Abstract
Flavobacteria are a poorly understood and speciated group of commensal bacteria and opportunistic pathogens. The psychrotroph Flavobacterium psychrophilum is the etiological agent of rainbow trout fry syndrome and bacterial cold water disease, septicemic diseases that heavily impact salmonids. Consequently, two verified but geographically diverse isolates were characterized phenotypically and biochemically. A facile typing system was devised which readily discriminated between closely related species and was verified against a pool of recent prospective isolates. F. psychrophilum was found to be enveloped in a loosely attached, strongly antigenic outer layer comprised of a predominant, highly immunogenic, low-molecular-mass carbohydrate antigen as well as several protein antigens. Surface-exposed antigens were visualized by a combination of immunoflourescence microscopy, immunogold transmission, and thin-section electron microscopy and were discriminated by Western blotting using rabbit antisera, by selective extraction with EDTA-polymyxin B agarose beads, and by extrinsic labeling of amines with sulfo–N-hydoxysuccinimide–biotin and glycosyl groups with biotin hydrazide. The predominant ∼16 kDa antigen was identified as low-molecular-mass lipopolysaccharide (LPS), whereas high-molecular-mass LPS containing O antigen was not as prevalent on whole cells but was abundant in culture supernatants. Rainbow trout convalescent antisera recognized both molecular mass classes of LPS as well as a predominant ∼20-kDa protein. This study represents the first description at the molecular level of the surface characteristics and potential vaccine targets of confirmed F. psychrophilum strains.
Certain Flavobacterium spp. are widespread opportunistic bacterial pathogens. The pathogenesis of Flavobacterium spp. is not well understood; in humans, however, they cause neonatal meningitis, catheter-associated bacteremia, and pneumonia and have also been associated with some cases of advanced human immunodeficiency virus disease (26, 37). Flavobacterium meningosepticum, which causes meningitis and pneumonia in humans, is also a known pathogen of birds (42). Flavobacterium spp. are also characterized by an atypical pattern of antimicrobial resistance (37).
Several species of these yellow-pigmented bacteria have been associated with diseased fish, including Flavobacterium columnare, Flavobacterium branchiophilum, Flavobacterium aquatile, Flavobacterium johnsoniae, and Flexibacter maritimus, as well as other unidentified gliding bacteria referred to as Cytophaga-like bacteria (4, 25, 31, 35, 39). Flavobacterium and Cytophaga spp. have also been found to exist naturally as part of salmonid microflora (9, 34). The heterogeneity of the genera Cytophaga, Flexibacter, and Flavobacterium has caused considerable confusion in differentiating these bacteria. Recent DNA-rRNA hybridization studies have shown that the genera Cytophaga and Flexibacter are highly polyphyletic, with most species being only distantly related to their respective type species and more closely related to F. aquatile, the type species of the genus Flavobacterium (6). The genus Flavobacterium was therefore amended to include those Cytophaga and Flexibacter species which show high DNA relatedness to F. aquatile (6).
The differentiation and confident identification of species in this family are important, especially for such economically important pathogens as Flavobacterium psychrophilum. Flavobacterium spp. are slow-growing, fastidious organisms, making their characterization both time-consuming and imprecise due to their weak response in standard nutritional tests. Thus, sensitive and specific methods of identifying F. psychrophilum are needed to enable early and accurate disease diagnosis and to facilitate reliable studies on pathogenesis.
F. psychrophilum (syn. Cytophaga psychrophila, Flexibacter psychrophilus) is the etiological agent of rainbow trout fry syndrome (RTFS) and bacterial cold water disease, septicemic infections which can cause significant early losses in hatchery-reared salmonids, particularly rainbow trout (Oncorhynchus mykiss) in Europe and coho salmon (Oncorhynchus kisutch) in North America. In the past decade, F. psychrophilum has emerged as a causative agent of severe RTFS mortality in Europe and is now known to affect salmonids worldwide (31, 36, 45). The host range appears to have broadened, with several more nonsalmonid fish species being affected (17, 22).
The molecular pathogenesis of F. psychrophilum is not well understood and is primarily limited to its exotoxins and plasmids (reviewed by Dalsgaard [13]). No vaccine is commercially available for RTFS, the development of which is presumably hindered by nutritional fastidiousness and speciation difficulties but also due to the early age of the fish most seriously affected. The greatest losses occur in immature salmonids (23) which are not fully immunocompetent and in which immunity is short lived (18). Infected fish can sometimes be successfully treated with antibiotics, but this treatment is disfavored due to high costs, short-term benefits, and potential for deleterious impact on human health and the environment. Therefore, the development of effective, inexpensive, easily administered vaccines has become an important goal.
This study describes a method to clearly differentiate F. psychrophilum from other closely related bacteria by using both randomly amplified polymorphic DNA (RAPD)-PCR and polyclonal antibodies against F. psychrophilum. Thus, using only accurately typed strains, we have for the first time identified several immunogenic cell surface molecules that may be involved in pathogenesis and that are potential vaccine targets. Careful characterization and elucidation of the molecular pathogenesis of F. psychrophilum are necessary preludes to rational vaccine development.
MATERIALS AND METHODS
Bacterial strains and isolation.
F. psychrophilum strains UP96/017 and 259–93 were originally isolated in Weymouth, England, and in Idaho, respectively, from moribund fish displaying typical symptoms of RTFS. Juvenile rainbow trout were experimentally infected by intraperitoneal (i.p.) injection (0.1 ml) of F. psychrophilum UP96/017 or 259–93 grown on modified Anacker and Ordal agar (MAOA) and resuspended in sterile phosphate-buffered saline (PBS), pH 7.4. F. psychrophilum was subsequently reisolated from kidney and spleen tissue of the infected fish postmortem. Tables 1 and 2 list all the strains used in this study.
TABLE 1.
F. psychrophilum strains and related type strains used in this study
| Straina | Fish | Origin |
|---|---|---|
| F. psychrophilum 259–93b | Rainbow trout | Idaho |
| F. psychrophilum UP96/017c | Rainbow trout | Dorset, England |
| F. psychrophilum 911209–2d | Rainbow trout | Denmark |
| F. psychrophilum ATCC 49418 | Coho salmon | Washington state |
| F. columnare ATCC 43622 | Salmonid fish | |
| Flexibacter maritimus ATCC 43397 | Black Sea brean | Japan |
ATCC, American Tissue Culture Collection, Manassas, Va.
Provided by S. LaPatra, Clear Springs Foods, Buhl, Idaho.
Provided by D. J. Alderman, CEFAS, Weymouth, United Kingdom.
Provided by E. Lorenzen, Statens Veterinaere Serumlaboratorium, Arhus, Denmark.
TABLE 2.
Yellow-pigmented bacterial isolates from diseased, juvenile salmonids located at freshwater hatcheries in British Columbia, Canada, from January to April 1997a
| Isolate | Fish | Tissue sampled |
|---|---|---|
| PBS9701 | Chinook salmon | Kidney |
| PBS9702 | Coho salmon | External lesion |
| PBS9703 | Coho salmon | Behind eye |
| PBS9704 | Coho salmon | Gills |
| PBS9705 | Coho salmon | Gills |
| PBS9706 | Cutthroat trout | Kidney |
| PBS9707 | Cutthroat trout | Kidney |
| PBS9708 | Coho salmon | Kidney |
| PBS9709 | Coho salmon | Kidney |
| PBS9710 | Coho salmon | Kidney |
| PBS9711 | Chinook salmon | Kidney |
| PBS9712 | Chinook salmon | Kidney |
| PBS9713 | Coho salmon | External lesion |
| PBS9714 | Coho salmon | Behind eye |
| PBS9716 | Coho salmon | Gills |
| PBS9716 | Coho salmon | Gills |
Strains kindly donated by D. Keiser, Pacific Biological Station, Nanaimo, British Columbia, Canada.
Media and growth conditions.
F. psychrophilum was routinely grown at 15°C on Anacker and Ordal medium (2), as modified by Bernardet (5). Broth medium (modified Anacker and Ordal broth [MAOB]) consisting of 0.5% tryptone (Gibco BRL, Rockville, Md.), 0.05% yeast extract (Gibco BRL), 0.02% sodium acetate, and 0.02% beef extract was solidified with 1.5% agar (Gibco BRL) when required (MAOA). Fish blood (3%) was added to MAOA to investigate its effect on growth. Cells were also grown in TYES broth (0.4% tryptone, 0.04% yeast extract, 0.05% CaCl2, and 0.05% MgSO4). Large-scale growth was achieved in a 35-liter Chemap fermenter (Chemap AG, Volketswil, Switzerland) containing 28 liters of MAT broth (TYES supplemented with 1% maltose and 0.02% sodium acetate) with stirring at 300 rpm and aeration at 20 liters min−1. Flexibacter maritimus was grown on MAT medium using Instant Ocean (Aquarium Systems, Mentor, Ohio) in place of deionized water.
Biochemical and physiological characterization of isolates.
The presence of characteristic flexirubin-type pigments in the bacterial cell wall was tested by the method of Reichenbach et al. (33): 20% KOH was added directly onto the MAOA culture; a positive reaction was indicated by a change in color from orange to brown. To test whether F. psychrophilum could absorb the aromatic sulfonated diazo dye, the heme analogue Congo red, cells were grown on MAOA supplemented with 30 μg of Congo red per ml. The presence of oxidase was tested with 1% tetramethyl-p-phenylenediamine dihydrochloride (20). The presence of catalase was tested with 3% H2O2 on a glass slide as previously described (38).
Antibody preparation.
A single New Zealand White rabbit was injected subcutaneously (s.c.) and intramuscularly (i.m.) with 200 μg of protein from formalin-fixed (5% formalin, overnight at 4°C) F. psychrophilum UP96/017 whole cells, grown on MAOA, and emulsified in Freund's complete adjuvant. Preimmune serum was collected prior to the primary immunization. The rabbit was boosted with a further 200 μg of fixed cells emulsified in Freund's incomplete adjuvant 3 weeks after the first injection. Immune serum was collected 2 weeks after the booster injection. The titer of the immune serum was determined by an enzyme-linked immunosorbent assay as previously described (12).
Rainbow trout convalescent serum was obtained from rainbow trout that survived challenge with F. psychrophilum 259–93. Rainbow trout (15 g) were injected with live F. psychrophilum (100 μl, 2.4 × 104 cells) from a 24-h MAT broth culture. Pooled rainbow trout serum from 45 surviving fish was obtained 5 weeks postchallenge. Serum was also obtained from unexposed fish injected with saline for use as a negative control.
Immunofluorescence microscopy.
F. psychrophilum cells were incubated in rabbit anti-F. psychrophilum primary antibody (1 h at room temperature [RT]) diluted 1/50 in PBS–5% fetal calf serum (FCS). Cells were washed twice with PBS–5% FCS, resuspended in goat anti-rabbit immunoglobulin G (IgG) conjugated with fluorescein isothiocyanate (FITC) (Caltag Labs, San Francisco, Calif.) diluted 1/40 in PBS–5% FCS and incubated (1 h, RT) in the dark. Cells were then resuspended in PBS and observed by fluorescent microscopy using barrier filters for FITC.
Electrophoresis.
Protein analyses of whole-cell lysates were carried out by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli (21), as modified by Ames (1). Samples were resolved in 12% polyacrylamide separating gels poured with 5% stacking gels. Molecular sizes were estimated according to the apparent molecular sizes of prestained protein markers.
Western blot analysis.
Bacterial cell proteins separated by SDS-PAGE were transferred onto nitrocellulose membranes by electroblotting at 50 mA/gel for 1 h in a semidry transblot apparatus (LKB Multiphor II; Pharmacia) as described by Towbin et al. (40). The membrane-immobilized immunogenic proteins were detected using either rabbit or rainbow trout serum raised against F. psychrophilum. The primary rabbit antibody (1/1,000 dilution) was detected using goat anti-rabbit IgG (1/4,000 dilution) conjugated to alkaline phosphatase (Caltag Labs) as outlined previously (12). Primary fish antibody (1/40 dilution) was incubated with the membrane overnight at 4°C and detected using a monoclonal antibody raised against purified rainbow trout Ig (1/100 dilution) (kindly donated by R. Beecroft, Immuno-Precise Antibodies Ltd., Victoria, British Columbia, Canada) followed by goat anti-mouse IgG1 conjugated to alkaline phosphatase (1/2,000 dilution) (Caltag Labs). Negative controls were carried out using preimmune serum as the primary antibody. The immunoreactive bands were visualized using 5-bromo-4-chloro-3-indolylphosphate (BCIP) and 4-Nitro Blue Tetrazolium chloride (Sigma, St. Louis, Mo.) as previously described (29).
Proteinase K treatment.
Digestion of material with proteinase K was carried out at a final concentration of 1 mg of proteinase K per ml at 65°C overnight.
Small-scale extraction of surface polysaccharide.
Polysaccharide samples suitable for SDS-PAGE were extracted using a modification of the method outlined by Valverde et al. (41) using EDTA-triethylamine (TEA)-polymyxin B (ETP). Approximately 20 mg of wet cells was gently resuspended in 50 μl of 100 mM EDTA (titrated with TEA to pH 7.0), incubated at RT for 15 min, and centrifuged (2 min at 10,000 × g). Fifty microliters of a 10% polymyxin B resin (Sigma) suspension in distilled H2O (dH2O) was added to the supernatant, incubated for 15 min at RT, and centrifuged (2 min at 10,000 × g). The pellet was resuspended in 100 μl of wash solution (100 mM KH2PO4–150 mM NaCl, pH 7), centrifuged once more (2 min at 10,000 × g), and finally resuspended in 50 μl of Laemmli sample buffer (21). Culture supernatant was collected from a 2-ml MAT broth culture (15°C, 6 days, 150 rpm) after centrifugation of 1 ml (1 min at 14,000 × g). Samples were boiled in Laemmli sample buffer and separated by SDS-PAGE.
Isolation of LPS.
Lipopolysaccharide (LPS) was isolated from 250 g (wet weight) of F. psychrophilum cells by the aqueous phenol extraction procedure (19).
Biotinylation of cell surface proteins.
Whole cells of F. psychrophilum were surface labeled using the extrinsic labeling reagent sulfo–N-hydroxysuccinimide (NHS)–biotin (Pierce, Rockford, Ill.). Cells were harvested from TYES broth, washed, and resuspended in PBS to 10 mg/ml (wet weight). For cell extract controls, cells were lysed by sonication at 40 W for four 15-s intervals. Sulfo-NHS-biotin (4 μl of a 1 mg/ml concentration in dimethyl sulfoxide) was added to 100-μl aliquots of the cells and the samples were incubated at RT for 1 min. The reaction was stopped with a 1,000-fold excess of glycine (pH 7.4). SDS-PAGE sample buffer was added and the mixture was boiled for 10 min. After electrophoresis, proteins were immobilized onto nitrocellulose membranes which were subsequently blocked in PBS–1% gelatin (1 h at RT). Biotin was detected by incubating the membranes with streptavidin-biotinylated alkaline phosphatase (0.5 mg of biotin per ml, 1/5,000 dilution) (Caltag Labs) in PBS–1% gelatin (1 h at RT). The biotinylated proteins were detected with 4-Nitro Blue Tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate as outlined above.
Immunogold electron microscopy. (i) Whole cells.
Cells were harvested from a 3-day, 15°C culture in TYES, resuspended in wash solution (10 mM Tris, 150 mM NaCl) and mounted on formvar-coated copper grids by floating the coated copper grids on drops of bacterial suspension for 1 min. Grids were floated on droplets of blocking buffer (Tris-NaCl–1% skim milk, 0.02% NaN3) for 40 min, washed three times, and incubated for 45 min with primary antibody diluted 1/1,000 in blocking buffer and then washed once more. Grids were then incubated for 30 min with 15-nm-gold-labeled protein A (Amersham, Frieburg, Germany) which was diluted 1/50 in blocking buffer. Grids were washed as before and stained for 30 s with 0.1% phosphotungstic acid and viewed with a Hitatchi 7000 transmission electron microscope at an accelerating voltage of 75 kV.
(ii) Thin sections.
Cells were harvested from a 48-h, 15°C culture in TYES, washed once in PBS, and centrifuged (10 s at 3000 × g). Primary fixation (4% paraformaldehyde, 1% glutaraldehyde, 150 mM NaCl, and 0.2 M Millonig's phosphate buffer [pH 7.4] [27]) was carried out for 1 h on ice. Cells were then washed three times in PBS before postfixation (1% osmium tetroxide, 150 mM NaCl, and Millonig's phosphate buffer) for 1 h at 4°C. After being washed once in PBS, cells were sequentially dehydrated in 95% ethanol, absolute ethanol, and then propylene oxide prior to being embedded in an Epon-Araldite epoxyy resin mix. Thin sections were cut using glass knives on an ultramicrotome and mounted on formvar-coated nickel grids. The labeling procedure was as follows: sections were washed on drops of dH2O (10 min), treated with 1% sodium meta-periodate (30 min), washed with dH2O (5 min), blocked with FCS (15 min), and diluted 1/20 with blocking buffer (0.5% bovine serum albumin [BSA] and 0.05% Tween 20 in PBS). The sections were then reacted with primary antibody (rabbit anti-F. psychrophilum polyclonal antiserum or preimmune serum), diluted 1/1,000 in blocking buffer for 1 h, and then washed (3 times for 5 min each) with blocking buffer. Secondary antibody, 5-nm-gold-conjugated goat anti-rabbit IgG (Cedar Lane Labs Ltd., Hornby, Ontario, Canada), diluted 1/50 with blocking buffer, was added and incubated for 1 h. The sections were subsequently washed (3 times for 5 min each) with blocking buffer followed by another wash (2 times for 5 min each) with PBS, postfixed using the primary fixative (15 min), rinsed on dH2O (5 min), stained with 2% aqueous uranyl acetate (30 min), and viewed with a Hitatchi 7000 transmission electron microscope at an accelerating voltage of 75 kV.
(iii) Culture supernatant.
Culture supernatants were collected and concentrated as indicated above. Formvar-coated copper grids were floated on concentrated culture supernatant (2 min) prior to labeling with immunogold as described above for whole cells.
KDO determination.
Dry F. psychrophilum cells were resuspended in 0.02 N H2SO4 and boiled for 20 min. The suspension was centrifuged (2 min at 13,000 × g) and the supernatant was tested for the presence of 2-keto-3-deoxyoctonate (KDO). Alternatively, cells were resuspended in 3% SDS (1 mg of proteinase K per ml was added when necessary), digested overnight at 65°C, and centrifuged (5 min at 13,000 × g). KDO was determined by the method of Weissbach and Hurwitz (44), as modified by Osborn (30) and Vincent and Cameron (43). Absorbance was scanned between 500 and 600 nm and was read at 548 nm (Ultrospec 3000; Pharmacia Biotech). Under these conditions, 1 mM KDO (Sigma) was calculated to give an absorbance value of 21.
Biotin labeling of glycosyl groups.
Biotinylation of 1,2 glycols and/or hydroxy carbonyl glycosides was carried out using the method of Doig et al. (14). F. psychrophilum whole cells were boiled in Laemmli sample buffer, separated by SDS-PAGE, and transferred onto a nitrocellulose membrane as described above. The membrane was washed in PBS for 10 min at RT. The blot was oxidized with 10 mM sodium meta-periodate in 50 mM sodium acetate buffer (pH 5.5) for 30 min at RT and then washed three times in PBS. Oxidized carbohydrate was reacted with 5 mM biotin hydrazide (Sigma) in 50 mM sodium acetate buffer (pH 5.5) for 1 h at RT. The membrane was washed three times with Tris-buffered saline (TBS), blocked with 1% BSA in TBS for 30 min, and washed a further three times in TBS. The blot was incubated for 1 h at RT with streptavidin-conjugated alkaline phosphatase diluted in TBS. After being washed three times in TBS the blot was developed as described above.
Extrinsic labeling of whole cells was done via a modification of the method of Aragon et al. (3). F. psychrophilum cells were suspended in 50 mM sodium acetate buffer (pH 5.5)–15 mM sodium meta-periodate (RT, 30 min). The oxidation reaction was stopped with 0.5 volumes of 80 mM sodium sulfite (RT, 15 min). Labeling was initiated by adding 0.5 volumes of 15 mM biotin hydrazide (Sigma) in 100 mM sodium acetate buffer, pH 5.5 (RT, 1 h). Cells were washed twice in PBS, separated by SDS-PAGE, and transferred onto a nitrocellulose membrane as described above. The presence of biotin was detected with streptavidin-conjugated alkaline phosphatase as described above.
Isolation of DNA.
Genomic DNA was isolated from F. psychrophilum using Chelex 100 resin (Bio-Rad, Hercules, Calif.) (D. Machander, Microtek International Ltd., Saanichton, British Columbia, Canada, personal communication). Colonies were picked and resuspended in 100 μl of Chelex bead suspension (5% Chelex in dH2O), boiled for 15 min, and centrifuged (30 s at 14,000 × g). DNA in 50 μl of supernatant was spectrophotometrically quantified (A260) and adjusted to 50 ng/μl.
RAPD-PCR.
Each 50-μl reaction mixture contained the following: 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM deoxyribonucleoside, 20 pmol of primer, 1.65 U of Taq DNA polymerase, and 50 ng of template DNA. The primers used were the following: 5′-TTCGCAGATCCCAACAACAA-3′ and 5′-CTAAGTACCGCCCCGATC-3′. Amplification was performed as follows: 1 cycle at 94°C for 3 min, 2 cycles at 94°C for 1 min, 47°C for 1 min and 72°C for 1 min; 41 cycles at 94°C for 1 min, 55°C for 1 min, and 72°C for 3 min, and 1 cycle at 72°C for 10 min. Thermocycling was performed in a PTC-100 programmable thermal cycler (MJ Research Inc., Waltham, Mass.). RAPD-PCR products were separated on a 1.5% agarose gel at 70 V for 45 min, stained with ethidium bromide, and photographed under UV light.
RESULTS
Strain characterization.
Field isolates of F. psychrophilum obtained from diseased and moribund fish were first characterized by a variety of standard tests and chosen as reference strains based on their detailed phenotypic and biochemical characteristics. The F. psychrophilum strains grew at temperatures from 4 to 25°C but more routinely grew at 15°C on MAOA containing 0.2 to 0.5% NaCl, and growth was inhibited at 1.0% NaCl. Colonies were yellow and spreading depending on the culture conditions. Growth on MAOA was enhanced by the inclusion of 3% fish or horse blood but was strongly inhibited in the presence of 30 μg of Congo red per ml. Additional tests confirmed that the reference strains were gram-negative, oxidase-negative, catalase-positive rods and possessed flexirubin-type pigments. Casein and gelatin were readily hydrolyzed, whereas agar and cellulose were not. Based on these criteria, two field isolates of F. psychrophilum, one from the United Kingdom (UP96/017) and one from the United States (259–93), were chosen as reference strains.
Strain typing by RAPD-PCR.
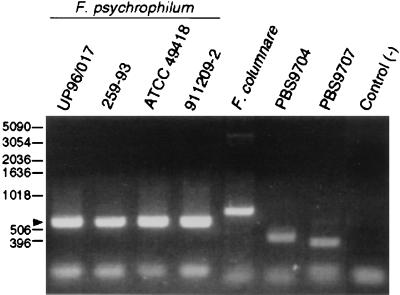
The aim of the RAPD-PCR was to find a method whereby F. psychrophilum isolates could be readily distinguished from related organisms isolated from diseased fish. RAPD-PCR “fingerprints” were generated for four strains of F. psychrophilum, including the type strain ATCC 49418 (Table 1). Initially, three sets of primers were used to generate the fingerprints. Each primer set produced identical RAPD-PCR profiles for both reference strains. The primer set chosen for further studies produced a single 600-bp band for all F. psychrophilum strains (Fig. 1, arrow), which was important given the diverse geographical origins of these strains. RAPD-PCR was performed using DNA from two related fish pathogens, Flavobacterium columnare and Flexibacter maritimus, as well as from 16 recent isolates of yellow-pigmented bacteria from diseased coho and chinook salmon (Table 2). The fish had presented symptoms of bacterial cold water disease and originated from four well-separated local hatcheries. The RAPD-PCR profiles of these 16 putative F. psychrophilum strains were compared with profiles of the reference species of F. psychrophilum strains from Idaho and Weymouth, United Kingdom. Six of the putative F. psychrophilum strains (PBS9701, PBS9702, PBS9703, PBS9708, PBS9709, and PBS9710) had the same RAPD-PCR profiles as the reference strains and were all characteristically unable to grow on 1% tryptone agar containing Congo red (30 μg/ml). Figure 1 shows examples of the characteristic RAPD-PCR profile identifying F. psychrophilum as well as typical non-F. psychrophilum reactions. Based on the RAPD-PCR fingerprints, six of the isolates were identified as F. psychrophilum, a finding confirmed by the previous phenotypic characteristics.
FIG. 1.
RAPD-PCR analysis of four geographically diverse F. psychrophilum strains, F. columnare, and two yellow-pigmented bacteria isolated from diseased salmonids. The PCR products were separated by electrophoresis through 1.5% agarose and stained with ethidium bromide. The negative control reaction mixture contained no template DNA. Molecular size standards (bp) are indicated on the left. The arrow indicates a 600-bp band obtained for all F. psychrophilum strains.
Immunochemical strain typing.
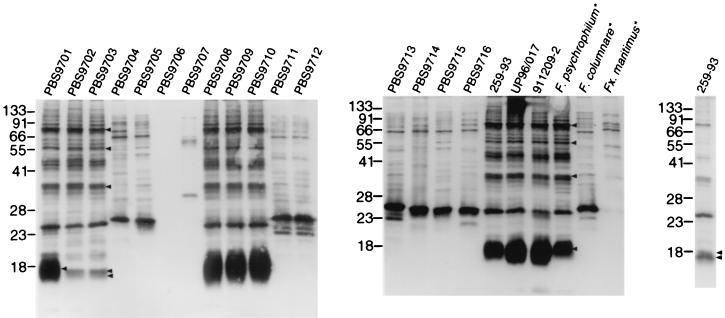
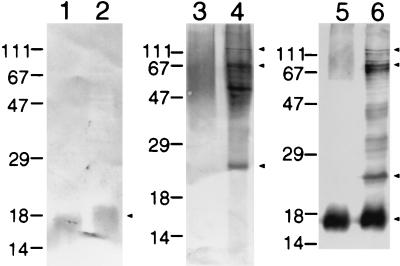
To further aid in the differentiation between F. psychrophilum isolates and related organisms recovered from diseased fish, Western blot analysis of four F. psychrophilum strains (Table 1) was carried out using polyclonal rabbit serum raised against F. psychrophilum UP96/017. The four geographically diverse F. psychrophilum strains had almost identical Western blot profiles (Fig. 2). To determine whether the 16 hatchery isolates possessed cellular antigens similar to or cross-reactive with those of the F. psychrophilum strains, whole-cell lysates of the 16 strains (Table 2) were prepared containing equal concentrations of cells. All strains, of equal concentrations, were resolved by Western blotting using rabbit polyclonal antiserum raised against F. psychrophilum UP96/017 (Fig. 2). When compared to the F. psychrophilum strains, the isolates were subsequently grouped based on their characteristic Western blot patterns. Six of the 16 strains possessed characteristically similar patterns with particularly common prominent bands from ∼16 kDa to ∼80 kDa, which were also present in F. psychrophilum strains but were not common to the other 10 strains (Fig. 2, arrows). The six strains were PBS9701, PBS9702, PBS9703, PBS9708, PBS9709, and PBS9710. The same six strains were also identified as F. psychrophilum following RAPD-PCR analysis. Although the same amount of material was loaded in each lane, some variation in band intensity was seen, particularly in the ∼16-kDa band. A fivefold dilution of F. psychrophilum strain 259–93 showed a doublet band at ∼16 kDa, as seen for strains PBS9702 and PBS9703. The Western blot profiles of F. columnare and Flexibacter maritimus showed considerable cross-reactivity with the anti-F. psychrophilum serum. No bands were observed when F. psychrophilum was reacted with preimmune serum (data not shown).
FIG. 2.
Western blot analysis of various yellow-pigmented bacteria isolated from diseased salmonids (PBS9701 to PBS9716), F. psychrophilum strains, and type strains of related bacterial fish pathogens. All strains analyzed were grown in MAT broth. Whole-cell lysates were separated by SDS-PAGE, blotted onto nitrocellulose, and reacted with anti-F. psychrophilum rabbit serum followed by immunochemical staining. All strains were reacted with rabbit preimmune serum as a control, which resulted in no bands (data not shown). The molecular mass standards (kDa) are indicated at the left of each blot. Arrows indicate antigens common to F. psychrophilum and field isolates. Asterisks indicate ATCC type strains. F., Flavobacterium; Fx., Flexibacter.
The grouping of these strains was also supported by their similar growth phenotypes: incubation time for colony formation, colonial morphologies, and the inability to grow in the presence of Congo red. These criteria could be consistently and reproducibly used for the presumptive identification of F. psychrophilum, but when combined with RAPD-PCR and immunochemical tests, they provide a reliable identification strategy.
Surface antigens of F. psychrophilum.
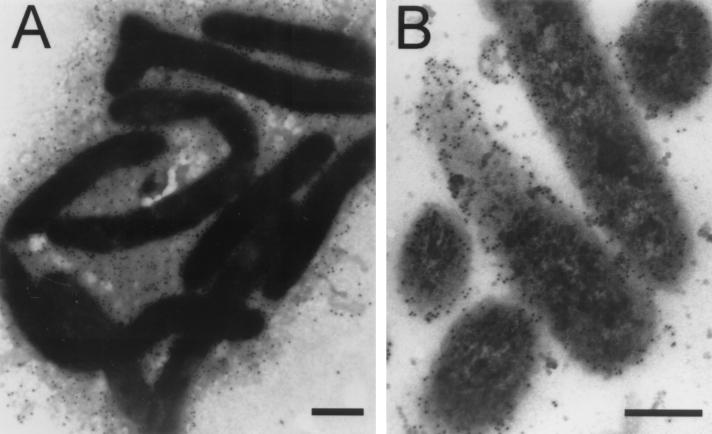
Immunoflourescence light microscopy using high-titer (∼106) rabbit anti-F. psychrophilum serum resulted in exceptionally bright and uniform surface labeling of F. psychrophilum strains UP96/017 and 259–93 (data not shown). Immunogold transmission electron microscopy showed rabbit anti-F. psychrophilum antibodies to be predominantly localized at the cell surface. TYES-grown F. psychrophilum 259–93 was shown to possess a prodigious outer layer surrounding these cells, heavily labeled with immunogold particles (Fig. 3A). Similar immunochemical labeling of thin sections confirmed that the gold particles were primarily localized at or near the bacterial surface (Fig. 3B).
FIG. 3.
Immunogold labeling of F. psychrophilum strain 259–93. (A) Cells grown in TYES and labeled directly with protein A gold (15-nm particles) after preincubation with rabbit anti-F. psychrophilum serum. Magnification, ×20,000 (bar = 0.5 μm). (B) Thin sections were labeled with goat anti-rabbit IgG conjugated to 5-nm gold particles after preincubation with rabbit anti-F. psychrophilum serum. Magnification, ×80,000 (bar = 0.2 μm).
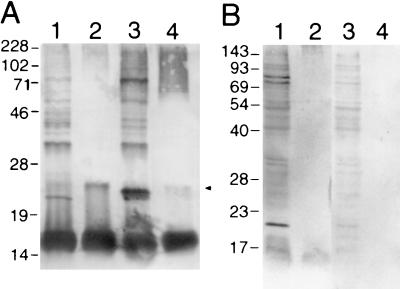
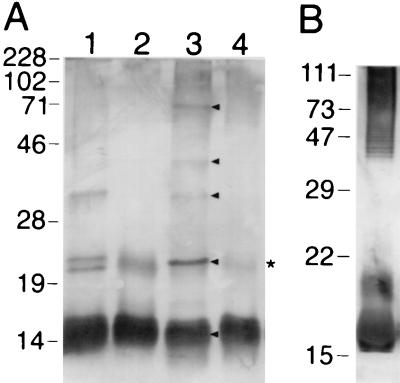
To discriminate the antigens of F. psychrophilum further, Western blotting was carried out using rabbit anti-F. psychrophilum serum. A major antigen with an apparent molecular mass of ∼16 kDa (Fig. 4A, lanes 1 and 3) as well as other less immunoreactive bands with apparent molecular masses of approximately 22, 24, 35, 47, 60, 74, and 100 kDa were readily identified. Lysed whole cells were then extensively digested with proteinase K to reveal those remaining antigens principally comprised of carbohydrate; two immunoreactive bands were clearly seen, a dominant one at ∼16 kDa and a weaker one at ∼25 kDa (Fig. 4A, lanes 2 and 4, arrow). High-molecular-mass, protein-free material (∼70 kDa to ∼200 kDa) seen in lane 4 was apparent as a fine banding ladder, indicative of repeating units, presumably of high-molecular-mass LPS O antigen. Rabbit preimmune serum used as a negative control was not reactive (data not shown).
FIG. 4.
Western blot analysis of F. psychrophilum 259–93. (A) F. psychrophilum was grown in MAOB (lanes 1 and 2) or TYES (lanes 3 and 4). In lanes 1 and 3 are whole-cell lysates, and in lanes 2 and 4 are proteinase K digests of intact cells. The samples were separated by SDS–12% PAGE and reacted with rabbit anti-F. psychrophilum serum. Whole cells did not react with rabbit preimmune sera. (B) F. psychrophilum was grown in TYES and reacted with pooled rainbow trout convalescent anti-F. psychrophilum sera (lanes 1 and 2) and naïve rainbow trout sera (lanes 3 and 4). In lanes 1 and 3 are whole-cell lysates, and in lanes 2 and 4 are proteinase K digests of intact cells. Molecular mass markers (kDa) are indicated on the left of each blot. The arrow indicates a proteinase K-resistant antigen.
The gel profile of immunoreactive species detected in either whole-cell lysates or proteinase K-digested samples could be altered somewhat depending on which growth medium was used. Western blots of TYES-grown cells had an extra band at ∼24 kDa which reacted strongly with rabbit anti-F. psychrophilum serum but was not present in MAOB-grown cells. Also, the two different media gave rise to strikingly different growth characteristics of F. psychrophilum: when shaken gently, TYES-grown cells formed large multicellular orange spheres, whereas cells grown in MAOB grew as a uniform suspension.
To determine which antigens promoted a humoral response in the salmonid host, Western blottings of F. psychrophilum cell lysates were carried out using convalescent rainbow trout anti-F. psychrophilum serum (Fig. 4B). Major immunoreactive proteins were seen with apparent molecular masses of ∼20, ∼75, and ∼83 kDa. An immunoreactive, proteinase K-resistant band was seen with an apparent molecular mass of ∼17 kDa. The negative control carried out with sera from healthy fish showed numerous but faint protein bands. These results suggest that the major proteinase K-resistant antigen recognized by rabbit anti-F. psychrophilum sera also reacts with convalescent rainbow trout anti-F. psychrophilum sera (summarized in Table 3).
TABLE 3.
Reaction of anti-F. psychrophilum sera with MAOB- and TYES-grown F. psychrophilum
| Antigena | Rabbit antiserum
|
Fish antiserum
|
|
|---|---|---|---|
| MAOB | TYES | TYES | |
| L-LPS (16 kDa) | + | + | + |
| H-LPS (∼100 kDa) | + | + | + |
| 20 kDa | + | + | + |
| ∼24 kDa | − | + | − |
| ∼22 kDa | + | + | − |
L-LPS, low-molecular-mass LPS; H-LPS, high-molecular-mass LPS.
Biotinylation of cell surface proteins.
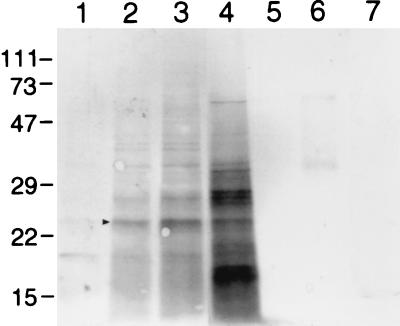
Cell surface-exposed proteins of F. psychrophilum, accessible to the anionic extrinsic probe sulfo-NHS-biotin, were separated by SDS-PAGE, labeled, and detected enzymatically after reaction with streptavidin-alkaline phosphatase (Fig. 5). As a control, sonicated cells were also labeled to show the differences between intact and disrupted cells, with the extra material labeled in the sonicated sample representing internal proteins or those surface proteins which were not accessible to the probe in intact cells. Since labeling with biotin over extended periods can result in some internalization of the probe, a set of timed labeling reactions was carried out. Direct comparison of the 1-min labeling reactions of intact and disrupted cells (Fig. 5, lanes 3 and 4, respectively) shows increased labeling of disrupted cells, indicating that the 1-min labeling reaction was enough to permit intracellular access of the probe. Labeling for 5 min resulted in a profile more similar to that of sonicated cells (data not shown); consequently, cells labeled for 1 min were chosen to more closely represent accessible surface proteins of F. psychrophilum. The major protein accessible to this probe had an apparent molecular mass of 24 kDa (Fig. 5, arrow). Other less prominent bands appeared at ∼27 and 33 kDa. No bands were detected following proteinase K digestion, thus confirming that the extrinsically labeled bands were indeed proteinaceous.
FIG. 5.
Biotin-labeled surface proteins of F. psychrophilum. Whole-cell lysates of F. psychrophilum grown in TYES. Cells were reacted with sulfo-NHS-biotin and separated by SDS–12% PAGE and proteins were detected by streptavidin-conjugated alkaline phosphatase. Lanes 1 through 3 show labeling at 0 s, 30 s, and 1 min, respectively. Lane 4 shows sonicated cells labeled for 1 min. Negative controls were carried out without cells (lane 5), without reagent (lane 6), and with proteinase K-treated cells (lane 7). The reagent control showed two faint bands with apparent molecular masses of ∼35 and ∼65 kDa. No labeling was apparent in the cell control. Proteinase K-treated cells showed a faint band at ∼15 kDa. Molecular mass markers (kDa) are indicated on the left. The arrow indicates the major protein accessible to this probe.
Extraction of the immunoreactive outer layer of F. psychrophilum and determination of LPS.
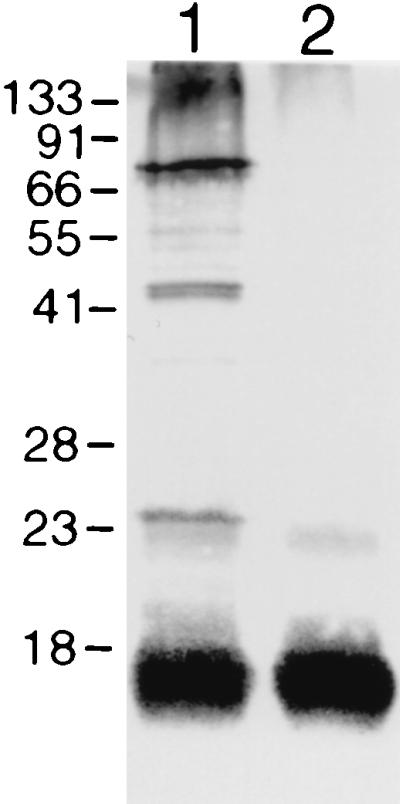
In order to selectively isolate some cell surface antigens, a nonlytic LPS extraction method was employed using polymyxin B (41). Western blot analysis of polymyxin B-bound polysaccharides, first detached by EDTA from cells grown in MAOB or TYES, revealed antigenic bands with apparent molecular masses of approximately 35, 40, and 70 kDa, with major bands at ∼16 and 23 to 25 kDa (Fig. 6A, lanes 1 and 3, arrows). As seen in Fig. 4, the use of different growth media resulted in somewhat altered antigenic profiles as seen by Western blotting of the surface material. In Fig. 6, lane 3, an extra band is seen at ∼40 kDa, and only one prominent band is seen between 23 and 25 kDa, whereas lane 1 shows two bands. However, after digestion with proteinase K, only the major band at ∼16 kDa and a fainter band at ∼22 kDa (Fig. 6A, asterisk) remained visible on Western blots (Fig. 6A, lanes 2 and 4). The effect of different growth media on the EDTA-extractable, polymyxin B-bound surface antigens of F. psychrophilum is shown in Fig. 6A, lanes 1 (MAOB) and 3 (TYES). The main ∼16-kDa, polymyxin B-extractable component that reacted with anti-F. psychrophilum serum was entirely resistant to proteinase K digestion and was equally present in cells grown on either media. An extra protein band (∼22 kDa) was seen in MAOB cells (Fig. 6A, lane 1) and a higher molecular mass (∼70 kDa) protein band was seen in TYES cells (Fig. 6A, lane 3), presumably representing anionic proteins binding to the polycationic beads.
FIG. 6.
(A) Western blot analysis of surface material extracted from F. psychrophilum 259–93. Cell surface material was derived from cells grown in MAOB (lanes 1 and 2) and TYES (lanes 3 and 4) by incubation with EDTA-TEA to dissociate LPS and by adsorption to polymyxin B resin followed by digestion with proteinase K (lanes 2 and 4). The extracts were separated by SDS–12% PAGE and reacted with rabbit anti-F. psychrophilum serum. Molecular mass markers (kDa) are indicated on the left. (B) Western blot of KDO-positive material from proteinase K-digested cells. Molecular mass markers (kDa) are indicated on the left. The arrow indicates the major antigens. The asterisk indicates proteinase K-resistant antigen.
To determine whether the surface carbohydrate of F. psychrophilum contained LPS, an assay for the typical LPS component, KDO, was employed on whole and proteinase K-treated TYES-grown cells. An equally positive reaction was obtained in both cases. The material recovered in the supernatant of proteinase K-treated cells was subjected to SDS-PAGE, transferred onto nitrocellulose, and visualized by Western blotting with rabbit anti-F. psychrophilum serum (Fig. 6B). The antigenic high-molecular-mass banding pattern and low-molecular-mass band are clearly indicative of LPS-containing O chain and core region lipooligosaccharide, respectively. The thiobarbituric acid assay for KDO was performed on the same material. The resulting solution had a maximum absorbance at 549 nm, which is in the expected range of 545 to 550 nm for KDO under these conditions (16). The amount of KDO obtained from dried cells was calculated to be in the range of 0.57 to 1.01% of dry weight.
LPS isolated by aqueous phenol extraction resulted in a yield of ∼10% of wet weight. The LPS was subjected to SDS-PAGE and visualized by Western blotting with both rabbit and convalescent rainbow trout anti-F. psychrophilum sera. Both high- and low-molecular-mass LPS were recognized by both sera (data not shown), as summarized in Table 3.
The culture supernatant was also found to contain antigens of similar molecular mass to those discovered by ETP LPS extraction (Fig. 7). Once again, a major antigenic band was seen at ∼16 kDa in both samples. The supernatant showed several protein bands at ∼24 kDa and between 45 and 75 kDa (Fig. 7, lane 1) which were not present in the proteinase K-treated sample (Fig. 7, lane 2). A faint band at ∼22 kDa was present both before and after proteinase K treatment. Western blot analysis of the proteinase K-treated culture supernatant revealed a binding pattern consistent with an LPS-like profile, comprised of a major band at ∼16 kDa, and high-molecular-mass material (Fig. 7, lane 2), characteristic of LPS O antigen oligomers.
FIG. 7.
Western blot characterization of the F. psychrophilum 259–93 MAT-grown culture supernatant, before (lane 1) and after (lane 2) proteinase K treatment. Molecular mass markers (kDa) are indicated on the left.
Immunogold analysis of culture supernatant revealed large aggregates of amorphous material which were labeled extensively with 15-nm protein A gold as well as smaller pieces (∼10 to 20 nm) of labeled antigenic material (data not shown). No antibody labeling was evident in the culture supernatant samples incubated with preimmune serum. Following proteinase K digestion of the concentrated supernatant, large aggregates were absent but small fragments that remained were associated with single gold particles (data not shown).
Biotinylation of glycosyl groups.
The presence of carbohydrate material of F. psychrophilum was investigated further by labeling periodate-treated cells with biotin hydrazide. Labeling was performed on fresh intact cells (extrinsic labeling) and for comparison on cellular material first immobilized on a nitrocellulose membrane prior to labeling (nonextrinsic). The methods of labeling employed here gave strikingly different results. Whole cells which were labeled extrinsically prior to SDS-PAGE showed that the majority of labeling occurred in high-molecular-mass material (Fig. 8, lanes 3 and 4). Major biotin hydrazide-labeled protein bands (those sensitive to proteinase digestion) had apparent molecular masses of 24, 53, and 72 kDa. Following proteinase K digestion, the molecular mass of the remaining, poorly resolving material was >45 kDa, likely high-molecular-mass LPS. However, cells which were labeled following transfer onto nitrocellulose showed labeling only in low-molecular-mass material in the range of ∼16 to 18 kDa (Fig. 8, lanes 1 and 2). Inexplicably, this band ran marginally lower following proteinase K digestion (Fig. 8, lanes 1, 2, 5, and 6). Western blot analysis of the biotin hydrazide-labeled material revealed that several of the prominent bands comigrate with major antigens, for example, at ∼16, 24, and ∼73 kDa.
FIG. 8.
Biotin hydrazide labeling of periodate-oxidized F. psychrophilum 259–93. Biotin hydrazide labeling of the transblot (intrinsic labeling) of SDS-PAGE of F. psychrophilum cells following proteinase K digestion (lane 1) and labeling of undigested cells (lane 2). Transblot of F. psychrophilum cells extrinsically labeled with biotin hydrazide following proteinase K digestion (lane 3) and undigested cells (lane 4). Lanes 5 and 6 show Western blots of the proteinase K-digested and undigested cells, respectively. Molecular mass markers (kDa) are indicated on the left. The arrows indicate the biotin hydrazide-labeled bands (lanes 1 to 4) which comigrated with major antigens (lanes 5 and 6).
DISCUSSION
The confusion over the taxonomy of Flavobacterium spp. mandated the development of reliable speciation tools. In this study, RAPD-PCR fingerprinting, Western blotting, and specific growth characteristics provided a means of readily distinguishing F. psychrophilum from numerous other closely related bacteria found in diseased salmonid fish. Recently, other techniques were described which also differentiate F. psychrophilum from related bacteria (10, 46). The various Western blot profiles of the different Flavobacterium species studied here showed that they could also be differentiated by using anti-F. psychrophilum polyclonal antiserum. This also highlights the fairly close immunological similarities between these species, given that they all reacted strongly with the antiserum. The combination of these techniques provides a facile and foolproof method of readily identifying F. psychrophilum. The general characteristics of the two reference strains of F. psychrophilum selected and characterized here are largely in agreement with published phenotypic characteristics of F. psychrophilum strains (7, 22, 24, 36) and differ significantly from other related strains (24).
The striking difference both in growth characteristics and antigenic profile observed when F. psychrophilum was grown in different media was likely due to the presence in TYES of MgSO4 and CaCl2, which are absent in MAOB. Conceivably, divalent cations facilitate aggregation by bridging acidic polysaccharides or proteins. The tendency of F. psychrophilum cells to aggregate in TYES broth may also be a result of increased levels of polysaccharide on the surface of these bacteria.
As a prelude to vaccine development, characterization of the surface antigens of F. psychrophilum, using both rabbit and trout antiserum, was carried out. Western blot analysis with rabbit anti-F. psychrophilum serum revealed approximately nine predominant antigens. The humoral response stimulated in fish was considerably weaker than that in the rabbit, with only four antigens eliciting a strong humoral response, as seen by Western blotting, all of which comigrated with antigens recognized by rabbit immune serum.
The nature of these antigens was elucidated by Western blot analysis of proteinase K-treated cells. The results initially suggested that three of the antigenic bands were carbohydrate, since they were apparently not susceptible to proteinase K digestion. However, the ∼22- to 24-kDa doublet band could eventually be digested away by proteinase K following ETP LPS extraction; therefore, these bands were thought to be protein, somewhat resistant to proteinase K.
To identify which antigens seen in the whole-cell preparations could be isolated from the cell surface, a gentle and nonlytic procedure (41) was adopted. Thus, EDTA-polymyxin B-extracted material was analyzed by Western blotting. Polymyxin B is a cationic, antibacterial peptide which avidly binds acidic polysaccharides, especially bacterial LPS, by forming a stable complex with the lipid A moiety (28). The high-titer antiserum generated against F. psychrophilum appears to be biased toward surface molecules based on thin-section immunogold electron microscopy. The highly immunoreactive SDS-PAGE band at ∼16 kDa is likely the predominant component of the unusually thick slime layer seen on the surface of these bacteria, because it is also found in abundance sloughed off in the culture medium and could also be obtained by this nonlytic LPS extraction method. LPS is typically released from gram-negative bacteria when cell surface Ca2+/Mg2+ is chelated. Western blots of culture supernatant (Fig. 7), particularly after proteinase K treatment, show a high-molecular-mass ladder, typical of repeating O antigen units of LPS. Thus, F. psychrophilum exhibits an LPS comprised of both low-molecular-mass oligosaccharide and higher-molecular-mass O antigen-containing polymers, confirmed by the positive reaction for KDO with both whole and proteinase K-treated cells. The amount of KDO present in F. psychrophilum was calculated to be in the range of 0.57 to 1.01%, consistent with that of a variety of gram-negative bacteria (43).
Further compositional analysis of F. psychrophilum LPS revealed an O chain composed of a repeating trisaccharide containing the very unusual sugar N-acylated bacillosamine, which may prove to be unique to F. psychrophilum and may serve as a specific target for diagnostic purposes (M. B. Perry, unpublished data).
The extrinsic primary amine labeling reagent, sulfo-NHS-biotin, revealed several surface proteins accessible to this probe, including a major surface protein with an apparent molecular mass of 24 kDa, which comigrated with a band also visualized by Western blotting. At least two other higher-molecular-mass bands were seen, one of which was immunogenic.
Following the labeling of periodate-treated intact cells with biotin hydrazide, only high-molecular-mass proteinase K-resistant material and a proteinase K-sensitive band at 24 kDa was accessible to the probe. Labeling of cells after SDS-PAGE and immunobilization on nitrocellulose, however, appeared to be far less sensitive and resulted in only a single band at ∼16 kDa. The revealed 24-kDa, apparently glycosylated band comigrated with a protein antigen seen on Western blots.
The ∼16-kDa component of the slime layer of F. psychrophilum has been shown here to be a major carbohydrate (LPS) antigen recognized by both the natural host and by rabbits. Data from Western blottings of proteinase K-treated surface material showed that the slime layer consists of both protein and carbohydrate. In analogy to other bacteria, this slime layer may be a pathogenic factor and may have a role in attachment to substrates, in the resistance to phagocytes, and in ensuring that degradative enzymes are kept in close contact with the substrate. The glycocalyx of Cytophaga sp. has been shown to be closely associated with proteases (11, 15). F. psychrophilum produces extracellular enzymes that degrade components of trout skin, muscle, and cartilage, lyse trout erythrocytes, and have fibrinogenase activity (8, 32). Further characterization of this outer layer may provide answers concerning the pathogenesis of F. psychrophilum, which still remains poorly understood.
This study represents the first molecular characterization of the surface of F. psychrophilum. The identification and characterization of important antigens, both cellular and extracellular, should lead to a greater understanding of the pathogenesis of this bacterium as well as being a prelude to the development of recombinant vaccines against F. psychrophilum, using some of the antigens revealed here as possible targets. The unusual structure of F. psychrophilum LPS is being further investigated with the aim of developing a simpler diagnostic tool for F. psychrophilum and to further elucidate the nature of its pathogenesis.
ACKNOWLEDGMENTS
This work was supported in part by a grant to W. W. Kay from the Canadian Bacterial Diseases Network and by Microtek International Ltd.
We thank R. Beecroft (Immuno-Precise Antibodies Ltd.) for anti-trout monoclonal antiserum, D. Machander (Microtek International Ltd.) for providing the RAPD-PCR protocol, D. Dolhaine (Microtek International Ltd.) for initial strain characterization, H. Croft for technical assistance, and S. K. Collinson, J. C. Thornton, M. A. Kuzyk, and A. P. White for helpful discussions.
REFERENCES
- 1.Ames G F-L. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. J Biol Chem. 1974;249:634–644. [PubMed] [Google Scholar]
- 2.Anacker R L, Ordal E J. Studies on the myxobacterium Chondrococcus columnaris. I. Serological studies. J Bacteriol. 1959;78:25–32. doi: 10.1128/jb.78.1.25-32.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aragon V, Diaz R, Moreno E, Moriyon I. Characterization of Brucella abortus and Brucella melitensis native haptens as outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J Bacteriol. 1996;178:1070–1079. doi: 10.1128/jb.178.4.1070-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin B, Stobie M. Recovery of yellow-pigmented bacteria from dead and moribund fish during outbreaks of rainbow trout, Oncorhynchus mykiss (Walblum), fry syndrome in England. J Fish Dis. 1991;14:677–682. [Google Scholar]
- 5.Bernardet J-F. Deoxyribonucleic acid relatedness and phenotypic characterization of Flexibacter columnaris sp. nov., nom. rev., Flexibacter psychrophilus sp. nov., nom. rev., and Flexibacter maritimus Wakabayashi, Hikida, and Masumura 1986. Int J Syst Bacteriol. 1989;39:346–354. [Google Scholar]
- 6.Bernardet J-F, Segers P, Vancanneyt M, Berthe F, Kersters K, Vandamme P. Cutting a gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (basonym, Cytophaga aquatilis Strohl and Tait 1978) Int J Syst Bacteriol. 1996;46:128–148. [Google Scholar]
- 7.Bernardet J-F, Kerouault B. Phenotypic and genomic studies of “Cytophaga psychrophila” isolated from diseased rainbow trout (Oncorhynchus mykiss) in France. Appl Environ Microbiol. 1989;55:1796–1800. doi: 10.1128/aem.55.7.1796-1800.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertolini J M, Wakabayashi H, Watral V G, Whipple M J, Rohvec J S. Electrophoretic detection of proteases from selected strains of Flexibacter psychrophilus and assessment of their variability. J Aquat Anim Health. 1994;6:224–233. [Google Scholar]
- 9.Cahill M M. Bacterial flora of fishes: a review. Microb Ecol. 1990;19:19–21. doi: 10.1007/BF02015051. [DOI] [PubMed] [Google Scholar]
- 10.Chakroun C, Urdact M C, Fuare D, Grimont F, Bernardet J-F. Random amplified polymorphic DNA analysis provides rapid differentiation among isolates of the fish pathogen Flavobacterium psychrophilum and among Flavobacterium species. Dis Aquat Org. 1997;31:187–196. [Google Scholar]
- 11.Christison J, Martin S M. Isolation and preliminary characterisation of an extracellular protease of Cytophaga sp. Can J Microbiol. 1971;17:1207–1216. doi: 10.1139/m71-193. [DOI] [PubMed] [Google Scholar]
- 12.Collinson S K, Emdy L, Müller K-H, Trust T J, Kay W W. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J Bacteriol. 1991;173:4773–4781. doi: 10.1128/jb.173.15.4773-4781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalsgaard I. Virulence mechanisms in Cytophaga psychrophila and other Cytophaga-like bacteria pathogenic for fish. Ann Rev Fish Dis. 1993;1993:127–144. [Google Scholar]
- 14.Doig P, Kinsella N, Guerry P, Trust T J. Characterization of a post-translational modification of Campylobacter flagellin: identification of a sero-specific glycosyl moiety. Mol Microbiol. 1996;19:379–387. doi: 10.1046/j.1365-2958.1996.370890.x. [DOI] [PubMed] [Google Scholar]
- 15.Duckworth M, Turvey J R. An extracellular agarase from a Cytophaga species. Biochem J. 1969;113:139–142. doi: 10.1042/bj1130139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerhardt P, editor. Manual methods of general bacteriology. Washington, D.C.: American Society for Microbiology; 1981. [Google Scholar]
- 17.Iida Y, Mizokami A. Outbreaks of coldwater disease in wild ayu and pale chub. Fish Pathol. 1996;31:157–164. [Google Scholar]
- 18.Johnson K A, Flynn J K, Amend D F. Duration of immunity in salmonids vaccinated by direct immersion with Yersinia ruckeri and Vibrio anguillarum bacterins. J Fish Dis. 1982;5:207–213. [Google Scholar]
- 19.Johnson K G, Perry M B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976;22:29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature. 1956;178:703. doi: 10.1038/178703a0. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Lehman J, Mock D, Sturenberg F J, Bernardet J-F. First isolation of Cytophaga psychrophila from a systematic disease in eel and cyprinids. Dis Aquat Org. 1991;10:217–220. [Google Scholar]
- 23.Lorenzen E. Ph.D. dissertation. Copenhagen, Denmark: Royal Veterinary and Agricultural University; 1994. [Google Scholar]
- 24.Lumsden J S, Ostland V E, Ferguson H W. Necrotic myositis in cage cultured rainbow trout, Oncorhynchus mykiss (Walblum), caused by Flexibacter psychrophilus. J Fish Dis. 1996;19:113–119. [Google Scholar]
- 25.Lumsden J S, Ostland V E, MacPhee D D, Ferguson H W. Production of a gill-associated and serum antibody by rainbow trout (Oncorhynchus mykiss) following immersion immunisation with acetone-killed Flavobacterium branchiophilum and the relationship to protection from experimental challenge. Fish Shellfish Immunol. 1995;5:151–165. [Google Scholar]
- 26.Manfredi R, Nanetti A, Ferri M, Mastroianni A, Coronado O V, Chiodo F. Flavobacterium spp. organisms as opportunistic bacterial pathogens during advanced HIV disease. J Infect. 1999;39:146–152. doi: 10.1016/s0163-4453(99)90007-5. [DOI] [PubMed] [Google Scholar]
- 27.Millonig G. Study on the factors which influence preservation of fine structure. In: Buffa P, editor. Symposium on electron microscopy. Rome, Italy: Consiglio Nazionale delle Ricerche; 1964. p. 347. [Google Scholar]
- 28.Morrison D C, Jacobs D M. Binding of polymyxin B to the lipid A portion of bacterial polysaccharides. Immunochemistry. 1976;13:813–819. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 29.Müller K-H, Trust T J, Kay W W. Fimbriation genes of Salmonella enteritidis. J Bacteriol. 1989;171:4648–4654. doi: 10.1128/jb.171.9.4648-4654.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osborn M J. Studies on the gram-negative cell wall. I. Evidence for the role of 2-keto-3-deoxyoctonate in the lipopolysaccharide of Salmonella typhimurium. Biochemistry. 1963;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostland V E, McGrogan D G, Ferguson H W. Cephalic osteochondritis and necrotic scleritis in intensively reared salmonids associated with Flexibacter psychrophilus. J Fish Dis. 1997;20:443–451. [Google Scholar]
- 32.Otis E J. M.S. thesis. Kingston: University of Rhode Island; 1984. [Google Scholar]
- 33.Reichenbach H, Kohl W, Achenbach A. The flexirubin type pigments, chemosystematically useful compounds. In: Reichenbach H, Weeks O B, editors. The Flavobacterium-Cytophaga group. Weinnheim, Germany: Verlag Chemie; 1981. pp. 101–108. [Google Scholar]
- 34.Ringø E, Strøm E, Tabachek J-A. Intestinal microflora of salmonids: a review. Aquac Res. 1995;26:773–789. [Google Scholar]
- 35.Rintamaki-kinnunen P, Bernardet J-F, Bloigu A. Yellow pigmented filamentous bacteria connected with framed salmonid fish mortality. Aquaculture. 1997;149:1–14. [Google Scholar]
- 36.Schmidtke L M, Carson J. Characteristics of Flexibacter psychrophilus isolated from Atlantic salmon in Australia. Dis Aquat Org. 1995;21:157–161. [Google Scholar]
- 37.Siegman-Igra Y, Schwartz D, Soferman G, Konforti N. Flavobacterium group IIb bacteremia: report of a case and review of Flavobacterium infections. Med Microbiol Immunol. 1987;176:103–111. doi: 10.1007/BF00200682. [DOI] [PubMed] [Google Scholar]
- 38.Smibert R M, Krieg N R. General characterization. In: Gerhardt P, editor. Manual of methods for general bacteriology. Washington, D.C.: American Society of Microbiology; 1981. p. 413. [Google Scholar]
- 39.Soltani M, Shanker S, Munday B L. Chemotherapy of Cytophaga/Flexibacter-like bacteria (CFLB) infections in fish: studies validating clinical efficacies of selected antimicrobials. J Fish Dis. 1995;18:555–565. [Google Scholar]
- 40.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valverde C, Hozbor D F, Lagares A. Rapid preparation of affinity-purified lipopolysaccharide samples for electrophoretic analysis. BioTechniques. 1997;22:230–236. doi: 10.2144/97222bm07. [DOI] [PubMed] [Google Scholar]
- 42.Vancanneyt M, Segers P, Hauben L, Hommez J, Devriese L A, Hoste B, Vandamme P, Kersters K. Flavobacterium meningosepticum, a pathogen in birds. J Clin Microbiol. 1994;32:2398–2403. doi: 10.1128/jcm.32.10.2398-2403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent W F, Cameron J A. Thiobarbiturate-reacting materials in microorganisms. J Bacteriol. 1967;93:156–158. doi: 10.1128/jb.93.1.156-158.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weissbach A, Hurwitz J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. J Biol Chem. 1959;234:705–709. [PubMed] [Google Scholar]
- 45.Wiklund T, Kaas K, Lonnstrom L, Dalsgaard I. Isolation of Cytophaga psychrophila (Flexibacter psychrophilus) from wild and farmed rainbow trout (Oncorhynchus mykiss) in Finland. Bull Eur Assoc Fish Pathol. 1994;14:44–46. [Google Scholar]
- 46.Wiklund T, Madsen L, Bruun M S, Dalsgaard I. Detection of Flavobacterium psychrophilum from fish tissue and water samples by PCR amplification. J Appl Microbiol. 2000;88:299–307. doi: 10.1046/j.1365-2672.2000.00959.x. [DOI] [PubMed] [Google Scholar]