Abstract
The present study investigated the associations of serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels with clinicopathological variables and survival outcomes in Libyan patients with pancreatic ductal adenocarcinoma (PDAC). The clinicopathological variables of 123 patients with PDAC registered at the National Cancer Institute in Misurata, Libya, between 2010 and 2018 were retrospectively analyzed. Blood samples from these patients were analyzed for serum CEA and CA19-9 levels before treatment by electrochemiluminescence immunoassay (double antibody sandwich ELISA) on a Roche cobas e 602 modules. The relationships between CA19-9 and CEA serum levels with clinicopathologic variables and survival outcomes were analyzed using the Kaplan-Meier method, log-rank test and Cox regression analyzes. Cut-off values for serum CEA and CA19-9 levels were 5 ng/ml and 400 U/ml, respectively. The median serum levels of all patients with PDAC for CEA and CA19-9 were 8 ng/ml (1.1-377 ng/ml) and 389 U/ml (1-10,050 U/ml), respectively. Tumors with higher serum CEA and CA19-9 levels were found in 63 and 48% of patients, respectively. Higher CEA and CA19-9 serum levels were significantly associated with more indicators of a malignant phenotype, including a surgically unresectable tumor, unevaluable lymph nodes, advanced stages and distant metastases. Regarding survival, patients with higher serum levels of the biomarkers CEA and CA19-9 had shorter overall survival rates (P<0.016 and (P<0.014, log-rank, respectively) and lower disease-free survival rates (P<0.002 and P<0.0001, log-rank, respectively). The present study demonstrated significant clinical and prognostic value of serum levels of biomarkers CEA and CA19-9 for Libyan patients with PDAC. Moreover, patients with PDAC with higher serum CEA and CA19-9 levels had more aggressive tumors, higher rates of disease recurrence and shorter overall survival rates and thus required more vigilant follow-up. Further multinational studies with larger PDAC cohorts are warranted to confirm these findings in terms of improved clinical decision making, more effective management and improved survival.
Keywords: pancreatic ductal adenocarcinoma, carcinoembryonic antigen, carbohydrate antigen 19-9, ELISA, expressions, prognosis, survival outcomes
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignancies and the fourth leading cause of cancer-related mortality (1). Despite advances in diagnostic and therapeutic strategies over the past two decades, the prognosis for patients with PDAC remains poor and the 5-year survival rate is ~6% (2,3). PDAC usually causes no detectable symptoms in early stages and the majority of patients are diagnosed in advanced stages with poor survival rates (4). The difficulty in identifying patients at high risk of metastasis and/or recurrence highlights the challenge of the unsatisfactory prognosis of patients with PDAC.
Computed tomography (CT) and magnetic resonance imaging (MRI) are currently the predominant imaging modalities for the preoperative diagnosis of patients with PDAC. These imaging techniques can classify pancreatic cancer into resectable, borderline resectable, locally advanced and metastatic (5). This radiological classification is based on local vascular invasion and the presence of parenchymatous and peritoneal metastases and allows patients to be classified for either upfront surgery or oncologic treatment (6,7). Surgical treatment of resectable pancreatic tumor remains the optimum treatment option for this cancer (8,9). Therefore, careful and appropriate preoperative staging is important in patients with pancreatic cancer.
As PDAC is a group of heterogeneous diseases with different biological and clinical characteristics, the identification of prognostic and predictive markers is clinically relevant for improved management of the disease. Some gene and protein candidates have been reported to be associated with the disease progression of PDAC (10,11). These biomarkers could also be useful to clarify molecular PDAC subtypes that appear anatomically similar to aiding clinical management (7,12).
In particular, serum tumor markers are non-invasive additional tools that are increasingly used in various cancers including PDAC, to advance understanding of cancer pathophysiology, improve molecular stratification and thus achieve improved outcomes. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are tumor markers associated with both diagnosis and prognosis of patients with PDAC (13-16). CEA, a glycoprotein with a molecular weight of 180-200 kDa, was first isolated from fetal colon and colorectal cancer tissue in 1965(17). CEA is overexpressed in several cancers, including colon, breast, lung and thyroid cancers (18-20). Moreover, serum CEA level is elevated in 30-60% of patients with PDAC (21,22) and it is even suggested to be an independent predictor of poor survival rates in patients with PDAC (23).
CA 19-9 is a tumor-associated antigen with a half-life of 4-8 days, whose epitope has been shown to be the sialylated Lewis antigen (24). CA19-9 is the only biomarker currently recommended in the National Comprehensive Cancer Network guidelines for clinical use in PDAC (25). However, when analyzing serum CA19-9 levels, in a clinical setting, several constraints should be considered. This is because CA19-9 may not have sufficient sensitivity and specificity in patients with certain metastases or obstructive jaundice (26). Moreover, sialylated Lewis antigen-negative individuals, who comprise ~5-10% of the population, have low or no CA19-9 secretion (27). Although CA19-9 is not accurate enough to be used for screening asymptomatic individuals for PDAC, it is currently the most useful blood test for distinguishing pancreatic cancer from chronic or recurrent pancreatitis, with a sensitivity of 70-90% and a specificity of 68-91% (28,29). It is also one of the most important prognostic factors for both patients with resectable disease and those with unresectable disease (30,31). In patients with PDAC, high expression of CA19-9 was associated with positive nodal status, an advanced disease and low survival rates (32). Measurement of CA19-9 as a prognostic factor provides valuable information to support therapeutic decision making, as patients with high preoperative CA19-9 levels are expected to have early recurrence. An elevated tumor marker value also implies a high probability of residual disease after resection (33). Preoperative serum levels of CEA and CA19-9 can be helpful to identify a subgroup of patients with poor outcomes after surgery (34). Currently, there are no biomarkers that can be discovered in the time interval between carcinogenesis and invasion, when the disease is potentially curable, due to low sensitivity and specificity in early detectable small tumors (35). CEA and CA19-9 still are the most studied biological markers for establishing both diagnosis and prognosis in PDAC (16,36,37). CEA and CA19-9 as biological markers are independent predictive factors for the presence of advanced PDAC with a positive predictive value of 91.0% for the combination of CEA (>7.0 ng/ml) and CA 19-9 (>305 U/ml) in predicting the presence of advanced disease (37). The aim of this study was to investigate the serum levels of CEA and CA19-9 and their associations with clinicopathological variables and survival outcomes in Libyan patients with PDAC.
Patients and methods
Clinicopathological data
The study group consisted of 123 patients with PDAC diagnosed between 2010 and 2018 at the National Cancer Institute in Misurata, Libya. Biological markers included serum levels of CEA and CA19-9, were determined in all patients before any treatment. The present study was conducted under research ethics approval by ethical committee at the National Cancer Institute, Misurata (Ethical Approval Number: EAN 6/2021). Written informed consent was obtained from all patients for surgical treatment, pathologic examinations and investigations performed according to the institutional guidelines of the National Cancer Institute, Misurata, Libya. Complete demographic and clinicopathological data included age, gender, family history, tumor location and size, lymph node status, stage, histological grade, type of treatment and follow-up data. These clinicopathological data were obtained from the patients' records and are summarized in Table I. The mean age of the patients was 61.2 years (range, 19-90 years). Tumor staging of pancreatic adenocarcinoma was evaluated according to the TNM classification (38). Radiological staging by Computed Tomography (CT) and/or Magnetic Resonance Imaging (MRI) was performed in all patients to assess tumor resectability. The extent of the tumor (local and distant) at the time of diagnosis was confirmed by imaging [CT, MRI, or Positron Emission Tomography (PET)]. Lymph node status was also assessed radiologically and positivity for malignancy was confirmed by histopathology of surgically resected nodes.
Table I.
Clinicopathological variables of 123 patients with pancreatic ductal adenocarcinoma.
| Variables | Number of patients (n=123) | Percent (%) |
|---|---|---|
| Age (years) | ||
| <50 | 24 | 19.5 |
| ≥50 | 99 | 80.5 |
| Sex | ||
| Male | 73 | 59.3 |
| Female | 50 | 40.7 |
| Family history | ||
| Positive | 6 | 4.9 |
| Negative | 117 | 95.1 |
| Tumor site | ||
| Head | 96 | 78.0 |
| Tail | 15 | 12.2 |
| Body | 12 | 9.8 |
| Surgical resectability | ||
| Resectable | 24 | 19.5 |
| Unresectable | 99 | 80.5 |
| Tumor size | ||
| T1 | 4 | 3.3 |
| T2 | 13 | 10.6 |
| T3 | 14 | 11.4 |
| T4 | 1 | 0.8 |
| Cannot be assessed | 91 | 74.4 |
| Lymph node status | ||
| Negative | 12 | 9.8 |
| Positive | 14 | 11.4 |
| Cannot be assessed | 97 | 78.9 |
| M | ||
| M0 | 27 | 22.0 |
| M1 | 96 | 78.0 |
| Histology grade | ||
| G1 | 6 | 4.9 |
| G2 | 30 | 24.4 |
| G3 | 87 | 70.7 |
| Stage | ||
| Stage 1 | 12 | 9.8 |
| Stage 2 | 12 | 9.8 |
| Stage 3 | 3 | 2.4 |
| Stage 4 | 96 | 78.0 |
| Systemic treatment | ||
| Adjuvant chemotherapy | 20 | 16.3 |
| Palliative chemotherapy | 81 | 65.9 |
| No treatment | 22 | 17.9 |
| (supportive therapy) |
The overall time to recurrence was estimated as the interval between the date of surgery and the date of recurrence (local and/or distant). Disease recurrence (local and distant) was confirmed by imaging (CT, MRI, or PET) performed when clinical symptoms suggestive of disease recurrence were present. Disease-free survival was defined as the time between initial treatment and last follow-up that patients survive without disease recurrence.
Treatment and follow-up
Approximately 21 patients were treated by pancreaticoduodenectomy (Whipple procedure), while palliative surgery was performed in three patients and no surgery was performed in 99 patients who had metastases at the time of diagnosis. However, fine needle aspiration biopsy with image guidance and endoscopic retrograde cholangiopancreatography were performed in these patients for histopathological diagnosis. In the National Cancer Institute in Misurata the following guidelines were established: Adjuvant combined chemotherapy (gemcitabine and oxaliplatin) was given to 18 patients while 2 patients received combined chemotherapy based on FOLFIRINOX (folinic acid, fluorouracil, irinotecan and oxaliplatin) and 81 patients received palliative chemotherapy with capecitabine and/or gemcitabine. In addition, 22 patients were not eligible for chemotherapy, so these patients did not receive chemotherapy. Patients were followed-up until death or the end of the observation period (until October 2018). The median duration of follow-up was 6 months (range 1-41 months). At the end of the follow-up period, 91 patients (74%) had succumbed to pancreatic cancer.
CEA and CA19-9 measurement
Prior to each treatment, approximately 5 ml of peripheral fasting blood was drawn from the forearm veins. The blood was immediately taken to the central laboratory of the National Cancer Institute in Misurata and then routinely centrifuged for 10 min at speed of 1,792 x g at 20-22˚C temperature. The serum samples were first stored at 4˚C. Then they were placed in polypropylene vials and stored at -80˚C. The concentrations of CEA and CA19-9 in serum were determined using an electrochemiluminescence immunoassay (double antibody sandwich ELISA, cat. nos. TM E-4131 and TM E-4531 respectively; Labor Diagnostika Nord GmbH & CoKG) on a Roche cobas e 602 modules (Roche Diagnostics). This technology uses a sandwich chemiluminescence immunoassay; Chemibeads contain a chemiluminescent dye and Sensibeads contain a photosensitizer dye. Biotinylated antibodies (1:100) and Chemibeads form sandwiches and immune complexes are formed by further addition of Sensibeads. A chemiluminescence reaction is initiated at 680 nm and finally the signal is detected at 612 nm (according to the manufacturer's instructions). The accuracy of internal and external quality controls was determined according to the guidelines of RiliBAeK (17). The detection limit and blank limit were as follows: CEA: 0.2 and 0.12 ng/ml, CA19-9: 2.0 and 1.0 U/ml, CA15-3: 1.0 and 0.3 U/ml, respectively. Roche's original ancillary reagents were used, including streptavidin-coated magnetic beads, anti-CEA monoclonal antibody and biotinylated anti-CA19-9 and anti-CEA monoclonal antibody and Ru-labelled anti-CA19-9.
Statistical analysis
Continuous variables were calculated using SPSS 26.0 for Windows (IBM Corp.). Frequency tables were analyzed using the χ2 or Fisher's exact tests to evaluate the power of association between categorical variables. Kaplan-Meier curves were constructed for survival rate analysis and differences between curves were analyzed using the log-rank test. Multivariate survival analysis for the outcome [overall survival and disease-free survival (DFS)] was performed using the proportional hazard Cox model in a backward stepwise manner with the log-likelihood ratio (L-R) significance test, using standard values for the entry and exclusion criteria. The cut-off point for CEA of 5 ng/ml and for CA19-9 of 400 U/ml was used to distinguish between high-expression and low-expression tumors as it provided the best results for prognosis prediction in this and other studies (39,40). The assumption of proportional hazards was controlled by log-minus-log (LML) survival plots. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient demographic and clinicopathologic variables. The demographic and clinicopathologic variables are shown in Table I. The mean age of the patients was 61.2 years (range, 19-90 years) and the majority of patients (80.5%) were >50 years old. Regarding sex distribution, PDAC was commoner in men (59.3%). A total of 4.9% of patients had a family history of pancreatic cancer and in 96 patients the tumors were located in the head of the pancreas (87%). The majority of patients (80.5%) had distant metastases or locally advanced unresectable disease. The most common T stage was Tx (74.4%), followed by T3, T2, T1 and T4 in decreasing frequency (11.4, 10.6 and 83.3 and 0.8%, respectively). A total of 14 patients (11.4%) had positive lymph nodes and negative lymph nodes were detected in 12 patients, while lymph node status could not be assessed in the majority of patients (78.9%). Most patients had high-grade tumors (70.7%). According to the AJCC staging system, 99 patients were at stage IV (78%), 3 patients were at stage III, 12 patients were at stage II and 12 patients were at stage I (38). Regarding treatment, 24 patients were treated by radical surgery, while palliative surgery was performed in three patients and no surgical intervention was performed in 99 patients. Adjuvant chemotherapy was performed in 20 patients, 81 patients received palliative chemotherapy and 22 patients were not eligible for chemotherapy.
General description of CEA and CA19-9 expression profiles
CEA and CA19-9 expressions at cut point of (5 ng/ml and 400 U/ml, respectively) are shown in Table II. The median CEA expression was 8 ng/ml (mean 22.8; range 1.1-377 ng/ml). CEA expression was low in 46 samples (<5 ng/ml) and high in 77 samples (≥5 ng/ml). The median value of CA19-9 was 389 U/ml (mean 762.4; range 1-10050 U/ml). CA19-9 was low in 64 samples (<400 U/ml) and high in 59 samples (≥400 U/ml). CEA expression was more frequent in tumors with high CA19-9 than in patients with low CA19-9 (P<0.0001).
Table II.
CEA and CA19-9 expression in Libyan pancreatic ductal adenocarcinoma. CEA level at cut point of 5 ng/ml and CA 19.9 level at cut point of 400 U/ml.
| Biological variables | Number of patients (n=123) | Percent (%) |
|---|---|---|
| CEA level (ng/ml) | ||
| <5 | 46 | 37.4 |
| ≥5 | 77 | 62.6 |
| CA 19-9 level (U/ml) | ||
| <400 | 64 | 52.0 |
| ≥400 | 59 | 48.0 |
CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen.
Correlation of CEA expression with clinicopathological variables
The significant correlations between CEA expression (<5 ng/ml vs. ≥5 ng/ml) and clinicopathological variables are shown in Table III. High CEA expression was significantly associated with surgically unresectable tumor (P<0.020), unevaluable lymph node status (P<0.007), advanced stages (P<0.0001) and distant metastases (P<0.002). However, age at diagnosis, gender, family history, tumor site, tumor size and histological grade showed no significant relationship with CEA expression.
Table III.
The association between CEA and CA19-9 expressions with clinicopathological variables in pancreatic adenocarcinoma cancer (n=123). Comparison between low CEA and CA19-9 expression group and high expression group.
| CEA expression (%) | CA19-9 expression (%) | ||||||
|---|---|---|---|---|---|---|---|
| Clinicopathological variable | Number | <5 ng/ml | ≥5 ng/ml | P-value | P-value | ||
| Age /years | 0.91 | 0.25 | |||||
| <50 | 24 | 37.5 | 62.5 | 62.5 | 37.5 | ||
| ≥50 | 99 | 37.4 | 62.6 | 49.5 | 50.5 | ||
| Sex | 0.38 | 0.91 | |||||
| Male | 73 | 34.2 | 65.8 | 52.1 | 47.9 | ||
| Female | 50 | 42.0 | 58.0 | 52.0 | 48.0 | ||
| Family history | 0.07 | 0.08 | |||||
| Positive | 6 | 16.7 | 83.3 | 33.3 | 66.7 | ||
| Negative | 117 | 38.5 | 61.5 | 53.0 | 47.0 | ||
| Site | 0.96 | 0.37 | |||||
| Head | 96 | 37.5 | 62.5 | 54.2 | 45.8 | ||
| Elsewhere | 27 | 37.0 | 63.0 | 44.4 | 55.6 | ||
| Surgical resectability | 0.020 | 0.020 | |||||
| Resectable | 24 | 58.3 | 41.7 | 75.0 | 25.0 | ||
| Unresectable | 99 | 32.3 | 67.7 | 46.5 | 53.5 | ||
| T stage | 0.092 | 0.091 | |||||
| T1 | 4 | 50.0 | 50.0 | 50.0 | 50.0 | ||
| T2 | 13 | 69.2 | 30.8 | 76.9 | 23.1 | ||
| T3 | 14 | 42.9 | 57.1 | 71.4 | 28.6 | ||
| T4 | 1 | 0.00 | 100 | 0.00 | 100 | ||
| Tx | 91 | 31.9 | 68.1 | 46.2 | 53.8 | ||
| Lymph node status | 0.007 | 0.001 | |||||
| Positive | 12 | 50.0 | 50.0 | 85.7 | 14.3 | ||
| Negative | 14 | 83.3 | 16.7 | 66.7 | 33.3 | ||
| Nx | 97 | 29.9 | 70.1 | 45.4 | 54.6 | ||
| Histological grade | 0.26 | 0.62 | |||||
| G1 | 6 | 33.3 | 66.7 | 50.0 | 50.0 | ||
| G2 | 30 | 50.0 | 50.0 | 60.0 | 40.0 | ||
| G3 | 87 | 33.3 | 66.7 | 49.4 | 50.6 | ||
| Stage | <0.0001 | <0.0001 | |||||
| Early stage | 24 | 70.8 | 29.2 | 83.3 | 16.7 | ||
| Late stage | 99 | 29.3 | 70.7 | 44.4 | 55.6 | ||
| Metastasis | 0.002 | 0.002 | |||||
| M0 | 27 | 63.0 | 37.0 | 77.8 | 22.2 | ||
| M1 | 96 | 30.2 | 69.8 | 44.8 | 55.2 | ||
Bold type indicates statistically significant values. CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen.
Correlation of CA19-9 expression with clinicopathological variables
The correlations between CA19-9 expression at the cut point of 400 U/ml and clinicopathological variables are shown in Table III. High CA19-9 expression was more common in patients with surgically unresectable tumor (P<0.02), unevaluable lymph node status (P<0.001), advanced stage (P<0.0001) and distant metastases (P<0.002). However, age at diagnosis, gender, family history, tumor site, tumor size and histological grade showed no significant association with CA19-9 expression.
Correlation of serum CEA and CA 19-9 expression patterns with patient survival outcomes
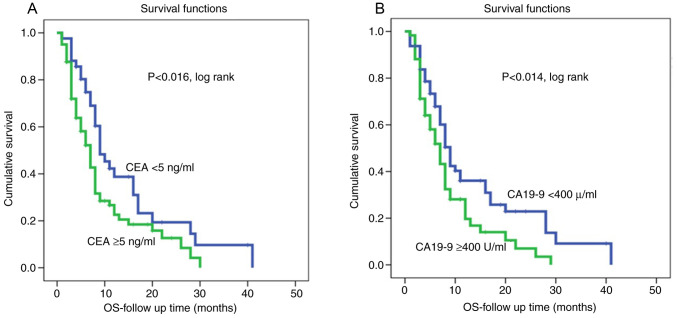
Univariate survival analyzes (survival rates) with CEA expression at a cut-off point of 5 ng/ml and CA19-9 at a cut-off point of 400 U/ml are shown in Table IV. The survival rate was 28.6% in patients with low CEA expression and 24.7% in patients with high expression profile (P<0.016). The low CA 19-9 expression group had an improved survival rate than the high expression group (31.3 and 20.3%, respectively) (P<0.014).
Table IV.
Univariate survival according to analysis of CEA expression (cut point of 5 ng/ml) and CA19-9 (cut point of 400 U/m) in Libyan pancreatic ductal adenocarcinoma (n=123).
| Survival analysis | ||||||
|---|---|---|---|---|---|---|
| Variables | Threshold | No of patients | Median survival (months) | Mean survival (months) | Survival rate (percent) | P-value |
| All patients | 123 | 6.00 | 8.55 | 26.0 | ||
| CEA level | 0.016 | |||||
| <5 | 46 | 8.00 | 11.02 | 28.6 | ||
| ≥5 | 77 | 5.00 | 7.27 | 24.7 | ||
| CA19-9 level | ||||||
| <400 | 64 | 7.00 | 9.89 | 31.3 | 0.014 | |
| ≥400 | 59 | 5.00 | 7.10 | 20.3 | ||
Bold type indicates statistically significant values. CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen.
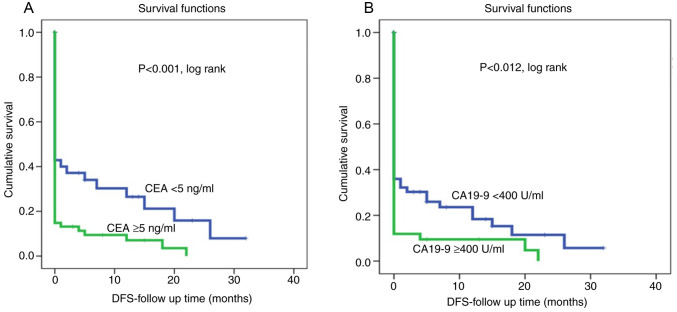
Kaplan-Meier survival curves for both CEA and CA19-9 levels showed that shorter survival was associated with high CEA and high CA19-9 levels (Fig. 1). On the other hand, patients with low CEA and CA19-9 levels were associated with a lower recurrence rate and therefore had longer disease-free survival (P<0.002 and P<0.0001, log rank, respectively, (Fig. 2). However, multivariate Cox regression analysis revealed that both serum CEA and CA19-9 levels were not independent markers of patient survival in relation to patient age, lymph node status, tumor grade and tumor stage.
Figure 1.
OS according to analysis of CEA expression and CA19-9 in pancreatic adenocarcinoma (Kaplan-Meier curves). (A) CEA level at cut point of 5 ng/ml. (B) CA 19-9 level at cut point of 400 U/ml. OS, overall survival; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen.
Figure 2.
DFS according to analysis of CEA expression and CA19-9 in pancreatic adenocarcinoma (Kaplan-Meier curves). (A) CEA level at cut point of 5 ng/ml. (B) CA 19-9 level at cut point of 400 U/ml. DFS, disease free survival; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen.
In addition, the correlation of surgical and/or systemic treatment with clinicopathologic variables and patient outcomes was observed in the present study, as shown in Table V. Patients who underwent radical surgical treatment were associated with small tumor size (P<0.0001), negative lymph node status (P<0.0001), early stage (P<0.0001), low expression of CEA level (P=0.020) and low expression of CA19-9 level (P=0.020). Survival was 37.5% in patients with resectable tumor and 23.2% in patients with non-resectable tumor (P<0.0001). Analysis using Kaplan-Meier survival curves for surgical resectability showed that shorter survival was associated with non-resectable tumor patients (P<0.0001). The best survival was observed in patients receiving adjuvant chemotherapy compared with patients receiving palliative chemotherapy or supportive care only (35.0, 27.3 and 23.5%, respectively).
Table V.
Surgical resectability accordance to demographic, clinicopathological and biological features in Libyan pancreatic ductal adenocarcinoma (n=123).
| Surgical resectability | ||||
|---|---|---|---|---|
| Clinicopathological variable | Number | Resectable | Unresectable | P-value |
| Age /years | 0.25 | |||
| <50 | 24 | 37.5 | 62.5 | |
| ≥50 | 99 | 37.4 | 49.5 | |
| Sex | 0.72 | |||
| Male | 73 | 20.5 | 79.5 | |
| Female | 50 | 18.0 | 82.0 | |
| Family history | 0.08 | |||
| Positive | 6 | 0.00 | 100.0 | |
| Negative | 117 | 20.5 | 79.5 | |
| Site | ||||
| Head | 96 | 19.8 | 80.2 | 0.88 |
| Elsewhere | 27 | 18.5 | 81,5 | |
| T stage | <0.0001 | |||
| T1 | 4 | 100.0 | 0.00 | |
| T2 | 13 | 69.2 | 30.8 | |
| T3 | 14 | 57.1 | 42.9 | |
| T4 | 1 | 0.00 | 100.0 | |
| Tx | 91 | 3.3 | 96.7 | |
| N | <0.0001 | |||
| Positive | 12 | 21.4 | 78.6 | |
| Negative | 14 | 83.3 | 3.1 | |
| Nx | 97 | 3.1 | 96.9 | |
| Histological grade | 0.62 | |||
| G1 | 6 | 83.3 | 16.7 | |
| G2 | 30 | 36.7 | 63.3 | |
| G3 | 87 | 9.2 | 90.8 | |
| Stage | <0.0001 | |||
| Early stage | 24 | 79.2 | 20.8 | |
| Late stage | 99 | 5.1 | 94.9 | |
| CEA level (ng/ml) | 0.020 | |||
| <5 | 46 | 30.4 | 69.6 | |
| ≥5 | 77 | 13.0 | 87.0 | |
| CA19-9 level (U/ml) | 0.020 | |||
| <400 | 64 | 28.1 | 71.9 | |
| ≥400 | 59 | 10.2 | 89.8 | |
Bold type indicates statistically significant values. CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen.
Discussion
PDAC is an aggressive heterogenous malignancy associated with a very poor prognosis and imposes an enormous burden on patients, their families and the healthcare system as a whole. Therefore, the identification and evaluation of diagnostic, prognostic and predictive markers is urgently needed for clinicians to achieve improved outcomes. Unfortunately, most patients are often diagnosed with metastatic disease or locally advanced, unresectable tumor (2-4,41). Although newer techniques have been used and target groups have been rigorously selected, screening programs are still ineffective for a large group and may be harmful, as noted in 2019(42). Therefore, regular screening with endoscopic ultrasound and MRI/CT imaging is recommended for individuals who are at high risk for genetic diseases (43,44). Therefore, treatment plans are based on the extent of the tumor, performance status of patients and various other clinical factors. However, not all patients benefit from conventional cancer therapy (45). Despite marked advances in treatment strategies, advanced PDAC cancer remains incurable and the goals of therapy range from relief of symptoms to prolonging survival. Of note, surgical treatment of a resectable tumor remains the best treatment option for this cancer (8,9). A number of studies suggest that multiple genes may correlate with PDAC patient outcomes (10,11). Moreover, tumor biology is heterogeneous in patients with PDAC with similar tumor anatomy (12,13). This highlights the importance of appropriate preoperative radiological PDAC staging and molecular and genetic stratification/classification to understand the biological behavior of this highly aggressive malignancy. Numerous studies have investigated the efficacy of various biological markers as diagnostic, predictive and prognostic markers in PDAC (22,23,34,38,46). Among them, CEA and CA19-9 are the most commonly used tumor biomarkers.
The present study performed a detailed retrospective analysis of 123 patients with PDAC diagnosed and treated at the National Cancer Institute, Misurata, Libya. CEA and CA19-9 expression levels at cut-off points (5 ng/ml and 400 U/ml, respectively) were found to be the most promising discriminators of both clinicopathological variables and survival outcomes.
In the cohort of present study, the median value of CEA in serum was 8 ng/ml and high CEA expression was detected in 63% of patients. The median value of CA19-9 in serum was 389 U/ml and high CA19-9 expression was detected in 48% of patients. In addition, the present study showed that the majority of Libyan patients had higher malignancy grade, such as locally advanced inoperable tumors, poorly undifferentiated tumors and distant metastases. Tumor size and lymph node status could not be determined in 75 and 79% of tumors, respectively. On the other hand, only 20% of patients had resectable tumors and 24 patients had early-stage PDAC at the time of diagnosis. These figures are consistent with other published data, which also reported that only 20% of patients diagnosed with PDAC were eligible for surgical resection and the majority of patients had advanced disease stage at the time of diagnosis (42,47-50).
Patients with high expression of CEA and/or CA19-9 are often associated with a worse prognosis. Therefore, increased expression of CEA and CA19-9 might indicate that the tumors are already at an advanced stage. Moreover, tumor progression was associated with higher levels of these tumor markers. Consistent with these findings, Lee et al (45) report that expression of CEA and CA19-9 is closely associated with tumor differentiation degree, lymph node metastasis and tumor progression. The aggressive behavior of PDAC, reflected in both unclear clinical symptoms at presentation and genetic heterogeneity of the tumor, may explain the advanced stages of the disease at diagnosis (41,45).
The present study also showed that the highly aggressive malignant phenotype of PDAC, manifested by surgically unresectable tumors, unevaluable lymph nodes, advanced stage and distant metastases, was significantly associated with high serum CEA and CA19-9 expression profiles. On the other hand, surgically resectable tumors, negative lymph nodes, early stage and low risk of metastasis were more common in the group with low serum CEA and CA19-9 expression. CEA expression was more frequent in tumors with high CA19-9 expression compared with those with low CA19-9 expression (P<0.0001). The most important finding of the present study was undoubtedly the significant correlation of CEA and CA19-9 expression with disease progression, especially overall survival and disease-free survival. The median follow-up time of the cohort study was six months and ~74% of patients had died of PDAC at the end of the follow-up period. Patients with low serum CEA and CA19-9 levels had a lower recurrence rate and lived longer than their counterparts with high serum levels. Analysis using Kaplan-Meier curves also showed that short survival was more common in the group with high CEA and CA19-9 levels, while the group with low CEA and CA19-9 levels had longer disease-free survival (P<0.002 and P<0.0001, respectively). These findings are consistent with the results of other previous studies (34,38,46), which indicated that Libyan patients with high expression of CEA and CA19-9 were associated with poor prognosis. Although associations between serum CEA and CA19-9 levels and treatment outcomes are found in numerous studies, some discrepancies are also reported. While some studies agreed with the findings of the present study and suggest that overexpression of CEA is associated with poor prognosis (6,38,46), others such as the study by Poruk et al (39) reported that CA19-9 is a useful biological marker in PDAC to predict disease extent, surgical resectability, disease progression and response to treatment. The present study showed that the combination of CEA and CA19-9 is more successful for prognosis prediction than a single biological marker (47-49). Therefore, Xu et al (50) showed that elevated CEA levels in combination with CA19-9 can significantly increase the prognostic efficacy of CA19-9. The present study confirmed this association and found that CEA expression was more frequent in tumors with high CA19-9 compared with patients with PDAC with low CA19-9.
The present study showed that the prognostic value of CEA and CA19-9 was almost the same and both markers did not to act as independent prognosticators for the outcome of patients with PDAC. However, van Manen et al (37) reported that both biological markers (CEA and CA19-9) were independent predictors for an advanced PDAC cohort from the Netherlands and that the predictive power of CEA was higher than that of CA19-9. These discrepancies could be due to several factors, including cohort size, diagnostic techniques (imaging and/or biopsies), genomic background of the cohort (ethnicity) and/or stage at diagnosis. However, other reports have found that elevated CEA and advanced stage are independent factors for poor survival of patients with PDAC (51,52).
The present study confirmed the role of both CEA (>8 ng/ml) and CA19-9 (>389 U/ml) serum levels as key markers for multidisciplinary management of Libyan patients with PDAC to aid decision making. Despite their significant associations with survival outcomes, neither marker was informative enough to serve as an independent predictor for advanced patients with PDAC.
The median serum levels of CEA and CA19-9 for all PDAC tumors were 8 ng/ml and 389 U/ml, respectively. Tumors with higher serum CEA and CA19-9 levels were found in 62.6 and 48% of PDAC cases, respectively. Significantly, patients with PDAC with higher CEA and CA19-9 serum levels had aggressive tumor grade, higher recurrence rate and shorter survival time and should be treated carefully. The prognostic value of CEA and CA19-9 was almost equivalent but not sufficient to be an independent prognosticator of patient outcome. An extended multinational study with a larger cohort is needed to confirm the prognostic value of both serum levels and tissue expression of these two promising markers.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
EE performed statistical analysis, designed the present study and drafted manuscript. MEd designed the study and collected demographic and clinicopathologic data and performed laboratory work and data analysis. OA, MEl and AJ collected and analyzed data and drafted the manuscript. MAS and MA performed data interpretation and analysis, drafting and proof reading and discussions. AB prepared the figures, reviewed the study, interpretated data and aided in drafting and proof reading of the manuscript. All authors critically reviewed and approved the final version of the manuscript. EE and MEl confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was conducted under research ethics approval by ethical committee at the National Cancer Institute, Misurata (Ethical Approval Number: EAN 6/2021). Written informed consent was obtained from all patients for surgical treatment, pathologic examinations and investigations performed according to the institutional guidelines of the National Cancer Institute, Misurata, Libya.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Petrushnko W, Gundara JS, De Reuver PR, O'Grady G, Samra JS, Mittal A. Systematic review of peri-operative prognostic biomarkers in pancreatic ductal adenocarcinoma. HPB (Oxford) 2016;18:652–663. doi: 10.1016/j.hpb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Board PATE. Pancreatic Cancer Treatment (PDQ@): Health professional version. National Cancer Institute, 2019. [Google Scholar]
- 5.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 6.Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Büchler M, Charnley RM, et al. Borderline resectable pancreatic cancer: A consensus statement by the international study group of pancreatic surgery (ISGPS) Surgery. 2014;155:977–988. doi: 10.1016/j.surg.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim SW, Kishiwada M, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2–11. doi: 10.1016/j.pan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Hidalgo M, Cascinu S, Kleeff J, Labianca R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL, Heinemann V. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology. 2015;15:8–18. doi: 10.1016/j.pan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Eberlin LS, Margulis K, Planell-Mendez I, Zare RN, Tibshirani R, Longacre TA, Jalali M, Norton JA, Poultsides GA. Pancreatic cancer surgical resection margins: Molecular assessment by mass spectrometry imaging. PLoS Med. 2016;13(e1002108) doi: 10.1371/journal.pmed.1002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piciucchi M, Capurso G, Valente R, Larghi A, Archibugi L, Signoretti M, Stigliano S, Zerboni G, Barucca V, La Torre M, et al. Early onset pancreatic cancer: Risk factors, presentation and outcome. Pancreatology. 2015;15:151–155. doi: 10.1016/j.pan.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Pontén F, Schwenk JM, Asplund A, Edqvist PH. The human protein atlas as a proteomic resource for biomarker discovery. J Intern Med. 2011;270:428–446. doi: 10.1111/j.1365-2796.2011.02427.x. [DOI] [PubMed] [Google Scholar]
- 12.Tzeng CW, Fleming JB, Lee JE, Xiao L, Pisters PW, Vauthey JN, Abdalla EK, Wolff RA, Varadhachary GR, Fogelman DR, et al. Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy. Ann Surg Oncol. 2012;19:2045–2053. doi: 10.1245/s10434-011-2211-4. [DOI] [PubMed] [Google Scholar]
- 13.Asaoka T, Miyamoto A, Maeda S, Tsujie M, Hama N, Yamamoto K, Miyake M, Haraguchi N, Nishikawa K, Hirao M, et al. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology. 2016;16:434–440. doi: 10.1016/j.pan.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Sasaki K, Furukawa H, Matsunaga K, Maeda A. Serum CA19-9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:977–985. doi: 10.1007/s11605-012-1859-9. [DOI] [PubMed] [Google Scholar]
- 15.Waraya M, Yamashita K, Katagiri H, Ishii K, Takahashi Y, Furuta K, Watanabe M. Preoperative serum CA19-9 and dissected peripancreatic tissue margin as determiners of long-term survival in pancreatic cancer. Ann Surg Oncol. 2009;16:1231–1240. doi: 10.1245/s10434-009-0415-7. [DOI] [PubMed] [Google Scholar]
- 16.Meng Q, Shi S, Liang C, Liang D, Xu W, Ji S, Zhang B, Ni Q, Xu J, Yu X. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: A systematic review and meta-analysis. Onco Targets Ther. 2017;10:4591–4598. doi: 10.2147/OTT.S145708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122:467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina R, Barak V, van Dalen A, Duffy MJ, Einarsson R, Gion M, Goike H, Lamerz R, Nap M, Sölétormos G, Stieber P. Tumor markers in breast cancer-European group on tumor markers recommendations. Tumour Biol. 2005;26:281–293. doi: 10.1159/000089260. [DOI] [PubMed] [Google Scholar]
- 19.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Juweid M, Sharkey RM, Behr T, Swayne LC, Rubin AD, Herskovic T, Hanley D, Markowitz A, Dunn R, Siegel J, et al. Improved detection of medullary thyroid cancer with radiolabeled antibodies to carcinoembryonic antigen. J Clin Oncol. 1996;14:1209–1217. doi: 10.1200/JCO.1996.14.4.1209. [DOI] [PubMed] [Google Scholar]
- 21.Nazli O, Bozdag AD, Tansug T, Kir R, Kaymak E. The diagnostic importance of CEA and CA 19-9 for the early diagnosis of pancreatic carcinoma. Hepatogastroenterology. 2000;47:1750–1752. [PubMed] [Google Scholar]
- 22.Satake K, Chung YS, Yokomatsu H, Nakata B, Tanaka H, Sawada T, Nishiwaki H, Umeyama K. A clinical evaluation of various tumor markers for the diagnosis of pancreatic cancer. Int J Pancreatol. 1990;7:25–36. doi: 10.1007/BF02924217. [DOI] [PubMed] [Google Scholar]
- 23.Imaoka H, Mizuno N, Hara K, Hijioka S, Tajika M, Tanaka T, Ishihara M, Hirayama Y, Hieda N, Yoshida T, et al. Prognostic impact of carcinoembryonic antigen (CEA) on patients with metastatic pancreatic cancer: A retrospective cohort study. Pancreatology. 2016;16:859–864. doi: 10.1016/j.pan.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes JM, Ching CK. Serum diagnostic tests for pancreatic cancer. Baillieres Clin Gastroenterol. 1990;4:833–852. doi: 10.1016/0950-3528(90)90022-9. [DOI] [PubMed] [Google Scholar]
- 25. doi: 10.6004/jnccn.2021.0017. National Comprehensive Cancer Network: NCCN clinical practive guideline in oncology (NCCN Guidelines®): Pancreatic adenocarcinoma. Version 1.2021, October, 2021. [DOI] [PubMed] [Google Scholar]
- 26.Hata S, Sakamoto Y, Yamamoto Y, Nara S, Esaki M, Shimada K, Kosuge T. Prognostic impact of postoperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2012;19:636–641. doi: 10.1245/s10434-011-2020-9. [DOI] [PubMed] [Google Scholar]
- 27.Takasaki H, Uchida E, Tempero MA, Burnett DA, Metzgar RS, Pour PM. Correlative study on expression of CA 19-9 and DU-PAN-2 in tumor tissue and in serum of pancreatic cancer patients. Cancer Res. 1988;48:1435–1438. [PubMed] [Google Scholar]
- 28.Aoki H, Ohnishi H, Hama K, Ishijima T, Satoh Y, Hanatsuka K, Ohashi A, Wada S, Miyata T, Kita H, et al. Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. Am J Physiol Cell Physiol. 2006;290:C1100–C1108. doi: 10.1152/ajpcell.00465.2005. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. International Association of Pancreatology. [DOI] [PubMed] [Google Scholar]
- 30.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi T, Kawa S, Tokoo M, Oguchi H, Kiyosawa K, Furuta S, Kanai M, Homma T. Comparative study of CA-50 (time-resolved fluoroimmunoassay), Span-1, and CA19-9 in the diagnosis of pancreatic cancer. Scand J Gastroenterol. 1991;26:787–797. doi: 10.3109/00365529108998600. [DOI] [PubMed] [Google Scholar]
- 32.Palmquist C, Dehlendorff C, Calatayud D, Hansen CP, Hasselby JP, Johansen JS. Prediction of unresectability and prognosis in patients undergoing surgery on suspicion of pancreatic cancer using carbohydrate antigen 19-9, interleukin 6, and YKL-40. Pancreas. 2020;49:53–61. doi: 10.1097/MPA.0000000000001466. [DOI] [PubMed] [Google Scholar]
- 33.Tian F, Appert HE, Myles J, Howard JM. Prognostic value of serum CA 19-9 levels in pancreatic adenocarcinoma. Ann Surg. 1992;215:350–355. doi: 10.1097/00000658-199204000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, Xu H, Wang W, Wu C, Chen Y, Yang J, Cen P, Xu J, Liu C, Long J, et al. A preoperative serum signature of CEA+/CA125+/CA19-9 ≥ 1000 U/ml indicates poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer. 2015;136:2216–2227. doi: 10.1002/ijc.29242. [DOI] [PubMed] [Google Scholar]
- 35.Chari ST, Kelly K, Hollingsworth MA, Thayer SP, Ahlquist DA, Andersen DK, Batra SK, Brentnall TA, Canto M, Cleeter DF, et al. Early detection of sporadic pancreatic cancer: Summative review. Pancreas. 2015;44:693–712. doi: 10.1097/MPA.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannis D, Moris D, Barbas AS. Diagnostic, predictive and prognostic molecular biomarkers in pancreatic cancer: An overview for clinicians. Cancers (Basel) 2021;13(1071) doi: 10.3390/cancers13051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Manen L, Groen JV, Putter H, Vahrmeijer AL, Swijnenburg RJ, Bonsing BA, Mieog JSD. Elevated CEA and CA19-9 serum levels independently predict advanced pancreatic cancer at diagnosis. Biomarkers. 2020;25:186–193. doi: 10.1080/1354750X.2020.1725786. [DOI] [PubMed] [Google Scholar]
- 38.Edge SB, Compton CC. The American joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 39.Poruk KE, Gay DZ, Brown K, Mulvihill JD, Boucher KM, Scaife CL, Firpo MA, Mulvihill SJ. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: Diagnostic and prognostic updates. Curr Mol Med. 2013;13:340–351. doi: 10.2174/1566524011313030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, Wang CF, Wang J, Tian YT, Liu Q, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164–169. doi: 10.1016/j.ejso.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 42.Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Curry SJ, Doubeni CA, Epling JW Jr, et al. Screening for pancreatic cancer: US preventive services task force reaffirmation recommendation statement. JAMA. 2019;322:438–444. doi: 10.1001/jama.2019.10232. US Preventive Services Task Force. [DOI] [PubMed] [Google Scholar]
- 43.He XY, Yuan YZ. Advances in pancreatic cancer research: Moving towards early detection. World J Gastroenterol. 2014;20:11241–11248. doi: 10.3748/wjg.v20.i32.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okano K, Suzuki Y. Strategies for early detection of resectable pancreatic cancer. World J Gastroenterol. 2014;20:11230–11240. doi: 10.3748/wjg.v20.i32.11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee KJ, Yi SW, Chung MJ, Park SW, Song SY, Chung JB, Park JY. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J. 2013;54:643–649. doi: 10.3349/ymj.2013.54.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 47.Reitz D, Gerger A, Seidel J, Kornprat P, Samonigg H, Stotz M, Szkandera J, Pichler M. Combination of tumour markers CEA and CA19-9 improves the prognostic prediction in patients with pancreatic cancer. J Clin Pathol. 2015;68:427–433. doi: 10.1136/jclinpath-2014-202451. [DOI] [PubMed] [Google Scholar]
- 48.Kim YC, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI, Shin JH. Can preoperative CA19-9 and CEA levels predict the resectability of patients with pancreatic adenocarcinoma? J Gastroenterol Hepatol. 2009;24:1869–1875. doi: 10.1111/j.1440-1746.2009.05935.x. [DOI] [PubMed] [Google Scholar]
- 49.Mehta J, Prabhu R, Eshpuniyani P, Kantharia C, Supe A. Evaluating the efficacy of tumor markers CA 19-9 and CEA to predict operability and survival in pancreatic malignancies. Trop Gastroenterol. 2010;31:190–194. [PubMed] [Google Scholar]
- 50.Xu HX, Liu L, Xiang JF, Wang WQ, Qi ZH, Wu CT, Liu C, Long J, Xu J, Ni QX, Yu XJ. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161:373–384. doi: 10.1016/j.surg.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Salmiheimo A, Mustonen H, Stenman UH, Puolakkainen P, Kemppainen E, Seppänen H, Haglund C. Systemic inflammatory response and elevated tumour markers predict worse survival in resectable pancreatic ductal adenocarcinoma. PLoS One. 2016;11(e0163064) doi: 10.1371/journal.pone.0163064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Distler M, Pilarsky E, Kersting S, Grützmann R. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas-a retrospective tumor marker prognostic study. Int J Surg. 2013;11:1067–1072. doi: 10.1016/j.ijsu.2013.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.