Abstract
As cut-outs from a graphene sheet, nanographenes (NGs) and graphene nanoribbons (GNRs) are ideal cases with which to connect the world of molecules with that of bulk carbon materials. While various top-down approaches have been developed to produce such nanostructures in high yields, in the present perspective, precision structural control is emphasized for the length, width, and edge structures of NGs and GNRs achieved by modern solution and on-surface syntheses. Their structural possibilities have been further extended from “flatland” to the three-dimensional world, where chirality and handedness are the jewels in the crown. In addition to properties exhibited at the molecular level, self-assembly and thin-film structures cannot be neglected, which emphasizes the importance of processing techniques. With the rich toolkit of chemistry in hand, NGs and GNRs can be endowed with versatile properties and functions ranging from stimulated emission to spintronics and from bioimaging to energy storage, thus demonstrating their multitalents in present and future materials science.
1. Introduction
Among classics of chemistry, the structure and properties of benzene come to mind, and aromaticity, despite or perhaps because of its somewhat diffuse definition, still ignites lively discussions.1−3 Those who fancy elegant multistep syntheses with new stereogenic centers may tend to look down at “flat” benzene structures. Many pharmaceuticals, however, are made with benzene functionalization as a key step.4 More importantly, the conjugated hexagon in benzene is a versatile module for the design of complex molecules such as linear oligophenylenes or disc-type polycyclic aromatic hydrocarbons (PAHs). These are by no means lacking the appeal of chirality, as is obvious from cases of atropisomerism and helicity,5−7 and there are many good reasons to even let them grow into helical polymers.
Rational assembly of regular building blocks by covalent or noncovalent bonding has become a widely employed protocol of modern chemistry, and this modular concept has made benzene an indispensable element in nanoscience and materials science. Further, the ability to visualize and manipulate nanosized molecules by scanning probe methods has stimulated a systematic increase in the size of “benzene” nanostructures in one, two, or three dimensions (1D, 2D, or 3D). A good case is that of hexa-peri-hexabenzocoronenes (HBCs) as soluble “superbenzenes,” for which early studies with scanning tunneling spectroscopy have allowed recording of current–potential curves at the single-molecule level on the path to emerging nanoelectronics.8,9
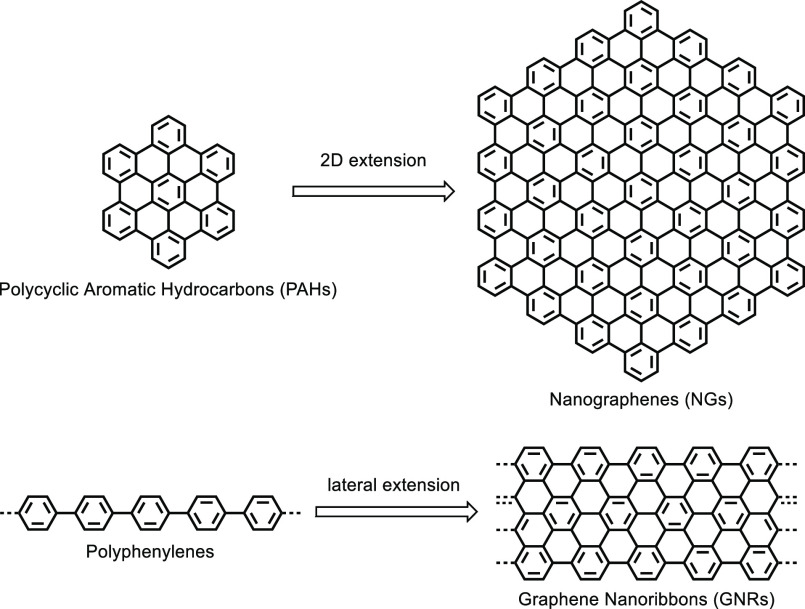
In the past decades, the world of carbon-rich polyphenylenes and PAHs has been extended toward carbon allotropes such as fullerenes, carbon nanotubes (CNTs), and graphene. While representing different dimensionalities, they are all made up of fused benzene rings and, remarkably enough, connect the realm of molecules with those of discrete particles and bulk materials. Our title compounds are nanographenes (NGs), ultralarge PAHs, and graphene nanoribbons (GNRs), ladder-type polyphenylenes (Scheme 1). Quite different ways of approaching GNRs are possible since they can be regarded either as (1) polyphenylenes extended laterally, (2) large PAHs grown into 1D, or (3) cut-outs from a graphene lattice.10 Indeed, various subunits have been carved out of graphene flakes by electron-beam lithography, while GNRs have been produced by slicing or squashing CNTs.11−13 The structural versatility of GNRs is outstanding considering not only variations of their length, width, and edge structure,14−18 but also heteroatom incorporation,19−21 nonplanarity, and helicity,22−26 as well as “drilling” of holes.27−29
Scheme 1. Representative Examples of Benzene-Based Graphenic Molecules.
These features are demanding challenges for synthesis, especially when structural precision is a major necessity. Carbon nanostructures such as NGs and GNRs hold promise for unprecedented physical properties, from exotic quantum states30,31 to stable biexcitonic states,32 and from spin transport33 to magnetism.34,35 Toward that end, GNRs must be made both structurally perfect and also narrow enough, and these two characteristics highlight the role of organic synthesis in graphene materials science. Solid-state and thin-film structures become important as well, and this emphasizes the importance of processing techniques such as gas-phase deposition or shear-mix exfoliation. Good cases include single sheets of NGs and GNRs, which are needed for defined van der Waals heterostructures with 2D materials.36
The vitality of research into NGs and GNRs is readily proven by the significant attention directed from various fields of chemistry, physics, biology, and materials science. What this perspectives article is meant to demonstrate is that precisely synthesized NGs and GNRs are astonishing multitalents in the field of functional carbon nanostructures.
2. Very Large – However, Still Perfect?
GNRs are defined as ribbon-shaped, quasi-1D graphenic nanostructures with aspect ratios larger than 10.37 From the viewpoint of polymer science, GNRs can be regarded as multistranded ladder polymers whose thermal and mechanical properties are expected to differ substantially from those of traditional single-stranded polymers.10,38,39 Additionally, various conjugation pathways arising in GNRs hold promise for special electronic band structures.40,41 Indeed, in the wake of the graphene hype, GNRs have attracted considerable attention from solid-state physics and materials science, which had an important electronic basis: despite its high charge carrier mobility, the vanishing band gap of graphene excluded widespread application as the semiconductor of field-effect transistors (FETs) due to unavoidable off-currents.42,43 In contrast, the geometric confinement prevailing in GNRs holds promise for finite and controllable band gaps. Theoretical studies of GNRs have revealed that their electronic properties, including band gaps and charge-carrier mobilities, depend critically on their width and edge structures.15,40,44,45 Materials scientists, recognizing the immense appeal of GNRs, have then employed various harsh methods of synthesis, including (1) lithographic46 and metal-nanoparticle catalyzed47 cutting of graphene sheets; (2) sonochemical extraction from expanded graphite;37 and (3) unzipping,48 plasma etching,11 and high-pressure squashing13 of CNTs. These methods have found appreciable attention, but lack the structural perfection needed for reliable band gap engineering. Precision polymer synthesis is therefore brought into play.
Synthesis of GNRs by consecutive fusion of small PAHs is unrealistic. Of widespread current use is a “polymerization–graphitization” protocol,49 in which branched polyphenylenes are made in a first step and then subjected to a chemical cyclodehydrogenation. Now the scope of cyclodehydrogenation is further broadened by the recent success of electrochemical methods.50,51 The branched polyphenylenes serve as carbon reservoirs; therefore, their topologies are crucial since the flattening process should neither leave holes of partially dehydrogenated spots nor produce spatial overlap of benzene rings. Scheme 2 presents some precursor polymers which document both the importance of a multibenzene “Lego” and the need for high molecular weights in the precursors.52−55 Transition-metal-catalyzed polycondensations, as demonstrated in Scheme 2b–c, suffer from unavoidable loss of functional groups,52−54 thus disturbing the perfect stoichiometries required for polycondensation and limiting the molecular weights, while repetitive Diels–Alder cycloadditions according to Scheme 2d can provide the targeted lengths of the polymers. The trick is to use an AB-type monomer 12 which contains the conjugated diene and an ethynyl group functioning as a dienophile. The structure of the resulting gulf-edged GNR 14 (4-gGNR, where “4” is the ribbon width defined by the number of carbon atoms across the ribbon) was firmly verified by infrared, Raman, ultraviolet–visible absorption, and nuclear magnetic resonance (NMR) spectroscopies, and an astonishing length of 600 nm was determined from dynamic light scattering experiments.55 Generating ultralarge GNRs via subsequent cyclodehydrogenation proceeds with a high degree of conversion and, surprisingly enough, provides solution-processable materials. This GNR synthesis certainly pushes the limits of molecular-based material synthesis and has been taken up by many research groups.56−59
Scheme 2. (a) Solution-Mediated Synthesis of GNR 4 through A2B2-Type Diels–Alder Polymerization;54 (b–c) Transition-Metal-Catalyzed Polycondensations;52,53 and (d) Diels–Alder Cycloadditions Used To Realize GNRs 8, 11, and 14 in Solution55.
The precursor polyphenylenes have multiple branches, but some of them are even dendritic. It is amazing that in seeking to extend PAHs into NGs, a new generation of dendrimers has been developed as polyphenylene dendrimers (PPDs) which consist only of twisted benzene units. Similar to the polyphenylene precursor 13, PPDs are also synthesized by repetitive Diels–Alder cycloadditions, but require a diethynyl-functionalized tetraphenylcyclopentadienone as an AB2-type branching reagent that carries two dienophilic units (Scheme 3a).60 Of course, the stepwise growth of higher dendrimer generation requires a protection–deprotection sequence, but it is the perfection of this protocol that guarantees high purity of PPDs as monodisperse polymers. The initial members of the series, as starting points for cyclodehydrogenation, are relatively small, as shown for C132 22 and C222 24 (Scheme 3b), but homologous dendrimers can be built up to the ninth generation with molecular weights of 1.9 MDa.61,62 There are many possible extensions into other fields of materials chemistry. One example is the transformation of PPDs with peripheral oligothienyl arms into networks upon electrochemical oxidative coupling.63 At more positive potentials, the dendrimer cores undergo partial flattening toward graphenic structures, which causes a dramatic increase in the electrical conductivity of the “graphene–thiophene” hybrid.
Scheme 3. (a) Synthesis of PPD 20 from AB2-Type Branching Reagent 16;60 (b) Syntheses of C132 and C222 by Cyclodehydrogenation from Relatively Small Dendrimers 22 and 24 with High Yields64,65.

Returning to the role of branched polyphenylenes as carbon reservoirs for precise synthesis of graphenic molecules, the critical step is cyclodehydrogenation to provide the much-needed quantitative flattening. Model reactions for small oligomers have led the way for investigations of cyclodehydrogenation efficiency. Astonishingly, far beyond the transformation of hexaphenylbenzene toward HBC, larger and larger precursors proceed with extremely high yields (Scheme 3b).64−66 Purification of such large NGs, which are practically insoluble, can only be done by washing with organic solvents to remove soluble precursors and byproducts, so it is critical to monitor the cyclodehydrogenations by high-resolution mass spectrometry. HBC, the starting member, can now be found as a widely accepted building block for an increasing number of complex unsaturated hydrocarbons used as chromophores and organic semiconductors.67−75
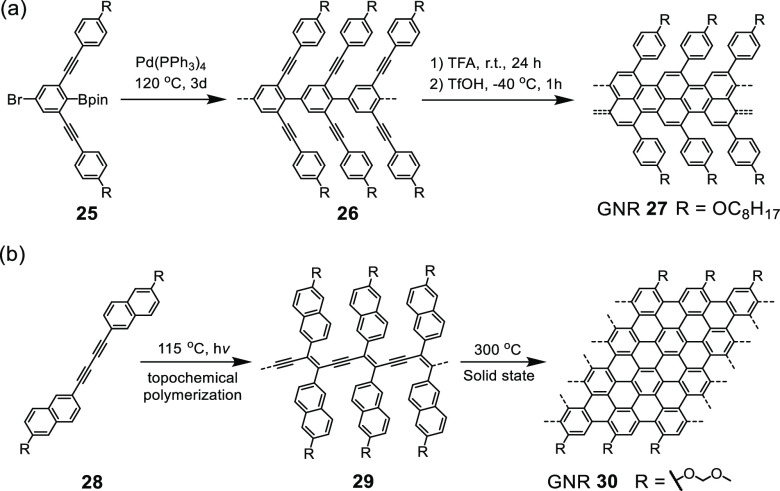
The Scholl reaction has played an indispensable role in quantitative cyclodehydrogenations of suitable precursors toward NGs and GNRs in solution, although sometimes unexpected rearrangements and/or inclusion of undesired halogens may occur.76−80 Alternatively, photocyclization of stilbene and related compounds,81 as well as annulative π-extension and dehydrative π-extension reactions,82−84 have been introduced as valuable tools for precise syntheses of larger and larger PAHs. With installation of halogens in the precursors at the ring-closing positions, cyclization can be further facilitated through intramolecular aryl–aryl coupling,85,86 but such halogenated precursors are synthetically more demanding. Efficient regioselective zipping of carbon–fluorine bonds via cyclodehydrofluorination on alumina provides new mechanisms for cyclizations providing PAHs and NGs.87,88 In addition to the “polymerization–graphitization” sequence, a few other protocols are being developed for GNR synthesis. An example is the Brønsted acid promoted nonoxidative benzannulation of polyalkynylated poly-para-phenylene precursors (Scheme 4a).89 Another one is the solid-phase topochemical polymerization of diacetylene precursor crystals with subsequent aromatization (Scheme 4b).90−92
Scheme 4. GNRs 27 and 30 Synthesized by (a) Brønsted Acid Promoted Nonoxidative Benzannulation89 and (b) Topochemical Polymerization90.
The essential question of whether such ultralarge carbon nanostructures are structurally perfect and reproducible relates to the methods of synthesis and characterization, both of which are still in need of further improvement. Regarding structure proof, while the whole toolbox of instrumental analysis must be employed, including crystal structure determinations, the macromolecular character and limited solubility of carbon nanostructures are severe obstacles. PAHs larger than HBC revealed such pronounced tendencies for aggregation that solution-NMR spectra could no longer be recorded, which emphasizes the need for various solid-state methods. Further, techniques such as scanning tunneling microscopy (STM) and noncontact atomic force microscopy (nc-AFM) have displayed chemical value in molecular structure visualizations with atomic precision.30,93−98 While these tools furnish nice graphics, deposition of molecules on metal surfaces and recording of micrographs might well overlook side products and defects beyond the limited visualization region. On the other hand, failures of graphene syntheses leave defects in the NG- and GNR-structures which, even if minor, may obstruct transport of charge carriers and hamper device performance, but go undetected in spectroscopic or microscopic analyses.
3. When Surfaces Come into Play
In addition to visualizing NGs and GNRs, nanoscience has also played a unique role in synthesis. Thereby, branched oligophenylene monomers equipped with two or more halogen substituents are deposited on metal surfaces by sublimation under ultrahigh vacuum (UHV) conditions. Carbon–halogen bonds are homolytically cleaved upon heating, which furnishes radical species prone to undergo clean polymerization. Further heating can cause cyclodehydrogenations leading to formation of flat graphenic species. The beauty of this approach is that (1) the processes can be monitored in situ by microscopies; (2) the radicals are not quenched by the solvent or air under UHV conditions; and (3) complex π-conjugated systems, including those that would not survive in solution, can be made and stabilized by interaction with the metal. The breakthrough in this direction was the synthesis of armchair-edged GNR 33 (7-AGNR, where, again, “7” is the ribbon width) from the dibromobianthryl 31 in our collaboration with group of Roman Fasel more than a decade ago (Figure 1).95 Excitingly, the structures of the target GNRs can be designed by the choice of monomer. This holds true for incorporation of heteroatoms or peripheral substituents,99−101 the nature of the edges such as the transition from armchair to zigzag peripheries,98 and the use of azulene-containing rather than all-benzenoid GNRs.102,103Scheme 5 can only provide some typical cases, but at present, the broad scope of this new concept is finding increasing attention.17,19,41,104−107 In addition to GNRs, more graphenic structures have been reported from on-surface syntheses, such as porous NGs,108,109 nanoporous graphene,28 and nonbenzenoid biphenylene networks,110 which are difficult or impossible to prepare via traditional solution chemistry.
Figure 1.

(a) Reaction schemes for 7-AGNR 33. (b) High-resolution STM image with a partly overlaid molecular model (blue) of 33. At the bottom left is a DFT-based STM simulation of 33 shown as a grayscale image. Reproduced with permission from ref (95). Copyright 2010 Springer Nature.
Scheme 5. Surface-Assisted Synthesis of (a) Heteroatom-Doped Chevron-Type GNR 36,99 (b) Zigzag-Edged GNR 39,98 and (c) Azulene-Containing GNRs 42 and 43(103).

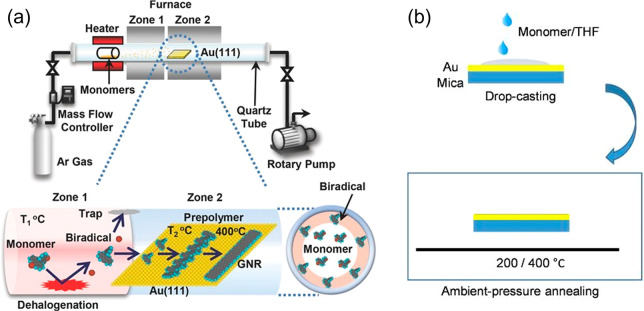
What speaks against this fascinating addition to the toolbox of organic and polymer synthesis is the sophisticated equipment required for surface physics and the extremely small scale, although some upscaling is possible by applying chemical vapor deposition (CVD) under less rigorous conditions.111−115 The reaction mechanism for the bottom-up GNR synthesis by CVD is similar to those of the UHV methods, except that trace amounts of oxygen can hardly be excluded in the CVD chamber; this reacts with the diradical intermediates to terminate the polymerization, thus leading to shorter and oxidized GNRs (Figure 2a).112 Therefore, it is essential to mix hydrogen with argon in the CVD growth process to suppress oxidation. A recent solution processing method has been developed to produce GNRs by drop-casting monomers on the reaction surface followed by annealing at ambient pressure (Figure 2b).116 In addition to the “polymerization–graphitization” protocol, control over GNR structures is realized through in situ growth of graphenic materials on certain catalytic templates, including germanium surfaces, hexagonal boron nitride trenches, nickel nanobars/films, and copper twin crystals.117−120 Recently, even a template-free CVD synthesis combining liquid copper and controlled etching by hydrogen has been demonstrated as a strategy for tunable growth, large scalability, and fewer defects in GNRs.121
Figure 2.
(a) Experimental CVD setup with an illustration of the presumed GNR growth process. Reproduced with permission from ref (112). Copyright 2014 John Wiley and Sons. (b) Schematic illustration of GNR synthesis through solution processing. Reproduced with permission from ref (116). Copyright 2017 The Chemical Society of Japan.
Despite the exciting progress in this field, metal-catalyzed on-surface reactions still face many unsolved mechanistic issues: (1) polymerization of diradical intermediates for repetitive CC-bond formation requires migration on the surface, which becomes increasingly difficult for higher oligomers; (2) end-capping by halogen or premature dehydrogenation may quench further reactions; and (3) the nature and surface structure of metals may become decisive, with metal adatoms coming into play as key reagents. Sublimation, even under UHV conditions, is limited by molecular size. In cases of ultralarge molecules, laser-supported deposition combined with soft-landing methods or deposition by vapor-phase transport has pushed the limits of processing in the gas phase.122,123 Further, control of on-surface GNR synthesis might comprise (1) the choice of the halogen substituent with different initiation temperatures; (2) the regiochemistry of asymmetric dihalo oligophenylenes; or (3) simultaneous introduction of different monomers, e.g., with electron-donating and electron-withdrawing substituents, which might furnish molecularly defined p–n junctions. Unlike solution synthesis, this on-surface chemistry is not troubled by solubility issues, but further applications of the GNRs in electronic devices will require lift-off from the surface and transfer to insulating substrates, either by etching of the metal surface or electrochemical delamination of the GNR films.104,112,113,124−126 These protocols, apart from necessitating a costly extra processing step, are still in need of further improvement. Therefore, establishing reliable on-surface synthetic methods directly on insulating substrates is critically important toward, for example, (opto)electronic and spintronic applications.127−130
4. Rising from Flatland
Using the branched polyphenylenes as carbon reservoirs, 3D precursors have been planarized to the graphenic “flatland”.131 Thereby, the topologies of the precursors in section 2 are designed to avoid spatial overlap of benzene rings during flattening. While this is crucial for accessing planar molecular nanocarbons, the cyclodehydrogenation reaction has also been successfully applied in the syntheses of nonplanar molecular structures despite the existing strain.132−139 By incorporating nonhexagonal rings into the “honeycomb” framework, curved NGs are obtained with bowl-shaped or saddle-shaped surfaces.140−144 In addition to the Scholl reaction, ring expansion, cyclotrimerization, intramolecular Friedel–Craft cyclization, Pd-catalyzed C–H arylation, and cascade radical photocyclization are also used to construct five-, seven-, or eight-membered rings.144−148 New opportunities for optical and electronic properties, especially chirality-related characteristics, have been demonstrated in various curved NGs with out-of-plane deformation of π-conjugation.137,149,150 The negatively curved NGs have the potential to self-assemble in organic solvents and serve as efficient gelators.133 The studies of curved NGs can also stimulate bottom-up syntheses and characterization of 3D carbon nanostructures,151 such as fullerenes,152−155 carbon schwarzites,140,156 Mackay crystals,157,158 and carbon nanosolenoids with Riemann surfaces.159 In a similar fashion, NGs can be twisted and bent by constructing the aliphatic chains as intramolecular bridges as in, for example, cyclophanes.160
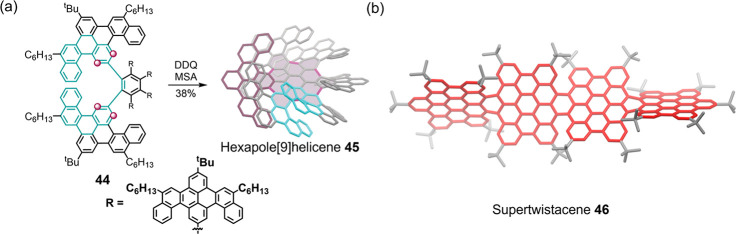
When “rising from the flatland”, chirality and handedness are no doubt the holy grail of synthesis. The resulting chiroptical features, such as circular dichroism and circularly polarized luminescence, are fascinating and have been studied for circularly polarized organic light-emitting diodes and chiral bioimaging applications.162,163 Chiral molecules not only interact with photons to produce chiroptical signals but also influence the spins of the electrons passing through the structures.164 This phenomenon, known as the chiral-induced spin-selectivity effect, has many potential applications in, for example, biorecognition as well as spintronics.165−167 Combinations of NGs and helicenes are therefore attracting increasing attention, and NGs can provide high hole mobility and an extra platform for chemical modifications.168−175 The chemistry of NGs, especially the cyclodehydrogenation reaction, has played a key role in substantially extending the π-conjugated systems of helicenes. This has provided multiple helical edges in NGs, such as hexapole [9]helicene 45 and supertwistacene 46 (Figure 3).135,161 The Scholl reaction can also deliver helical structures regioselectively from naphthalene, phenanthrene, furan, and thiadiazole building blocks137,176−178 in which the position with higher electron density appears to favor cyclodehydrogenation. Besides the Scholl reaction, the transition-metal-catalyzed [2 + 2 + 2] cycloaddition is another powerful tool to synthesize helical NGs.179,180 The π-extended helicenes, sometimes referred to as superhelicenes, possess intriguing mechanical, electronic, magnetic, and spin properties as nanosprings181 and nanosolenoids.149 In addition to the neutral π-extended helicenes, the charged species obtained through metal reduction offer new possibilities to engineer the geometry, aromaticity, and electronic structures.182−185
Figure 3.
Molecular models of NGs with multiple helical edges: (a) Synthesis of hexapole[9]helicene 45 from 44 via Scholl Reaction (reaction positions are highlighted with purple circles). Reproduced with permission from ref (161). Copyright 2018 John Wiley and Sons. (b) Supertwistacene 46. Reproduced with permission from ref (135). Copyright 2020 American Chemical Society.
“Superhelicenes” are expected to be superior to conventional helicenes in view of their enhanced chiroptical and electronic properties. A further extension from helical NGs to helical GNRs is expected to provide amplified chirality and electron conductivity due to the polymeric nature of GNRs. Precise structural control of nonplanar GNRs, including their size, length, edge structure, and handedness, is expected to be more demanding than that of their small molecular analogs, partly due to the strain accumulated along the polymer backbone. A smart strategy is to utilize the steric hindrance on the edge to create nonplanarity only on the periphery, as demonstrated by the cove-18,24 and fjord-edge26 GNRs reported recently (Figure 4a–c). While the nonplanar edge structures can be disclosed by X-ray crystallographic analyses of the model compounds 56 and 57, the corresponding GNRs 55 are only tentatively envisioned to possess a single site of chirality on the edges (only M or only P) in the most stable geometry (Figure 4d).
Figure 4.
Synthetic routes toward (a–b) Cove-edged GNRs 49, 52 and (c) Fjord-edged GNR 55. (d) X-ray crystallographic analyses of fjord-edged model compounds 56 and 57, as well as geometrical envisioning of the corresponding GNRs 55. Reproduced with permission from ref (26). Copyright 2021 American Chemical Society.
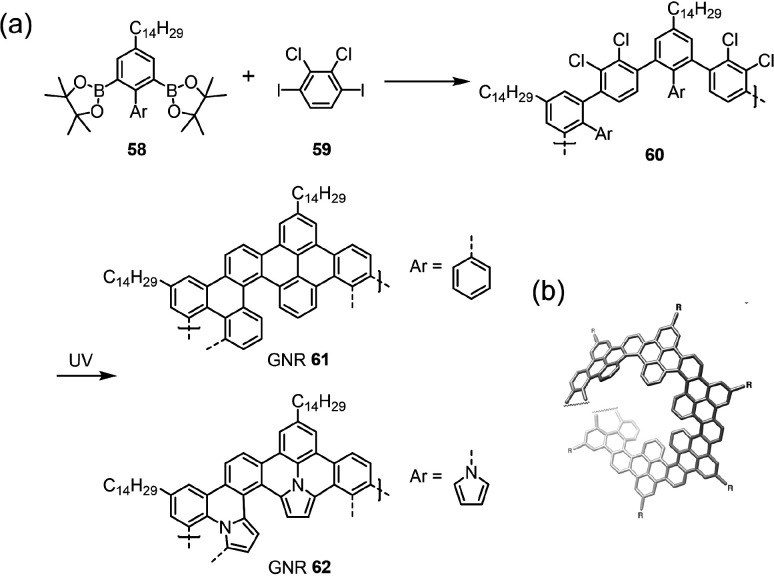
Such multihelicity on the edge together with the intrinsic polydisperse nature of the GNRs will unavoidably prevent chiral separation and investigations of their chiral properties. The helicene-like GNRs 61 and 62 synthesized by the photochemical cyclodehydrochlorination of the chlorinated polyphenylene precursors provide promising examples of chiral GNRs with single-handedness (Figure 5).22,25 Unfortunately, the helicity is created during the cyclodehydrochlorination without chiral selectivity, meaning that the obtained GNRs are still racemic mixtures. There is, thus, plenty of room to adopt state-of-the-art asymmetric syntheses to fully unleash the potential of chiral GNRs.186
Figure 5.
(a) Helically coiled GNRs 61 and 62 from Suzuki polymerization followed by a photochemical cyclodehydrochlorination reaction. (b) Helical structure of GNR 61 simulated by DFT calculations.
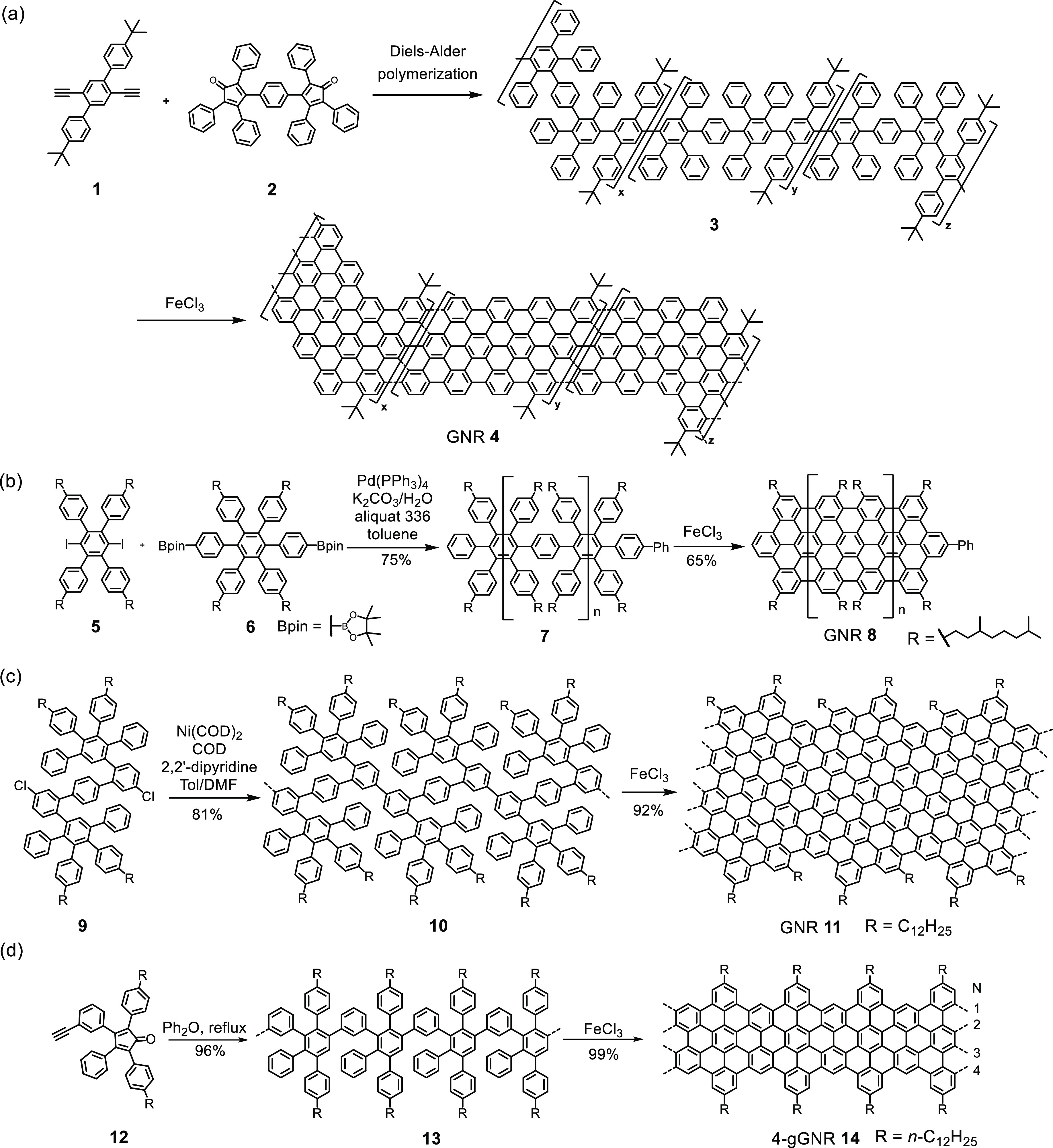
In addition to the helical carbon structures, carbon nanorings and nanobelts, which are regarded as the molecular subunits of CNTs, are related cases that comprise closed loops of polyphenylenes and fully fused benzene rings, respectively.86,187−191 Their optoelectronic properties can be engineered by modifying the ring size, width, and edge structures.191,192 Chirality can also be found in carbon nanorings and nanobelts due to the absence of an inversion center and symmetry plane.193 Even more complicated ring-shaped carbon species are highly twisted macrocycles (figure-eight, Moebius ring, etc.) and trefoil knots.194−196 These nanocarbons have attracted chemists for decades, not only because of their appeal as synthetic showcases but also due to their unique properties and applications in supramolecular chemistry and optoelectronics.191,192 For instance, the crystalline [10]cycloparaphenylene–iodine complex furnished electrical stimuli-responsive white light emission and “turn on” electrical conductivity (Figure 6a).197 Another convincing example is the highest organic luminescence dissymmetry factor (glum = 0.152) obtained from a tubular molecule, (12,8)-[4]cyclo-2,8-chrysenylene 64 and 65 ((12,8)-[4]CC) in Figure 6b–c) with an emission quantum yield of 80%.198 Using theoretical calculations, the dissymmetry factor was found to benefit directly from the large magnetic dipole transition moment parallel to its unique cylinder topology.
Figure 6.
(a) Electric-stimulus-induced phase transition of the [10]cycloparaphenylene-iodine complex and turn-on electrical conductivity as well as white light emission. Reproduced with permission from ref (197). Copyright 2017 John Wiley and Sons. (b) Structure of (P)- and (M)-(12,8)-[4]cyclo-2,8-chrysenylene. (c) Circularly polarized luminescence spectra of 64 and 65 in toluene solution. Reproduced with permission from ref (198). Copyright 2017 National Academy of Sciences.
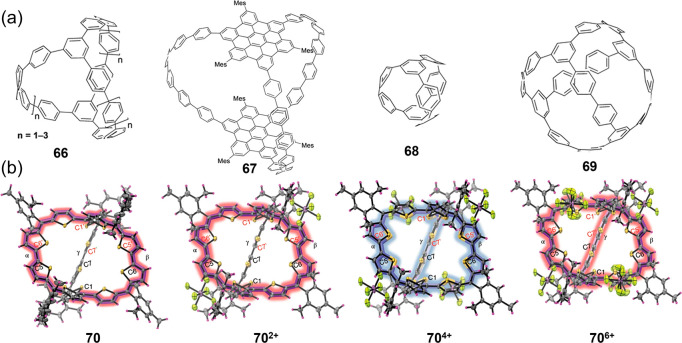
When multiple rings are interconnected, 3D hydrocarbon-based molecular cages and nanotubes can be constructed with permanent holes, which allow applications in host–guest chemistry, chemical sensing, and gas adsorption/separation.200−202 The “perfect” example is, of course, the famous fullerene C60, while there are many more hydrocarbon cages that have been synthesized by various carbon–carbon coupling reactions (Figure 7a).203−208 Using benzene as the repeating unit, the aromaticity in 3D π-conjugated molecules appears to be a challenging topic. One notable example is Hirsch’s 2(n+1)2 spherical aromaticity rule for fullerenes, where π-electrons are delocalized through the whole spherical framework.209,210 A recent example is the C3 symmetrical and fully conjugated molecular cage 70 synthesized by connecting two carbon-centered radicals with three identical conjugated bridges (Figure 7b). Different types of aromaticity (Hückel, Baird, and 3D global aromaticity) occur in this cage with different oxidation states.199
Figure 7.
(a) Spherical carbon nanocages 66–69. (b) X-ray crystallographic structures of the threefold symmetrical and fully conjugated molecular cage 70 with different oxidation states. Reproduced with permission from ref (199). Copyright 2020 Springer Nature.
5. Access to High-Spin Materials
The characteristic structural features of GNRs, which have been introduced above, are all relevant for the electronic band structure of GNRs and, in turn, offer enormous opportunities for fine-tuning their photophysical and optoelectronic properties. This has been extensively described in other review articles.17,19,38,105 Herein, another important method for electronic structure control is emphasized, that is, bringing spins to molecular graphenic structures. Controlling quantum degrees of freedom is an important challenge of physics, and “quantum matter” has been realized in, for example, ion traps or arrays of cold atoms, but often the underlying coupling energies remain low. Attention has therefore been directed to π-radicals as constituents of highly entangled spin chains or superlattices with strong interactions that originate from unpaired π-electrons or partially filled π-bands.33,98,211
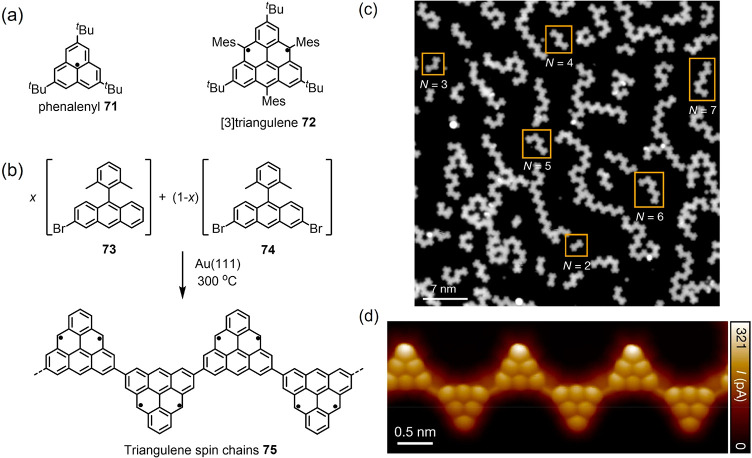
Such building blocks are, for example, the phenalenyl 71 and [3]triangulenyl 72 systems (Figure 8a), which can be kinetically stabilized in the crystalline state by introducing bulky groups (tert-butyl or mesityl substituents).213,214 Compared to solution-mediated syntheses, these radicals can be synthesized and made more persistent by thermal CH-cleavage from closed-shell precursors on metal surfaces. This concept can go even further since the triangulenyl lattice has been made via metal catalysis by ring closure from 9-(2,6-dimethylphenyl)anthracene and the triangulenyls assembled into networks when using halogen-substituted derivatives (Figure 8b–d).212,215
Figure 8.
(a) Structures of phenalenyl 71 and [3]triangulene 72. (b) On-surface synthesis of triangulenyl spin chains using a precursor mixture of 73 + 74. (c) Overview STM image after annealing the precursor mixture (x = 0.2) on Au(111) at 300 °C. (d) Bond-resolved STM images of triangulenyl spin chains 75. Reproduced with permission from ref (212). Copyright 2021 Springer Nature.
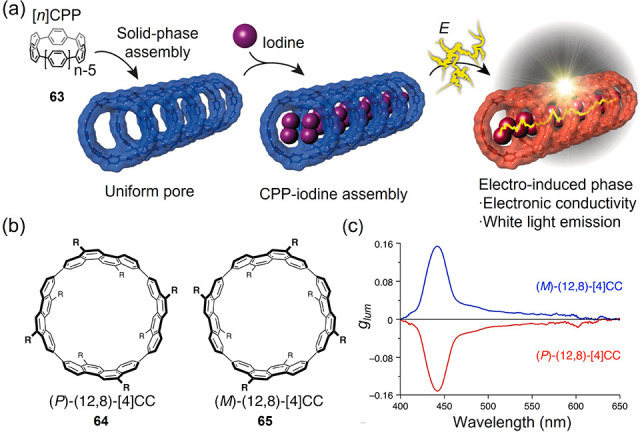
Another way to access high-spin structures and nontrivial magnetism is the design of zigzag-edges as occurring in the new rhombus-shaped discs [4]- and [5]-rhombene (77 and 79, Figure 9).216 Scanning tunneling spectroscopy (STS) analysis of [4]- and [5]-rhombene revealed an emergent magnetic spin-singlet ground state with increasing nanographene size, and a magnetic exchange coupling of more than 100 meV, significantly exceeding the room temperature Landauer limit.
Figure 9.
(a) Synthetic schemes for [4]- and [5]-rhombenes. (b) dI/dV spectra of 77 with HOMO and LUMO resonances at −330 mV and +400 mV, respectively. (c) Background-subtracted dI/dV spectrum of 79 revealing inelastic excitation steps. Reproduced with permission from ref (216). Copyright 2021 Springer Nature.
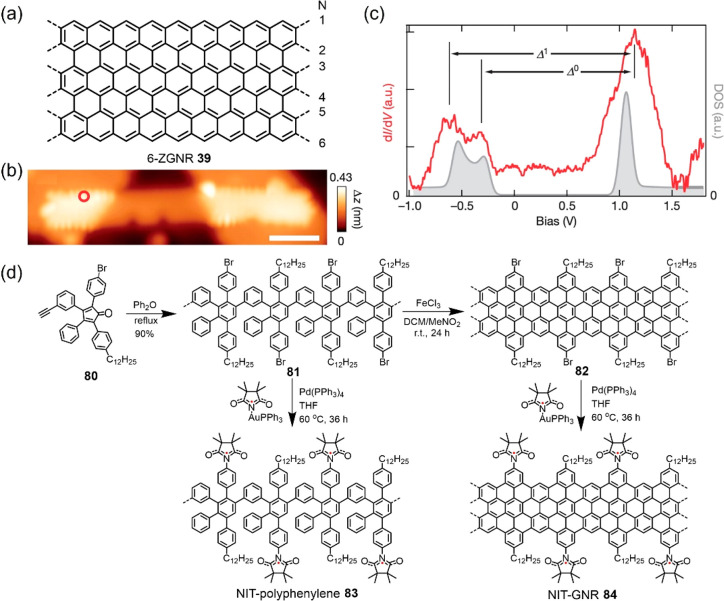
Extending this concept to GNRs has prompted the synthesis of zigzag-edged GNR 39 (6-ZGNR, where “6” is the ribbon width as above), which was shown by density functional theory (DFT) calculations to possess extremely low band gaps as well as edge-localized electronic states with energy splittings.98 These features lead to magnetism and spin-filtering behavior (Figure 10).98,217−219 Without doubt, magnetic edges in GNRs and controlled manipulation of them would define a breakthrough for spintronics and quantum computing. On-surface syntheses of such GNRs, as sketched within Scheme 5b in Section 3, imply CC-bond formation by methyl-aryl coupling next to electrocyclic ring closure by aryl–aryl coupling, which highlights the importance of precursor design. The synthesis of the U-shaped precursor dibenzo[a,j]anthracene 37 with additional phenyl and/or methyl substituents is a convincing case of GNR-edge control.220 The chemical instability of ZGNRs and the importance of spins delocalized over GNRs have suggested yet another approach depicted in Figure 10d.221 There, stable GNRs 84 are synthesized and their peripheries are decorated with persistent radicals that can partially delocalize spin density onto the GNR. Characterization of the resulting conjugates by electron spin resonance (ESR) spectroscopy will be mentioned in section 7.
Figure 10.
(a) Structure of 6-ZGNR 39. (b) STM topography image of 6-ZGNR 39 bridging between two NaCl monolayer islands. (c) Differential conductance (dI/dV) spectrum (red) taken at the zigzag edge marked by the red circle in (b), and the quasiparticle density of states (DOS; gray). Reproduced with permission from ref (98). Copyright 2016 Springer Nature. (d) Synthetic route to NIT-GNR 84 decorated with nitronyl nitroxide (NIT) radicals at the periphery.
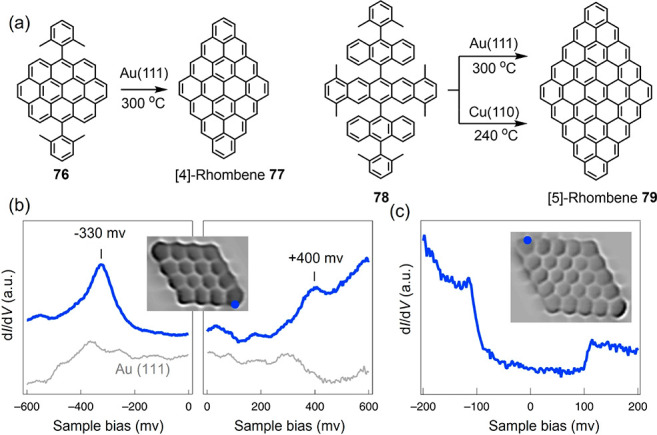
Another concept with relevance for quantum applications is formation of spins in a semiconductor when starting with defects.27,223−225 One possibility is to enclose five- or seven-membered rings in the hexagonal matrix.27,226,227 In graphene, dislocations giving rise to two pentagon–heptagon pairs, known as a Stone–Wales defect,228 can still migrate around, whereas the present molecularly defined graphene chemistry can provide static and carefully engineered defects. While azulene-based GNRs have been mentioned above, there is much to learn from NGs and defined oligomers. Recent cases are the bis-pentagon derivatives of perylene 85,229 bisanthene 86,230 and HBC 87, 88(231) (Figure 11a). A comparison of the para- or meta-fused biradicals in HBC (87 and 88 in Figure 11a, respectively), which are formed by oxidation of the corresponding dianions, is revealing. When attempting to describe the bonding situation in NGs by closed-shell Kekulé structures, the number of six-membered rings maintaining their resonance stabilization is the decisive factor, and a singlet-ground state structure of 87 would cost too many benzene resonance energies. Pentagon structures are also accessible in on-surface protocols when a methyl group is placed inside a bay region, as in the case of polyphenylenes and GNRs.232 The substituted polyphenylenes 89 and 90 containing methyl and methylene groups yield chevron-type GNRs that are further fused into nanoporous graphene on the surface (Figure 11b).222
Figure 11.
(a) Structures of bis-pentagon derivatives of perylene 85, bisanthene 86, and HBC 87–88. (b) On-surface synthesis of a pentagon structure through cyclization of polyphenylenes 89 and 90 with methyl and methylene groups. Reproduced with permission from ref (222). Copyright 2020 American Chemical Society.
6. Size Matters
The slogan “size matters” is commonly put forward in advertising the chemistry of nanoparticles such as, for example, polymer latex particles or inorganic quantum dots. Critical features are the surface-to-volume ratio or quantum confinement effects. However, size also matters when one proceeds from PAHs to increasingly larger NGs, which leads to bathochromic shifts of optical absorption bands.64 Tailoring the HOMO–LUMO energy gaps of NGs not only by size but also by edge and topology233−235 provides access to near-infrared absorbers, in particular, in conjunction with attached auxochromic carboxamide groups. The latter are of enormous technical importance in diverse areas such as laser welding, security printing, photodynamic, and photothermal tumor therapies.236−240
The optical properties of GNRs stand in sharp contrast to those of graphene. Whereas visible light is absorbed by graphene independent of the wavelength, absorption by GNRs varies with the width and edge structure of ribbons that can be tuned chemically.23,241,242 Even more exciting aspects are the dynamics of their excited states. While recombination of excitons leads to single-photon emission from GNRs,243 stimulated emission resulting from biexcitons has also been reported for GNRs under high-excitation conditions, thus opening up opportunities in lasing applications.32 In addition, NGs with zigzag edges such as the dibenzo[hi, st]ovalene (DBOV, 91) and peri-acenoacenes 92–96 help to discover the underlying dynamics of stimulated emission, and one may well envisage opportunities for light amplification in tunable lasers or light-emitting diodes (Figure 12).244−248
Figure 12.
Representative NGs displaying stimulated emission: (a) Dibenzo[hi, st]ovalene 91. (b) Peri-acenoacenes 92–96.
The molecule and particle worlds are closely connected in the case of so-called graphene quantum dots (GQDs), which contain graphene sheets within the nanometer length scale and exhibit quantum confinement as well as edge effects.250 While several strategies have been employed in making GQDs, such as solvothermal routes,251 opening of fullerene cages,252 and modern lithography techniques,253 synthesis of monodisperse GQDs with defined morphology is still a critical issue. NGs are, in a sense, molecularly defined GQDs but can also be used as a source for bottom-up GQD-fabrication by a sequence involving columnar stacking, pyrolysis, and exfoliation (Figure 13).249,254
Figure 13.

Processing diagram for the preparation of photoluminescent GQDs by using HBC as the carbon source. Reproduced with permission from ref (249). Copyright 2011 American Chemical Society.
As was mentioned above, formation of monolayers or thin films is a major prerequisite in many nanoscience experiments and in device fabrication. Processing in solution mostly requires attachment of alkyl chains to the aromatic cores, in particular branched ones, which enhance solubility due to increased solution entropy in organic solvents. This will also result in nanophase separation between the “hard” aromatic disc and the “soft” aliphatic periphery and promote the formation of mesophases.255−257 Liquid-crystalline phases have been considered for improving charge-carrier transport. While the hexaalkoxy triphenylenes possess an extremely narrow mesophase width, the discotic mesophase of hexaalkyl HBCs can persist in a wide temperature range from 110 to 420 °C, which offers much better opportunities for device fabrication.258−260 The size, shape, and periphery of discotics are decisive. This is easily understood by considering that the preferred angle of twist in a columnar stack is not necessarily the one delivering the strongest electronic coupling between the layers.
There are many more similar size effects of graphenic layers at different levels of technology,45,129,261−263 such as in attempts at minimizing friction.264 Lubrication with graphite or 2D materials is well-known, but only precise GNRs offer defined nanocontacts on substrates.265 GNRs have been shifted around on gold surfaces by an AFM tip with a nearly superlubric motion, and one relevant feature is, again, the size of the ribbons.265
7. Changing the World of Electronics
In seeking an FET with appropriate performance and switching behavior, GNRs with sufficient length and narrow width are needed to achieve a low band gap. Moreover, the mobility of charge-carrier transport, a decisive process of an FET, is often sensitively dependent on the degree of order in semiconducting materials. To improve device performance, an FET can be built from low-band gap UHV-grown 9-AGNR 97 which is transferred from gold to a hafnium oxide gate dielectric. In sharp contrast to the low ratios observed for graphene, a high on/off ratio of 105 has been achieved from this GNR-based FET device (Figure 14).266 Casting networks from solution-made GNRs are much easier and more robust, but the achievable mobilities are largely determined by interribbon transport.267−270 In a single-ribbon experiment, however, the current is mainly limited by tunneling through the Schottky barrier at the contact with the electrodes.37,113,266,271 To reduce this contact resistance, the use of graphene electrodes proves to be an effective strategy.272 A comparison of FETs using gapless graphene and GNRs was provided in a recent review.104,273
Figure 14.
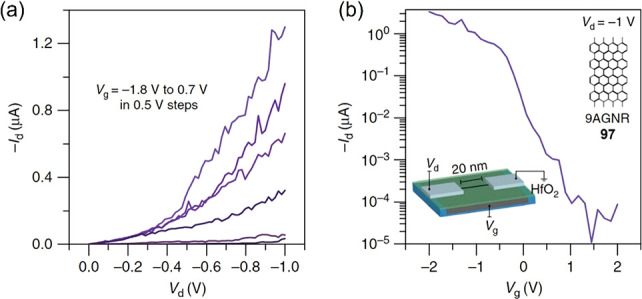
(a) Id–Vd and (b) Id–Vg characteristics of the untrascaled, high-performance 9-AGNR 97 FET at room temperature. Adapted with permission from ref (266). Copyright 2017 Springer Nature, http://creativecommons.org/licenses/by/4.0/.
When proceeding toward other applications, a closer look at theoretical work is appropriate. As expected, changes in band gaps as a function of length, width, and edge structure have stood in the foreground, but additional features have come into play as well, such as the role of end states, noncontinuous changes of properties with length, and the possible influence of a metal substrate on band gaps.15,23,31,45 In the literature, experimental and theoretical values of GNR band gaps differ widely, which is partly due to the lack of information on methods of synthesis and thus structural perfection or unspecified length.15,23
Before looking at spin-bearing GNRs,98 whose synthesis has been described in section 5, another theoretical GNR study, which is relevant to device application, should be mentioned. This is the response of a GNR to external effects such as a transverse electrical field. This can induce the transition of a semiconductor to a semimetal and thus furnish Dirac Fermions in a semiconductor.274 Returning to molecular control, the NIT-GNR 84 (Figure 15a–b) carrying delocalized spins without having a zigzag edge has been subjected to time-resolved ESR spectroscopy. These experiments, which were performed in the group of Lapo Bogani, describe the evolution of a spin and, above all, yield ultrahigh spin coherence times in the microsecond range even at room temperature.221 These results were obtained for a stable material under ambient conditions and hold enormous promise for quantum operations executed by single-electron transport combined with electrical detection of spins.
Figure 15.

(a) Structures of NIT-polyphenylene 83 and NIT-GNR 84. (b) Multifrequency ESR spectra for NIT-polyphenylene 83 (green) and NIT-GNR 84 (red), along with simulations (black), plotted against the magnetic field from the edge-state resonance. Reproduced with permission from ref (221). Copyright 2018 Springer Nature.
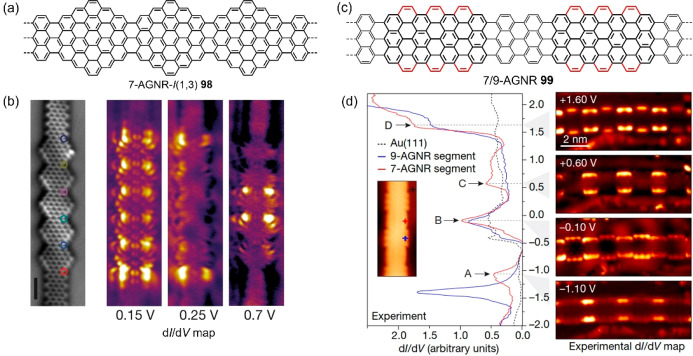
It is fascinating that the topics of greatest interest in condensed matter physics are closely connected with syntheses of robust, but atomically precise GNRs possessing different edge structures. The reason is obvious: chemistry offers the potential of even engineering topological electronic phases and this provides access to exotic quantum states that are essential in spintronics or quantum computing. The theoretical basis of this concept is provided by the Su–Schrieffer–Heeger model,275 which has been extended from the classical description of polyacetylene to that of GNRs (Figure 16a) such as edge extended 7-AGNR-I(1,3) 98.30 STS can verify controlled periodic coupling of topological boundary states and thus prove the existence of quasi-1D quantum phases (Figure 16b). The recent literature provides many similar cases of monitoring the rise of topological properties by material synthesis,31,98 such as GNR-based topological insulators.276 The 7/9-AGNR 99 are fabricated by partially adding K-regions to the armchair edge of 7-AGNRs (Figure 16c–d) to manifest nontrivial 1D topological phases.31 Thereby, important additions to structural control of GNRs, apart from width and edge, are syntheses of semimetallic chiral GNRs.211
Figure 16.
Chemical structures of (a) 7-AGNR-I(1,3) 98 and (c) 7/9-AGNR 99 exhibiting topological electronic phases. (b) Constant-height nc-AFM image and experimental dI/dV maps of 7-AGNR-I(1,3) 98 on Au(111). (d) dI/dV spectra taken at the locations indicated by the corresponding color markers, and constant-current dI/dV experimental maps of 7/9-AGNR 99 on Au(111). Reproduced with permission from refs (30, 31). Copyright 2018 Springer Nature.
Uncovering the many electronic functions of NGs and GNRs has led the community a long way. What begins with opening of a band gap and the associated engineering finds its highlights with the creation of exotic quantum states and new opportunities for physics, but physics, in turn, must now put these molecules to work.
8. Synthetic Carbon Materials in the World of Biology
The common belief that synthetic carbon nanostructures are strictly “nonnatural” can be easily proven wrong with their powerful roles in many bioapplications. Dendrimers have often been employed as molecularly defined “functional nanoparticles”.277−279 Therefore, carbon-rich polyphenylene dendrimers (PPDs) not only serve as precursors for syntheses of NGs but also stand out due to their shape-persistent arms.280 This structural feature, which results from the presence of stiff polyphenylene chains, promises precise nanosite definition when functional groups such as chromophores are placed at the core, the scaffold, or the surface.281,282 An example of outstanding biological importance is peripheral functionalization with alternating “patches” of polar and nonpolar groups. The semirigidity prevents clustering as a result of conformational changes within the dendrimer interior. This alternating array of polar and nonpolar functions furnishes solubility in both aqueous and organic solvents with unique cell permeability.283,284 Another surprising result is the aggregation behavior seen with charged particles such as viruses. Adenoviruses, for example, which are considered vectors for DNA transfection, can be decorated for uptake into tumor cells by aggregation with patched PPDs.285
Functionalized PPDs can also serve as building blocks for the assembly process. Examples are layer-by-layer deposition of PPDs with oppositely charged surfaces or nuclear staining using electrolyte-electrolyte and histone interactions to form thin films for high-sensitivity DNA detection.286−289 PPDs with peripheral thiomethyl groups give rise to ordered and porous gold nanoparticle–dendrimer composites in the solid state, and they are used in sensor applications due to their strong plasmon band.290 These accomplishments are rooted in the combination of advanced synthesis and the versatility of functionalized PPDs in tailoring their self-assembly properties. Similarly, networks can be formed from CNTs and dodecyl-substituted HBCs or other discotics to yield chemiresistors and cross-reactive arrays, which display excellent discrimination between volatile organic compounds in exhaled breath and serve as cost-effective, portable, and noninvasive diagnostic tools for detecting cancer and neurodegenerative diseases.291−293
Precise control of photophysical and biological properties such as DNA-binding of synthetic NGs has provided a great variety of dyes as reporter molecules and imaging agents for biomedicine.240,296−298 For example, inspired by the excellent photophysical properties of DBOV 91 discussed in section 6, selective nitrogen-doping was achieved to produce N-DBOV 100 with strong luminescence (Figure 17a). Due to the presence of nitrogen atoms, N-DBOV exhibits a surprising pH-responsive blinking effect which enables pH-sensitive superresolution imaging.294 Another example is the important area of cancer theranostics realized by the combination of diagnosis (luminescence and photoacoustic imaging) and therapy (chemical, photodynamic, and photothermal therapy) using rylenecarboximides derivatives. The poly(ethylene glycol)-functionalized quaterrylenediimide (P(QDI), 101) self-assembles into QDI-nanoparticles (QDI-NPs) with sizes of approximately 10 nm in aqueous solution (Figure 17b), which allows high-resolution photoacoustic imaging and efficient photothermal cancer therapy upon NIR laser irradiation.295 Further improvement of penetration depth, realization of rapid and early diagnoses of some critical diseases, and targeted therapy can well be expected in the future.
Figure 17.

(a) N-doped DBOV 100 with pH-dependent blinking properties. Reproduced with permission from ref (294). Copyright 2021 American Chemical Society. (b) Self-assembling QDI-NPs with high-resolution photoacoustic imaging and efficient photothermal cancer therapy. Reproduced with permission from ref (295). Copyright 2019 John Wiley and Sons.
Porous graphenic nanostructures and other 2D materials have attracted considerable attention for sieving or sensing applications, including potential nucleic-acid sensing and DNA sequencing.299−301 It has been theoretically demonstrated that DNA nucleobases inserted into the nanopores of GNRs lead to unique changes in device conductance and allow DNA sequencing.302 While such biosensing devices have been proven experimentally with top-down preparation of a nanopore GNR with undefined edge structures,303 measurement sensitivity can be drastically enhanced by using zigzag-edged or topologically insulating GNRs,302 which are the synthetic challenges to be approached in the future.
Applications of GNRs in biological systems rely mainly on their luminescence and the presence of substituents for further functionalization. Grafting of polyamide or polyethylenimine (PEI) chains to GNRs delivers conjugates serving as carriers for gene therapy because of their notable affinities and specificities toward somatic cells and proteins.304 A remarkable study involved PEI-grafted GNR (PEI-g-GNR) as an effective gene vector for the locked nucleic acid modified molecular beacon (LNA-m-MB). The large surface area and high charge density of PEI-g-GNR protect the LNA-m-MB probes from nuclease digestion or binding interactions with proteins. The resulting complex LNA-m-MB/PEI-g-GNR leads to high transfection efficiency, which is favorable for sensitive detection of the recognized target microRNA, implying potential applications in gene therapy.305 The challenges for use of GNRs in the biomedical field can thus be summarized as reproducibility of the material, higher drug loading capacity, and lower toxicity, but all of these require controllable and atomically precise syntheses of GNRs.
9. New Opportunities in Energy Technologies
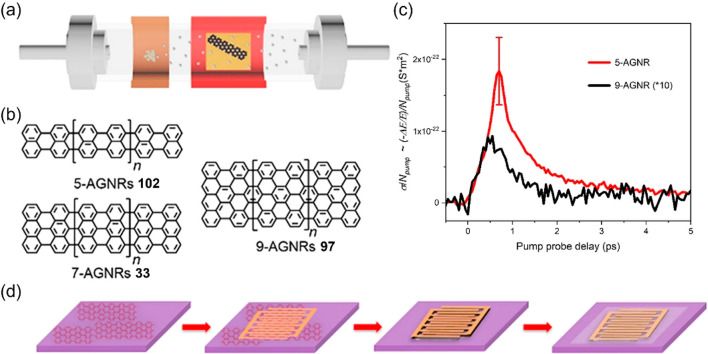
A common way of thinking in carbon nanostructure research is centered around the role of NGs and GNRs as “graphene models” whose sizes are increased to allow them to approach the behavior of graphene. This is probably missing the most important feature that was already discussed above: GNRs offer properties that graphene does not.106 This claim can be illustrated in the domain of energy technologies. What is needed for charge storage is high capacitance, high charging–discharging rates, and long cycling life.306 The layered structures of graphene, which are often fabricated as hybrids with conducting polymers, are particularly well suited for energy storage in supercapacitors due to the rapid influx of counterions.307 Indeed, graphenes obtained by electrochemically assisted exfoliation of graphite possess high energy densities and high power densities according to their Ragone plots.308,309 Moreover, GNRs prepared via a bottom-up CVD approach are superior to graphene with its large basal plane.8 Employing 5-, 7-, and 9-AGNRs as electrode materials for microsupercapacitors gives an excellent volumetric capacitance of 307 F cm–3 and ultrahigh power densities of up to 2000 W cm–3, and the narrowest ribbon is the best. This electrochemical performance of microsupercapacitors can be rationalized by the largely increased edges and the high charge-carrier mobilities, as determined by pump–probe terahertz spectroscopy (Figure 18).310
Figure 18.
(a–b) Schematic illustration of the growth of 5-AGNR 102, 7-AGNR 33, and 9-AGNR 97 via CVD. (c) Time-resolved complex terahertz photoconductivity of 5-AGNR 102 and 9-AGNR 97 normalized to the absorbed photon density. (d) Schematic illustration for device fabrication of microsupercapacitors based on GNR films. Reproduced with permission from ref (310). Copyright 2020 American Chemical Society.
“Segmenting” the graphene sheet is a matter of not only cutting out smaller subunits but also avoiding stacking, and that is why obstructing aggregation of GNRs is equally important before putting them to work in charge storage. Alkyl substituents can improve the solubility of NGs and GNRs, but they also “dilute” desired electronic properties with regard to applications in charge-storage devices.311 GNRs without solubilizing groups have also been prepared in solution on a gram scale, but these insoluble GNRs can only be processed by strong sonication, which is known to break them down and shorten their lengths.58,312,313 Therefore, although it may not appear too appealing from the perspective of atom economy, thermal removal of alkyl chains after film formation has become an established protocol.268
To realize practical applications in energy technologies, however, one may not need precisely defined edges. Instead, robust, high-yielding syntheses and cheaper processing protocols would be in urgent demand. Understandably, therefore, top-down approaches, including lithographic cutting of graphene, sonochemical treatment of graphite in solution, unzipping of CNTs, and most importantly, pyrolysis, have been widely applied to prepare graphenic nanostructures.315−317 From the perspectives of organic and polymer chemistry, pyrolytic processes may be considered undefined techniques with mostly unknown reaction mechanisms, but they are of immense value when, for example, encapsulating metal, metal oxide, or silicon particles for morphologically stable anode materials of batteries.318 Producing graphite or carbon nanofibers from asphalt-like solid pitches is a major industrial challenge.319,320 One inspiring case is the phase-forming superphenalene derivative 103, which is spin-coated on substrates and then heated at 1100 °C in an argon atmosphere.321 The resulting conductive carbon films are transparent over the wavelength range 260 to 800 nm322 and thus offer suitable “window electrodes” for photovoltaic or electroluminescent devices (Figure 19).314 Fabrication of large-scale and ultrathin graphene films by CVD or reduction of graphene oxide has also been employed toward that end,323,324 but spin-coating of large, yet soluble, NGs and subsequent pyrolysis of the NG films at temperatures above 1000 °C offer a unique alternative.325
Figure 19.

Bottom-up chemical approach used to synthesize a transparent graphene film from superphenalene derivative 103 and application of the graphene as a window electrode in an organic solar cell. Reproduced with permission from ref (314). Copyright 2008 John Wiley and Sons.
Ionothermally induced polymerization of small aromatic molecules at a high temperature can also lead to formation of porous polymer networks with high surface areas, adjustable pore sizes, controllable chemical structures, and adaptable chemical functionalities.326,327 Treatment of terephthalonitrile 104 at 400 °C provides a covalent triazine-based framework with poor electrical conductivity. Heating to 550 °C, however, produces nitrogen-rich networks (TNNs) serving as high-performance electrode materials for supercapacitors (Figure 20).328
Figure 20.
(a) Ionothermally induced synthesis of TNNs from terephthalonitrile 104. (b) High-resolution TEM and STEM images of TNNs. (c) Typical N 1s XPS spectrum of TNNs with four distinct nitrogen configurations. (d) Specific capacitances at various current densities. Reproduced with permission from ref (328). Copyright 2012 Royal Society of Chemistry.
What these few energy applications point out is that economically feasible protocols and more demanding syntheses can well go hand in hand.
10. Conclusions and Outlook
NGs and GNRs, the title structures of this article, highlight the enormous versatility of organic chemistry in realizing π-conjugation, which also stands at the origin of organic electronic materials due to their ability to interact with light and undergo electron transfer. The prototypes of π-conjugation are oligoenes, their cyclic congeners (known as annulenes), and simple PAHs. Further, fundamental features such as the HOMO–LUMO energy gap, orbital symmetry, or orbital degeneracy have proven to be indispensable tools in understanding or even predicting material properties. Currently, an increasing number of theoretical models are used to demonstrate the electronic and quantum properties of NGs and GNRs, such as the occurrence of topological phases; this, in turn, stimulates design of more unprecedented graphenic molecules. The current literature on NGs and GNRs offers ample examples of the lively interplay of theory and experiment.329,330
When considering a homologous series of oligomers, polyacetylene might appear as the logical end point for extending oligoene chains. However, what is theoretically enlightening is much less straightforward in synthesis. Oligoenes, when made in solution, appear to be extremely unstable, while the corresponding “polyene”, namely, polyacetylene, has been obtained by catalyzed polymerization of acetylene as a solid and thus profited from its lattice energy.331 There is thus no trivial connection between small conjugated molecules and their related polymeric materials. While Geim’s and Novoselov’s ingenious experiment on peeling off graphene flakes from graphite with scotch-tape has opened the groundbreaking world of 2D electronic materials,253,332−335 this approach does not lay the ground for robust fabrication protocols, nor does continuous extension of NGs create a feasible transition to graphene materials. An important additional aspect is that many conjugated polymers in the early science and technology of “synthetic metals” excelled due to their tremendous increase of electrical conductivity upon doping,336 but not from their structural precision. Structural precision, however, is a key requirement when targeting unconventional properties of GNRs.
These GNRs establish a class of quasi-1D semiconductors which may close the gap between linear conjugated polymers and graphene. GNRs offer ample opportunities for band gap and bandwidth engineering, which can control the performance of electronic devices. However, the chain-type structures of conjugated polymers allow band gap engineering as well, and low band gap polymers have been long sought targets. GNRs have the potential of maintaining the high charge-carrier mobilities of graphene while at the same time furnishing a finite band gap. Nevertheless, the real value of GNRs lies in the formation of exotic quantum states as the starting point for future quantum technologies. The underlying principles may well go beyond the capacity of a chemist, but these exciting features, such as the creation of zigzag edges, defined defects, and nonplanarity, are intimately connected to the power of chemistry. What is needed, therefore, is a more general concept of synthesis in the future. Zigzag edges or high-spin structures may not persist when formed in solution or under ambient conditions, thus requiring the stabilization of a metal surface or demanding UHV conditions. Therefore, keeping spins apart for “stabilization” will conflict with the need for strong magnetic exchange coupling. In view of future defect engineering, the most promising directions may emerge from the precise synthesis of NGs and GNRs embedding pentagon–heptagon pairs, which offer a whole new landscape of planar yet, nonbenzenoid carbon structures with unique properties. When nonplanarity is introduced, such as in helicenes and cyclacenes,337 the NGs and GNRs can “rise” from flatland with interesting chiral optoelectronic perspectives. Due to their macromolecular character and limited solubility, development of more advanced characterization methods for ultralarge NGs and GNRs is necessary. The importance of solid structures and their packing modes has been emphasized above, but many applications of NGs and GNRs require deposition on insulating substrates, and adequate transfer procedures are another need for future device fabrication.
Tightly connected with this refined understanding of materials synthesis is its combination with nanoscience. Synthesis of NGs and GNRs on surfaces goes far beyond visualization and in situ structure characterization, as was convincingly proven by detection of topological phases via STS or determination of magnetic exchange coupling through inelastic electron tunneling spectroscopy.338
Other carbon nanostructures such as nanodiamonds also open breakthroughs in physics as well as life science.339,340 Similarly, the above applications of NGs and GNRs in sensing, diagnostics, and therapy originate from fundamental electronic properties and their subtle dependence upon assembly and interfacing processes. On the other hand, the growing roles of NGs and GNRs as multitalents of physics and life sciences by no means imply that the demanding chemistry of NGs and GNRs has come to an end. Thus, controlling the multihelicity of larger and larger graphenic molecules is still in its infancy and the importance of porphyrins and phthalocyanines as chromophores and redox units strongly suggests their incorporation into growing graphene honeycomb molecules. Perfluorination of NG-edges or perhydrogenation of NGs toward nanographanes would be further challenges. In view of practical applications, upscaling of bottom-up synthesis is critically important, and minimum reaction steps and low-cost reagents should be considered.
Another question is whether fine-tuning of electronic structures will always require a new bottom-up synthesis. A good case has been made above by covalently attaching small molecules such as chromophores or stable free radicals to the periphery of parent NGs and GNRs to allow exciton or spin transfer. Beyond covalent bonding, small π-conjugated molecules can be deposited on GNRs to modify their electronic structures and achieve ordered supramolecular arrangements. The above-mentioned porphyrins or phthalocyanines offer defined anchor points for binding of guest molecules and, thus, out-of-plane growth. Likewise, while holey graphenes have found much attention, similar approaches hold enormous promise for NGs and GNRs, and selective binding of guest molecules in holes or at heteroatoms will be increasingly adopted for sensing purposes.
GNRs are available not only from sophisticated syntheses but also from harsh top-down methods. One is tempted to emphasize the need for precision synthesis in producing, for example, unobstructed edge structures or defined defects. There are, however, materials properties that may not need this high precision, thus allowing more relaxed and cheaper methods. Independent of how they are made, with their unprecedented properties, NGs and GNRs now emerge as components of new technologies. This point, however, would have never been reached without pioneers such as Erich Clar and Erich Hückel, who recognized the fascinating structure–property relationships of basic conjugated molecules.
Acknowledgments
Our cordial thanks go to all members of the group and to all our collaboration partners. This perspective is financially supported by the Max Planck Society, the Johannes Gutenberg-Universität Mainz (JGU) through the Gutenberg Research College, and start-up funding at the Chinese University of Hong Kong, Shenzhen (UDF01002468). Y.G. and Z.Q. acknowledge support from the Alexander von Humboldt Foundation.
Open access funded by Max Planck Society.
The authors declare no competing financial interest.
References
- Hafner K. August Kekulé—The Architect of Chemistry Commemorating the 150th Anniversary of His Birth. Angew. Chem., Int. Ed. 1979, 18 (9), 641–651. 10.1002/anie.197906413. [DOI] [Google Scholar]
- Watson M. D.; Fechtenkötter A.; Müllen K. Big Is Beautiful-“Aromaticity” Revisited from the Viewpoint of Macromolecular and Supramolecular Benzene Chemistry. Chem. Rev. 2001, 101 (5), 1267–1300. 10.1021/cr990322p. [DOI] [PubMed] [Google Scholar]
- Martín N.; Scott L. T. Challenges in aromaticity: 150 years after Kekulé’s benzene. Chem. Soc. Rev. 2015, 44 (18), 6397–6400. 10.1039/C5CS90085A. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J.; Yamaguchi A. D.; Itami K. C-H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem., Int. Ed. 2012, 51 (36), 8960–9009. 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- Bringmann G.; Price Mortimer A. J.; Keller P. A.; Gresser M. J.; Garner J.; Breuning M. Atroposelective Synthesis of Axially Chiral Biaryl Compounds. Angew. Chem., Int. Ed. 2005, 44 (34), 5384–5427. 10.1002/anie.200462661. [DOI] [PubMed] [Google Scholar]
- Classics in hydrocarbon chemistry; Hopf H., Ed.; Wiley-VCH: Weinheim, 2000. [Google Scholar]
- Fascinating molecules in organic chemistry; Fritz V., Ed.; Wiley & Sons: New York, 1992. [Google Scholar]
- Müllen K.; Rabe J. P. Nanographenes as Active Components of Single-Molecule Electronics and How a Scanning Tunneling Microscope Puts Them To Work. Acc. Chem. Res. 2008, 41 (4), 511–520. 10.1021/ar7001446. [DOI] [PubMed] [Google Scholar]
- Jäckel F.; Watson M. D.; Müllen K.; Rabe J. P. Prototypical Single-Molecule Chemical-Field-Effect Transistor with Nanometer-Sized Gates. Phys. Rev. Lett. 2004, 92 (18), 188303. 10.1103/PhysRevLett.92.188303. [DOI] [PubMed] [Google Scholar]
- Wang X.-Y.; Narita A.; Müllen K. Precision synthesis versus bulk-scale fabrication of graphenes. Nat. Rev. Chem. 2018, 2 (1), 0100. 10.1038/s41570-017-0100. [DOI] [Google Scholar]
- Jiao L.; Zhang L.; Wang X.; Diankov G.; Dai H. Narrow graphene nanoribbons from carbon nanotubes. Nature 2009, 458 (7240), 877–80. 10.1038/nature07919. [DOI] [PubMed] [Google Scholar]
- Jiao L.; Wang X.; Diankov G.; Wang H.; Dai H. Facile synthesis of high-quality graphene nanoribbons. Nat. Nanotechnol. 2010, 5 (5), 321–325. 10.1038/nnano.2010.54. [DOI] [PubMed] [Google Scholar]
- Chen C.; Lin Y.; Zhou W.; Gong M.; He Z.; Shi F.; Li X.; Wu J. Z.; Lam K. T.; Wang J. N.; Yang F.; Zeng Q.; Guo J.; Gao W.; Zuo J.-M.; Liu J.; Hong G.; Antaris A. L.; Lin M.-C.; Mao W. L.; Dai H. Sub-10-nm graphene nanoribbons with atomically smooth edges from squashed carbon nanotubes. Nat. Electron 2021, 4 (9), 653–663. 10.1038/s41928-021-00633-6. [DOI] [Google Scholar]
- Son Y.-W.; Cohen M. L.; Louie S. G. Energy Gaps in Graphene Nanoribbons. Phys. Rev. Lett. 2006, 97 (21), 216803. 10.1103/PhysRevLett.97.216803. [DOI] [PubMed] [Google Scholar]
- Han M. Y.; Özyilmaz B.; Zhang Y.; Kim P. Energy Band-Gap Engineering of Graphene Nanoribbons. Phys. Rev. Lett. 2007, 98 (20), 206805. 10.1103/PhysRevLett.98.206805. [DOI] [PubMed] [Google Scholar]
- Chen Y.-C.; de Oteyza D. G.; Pedramrazi Z.; Chen C.; Fischer F. R.; Crommie M. F. Tuning the Band Gap of Graphene Nanoribbons Synthesized from Molecular Precursors. ACS Nano 2013, 7 (7), 6123–6128. 10.1021/nn401948e. [DOI] [PubMed] [Google Scholar]
- Narita A.; Wang X. Y.; Feng X.; Mullen K. New advances in nanographene chemistry. Chem. Soc. Rev. 2015, 44 (18), 6616–6643. 10.1039/C5CS00183H. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li B.-W.; Tan Y.-Z.; Giannakopoulos A.; Sanchez-Sanchez C.; Beljonne D.; Ruffieux P.; Fasel R.; Feng X.; Müllen K. Toward Cove-Edged Low Band Gap Graphene Nanoribbons. J. Am. Chem. Soc. 2015, 137 (18), 6097–6103. 10.1021/jacs.5b03017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Y.; Yao X.; Narita A.; Mullen K. Heteroatom-Doped Nanographenes with Structural Precision. Acc. Chem. Res. 2019, 52 (9), 2491–2505. 10.1021/acs.accounts.9b00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen G. D.; Toma F. M.; Cao T.; Pedramrazi Z.; Chen C.; Rizzo D. J.; Joshi T.; Bronner C.; Chen Y.-C.; Favaro M.; Louie S. G.; Fischer F. R.; Crommie M. F. Bottom-Up Synthesis of N = 13 Sulfur-Doped Graphene Nanoribbons. J. Phys. Chem. C 2016, 120 (5), 2684–2687. 10.1021/acs.jpcc.5b09986. [DOI] [Google Scholar]
- Rizzo D. J.; Wu M.; Tsai H.-Z.; Marangoni T.; Durr R. A.; Omrani A. A.; Liou F.; Bronner C.; Joshi T.; Nguyen G. D.; Rodgers G. F.; Choi W.-W.; Jørgensen J. H.; Fischer F. R.; Louie S. G.; Crommie M. F. Length-Dependent Evolution of Type II Heterojunctions in Bottom-Up-Synthesized Graphene Nanoribbons. Nano Lett. 2019, 19 (5), 3221–3228. 10.1021/acs.nanolett.9b00758. [DOI] [PubMed] [Google Scholar]
- Daigle M.; Miao D.; Lucotti A.; Tommasini M.; Morin J. F. Helically Coiled Graphene Nanoribbons. Angew. Chem., Int. Ed. 2017, 56 (22), 6213–6217. 10.1002/anie.201611834. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Xie P.; De Corato M.; Ruini A.; Zhao S.; Meggendorfer F.; Straaso L. A.; Rondin L.; Simon P.; Li J.; Finley J. J.; Hansen M. R.; Lauret J. S.; Molinari E.; Feng X.; Barth J. V.; Palma C. A.; Prezzi D.; Mullen K.; Narita A. Bandgap Engineering of Graphene Nanoribbons by Control over Structural Distortion. J. Am. Chem. Soc. 2018, 140 (25), 7803–7809. 10.1021/jacs.8b02209. [DOI] [PubMed] [Google Scholar]
- Niu W.; Ma J.; Soltani P.; Zheng W.; Liu F.; Popov A. A.; Weigand J. J.; Komber H.; Poliani E.; Casiraghi C.; Droste J.; Hansen M. R.; Osella S.; Beljonne D.; Bonn M.; Wang H. I.; Feng X.; Liu J.; Mai Y. A Curved Graphene Nanoribbon with Multi-Edge Structure and High Intrinsic Charge Carrier Mobility. J. Am. Chem. Soc. 2020, 142 (43), 18293–18298. 10.1021/jacs.0c07013. [DOI] [PubMed] [Google Scholar]
- Miao D.; Di Michele V.; Gagnon F.; Aumaitre C.; Lucotti A.; Del Zoppo M.; Lirette F.; Tommasini M.; Morin J. F. Pyrrole-Embedded Linear and Helical Graphene Nanoribbons. J. Am. Chem. Soc. 2021, 143 (30), 11302–11308. 10.1021/jacs.1c05616. [DOI] [PubMed] [Google Scholar]
- Yao X.; Zheng W.; Osella S.; Qiu Z.; Fu S.; Schollmeyer D.; Müller B.; Beljonne D.; Bonn M.; Wang H. I.; Müllen K.; Narita A. Synthesis of Nonplanar Graphene Nanoribbon with Fjord Edges. J. Am. Chem. Soc. 2021, 143 (15), 5654–5658. 10.1021/jacs.1c01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr T. G.; Urgel J. I.; Eimre K.; Liu J.; Di Giovannantonio M.; Mishra S.; Berger R.; Ruffieux P.; Pignedoli C. A.; Fasel R.; Feng X. On-Surface Synthesis of Non-Benzenoid Nanographenes by Oxidative Ring-Closure and Ring-Rearrangement Reactions. J. Am. Chem. Soc. 2020, 142 (31), 13565–13572. 10.1021/jacs.0c05668. [DOI] [PubMed] [Google Scholar]
- Moreno C.; Vilas-Varela M.; Kretz B.; Garcia-Lekue A.; Costache Marius V.; Paradinas M.; Panighel M.; Ceballos G.; Valenzuela Sergio O.; Peña D.; Mugarza A. Bottom-up synthesis of multifunctional nanoporous graphene. Science 2018, 360 (6385), 199–203. 10.1126/science.aar2009. [DOI] [PubMed] [Google Scholar]
- Pawlak R.; Liu X.; Ninova S.; D’Astolfo P.; Drechsel C.; Sangtarash S.; Häner R.; Decurtins S.; Sadeghi H.; Lambert C. J.; Aschauer U.; Liu S.-X.; Meyer E. Bottom-up Synthesis of Nitrogen-Doped Porous Graphene Nanoribbons. J. Am. Chem. Soc. 2020, 142 (29), 12568–12573. 10.1021/jacs.0c03946. [DOI] [PubMed] [Google Scholar]
- Groning O.; Wang S.; Yao X.; Pignedoli C. A.; Borin Barin G.; Daniels C.; Cupo A.; Meunier V.; Feng X.; Narita A.; Mullen K.; Ruffieux P.; Fasel R. Engineering of robust topological quantum phases in graphene nanoribbons. Nature 2018, 560 (7717), 209–213. 10.1038/s41586-018-0375-9. [DOI] [PubMed] [Google Scholar]
- Rizzo D. J.; Veber G.; Cao T.; Bronner C.; Chen T.; Zhao F.; Rodriguez H.; Louie S. G.; Crommie M. F.; Fischer F. R. Topological band engineering of graphene nanoribbons. Nature 2018, 560 (7717), 204–208. 10.1038/s41586-018-0376-8. [DOI] [PubMed] [Google Scholar]
- Soavi G.; Dal Conte S.; Manzoni C.; Viola D.; Narita A.; Hu Y.; Feng X.; Hohenester U.; Molinari E.; Prezzi D.; Müllen K.; Cerullo G. Exciton-exciton annihilation and biexciton stimulated emission in graphene nanoribbons. Nat. Commun. 2016, 7 (1), 11010. 10.1038/ncomms11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazyev O. V. Emergence of magnetism in graphene materials and nanostructures. Rep. Prog. Phys. 2010, 73 (5), 056501. 10.1088/0034-4885/73/5/056501. [DOI] [Google Scholar]
- Song S.; Su J.; Telychko M.; Li J.; Li G.; Li Y.; Su C.; Wu J.; Lu J. On-surface synthesis of graphene nanostructures with π-magnetism. Chem. Soc. Rev. 2021, 50 (5), 3238–3262. 10.1039/D0CS01060J. [DOI] [PubMed] [Google Scholar]
- Pizzochero M.; Barin G. B.; Č̂erņevičs K. n.; Wang S.; Ruffieux P.; Fasel R.; Yazyev O. V. Edge Disorder in Bottom-Up Zigzag Graphene Nanoribbons: Implications for Magnetism and Quantum Electronic Transport. J. Phys. Chem. Lett. 2021, 12 (19), 4692–4696. 10.1021/acs.jpclett.1c00921. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Qiu H.; Fu S.; Wang C.; Yao X.; Dixon A. G.; Campidelli S.; Pavlica E.; Bratina G.; Zhao S.; Rondin L.; Lauret J. S.; Narita A.; Bonn M.; Mullen K.; Ciesielski A.; Wang H. I.; Samori P. Solution-Processed Graphene-Nanographene van der Waals Heterostructures for Photodetectors with Efficient and Ultralong Charge Separation. J. Am. Chem. Soc. 2021, 143 (41), 17109–17116. 10.1021/jacs.1c07615. [DOI] [PubMed] [Google Scholar]
- Li X.; Wang X.; Zhang L.; Lee S.; Dai H. Chemically Derived, Ultrasmooth Graphene Nanoribbon Semiconductors. Science 2008, 319 (5867), 1229–1232. 10.1126/science.1150878. [DOI] [PubMed] [Google Scholar]
- Qiu Z.; Hammer B. A. G.; Müllen K. Conjugated polymers - Problems and promises. Prog. Polym. Sci. 2020, 100, 101179. 10.1016/j.progpolymsci.2019.101179. [DOI] [Google Scholar]
- Yano Y.; Mitoma N.; Ito H.; Itami K. A Quest for Structurally Uniform Graphene Nanoribbons: Synthesis, Properties, and Applications. J. Org. Chem. 2020, 85 (1), 4–33. 10.1021/acs.joc.9b02814. [DOI] [PubMed] [Google Scholar]
- Obradovic B.; Kotlyar R.; Heinz F.; Matagne P.; Rakshit T.; Giles M. D.; Stettler M. A.; Nikonov D. E. Analysis of graphene nanoribbons as a channel material for field-effect transistors. Appl. Phys. Lett. 2006, 88 (14), 142102. 10.1063/1.2191420. [DOI] [Google Scholar]
- Narita A.; Chen Z.; Chen Q.; Müllen K. Solution and on-surface synthesis of structurally defined graphene nanoribbons as a new family of semiconductors. Chem. Sci. 2019, 10 (4), 964–975. 10.1039/C8SC03780A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meric I.; Han M. Y.; Young A. F.; Ozyilmaz B.; Kim P.; Shepard K. L. Current saturation in zero-bandgap, top-gated graphene field-effect transistors. Nat. Nanotechnol. 2008, 3 (11), 654–659. 10.1038/nnano.2008.268. [DOI] [PubMed] [Google Scholar]
- Avouris P. Graphene: Electronic and Photonic Properties and Devices. Nano Lett. 2010, 10 (11), 4285–4294. 10.1021/nl102824h. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhao R.; Yang M.; Liu Z.; Liu Z. Inverse relationship between carrier mobility and bandgap in graphene. J. Chem. Phys. 2013, 138 (8), 084701. 10.1063/1.4792142. [DOI] [PubMed] [Google Scholar]
- Nakada K.; Fujita M.; Dresselhaus G.; Dresselhaus M. S. Edge state in graphene ribbons: Nanometer size effect and edge shape dependence. Phys. Rev. B 1996, 54 (24), 17954–17961. 10.1103/PhysRevB.54.17954. [DOI] [PubMed] [Google Scholar]
- Tapasztó L.; Dobrik G.; Lambin P.; Biró L. P. Tailoring the atomic structure of graphene nanoribbons by scanning tunnelling microscope lithography. Nat. Nanotechnol. 2008, 3 (7), 397–401. 10.1038/nnano.2008.149. [DOI] [PubMed] [Google Scholar]
- Datta S. S.; Strachan D. R.; Khamis S. M.; Johnson A. T. C. Crystallographic Etching of Few-Layer Graphene. Nano Lett. 2008, 8 (7), 1912–1915. 10.1021/nl080583r. [DOI] [PubMed] [Google Scholar]
- Kosynkin D. V.; Higginbotham A. L.; Sinitskii A.; Lomeda J. R.; Dimiev A.; Price B. K.; Tour J. M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458 (7240), 872–876. 10.1038/nature07872. [DOI] [PubMed] [Google Scholar]
- Narita A.; Feng X.; Mullen K. Bottom-up synthesis of chemically precise graphene nanoribbons. Chem. Rec. 2015, 15 (1), 295–309. 10.1002/tcr.201402082. [DOI] [PubMed] [Google Scholar]
- Zeng C.; Zheng W.; Xu H.; Osella S.; Ma W.; Wang H. I.; Qiu Z.; Otake K. I.; Ren W.; Cheng H.; Mullen K.; Bonn M.; Gu C.; Ma Y. Electrochemical Deposition of a Single-Crystalline Nanorod Polycyclic Aromatic Hydrocarbon Film with Efficient Charge and Exciton Transport. Angew. Chem., Int. Ed. 2021, e202115389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C.; Wang B.; Zhang H.; Sun M.; Huang L.; Gu Y.; Qiu Z.; Mullen K.; Gu C.; Ma Y. Electrochemical Synthesis, Deposition, and Doping of Polycyclic Aromatic Hydrocarbon Films. J. Am. Chem. Soc. 2021, 143 (7), 2682–2687. 10.1021/jacs.0c13298. [DOI] [PubMed] [Google Scholar]
- Schwab M. G.; Narita A.; Hernandez Y.; Balandina T.; Mali K. S.; De Feyter S.; Feng X.; Mullen K. Structurally defined graphene nanoribbons with high lateral extension. J. Am. Chem. Soc. 2012, 134 (44), 18169–18172. 10.1021/ja307697j. [DOI] [PubMed] [Google Scholar]
- Yang X.; Dou X.; Rouhanipour A.; Zhi L.; Räder H. J.; Müllen K. Two-Dimensional Graphene Nanoribbons. J. Am. Chem. Soc. 2008, 130 (13), 4216–4217. 10.1021/ja710234t. [DOI] [PubMed] [Google Scholar]
- Wu J.; Gherghel L.; Watson M. D.; Li J.; Wang Z.; Simpson C. D.; Kolb U.; Müllen K. From Branched Polyphenylenes to Graphite Ribbons. Macromolecules 2003, 36 (19), 7082–7089. 10.1021/ma0257752. [DOI] [Google Scholar]
- Narita A.; Feng X.; Hernandez Y.; Jensen S. A.; Bonn M.; Yang H.; Verzhbitskiy I. A.; Casiraghi C.; Hansen M. R.; Koch A. H.; Fytas G.; Ivasenko O.; Li B.; Mali K. S.; Balandina T.; Mahesh S.; De Feyter S.; Mullen K. Synthesis of structurally well-defined and liquid-phase-processable graphene nanoribbons. Nat. Chem. 2014, 6 (2), 126–32. 10.1038/nchem.1819. [DOI] [PubMed] [Google Scholar]
- Takahashi A.; Lin C.-J.; Ohshimizu K.; Higashihara T.; Chen W.-C.; Ueda M. Synthesis and characterization of novel polythiophenes with graphene-like structures via intramolecular oxidative coupling. Polym. Chem. 2012, 3 (2), 479–485. 10.1039/C1PY00501D. [DOI] [Google Scholar]
- Kim K. T.; Jung J. W.; Jo W. H. Synthesis of graphene nanoribbons with various widths and its application to thin-film transistor. Carbon 2013, 63, 202–209. 10.1016/j.carbon.2013.06.074. [DOI] [Google Scholar]
- Vo T. H.; Shekhirev M.; Kunkel D. A.; Morton M. D.; Berglund E.; Kong L.; Wilson P. M.; Dowben P. A.; Enders A.; Sinitskii A. Large-scale solution synthesis of narrow graphene nanoribbons. Nat. Commun. 2014, 5, 3189. 10.1038/ncomms4189. [DOI] [PubMed] [Google Scholar]
- Li G.; Yoon K.-Y.; Zhong X.; Zhu X.; Dong G. Efficient Bottom-Up Preparation of Graphene Nanoribbons by Mild Suzuki-Miyaura Polymerization of Simple Triaryl Monomers. Chem.—Eur. J. 2016, 22 (27), 9116–9120. 10.1002/chem.201602007. [DOI] [PubMed] [Google Scholar]
- Bernhardt S.; Kastler M.; Enkelmann V.; Baumgarten M.; Müllen K. Pyrene as Chromophore and Electrophore: Encapsulation in a Rigid Polyphenylene Shell. Chem.—Eur. J. 2006, 12 (23), 6117–6128. 10.1002/chem.200500999. [DOI] [PubMed] [Google Scholar]
- Nguyen T.-T.-T.; Baumgarten M.; Rouhanipour A.; Räder H. J.; Lieberwirth I.; Müllen K. Extending the Limits of Precision Polymer Synthesis: Giant Polyphenylene Dendrimers in the Megadalton Mass Range Approaching Structural Perfection. J. Am. Chem. Soc. 2013, 135 (11), 4183–4186. 10.1021/ja311430r. [DOI] [PubMed] [Google Scholar]
- Räder H. J.; Nguyen T.-T.-T.; Müllen K. MALDI-TOF Mass Spectrometry of Polyphenylene Dendrimers up to the Megadalton Range. Elucidating Structural Integrity of Macromolecules at Unrivaled High Molecular Weights. Macromolecules 2014, 47 (4), 1240–1248. 10.1021/ma402347y. [DOI] [Google Scholar]
- John H.; Bauer R.; Espindola P.; Sonar P.; Heinze J.; Müllen K. 3D-Hybrid Networks with Controllable Electrical Conductivity from the Electrochemical Deposition of Terthiophene-Functionalized Polyphenylene Dendrimers. Angew. Chem., Int. Ed. 2005, 44 (16), 2447–2451. 10.1002/anie.200462378. [DOI] [PubMed] [Google Scholar]
- Iyer V. S.; Wehmeier M.; Brand J. D.; Keegstra M. A.; Müllen K. From Hexa-peri-hexabenzocoronene to “Superacenes. Angew. Chem., Int. Ed. 1997, 36 (15), 1604–1607. 10.1002/anie.199716041. [DOI] [Google Scholar]
- Simpson C. D.; Brand J. D.; Berresheim A. J.; Przybilla L.; Räder H. J.; Müllen K. Synthesis of a Giant 222 Carbon Graphite Sheet. Chem.—Eur. J. 2002, 8 (6), 1424–1429. . [DOI] [PubMed] [Google Scholar]
- Rieger R.; Müllen K. Forever young: polycyclic aromatic hydrocarbons as model cases for structural and optical studies. J. Phys. Org. Chem. 2010, 23 (4), 315–325. [Google Scholar]
- Weil T.; Wiesler U. M.; Herrmann A.; Bauer R.; Hofkens J.; De Schryver F. C.; Müllen K. Polyphenylene Dendrimers with Different Fluorescent Chromophores Asymmetrically Distributed at the Periphery. J. Am. Chem. Soc. 2001, 123 (33), 8101–8108. 10.1021/ja010579g. [DOI] [PubMed] [Google Scholar]
- Qu J.; Pschirer N. G.; Liu D.; Stefan A.; De Schryver F. C.; Müllen K. Dendronized Perylenetetracarboxdiimides with Peripheral Triphenylamines for Intramolecular Energy and Electron Transfer. Chem.—Eur. J. 2004, 10 (2), 528–537. 10.1002/chem.200304994. [DOI] [PubMed] [Google Scholar]
- Golshan M.; Rostami-Tapeh-Esmail E.; Salami-Kalajahi M.; Roghani-Mamaqani H. A review on synthesis, photophysical properties, and applications of dendrimers with perylene core. Eur. Polym. J. 2020, 137, 109933. 10.1016/j.eurpolymj.2020.109933. [DOI] [Google Scholar]
- Yen H. J.; Tsai H.; Zhou M.; Holby E. F.; Choudhury S.; Chen A.; Adamska L.; Tretiak S.; Sanchez T.; Iyer S.; Zhang H.; Zhu L.; Lin H.; Dai L.; Wu G.; Wang H. L. Structurally Defined 3D Nanographene Assemblies via Bottom-Up Chemical Synthesis for Highly Efficient Lithium Storage. Adv. Mater. 2016, 28 (46), 10250–10256. 10.1002/adma.201603613. [DOI] [PubMed] [Google Scholar]
- Zeng Z.; Guan Z.; Xu Q.-H.; Wu J. Octupolar Polycyclic Aromatic Hydrocarbons as New Two-Photon Absorption Chromophores: Synthesis and Application for Optical Power Limiting. Chem.—Eur. J. 2011, 17 (14), 3837–3841. 10.1002/chem.201003235. [DOI] [PubMed] [Google Scholar]
- Reger D.; Haines P.; Heinemann F. W.; Guldi D. M.; Jux N. Oxa[7]superhelicene: A π-Extended Helical Chromophore Based on Hexa-peri-hexabenzocoronenes. Angew. Chem., Int. Ed. 2018, 57 (20), 5938–5942. 10.1002/anie.201800585. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y.; Fukushima T.; Suna Y.; Ishii N.; Saeki A.; Seki S.; Tagawa S.; Taniguchi M.; Kawai T.; Aida T. Photoconductive Coaxial Nanotubes of Molecularly Connected Electron Donor and Acceptor Layers. Science 2006, 314 (5806), 1761–1764. 10.1126/science.1134441. [DOI] [PubMed] [Google Scholar]
- van de Craats A. M.; Stutzmann N.; Bunk O.; Nielsen M. M.; Watson M.; Müllen K.; Chanzy H. D.; Sirringhaus H.; Friend R. H. Meso-Epitaxial Solution-Growth of Self-Organizing Discotic Liquid-Crystalline Semiconductors. Adv. Mater. 2003, 15 (6), 495–499. 10.1002/adma.200390114. [DOI] [Google Scholar]
- Fleischmann E.-K.; Zentel R. Liquid-Crystalline Ordering as a Concept in Materials Science: From Semiconductors to Stimuli-Responsive Devices. Angew. Chem., Int. Ed. 2013, 52 (34), 8810–8827. 10.1002/anie.201300371. [DOI] [PubMed] [Google Scholar]
- Rempala P.; Kroulík J.; King B. T. A Slippery Slope: Mechanistic Analysis of the Intramolecular Scholl Reaction of Hexaphenylbenzene. J. Am. Chem. Soc. 2004, 126 (46), 15002–15003. 10.1021/ja046513d. [DOI] [PubMed] [Google Scholar]
- Rempala P.; Kroulík J.; King B. T. Investigation of the Mechanism of the Intramolecular Scholl Reaction of Contiguous Phenylbenzenes. J. Org. Chem. 2006, 71 (14), 5067–5081. 10.1021/jo0526744. [DOI] [PubMed] [Google Scholar]
- Zhai L.; Shukla R.; Wadumethrige S. H.; Rathore R. Probing the Arenium-Ion (ProtonTransfer) versus the Cation-Radical (Electron Transfer) Mechanism of Scholl Reaction Using DDQ as Oxidant. J. Org. Chem. 2010, 75 (14), 4748–4760. 10.1021/jo100611k. [DOI] [PubMed] [Google Scholar]
- Ponugoti N.; Parthasarathy V. Rearrangements in Scholl Reaction. Chem.—Eur. J. 2022, 28, e202103530 10.1002/chem.202103530. [DOI] [PubMed] [Google Scholar]
- Miao Q. Rearrangements come to Scholl. Nat. Rev. Chem. 2021, 5 (9), 602–603. 10.1038/s41570-021-00308-y. [DOI] [PubMed] [Google Scholar]
- Mallory F. B.; Wood C. S.; Gordon J. T. Photochemistry of Stilbenes. III. Some Aspects of the Mechanism of Photocyclization to Phenanthrenes. J. Am. Chem. Soc. 1964, 86 (15), 3094–3102. 10.1021/ja01069a025. [DOI] [Google Scholar]
- Ito H.; Ozaki K.; Itami K. Annulative π-Extension (APEX): Rapid Access to Fused Arenes, Heteroarenes, and Nanographenes. Angew. Chem., Int. Ed. 2017, 56 (37), 11144–11164. 10.1002/anie.201701058. [DOI] [PubMed] [Google Scholar]
- Ito H.; Segawa Y.; Murakami K.; Itami K. Polycyclic Arene Synthesis by Annulative π-Extension. J. Am. Chem. Soc. 2019, 141 (1), 3–10. 10.1021/jacs.8b09232. [DOI] [PubMed] [Google Scholar]
- Lungerich D.; Papaianina O.; Feofanov M.; Liu J.; Devarajulu M.; Troyanov S. I.; Maier S.; Amsharov K. Dehydrative π-extension to nanographenes with zig-zag edges. Nat. Commun. 2018, 9 (1), 4756. 10.1038/s41467-018-07095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X. H.; Jester S.-S.; Höger S. Synthesis and Aggregates of Phenylene-Ethynylene Substituted Polycyclic Aromatic Compounds. Macromolecules 2004, 37 (19), 7065–7068. 10.1021/ma048728d. [DOI] [Google Scholar]
- Povie G.; Segawa Y.; Nishihara T.; Miyauchi Y.; Itami K. Synthesis of a carbon nanobelt. Science 2017, 356 (6334), 172–175. 10.1126/science.aam8158. [DOI] [PubMed] [Google Scholar]
- Steiner A.-K.; Amsharov K. Y. The Rolling-Up of Oligophenylenes to Nanographenes by a HF-Zipping Approach. Angew. Chem., Int. Ed. 2017, 56 (46), 14732–14736. 10.1002/anie.201707272. [DOI] [PubMed] [Google Scholar]
- Amsharov K. Y.; Kabdulov M. A.; Jansen M. Facile Bucky-Bowl Synthesis by Regiospecific Cove-Region Closure by HF Elimination. Angew. Chem., Int. Ed. 2012, 51 (19), 4594–4597. 10.1002/anie.201200516. [DOI] [PubMed] [Google Scholar]
- Yang W.; Lucotti A.; Tommasini M.; Chalifoux W. A. Bottom-Up Synthesis of Soluble and Narrow Graphene Nanoribbons Using Alkyne Benzannulations. J. Am. Chem. Soc. 2016, 138 (29), 9137–44. 10.1021/jacs.6b03014. [DOI] [PubMed] [Google Scholar]
- Jordan R. S.; Wang Y.; McCurdy R. D.; Yeung M. T.; Marsh K. L.; Khan S. I.; Kaner R. B.; Rubin Y. Synthesis of Graphene Nanoribbons via the Topochemical Polymerization and Subsequent Aromatization of a Diacetylene Precursor. Chem. 2016, 1 (1), 78–90. 10.1016/j.chempr.2016.06.010. [DOI] [Google Scholar]
- Jordan R. S.; Li Y. L.; Lin C. W.; McCurdy R. D.; Lin J. B.; Brosmer J. L.; Marsh K. L.; Khan S. I.; Houk K. N.; Kaner R. B.; Rubin Y. Synthesis of N = 8 Armchair Graphene Nanoribbons from Four Distinct Polydiacetylenes. J. Am. Chem. Soc. 2017, 139 (44), 15878–15890. 10.1021/jacs.7b08800. [DOI] [PubMed] [Google Scholar]
- Li Y. L.; Zee C. T.; Lin J. B.; Basile V. M.; Muni M.; Flores M. D.; Munarriz J.; Kaner R. B.; Alexandrova A. N.; Houk K. N.; Tolbert S. H.; Rubin Y. Fjord-Edge Graphene Nanoribbons with Site-Specific Nitrogen Substitution. J. Am. Chem. Soc. 2020, 142 (42), 18093–18102. 10.1021/jacs.0c07657. [DOI] [PubMed] [Google Scholar]
- Wang X.-Y.; Richter M.; He Y.; Björk J.; Riss A.; Rajesh R.; Garnica M.; Hennersdorf F.; Weigand J. J.; Narita A.; Berger R.; Feng X.; Auwärter W.; Barth J. V.; Palma C.-A.; Müllen K. Exploration of pyrazine-embedded antiaromatic polycyclic hydrocarbons generated by solution and on-surface azomethine ylide homocoupling. Nat. Commun. 2017, 8 (1), 1948. 10.1038/s41467-017-01934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q.; Hu Y.; Niu K.; Zhang H.; Yang B.; Ebeling D.; Tschakert J.; Cheng T.; Schirmeisen A.; Narita A.; Müllen K.; Chi L. Benzo-Fused Periacenes or Double Helicenes? Different Cyclodehydrogenation Pathways on Surface and in Solution. J. Am. Chem. Soc. 2019, 141 (18), 7399–7406. 10.1021/jacs.9b01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J.; Ruffieux P.; Jaafar R.; Bieri M.; Braun T.; Blankenburg S.; Muoth M.; Seitsonen A. P.; Saleh M.; Feng X.; Mullen K.; Fasel R. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 2010, 466 (7305), 470–3. 10.1038/nature09211. [DOI] [PubMed] [Google Scholar]
- Talirz L.; Söde H.; Dumslaff T.; Wang S.; Sanchez-Valencia J. R.; Liu J.; Shinde P.; Pignedoli C. A.; Liang L.; Meunier V.; Plumb N. C.; Shi M.; Feng X.; Narita A.; Müllen K.; Fasel R.; Ruffieux P. On-Surface Synthesis and Characterization of 9-Atom Wide Armchair Graphene Nanoribbons. ACS Nano 2017, 11 (2), 1380–1388. 10.1021/acsnano.6b06405. [DOI] [PubMed] [Google Scholar]
- Di Giovannantonio M.; Urgel J. I.; Beser U.; Yakutovich A. V.; Wilhelm J.; Pignedoli C. A.; Ruffieux P.; Narita A.; Müllen K.; Fasel R. On-Surface Synthesis of Indenofluorene Polymers by Oxidative Five-Membered Ring Formation. J. Am. Chem. Soc. 2018, 140 (10), 3532–3536. 10.1021/jacs.8b00587. [DOI] [PubMed] [Google Scholar]
- Ruffieux P.; Wang S.; Yang B.; Sanchez-Sanchez C.; Liu J.; Dienel T.; Talirz L.; Shinde P.; Pignedoli C. A.; Passerone D.; Dumslaff T.; Feng X.; Mullen K.; Fasel R. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 2016, 531 (7595), 489–92. 10.1038/nature17151. [DOI] [PubMed] [Google Scholar]
- Bronner C.; Stremlau S.; Gille M.; Brausse F.; Haase A.; Hecht S.; Tegeder P. Aligning the band gap of graphene nanoribbons by monomer doping. Angew. Chem., Int. Ed. 2013, 52 (16), 4422–5. 10.1002/anie.201209735. [DOI] [PubMed] [Google Scholar]
- Kawai S.; Saito S.; Osumi S.; Yamaguchi S.; Foster A. S.; Spijker P.; Meyer E. Atomically controlled substitutional boron-doping of graphene nanoribbons. Nat. Commun. 2015, 6, 8098. 10.1038/ncomms9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S.; Nakatsuka S.; Hatakeyama T.; Pawlak R.; Meier T.; Tracey J.; Meyer E.; Foster A. S. Multiple heteroatom substitution to graphene nanoribbon. Sci. Adv. 2018, 4 (4), eaar7181 10.1126/sciadv.aar7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou I. C.-Y.; Sun Q.; Eimre K.; Di Giovannantonio M.; Urgel J. I.; Ruffieux P.; Narita A.; Fasel R.; Müllen K. On-Surface Synthesis of Unsaturated Carbon Nanostructures with Regularly Fused Pentagon-Heptagon Pairs. J. Am. Chem. Soc. 2020, 142 (23), 10291–10296. 10.1021/jacs.0c03635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q.; Martin-Jimenez D.; Ebeling D.; Krug C. K.; Brechmann L.; Kohlmeyer C.; Hilt G.; Hieringer W.; Schirmeisen A.; Gottfried J. M. Nanoribbons with Nonalternant Topology from Fusion of Polyazulene: Carbon Allotropes beyond Graphene. J. Am. Chem. Soc. 2019, 141 (44), 17713–17720. 10.1021/jacs.9b08060. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Narita A.; Mullen K. Graphene Nanoribbons: On-Surface Synthesis and Integration into Electronic Devices. Adv. Mater. 2020, 32 (45), 2001893. 10.1002/adma.202001893. [DOI] [PubMed] [Google Scholar]
- Qiu Z.; Narita A.; Müllen K. Spiers Memorial Lecture Carbon nanostructures by macromolecular design - from branched polyphenylenes to nanographenes and graphene nanoribbons. Faraday Discuss. 2021, 227 (0), 8–45. 10.1039/D0FD00023J. [DOI] [PubMed] [Google Scholar]
- Talirz L.; Ruffieux P.; Fasel R. On-Surface Synthesis of Atomically Precise Graphene Nanoribbons. Adv. Mater. 2016, 28 (29), 6222–31. 10.1002/adma.201505738. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Yu G. Modified Engineering of Graphene Nanoribbons Prepared via On-Surface Synthesis. Adv. Mater. 2020, 32 (6), 1905957. 10.1002/adma.201905957. [DOI] [PubMed] [Google Scholar]
- Di Giovannantonio M.; Yao X.; Eimre K.; Urgel J. I.; Ruffieux P.; Pignedoli C. A.; Müllen K.; Fasel R.; Narita A. Large-Cavity Coronoids with Different Inner and Outer Edge Structures. J. Am. Chem. Soc. 2020, 142 (28), 12046–12050. 10.1021/jacs.0c05268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K.; Urgel J. I.; Eimre K.; Di Giovannantonio M.; Keerthi A.; Komber H.; Wang S.; Narita A.; Berger R.; Ruffieux P.; Pignedoli C. A.; Liu J.; Müllen K.; Fasel R.; Feng X. On-Surface Synthesis of a Nonplanar Porous Nanographene. J. Am. Chem. Soc. 2019, 141 (19), 7726–7730. 10.1021/jacs.9b03554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q.; Yan L.; Tripp Matthias W.; Krejčí O.; Dimosthenous S.; Kachel Stefan R.; Chen M.; Foster Adam S.; Koert U.; Liljeroth P.; Gottfried J. M. Biphenylene network: A nonbenzenoid carbon allotrope. Science 2021, 372 (6544), 852–856. 10.1126/science.abg4509. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Wang H. I.; Bilbao N.; Teyssandier J.; Prechtl T.; Cavani N.; Tries A.; Biagi R.; De Renzi V.; Feng X.; Klaui M.; De Feyter S.; Bonn M.; Narita A.; Mullen K. Lateral Fusion of Chemical Vapor Deposited N = 5 Armchair Graphene Nanoribbons. J. Am. Chem. Soc. 2017, 139 (28), 9483–9486. 10.1021/jacs.7b05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi H.; Kawagoe Y.; Hirano Y.; Iruka T.; Yano M.; Nakae T. Width-controlled sub-nanometer graphene nanoribbon films synthesized by radical-polymerized chemical vapor deposition. Adv. Mater. 2014, 26 (24), 4134–4138. 10.1002/adma.201305034. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Zhang W.; Palma C. A.; Lodi Rizzini A.; Liu B.; Abbas A.; Richter N.; Martini L.; Wang X. Y.; Cavani N.; Lu H.; Mishra N.; Coletti C.; Berger R.; Klappenberger F.; Klaui M.; Candini A.; Affronte M.; Zhou C.; De Renzi V.; Del Pennino U.; Barth J. V.; Rader H. J.; Narita A.; Feng X.; Mullen K. Synthesis of Graphene Nanoribbons by Ambient-Pressure Chemical Vapor Deposition and Device Integration. J. Am. Chem. Soc. 2016, 138 (47), 15488–15496. 10.1021/jacs.6b10374. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Wang H. I.; Teyssandier J.; Mali K. S.; Dumslaff T.; Ivanov I.; Zhang W.; Ruffieux P.; Fasel R.; Rader H. J.; Turchinovich D.; De Feyter S.; Feng X.; Klaui M.; Narita A.; Bonn M.; Mullen K. Chemical Vapor Deposition Synthesis and Terahertz Photoconductivity of Low-Band-Gap N = 9 Armchair Graphene Nanoribbons. J. Am. Chem. Soc. 2017, 139 (10), 3635–3638. 10.1021/jacs.7b00776. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H.; Song S.; Kojima T.; Nakae T. Homochiral polymerization-driven selective growth of graphene nanoribbons. Nat. Chem. 2017, 9 (1), 57–63. 10.1038/nchem.2614. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Berger R.; Müllen K.; Narita A. On-surface Synthesis of Graphene Nanoribbons through Solution-processing of Monomers. Chem. Lett. 2017, 46 (10), 1476–1478. 10.1246/cl.170606. [DOI] [Google Scholar]
- Way A. J.; Jacobberger R. M.; Arnold M. S. Seed-Initiated Anisotropic Growth of Unidirectional Armchair Graphene Nanoribbon Arrays on Germanium. Nano Lett. 2018, 18 (2), 898–906. 10.1021/acs.nanolett.7b04240. [DOI] [PubMed] [Google Scholar]
- Chen L.; He L.; Wang H. S.; Wang H.; Tang S.; Cong C.; Xie H.; Li L.; Xia H.; Li T.; Wu T.; Zhang D.; Deng L.; Yu T.; Xie X.; Jiang M. Oriented graphene nanoribbons embedded in hexagonal boron nitride trenches. Nat. Commun. 2017, 8 (1), 14703. 10.1038/ncomms14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T.; Hatakeyama R. Site- and alignment-controlled growth of graphene nanoribbons from nickel nanobars. Nat. Nanotechnol. 2012, 7 (10), 651–656. 10.1038/nnano.2012.145. [DOI] [PubMed] [Google Scholar]
- Hayashi K.; Sato S.; Ikeda M.; Kaneta C.; Yokoyama N. Selective Graphene Formation on Copper Twin Crystals. J. Am. Chem. Soc. 2012, 134 (30), 12492–12498. 10.1021/ja300811p. [DOI] [PubMed] [Google Scholar]
- Cai L.; He W.; Xue X.; Huang J.; Zhou K.; Zhou X.; Xu Z.; Yu G. In situ growth of large-area and self-aligned graphene nanoribbon arrays on liquid metal. Natl. Sci. Rev. 2021, 8 (12), nwaa298. 10.1093/nsr/nwaa298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas A. N.; Liu B.; Narita A.; Dossel L. F.; Yang B.; Zhang W.; Tang J.; Wang K. L.; Rader H. J.; Feng X.; Mullen K.; Zhou C. Vapor-phase transport deposition, characterization, and applications of large nanographenes. J. Am. Chem. Soc. 2015, 137 (13), 4453–4459. 10.1021/ja513207e. [DOI] [PubMed] [Google Scholar]
- Räder H. J.; Rouhanipour A.; Talarico A. M.; Palermo V.; Samorì P.; Müllen K. Processing of giant graphene molecules by soft-landing mass spectrometry. Nat. Mater. 2006, 5 (4), 276–280. 10.1038/nmat1597. [DOI] [PubMed] [Google Scholar]
- Cai J.; Pignedoli C. A.; Talirz L.; Ruffieux P.; Sode H.; Liang L.; Meunier V.; Berger R.; Li R.; Feng X.; Mullen K.; Fasel R. Graphene nanoribbon heterojunctions. Nat. Nanotechnol. 2014, 9 (11), 896–900. 10.1038/nnano.2014.184. [DOI] [PubMed] [Google Scholar]
- Gao L.; Ren W.; Xu H.; Jin L.; Wang Z.; Ma T.; Ma L.-P.; Zhang Z.; Fu Q.; Peng L.-M.; Bao X.; Cheng H.-M. Repeated growth and bubbling transfer of graphene with millimetre-size single-crystal grains using platinum. Nat. Commun. 2012, 3 (1), 699. 10.1038/ncomms1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina A.; Jia X.; Ho J.; Nezich D.; Son H.; Bulovic V.; Dresselhaus M. S.; Kong J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9 (1), 30–35. 10.1021/nl801827v. [DOI] [PubMed] [Google Scholar]
- Kolmer M.; Steiner A.-K.; Izydorczyk I.; Ko W.; Engelund M.; Szymonski M.; Li A.-P.; Amsharov K. Rational synthesis of atomically precise graphene nanoribbons directly on metal oxide surfaces. Science 2020, 369 (6503), 571–575. 10.1126/science.abb8880. [DOI] [PubMed] [Google Scholar]
- Amsharov K. Cyclodehydrofluorination of fluoroarenes on metal oxides: Toward bottom-up synthesis of carbon nanostructures on insulating surfaces. Phys. Status Solidi B 2016, 253 (12), 2473–2477. 10.1002/pssb.201600211. [DOI] [Google Scholar]
- Wang S.; Talirz L.; Pignedoli C. A.; Feng X.; Mullen K.; Fasel R.; Ruffieux P. Giant edge state splitting at atomically precise graphene zigzag edges. Nat. Commun. 2016, 7, 11507. 10.1038/ncomms11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmer M.; Zuzak R.; Steiner A. K.; Zajac L.; Engelund M.; Godlewski S.; Szymonski M.; Amsharov K. Fluorine-programmed nanozipping to tailored nanographenes on rutile TiO2 surfaces. Science 2019, 363 (6422), 57–60. 10.1126/science.aav4954. [DOI] [PubMed] [Google Scholar]
- Flatland; Abbott E. A., Princeton University Press: 2015. [Google Scholar]
- Fernandez-Garcia J. M.; Evans P. J.; Medina Rivero S.; Fernandez I.; Garcia-Fresnadillo D.; Perles J.; Casado J.; Martin N. pi-Extended Corannulene-Based Nanographenes: Selective Formation of Negative Curvature. J. Am. Chem. Soc. 2018, 140 (49), 17188–17196. 10.1021/jacs.8b09992. [DOI] [PubMed] [Google Scholar]
- Kato K.; Takaba K.; Maki-Yonekura S.; Mitoma N.; Nakanishi Y.; Nishihara T.; Hatakeyama T.; Kawada T.; Hijikata Y.; Pirillo J.; Scott L. T.; Yonekura K.; Segawa Y.; Itami K. Double-Helix Supramolecular Nanofibers Assembled from Negatively Curved Nanographenes. J. Am. Chem. Soc. 2021, 143 (14), 5465–5469. 10.1021/jacs.1c00863. [DOI] [PubMed] [Google Scholar]
- Liu B.; Bockmann M.; Jiang W.; Doltsinis N. L.; Wang Z. Perylene Diimide-Embedded Double [8]Helicenes. J. Am. Chem. Soc. 2020, 142 (15), 7092–7099. 10.1021/jacs.0c00954. [DOI] [PubMed] [Google Scholar]
- Ma S.; Gu J.; Lin C.; Luo Z.; Zhu Y.; Wang J. Supertwistacene: A Helical Graphene Nanoribbon. J. Am. Chem. Soc. 2020, 142 (39), 16887–16893. 10.1021/jacs.0c08555. [DOI] [PubMed] [Google Scholar]
- Qiu Z.; Asako S.; Hu Y.; Ju C. W.; Liu T.; Rondin L.; Schollmeyer D.; Lauret J. S.; Mullen K.; Narita A. Negatively Curved Nanographene with Heptagonal and [5]Helicene Units. J. Am. Chem. Soc. 2020, 142 (35), 14814–14819. 10.1021/jacs.0c05504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z.; Ju C. W.; Frederic L.; Hu Y.; Schollmeyer D.; Pieters G.; Mullen K.; Narita A. Amplification of Dissymmetry Factors in pi-Extended [7]- and [9]Helicenes. J. Am. Chem. Soc. 2021, 143 (12), 4661–4667. 10.1021/jacs.0c13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X.; Pedersen S. K.; Aranda D.; Yang J.; Wiscons R. A.; Pittelkow M.; Steigerwald M. L.; Santoro F.; Schuster N. J.; Nuckolls C. Chirality Amplified: Long, Discrete Helicene Nanoribbons. J. Am. Chem. Soc. 2021, 143 (2), 983–991. 10.1021/jacs.0c11260. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Guo X.; Li Y.; Wang J. Fusing of Seven HBCs toward a Green Nanographene Propeller. J. Am. Chem. Soc. 2019, 141 (13), 5511–5517. 10.1021/jacs.9b01266. [DOI] [PubMed] [Google Scholar]
- Pun S. H.; Miao Q. Toward Negatively Curved Carbons. Acc. Chem. Res. 2018, 51 (7), 1630–1642. 10.1021/acs.accounts.8b00140. [DOI] [PubMed] [Google Scholar]
- Fernández-García J. M.; Evans P. J.; Filippone S.; Herranz M. Á.; Martín N. Chiral Molecular Carbon Nanostructures. Acc. Chem. Res. 2019, 52 (6), 1565–1574. 10.1021/acs.accounts.9b00144. [DOI] [PubMed] [Google Scholar]
- Liu J.; Ma J.; Zhang K.; Ravat P.; Machata P.; Avdoshenko S.; Hennersdorf F.; Komber H.; Pisula W.; Weigand J. J.; Popov A. A.; Berger R.; Müllen K.; Feng X. π-Extended and Curved Antiaromatic Polycyclic Hydrocarbons. J. Am. Chem. Soc. 2017, 139 (22), 7513–7521. 10.1021/jacs.7b01619. [DOI] [PubMed] [Google Scholar]
- Muzammil E. M.; Halilovic D.; Stuparu M. C. Synthesis of corannulene-based nanographenes. Commun. Chem. 2019, 2 (1), 58. 10.1038/s42004-019-0160-1. [DOI] [Google Scholar]
- Matsubara S.; Koga Y.; Segawa Y.; Murakami K.; Itami K. Creation of negatively curved polyaromatics enabled by annulative coupling that forms an eight-membered ring. Nat. Catal 2020, 3 (9), 710–718. 10.1038/s41929-020-0487-0. [DOI] [Google Scholar]
- Majewski M. A.; Stȩpień M. Bowls, Hoops, and Saddles: Synthetic Approaches to Curved Aromatic Molecules. Angew. Chem., Int. Ed. 2019, 58 (1), 86–116. 10.1002/anie.201807004. [DOI] [PubMed] [Google Scholar]
- Ejlli B.; Nußbaum P.; Rominger F.; Freudenberg J.; Bunz U. H. F.; Müllen K. Benzo-fused Tri[8]annulenes as Molecular Models of Cubic Graphite. Angew. Chem., Int. Ed. 2021, 60 (37), 20220–20224. 10.1002/anie.202106233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z. L.; Chen X. W.; Huang Y. D.; Wei R. J.; Chu K. S.; Zhao X. J.; Tan Y. Z. Nanographene with Multiple Embedded Heptagons: Cascade Radical Photocyclization. Angew. Chem., Int. Ed. 2022, 61 (18), e202116955 10.1002/anie.202116955. [DOI] [PubMed] [Google Scholar]
- Zhang X.-S.; Huang Y.-Y.; Zhang J.; Meng W.; Peng Q.; Kong R.; Xiao Z.; Liu J.; Huang M.; Yi Y.; Chen L.; Fan Q.; Lin G.; Liu Z.; Zhang G.; Jiang L.; Zhang D. Dicyclohepta[ijkl,uvwx]rubicene with Two Pentagons and Two Heptagons as a Stable and Planar Non-benzenoid Nanographene. Angew. Chem., Int. Ed. 2020, 59 (9), 3529–3533. 10.1002/anie.201914416. [DOI] [PubMed] [Google Scholar]
- Xu F.; Yu H.; Sadrzadeh A.; Yakobson B. I. Riemann Surfaces of Carbon as Graphene Nanosolenoids. Nano Lett. 2016, 16 (1), 34–9. 10.1021/acs.nanolett.5b02430. [DOI] [PubMed] [Google Scholar]
- Castro-Fernandez S.; Cruz C. M.; Mariz I. F. A.; Marquez I. R.; Jimenez V. G.; Palomino-Ruiz L.; Cuerva J. M.; Macoas E.; Campana A. G. Two-Photon Absorption Enhancement by the Inclusion of a Tropone Ring in Distorted Nanographene Ribbons. Angew. Chem., Int. Ed. 2020, 59 (18), 7139–7145. 10.1002/anie.202000105. [DOI] [PubMed] [Google Scholar]
- Urieta-Mora J.; Krug M.; Alex W.; Perles J.; Fernandez I.; Molina-Ontoria A.; Guldi D. M.; Martin N. Homo and Hetero Molecular 3D Nanographenes Employing a Cyclooctatetraene Scaffold. J. Am. Chem. Soc. 2020, 142 (9), 4162–4172. 10.1021/jacs.9b10203. [DOI] [PubMed] [Google Scholar]
- Scott L. T.; Boorum M. M.; McMahon B. J.; Hagen S.; Mack J.; Blank J.; Wegner H.; de Meijere A. A Rational Chemical Synthesis of C60. Science 2002, 295 (5559), 1500–1503. 10.1126/science.1068427. [DOI] [PubMed] [Google Scholar]
- Boorum M. M.; Vasil’ev Y. V.; Drewello T.; Scott L. T. Groundwork for a Rational Synthesis of C60: Cyclodehydrogenation of a C60H30 Polyarene. Science 2001, 294 (5543), 828–831. 10.1126/science.1064250. [DOI] [PubMed] [Google Scholar]
- Otero G.; Biddau G.; Sánchez-Sánchez C.; Caillard R.; López M. F.; Rogero C.; Palomares F. J.; Cabello N.; Basanta M. A.; Ortega J.; Méndez J.; Echavarren A. M.; Pérez R.; Gómez-Lor B.; Martín-Gago J. A. Fullerenes from aromatic precursors by surface-catalysed cyclodehydrogenation. Nature 2008, 454 (7206), 865–868. 10.1038/nature07193. [DOI] [PubMed] [Google Scholar]
- Lungerich D.; Hoelzel H.; Harano K.; Jux N.; Amsharov K. Y.; Nakamura E. A Singular Molecule-to-Molecule Transformation on Video: The Bottom-Up Synthesis of Fullerene C60 from Truxene Derivative C60H30. ACS Nano 2021, 15 (8), 12804–12814. 10.1021/acsnano.1c02222. [DOI] [PubMed] [Google Scholar]
- Segawa Y.; Ito H.; Itami K. Structurally uniform and atomically precise carbon nanostructures. Nat. Rev. Mater. 2016, 1 (1), 15002. 10.1038/natrevmats.2015.2. [DOI] [Google Scholar]
- Mackay A. L.; Terrones H. Diamond from graphite. Nature 1991, 352 (6338), 762–762. 10.1038/352762a0.1881433 [DOI] [Google Scholar]
- Terrones H., Fullerenes and beyond: Complexity, morphology, and functionality in closed carbon nanostructures. In Springer Handbook of Nanomaterials, Ed.; Springer: Berlin, 2013; pp 83–104. [Google Scholar]
- Wang J.; Zhu Y.; Zhuang G.; Wu Y.; Wang S.; Huang P.; Sheng G.; Chen M.; Yang S.; Greber T.; Du P. Synthesis of a magnetic pi-extended carbon nanosolenoid with Riemann surfaces. Nat. Commun. 2022, 13 (1), 1239. 10.1038/s41467-022-28870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modern cyclophane chemistry; Gleiter R.; Hopf H., Eds.; John Wiley & Sons: Weinheim, 2006. [Google Scholar]
- Wang Y.; Yin Z.; Zhu Y.; Gu J.; Li Y.; Wang J. Hexapole [9]Helicene. Angew. Chem., Int. Ed. 2019, 58 (2), 587–591. 10.1002/anie.201811706. [DOI] [PubMed] [Google Scholar]
- Sang Y.; Han J.; Zhao T.; Duan P.; Liu M. Circularly Polarized Luminescence in Nanoassemblies: Generation, Amplification, and Application. Adv. Mater. 2020, 32 (41), 1900110 10.1002/adma.201900110. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Li S.; Dong X.-Y.; Zang S.-Q. Circularly polarized luminescence of agglomerate emitters. Aggregate 2021, 2 (5), e48 10.1002/agt2.48. [DOI] [Google Scholar]
- Kiran V.; Mathew S. P.; Cohen S. R.; Hernandez Delgado I.; Lacour J.; Naaman R. Helicenes-A New Class of Organic Spin Filter. Adv. Mater. 2016, 28 (10), 1957–62. 10.1002/adma.201504725. [DOI] [PubMed] [Google Scholar]
- Michaeli K.; Kantor-Uriel N.; Naaman R.; Waldeck D. H. The electron’s spin and molecular chirality - how are they related and how do they affect life processes?. Chem. Soc. Rev. 2016, 45 (23), 6478–6487. 10.1039/C6CS00369A. [DOI] [PubMed] [Google Scholar]
- Naaman R.; Paltiel Y.; Waldeck D. H. Chiral molecules and the electron spin. Nat. Rev. Chem. 2019, 3 (4), 250–260. 10.1038/s41570-019-0087-1. [DOI] [Google Scholar]
- Göhler B.; Hamelbeck V.; Markus T. Z.; Kettner M.; Hanne G. F.; Vager Z.; Naaman R.; Zacharias H. Spin Selectivity in Electron Transmission Through Self-Assembled Monolayers of Double-Stranded DNA. Science 2011, 331 (6019), 894–897. 10.1126/science.1199339. [DOI] [PubMed] [Google Scholar]
- Reger D.; Haines P.; Amsharov K. Y.; Schmidt J. A.; Ullrich T.; Bönisch S.; Hampel F.; Görling A.; Nelson J.; Jelfs K. E.; Guldi D. M.; Jux N. A Family of Superhelicenes: Easily Tunable, Chiral Nanographenes by Merging Helicity with Planar π Systems. Angew. Chem., Int. Ed. 2021, 60 (33), 18073–18081. 10.1002/anie.202103253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Garcia P.; Fernandez-Garcia J. M.; Fernandez I.; Perles J.; Martin N. Helically Arranged Chiral Molecular Nanographenes. J. Am. Chem. Soc. 2021, 143 (30), 11864–11870. 10.1021/jacs.1c05977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakakuki Y.; Hirose T.; Sotome H.; Miyasaka H.; Matsuda K. Hexa- peri-hexabenzo[7]helicene: Homogeneously pi-Extended Helicene as a Primary Substructure of Helically Twisted Chiral Graphenes. J. Am. Chem. Soc. 2018, 140 (12), 4317–4326. 10.1021/jacs.7b13412. [DOI] [PubMed] [Google Scholar]
- Evans P. J.; Ouyang J.; Favereau L.; Crassous J.; Fernandez I.; Perles J.; Martin N. Synthesis of a Helical Bilayer Nanographene. Angew. Chem., Int. Ed. 2018, 57 (23), 6774–6779. 10.1002/anie.201800798. [DOI] [PubMed] [Google Scholar]
- Liu G.; Koch T.; Li Y.; Doltsinis N. L.; Wang Z. Nanographene Imides Featuring Dual-Core Sixfold [5]Helicenes. Angew. Chem., Int. Ed. 2019, 58 (1), 178–183. 10.1002/anie.201810734. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Song I.; Ahn J.; Han M.; Linares M.; Surin M.; Zhang H. J.; Oh J. H.; Lin J. pi-Extended perylene diimide double-heterohelicenes as ambipolar organic semiconductors for broadband circularly polarized light detection. Nat. Commun. 2021, 12 (1), 142. 10.1038/s41467-020-20390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz C. M.; Castro-Fernandez S.; Macoas E.; Cuerva J. M.; Campana A. G. Undecabenzo[7]superhelicene: A Helical Nanographene Ribbon as a Circularly Polarized Luminescence Emitter. Angew. Chem., Int. Ed. 2018, 57 (45), 14782–14786. 10.1002/anie.201808178. [DOI] [PubMed] [Google Scholar]
- Berezhnaia V.; Roy M.; Vanthuyne N.; Villa M.; Naubron J. V.; Rodriguez J.; Coquerel Y.; Gingras M. Chiral Nanographene Propeller Embedding Six Enantiomerically Stable [5]Helicene Units. J. Am. Chem. Soc. 2017, 139 (51), 18508–18511. 10.1021/jacs.7b07622. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Wang X. Y.; Peng P. X.; Wang X. C.; Cao X. Y.; Feng X.; Mullen K.; Narita A. Benzo-Fused Double [7]Carbohelicene: Synthesis, Structures, and Physicochemical Properties. Angew. Chem., Int. Ed. 2017, 56 (12), 3374–3378. 10.1002/anie.201610434. [DOI] [PubMed] [Google Scholar]
- Chang H.; Liu H.; Dmitrieva E.; Chen Q.; Ma J.; He P.; Liu P.; Popov A. A.; Cao X. Y.; Wang X. Y.; Zou Y.; Narita A.; Mullen K.; Peng H.; Hu Y. Furan-containing double tetraoxa[7]helicene and its radical cation. Chem. Commun. 2020, 56 (96), 15181–15184. 10.1039/D0CC06970A. [DOI] [PubMed] [Google Scholar]
- Yang H.; Zhang L.; Jiao L. N-Methylanilines as Simple and Efficient Promoters for Radical-Type Cross-Coupling Reactions of Aryl Iodides. Chem.—Eur. J. 2017, 23 (1), 65–69. 10.1002/chem.201604602. [DOI] [PubMed] [Google Scholar]
- Hosokawa T.; Takahashi Y.; Matsushima T.; Watanabe S.; Kikkawa S.; Azumaya I.; Tsurusaki A.; Kamikawa K. Synthesis, Structures, and Properties of Hexapole Helicenes: Assembling Six [5]Helicene Substructures into Highly Twisted Aromatic Systems. J. Am. Chem. Soc. 2017, 139 (51), 18512–18521. 10.1021/jacs.7b07113. [DOI] [PubMed] [Google Scholar]
- Kiel G. R.; Patel S. C.; Smith P. W.; Levine D. S.; Tilley T. D. Expanded Helicenes: A General Synthetic Strategy and Remarkable Supramolecular and Solid-State Behavior. J. Am. Chem. Soc. 2017, 139 (51), 18456–18459. 10.1021/jacs.7b10902. [DOI] [PubMed] [Google Scholar]
- Zhan H.; Zhang Y.; Yang C.; Zhang G.; Gu Y. Graphene helicoid as novel nanospring. Carbon 2017, 120, 258–264. 10.1016/j.carbon.2017.05.044. [DOI] [Google Scholar]
- Zhou Z.; Fernandez-Garcia J. M.; Zhu Y.; Evans P. J.; Rodriguez R.; Crassous J.; Wei Z.; Fernandez I.; Petrukhina M. A.; Martin N. Site-Specific Reduction-Induced Hydrogenation of a Helical Bilayer Nanographene with K and Rb Metals: Electron Multiaddition and Selective Rb(+) Complexation. Angew. Chem., Int. Ed. 2022, 61 (10), e202115747 10.1002/anie.202116013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Zhu Y.; Fernández-García J. M.; Wei Z.; Fernández I.; Petrukhina M. A.; Martín N. Stepwise reduction of a corannulene-based helical molecular nanographene with Na metal. Chem. Commun. 2022, 58, 5574. 10.1039/D2CC00971D. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Fu L.; Hu Y.; Wang X. Y.; Wei Z.; Narita A.; Mullen K.; Petrukhina M. A. Compressing Double [7]Helicene by Successive Charging with Electrons. Angew. Chem., Int. Ed. 2020, 59 (37), 15923–15927. 10.1002/anie.202005852. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Wang X. Y.; Wei Z.; Mullen K.; Petrukhina M. A. Charging OBO-Fused Double [5]Helicene with Electrons. Angew. Chem., Int. Ed. 2019, 58 (42), 14969–14973. 10.1002/anie.201908658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asymmetric synthetic methodology; Ager D. J., East M. B., Eds.; CRC Press: Boca Raton, FL, 2020. [Google Scholar]
- Cheung K. Y.; Gui S.; Deng C.; Liang H.; Xia Z.; Liu Z.; Chi L.; Miao Q. Synthesis of Armchair and Chiral Carbon Nanobelts. Chem. 2019, 5 (4), 838–847. 10.1016/j.chempr.2019.01.004. [DOI] [Google Scholar]
- Zhu J.; Han Y.; Ni Y.; Li G.; Wu J. Facile Synthesis of Nitrogen-Doped [(6.)m8]nCyclacene Carbon Nanobelts by a One-Pot Self-Condensation Reaction. J. Am. Chem. Soc. 2021, 143 (7), 2716–2721. 10.1021/jacs.1c00409. [DOI] [PubMed] [Google Scholar]
- Cheung K. Y.; Watanabe K.; Segawa Y.; Itami K. Synthesis of a zigzag carbon nanobelt. Nat. Chem. 2021, 13 (3), 255–259. 10.1038/s41557-020-00627-5. [DOI] [PubMed] [Google Scholar]
- Omachi H.; Segawa Y.; Itami K. Synthesis of Cycloparaphenylenes and Related Carbon Nanorings: A Step toward the Controlled Synthesis of Carbon Nanotubes. Acc. Chem. Res. 2012, 45 (8), 1378–1389. 10.1021/ar300055x. [DOI] [PubMed] [Google Scholar]
- Lewis S. E. Cycloparaphenylenes and related nanohoops. Chem. Soc. Rev. 2015, 44 (8), 2221–2304. 10.1039/C4CS00366G. [DOI] [PubMed] [Google Scholar]
- Li Y.; Kono H.; Maekawa T.; Segawa Y.; Yagi A.; Itami K. Chemical Synthesis of Carbon Nanorings and Nanobelts. Acc. Mater. Res. 2021, 2 (8), 681–691. 10.1021/accountsmr.1c00105. [DOI] [Google Scholar]
- Rickhaus M.; Mayor M.; Juríček M. Chirality in curved polyaromatic systems. Chem. Soc. Rev. 2017, 46 (6), 1643–1660. 10.1039/C6CS00623J. [DOI] [PubMed] [Google Scholar]
- Fan W.; Matsuno T.; Han Y.; Wang X.; Zhou Q.; Isobe H.; Wu J. Synthesis and Chiral Resolution of Twisted Carbon Nanobelts. J. Am. Chem. Soc. 2021, 143 (39), 15924–15929. 10.1021/jacs.1c08468. [DOI] [PubMed] [Google Scholar]
- Krzeszewski M.; Ito H.; Itami K. Infinitene: A Helically Twisted Figure-Eight [12]Circulene Topoisomer. J. Am. Chem. Soc. 2022, 144 (2), 862–871. 10.1021/jacs.1c10807. [DOI] [PubMed] [Google Scholar]
- Segawa Y.; Kuwayama M.; Hijikata Y.; Fushimi M.; Nishihara T.; Pirillo J.; Shirasaki J.; Kubota N.; Itami K. Topological molecular nanocarbons: All-benzene catenane and trefoil knot. Science 2019, 365 (6450), 272–276. 10.1126/science.aav5021. [DOI] [PubMed] [Google Scholar]
- Ozaki N.; Sakamoto H.; Nishihara T.; Fujimori T.; Hijikata Y.; Kimura R.; Irle S.; Itami K. Electrically Activated Conductivity and White Light Emission of a Hydrocarbon Nanoring-Iodine Assembly. Angew. Chem., Int. Ed. 2017, 56 (37), 11196–11202. 10.1002/anie.201703648. [DOI] [PubMed] [Google Scholar]
- Sato S.; Yoshii A.; Takahashi S.; Furumi S.; Takeuchi M.; Isobe H. Chiral intertwined spirals and magnetic transition dipole moments dictated by cylinder helicity. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (50), 13097. 10.1073/pnas.1717524114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y.; Gopalakrishna T. Y.; Phan H.; Kim T.; Herng T. S.; Han Y.; Tao T.; Ding J.; Kim D.; Wu J. 3D global aromaticity in a fully conjugated diradicaloid cage at different oxidation states. Nat. Chem. 2020, 12 (3), 242–248. 10.1038/s41557-019-0399-2. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Mastalerz M. Organic cage compounds - from shape-persistency to function. Chem. Soc. Rev. 2014, 43 (6), 1934–1947. 10.1039/C3CS60358J. [DOI] [PubMed] [Google Scholar]
- Hasell T.; Cooper A. I. Porous organic cages: soluble, modular and molecular pores. Nat. Rev. Mater. 2016, 1 (9), 16053. 10.1038/natrevmats.2016.53. [DOI] [Google Scholar]
- Sun Z.; Ikemoto K.; Fukunaga T. M.; Koretsune T.; Arita R.; Sato S.; Isobe H. Finite phenine nanotubes with periodic vacancy defects. Science 2019, 363 (6423), 151–155. 10.1126/science.aau5441. [DOI] [PubMed] [Google Scholar]
- Matsui K.; Segawa Y.; Itami K. All-benzene carbon nanocages: size-selective synthesis, photophysical properties, and crystal structure. J. Am. Chem. Soc. 2014, 136 (46), 16452–8. 10.1021/ja509880v. [DOI] [PubMed] [Google Scholar]
- Kayahara E.; Iwamoto T.; Takaya H.; Suzuki T.; Fujitsuka M.; Majima T.; Yasuda N.; Matsuyama N.; Seki S.; Yamago S. Synthesis and physical properties of a ball-like three-dimensional pi-conjugated molecule. Nat. Commun. 2013, 4, 2694. 10.1038/ncomms3694. [DOI] [PubMed] [Google Scholar]
- Ni Y.; Gordillo-Gámez F.; Peña Alvarez M.; Nan Z.; Li Z.; Wu S.; Han Y.; Casado J.; Wu J. A Chichibabin’s Hydrocarbon-Based Molecular Cage: The Impact of Structural Rigidity on Dynamics, Stability, and Electronic Properties. J. Am. Chem. Soc. 2020, 142 (29), 12730–12742. 10.1021/jacs.0c04876. [DOI] [PubMed] [Google Scholar]
- Sato H.; Bender J. A.; Roberts S. T.; Krische M. J. Helical Rod-like Phenylene Cages via Ruthenium Catalyzed Diol-Diene Benzannulation: A Cord of Three Strands. J. Am. Chem. Soc. 2018, 140 (7), 2455–2459. 10.1021/jacs.8b00131. [DOI] [PubMed] [Google Scholar]
- Cui S.; Zhuang G.; Lu D.; Huang Q.; Jia H.; Wang Y.; Yang S.; Du P. A Three-Dimensional Capsule-like Carbon Nanocage as a Segment Model of Capped Zigzag [12,0] Carbon Nanotubes: Synthesis, Characterization, and Complexation with C70. Angew. Chem., Int. Ed. 2018, 57 (30), 9330–9335. 10.1002/anie.201804031. [DOI] [PubMed] [Google Scholar]
- Hayase N.; Nogami J.; Shibata Y.; Tanaka K. Synthesis of a Strained Spherical Carbon Nanocage by Regioselective Alkyne Cyclotrimerization. Angew. Chem., Int. Ed. 2019, 58 (28), 9439–9442. 10.1002/anie.201903422. [DOI] [PubMed] [Google Scholar]
- Hirsch A.; Chen Z.; Jiao H. Spherical Aromaticity in Ih Symmetrical Fullerenes: The 2(N+1)2 Rule. Angew. Chem., Int. Ed. 2000, 39 (21), 3915–3917. . [DOI] [PubMed] [Google Scholar]
- Bühl M.; Hirsch A. Spherical Aromaticity of Fullerenes. Chem. Rev. 2001, 101 (5), 1153–1184. 10.1021/cr990332q. [DOI] [PubMed] [Google Scholar]
- Li J.; Sanz S.; Corso M.; Choi D. J.; Peña D.; Frederiksen T.; Pascual J. I. Single spin localization and manipulation in graphene open-shell nanostructures. Nat. Commun. 2019, 10 (1), 200. 10.1038/s41467-018-08060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S.; Catarina G.; Wu F.; Ortiz R.; Jacob D.; Eimre K.; Ma J.; Pignedoli C. A.; Feng X.; Ruffieux P.; Fernández-Rossier J.; Fasel R. Observation of fractional edge excitations in nanographene spin chains. Nature 2021, 598 (7880), 287–292. 10.1038/s41586-021-03842-3. [DOI] [PubMed] [Google Scholar]
- Arikawa S.; Shimizu A.; Shiomi D.; Sato K.; Shintani R. Synthesis and Isolation of a Kinetically Stabilized Crystalline Triangulene. J. Am. Chem. Soc. 2021, 143 (46), 19599–19605. 10.1021/jacs.1c10151. [DOI] [PubMed] [Google Scholar]
- Goto K.; Kubo T.; Yamamoto K.; Nakasuji K.; Sato K.; Shiomi D.; Takui T.; Kubota M.; Kobayashi T.; Yakusi K.; Ouyang J. A Stable Neutral Hydrocarbon Radical: Synthesis, Crystal Structure, and Physical Properties of 2,5,8-Tri-tert-butyl-phenalenyl. J. Am. Chem. Soc. 1999, 121 (7), 1619–1620. 10.1021/ja9836242. [DOI] [Google Scholar]
- Hieulle J.; Castro S.; Friedrich N.; Vegliante A.; Lara F. R.; Sanz S.; Rey D.; Corso M.; Frederiksen T.; Pascual J. I.; Peña D. On-Surface Synthesis and Collective Spin Excitations of a Triangulene-Based Nanostar. Angew. Chem., Int. Ed. 2021, 60 (48), 25224–25229. 10.1002/anie.202108301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S.; Yao X.; Chen Q.; Eimre K.; Gröning O.; Ortiz R.; Di Giovannantonio M.; Sancho-García J. C.; Fernández-Rossier J.; Pignedoli C. A.; Müllen K.; Ruffieux P.; Narita A.; Fasel R. Large magnetic exchange coupling in rhombus-shaped nanographenes with zigzag periphery. Nat. Chem. 2021, 13 (6), 581–586. 10.1038/s41557-021-00678-2. [DOI] [PubMed] [Google Scholar]
- Yang L.; Cohen M. L.; Louie S. G. Magnetic Edge-State Excitons in Zigzag Graphene Nanoribbons. Phys. Rev. Lett. 2008, 101 (18), 186401. 10.1103/PhysRevLett.101.186401. [DOI] [PubMed] [Google Scholar]
- Son Y.-W.; Cohen M. L.; Louie S. G. Half-metallic graphene nanoribbons. Nature 2006, 444 (7117), 347–349. 10.1038/nature05180. [DOI] [PubMed] [Google Scholar]
- Wimmer M.; Adagideli I.; Berber S.; Tomanek D.; Richter K. Spin currents in rough graphene nanoribbons: universal fluctuations and spin injection. Phys. Rev. Lett. 2008, 100 (17), 177207. 10.1103/PhysRevLett.100.177207. [DOI] [PubMed] [Google Scholar]
- Shinde P. P.; Liu J.; Dienel T.; Gröning O.; Dumslaff T.; Mühlinghaus M.; Narita A.; Müllen K.; Pignedoli C. A.; Fasel R.; Ruffieux P.; Passerone D. Graphene nanoribbons with mixed cove-cape-zigzag edge structure. Carbon 2021, 175, 50–59. 10.1016/j.carbon.2020.12.069. [DOI] [Google Scholar]
- Slota M.; Keerthi A.; Myers W. K.; Tretyakov E.; Baumgarten M.; Ardavan A.; Sadeghi H.; Lambert C. J.; Narita A.; Mullen K.; Bogani L. Magnetic edge states and coherent manipulation of graphene nanoribbons. Nature 2018, 557 (7707), 691–695. 10.1038/s41586-018-0154-7. [DOI] [PubMed] [Google Scholar]
- Jacobse P. H.; McCurdy R. D.; Jiang J.; Rizzo D. J.; Veber G.; Butler P.; Zuzak R.; Louie S. G.; Fischer F. R.; Crommie M. F. Bottom-up Assembly of Nanoporous Graphene with Emergent Electronic States. J. Am. Chem. Soc. 2020, 142 (31), 13507–13514. 10.1021/jacs.0c05235. [DOI] [PubMed] [Google Scholar]
- Banhart F.; Kotakoski J.; Krasheninnikov A. V. Structural Defects in Graphene. ACS Nano 2011, 5 (1), 26–41. 10.1021/nn102598m. [DOI] [PubMed] [Google Scholar]
- Yazyev O. V.; Louie S. G. Electronic transport in polycrystalline graphene. Nat. Mater. 2010, 9 (10), 806–809. 10.1038/nmat2830. [DOI] [PubMed] [Google Scholar]
- Araujo P. T.; Terrones M.; Dresselhaus M. S. Defects and impurities in graphene-like materials. Mater. Today 2012, 15 (3), 98–109. 10.1016/S1369-7021(12)70045-7. [DOI] [Google Scholar]
- Mishra S.; Lohr T. G.; Pignedoli C. A.; Liu J.; Berger R.; Urgel J. I.; Müllen K.; Feng X.; Ruffieux P.; Fasel R. Tailoring Bond Topologies in Open-Shell Graphene Nanostructures. ACS Nano 2018, 12 (12), 11917–11927. 10.1021/acsnano.8b07225. [DOI] [PubMed] [Google Scholar]
- Fei Y.; Fu Y.; Bai X.; Du L.; Li Z.; Komber H.; Low K.-H.; Zhou S.; Phillips D. L.; Feng X.; Liu J. Defective Nanographenes Containing Seven-Five-Seven (7–5-7)-Membered Rings. J. Am. Chem. Soc. 2021, 143 (5), 2353–2360. 10.1021/jacs.0c12116. [DOI] [PubMed] [Google Scholar]
- Stone A. J.; Wales D. J. Theoretical studies of icosahedral C60 and some related species. Chem. Phys. Lett. 1986, 128 (5), 501–503. 10.1016/0009-2614(86)80661-3. [DOI] [Google Scholar]
- Zou Y.; Zeng W.; Gopalakrishna T. Y.; Han Y.; Jiang Q.; Wu J. Dicyclopenta[4,3,2,1-ghi:4′,3′,2′,1′-pqr]perylene: A Bowl-Shaped Fragment of Fullerene C70 with Global Antiaromaticity. J. Am. Chem. Soc. 2019, 141 (18), 7266–7270. 10.1021/jacs.9b03169. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Gopalakrishna T. Y.; Phan H.; Herng T. S.; Dong S.; Ding J.; Chi C. Cyclopenta Ring Fused Bisanthene and Its Charged Species with Open-Shell Singlet Diradical Character and Global Aromaticity/ Anti-Aromaticity. Angew. Chem., Int. Ed. 2017, 56 (38), 11415–11419. 10.1002/anie.201704805. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Baumgarten M.; Wagner M.; Hu Y.; Hou I. C.-Y.; Narita A.; Müllen K. Dicyclopentaannelated Hexa-peri-hexabenzocoronenes with a Singlet Biradical Ground State. Angew. Chem., Int. Ed. 2021, 60 (20), 11300–11304. 10.1002/anie.202102932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.; Di Giovannantonio M.; Urgel J. I.; Pignedoli C. A.; Ruffieux P.; Müllen K.; Fasel R.; Narita A. On-surface activation of benzylic C-H bonds for the synthesis of pentagon-fused graphene nanoribbons. Nano Res. 2021, 14 (12), 4754–4759. 10.1007/s12274-021-3419-2. [DOI] [Google Scholar]
- Kastler M.; Schmidt J.; Pisula W.; Sebastiani D.; Müllen K. From Armchair to Zigzag Peripheries in Nanographenes. J. Am. Chem. Soc. 2006, 128 (29), 9526–9534. 10.1021/ja062026h. [DOI] [PubMed] [Google Scholar]
- Mishra S.; Beyer D.; Eimre K.; Kezilebieke S.; Berger R.; Gröning O.; Pignedoli C. A.; Müllen K.; Liljeroth P.; Ruffieux P.; Feng X.; Fasel R. Topological frustration induces unconventional magnetism in a nanographene. Nat. Nanotechnol. 2020, 15 (1), 22–28. 10.1038/s41565-019-0577-9. [DOI] [PubMed] [Google Scholar]
- Gu Y.; Wu X.; Gopalakrishna T. Y.; Phan H.; Wu J. Graphene-like Molecules with Four Zigzag Edges. Angew. Chem., Int. Ed. 2018, 57 (22), 6541–6545. 10.1002/anie.201802818. [DOI] [PubMed] [Google Scholar]
- Grimsdale A. C.; Müllen K. The Chemistry of Organic Nanomaterials. Angew. Chem., Int. Ed. 2005, 44 (35), 5592–5629. 10.1002/anie.200500805. [DOI] [PubMed] [Google Scholar]
- Pschirer N. G.; Kohl C.; Nolde F.; Qu J.; Müllen K. Pentarylene- and Hexarylenebis(dicarboximide)s: Near-Infrared-Absorbing Polyaromatic Dyes. Angew. Chem., Int. Ed. 2006, 45 (9), 1401–1404. 10.1002/anie.200502998. [DOI] [PubMed] [Google Scholar]
- Avlasevich Y.; Müllen K. Dibenzopentarylenebis(dicarboximide)s: Novel near-infrared absorbing dyes. Chem. Commun. 2006, (42), 4440–4442. 10.1039/B610318A. [DOI] [PubMed] [Google Scholar]
- Fabian J.; Nakazumi H.; Matsuoka M. J. C. R. Near-infrared absorbing dyes. Chem. Rev. 1992, 92 (6), 1197–1226. 10.1021/cr00014a003. [DOI] [Google Scholar]
- Ji C.; Cheng W.; Yuan Q.; Müllen K.; Yin M. From Dyestuff Chemistry to Cancer Theranostics: The Rise of Rylenecarboximides. Acc. Chem. Res. 2019, 52 (8), 2266–2277. 10.1021/acs.accounts.9b00221. [DOI] [PubMed] [Google Scholar]
- Zhao S.; Rondin L.; Delport G.; Voisin C.; Beser U.; Hu Y.; Feng X.; Müllen K.; Narita A.; Campidelli S.; Lauret J. S. Fluorescence from graphene nanoribbons of well-defined structure. Carbon 2017, 119, 235–240. 10.1016/j.carbon.2017.04.043. [DOI] [Google Scholar]
- Hsu H.; Reichl L. E. Selection rule for the optical absorption of graphene nanoribbons. Phys. Rev. B 2007, 76 (4), 045418. 10.1103/PhysRevB.76.045418. [DOI] [Google Scholar]
- Ma C.; Xiao Z.; Puretzky A. A.; Wang H.; Mohsin A.; Huang J.; Liang L.; Luo Y.; Lawrie B. J.; Gu G.; Lu W.; Hong K.; Bernholc J.; Li A. P. Engineering Edge States of Graphene Nanoribbons for Narrow-Band Photoluminescence. ACS Nano 2020, 14 (4), 5090–5098. 10.1021/acsnano.0c01737. [DOI] [PubMed] [Google Scholar]
- Paternò G. M.; Chen Q.; Wang X.-Y.; Liu J.; Motti S. G.; Petrozza A.; Feng X.; Lanzani G.; Müllen K.; Narita A.; Scotognella F. Synthesis of Dibenzo[hi,st]ovalene and Its Amplified Spontaneous Emission in a Polystyrene Matrix. Angew. Chem., Int. Ed. 2017, 56 (24), 6753–6757. 10.1002/anie.201700730. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Thoms S.; Stöttinger S.; Schollmeyer D.; Müllen K.; Narita A.; Basché T. Dibenzo[hi,st]ovalene as Highly Luminescent Nanographene: Efficient Synthesis via Photochemical Cyclodehydroiodination, Optoelectronic Properties, and Single-Molecule Spectroscopy. J. Am. Chem. Soc. 2019, 141 (41), 16439–16449. 10.1021/jacs.9b08320. [DOI] [PubMed] [Google Scholar]
- Muñoz-Mármol R.; Gordillo F.; Bonal V.; Villalvilla J. M.; Boj P. G.; Quintana J. A.; Ross A. M.; Paternò G. M.; Scotognella F.; Lanzani G.; Derradji A.; Sancho-García J. C.; Gu Y.; Wu J.; Casado J.; Díaz-García M. A. Near-Infrared Lasing in Four-Zigzag Edged Nanographenes by 1D versus 2D Electronic π-Conjugation. Adv. Funct. Mater. 2021, 31 (41), 2105073. 10.1002/adfm.202105073. [DOI] [Google Scholar]
- Bonal V.; Munoz-Marmol R.; Gordillo Gamez F.; Morales-Vidal M.; Villalvilla J. M.; Boj P. G.; Quintana J. A.; Gu Y.; Wu J.; Casado J.; Diaz-Garcia M. A. Solution-processed nanographene distributed feedback lasers. Nat. Commun. 2019, 10 (1), 3327. 10.1038/s41467-019-11336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y.; Muñoz-Mármol R.; Fan W.; Han Y.; Wu S.; Li Z.; Bonal V.; Villalvilla J. M.; Quintana J. A.; Boj P. G.; Díaz-García M. A.; Wu J. Peri-Acenoacene for Solution Processed Distributed Feedback Laser: The Effect of 1,2-Oxaborine Doping. Adv. Optical Mater. 2022, 10 (7), 2102782. 10.1002/adom.202102782. [DOI] [Google Scholar]
- Liu R.; Wu D.; Feng X.; Müllen K. Bottom-Up Fabrication of Photoluminescent Graphene Quantum Dots with Uniform Morphology. J. Am. Chem. Soc. 2011, 133 (39), 15221–15223. 10.1021/ja204953k. [DOI] [PubMed] [Google Scholar]
- Zheng X. T.; Ananthanarayanan A.; Luo K. Q.; Chen P. Glowing Graphene Quantum Dots and Carbon Dots: Properties, Syntheses, and Biological Applications. Small 2015, 11 (14), 1620–1636. 10.1002/smll.201402648. [DOI] [PubMed] [Google Scholar]
- Zhu S.; Zhang J.; Qiao C.; Tang S.; Li Y.; Yuan W.; Li B.; Tian L.; Liu F.; Hu R.; Gao H.; Wei H.; Zhang H.; Sun H.; Yang B. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47 (24), 6858–6860. 10.1039/c1cc11122a. [DOI] [PubMed] [Google Scholar]
- Lu J.; Yeo P. S. E.; Gan C. K.; Wu P.; Loh K. P. Transforming C60 molecules into graphene quantum dots. Nat. Nanotechnol. 2011, 6 (4), 247–252. 10.1038/nnano.2011.30. [DOI] [PubMed] [Google Scholar]
- Ponomarenko L. A.; Schedin F.; Katsnelson M. I.; Yang R.; Hill E. W.; Novoselov K. S.; Geim A. K. Chaotic Dirac Billiard in Graphene Quantum Dots. Science 2008, 320 (5874), 356–358. 10.1126/science.1154663. [DOI] [PubMed] [Google Scholar]
- Bacon M.; Bradley S. J.; Nann T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2014, 31 (4), 415–428. 10.1002/ppsc.201300252. [DOI] [Google Scholar]
- Fechtenkötter A.; Tchebotareva N.; Watson M.; Müllen K. Discotic liquid crystalline hexabenzocoronenes carrying chiral and racemic branched alkyl chains: supramolecular engineering and improved synthetic methods. Tetrahedron 2001, 57 (17), 3769–3783. 10.1016/S0040-4020(01)00252-6. [DOI] [Google Scholar]
- Craats A. M. v. d.; Warman J. M.; Fechtenkötter A.; Brand J. D.; Harbison M. A.; Müllen K. Record Charge Carrier Mobility in a Room-Temperature Discotic Liquid-Crystalline Derivative of Hexabenzocoronene. Adv. Mater. 1999, 11 (17), 1469–1472. . [DOI] [Google Scholar]
- Wu J.; Pisula W.; Müllen K. Graphenes as Potential Material for Electronics. Chem. Rev. 2007, 107 (3), 718–747. 10.1021/cr068010r. [DOI] [PubMed] [Google Scholar]
- Ito S.; Wehmeier M.; Brand J. D.; Kübel C.; Epsch R.; Rabe J. P.; Müllen K. Synthesis and Self-Assembly of Functionalized Hexa-peri-hexabenzocoronenes. Chem.—Eur. J. 2000, 6 (23), 4327–4342. . [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S. Discotic liquid crystals. A brief review. Liq. Cryst. 1993, 14 (1), 3–14. 10.1080/02678299308027301. [DOI] [Google Scholar]
- Kumar S. Recent developments in the chemistry of triphenylene-based discotic liquid crystals. Liq. Cryst. 2004, 31 (8), 1037–1059. 10.1080/02678290410001724746. [DOI] [Google Scholar]
- Masrour R.; Bahmad L.; Benyoussef A. Size effect on magnetic properties of a nano-graphene bilayer structure: A Monte Carlo study. J. Magn. Magn. Mater. 2012, 324 (23), 3991–3996. 10.1016/j.jmmm.2012.06.048. [DOI] [Google Scholar]
- Yang Y.; Murali R. Impact of Size Effect on Graphene Nanoribbon Transport. IEEE Electron Device Lett. 2010, 31 (3), 237–239. 10.1109/LED.2009.2039915. [DOI] [Google Scholar]
- Golor M.; Wessel S.; Schmidt M. J. Quantum Nature of Edge Magnetism in Graphene. Phys. Rev. Lett. 2014, 112 (4), 046601. 10.1103/PhysRevLett.112.046601. [DOI] [PubMed] [Google Scholar]
- Zhai W.; Zhou K. Nanomaterials in Superlubricity. Adv. Funct. Mater. 2019, 29 (28), 1806395. 10.1002/adfm.201806395. [DOI] [Google Scholar]
- Kawai S.; Benassi A.; Gnecco E.; Söde H.; Pawlak R.; Feng X.; Müllen K.; Passerone D.; Pignedoli C. A.; Ruffieux P.; Fasel R.; Meyer E. Superlubricity of graphene nanoribbons on gold surfaces. Science 2016, 351 (6276), 957–961. 10.1126/science.aad3569. [DOI] [PubMed] [Google Scholar]
- Llinas J. P.; Fairbrother A.; Borin Barin G.; Shi W.; Lee K.; Wu S.; Yong Choi B.; Braganza R.; Lear J.; Kau N.; Choi W.; Chen C.; Pedramrazi Z.; Dumslaff T.; Narita A.; Feng X.; Mullen K.; Fischer F.; Zettl A.; Ruffieux P.; Yablonovitch E.; Crommie M.; Fasel R.; Bokor J. Short-channel field-effect transistors with 9-atom and 13-atom wide graphene nanoribbons. Nat. Commun. 2017, 8 (1), 633. 10.1038/s41467-017-00734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter N.; Chen Z.; Tries A.; Prechtl T.; Narita A.; Müllen K.; Asadi K.; Bonn M.; Kläui M. Charge transport mechanism in networks of armchair graphene nanoribbons. Sci. Rep 2020, 10 (1), 1988. 10.1038/s41598-020-58660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas A. N.; Liu G.; Narita A.; Orosco M.; Feng X.; Müllen K.; Zhou C. Deposition, Characterization, and Thin-Film-Based Chemical Sensing of Ultra-long Chemically Synthesized Graphene Nanoribbons. J. Am. Chem. Soc. 2014, 136 (21), 7555–7558. 10.1021/ja502764d. [DOI] [PubMed] [Google Scholar]
- Gao J.; Uribe-Romo F. J.; Saathoff J. D.; Arslan H.; Crick C. R.; Hein S. J.; Itin B.; Clancy P.; Dichtel W. R.; Loo Y.-L. Ambipolar Transport in Solution-Synthesized Graphene Nanoribbons. ACS Nano 2016, 10 (4), 4847–4856. 10.1021/acsnano.6b00643. [DOI] [PubMed] [Google Scholar]
- Zschieschang U.; Klauk H.; Müeller I. B.; Strudwick A. J.; Hintermann T.; Schwab M. G.; Narita A.; Feng X.; Müellen K.; Weitz R. T. Electrical Characteristics of Field-Effect Transistors based on Chemically Synthesized Graphene Nanoribbons. Adv. Electron. Mater. 2015, 1 (3), 1400010. 10.1002/aelm.201400010. [DOI] [Google Scholar]
- Bennett P. B.; Pedramrazi Z.; Madani A.; Chen Y.-C.; de Oteyza D. G.; Chen C.; Fischer F. R.; Crommie M. F.; Bokor J. Bottom-up graphene nanoribbon field-effect transistors. Appl. Phys. Lett. 2013, 103 (25), 253114. 10.1063/1.4855116. [DOI] [Google Scholar]
- Candini A.; Martini L.; Chen Z.; Mishra N.; Convertino D.; Coletti C.; Narita A.; Feng X.; Müllen K.; Affronte M. High Photoresponsivity in Graphene Nanoribbon Field-Effect Transistor Devices Contacted with Graphene Electrodes. J. Phys. Chem. C 2017, 121 (19), 10620–10625. 10.1021/acs.jpcc.7b03401. [DOI] [Google Scholar]
- Ponomarenko V. P.; Popov V. S.; Popov S. V. Graphene Structures-Based 2D Nanotransistors (Review). J. Commun. Technol. Electron. 2021, 66 (9), 1108–1122. 10.1134/S1064226921090138. [DOI] [Google Scholar]
- Pizzochero M.; Tepliakov N. V.; Mostofi A. A.; Kaxiras E. Electrically Induced Dirac Fermions in Graphene Nanoribbons. Nano Lett. 2021, 21 (21), 9332–9338. 10.1021/acs.nanolett.1c03596. [DOI] [PubMed] [Google Scholar]
- Su W. P.; Schrieffer J. R.; Heeger A. J. Solitons in Polyacetylene. Phys. Rev. Lett. 1979, 42 (25), 1698–1701. 10.1103/PhysRevLett.42.1698. [DOI] [Google Scholar]
- Cao T.; Zhao F.; Louie S. G. Topological Phases in Graphene Nanoribbons: Junction States, Spin Centers, and Quantum Spin Chains. Phys. Rev. Lett. 2017, 119 (7), 076401. 10.1103/PhysRevLett.119.076401. [DOI] [PubMed] [Google Scholar]
- Grayson S. M.; Fréchet J. M. J. Convergent Dendrons and Dendrimers: from Synthesis to Applications. Chem. Rev. 2001, 101 (12), 3819–3868. 10.1021/cr990116h. [DOI] [PubMed] [Google Scholar]
- Abbasi E.; Aval S. F.; Akbarzadeh A.; Milani M.; Nasrabadi H. T.; Joo S. W.; Hanifehpour Y.; Nejati-Koshki K.; Pashaei-Asl R. Dendrimers: synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9 (1), 247. 10.1186/1556-276X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögtle F.; Gestermann S.; Hesse R.; Schwierz H.; Windisch B. Functional dendrimers. Prog. Polym. Sci. 2000, 25 (7), 987–1041. 10.1016/S0079-6700(00)00017-4. [DOI] [Google Scholar]
- Petekidis G.; Vlassopoulos D.; Galda P.; Rehahn M.; Ballauff M. Determination of Chain Conformation of Stiff Polymers by Depolarized Rayleigh Scattering in Solution. Macromolecules 1996, 29 (27), 8948–8953. 10.1021/ma961128x. [DOI] [Google Scholar]
- Yin M.; Shen J.; Gropeanu R.; Pflugfelder G. O.; Weil T.; Mullen K. Fluorescent core/shell nanoparticles for specific cell-nucleus staining. Small 2008, 4 (7), 894–8. 10.1002/smll.200701107. [DOI] [PubMed] [Google Scholar]
- Yin M.; Shen J.; Pflugfelder G. O.; Müllen K. A Fluorescent Core-Shell Dendritic Macromolecule Specifically Stains The Extracellular Matrix. J. Am. Chem. Soc. 2008, 130 (25), 7806–7807. 10.1021/ja8022362. [DOI] [PubMed] [Google Scholar]
- Stangenberg R.; Wu Y.; Hedrich J.; Kurzbach D.; Wehner D.; Weidinger G.; Kuan S. L.; Jansen M. I.; Jelezko F.; Luhmann H. J.; Hinderberger D.; Weil T.; Müllen K. A Polyphenylene Dendrimer Drug Transporter with Precisely Positioned Amphiphilic Surface Patches. Adv. Healthcare Mater. 2015, 4 (3), 377–384. 10.1002/adhm.201400291. [DOI] [PubMed] [Google Scholar]
- Stangenberg R.; Saeed I.; Kuan S. L.; Baumgarten M.; Weil T.; Klapper M.; Müllen K. Tuning Polarity of Polyphenylene Dendrimers by Patched Surface Amphiphilicity—Precise Control over Size, Shape, and Polarity. Macromol. Rapid Commun. 2014, 35 (2), 152–160. 10.1002/marc.201300671. [DOI] [PubMed] [Google Scholar]
- Wagner J.; Li L.; Simon J.; Krutzke L.; Landfester K.; Mailänder V.; Müllen K.; Ng D. Y. W.; Wu Y.; Weil T. Amphiphilic Polyphenylene Dendron Conjugates for Surface Remodeling of Adenovirus 5. Angew. Chem., Int. Ed. 2020, 59 (14), 5712–5720. 10.1002/anie.201913708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M.; Ding K.; Gropeanu R. A.; Shen J.; Berger R.; Weil T.; Müllen K. Dendritic Star Polymers for Efficient DNA Binding and Stimulus-Dependent DNA Release. Biomacromolecules 2008, 9 (11), 3231–3238. 10.1021/bm800797j. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Yin M.; Müllen K.; Knoll W. LbL-assembled multilayer films of dendritic star polymers: surface morphology and DNA hybridization detection. J. Mater. Chem. 2012, 22 (16), 7880–7886. 10.1039/c2jm15931g. [DOI] [Google Scholar]
- Feng C. L.; Yin M.; Zhang D.; Zhu S.; Caminade A. M.; Majoral J. P.; Müllen K. Fluorescent Core-Shell Star Polymers Based Bioassays for Ultrasensitive DNA Detection by Surface Plasmon Fluorescence Spectroscopy. Macromol. Rapid Commun. 2011, 32 (8), 679–683. 10.1002/marc.201000788. [DOI] [PubMed] [Google Scholar]
- Yin M.; Feng C.; Shen J.; Yu Y.; Xu Z.; Yang W.; Knoll W.; Müllen K. Dual-Responsive Interaction to Detect DNA on Template-Based Fluorescent Nanotubes. Small 2011, 7 (12), 1629–1634. 10.1002/smll.201100187. [DOI] [PubMed] [Google Scholar]
- Taubert A.; Wiesler U.-M.; Müllen K. Dendrimer-controlled one-pot synthesis of gold nanoparticles with a bimodal size distribution and their self-assembly in the solid state. J. Mater. Chem. 2003, 13 (5), 1090–1093. 10.1039/b207895c. [DOI] [Google Scholar]
- Ionescu R.; Broza Y.; Shaltieli H.; Sadeh D.; Zilberman Y.; Feng X.; Glass-Marmor L.; Lejbkowicz I.; Müllen K.; Miller A.; Haick H. Detection of Multiple Sclerosis from Exhaled Breath Using Bilayers of Polycyclic Aromatic Hydrocarbons and Single-Wall Carbon Nanotubes. ACS Chem. Neurosci. 2011, 2 (12), 687–693. 10.1021/cn2000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman Y.; Tisch U.; Shuster G.; Pisula W.; Feng X.; Müllen K.; Haick H. Carbon Nanotube/Hexa-peri-hexabenzocoronene Bilayers for Discrimination Between Nonpolar Volatile Organic Compounds of Cancer and Humid Atmospheres. Adv. Mater. 2010, 22 (38), 4317–4320. 10.1002/adma.201001275. [DOI] [PubMed] [Google Scholar]
- Zilberman Y.; Ionescu R.; Feng X.; Müllen K.; Haick H. Nanoarray of Polycyclic Aromatic Hydrocarbons and Carbon Nanotubes for Accurate and Predictive Detection in Real-World Environmental Humidity. ACS Nano 2011, 5 (8), 6743–6753. 10.1021/nn202314k. [DOI] [PubMed] [Google Scholar]
- Jin E.; Yang Q.; Ju C.-W.; Chen Q.; Landfester K.; Bonn M.; Müllen K.; Liu X.; Narita A. A Highly Luminescent Nitrogen-Doped Nanographene as an Acid- and Metal-Sensitive Fluorophore for Optical Imaging. J. Am. Chem. Soc. 2021, 143 (27), 10403–10412. 10.1021/jacs.1c04880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.; Zhang S.; Li J.; Wei J.; Müllen K.; Yin M. A Water-Soluble, NIR-Absorbing Quaterrylenediimide Chromophore for Photoacoustic Imaging and Efficient Photothermal Cancer Therapy. Angew. Chem., Int. Ed. 2019, 58 (6), 1638–1642. 10.1002/anie.201810541. [DOI] [PubMed] [Google Scholar]
- Lin H. A.; Sato Y.; Segawa Y.; Nishihara T.; Sugimoto N.; Scott L. T.; Higashiyama T.; Itami K. A Water-Soluble Warped Nanographene: Synthesis and Applications for Photoinduced Cell Death. Angew. Chem., Int. Ed. 2018, 57 (11), 2874–2878. 10.1002/anie.201713387. [DOI] [PubMed] [Google Scholar]
- Yamato K.; Sekiya R.; Suzuki K.; Haino T. Near-Infrared-Emitting Nitrogen-Doped Nanographenes. Angew. Chem., Int. Ed. 2019, 58 (27), 9022–9026. 10.1002/anie.201901510. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Liu Y.; Xiong X.-Q.; Peng L.-H.; Gan L.; Chen C.-F.; Xu H.-B. Three-Dimensional Nanographene Based on Triptycene: Synthesis and Its Application in Fluorescence Imaging. Org. Lett. 2012, 14 (23), 5912–5915. 10.1021/ol302833t. [DOI] [PubMed] [Google Scholar]
- Qiu H.; Zhou W.; Guo W. Nanopores in Graphene and Other 2D Materials: A Decade’s Journey toward Sequencing. ACS Nano 2021, 15 (12), 18848–18864. 10.1021/acsnano.1c07960. [DOI] [PubMed] [Google Scholar]
- Heerema S. J.; Dekker C. Graphene nanodevices for DNA sequencing. Nat. Nanotechnol. 2016, 11 (2), 127–136. 10.1038/nnano.2015.307. [DOI] [PubMed] [Google Scholar]
- Venkatesan B. M.; Bashir R. Nanopore sensors for nucleic acid analysis. Nat. Nanotechnol. 2011, 6 (10), 615–624. 10.1038/nnano.2011.129. [DOI] [PubMed] [Google Scholar]
- Saha K. K.; Drndić M.; Nikolić B. K. DNA Base-Specific Modulation of Microampere Transverse Edge Currents through a Metallic Graphene Nanoribbon with a Nanopore. Nano Lett. 2012, 12 (1), 50–55. 10.1021/nl202870y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversi F.; Raillon C.; Benameur S. M.; Liu K.; Khlybov S.; Tosun M.; Krasnozhon D.; Kis A.; Radenovic A. Detecting the translocation of DNA through a nanopore using graphene nanoribbons. Nat. Nanotechnol. 2013, 8 (12), 939–945. 10.1038/nnano.2013.240. [DOI] [PubMed] [Google Scholar]
- Shende P.; Augustine S.; Prabhakar B. A review on graphene nanoribbons for advanced biomedical applications. Carbon Lett. 2020, 30 (5), 465–475. 10.1007/s42823-020-00125-1. [DOI] [Google Scholar]
- Dong H.; Ding L.; Yan F.; Ji H.; Ju H. The use of polyethylenimine-grafted graphene nanoribbon for cellular delivery of locked nucleic acid modified molecular beacon for recognition of microRNA. Biomaterials 2011, 32 (15), 3875–3882. 10.1016/j.biomaterials.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Wang G.; Zhang L.; Zhang J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41 (2), 797–828. 10.1039/C1CS15060J. [DOI] [PubMed] [Google Scholar]
- Bonaccorso F.; Colombo L.; Yu G.; Stoller M.; Tozzini V.; Ferrari A. C.; Ruoff R. S.; Pellegrini V. 2D materials. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347 (6217), 1246501. 10.1126/science.1246501. [DOI] [PubMed] [Google Scholar]
- Parvez K.; Wu Z.-S.; Li R.; Liu X.; Graf R.; Feng X.; Müllen K. Exfoliation of Graphite into Graphene in Aqueous Solutions of Inorganic Salts. J. Am. Chem. Soc. 2014, 136 (16), 6083–6091. 10.1021/ja5017156. [DOI] [PubMed] [Google Scholar]
- Zhou F.; Huang H.; Xiao C.; Zheng S.; Shi X.; Qin J.; Fu Q.; Bao X.; Feng X.; Müllen K.; Wu Z.-S. Electrochemically Scalable Production of Fluorine-Modified Graphene for Flexible and High-Energy Ionogel-Based Microsupercapacitors. J. Am. Chem. Soc. 2018, 140 (26), 8198–8205. 10.1021/jacs.8b03235. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Chen Z.; Wang C.; Wang H. I.; Wuttke M.; Wang X.-Y.; Bonn M.; Chi L.; Narita A.; Müllen K. Bottom-Up, On-Surface-Synthesized Armchair Graphene Nanoribbons for Ultra-High-Power Micro-Supercapacitors. J. Am. Chem. Soc. 2020, 142 (42), 17881–17886. 10.1021/jacs.0c06109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Hu Y.; Zheng W.; Wang C.; Baaziz W.; Richard F.; Ersen O.; Bonn M.; Wang H. I.; Narita A.; Ciesielski A.; Müllen K.; Samorì P. Untying the Bundles of Solution-Synthesized Graphene Nanoribbons for Highly Capacitive Micro-Supercapacitors. Adv. Funct. Mater. 2022, 32, 2109543. 10.1002/adfm.202109543. [DOI] [Google Scholar]
- Vo T. H.; Shekhirev M.; Kunkel D. A.; Orange F.; Guinel M. J. F.; Enders A.; Sinitskii A. Bottom-up solution synthesis of narrow nitrogen-doped graphene nanoribbons. Chem. Commun. 2014, 50 (32), 4172–4174. 10.1039/C4CC00885E. [DOI] [PubMed] [Google Scholar]
- Mehdi Pour M.; Lashkov A.; Radocea A.; Liu X.; Sun T.; Lipatov A.; Korlacki R. A.; Shekhirev M.; Aluru N. R.; Lyding J. W.; Sysoev V.; Sinitskii A. Laterally extended atomically precise graphene nanoribbons with improved electrical conductivity for efficient gas sensing. Nat. Commun. 2017, 8 (1), 820. 10.1038/s41467-017-00692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Zhi L.; Tsao N.; Tomović Ž.; Li J.; Müllen K. Transparent Carbon Films as Electrodes in Organic Solar Cells. Angew. Chem., Int. Ed. 2008, 47 (16), 2990–2992. 10.1002/anie.200704909. [DOI] [PubMed] [Google Scholar]
- Frackowiak E.; Béguin F. Electrochemical storage of energy in carbon nanotubes and nanostructured carbons. Carbon 2002, 40 (10), 1775–1787. 10.1016/S0008-6223(02)00045-3. [DOI] [Google Scholar]
- Yang Z.; Ren J.; Zhang Z.; Chen X.; Guan G.; Qiu L.; Zhang Y.; Peng H. Recent Advancement of Nanostructured Carbon for Energy Applications. Chem. Rev. 2015, 115 (11), 5159–5223. 10.1021/cr5006217. [DOI] [PubMed] [Google Scholar]
- Cui G.; Hu Y.-S.; Zhi L.; Wu D.; Lieberwirth I.; Maier J.; Müllen K. A One-Step Approach Towards Carbon-Encapsulated Hollow Tin Nanoparticles and Their Application in Lithium Batteries. Small 2007, 3 (12), 2066–2069. 10.1002/smll.200700350. [DOI] [PubMed] [Google Scholar]
- Yang S.; Feng X.; Ivanovici S.; Müllen K. Fabrication of Graphene-Encapsulated Oxide Nanoparticles: Towards High-Performance Anode Materials for Lithium Storage. Angew. Chem., Int. Ed. 2010, 49 (45), 8408–8411. 10.1002/anie.201003485. [DOI] [PubMed] [Google Scholar]
- Yoon S. B.; Chai G. S.; Kang S. K.; Yu J.-S.; Gierszal K. P.; Jaroniec M. Graphitized Pitch-Based Carbons with Ordered Nanopores Synthesized by Using Colloidal Crystals as Templates. J. Am. Chem. Soc. 2005, 127 (12), 4188–4189. 10.1021/ja0423466. [DOI] [PubMed] [Google Scholar]
- Jian K. Q.; Shim H. S.; Schwartzman A.; Crawford G. P.; Hurt R. H. Orthogonal Carbon Nanofibers by Template-Mediated Assembly of Discotic Mesophase Pitch. Adv. Mater. 2003, 15 (2), 164–167. 10.1002/adma.200390036. [DOI] [Google Scholar]
- Tomović Ž.; Watson M. D.; Müllen K. Superphenalene-Based Columnar Liquid Crystals. Angew. Chem., Int. Ed. 2004, 43 (6), 755–758. 10.1002/anie.200352855. [DOI] [PubMed] [Google Scholar]
- Chhowalla M.; Wang H.; Sano N.; Teo K. B. K.; Lee S. B.; Amaratunga G. A. J. Carbon Onions: Carriers of the 217.5 nm Interstellar Absorption Feature. Phys. Rev. Lett. 2003, 90 (15), 155504. 10.1103/PhysRevLett.90.155504. [DOI] [PubMed] [Google Scholar]
- Kim K. S.; Zhao Y.; Jang H.; Lee S. Y.; Kim J. M.; Kim K. S.; Ahn J.-H.; Kim P.; Choi J.-Y.; Hong B. H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457 (7230), 706–710. 10.1038/nature07719. [DOI] [PubMed] [Google Scholar]
- Eda G.; Fanchini G.; Chhowalla M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3 (5), 270–274. 10.1038/nnano.2008.83. [DOI] [PubMed] [Google Scholar]
- Liang Y.; Frisch J.; Zhi L.; Norouzi-Arasi H.; Feng X.; Rabe J. P.; Koch N.; Müllen K. Transparent, highly conductive graphene electrodes from acetylene-assisted thermolysis of graphite oxide sheets and nanographene molecules. Nanotechnology 2009, 20 (43), 434007. 10.1088/0957-4484/20/43/434007. [DOI] [PubMed] [Google Scholar]
- Li X.; Song Q.; Hao L.; Zhi L. Graphenal Polymers for Energy Storage. Small 2014, 10 (11), 2122–2135. 10.1002/smll.201303717. [DOI] [PubMed] [Google Scholar]
- Kuhn P.; Forget A.; Su D.; Thomas A.; Antonietti M. From Microporous Regular Frameworks to Mesoporous Materials with Ultrahigh Surface Area: Dynamic Reorganization of Porous Polymer Networks. J. Am. Chem. Soc. 2008, 130 (40), 13333–13337. 10.1021/ja803708s. [DOI] [PubMed] [Google Scholar]
- Hao L.; Luo B.; Li X.; Jin M.; Fang Y.; Tang Z.; Jia Y.; Liang M.; Thomas A.; Yang J.; Zhi L. Terephthalonitrile-derived nitrogen-rich networks for high performance supercapacitors. Energy Environ. Sci. 2012, 5 (12), 9747–9751. 10.1039/c2ee22814a. [DOI] [Google Scholar]
- Graphene chemistry: theoretical perspectives; Jiang D.-e., Chen Z., Ed.; John Wiley & Sons: Chichester, West Sussex, 2013. [Google Scholar]
- Carbon Nanomaterial Electronics: Devices and Applications; Hazra A., Goswami R., Ed.; Springer: Singapore, 2021. [Google Scholar]
- Shirakawa H.; Louis E. J.; MacDiarmid A. G.; Chiang C. K.; Heeger A. J. Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH). J. Chem. Soc., Chem. Commun. 1977, (16), 578–580. 10.1039/c39770000578. [DOI] [Google Scholar]
- Novoselov K. S.; Fal′ko V. I.; Colombo L.; Gellert P. R.; Schwab M. G.; Kim K. A roadmap for graphene. Nature 2012, 490 (7419), 192–200. 10.1038/nature11458. [DOI] [PubMed] [Google Scholar]
- Geim A. K.; Novoselov K. S. The rise of graphene. Nat. Mater. 2007, 6 (3), 183–191. 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- Schedin F.; Geim A. K.; Morozov S. V.; Hill E. W.; Blake P.; Katsnelson M. I.; Novoselov K. S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6 (9), 652–655. 10.1038/nmat1967. [DOI] [PubMed] [Google Scholar]
- Novoselov K. S.; Geim A. K.; Morozov S. V.; Jiang D.; Zhang Y.; Dubonos S. V.; Grigorieva I. V.; Firsov A. A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306 (5696), 666–669. 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- Chiang C. K.; Fincher C. R.; Park Y. W.; Heeger A. J.; Shirakawa H.; Louis E. J.; Gau S. C.; MacDiarmid A. G. Electrical Conductivity in Doped Polyacetylene. Phys. Rev. Lett. 1977, 39 (17), 1098–1101. 10.1103/PhysRevLett.39.1098. [DOI] [Google Scholar]
- Shi T.-H.; Wang M.-X. Zigzag Hydrocarbon Belts. CCS Chem. 2021, 3 (2), 916–931. 10.31635/ccschem.020.202000287. [DOI] [Google Scholar]
- Li J.; Merino-Díez N.; Carbonell-Sanromà E.; Vilas-Varela M.; de Oteyza D. G.; Peña D.; Corso M.; Pascual J. I. Survival of spin state in magnetic porphyrins contacted by graphene nanoribbons. Sci. Adv. 2018, 4 (2), eaaq0582 10.1126/sciadv.aaq0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Jelezko F.; Plenio M. B.; Weil T. Diamond Quantum Devices in Biology. Angew. Chem., Int. Ed. 2016, 55 (23), 6586–6598. 10.1002/anie.201506556. [DOI] [PubMed] [Google Scholar]
- Schrand A. M.; Hens S. A. C.; Shenderova O. A. Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34 (1–2), 18–74. 10.1080/10408430902831987. [DOI] [Google Scholar]