Abstract
Introduction:
Despite advances in and increased adoption of technology, glycemic outcomes for individuals with type 1 diabetes (T1D) have not improved. Access to care is limited for many, in part due to a shortage of endocrinologists and their concentration in urban areas. Managing T1D via telehealth has potential to improve glycemic outcomes, as the barriers of travel-related time and cost are mitigated.
Methods:
Our endocrine telehealth program started in 2013 and currently provides care to nine rural community hospitals in Nebraska and Iowa. A retrospective cohort study was performed to evaluate glycemic outcomes in people with T1D who received care at these telehealth clinics from 2013-2019. Data were collected on age, race, gender, prior diabetes provider, use of diabetes technology, and A1c values over time.
Results:
One hundred thirty-nine individuals were followed for an average duration of 32 months (range 4-69 months). Sixty-six percent of people were previously under the care of an endocrinologist. The most common therapeutic action, in addition to insulin adjustment, was addition of a CGM (52%). Each year in telemedicine care was associated with a decline of 0.13% in A1c (95% CI: −0.20, −0.06). There was no association between A1c and age or gender. When stratifying by previous diabetes provider, all groups had a statistically significant decline in A1c, even those with a previous endocrine provider. There was no statistically significant decline in A1c based on addition of technology.
Conclusion:
We have shown that traditional telehealth visits are an effective way to provide care for people with T1D long-term and may provide distinct advantages to home telehealth visits.
Keywords: Diabetes, technology, telehealth, telemedicine, Type 1 diabetes, rural health
Introduction
Despite impressive advances in development and adoption of technology involving subcutaneous insulin pumps and continuous glucose monitors (CGMs), glycemic outcomes for individuals with type 1 diabetes (T1D) have not improved. 1 One of the contributing factors to the observed lack of progress in achieving glycemic goals is limited access to care. Not only is there a shortage of endocrinologists, but most endocrinologists practice in urban areas, leaving those living rural areas at a disadvantage. Many individuals have personal and economic barriers that prevent their attendance at regular quarterly office visits, making it more difficult to achieve treatment goals. 2 Managing T1D via telehealth has the potential to improve adherence and may improve outcomes, as the barriers of travel-related time and cost are mitigated.
Defining Telehealth
Definitions around telehealth interventions are inconsistent and not standardized. The department of Health and Human Services defines telehealth—sometimes called telemedicine—as the use of electronic information and telecommunication technologies to provide care when the patient and provider are not in the same place at the same time. 3 This includes a variety of interventions, such as phone calls, video calls, secure messages through patient portals, emails, secure file exchange, and remote patient monitoring.
For this paper, we are defining telehealth as a synchronous audio-video visit between a patient and provider (here on referred to as “telehealth visit”). Prior to the COVID-19 pandemic, video visits were reimbursable by CMS when they occurred in a designated rural site (designated as a Health Professional Shortage Area) at an originating site such as a hospital or clinic. In this paper, we will refer to this visit type as a “traditional telehealth visit.” After the declaration of the public health emergency in spring 2020, home-based video visits became reimbursable, recognizing home as a place of service. We will refer to these home-based telehealth visits as “home telehealth visits.”
Current evidence
Current available data describing the impact of telemedicine on outcomes in patient with T1D are limited. While a recent meta-analysis on T1D and telemedicine reviewing thirty-eight studies showed a small positive impact on hemoglobin A1c (A1c) (mean reduction of 0.18%), only three of the studies involved a tele-consultation intervention, the rest focusing on education, case management and remote monitoring. Studies involved small numbers of patients followed over short periods of time, with sample size ranging from 10 to 180 participants, and the length of intervention ranging from two weeks to twelve months. 4 Furthermore, reviewing the three studies labeled as “teleconsultation,” none provided a solely provider-driven telehealth intervention. Two studies alternated in-person and telehealth visits over a six-month period with intervention group sizes of sixteen and fifty-four.5,6 The third study focused on an insulin adjustment software rather than telehealth. 7
The inconsistency in definitions around telehealth interventions make understanding the impact of a specific intervention challenging. Many interventions defined as telehealth involve asynchronous remote monitoring of glucose data with periodic check-ins with patients via phone, which are not formal office visits. Other studies include office visits delivered via telehealth, but these visits alternate with standard in-person visits.8,9
Most data on the effects of telemedicine on diabetes outcomes focus on type 2 diabetes (T2D), with fewer studies involving patients with T1D. 10 Several available studies focusing on telehealth visits for individuals with T1D involve VA and military populations, potentially limiting their applicability to the general population.11,12 Change in A1c in those with T1D, when reported, often shows non-inferiority or slightly favors telehealth interventions but is lackluster.4,10,13,14
In this paper, we report glycemic outcome data from the longest to date studied telehealth intervention under the traditional telehealth visit model for T1D. This intervention was provided as a routine clinical model of care, with no grant support.
Methods
Description of Our Telehealth Program
In order to better meet healthcare needs of our predominantly rural state of Nebraska, we started our endocrine telehealth program at the University of Nebraska Medical Center in 2013. The program started at a single site with one provider and over eight years has expanded to nine community hospitals in Nebraska and western Iowa with a team of four providers. To ensure program sustainability and reimbursement by CMS, sites were selected in designated rural areas and occurred in medical facilities. Regarding other payers, Nebraska Medicaid has had payment parity for telehealth since the passing of the Nebraska Telehealth Act in 1999, and we have found that the majority of private payers in our area reimburse in accordance with CMS guidelines.
Care was provided in the form of synchronous office visits delivered by secure video conferencing technology. The provider was located at the academic medical center while the patient was located in a clinical exam room at a rural community hospital. In these general endocrinology telehealth clinics, individuals with diabetes accounted for about fifty percent of the visits. Number of visits per year increased steadily from 254 in calendar year 2014 to 2068 in calendar year 2019.
Model of Care
Of our nine current sites, seven had no prior endocrine care, and eight sites were new to telehealth. For the first two years of our program, we provided a mix of regular telehealth clinics and quarterly in-person outreach clinics. As our program expanded to additional locations, we pivoted and became a purely telehealth program.
The academic medical center team consists of three board certified endocrinologists and one physician assistant. There is also a dedicated RN, certified diabetes care and education specialist (CDCES) case manager located at the University site, assigned full time to the program. Their role is to facilitate clinics, receive patient information before and after the visits, be available to patients for interim issues, and communicate with each site. At each rural site, support staff varies. Two of our locations employ advanced practice providers (APPs), who often co-manage patients with the academic endocrinologist. At seven sites, there is a hospital employed CDCES that is used as a local resource. One rural site shares the same electronic medical record (EMR) as our academic center, while the remaining eight sites utilize a different EMR. Data is shared via secure scanning and emailing of documents. Local sites manage the schedule and submit schedules to the academic medical center one to two weeks prior to the clinic date, which are then entered into the EMR under a telehealth-specific department. The endocrine provider bills a professional fee, while the local site bills and collects the facility fee for each visit. Patients are able to contact the RN, CDCES case manager at the academic medical center for any interim blood sugar issues, but we often utilize a local CDCES for injection teaching, scheduled interim blood sugar reviews, downloading technology and training or troubleshooting with new technology.
Data Collection and Analysis
This is a retrospective cohort study, evaluating glycemic outcomes in a subgroup of patients with T1D receiving routine clinical care via site-to site telehealth. Patients with T1D seen for three or more telehealth visits over at least six months of time at any telehealth clinic location were included in the analysis. Diagnosis of T1D was confirmed by the academic endocrine team. Patients were stratified by prior diabetes provider and use of diabetes technology. This study was approved by the University of Nebraska Medical Center Institutional Review Board.
Data collected on all people seen via telemedicine for three or more visits from 1/1/2013-10/29/2019 with a visit diagnosis code for T1D (E11.xx) included:
Age
Race
Gender
Diabetes treatment choice at initial and most recent visit
Accuracy of diabetes diagnosis (whether diagnosis was changed to T1D after initial visit)
Provider previously managing T1D
A1c at initial visit and all subsequent A1c values
Number and dates of office visits
Random coefficient regression analysis was used to assess the impact of telemedicine on A1c trend and differentials by subgroups.
Results
One hundred thirty-nine individuals met our inclusion criteria. Average duration of follow-up, as defined by time between first and last A1c value, was thirty-two months. Demographic data are presented in Table 1. Eighty-four percent of our patients were seen only via telehealth, meaning they never had an in-person visit while they were under our care. Sixteen percent of people had a mix of in person and telehealth visits due to personal preference.
Table 1.
Baseline Demographic Data on Cohort with T1D Seen in Traditional Telehealth Clinics.
| N = 139 | |
|---|---|
| % female | 57.6% |
| % non-Hispanic White | 97% |
| Average age at first visit (y) | 44.5 (16-94) |
| Average # of A1c values | 8.3 |
| Average duration between first & last A1c (m) | 31.7 (4-69) |
| % with diagnosis changed from T2D to T1D | 10 (n = 14) |
| % seen only via telemedicine | 84 (n = 117) |
Initial treatment regimen is listed in Table 2. Forty-one percent of people (n = 57) were on multiple daily injections (MDI) at their initial visit, with 7% (n = 4) of that group on a continuous glucose monitor (CGM).
Table 2.
Description of Initial Diabetes Treatment Regimen.
| Initial treatment | # (%) |
|---|---|
| MDI + POC | 53 (38.1) |
| MDI + CGM | 4 (2.9) |
| Pump + POC | 69 (49.6) |
| Pump + CGM | 10 (7.2) |
| MDI + oral agents | 1 (0.7) |
| Oral agents | 2 (1.4) |
Abbreviations: CGM, continuous glucose monitor; MDI, multiple daily injections; POC, point of care glucose monitoring; Pump, subcutaneous continuous insulin infusion.
Fifty-seven percent (n = 79) were using an insulin pump at their initial visit, with 13% (n = 10) of this group also on a CGM. Comparing the treatment regimen at the first versus most recent visit, the most common therapeutic action was addition of a CGM, which occurred in 52% of patients (Table 3).
Table 3.
Description of Technology Intervention for the Cohort.
| Added technology | # (%) |
|---|---|
| No change | 54 (41.9) |
| Added CGM | 67 (51.9) |
| Added pump | 2 (1.6) |
| Added pump & CGM | 6 (4.7) |
Abbreviations: CGM, continuous glucose monitor; Pump, subcutaneous continuous insulin infusion.
Information on prior diabetes provider is shown in Table 4. The majority of patients (66%, n = 92) were previously under the care of an endocrinologist.
Table 4.
Description of Prior Healthcare Provider Managing T1D.
| Prior diabetes provider | # (%) |
|---|---|
| Private practice endocrinologist | 59 (42.5) |
| PCP | 44 (31.7) |
| Academic endocrinologist | 33 (23.7) |
| No prior provider | 3 (2.2) |
Abbreviation: PCP, primary care provider.
Glycemic control
To evaluate changes in glycemic control, we followed A1c values longitudinally. The mean initial A1c was 8.4%. Each year in telemedicine care was associated with a decline of 0.13% in A1c (95% CI: -0.20, -0.06), with the mean A1c at the final/most recent visit was 8.0% (Figure 1).
Figure 1.
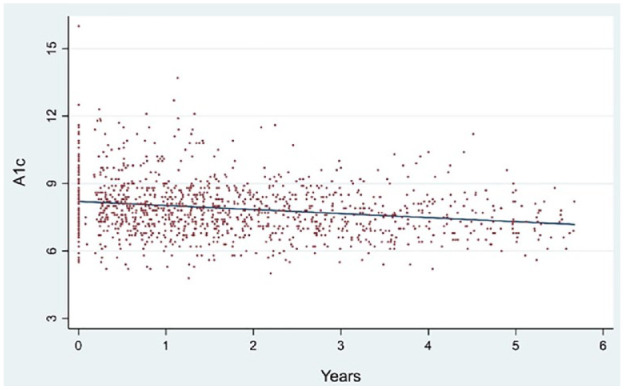
Alc trend between first and last telemedicine visit (n = 139 patients & 1,224 data points).
In order to evaluate the true effect of the program as a health care delivery model, as opposed to the expertise of a trained academic endocrinologist, we separately evaluated the A1c change over time according to the previous diabetes provider (academic endocrinologist, community endocrinologist, primary care provider) and found a statistically significant decline in A1c across all groups (Figure 2). Even for patients previously seen by an academic endocrinologist, the decline in A1c remained statistically significant (P < .001). We did not find an association between A1c and age or gender. Among those with poor control, as defined by initial a1c > 9%, those previously treated by their primary care provider had a steeper decline in a1c over time (Figure 3).
Figure 2.
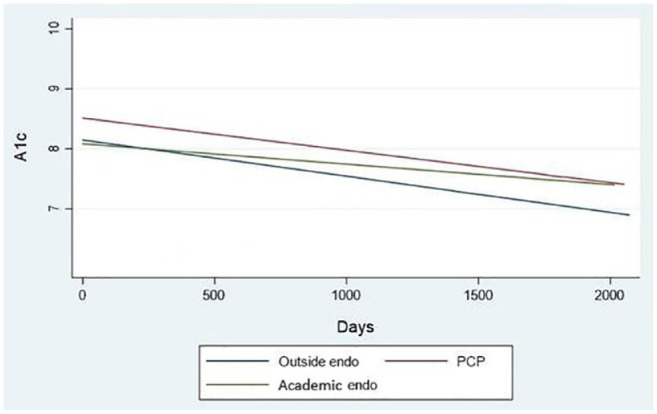
Alc trend between first and last telemedicine visit by prior provider.
Figure 3.
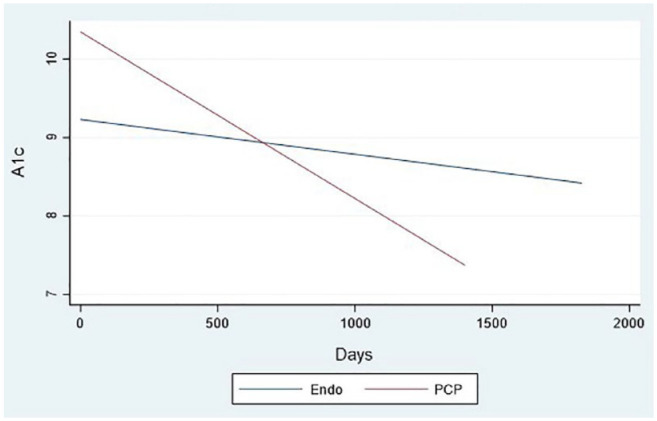
Alc trend by prior provider among patients with A1c > 9 (n = 34 patients).
Change in treatment is described in Table 3. Forty two percent of people had intensification of their initial treatment without a change in technology, while 58% (n = 75) had technology added. For those that had technology added to their treatment plan, this included a CGM 97% of the time. There was not a statistically significant decline in A1c based on addition of technology (Figure 4).
Figure 4.
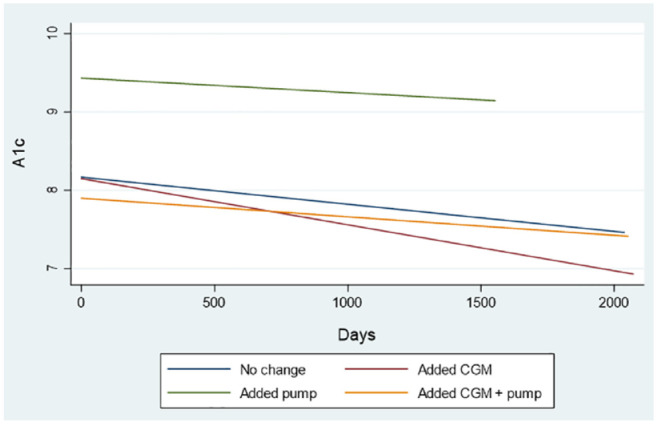
Alc trend between first and last telemedicine visit by change in treatment.
Discussion
We present the longest follow up of the largest cohort of people with T1D receiving care via telehealth reported in the literature thus far. Our findings support the concept that T1D can be effectively managed via telemedicine over a long duration, not just as a temporary measure. The size of our group, duration of care, and the consistency of the intervention helps address a gap in current literature.
A significant decline in A1c was seen irrespective of addition of diabetes technology (Figure 1). As expected, patients with uncontrolled diabetes (defined as A1c > 9%) benefited more from our telehealth intervention compared to those with lower A1c values (Figure 3). A more surprising finding is that even patients previously managed by an academic endocrinologist had a statistically significant decline in A1c over time. However, clinically this decline appears less impressive. This may be related to the lower baseline A1c reflecting the intensive management patients were already receiving at the academic medical center, which has the support of an ADA-certified diabetes education program and case management system.
The adoption of diabetes technology alone is insufficient to achieve improvement of A1c on a population scale, as shown by recent T1D Exchange data. 1 We believe our success in consistently lowering a1c values over time is due to this particular model of care – not necessarily adding technology or changing to an academic endocrinologist. Potential reasons for improvement include increased frequency of visits due to visits being more convenient with less travel time as well as more frequent and effective collaboration with a local CDCES. Even though 66% of our patients were previously under the care of an endocrinologist before transferring to our traditional telehealth clinics, in most of those instances, the prior endocrinologist had left the area. For others, the access was limited due to time and travel requirements. One weakness of our data is the lack of information regarding visit frequency prior to this intervention—therefore we were not able to determine if visit frequency improved. We also do not have data about frequency of in-person local CDCES visits (due to our lack of access to their electronic health records), which we believe played a role in improved glycemic outcomes.
We believe that the most important key to the success of these clinics has been the relationship between the patient, the provider and the local champion, most commonly an RN and/or CDCES. Having a consistent, familiar face greet them and occasionally join them during the appointment helps make what could be perceived as an impersonal visit as more comfortable and familiar. This visit type is especially important for people with lower levels of digital literacy, those without a connected device and those without reliable home internet access. We also believe that in these smaller rural communities where our clinics take place (populations ranging from 2000-25000), there is a preference from patients to meet with a member of their own community.
Our clinics have been extremely successful with high patient satisfaction (data not shown). In 2017, we collected patient satisfaction data at our 3 largest telehealth clinic locations from 310 patients, seen for a variety of general endocrine issues. Ninety-nine percent rated the quality of the care as good or very good, while 98% would recommend telehealth to a friend or relative. Most importantly, 42% of respondents stated that if a telehealth clinic was not an option, they would not have traveled to seek specialty care, highlighting the important access these clinics provide. Eighty-three percent of those surveyed missed less than 2 hours of work or school for their appointment (with 59% missing less than 1 hour).
While our data above helps support the concept that T1D can be effectively managed via telehealth, our focus was on traditional telehealth visits. This is because, prior to the COVID-19 pandemic, this was the only setting in which telehealth clinic visits were reimbursed. During the COVID-19 pandemic, the number of telehealth visits at our center as well as across the world grew exponentially. Our model of care for rural patients did not change during COVID; we continued clinic-based telehealth, seeing patients in our rural clinics with precautions in place. This is in contrast to the overwhelming majority of telehealth visits occurring after March 2020 were not clinic-based visits, but home-based visits.
Advantages and Disadvantages of Different Telehealth Models
We believe that a clinic-based telehealth model has unique value and offers advantages that home-based models cannot provide.
Benefits of home-based telehealth include convenience, savings of time and travel costs, and the potential to improve attendance. However, despite these important advantages, several downsides exist. These visits require a certain degree of digital literacy, internet access, a stable internet connection, and a connected device with a camera, criteria that many people do not have. There is real concern that continued promotion of home video visits may further widen already existing health disparities in diabetes.
What we perceive as a major benefit of clinic-based telehealth visits is the lack of dependence on personal technology access. Patients still save time and travel fewer miles when compared to traveling to the specialist’s office. Onsite staff obtain vital signs, and patients can have labs drawn before or after their appointment. Patients can bring blood sugar data to the appointment to have transmitted to their provider. Having these clinics located in community hospitals with hospital-affiliated primary care clinics often leads to more referrals and a better relationship with local providers.
Benefits to the local hospital include the promotion of specialty care to their community and additional revenue from ancillary services such as laboratory charges and radiology studies. In several of our locations, the local champion has started a professional CGM program. This not only generates new revenue for the hospital or clinic, but it also allows hospital affiliated primary care practices to experience the benefits of CGM.
When traditional telehealth clinics are supported by a local CDCES, therapeutic benefits are enhanced and relationships are fostered between patients and local educators. CDCESs benefit as well, through expanded patient volumes and exposure to newer diabetes technology and medications that local providers may be slower to adopt. For traditional telehealth clinics that include APP support, patients benefit from the combination of a local provider working with an academic specialty provider, and the APP gets additional mentoring.
Conclusions
In conclusion, we have shown that traditional telehealth visits are an effective way to provide long-term care for people with T1D living in rural areas and may provide distinct advantages to home telehealth visits.
Acknowledgments
The authors would like to thank Padmaja Akkireddy, MBBS and Lisa Kuechenmeister, PA for their assistance with data collection and care provided in telehealth clinics, as well as Vonnetta Byington, CRA, Cyrus Desouza, MBBS and Geri Hansen, MSN, RN for their administrative support. We are also grateful to Andrea Hoge, RN, BSN, CDCES, Deann Carpenter, DNP, APRN, CDCES, and Sara Gaul, MSN, RN for their contributions to telehealth clinic development and patient care.
Footnotes
Abbreviations: Type 1 Diabetes (T1D), continuous glucose monitor (CGM), hemoglobin A1c (A1c), type 2 diabetes (T2D), certified diabetes care and education specialist (CDCES), advanced practice providers (APPs), electronic medical record (EMR), multiple daily injections (MDI), primary care provider (PCP).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Leslie A Eiland  https://orcid.org/0000-0002-4322-953X
https://orcid.org/0000-0002-4322-953X
References
- 1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker AF, Hood KK, Gurka MJ, et al. Barriers to technology use and endocrinology care for underserved communities with type 1 diabetes. Diabetes Care. 2021;44(7):1480-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. TELEHEALTH.HHS.GOV. What is telehealth? 2021. Updated May 4, 2021. Accessed May 14 2021. https://telehealth.hhs.gov/patients/understanding-telehealth/
- 4. Lee SWH, Ooi L, Lai YK. Telemedicine for the management of glycemic control and clinical outcomes of type 1 diabetes mellitus: a systematic review and meta-analysis of randomized controlled studies. Front Pharmacol. 2017;8:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Esmatjes E, Jansa M, Roca D, et al. The efficiency of telemedicine to optimize metabolic control in patients with type 1 diabetes mellitus: telemed study. Diabetes Technol Ther. 2014;16(7):435-441. [DOI] [PubMed] [Google Scholar]
- 6. Jansa M, Vidal M, Viaplana J, et al. Telecare in a structured therapeutic education programme addressed to patients with type 1 diabetes and poor metabolic control. Diabetes Res Clin Pract. 2006;74(1):26-32. [DOI] [PubMed] [Google Scholar]
- 7. Charpentier G, Benhamou PY, Dardari D, et al. The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 Study). Diabetes Care. 2011;34(3):533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yaron M, Sher B, Sorek D, et al. A randomized controlled trial comparing a telemedicine therapeutic intervention with routine care in adults with type 1 diabetes mellitus treated by insulin pumps. Acta Diabetol. 2019;56(6):667-673. [DOI] [PubMed] [Google Scholar]
- 9. Crossen S, Glaser N, Sauers-Ford H, Chen S, Tran V, Marcin J. Home-based video visits for pediatric patients with poorly controlled type 1 diabetes. J Telemed Telecare. 2020;26(6):349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faruque LI, Wiebe N, Ehteshami-Afshar A, et al. Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ. 2017;189(9):E341-E364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi YS, Cucura J, Jain R, Berry-Caban C. Telemedicine in US Army soldiers with type 1 diabetes. J Telemed Telecare. 2015;21(7):392-395. [DOI] [PubMed] [Google Scholar]
- 12. Xu T, Pujara S, Sutton S, Rhee M. Telemedicine in the management of type 1 diabetes. Prev Chronic Dis. 2018;15:E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kompala T, Neinstein AB. Telehealth in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2021;28(1):21-29. [DOI] [PubMed] [Google Scholar]
- 14. Tchero H, Kangambega P, Briatte C, Brunet-Houdard S, Retali GR, Rusch E. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J E Health. 2019;25(7):569-583. [DOI] [PubMed] [Google Scholar]


