Abstract
The present study aimed to investigate the relationship between BMI and the prostate cancer (PCa) risk at biopsy in Italian men. Retrospective analyses of the clinical data of 2,372 consecutive men undergoing ultrasound-guided multicore (≥10) prostate biopsy transrectally between May 2010 and December 2018 were performed. BMIs were categorized, according to Western countries' classification of obesity, as follows: <18.5 kg/m2 (underweight), 18.5-24.99 kg/m2 (normal weight), 25-30 kg/m2 (overweight) and >30 kg/m2 (obese). The distribution of patients undergoing biopsy was compared with a model population from the official survey data. Patient characteristics and the relationships between characteristics were investigated using correlation analysis, ANOVA, Kruskal-Wallis and Dunn's tests. The present study estimated the influence on cancer incidence not only of BMI but also of other patient characteristics using multi-variable logistic modelling and compared, using the models, the expected outcomes for patients who differed only in BMI. From a sample of 2,372 men, the present study enrolled 1,079 men due to a lack of clinical data [such as prostate specific antigen (PSA) and BMI data] in the other patients undergoing prostate biopsy. Their distribution was significantly different from the model distribution with the probability of undergoing biopsy increasing with increasing BMI. The median age was 69.4 years. The median BMI was 26.4 kg/m2, while the median PSA level was 7.60 ng/ml. In total, the biopsies detected PCa in 320 men (29.7%) and high-grade PCa (HGPCa) in 218 men (20.2%). Upon applying the aforementioned Western countries' criteria for BMI categories, there were 4 (0.4%) underweight, 318 (29.5%) of normal weight, 546 (50.6%) overweight, and 211 (19.6%) obese patients. ANOVA/Kruskal-Wallis tests revealed that overweight and obese men were younger than the normal-weight men, while there was no statistical difference in their PSA values. Furthermore, 29.3% of normal-weight men, 29.5% of overweight men and 29.9% of obese men were diagnosed with PCa, while 19.5% of normal-weight men, 20.1% of overweight men and 21.8% of obese men were affected by severe cancer. BMI was found to be positively correlated with PCa risk and negatively correlated with both age and PSA level. Age and PSA level were both positively correlated with PCa risk, while digital rectal examination (DRE) outcome was strongly indicative of PCa discovery if the test outcome was positive. Logistics models attributed a positive coefficient to BMI when evaluated against both PCa risk and HGPCa risk. In patients having a negative DRE outcome who differed only in BMI, logistic regression showed a 60% increased risk of PCa diagnosis in obese patients compared with in normal-weight patients. This risk difference increased when other characteristics were less indicative of PCa (younger age/lower PSA), while it decreased when patient characteristics were more indicative (older age/higher PSA, positive DRE). In conclusion, the present study demonstrated that, in men with higher BMIs, the risk of PCa is higher. The relative difference in risk between low and high BMI is most pronounced in younger patients having a lower PSA level and a negative DRE outcome.
Keywords: prostate cancer, high-grade prostatic disease, body mass index
Introduction
Prostate cancer (PCa) is the second most common cancer in the male population, with a prevalence of 59% (48-71%) in men over 79 years of age (1). The incidence of PCa diagnosis varies widely between different geographic areas, reaching peaks in Australia/New Zealand, North America and Western and Northern Europe. The greater incidence in these geographical areas is largely due to the use of prostate specific antigen (PSA) and the aging of the population. On the contrary, the incidence is low in eastern and central-southern Asia while the rates in eastern and southern Europe, once low, show a steady increase (2,3). PCa mortality rates are generally high in populations of African descent, intermediate in the United States and very low in Asia (2).
Having no symptoms except in very advanced stages (such as hematuria and urinary obstruction), PCa is usually suspected on the result of rectal examination (DRE) and/or PSA level. The definitive diagnosis is histopathological in prostate biopsy specimens or from pathological pieces resulting from surgical treatment for benign prostatic hypertrophy.
The increase in the incidence of PCa, in the presence of a family history or a racial background, suggests a genetic predisposition (4,5). However, only a small subpopulation of men with PCa truly have a hereditary disease, defined as three or more affected relatives or at least two relatives who have developed early-onset PCa (<55 years) (5). Although the disease is diagnosed six to seven years earlier than average, the aggression and the clinical course do not seem to differ (5,6). On the contrary, in men of African origin there is a higher incidence of disease with a generally more aggressive course (7). Genomic studies have identified 100 susceptibility loci contributing to the risk of PCa, explaining approximately 39% of the familial risk for this disease (8,9). In addition, a 12% incidence of germline mutations in genes that mediate DNA repair processes was found among men with metastatic disease (10). Germinal mutations in genes such as BRCA 1/2 and HOXB13 have been associated with an increased risk of PCa (11,12).
Numerous exogenous/environmental factors have been associated with the risk of developing PCa or discussed as etiologically important for progression from latent to clinically significant disease (13). A study of Japanese men showed a lower risk of PCa than men in the Western world. However, after moving to California, their risk of PCa increased, becoming similar to that of American men, implying an involvement of environmental or dietary factors (14). In this regard, it is very important to assess the role of obesity which is an increasing health problem; it affects more than 300 million people worldwide (15). In epidemiological and basic research studies, obesity has been repeatedly linked to the development of different cancers (16,17). As a cancer risk factor, obesity is second only to tobacco consumption (18) and is linked to increased mortality from all cancers, including PCa (19).
Among the reasons for cancer-related deaths are obesity-mediated delays in diagnosis (due to the insufficiencies of our testing) and the underestimation of disease severity due to obesity. The obesity-mediated delay in PCa diagnosis is due to the relative association between obesity and lower PSA (prostate-specific antigen) levels found in this category of patients (20-28). It is possible that this inverse association is due to hemodilution along with increased blood volume and lower testosterone levels in obese patients (29). Moreover, several studies have argued that obesity is a possible impediment to cancer screening in general (30-32). Thus, obese patients are affected by occult locally advanced disease, even with relatively low PSA levels, stage for stage (33,34). Several studies in Western populations have shown that progression and prognosis in PCa, which causes more than a quarter of a million deaths worldwide every year (35), are inversely correlated with the patient's body mass index (36,37). For example, in a study that involved a free prostate screening program in North Carolina, Price et al (28) found that PSA and body mass index were inversely related. Since then, however, several reports have shown conflicting results. Some studies have not found associations (38-40), but most have suggested an inverse relationship between BMI and PSA (20-28). A previous meta-analysis showed an association of a BMI over 30 with a 15 and 37% higher risk of PCa and high-grade PCa detection, respectively, at biopsy (41). In addition, MacInnis and English (42), in their meta-analysis and systematic review involving 31 cohort and 25 case-control studies, found that BMI was a weak, but statistically significant, predictor of tumor risk (relative risk: 1.05 per 5 kg/m2 increment; 95% confidence interval: 1.01-1.08).
To further investigate the relationship between BMI and PCa and HGPCa detection at biopsy in a Western population, we conducted a retrospective analysis not only of BMI but also of PCa and high-grade PCa risk at biopsy. The impact of this study could be extensive, as revealing the true impact of BMI on biopsy results could be helpful for current PCa screening strategies for patients with different body weights. We supposed that, after controlling for clinical characteristics, we would find not only an inverse association between the prebiopsy PSA level and the BMI but also a significant direct relationship between PCa risk and BMI.
Patients and methods
Study population and study variables
Once patients gave informed consent for us to obtain their clinical data before their biopsies, we retrospectively reviewed the clinical data of 2372 consecutive patients undergoing transrectal ultrasound (TRUS)-guided initial multicore (≥10) prostate biopsies between May 2010 and December 2018 at Department of Urology of Umberto I Hospital in Nocera Inferiore. The biopsy indications included three conditions: (1) elevated PSA levels (≥4 ng/ml); (2) digital rectal examination (DRE) findings suggestive of malignancy; and (3) both. All the multicore needle biopsies were performed under TRUS guidance. The exclusion criteria were as follows: (1) previous prostate biopsy, (2) prostate surgery, (3) current or previous therapy with 5-alpha-reductase inhibitors, and (4) a known diagnosis of PCa. Additionally, patients with unavailable BMI data, dates of birth or Gleason scores were also excluded, leaving a total study population of 1,079 patients. All the men showed at least one measured serum PSA level and underwent DRE. Data on age, pre-biopsy BMI, pre-treatment PSA, DRE, diagnostic imaging and pathological results of prostate biopsy were collected retrospectively. The BMI (kg/m2) was calculated as weight in kilograms divided by height in meters squared. All the men were divided into four groups depending on their BMIs, which were calculated according to the criteria and classification of obesity in Western countries as follows: underweight (<18.5 kg/m2), normal weight (18.5-24.99 kg/m2), overweight (25-30 kg/m2), and obese (>30 kg/m2). Prostate biopsy was performed transrectally and guided by ultrasound, with the patient placed in the left lateral position. The prostate volume was measured using the ellipsoid formula. A 12-core or 20-core systematic biopsy was performed depending on the urologist's assessment of the prostate volume and DRE findings. The core specimens were examined by an expert pathologist in our institution. When diagnosed at biopsy, PCa was scored according to the Gleason grading system as follows: 0 <GS ≤7 (3 + 4) indicated low-grade PCa (considered clinically insignificant), while GS ≥7 (4 + 3) indicated high-grade PCa (considered clinically significant).
Statistical analysis
Differences in the patients' characteristics (age, BMI and PSA), overall PCa detection rate and HGPCa detection rate across the BMI categories were evaluated. Normality was evaluated from histograms and Shapiro-Wilks (SW) tests while uniformity of variance was evaluated using Levene's test after which the analysis proceeded in both parametric and non-parametric fashion using one-way ANOVA (with Bonferroni correction as the post hoc test), or Kruskal-Wallis and Dunn's test to determine the significance of the observed variations in patients' characteristics. Correlation between the patients' characteristics was evaluated using Pearson's and Spearman's tests. The effect of BMI on the distribution of patients undergoing biopsy was determined by the chi-squared goodness of fit testing against a model distribution built from data obtained from population surveys. In addition to linear and rank-order correlation, multivariate logistic modeling was used to determine the effect of the patients' characteristics on the biopsy outcome. Modeling was divided in two groups: the first group of models was performed on no cancer/cancer result, while the second group of models was performed on PCa/HGPCa result provided that a cancer is detected. Within each group a leave-one-out approach was used to determine the impact of omitted characteristics on the model's performance, which was described by means of area under receiver operating characteristic curve (ROC AUC), sensitivity and specificity. Since there were only four patients in the underweight BMI category, these patients were neglected in the statistical analyses because of their lower statistical significance.
Results
Patient BMI distribution vs. modeled distribution
A BMI distribution under the hypothesis that BMI has no effect on the probability of being included in the study was calculated from data obtained from ISTAT for the population in the Campania region. The model was weighed for the age of the patients under observation and the number of patients in each year under the study. The results from Chi squared goodness of fit testing are reported in Table I and show that there is a statistically significant difference between the observed and modeled distributions with the obesity and overweight categories that are over-represented in the sample and the normal-weight category being under-represented.
Table I.
Number of patients per category in modelled and observed cases and χ2 test results.
| Parameter | Underweight | Normal-weight | Overweight | Obese |
|---|---|---|---|---|
| Expected patients from model distribution, n (%) | 7 (0.7) | 375 (34.8) | 532 (49.3) | 164 (15.2) |
| Observed patients, n (%) | 4 (0.4) | 318 (29.5) | 546 (50.6) | 211 (19.6) |
| Difference in observed vs. modeled, % | -0.3 | -5.3 | +1.3 | +4.4 |
| χ2 test statistic (P-value) | 10.46 (0.015) |
Patient characteristics
The baseline characteristics of the overall study population are presented in Table II. For the 1,079 patients enrolled in this study, the median age was 69.4 years, the median BMI was 26.4 kg/m2 and the median PSA level was 7.6 ng/ml. In total, PCa was detected at biopsy in 320 men (29.7%), while HGPCa was found in 218 men (20.2%). It is worth noting that the patients affected by HGPCa were not included in the evaluation of patients within the PCa subgroup. Upon applying the aforementioned Western countries' criteria for BMI categories, there were 4 (0.4%) underweight (UW) patients, 318 (29.5%) normal-weight (NW) patients, 546 (50.6%) overweight patients (OW) and 211 (19.6%) obese (OB) patients.
Table II.
Clinical characteristics and results of biopsy stratified by BMI.
| Variable | Overall | Underweight (BMI <18.5) | Normal weight (18.5≤ BMI <25) | Overweight (25≤ BMI <30) | Obese (30≤ BMI) |
|---|---|---|---|---|---|
| Patients, n (%) | 1,079 | 4 (0.4) | 318 (29.5) | 546 (50.6) | 211 (19.6) |
| BMI, kg/m2 | |||||
| Mean (SD) | 27.0 (3.5) | 17.8 (0.7) | 23.5 (1.2) | 27.1 (1.4) | 32.5 (2.7) |
| Median (IQR) | 26.4 (24.6-29.1) | 18.0 (17.4-18.4) | 23.9 (22.8-24.4) | 27.0 (25.8-28.2) | 31.8 (30.9-33.3) |
| Range | 16.9-50.4 | 16.9-18.4 | 18.9-24.9 | 25.0-29.8 | 30.0-50.4 |
| Age, years | |||||
| Mean (SD) | 69.4 (7.8) | 70.9 (6.8) | 70.2 (8.3) | 69.2 (7.7) | 68.1 (7.4) |
| Median (IQR) | 69.4 (63.8-75.4) | 72.0 (69.2-73.7) | 71.0 (65.5-76.6) | 68.5 (63.2-75.3) | 68.7 (63.2-73.4) |
| Range | 42.9-93.5 | 61.7-78.0 | 42.9-93.5 | 44.7-88.2 | 51.1-86.1 |
| PSA, ng/ml | |||||
| Mean (SD) | 14.1 (35.1) | 22.6 (18.8) | 15.0 (45.6) | 14.3 (33.4) | 11.9 (15.9) |
| Median (IQR) | 7.6 (5.3-11.3) | 23.8 (10.4-36.0) | 7.9 (5.4-12.5) | 7.3 (5.25-10.7) | 8.15 (5.4-11.5) |
| Range | 0.01-750 | 0.8-42 | 0.1-750 | 0.01-522 | 0.1-134 |
| PCa detected from biopsy, n (%) | 320 (29.7) | 3(75) | 93 (29.3) | 161 (29.5) | 63 (29.9) |
| HGPCa detected from biopsy, n (%) | 218 (20.2) | 0 (0) | 62 (19.5) | 110 (20.1) | 46 (21.8) |
HGPCa, high-grade prostate cancer; IQR, interquartile range; PCa, prostate cancer; PSA, prostate specific antigen.
BMI and PCa detection
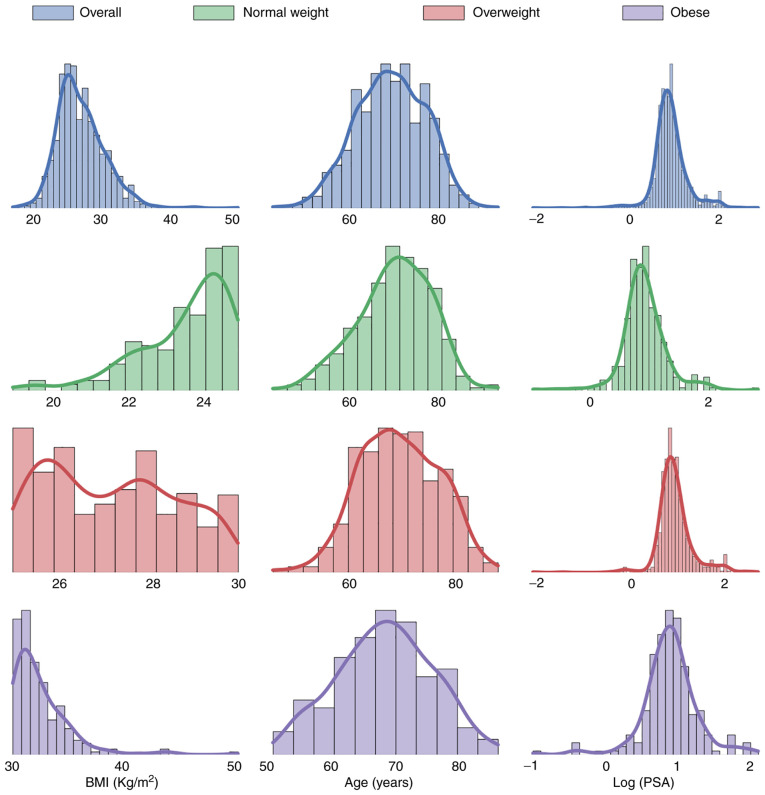
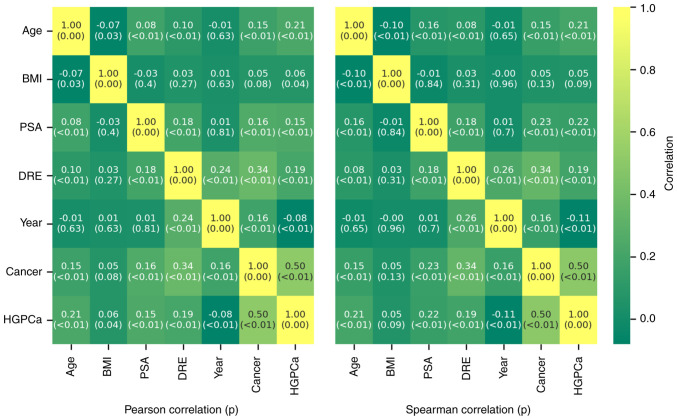
Fig. 1 shows the risk of overall cancer detection at biopsy across the BMI categories. The percentage of men diagnosed with PCa at biopsy was 75% in the underweight group, 29.3% in the normal-weight group, 29.5% in the overweight group and 29.9% in the obese group. With regard to high-grade PCa, 0% of the underweight, 19.5% of the normal-weight, 20.1% of the overweight and 21.8% of the obese men were affected. In these cases, the percentages were calculated not with respect to the total number of patients in each cancer class, but with respect to the total number of patients in each BMI class. Histograms and kernel density estimates were calculated and are plotted in Fig. 2. The plots show the patients' characteristics overall and among BMI categories. Due to the very wide range of values taken by PSA level a logarithmic (log) transformation on this parameter was applied in order to obtain a distribution closer to a gaussian. Shapiro-Wilk's (SW) test, Levene's (L) test, one way ANOVA (OWA) test Kruskal-Wallis (KW) and Dunn's test results are shown in Table III. Across the BMI categories, the hypotheses of normal distribution could be rejected according to the SW test in the NW and OW categories for age distribution and in all the categories for log(PSA) distribution. Across all categories, for age and log(PSA), the hypotheses of equal variances could not be rejected. The OWA and KW/Dunn's tests indicated that the obese men were younger than the normal-weight patients and this result was statistically significant, as shown in Table III. PSA, analyzed as log(PSA), did not return statistically significant differences across the categories. Parameter correlation results given in Fig. 3 show that BMI has a very low positive correlation with the cancer outcome and the HGPCa. PSA, Age and DRE have a stronger correlation against the outcomes in both cases. BMI has a very low negative correlation with age and PSA while the inverse is true for DRE. The results are true both under Pearson's test and under Spearman's.
Figure 1.
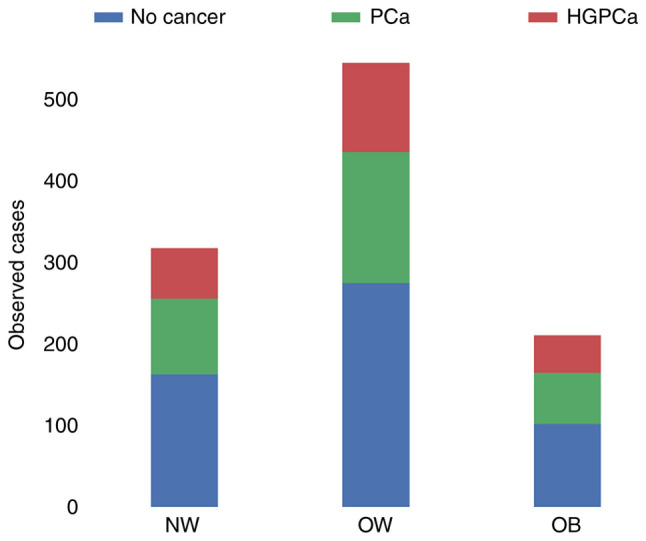
Biopsy outcomes across the BMI categories. HGPCa, high-grade prostate cancer; NW, normal-weight; OB, obese; OW, overweight; PCa, prostate cancer.
Figure 2.
BMI, age, and log(PSA) histograms and kernel density estimates according to BMI distribution. PSA, prostate specific antigen.
Table III.
Shapiro-Wilks, Levene, one-way ANOVA, Kruskal-Wallis and Dunn's test results across BMI categories.
| Test | Variable | Category | Test result (P-value) |
|---|---|---|---|
| Shapiro-Wilks | Age | NW | 0.988 (0.011) |
| Shapiro-Wilks | Age | OW | 0.992 (0.009) |
| Shapiro-Wilk | Age | OB | 0.992 (0.300) |
| Shapiro-Wilks | log(PSA) | NW | 0.903 (<0.001) |
| Shapiro-Wilks | log(PSA) | OW | 0.832 (<0.001) |
| Shapiro-Wilks | log(PSA) | OB | 0.909 (<0.001) |
| Levene's | Age | NW vs. OW | 0.631 (0.427) |
| Levene's | Age | NW vs. OB | 2.188 (0.138) |
| Levene's | Age | OW vs. OB | 1.075 (0.300) |
| Levene's | log(PSA) | NW vs. OW | 0.631 (0.427) |
| Levene's | log(PSA) | NW vs. OB | 2.188 (0.138) |
| Levene's | log(PSA) | OW vs. OB | 1.075 (0.300) |
| One-way ANOVA | Age | NW vs. OW vs. OB | 3.252 (0.039) |
| One-way ANOVA | log(PSA) | NW vs. OW vs. OB | 0.107 (0.898) |
| One-way ANOVA, Bonferroni post hoc | Age | NW vs. OW | 3.374 (0.200) |
| One-way ANOVA, Bonferroni post hoc | Age | NW vs. OB | 5.775 (0.0498) |
| One-way ANOVA, Bonferroni post hoc | Age | OW vs. OB | 1.177 (0.834) |
| Kruskal-Wallis | Age | NW vs. OW vs. OB | 8.55 (0.014) |
| Kruskal-Wallis | log(PSA) | NW vs. OW vs. OB | 1.687 (0.430) |
| Dunn's | Age | NW vs. OW | 0.065 |
| Dunn's | Age | NW vs. OB | 0.020 |
| Dunn's | Age | OW vs. OB | 0.984 |
NW, normal-weight; OB, obese; OW, overweight; PSA, prostate specific antigen.
Figure 3.
Pearson correlation (left) and Spearman correlation (right) of patient characteristics and outcomes. DRE, digital rectal examination; HGPCa, high-grade prostate cancer; PSA, prostate specific antigen.
Multiple logistic modeling of PCa and HGPCa outcomes
The results obtained from logistic modeling are shown in Table IV, Table V, Table VI and Table VII. In the cancer vs. no cancer models the BMI parameter estimates are always positive when included; however, omitting BMI, it does not have an effect on the performance of the model. Model performance as determined by AUC ROC is affected mostly by the removal of log(PSA) and DRE with a simultaneous drop in specificity for the former and sensitivity for the latter. In general, there is an increase in a cancer diagnosis at biopsy with increasing BMI, PSA level, Age, Year of examination and positive DRE findings. For the model discriminating between HGPCa and PCa, given a cancer diagnosis, the results were in general statistically more uncertain. In this case the model indicated that the ratio of HGPCa/PCa was decreasing as the years under study progressed and increased with an increase in BMI, age, PSA level and positive DRE findings.
Table IV.
Parameter estimates and model performance statistics from multiple logistic modeling for prediction of cancer presence vs. absence.
| Model | Intercept (P-value) | BMI (P-value) | log(PSA) (P-value) | Age (P-value) | DRE (P-value) | Year (P-value) | Median residual | AUC ROC | Specificity (0.5 threshold) | Sensitivity (0.5 threshold) |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | -5.238 (<0.001) | 0.036 (0.064) | 1.321 (<0.001) | 0.034 (<0.001) | 1.385 (<0.001) | 0.153 (0.001) | 0.012 | 0.729 | 0.566 | 0.8 |
| Model 2 | -4.180 (<0.001) | Omitted | 1.319 (<0.001) | 0.033 (<0.001) | 1.390 (<0.001) | 0.153 (0.001) | 0.002 | 0.729 | 0.566 | 0.783 |
| Model 3 | -4.316 (<0.001) | 0.035 (0.065) | Omitted | 0.038 (<0.001) | 1.522 (<0.001) | 0.137 (0.003) | -0.144 | 0.701 | 0.508 | 0.8 |
| Model 4 | -2.793 (<0.001) | 0.030 (0.112) | 1.406 (<0.001) | Omitted | 1.412 (<0.001) | 0.144 (0.002) | 0.03 | 0.716 | 0.55 | 0.798 |
| Model 5 | -5.580 (<0.001) | 0.038 (0.040) | 1.512 (<0.001) | 0.037 (<0.001) | Omitted | 0.236 (<0.001) | 0.058 | 0.685 | 0.615 | 0.654 |
| Model 6 | -4.756 (<0.001) | 0.035 (0.065) | 1.289 (<0.001) | 0.033 (<0.001) | 1.493 (<0.001) | Omitted | 0.025 | 0.721 | 0.563 | 0.774 |
AUC, area under the curve; DRE, digital rectal examination; PSA, prostate specific antigen; ROC, receiver operating characteristic.
Table V.
Parameter estimates and model performance statistics from multiple logistic modeling for prediction of HGPCa vs. PCa conditional on cancer being present.
| Model | Intercept (P-value) | BMI (P-value) | log(PSA) (P-value) | Age (P-value) | DRE (P-value) | Year (P-value) | Median residual | AUC ROC | Specificity (at 0.5 threshold) | Sensitivity (at 0.5 threshold) |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 7 | -4.319 (<0.001) | 0.025 (0.311) | 0.562 (0.015) | 0.048 (<0.001) | 0.248 (0.229) | -0.285 (<0.001) | -0.718 | 0.675 | 0.313 | 0.856 |
| Model 8 | -3.549 (<0.001) | Omitted | 0.565 (0.015) | 0.044 (0.001) | 0.256 (0.215) | -0.285 (<0.001) | -0.728 | 0.676 | 0.294 | 0.871 |
| Model 9 | -4.058 (<0.001) | 0.026 (0.295) | Omitted | 0.049 (<0.001) | 0.371 (0.062) | -0.296 (<0.001) | -0.730 | 0.66 | 0.294 | 0.880 |
| Model 10 | -0.975 (0.176) | 0.016 (0.517) | 0.668 (0.004) | Omitted | 0.272 (0.181) | -0.295 (<0.001) | -0.760 | 0.654 | 0.229 | 0.913 |
| Model 11 | -4.412 (<0.001) | 0.026 (0.289) | 0.632 (0.005) | 0.045 (<0.001) | Omitted | -0.260 (<0.001) | -0.718 | 0.671 | 0.328 | 0.874 |
| Model 12 | -5.117 (<0.001) | 0.024 (0.330) | 0.636 (0.006) | 0.047 (<0.001) | -0.034 (0.857) | Omitted | -0.787 | 0.639 | 0.199 | 0.892 |
AUC, area under the curve; DRE, digital rectal examination; HGPCa, high-grade prostate cancer; PCa, prostate cancer; PSA, prostate specific antigen; ROC, receiver operating characteristic.
Table VI.
Confidence intervals (95%) for the variable coefficients estimated by the logistic regression models for prediction of cancer presence vs. absence.
| Model | Intercept | BMI | log(PSA) | Age | DRE | Year |
|---|---|---|---|---|---|---|
| Model 1 | -6.972, -3.547 | -0.002, 0.074 | 0.909, 1.758 | 0.017, 0.052 | 1.061, 1.720 | 0.062, 0.246 |
| Model 2 | -5.475, -2.916 | Omitted | 0.908, 1.757 | 0.016, 0.050 | 1.066, 1.724 | 0.061, 0.245 |
| Model 3 | -5.983, -2.686 | -0.002, 0.072 | Omitted | 0.021, 0.056 | 1.206, 1.848 | 0.047, 0.227 |
| Model 4 | -3.936, -1.677 | -0.007, 0.068 | 0.990, 1.846 | Omitted | 1.090, 1.744 | 0.053, 0.235 |
| Model 5 | -7.250, -3.955 | 0.002, 0.075 | 1.120, 1.930 | 0.021, 0.054 | Omitted | 0.149, 0.325 |
| Model 6 | -6.444, -3.104 | -0.002, 0.073 | 0.881, 1.723 | 0.015, 0.050 | 1.176, 1.821 | Omitted |
DRE, digital rectal examination; PSA, prostate specific antigen.
Table VII.
Confidence intervals (95%) for the variable coefficients estimated by the logistic regression models for prediction of HGPCa vs. PCa conditional on cancer being present.
| Model | Intercept | BMI | log(PSA) | Age | DRE | Year |
|---|---|---|---|---|---|---|
| Model 7 | -6.692, -2.004 | -0.023, 0.073 | 0.112, 1.022 | 0.020, 0.070 | -0.156, 0.654 | -0.418, -0.157 |
| Model 8 | -5.370, -1.781 | Omitted | 0.115, 1.025 | 0.019, 0.069 | -0.148, 0.661 | -0.418, -0.157 |
| Model 9 | -6.413, -1.759 | -0.022, 0.074 | Omitted | 0.024, 0.074 | -0.018, 0.763 | -0.428, -0.168 |
| Model 10 | -2.391, 0.445 | -0.032, 0.063 | 0.221, 1.127 | Omitted | -0.127, 0.672 | -0.426, -0.168 |
| Model 11 | -6.778, -2.102 | -0.022, 0.074 | 0.195, 1.079 | 0.021, 0.071 | Omitted | -0.384, -0.139 |
| Model 12 | -7.440, -2.870 | -0.024, 0.071 | 0.188, 1.094 | 0.023, 0.072 | -0.413, 0.340 | Omitted |
DRE, digital rectal examination; HGPCa, high-grade prostate cancer; PCa, prostate cancer; PSA, prostate specific antigen.
Discussion
The impact of body weight gain on PCa detection is a global health problem, affecting not only Western countries but also Asian countries due to increasingly prevalent unhealthy lifestyle changes. The increasing obesity rates and higher incidence of PCa in Asia have inspired several studies to address the correlation between BMI and PCa detection among Asian populations (43-47). Furthermore, Lavalette et al (48) found that men with a normal BMI at age 20 developing overweight or obesity during adulthood were at increased risk of aggressive PCa compared to men who maintained a normal BMI. These results emphasized the importance of maintaining a healthy BMI throughout adulthood. Then, according to Michael et al (49), regardless of exercise, higher BMI is linked with higher risk of aggressive PC while exercise is unrelated to PC risk. Therefore, in this study, we evaluated the biological association between a higher body mass index and an increased risk of PCa development. We initially constructed a model for the BMI distribution of the patients under study under the hypothesis that BMI did not affect the chance of passing the inclusion criteria for this study. Chi squared goodness of fit testing indicated that this model distribution was significantly different than the observed patient distribution. This difference is correlated with BMI as the higher BMI categories (OW, OB) showed an increase in inclusion from the model distribution with the lower BMI categories showing a decrease. This is significant because the parameters for inclusion themselves (PSA, DRE) were shown to be positively correlated with the probability of detecting PCa or HGPCa at biopsy. We further hypothesized that several factors, such as PSA levels, can play an important role in PCa detection; specifically, we hypothesized that increases in BMI are inversely correlated with serum PSA levels (50,51). This inverse relation may be linked to the low testosterone levels in obese patients (40) or to larger plasma volumes as a hemodilution effect (29). In either case, the lower PSA levels in obese men could obscure the presence of PCa. In an attempt to test this hypothesis, we investigated the associations among BMI, PSA levels, age at biopsy, DRE and PCa risk. In particular, correlation tests showed that BMI, PSA, age and DRE were positively correlated with cancer detection. The same tests showed that BMI was negatively correlated with both age, as found in other studies (44), and PSA. Upon further investigation with ANOVA, Kurskal-Wallis and Dunn's tests the age distribution among the normal-weight and obese categories was found to be significantly different; in particular, Dunn's test showed that difference in the median age between normal-weight patients and obese patients was statistically significant i.e., the obese patients were younger. It is interesting to note that, although age and PSA levels decrease with increasing BMI category, the rate of cancer detection rises slightly. This suggests that BMI, or a factor positively correlated with it, is driving cancer detection and compensating for the age and PSA level decrease. Furthermore, higher BMI contributes to creating a more favorable biological microenvironment for cancer onset and growth. According to some studies, men with a higher BMI are likely to produce less testosterone, resulting in PCa that is less androgen-dependent and, consequently, more aggressive (52). About this, in his prospective, hospital-based, cross-sectional study involving consecutive patients with PCa, Nwadi et al (53) found that the BMI of patients with PCa correlated positively with their Gleason score. Excessive adiposity might also result in the secretion of different adipokines and inflammatory cytokines, which may promote tumor growth (54). In addition, obese men usually have high levels of insulin and insulin-like growth factor 1 (IGF-1), both of which can inhibit apoptosis and encourage carcinogenesis (55). Prostate biopsy is usually prompted by either an abnormal DRE finding suggestive of cancer or, more often, an elevated PSA blood value. Factors such as patients' ages can potentially hide the impact of BMI on PCa detection at biopsy, as has also been evidenced by previous studies (40,56). Logistic models allow us to estimate the influence that BMI has on cancer detection by controlling for such factors. Table VIII shows the results from Model 1 (full model) on 'median patients' where the patients have median characteristics and vary only in their BMI. Two cases, one with a positive DRE outcome and one with a negative DRE outcome, are considered. The above results suggest that BMI has a substantial incidence on PCa detection, especially in those patients where the expectation of finding PCa would be lower such as those with a negative DRE finding (as shown in table) or have a lower age or PSA level. In patients with positive DRE result, the increase in BMI still contributes positively to the likelihood of cancer detection; however, the relative difference between normal weight and obese patients is less marked due to the high baseline detection associated with a positive DRE outcome. When estimating the influence of BMI on the rate of HGPCa when cancer is detected, the results from Table VI. show that BMI has a positive coefficient as well. The result is, however, statistically insignificant; this fact indicates that, although it can be concluded that BMI drives cancer detection in general, it cannot be concluded with certainty that HGPCa is driven more than, or at the expense of, PCa. These results suggest, however, that a larger study might confirm this, provided the trends are similar. The present study presents some limitations that must be taken into account. First, the study was carried out in a single center and, for this reason, it might not represent the whole population of Italy. However, to the best of our knowledge, there has not, to date, been a single-center Italian study that has evaluated such a large number of patients undergoing prostate biopsy. Therefore, our study is the first of its kind. Second, the prebiopsy PSA levels were collected over eight years, making our findings susceptible to laboratory heterogeneity. Nonetheless, this level of variation was taken into account by controlling for the year in which the measurement was done. Third, BMI (calculated from self-reported height and weight) might be an imperfect measure of obesity, leading to potential deviations from patients' true measurements. It was impossible to distinguish fat from muscle with the BMI, whereas the waist circumference (57), waist-to-hip ratio (58) and percentage of visceral adipose tissue (59) have recently been shown to be better obesity indicators. Furthermore, according to Choi et al (60), there is a discrepancy in the trend of PCa development according to BMI among the groups with different categories for waist circumference: higher the waist circumference category, the stronger was the association with BMI. Nonetheless, BMI is a convenient alternative to other obesity indicators and has a universal measurement standard. Moreover, its association with PSA has been thoroughly studied, making it a generalizable and convenient proxy for obesity. Finally, the study failed to adjust for additional confounding factors that could possibly be associated with PSA levels, such as the duration of obesity, medication use, comorbidities, daily diet and exercise. When considering the implications of this study on a random sample of the population the effect of BMI on PCa outcome is likely to be underestimated due to the effect of the inclusion criteria.
Table VIII.
Model patient characteristics and probability of cancer detection based on Model 1.
| Patient characteristics | BMI, kg/m2 | Age, years | log(PSA) | Year | DRE | Probability of cancer detection, % | Relative risk |
|---|---|---|---|---|---|---|---|
| Normal weight, negative DRE | 23.9 | 69.4 | 0.881 | 2 | 0 (negative) | 22.1 | 1.00 |
| Overweight, negative DRE | 27.0 | 69.4 | 0.881 | 2 | 0 (negative) | 26.8 | 1.21 |
| Obese, negative DRE | 31.8 | 69.4 | 0.881 | 2 | 0 (negative) | 35.3 | 1.60 |
| Normal weight, positive DRE | 23.9 | 69.4 | 0.881 | 2 | 1 (positive) | 87.3 | 3.96 |
| Overweight, positive DRE | 27.0 | 69.4 | 0.881 | 2 | 1 (positive) | 89.9 | 4.07 |
| Obese, positive DRE | 31.8 | 69.4 | 0.881 | 2 | 1 (positive) | 93.0 | 4.21 |
DRE, digital rectal examination; PSA, prostate specific antigen.
In conclusion, taking into account the above limitations, our results still have important implications for PCa screening and detection for obese patients. In the current study, the risk of developing PCa was found to be higher in men with higher BMI with significantly younger obese patients suffering from similar levels of pathology as older normal-weight patients. In model patients with median characteristics, the relative risk analysis of cancer diagnosis shows that for patients with a negative DRE result, obesity accounts for a 60% increased risk. Instead, when the DRE outcome is positive, the increase is subdued due to the high baseline risk in this scenario. This suggests that obese patients are more likely to be affected by advanced disease at a younger age and, hence, might benefit from more aggressive treatment options.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
RB was the major contributor in writing the manuscript. RB, GM and GN performed the prostate biopsies. CC, CG and ABF performed the statistical analysis of the data. CD and FP made substantial contributions to conception and design of this study. RB and GM confirm the authenticity of all the raw data. RS, UDM, OI and UP interpreted the patient data regarding urological disease. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki. However, ethics approval was not applicable because this was a retrospective study that did not include procedures outside of common and correct clinical practice. Furthermore, all patients included in the study gave their written informed consent for the processing of medical data.
Patient consent for publication
Written informed consent was obtained from all patients for the publication of the present study and for processing their medical data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bell KJ, Del Mar C, Wright G, Dickinson J, Glasziou P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int J Cancer. 2015;137:1749–1757. doi: 10.1002/ijc.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: Perspectives from autopsy studies. Can J Urol. 2008;15:3866–3871. [PMC free article] [PubMed] [Google Scholar]
- 4.Jansson KF, Akre O, Garmo H, Bill-Axelson A, Adolfsson J, Stattin P, Bratt O. Concordance of tumor differentiation among brothers with prostate cancer. Eur Urol. 2012;62:656–561. doi: 10.1016/j.eururo.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Hemminki K. Familial risk and familial survival in prostate cancer. World J Urol. 2012;30:143–148. doi: 10.1007/s00345-011-0801-1. [DOI] [PubMed] [Google Scholar]
- 6.Randazzo M, Müller A, Carlsson S, Eberli D, Huber A, Grobholz R, Manka L, Mortezavi A, Sulser T, Recker F, Kwiatkowski M. A positive family history as a risk factor for prostate cancer in a population-based study with organised prostate-specific antigen screening: Results of the Swiss European randomised study of screening for prostate cancer (ERSPC, Aarau) BJU Int. 2016;117:576–583. doi: 10.1111/bju.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan DS, Mok TS, Rebbeck TR. Cancer genomics: Diversity and disparity across ethnicity and geography. J Clin Oncol. 2016;34:91–101. doi: 10.1200/JCO.2015.62.0096. [DOI] [PubMed] [Google Scholar]
- 8.Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J, Jugurnauth-Little S, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45:385–391. doi: 10.1038/ng.2560. 391e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin Al Olama A, Dadaev T, Hazelett DJ, Li Q, Leongamornlert D, Saunders EJ, Stephens S, Cieza-Borrella C, Whitmore I, Benlloch Garcia S, et al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum Mol Genet. 2015;24:5589–5602. doi: 10.1093/hmg/ddv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–453. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch HT, Kosoko-Lasaki O, Leslie SW, Rendell M, Shaw T, Snyder C, D'Amico AV, Buxbaum S, Isaacs WB, Loeb S, et al. Screening for familial and hereditary prostate cancer. Int J Cancer. 2016;138:2579–2591. doi: 10.1002/ijc.29949. [DOI] [PubMed] [Google Scholar]
- 12.Ewing CM, Ray AM, Lange EM, Zuhlke KA, Robbins CM, Tembe WD, Wiley KE, Isaacs SD, Johng D, Wang Y, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366:141–149. doi: 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitzmann MF, Rohrmann S. Risk factors for the onset of prostatic cancer: Age, location, and behavioral correlates. Clin Epidemiol. 2012;4:1–11. doi: 10.2147/CLEP.S16747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breslow N, Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B, Sternby NH, Tulinius H. Latent carcinoma of prostate at autopsy in seven areas. The international agency for research on cancer, Lyons, France. Int J Cancer. 1977;20:680–688. doi: 10.1002/ijc.2910200506. [DOI] [PubMed] [Google Scholar]
- 15. Global Health Risks: Mortality and burden of disease attributable to selected major risks. World Health Organisation: Geneva, Switzerland, 2019. [Google Scholar]
- 16.Kaaks R, Kühn T. Epidemiology: Obesity and cancer-the evidence is fattening up. Nat Rev Endocrinol. 2014;10:644–645. doi: 10.1038/nrendo.2014.168. [DOI] [PubMed] [Google Scholar]
- 17.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Cancer Society: Cancer facts and figures. American Cancer Society: Atlanta, GA, USA, 2012. [Google Scholar]
- 19.Buschemeyer WC III, Freedland SJ. Obesity and prostate cancer: Epidemiology and clinical implications. Eur Urol. 2007;52:331–343. doi: 10.1016/j.eururo.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 20.Culp S, Porter M. The effect of obesity and lower serum prostate-specific antigen levels on prostate-cancer screening results in American men. BJU Int. 2009;104:1457–1461. doi: 10.1111/j.1464-410X.2009.08646.x. [DOI] [PubMed] [Google Scholar]
- 21.Baillargeon J, Pollock BH, Kristal AR, Bradshaw P, Hernandez J, Basler J, Higgins B, Lynch S, Rozanski T, Troyer D, Thompson I. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103:1092–1095. doi: 10.1002/cncr.20856. [DOI] [PubMed] [Google Scholar]
- 22.Freedland SJ, Terris MK, Platz EA, Presti JC Jr. Body mass index as a predictor of prostate cancer: Development versus detection on biopsy. Urology. 2005;66:108–113. doi: 10.1016/j.urology.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 23.Fowke JH, Signorello LB, Chang SS, Matthews CE, Buchowski MS, Cookson MS, Ukoli FM, Blot WJ. Effects of obesity and height on prostate-specific antigen (PSA) and percentage of free PSA levels among African-American and Caucasian men. Cancer. 2006;107:2361–2367. doi: 10.1002/cncr.22249. [DOI] [PubMed] [Google Scholar]
- 24.Beebe-Dimmer JL, Faerber GJ, Morgenstern H, Werny D, Wojno K, Halstead-Nussloch B, Cooney KA. Body composition and serum prostate-specific antigen: Review and findings from Flint men's health study. Urology. 2008;71:554–560. doi: 10.1016/j.urology.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rundle A, Neugut AI. Obesity and screening PSA levels among men undergoing an annual physical exam. Prostate. 2008;68:373–380. doi: 10.1002/pros.20704. [DOI] [PubMed] [Google Scholar]
- 26.Grubb RL III, Black A, Izmirlian G, Hickey TP, Pinsky PF, Mabie JE, Riley TL, Ragard LR, Prorok PC, Berg CD, et al. Serum prostate-specific antigen hemodilution among obese men undergoing screening in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2009;18:748–751. doi: 10.1158/1055-9965.EPI-08-0938. [DOI] [PubMed] [Google Scholar]
- 27.Barqawi AB, Golden BK, O'Donnell C, Brawer MK, Crawford ED. Observed effect of age and body mass index on total and complexed PSA: Analysis from a national screening program. Urology. 2005;65:708–712. doi: 10.1016/j.urology.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 28.Price MM, Hamilton RJ, Robertson CN, Butts MC, Freedland SJ. Body mass index, prostate-specific antigen, and digital rectal examination findings among participants in a prostate cancer screening clinic. Urology. 2008;71:787–791. doi: 10.1016/j.urology.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Bañez LL, Hamilton RJ, Partin AW, Vollmer RT, Sun L, Rodriguez C, Wang Y, Terris MK, Aronson WJ, Presti JC Jr, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275–2280. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 30.Fontaine KR, Heo M, Allison DB. Obesity and prostate cancer screening in the USA. Public Health. 2005;119:694–698. doi: 10.1016/j.puhe.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Fowke JH, Signorello LB, Underwood W III, Ukoli FA, Blot WJ. Obesity and prostate cancer screening among African-American and Caucasian men. Prostate. 2006;66:1371–1380. doi: 10.1002/pros.20377. [DOI] [PubMed] [Google Scholar]
- 32.Scales CD Jr, Curtis LH, Norris RD, Schulman KA, Dahm P, Moul JW. Relationship between body mass index and prostate cancer screening in the United States. J Urol. 2007;177:493–498. doi: 10.1016/j.juro.2006.09.059. [DOI] [PubMed] [Google Scholar]
- 33.Engeland A, Tretli S, Bjørge T. Height, body mass index, and prostate cancer: A follow-up of 950000 Norwegian men. Br J Cancer. 2003;89:1237–1242. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson SO, Wolk A, Bergström R, Adami HO, Engholm G, Englund A, Nyrén O. Body size and prostate cancer: A 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–389. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 35.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 36.Freedland SJ, Bañez LL, Sun LL, Fitzsimons NJ, Moul JW. Obese men have higher-grade and larger tumors: An analysis of the duke prostate center database. Prostate Cancer Prostatic Dis. 2009;12:259–263. doi: 10.1038/pcan.2009.11. [DOI] [PubMed] [Google Scholar]
- 37.Hu MB, Xu H, Bai PD, Jiang HW, Ding Q. Obesity has multifaceted impact on biochemical recurrence of prostate cancer: A dose-response meta-analysis of 36,927 patients. Med Oncol. 2014;31(829) doi: 10.1007/s12032-013-0829-8. [DOI] [PubMed] [Google Scholar]
- 38.Hutterer G, Perrotte P, Gallina A, Walz J, Jeldres C, Traumann M, Suardi N, Saad F, Bénard F, Valiquette L, et al. Body mass index does not predict prostate-specific antigen or percent free prostate-specific antigen in men undergoing prostate cancer screening. Eur J Cancer. 2007;43:1180–1187. doi: 10.1016/j.ejca.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Capitanio U, Perrotte P, Hutterer GC, Suardi N, Jeldres C, Shariat SF, Duclos A, Arjane P, Montorsi F, Karakiewicz PI. Effect of body mass index on prostate-specific antigen and percentage free prostate-specific antigen: Results from a prostate cancer screening cohort of 1490 men. Int J Urol. 2009;16:91–95. doi: 10.1111/j.1442-2042.2008.02192.x. [DOI] [PubMed] [Google Scholar]
- 40.Freedland SJ, Platz EA, Presti JC Jr, Aronson WJ, Amling CL, Kane CJ, Terris MK. Obesity, serum prostate specific antigen and prostate size: Implications for prostate cancer detection. J Urol. 2006;175:500–504. doi: 10.1016/S0022-5347(05)00162-X. [DOI] [PubMed] [Google Scholar]
- 41.Hu MB, Liu SH, Jiang HW, Bai PD, Ding Q. Obesity affects the biopsy-mediated detection of prostate cancer, particularly high-grade prostate cancer: A dose-response meta-analysis of 29,464 Patients. PLoS One. 2014;9(e106677) doi: 10.1371/journal.pone.0106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: Systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi T, Mitsumori K, Nishizawa K, Kawahara T, Ogura K, Ide Y. Association between body mass index and prostate cancer detection rates in Japanese urologic patients. Urology. 2005;66:130–134. doi: 10.1016/j.urology.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 44.Masuda H, Kagawa M, Kawakami S, Numao N, Matsuoka Y, Yokoyama M, Yamamoto S, Yonese J, Fukui I, Kihara K. Body mass index influences prostate cancer risk at biopsy in Japanese men. Int J Urol. 2013;20:701–707. doi: 10.1111/iju.12023. [DOI] [PubMed] [Google Scholar]
- 45.Park J, Cho SY, Lee SB, Son H, Jeong H. Obesity is associated with higher risk of prostate cancer detection in a biopsy population in Korea. BJU Int. 2014;114:891–895. doi: 10.1111/bju.12600. [DOI] [PubMed] [Google Scholar]
- 46.Lee SE, Hong SK, Park HZ, Chang JS, Yoon CY, Byun SS, Abdullajanov M. Higher body mass index is associated with lower risk of prostate cancer detection via multi (≥12)-core prostate biopsy in Korean men. Urology. 2010;76:1063–1066. doi: 10.1016/j.urology.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 47.Oh JJ, Jeong SJ, Lee BK, Jeong CW, Byun SS, Hong SK, Lee SE. Does obesity affect the accuracy of prostate-specific antigen (PSA) for predicting prostate cancer among men undergoing prostate biopsy. BJU Int. 2013;112:E265–E271. doi: 10.1111/j.1464-410X.2012.11766.x. [DOI] [PubMed] [Google Scholar]
- 48.Lavalette C, Cordina Duverger E, Artaud F, Rébillard X, Lamy PJ, Trétarre B, Cénée S, Menegaux F. Body mass index trajectories and prostate cancer risk: Results from the EPICAP study. Cancer Med. 2020;9:6421–6429. doi: 10.1002/cam4.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michael J, Oyekunle T, Howard L, De Hoedt A, Hoyo C, Grant D, Freedland S. Interplay between exercise and BMI; results from an equal access, racially diverse biopsy study. Cancer Causes Control. 2019;30:13–20. doi: 10.1007/s10552-018-1104-2. [DOI] [PubMed] [Google Scholar]
- 50.Werny DM, Thompson T, Saraiya M, Freedman D, Kottiri BJ, German RR, Wener M. Obesity is negatively associated with prostate-specific antigen in U.S. men, 2001-2004. Cancer Epidemiol Biomarkers Prev. 2007;16:70–76. doi: 10.1158/1055-9965.EPI-06-0588. [DOI] [PubMed] [Google Scholar]
- 51.Kim YJ, Han BK, Hong SK, Byun SS, Kim WJ, Lee SE. Body mass index influences prostate-specific antigen in men younger than 60 years of age. Int J Urol. 2007;14:1009–1012. doi: 10.1111/j.1442-2042.2007.01879.x. [DOI] [PubMed] [Google Scholar]
- 52.Isom-Batz G, Bianco FJ Jr, Kattan MW, Mulhall JP, Lilja H, Eastham JA. Testosterone as a predictor of pathological stage in clinically localized prostate cancer. J Urol. 2005;173:1935–1937. doi: 10.1097/01.ju.0000158040.33531.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nwadi UV, Nwofor AME, Oranusi CK, Orakwe JC, Obiesie EA, Mbaeri TU, Abiahu JA, Mbonu OO. Correlation between body mass index and gleason score in men with prostate cancer in Southeastern Nigeria. Niger J Surg. 2021;27:22–27. doi: 10.4103/njs.NJS_66_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price RS, Cavazos DA, De Angel RE, Hursting SD, deGraffenried LA. Obesity-related systemic factors promote an invasive phenotype in prostate cancer cells. Prostate Cancer Prostatic Dis. 2012;15:135–143. doi: 10.1038/pcan.2011.54. [DOI] [PubMed] [Google Scholar]
- 55.Nandeesha H. Insulin: A novel agent in the pathogenesis of prostate cancer. Int Urol Nephrol. 2009;41:267–272. doi: 10.1007/s11255-008-9440-x. [DOI] [PubMed] [Google Scholar]
- 56.Fowke JH, Motley SS, Cookson MS, Concepcion R, Chang SS, Wills ML, Smith JA Jr. The association between body size, prostate volume and prostate-specific antigen. Prostate Cancer Prostatic Dis. 2007;10:137–142. doi: 10.1038/sj.pcan.4500924. [DOI] [PubMed] [Google Scholar]
- 57.Park JH, Cho BL, Kwon HT, Lee CM, Han HJ. Effect of body mass index and waist circumference on prostate specific antigen and prostate volume in a generally healthy Korean population. J Urol. 2009;182:106–111. doi: 10.1016/j.juro.2009.02.130. [DOI] [PubMed] [Google Scholar]
- 58.Yang CY, Peng CY, Liu YC, Chen WZ, Chiou WK. Surface anthropometric indices in obesity-related metabolic diseases and cancers. Chang Gung Med J. 2011;34:1–22. [PubMed] [Google Scholar]
- 59.Qu YY, Dai B, Kong YY, Chang K, Ye DW, Yao XD, Zhang SL, Zhang HL, Yang WY. Influence of obesity on localized prostate cancer patients treated with radical prostatectomy. Asian J Androl. 2013;15:747–752. doi: 10.1038/aja.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi JB, Myong JP, Lee Y, Kim I, Kim JH, Hong SH, Ha US. Does increased body mass index lead to elevated prostate cancer risk? It depends on waist circumference. BMC Cancer. 2020;20(589) doi: 10.1186/s12885-020-07089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.