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purtill D, Antonenas V, Chiappini P, Tong D, O’Flaherty E, Bajel A, et al. Variable CD34+ recovery of cryopreserved allogeneic HPC products: transplant implications during the COVID-19 pandemic. Blood Adv. 2020;4:4147–50. doi: 10.1182/bloodadvances.2020002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avery S, Shi W, Lubin M, Gonzales AM, Heller G, Castro-Malaspina H, et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117:3277–85. doi: 10.1182/blood-2010-08-300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Acker JP, Cabuhat M, Letcher B, Larrat L, McGann LE. Association of post-thaw viable CD34 þ cells and CFU-GM with time to hematopoietic engraftment. Bone Marrow Transpl. 2005;35:881–7. doi: 10.1038/sj.bmt.1704926. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Kim S, Kim H, Baek EJ, Jin H, Kim J, et al. Post-thaw viable CD34(+) cell count is a valuable predictor of haematopoietic stem cell engraftment in autologous peripheral blood stem cell transplantation. Vox Sang. 2008;94:146–52. doi: 10.1111/j.1423-0410.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- 16.Purtill D, Smith K, Devlin S, Meagher R, Tonon J, Lubin M, et al. Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124:2905–12. doi: 10.1182/blood-2014-03-566216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanza F, Saccardi R, Seghatchian J. New horizons on stem cell cryopreservation through the artificial eyes of CD34 using modern flow cytometry tools. Transfus Apher Sci. 2020;59:102785. doi: 10.1016/j.transci.2020.102785. [DOI] [PubMed] [Google Scholar]
- 18.Berens C, Heine A, Müller J, Held SA, Mayer K, Brossart P, et al. Variable resistance to freezing and thawing of CD34-positive stem cells and lymphocyte subpopulations in leukapheresis products. Cytotherapy. 2016;18:1325–31. doi: 10.1016/j.jcyt.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Fisher V, Khuu H, David-Ocampo V, Byrne K, Pavletic S, Bishop M, et al. Analysis of the recovery of cryopreserved and thawed CD34+ and CD3+ cells collected for hematopoietic transplantation. Transfusion. 2014;54:1088–92. doi: 10.1111/trf.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiercinska E, Schlipfenbacher V, Bug G, Bader P, Verbeek M, Seifried E, et al. Allogeneic transplant procurement in the times of COVID-19: Quality report from the central European cryopreservation site. J Transl Med. 2021;19:145. doi: 10.1186/s12967-021-02810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy OL, Stroncek DF, Panch SR. Improving CAR T cell therapy by optimizing critical quality attributes. Semin Hematol. 2020;57:33–38. doi: 10.1053/j.seminhematol.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo KH, Lee SH, Kim HJ, Sung KW, Jung HL, Cho EJ, et al. The impact of post-thaw colony-forming units-granulocyte/macrophage on engraftment following unrelated cord blood transplantation in pediatric recipients. Bone Marrow Transpl. 2007;39:515–21. doi: 10.1038/sj.bmt.1705629. [DOI] [PubMed] [Google Scholar]
- 23.Castillo N, Garcia-Cadenas I, Barba P, Martino R, Azqueta C, Ferra C, et al. Post-thaw viable CD45+ cells and clonogenic efficiency are associated with better engraftment and outcomes after single cord blood transplantation in adult patients with malignant disease. Biol Blood Marrow Transpl. 2015;21:2167–72. doi: 10.1016/j.bbmt.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Ings SJ, Balsa C, Leverett D, Mackinnon S, Linch DC, Watts MJ. Peripheral blood stem cell yield in 400 normal donors mobilised with granulocyte colony-stimulating factor (G-CSF): impact of age, sex, donor weight and type of G-CSF used. Br J Haematol. 2006;134:517–25. doi: 10.1111/j.1365-2141.2006.06223.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.