Abstract
Islet autoantibodies, including autoantibodies directed against the 65kDa isoform of glutamate decarboxylase (GAD65Ab), are present in the majority of patients with newly diagnosed type 1 diabetes (T1D). Whereas these autoantibodies are historically viewed as an epiphenomenon of the autoimmune response with no significant pathogenic function, we consider in this study the possibility that they impact the major islet function, namely glucose‐stimulated insulin secretion. Two human monoclonal GAD65Ab (GAD65 mAb) (b78 and b96.11) were investigated for uptake by live rat beta cells, subcellular localization and their effect on glucose‐stimulated insulin secretion. The GAD65 mAbs were internalized by live pancreatic beta cells, where they localized to subcellular structures in an epitope‐specific manner. Importantly, GAD65 mAb b78 inhibited, while GAD65 mAb b96.11 enhanced, glucose‐stimulated insulin secretion (GSIS). These opposite effects on GSIS rule out non‐specific effects of the antibodies and suggest that internalization of the antibody leads to epitope‐specific interaction with intracellular machinery regulating insulin granule release. The most likely explanation for the alteration of GSIS by GAD65 Abs is via changes in GABA release due to inhibition or change in GAD65 enzyme activity. This is the first report indicating an active role of GAD65Ab in the pathogenesis of T1D.
Keywords: autoantibodies, GAD65, insulin secretion
1. INTRODUCTION
Type 1 diabetes (T1D), an autoimmune disease that requires lifelong injections of insulin and is associated with increased risk for multiple complications, has been extensively studied. However, the role of autoantibodies (AutoAbs) in the development of T1D is the subject of ongoing debate. AutoAbs directed against islet cell antigens, including insulin and the 65 kDa isoform of glutamate decarboxylase (GAD65), are found in the majority of individuals diagnosed with T1D. 1 Islet AutoAb in T1D are generally considered an epiphenomenon with no pathogenic role. While publications in the early 80s indicated an effect of islet AutoAbs on insulin secretion, the results were inconclusive. 2 , 3 Passive transfer of islet AutoAbs derived from children diagnosed with T1D increased insulin secretion in perifused mouse islets 2 ; however, incubation of dispersed rat islets with islet AutoAbs led to an decrease of insulin secretion. 3
GAD65 is not only expressed in the beta cells of pancreatic islets but also in the central nervous system (CNS), 4 , 5 where it is function as one of two enzymes decarboxylating glutamate to yield the inhibitory neurotransmitter gamma‐butyric acid (GABA) is better understood. Moreover, GAD65’s association with the cytosolic face of GABAergic synaptic vesicles (SVs) 6 , 7 is essential for axonal transport of the vesicles from the Golgi apparatus to nerve terminals 8 , 9 and facilitates GABA’s inhibitory effects at nerve synapses. Individuals suffering from neuromuscular autoimmune diseases, including Stiff Person Syndrome and autoimmune Cerebellar Ataxia, present with high titres of GAD65Ab both in the circulation and in the CNS. 1 , 10 , 11 The intracellular location of GAD65 called into question whether GAD65Ab could play a pathogenic role, since antibodies are not thought to cross plasma membranes. 12 However, we have shown that GAD65Ab are internalized and retained by live neurons. 13 , 14 Furthermore, GAD65Ab injected into the cerebellum of rodents localize into Purkinje cells. 14 , 15 Mechanistically, GAD65Ab interferes with the binding of GAD65 to GABAergic SVs, and the subsequent transport of the vesicles to the synapsis. 14 Importantly, the above observations were replicated with human monoclonal GAD65Ab b78 that recognizes a specific conformational epitope, while a monoclonal GAD65Ab of a different epitope specificity (b96.11) had little or no effect on GABAergic neurotransmission. 14 , 16
Similar to neurons, in pancreatic beta cells GAD65 binds to GABAergic synaptic‐like microvesicles (SLMVs), 17 , 18 which differ from insulin secretory granules. 17 , 19 Despite a number of studies investigating the role of GABA on insulin secretion, 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 a clear role of GAD65 and GABA in the regulation of insulin secretion has not been established. Based on the above described studies showing a pathogenic role of GAD65Ab in neuromuscular diseases, we investigated whether GAD65Ab may impact insulin secretion in dispersed rat islet cells.
2. MATERIALS AND METHODS
2.1. Monoclonal antibodies
Monoclonal antibodies b96.11 and b78 were derived from a patient with autoimmune polyendocrine syndrome type 1 and recognize epitopes located at amino acid residues 308‐365 and 451‐585 respectively. 28 , 29 Mouse monoclonal antibody AE6D9 recognizes epitopes located at the A chain of insulin. 30 Human monoclonal antibody HAA1 (ATCC Manassas VA, USA; ATCC number: HB‐8534) is directed against Blood group A antigen and served as an isotype control for mAb b96.11 and b78. B‐cell lines and hybridoma were grown under standard conditions, and the antibodies were purified from cell supernatant using Protein G Sepharose (Catalog number 101242, Invitrogen). Monoclonal antibodies b78 and HAA1 were labelled with Alexa Fluor 647 (AF 647) (Catalog number A20173, Invitrogen), while b96.11 and AE6D9 were labelled with Alexa Fluor 488 (AF 488) (Catalog number A20181, Invitrogen) according to the manufacturer's instructions. TGN‐38 antibody (2F7.1) (catalog number NB300‐575SS) was obtained from Novus Biologicals (Littleton, CO).
2.2. Rat islet isolation and culture
Rat islets used in this study are an excellent model for testing the effects of treatments in beta cell function. Secretion of insulin from rat islets responds to all physiologic agents in vitro as they do in vivo in both humans and rats including glucose, amino acids, fatty acids, neurotransmitters (acetylcholine), incretins (GLP‐1), other hormones (somatostatin and glucagon) and sulfonylureas. Moreover, human islet quality and characteristics are very variable relative to inbred rodent models which tend to be very reproducible. 31
Islets were harvested from Sprague Dawley male rats (≈250 g, Envigo/Harlan, Indianapolis, IN)) anaesthetized by intraperitoneal injection of sodium pentobarbital (35 mg/230 g rat). Subsequently, islets were prepared by injecting collagenase (10 ml of 0.23 mg/ml Liberase, catalog number 05339880001; Roche Molecular Biochemicals, Indianapolis, IN) into the pancreatic duct and surgically removing the pancreas. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington. The pancreata were placed into 15‐ml conical tubes containing 5 ml of 0.23 mg/ml Liberase and incubated at 37°C for 30 min. The digestate was filtered (400 µm stainless steel screen), rinsed (Hank's buffered salt solution, pH 7.4 ± 0.1, catalog number 14025, Gibco Grand Island, NY) and purified in a gradient solution of OptiPrep (catalog number NC9182490, Fisher Scientific). 32 Islets were cultured for 18‐24 h prior to the experiments in RPMI media 1640 (catalog number 21875034, Gibco) supplemented with 10% heat‐inactivated foetal bovine serum (FBS) (catalog number S11195H, Atlanta Biologicals). 33
2.3. Glucose‐stimulated insulin secretion
When assessing the effect of antibodies on GSIS, isolated islets were cultured for 72 h in the presence or absence of antibodies and glibenclamide (1 µM) (catalog number CAS 10238‐21‐8, R&D, Minnesota), acetylcholine (10 µM) (catalog number A2261, Sigma‐Aldrich) and glucagon like peptide‐1 (GLP‐1) (100 nM) (catalog number G9416, Sigma‐Aldrich) as indicated (60 islets/condition). At the end of this incubation period, insulin secretion rate (ISR) was determined statically as described previously. 34 Briefly, islets were handpicked into a petri dish containing Krebs‐Ringer Bicarbonate Buffer, pH 7.4 ± 0.1, 0.1% BSA (catalog number A3059, Sigma‐Aldrich), and 3 mM glucose (catalog number G8270, Sigma‐Aldrich) and pre‐incubated at 37°C/5% CO2 for 60 min. Subsequently, islets were picked into wells of 96‐well plates (10 islets/well) containing desired amounts of glucose (either 3 or 20 mM) and antibody (2.6 µM) (n = 4 for each condition) and incubated for an additional 60 min. At the end of this period, supernatant was collected and later assayed for insulin utilizing a rat insulin radioimmunoassay RIA kit (catalog number RI‐13K, Millipore Sigma).
2.4. Islet single‐cell cultures
Rat islets were dissociated into single cells as previously described. 35 Briefly, dissociated islet cells was accomplished by gentle trituration of islets suspended in a phosphate‐buffered saline solution pH 7.4 ± 0.1 containing 0.125% trypsin/0.05 mM EDTA (catalog number 25 200 056, Gibco) every minute (using a 1 ml pipet) until the islets were visibly dispersed (about 5‐10 min). The trypsin was then deactivated by the addition of heat‐inactivated FBS.
2.5. Confocal microscopy
Primary rat islet cells derived from dissociated whole islets were cultured as monolayers on coverslips at 50‐100,000 cells/well to allow for high‐resolution confocal microscopy. Cells were fixed with 4% paraformaldehyde (catalog number J61899‐AK, Alfa Aesar) for 30 mins, blocked and permeabilized in PBS (catalog number 10010023, Gibco) with 0.3% Triton ‐100 (catalog number CAS 9002‐93‐1, Sigma‐Aldrich) and 10% FBS (catalog number S11195H, Atlanta Biologicals) for 1 h. Cells were incubated with fluorescently labelled antibodies (2 µg/ml) for 2 h. For live‐cell imaging, cells were incubated for the indicated periods of time with fluorescently labelled antibodies prior to confocal imaging. Confocal imaging was carried out using a Leica TCS SP8 confocal microscope with a 40×, 1.3 NA oil immersion objective. All images for quantification within a single experiment were captured with the same laser power and detector gain.
2.6. Statistical analysis
GSIS responses were compared within treatment groups. Friedman tests for multiple comparisons were performed with the GraphPad software (GraphPad Software Inc, San Diego, CA). A p‐value of <0.05 was considered as significant.
3. RESULTS
3.1. GAD65‐specific mAb show distinct staining in dispersed rat islets
To determine the respective binding pattern for GAD65 mAb b78 and b96.11, fixed and permeabilized dispersed islet cells were stained with fluorescent GAD65 mAb and control antibody HAA1 (Blood group A antigen) and their localization was assessed by confocal microscopy (Figure 1).
FIGURE 1.
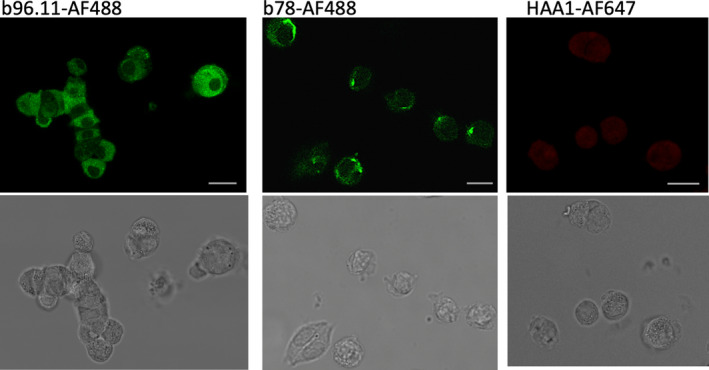
Confocal imaging of dispersed islet cells incubated with mAbs b96.11, b78 and HAA1 conjugated to the indicated fluorochromes. Dispersed islet cells were grown on coverslips at 50‐100,000 cells/well, fixed and permeabilized, and incubated with 2μg/ml of antibody for 2 h. Top row of panels: fluorescent images. Lower row of panels: bright field images of the respective cells shown in the above panel. Roughly 100 cells were analyzed for each image with ~60% showing positive staining with GAD65 mAb. The images are typical individual cells found within a single set of dispersed islets and are representative of similar analyses of six independent rat islet isolations. Scale bar is 10 microns in length
Distinct staining pattern for different GAD65 mAbs was observed. B78 showed a diffuse staining of the cytoplasm, with enhanced staining of structures adjacent to the nucleus, while b96.11 showed punctate staining located in the cytosol. Neither of the GAD65 mAbs stained the area of the nucleus. Control antibody HAA1 showed no significant staining.
3.2. Binding of GAD65Ab is specific for beta cells
Next, we endeavoured to ascertain whether GAD65 mAb staining was preferentially associating with pancreatic beta cells by comparing staining for GAD65 mAb to the other major type of cell in the islets, the alpha cell. Alpha cells are less than half the size of beta cells and are thus identifiable even without staining for glucagon. 36 , 37 The dispersed islets were co‐stained with GAD65 mAb and insulin‐specific monoclonal antibody AE6D9 (Figure 2).
FIGURE 2.
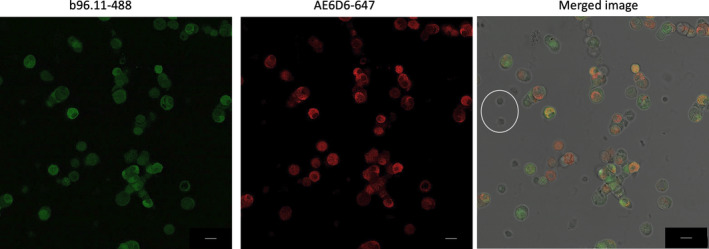
Confocal imaging of single islet cells incubated with GAD65 mAb b96.11‐AF488 (left panel) and insulin mAb AE6D9‐647 (middle panel). Dispersed islet cells were grown on coverslips at 50‐100,000 cells/well, fixed and permeabilized, and incubated with 2μg/ml of antibody for 2 h. The panel on the right presents an overlay of both stains on a bright field image. Roughly 100 cells were analyzed for each image with ~60% showing positive staining with GAD65 mAb and/or insulin. The images are typical individual cells found within a single set of dispersed islets and are representative of similar analyses of four independent rat islet isolations. Two alpha cells are circled for size comparison to the larger beta cells. Scale bar is 10 microns in length
Staining with GAD65 mAb b96.11 resulted in a strong fluorescence signal by beta cells, whereas alpha cells remained largely unstained. Although not all insulin‐positive cells stained for b96.11‐488, all cells that stained for GAD65 also stained for insulin. No staining of delta and epsilon cells was observed (data not shown).
3.3. GAD65 mAb b78 associated with the trans‐Golgi Network
GAD65 mAb b78 showed distinct staining of a subcellular structure, located close to the cell nucleus, while GAD65 mAb b96.11 resulted in the previously reported staining of vesicular structures. 17 To confirm that the b78 stained structure is the trans‐Golgi Network (TGN), we incubated fixed and permeabilized dispersed rat islet cells with b78 and TGN‐specific monoclonal antibody TGN‐38 (Figure 3) and staining was visualized by confocal imaging.
FIGURE 3.
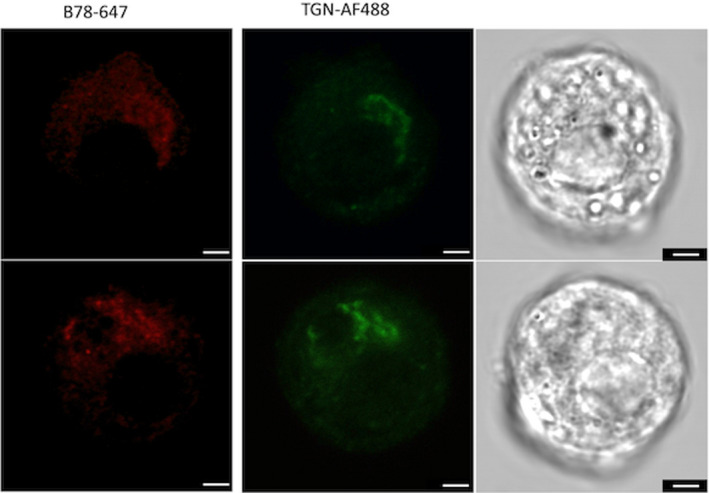
Confocal imaging of dispersed islets incubated with GAD65 mAb b78‐AF647 and TGN‐AF488. Bright field images of the respective cells are shown in the right panels. Dispersed islet cells were grown on coverslips at 50‐100,000 cells/well, fixed and permeabilized, and incubated with 2μg/ml of antibody for 2 h. Roughly 100 cells were analyzed. The images are typical of individual cells found within a single set of dispersed islets and are representative of similar analyses of three independent rat islet isolations. Scale bar is 2.5 microns in length
Staining with b78 was localized to the same subcellular structure that was bound by TGN‐specific mAb TGN‐38, confirming that b78 recognizes predominately GAD65 associated with the TGN.
3.4. GAD65 mAb are taken up by live pancreatic beta cells
To test uptake of GAD65 mAb, rat islet cells were incubated with fluorescent labelled GAD65 mAb b96.11. Fluorescence was recorded after 1, 2, 3, 4, 5 and 6 days (Figure 4).
FIGURE 4.
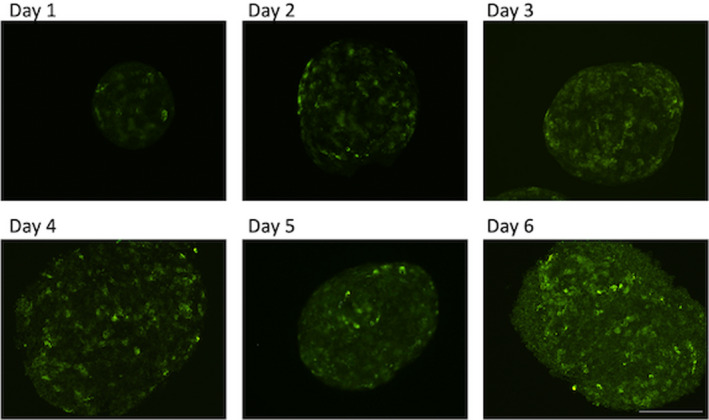
Pancreatic rat islets were incubated for the indicated times with GAD65 mAb b96.11‐AF488 (2.6 μM). The images are representative of similar analyses of three independent rat islet isolations. Scale bar is 50 microns in length
We observed a time‐dependent increase of uptake for b96.11. The strongest uptake was observed after 6 days. Similar kinetics were observed for b78, while control antibody HAA1 showed no uptake (data not shown). Staining of subcellular structures in live dispersed islets with fluorescent labelled GAD65 mAb revealed staining pattern similar to that observed in fixed and permeabilized cells. Co‐staining with cell viability markers showed that pancreatic beta cells were viable after uptake of monoclonal antibodies (data not shown).
3.5. GAD65 mAb modulate GSIS in an epitope‐specific manner
To evaluate the effect of GAD65 mAb on beta cell secretory function, we pre‐incubated live rat islet cells with GAD65 mAb for 72 h alone, or in the presence of agents designed to degranulate the islet insulin stores (either glibenclamide alone or glibenclamide plus acetylcholine and GLP‐1) and subsequently measured glucose‐stimulated insulin secretion (GSIS) (Figure 5).
FIGURE 5.
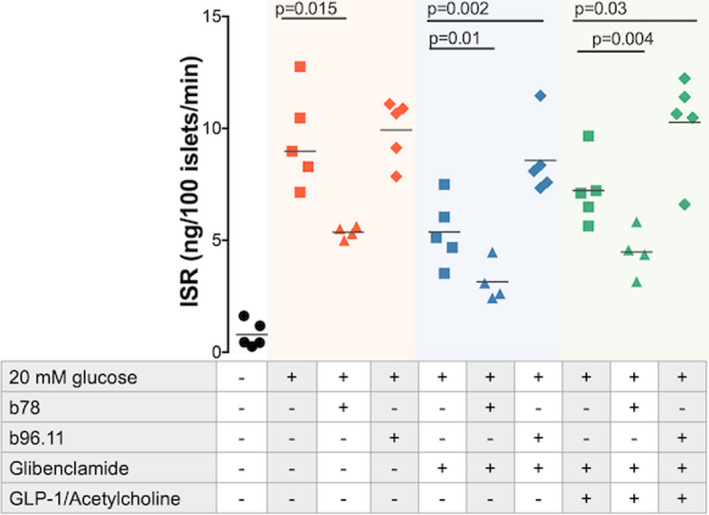
Effect of antibodies on GSIS. Islets were pre‐incubated for 72 h in the presence of the indicated antibodies and reagents. Insulin secretion in the presence of 5mM glucose indicates the unstimulated (baseline) insulin secretion (black filled circles). Insulin secretion rate (ISR) is measured in the absence of antibody (filled squares), in the presence of b96.11 (2.6 μM) (triangles) and in the presence of b78 (2.6 μM) (diamonds). GSIS is measured in the absence (red symbols) and presence of glibenclamide (blue symbols) and presence of glibenclamide plus acetylcholine and GLP‐1 (green symbols). Insulin secretion was measured in the presence of 3 or 20 mM glucose (see Methods section for details). Data are plotted as the ratio of insulin secretion at 20 mM glucose over that at 3 mM glucose. The results of five independent experiments are presented. For some experiments, the data points overlap, so that not all five results are visible. The median is presented by the horizontal bar
Glibenclamide is a powerful secretagogue, which does not promote compensatory proinsulin biosynthesis. 38 , 39 Acetylcholine and GLP‐1 both stimulate insulin secretion. 40 We performed experiments after pre‐incubation with the above secretagogues, to evaluate the effect of antibodies on insulin secretion at different states of degranulation.
GSIS after pre‐incubation with GAD65 mAb b78 alone was significantly lower than that of the control (median 8.6 vs. 5.4) (p = .015), while GAD65 mAb b96.11 had no effect on GSIS. The inhibitory effect of GAD65 mAb b78 was also observed when cells were pre‐incubated with secretagogues (Figure 5) (median 4.9 vs 2.8 pre‐incubation with glibenclamide) (p = 0.01) and (7.1 vs 4.5 pre‐incubation with Glib/Ach/GLP‐1) (p = 0.004). Moreover, the GSIS in the presence of GAD65 mAb b96.11 was significantly higher compared to that from control islets (4.9 vs 7.8 pre‐incubation with glibenclamide) (p = 0.01) (7.1 vs. 12.2 pre‐incubation with Glib/Ach/GLP‐1) (p = 0.03).
4. DISCUSSION
We present data, indicating that GAD65 mAb are internalized by living pancreatic beta cells and associate with specific subcellular structures. The antibody uptake is not restricted to GAD65Ab but was also observed for insulin‐specific Ab (data not shown). Antibody uptake by living cells has been demonstrated for different cell types (including Purkinje cells, 15 , 41 fibroblasts, 42 epithelial cells 43 and neurons 44 ), both in vitro 42 , 43 , 45 and in vivo. 15 , 41 , 46 Importantly, many autoimmune diseases are accompanied by autoantibodies targeting intracellular targets (reviewed in 47 ). While the mechanism of internalization has been determined for some autoantibodies (including interaction of basic residues in the CDR of autoantibodies with a negatively charged cell surface, 48 Fc receptor‐mediated entry, 49 clathrin‐mediated endocytosis 50 or caveolae/raft‐dependent endocytosis 51 ), other studies were not able to determine the mechanism of penetration, 42 or did not report it. 41 , 45 We investigated several possible uptake mechanisms using specific inhibitors: clathrin‐mediated endocytosis (Pitstop 2), caveolae‐mediated endocytosis (Filipin III), actin dependent endocytosis (Cytochalasin D) and Fc‐mediated uptake (Fc blocker Human TruStain FcX). None of the tested inhibitors had a significant effect on antibody uptake (data not shown). Thus, the mechanism involved in the uptake of autoantibodies to live beta cells remains to be determined.
Moreover, we identified specific GAD65 mAb enhancing GSIS, while GAD65 mAb with other epitope specificities resulted in a suppression of GSIS. These results may explain some of the earlier contradicting findings of the effect of GAD65Ab on insulin secretion. 2 , 3 Our previous investigations into the role of GAD65 mAb in GABAergic neurotransmission already revealed epitope‐specific effects and support these new findings. 13 , 52 Specifically, we found that GAD65 mAb b78 associates with GAD65 present on the trans‐Golgi Network and significantly inhibits GSIS, while GAD65 mAb b96.11 associates with GAD65 present on synaptic‐like microvesicles (SLMVs) and significantly stimulates GSIS. The respective association of the GAD65 mAbs confirms previous studies localizing GAD65 to the Golgi and to SLMVs. 17 , 53
GAD65 mAb‐mediated interference with GSIS is particularly strong in islets that have been partially depleted of their insulin granules and therefore rely on the replenishment of the large dense core vesicles (LDCVs). Since GAD65 is not directly associated with the LDCVs, the modulation of GSIS is unlikely the outcome of a direct modulation of insulin release from vesicles but may be mediated indirectly through modulation of GABA release or GABA levels. Insulin secretion is controlled by multiple factors in addition to extracellular glucose concentration. GABA‐mediated paracrine communication between islet cells has been suggested in the regulation of insulin secretion. 20 , 21 GABA is present at high levels in beta cells 19 and is released at a constant level via a glucose‐independent pathway. 17 , 54 Released GABA can regulate alpha and beta cell function via GABAA receptors expressed on both cell types and GABAB receptors expressed on beta cells only. 22 , 55 Activation of GABAA receptors present on alpha cells results in membrane hyperpolarization and inhibition of glucagon secretion, thereby blocking insulin secretion. 22 , 23 The effect of GABA on beta cells is depended on the extracellular glucose concentration. 26 , 27 At high glucose concentrations, GABA induces hyperpolarization and suppresses the secretion of insulin in an autocrine negative feedback loop. 27 , 56 Association of GAD65 mAb b96.11 with GAD65 present on the surface of GABAergic SLMVs may interfere with the proper release of GABA, thereby preventing the above downregulation of insulin secretion. This effect will be particularly relevant in the event when GABAergic SLMVs need to be replenished, for example after degranulation of beta cells, because GABAergic SLMVs need to be transported towards insulin secretion sites. Thus, the observation that b96.11 affects GSIS particularly after degranulation supports this hypothesis.
An alternative scenario considers the effect of intracellular GABA levels on insulin secretion. The majority of GABA in the beta cells is located in the cytosol and not associated with LDCVs or SLMVs. 19 , 57 Cytosolic GABA may enter the ‘GABA shunt’ after conversion into succinic semialdehyde (SSA) catalysed by the GABA transaminase T (GABA‐T). 58 , 59 Inhibition of GAD activity and GABA‐T has been demonstrated to reduce GSIS. 19 , 57 It is thus feasible that inhibition of GAD65 enzymatic activity by GAD65 mAb b78 11 reduces the intracellular GABA concentration and thereby interferes with GSIS. In contrast, GAD65 mAb b96.11 does not inhibit GAD65 enzyme activity 11 and will therefore not reduce GSIS.
Additional studies to investigate the mechanisms involved are underway.
To our knowledge, this is the first report showing a direct effect of GAD65 Ab on beta cell function, which may open a new line of investigations into the pathogenesis of T1D. To date, the presence of antibodies in pancreatic islets and specifically in beta cells has not been demonstrated. This may be due to the low number of intact beta cells in T1D patients, or low signal intensity. Immunoglobulin deposits in patient biopsies have been observed for few autoimmune diseases 60 , 61 , 62 and in neurons of normal rats. 63 However, the staining is often sporadic. 43 We are currently in the process of procuring pancreatic sections from new onset T1D patients to further evaluate this aspect. Other aspects of these antibodies, including titre, affinity and isotypes, will need to be considered and in vivo studies are planned to understand the relevance of our findings regarding beta cell function and T1D pathogenesis.
ACKNOWLEDGEMENT
None.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
VK, JRR and QH performed experiments; VK and JRR collected the data; CSH, IRS and WW analysed the data and interpreted the data, CSHs and IRS conceived and designed the study, CSH, IRS and WW supervised the study; CSH wrote the paper; CSH, IRS, WW, VK, QH and JRR approved the final version of the manuscript.
Kamat V, Radtke JR, Hu Q, Wang W, Sweet IR, Hampe CS. Autoantibodies directed against glutamate decarboxylase interfere with glucose‐stimulated insulin secretion in dispersed rat islets. Int J Exp Path. 2022;103:140–148. doi: 10.1111/iep.12437
Funding information
The insulin secretion assays for this project were carried out by the Cell Function Analysis Core of the Diabetes Research Center (DK017047).
REFERENCES
- 1. Couper JJ, Haller MJ, Ziegler AG, Knip M, Ludvigsson J, Craig ME. Phases of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2014;15:18‐25. [DOI] [PubMed] [Google Scholar]
- 2. Ziegler M, Lernmark Å. Insulin release from mouse islets perifused with serum IgG from newly insulin‐dependent diabetics. Acta Biol Med Ger. 1982;41:1123‐1127. [PubMed] [Google Scholar]
- 3. Kitagawa Y, Kanatsuna T, Kajiyama S, et al. Islet cell surface antibodies preferentially inhibit glucose‐stimulated insulin release in vitro. Diabetes Res Clin Pract. 1990;9:7‐13. [DOI] [PubMed] [Google Scholar]
- 4. Kaufman DL, Houser CR, Tobin AJ. Two forms of the γ‐aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christgau S, Schierbeck H, Aanstoot HJ, et al. Pancreatic β cells express two autoantigenic forms of glutamic acid decarboxylase, a 65‐kDa hydrophilic form and a 64‐kDa amphiphilic form which can be both membrane‐bound and soluble. J Biol Chem. 1991;266:21257‐21264. [PubMed] [Google Scholar]
- 6. Kanaani J, Cianciaruso C, Phelps EA, et al. Compartmentalization of GABA synthesis by GAD67 differs between pancreatic beta cells and neurons. PLoS One. 2015;10:1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanaani J, Patterson G, Schaufele F, Lippincott‐Schwartz J, Baekkeskov S. A palmitoylation cycle dynamically regulates partitioning of the GABA‐synthesizing enzyme GAD65 between ER‐Golgi and post‐Golgi membranes. J Cell Sci. 2008;121:437‐449. [DOI] [PubMed] [Google Scholar]
- 8. Kanaani J, El‐Husseini AED, Aguilera‐Moreno A, Diacovo JM, Bredt DS, Baekkeskov S. A combination of three distinct trafficking signals mediates axonal targeting and presynaptic clustering of GAD65. J Cell Biol. 2002;158:1229‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buddhala C, Suarez M, Modi J, et al. Calpain cleavage of brain glutamic acid decarboxylase 65 is pathological and impairs GABA neurotransmission. PLoS One. 2012;7:e33002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manto M, Mitoma H, Hampe CS. Anti‐GAD antibodies and the cerebellum: where do we stand? Cerebellum. 2019;18(2):153‐156. http://www.ncbi.nlm.nih.gov/pubmed/30343467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raju R, Foote J, Banga JP, et al. Analysis of GAD65 autoantibodies in Stiff‐Person Syndrome patients. J Immunol. 2005;175:7755‐7762. [DOI] [PubMed] [Google Scholar]
- 12. Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7:327‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hampe CS, Petrosini L, De Bartolo P, et al. Monoclonal antibodies to 65kDa glutamate decarboxylase induce epitope specific effects on motor and cognitive functions in rats. Orphanet J Rare Dis. 2013;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manto M, Honnorat J, Hampe CS, et al. Disease‐specific monoclonal antibodies targeting glutamate decarboxylase impair GABAergic neurotransmission and affect motor learning and behavioral functions. Front Behav Neurosci. 2015;9:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vega‐Flores G, Rubio SE, Jurado‐Parras MT, et al. The GABAergic septohippocampal pathway is directly involved in internal processes related to operant reward learning. Cereb Cortex. 2014;24:2093‐2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manto MU, Hampe CS, Rogemond V, Honnorat J. Respective implications of glutamate decarboxylase antibodies in stiff person syndrome and cerebellar ataxia. Orphanet J Rare Dis. 2011;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reetz A, Solimena M, Matteoli M, Folli F, Takei K, De Camilli P. GABA and pancreatic beta‐cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic‐like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991;10:1275‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christgau S, Aanstoot HJ, Schierbeck H, et al. Membrane anchoring of the autoantigen GAD65 to microvesicles in pancreatic β‐cells by palmitoylation in the NH2‐terminal domain. J Cell Biol. 1992;118:309‐320. doi: 10.1083/jcb.118.2.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sorenson RL, Garry DG, Brelje TC. Structural and functional considerations of GABA in islets of Langerhans. Beta‐cells and nerves. Diabetes. 1991;40:1365‐1374. [DOI] [PubMed] [Google Scholar]
- 20. Braun M, Ramracheya R, Rorsman P. Autocrine regulation of insulin secretion. Diabetes Obes Metab. 2012;14:143‐151. [DOI] [PubMed] [Google Scholar]
- 21. Shi Y, Kanaani J, Menard‐Rose V, et al. Increased expression of GAD65 and GABA in pancreatic β‐cells impairs first‐phase insulin secretion. Am J Physiol‐Endocrinol. Metab. 2000;279:684‐694. [DOI] [PubMed] [Google Scholar]
- 22. Xu E, Kumar M, Zhang Y, et al. Intra‐islet insulin suppresses glucagon release via GABA‐GABAA receptor system. Cell Metab. 2006;3:47‐58. [DOI] [PubMed] [Google Scholar]
- 23. Wendt A, Birnir B, Buschard K, et al. Glucose inhibition of glucagon secretion from rat α‐cells is mediated by GABA released from neighboring β‐cells. Diabetes. 2004;53:1038‐1045. [DOI] [PubMed] [Google Scholar]
- 24. Dong H, Kumar M, Zhang Y, et al. Gamma‐aminobutyric acid up‐ and downregulates insulin secretion from beta cells in concert with changes in glucose concentration. Diabetologia. 2006;49:697‐705. [DOI] [PubMed] [Google Scholar]
- 25. Faraji F, Ghasemi A, Motamedi F, Zahediasl S. Time‐dependent effect of GABA on glucose‐stimulated insulin secretion from isolated islets in rat. Scand J Clin Lab Invest. 2011;71:462‐466. [DOI] [PubMed] [Google Scholar]
- 26. Braun M, Wendt A, Birnir B, et al. Regulated exocytosis of GABA‐containing synaptic‐like microvesicles in pancreatic β‐cells. J Gen Physiol. 2004;123:191‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brice NL, Varadi A, Ashcroft SJH, Molnar E. Metabotropic glutamate and GABA(B) receptors contribute to the modulation of glucose‐stimulated insulin secretion in pancreatic beta cells. Diabetologia. 2002;45:242‐252. [DOI] [PubMed] [Google Scholar]
- 28. Tremble J, Morgenthaler NG, Vlug A, et al. Human B cells secreting immunoglobulin G to glutamic acid decarboxylase‐ 65 from a nondiabetic patient with multiple autoantibodies and Graves’ disease: a comparison with those present in type 1 diabetes. J Clin Endocrinol Metab. 1997;82:2664‐2670. [DOI] [PubMed] [Google Scholar]
- 29. Fenalti G, Hampe CS, Arafat Y, et al. COOH‐terminal clustering of autoantibody and T‐cell determinants on the structure of GAD65 provide insights into the molecular basis of autoreactivity. Diabetes. 2008;57:1293‐1301. [DOI] [PubMed] [Google Scholar]
- 30. Schroer JA, Bender T, Feldmann RJ, Kim KJ. Mapping epitopes on the insulin molecule using monoclonal antibodies. Eur J Immunol. 1983;13:693‐700. [DOI] [PubMed] [Google Scholar]
- 31. Sweet IR, Gilbert M, Jensen R, et al. Glucose stimulation of cytochrome C reduction and oxygen consumption as assessment of human islet quality. Transplantation. 2005;80:1003‐1011. [DOI] [PubMed] [Google Scholar]
- 32. Matsumoto S, Kirchhof N. Immediate reversal of diabetes in primates following intraportal transplantation of porcine islets purified on a new histidine‐lactobionate‐iodixanol gradient. Transplantation. 1999;67:S220. [Google Scholar]
- 33. Sweet IR, Cook DL, DeJulio E, et al. Regulation of ATP/ADP in pancreatic islets. Diabetes. 2004;53:401‐409. [DOI] [PubMed] [Google Scholar]
- 34. Jung SR, Reed BJ, Sweet IR. A highly energetic process couples calcium influx through L‐type calcium channels to insulin secretion in pancreatic β‐cells. Am J Physiol‐Endocrinol Metab. 2009;297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neal AS, Rountree AM, Radtke JR, et al. A method for high‐throughput functional imaging of single cells within heterogeneous cell preparations. Sci Rep. 2016;6:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: a comparative study. Islets. 2009;1:129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zimny ML, Blackard WG. The surface structure of isolated pancreatic islet cells. Cell Tissue Res. 1975;164:467‐471. [DOI] [PubMed] [Google Scholar]
- 38. Borg LA, Andersson A. Long‐term effects of glibenclamide on the insulin production, oxidative metabolism and quantitative ultrastructure of mouse pancreatic islets maintained in tissue culture at different glucose concentrations. Acta Diabetol Lat. 1981;18:65‐83. [DOI] [PubMed] [Google Scholar]
- 39. Ling Z, Wang Q, Stangé G, In’t Veld P, Pipeleers D. Glibenclamide treatment recruits β‐cell subpopulation into elevated and sustained basal insulin synthetic activity. Diabetes. 2006;55:78‐85. [PubMed] [Google Scholar]
- 40. Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic β‐cell function. Endocr Rev. 2001;22:565‐604. [DOI] [PubMed] [Google Scholar]
- 41. Greenlee JE, Burns JB, Rose JW, Jaeckle KA, Clawson S. Uptake of systemically administered human anticerebellar antibody by rat Purkinje cells following blood‐brain barrier disruption. Acta Neuropathol. 1995;89:341‐345. [DOI] [PubMed] [Google Scholar]
- 42. Sali AD, Karakasiliotis I, Evangelidou M, Avrameas S, Lymberi P. Immunological evidence and regulatory potential for cell‐penetrating antibodies in intravenous immunoglobulin. Clin. Transl. Immunol. [Internet] 2015;4(10):e42. doi: 10.1038/cti.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Golan T, Gharavi A, Elkon K. Penetration of autoantibodies into living epithelial cells. J Investig Dermatol. 1993;100(3):316‐322. [DOI] [PubMed] [Google Scholar]
- 44. Douglas JN, Gardner LA, Levin MC. Antibodies to an intracellular antigen penetrate neuronal cells and cause deleterious effects. J. Clin Cell. Immunol. 2013;4:1‐7. doi: 10.4172/2155-9899.1000134 [DOI] [Google Scholar]
- 45. Hill KE, Clawson SA, Rose JW, Carlson NG, Greenlee JE. Cerebellar Purkinje cells incorporate immunoglobulins and immunotoxins in vitro: Implications for human neurological disease and immunotherapeutics. J Neuroinflammation. 2009;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abedi‐Valugerdi M, Hu H, Möller G. Mercury‐induced anti‐nucleolar autoantibodies can transgress the membrane of living cells in vivo and in vitro. Int Immunol. 1999;11:605‐615. [DOI] [PubMed] [Google Scholar]
- 47. Burbelo PD, Iadarola MJ, Keller JM, Warner BM. Autoantibodies targeting intracellular and extracellular proteins in autoimmunity. Front Immunol. 2021;12:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song YC, Sun GH, Lee TP, et al. Arginines in the CDR of anti‐dsDNA autoantibodies facilitate cell internalization via electrostatic interactions. Eur J Immunol. 2008;38:3178‐3190. [DOI] [PubMed] [Google Scholar]
- 49. Lisi S, Sisto M, Soleti R, et al. Fcγ receptors mediate internalization of anti‐Ro and anti‐La autoantibodies from Sjögren’s syndrome and apoptosis in human salivary gland cell line A‐253. J Oral Pathol Med. 2007;36(9):511‐523. doi: 10.1111/j.1600-0714.2007.00563.x [DOI] [PubMed] [Google Scholar]
- 50. Choi DK, Bae J, Shin SM, Shin JY, Kim S, Kim YS. A general strategy for generating intact, full‐length IgG antibodies that penetrate into the cytosol of living cells. Mabs. 2014;6:1402‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jang JY, Jeong JG, Jun HR, et al. A nucleic acid‐hydrolyzing antibody penetrates into cells via caveolae‐mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell Mol Life Sci. 2009;66:1985‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mitoma H, Manto M, Hampe CS. Pathogenic roles of glutamic acid decarboxylase 65 autoantibodies in cerebellar ataxias. J Immunol. Res. 2017;2017:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Phelps EA, Cianciaruso C, Michael IP, et al. Aberrant accumulation of the diabetes autoantigen GAD65 in Golgi membranes in conditions of er stress and autoimmunity. Diabetes. 2016;65:2686‐2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rorsman P, Berggren P, Bokvist K, et al. Glucose‐inhibition of glucagon secretion involves activation of GABAA‐receptor chloride channels. Nature. 1989;341:233‐236. [DOI] [PubMed] [Google Scholar]
- 55. Braun M, Ramracheya R, Bengtsson M, et al. γ‐aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic β‐cells. Diabetes. 2010;59:1694‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gu XH, Kurose T, Kato S, et al. Suppressive effect of GABA on insulin secretion from the pancreatic beta‐cells in the rat. Life Sci. 1993;52:687‐694. [DOI] [PubMed] [Google Scholar]
- 57. Pizarro‐Delgado J, Braun M, Hernández‐Fisac I, Martín‐Del‐Río R, Tamarit‐Rodriguez J. Glucose promotion of GABA metabolism contributes to the stimulation of insulin secretion in β‐cells. Biochem J. 2010;431:381‐389. [DOI] [PubMed] [Google Scholar]
- 58. Hernández‐Fisac I, Fernández‐Pascual S, Ortsäter H, et al. Oxo‐4‐methylpentanoic acid directs the metabolism of GABA into the Krebs cycle in rat pancreatic islets. Biochem J. 2006;400:81‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang C, Kerckhofs K, Van De Casteele M, Smolders I, Pipeleers D, Ling Z. Glucose inhibits GABA release by pancreatic β‐cells through an increase in GABA shunt activity. Am J Physiol‐Endocrinol. Metab. 2006;290:494‐500. [DOI] [PubMed] [Google Scholar]
- 60. Tan E, Kunkel H. An immunofluorescent study of the skin lesions in systemic lupus erythematosus. Arthritis Rheum. 1966;9:37‐47. [DOI] [PubMed] [Google Scholar]
- 61. Böhm I. IgG deposits can be detected in cell nuclei of patients with both lupus erythematosus and malignancy. Clin Rheumatol. 2007;26:1877‐1882. [DOI] [PubMed] [Google Scholar]
- 62. Borges LF, Busis NA. Intraneuronal accumulation of myeloma proteins. Arch Neurol. 1985;42:690‐694. [DOI] [PubMed] [Google Scholar]
- 63. Fabian RH, Ritchie TC. Intraneuronal IgG in the central nervous system. J Neurol Sci. 1986;73:257‐267. [DOI] [PubMed] [Google Scholar]


