Abstract
Introduction:
The first meeting of the Integration of Continuous Glucose Monitor Data into the Electronic Health Record (iCoDE) project, organized by Diabetes Technology Society, took place virtually on January 27, 2022.
Methods:
Clinicians, government officials, data aggregators, attorneys, and standards experts spoke in panels and breakout groups. Three themes were covered: 1) why digital health data integration into the electronic health record (EHR) is needed, 2) what integrated continuously monitored glucose data will look like, and 3) how this process can be achieved in a way that will satisfy clinicians, healthcare organizations, and regulatory experts.
Results:
The meeting themes were addressed within eight sessions: 1) What Do Inpatient Clinicians Want to See With Integration of CGM Data into the EHR?, 2) What Do Outpatient Clinicians Want to See With Integration of CGM Data into the EHR?, 3) Why Are Data Standards and Guidances Useful?, 4) What Value Can Data Integration Services Add?, 5) What Are Examples of Successful Integration?, 6) Which Privacy, Security, and Regulatory Issues Must Be Addressed to Integrate CGM Data into the EHR?, 7) Breakout Group Discussions, and 8) Presentation of Breakout Group Ideas.
Conclusions:
Creation of data standards and workflow guidance are necessary components of the Integration of Continuous Glucose Monitor Data into the Electronic Health Record (iCoDE) standard project. This meeting, which launched iCoDE, will be followed by a set of working group meetings intended to create the needed standard.
Keywords: continuous glucose monitor, data integration, diabetes, digital health, electronic health record, standard
Introduction
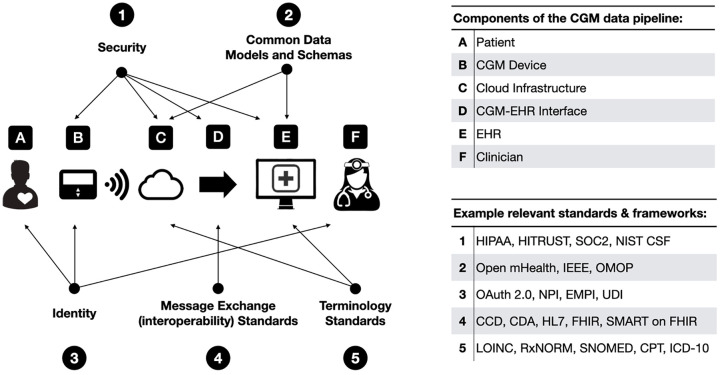
The Integration of Continuous Glucose Monitor Data into the Electronic Health Record (iCoDE) project is a program intended to develop data standards and implementation policies to integrate continuous glucose monitor (CGM) data into the electronic health record (EHR). 1 The CGM data pipeline from patient to clinician is complex, but there are a number of opportunities along the way to adopt, adapt, or develop standards and define best practices that can significantly improve data access and drive adoption (Figure 1). Different sensors are currently being used for physiologic monitoring of cardiac, neurologic, gastrointestinal, pulmonary, orthopedic, and other conditions. Lessons learned from the iCoDE project will likely be able to be adapted to support other data integration efforts. Thus, it is expected that the iCoDE standard will not only facilitate the use of CGM technology for helping persons with diabetes (PWD), but it will break down a significant barrier to interconnected data to facilitate the use of all digital health tools for a variety of medical purposes.
Figure 1.
Opportunities to adopt, adapt, or develop standards and best practices in the CGM data pipeline.
Abbreviations: CCD, Continuity of Care Documents; CDA, Clinical Document Architecture; CGM, continuous glucose monitor; CPT, Current Procedural Terminology; EHR, electronic health record; EMPI, Enterprise Master Patient Index; FHIR, Fast Healthcare Interoperability Resources; HIPAA, Health Insurance Portability and Accountability Act; HL7, Health Level 7; ICD-10, International Classification of Diseases 10th Revision; IEEE, Institute of Electrical and Electronics Engineers; LOINC, Logical Observation Identifiers Names and Codes; NIST CSF, National Institute of Standards and Technology Cybersecurity Framework; NPI, National Provider Identifier; OMOP, Observational Medical Outcomes Partnership; SMART, Substitutable Medical Applications, Reusable Technologies; SNOMED, Systemized Nomenclature of Medicine; SOC2, System and Organization Controls type 2 – Trust Services Criteria; UDI, Unique Device Identifier.
The iCoDE project consists of key stakeholders who are committed to advancing the field of diabetes digital health by developing this standard to break the barrier of wearable sensor data failing to become part of the EHR. Steering Committee members of the iCoDE project come from the United States (US), Canada, Denmark, and Japan and include experts in the presentation, coding, regulation, analysis, and clinical use of CGMs. These CGM data experts represent (1) US government agencies, (2) CGM manufacturing companies, (3) manufacturers of other diabetes hardware and software products, (4) EHR database companies, (5) professional organizations, (6) PWD, and (7) academics in such disciplines as medicine, nursing, law, information technology (IT), ontology, and cybersecurity. The iCoDE Steering Committee met on January 27, 2022 to discuss the current status and necessary future developments in the integration of CGM data into the EHR. The meeting, presented by Diabetes Technology Society (DTS), covered eight topics (Table 1) and was chaired by David C. Klonoff, MD (Mills-Peninsula Medical Center and University of California, San Francisco) and Juan Espinoza, MD (Children’s Hospital of Los Angeles, University of Southern California).
Table 1.
Agenda of the Meeting, With a List of the Session Topics.
| Session 1: What Do Inpatient Clinicians Want to See With Integration of CGM Data into the EHR? |
| Session 2: What Do Outpatient Clinicians Want to See With Integration of CGM Data into the EHR? |
| Session 3: Why Are Data Standards and Guidances Are Useful? |
| Session 4: What Value Can Data Integration Services Add? |
| Session 5: What Are Examples of Successful Integration? |
| Session 6: Which Privacy, Security, and Regulatory Issues Must Be Addressed to Integrate CGM Data into the EHR? |
| Session 7: Breakout Group Discussions |
| Session 8: Presentation of Breakout Group Ideas |
Abbreviations: CGM, continuous glucose monitor; EHR, electronic health record.
iCoDE and US Federal Policy Toward Patient-Generated Health Data
The US Department of Health and Human Services, Office of the National Coordinator for Health Information Technology (HHS ONC HIT) is the US government’s principal federal agency for coordinating nationwide efforts to implement and use the most advanced health IT and electronic exchange of health information. The iCoDE project is following policies advocated by this agency for CGM data, which is a form of patient-generated health data. The HHS ONC HIT released the most updated version of the Patient Engagement Playbook in 2019. 2 According to this playbook, patient-generated health data can fill information gaps, reduce hospital readmissions, and promote efficient diagnosis of illnesses.
The HHS ONC HIT believes that patient-generated health data is a growing opportunity because more than four in ten people with mobile devices use them to track their health goals, three in ten people own a health monitoring device, and two in ten people with mobile devices or health monitoring devices share and discuss data from these devices with healthcare professionals (HCPs). 3 The framework underlying the iCoDE project to facilitate interoperable integration of CGM data (as an example of patient-generated health data) into the EHR is in line with the direction for healthcare that this agency is promoting.
Session 1: What Do Inpatient Clinicians Want to See With Integration of CGM Data into the EHR?
Moderator
Elias K. Spanakis, MD
University of Maryland, Baltimore, MD, USA
Speakers
Eileen Faulds, PhD, MS, RN
The Ohio State University, Columbus, OH, USA
Jane Jeffrie Seley, DNP, MPH, MSN, GNP, BC-ADM, CDCES, CDTC, FADCES
New York-Presbyterian/Weill Cornell Medicine, New York, NY, USA
Guillermo E. Umpierrez, MD, CDCES, FACE, MACP
Emory University, Atlanta, GA, USA
Amisha Wallia, MD, MS
Northwestern University, Chicago, IL, USA
CGMs represent a promising system that can be utilized in the hospital setting to transmit glucose to the nursing station, reduce inpatient hypoglycemia, 4 and assist in titration of intravenous insulin. The use of CGMs can reduce the need for point-of-care testing and personal protective equipment use.5-7 One of the greatest limitations that CGMs currently face is the lack of automatic integration of CGM data into the EHR.8,9 This lack of integration is an important obstacle that must be overcome to see successful implementation of CGMs in the hospital. CGM “alarms” that lead to treatment interventions can result in medical legal action if the data is not properly documented. 10 CGM data integration can mimic that of cardiac telemetry by using discrete CGM values, glycemic trends, and hypoglycemic and hyperglycemic alarms in a similar way that heart rate, cardiac telemetry graphs, and alarms are used. CGM reports need to be standardized and be extremely simple to use. These reports can be similar to the current Ambulatory Glucose Profile (AGP) report. They may include novel, derived composite metrics like the Glycemic Risk Assessment Diabetes Equation 11 and the Glycemia Risk Index (GRI), 12 as well as insulin dosing data. Importantly, this data must be portable and compatible with a variety of EHR systems. These reports should be available not only to nurses or to medical providers, but also to every HCP who is involved in the care of patients. It is also fundamental to provide education to HCPs. Finally, it is important to develop an infrastructure that will support integration of CGM data into the EHR irrespective of whether the data originates from hospital-based CGMs or from outpatient CGMs.
Session 2: What Do Outpatient Clinicians Want to See With Integration of CGM Data into the EHR?
Moderator
Juan Espinoza, MD, FAAP
Children’s Hospital Los Angeles, University of Southern California, Los Angeles, CA, USA
Speakers
Wei-An (Andy) Lee, DO
LAC+USC Medical Center, Los Angeles, CA, USA
Charlotte Niznik, APRN, CDCES
Northwestern University, Chicago, IL, USA
Maya Payne, MD
Naval Medical Center Portsmouth, Portsmouth, VA, USA
Viral N. Shah, MD
Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Jennifer L. Sherr, MD, PhD
Yale University, New Haven, CT, USA
Access to CGM data in outpatient settings can be both time consuming and inefficient. Current practices require clinics to ensure that patients are connected to multiple portals and sites to access their own health data. Sometimes, clinic staff provides technical support for patients. Once patient data is in the manufacturer’s portal, it is either (1) manually transcribed into the EHR (e.g., in a note), (2) printed and scanned, (3) inserted via a snipped image, or (4) uploaded as a Portable Document Format (PDF) file. These methods are time intensive and resource dependent. Integration of CGM data into the EHR could help improve and streamline clinic workflows, freeing up staff and clinicians to other aspects of patient care.
A standardized approach to diabetes care and data collection would ensure that HCPs are obtaining the same data forms and would additionally make CGM data more accessible for research and study recruitment when analyzing EHRs. Clinicians would also like to see modifiable CGM thresholds for AGP reports, such as altering time and target ranges, to individualize patient care. To further support CGM data integration, both HCPs and patients may benefit from tools to access and analyze longitudinal trends. Through automatic data uploading and interpretable visualization of longitudinal data, both patients and HCPs can more easily retrieve data to compare patterns and progress longitudinally. CGM data could be analyzed alongside biomarkers such as hemoglobin A1C over different time periods to assess response to treatment and changes over time. With increased data availability, training will be required for every member of the patient’s care team (i.e., nutritionist, pharmacist, diabetes nurse educator, etc.) to interpret values and trends and incorporate them into treatment plans.
Outpatient clinicians also desire to see improvements and efforts toward prevention of data loss due to changes in devices or health information systems. CGM data must be integrated within and transferable across different EHR systems to ensure consistent access for HCPs as well as patients themselves. It is currently difficult to introduce external records across various EHR systems. Data transparency across healthcare systems is necessary to allow HCPs to make informed clinical decisions regarding patient care based on all available data. Uploading CGM data into the EHR is time consuming, and thereby competes with valuable time allocated for patient visits. CGM data integration, access, visualization, and interpretation must be made simple and beneficial for patients; better patient care and experience should drive integration efforts. Ultimately, automatic CGM data integration into the EHR will be pivotal to optimizing care for PWD.
Session 3: Why Are Data Standards and Guidances Are Useful?
Moderator
Charisse Madlock-Brown, PhD, MLS
University of Tennessee Health Science Center, Memphis, TN, USA
Speakers
Carole Carey, MEngSc (Computer Eng)
Engineering in Medicine and Biology Technical Committee on Standards, IEEE, Piscataway, NJ, USA
Gora Datta, FHL7, VS, SMIEEE, SMACM
Founding Co-Chair HL7 Mobile Health
Founding Convenor ISO/TC215 Traditional Medicine
Founding Chair IEEE Healthcare: Blockchain & AI
Tom Haskell, BS
Cerner Enviza, Kansas City, MO, USA
Standards Committees in the IEEE (Institute of Electrical and Electronics Engineers) take responsibility for the scope and technical content of standards and provide oversight to specific working groups pertinent to the field of interest of the Society or organizational unit that develops the standards. Diabetes Technology Society (DTS) developed the DTS Cybersecurity Standard for Connected Diabetes Devices (DTSec) 13 and the DTS Mobile Platform Controlling a Diabetes Device Security and Safety Standard (DTMoSt). 14 These are now being reformatted by the IEEE P2621 Healthcare Device Security Assurance Working Group into three industry voluntary consensus standards for diabetes device security. 15 With over 350,000 different mobile health applications on the market currently, it is important to have some type of governance over them. 16 Standards have been developed to discuss the functional requirements of mobile health applications related to data transfer from Internet of Things devices and unique identifiers to track users. Data standards are also important for quality research, and as standards are developed for specific use cases, data harmonization will be vital. In addition, engaging with various stakeholders, such as users, manufacturers, academics, and government agencies, can help promote the use of standards.
For successful data standards development, it is crucial to ensure organizations have the resources to train new committee members and to ensure that committees maintain regular meetings to keep people engaged. Consensus-driven governance can help ensure projects will represent diverse perspectives and will result in equitable interoperability standards with multiple stakeholders. Finally, considering the scope of a standard for application only to a US audience or to an international audience will be helpful to plan the scope of the standard.
Session 4: What Value Can Data Integration Services Add?
Moderator
Siavash Sarlati, MD
Anthem, Inc, Indianapolis, IN, USA
Speakers
Ed Deng, BS
Health2Sync, Taipei, Taiwan
Mark Clements, MD, PhD, CPI, FAAP
Children’s Mercy Hospitals & Clinics, Kansas City, MO, USA
CaroLynn Brinckerhoff, BA
Redox, Madison, WI, USA
Yael Shtrit, MSc, MBA
DreaMed, Petah Tikva, Israel
Data integration can help improve disease management. The integration of regular blood glucose monitoring values, whether directly from a CGM or user entered, with the EHR can create better insights for both patients and HCPs. This kind of data integration can drive automated feedback and dashboards like those seen on the Health2Sync (Taipei, Taiwan) application, which uses Open Authorization 2.0 to overcome certain EHR interoperability barriers. 17 CGM data integration can improve self-management and drive a more personalized and engaged therapeutic relationship with HCPs and healthcare systems. Integrated systems can open the door to higher quality and more cost-effective care, more efficient clinical trials, and better monitoring and evaluation of clinical programs and pilots. DreaMed (Petah Tikva, Israel) provides a decision support tool that helps HCPs analyze actionable data to create and execute treatment plans. The treatment plan, CGM data, and insulin data are then integrated into the EHR in a searchable manner. Redox (Madison, Wisconsin, USA) takes a stepwise approach to fulfill the remote patient monitoring integration needs of clients. It is useful to take a phased approach that starts with the ability to manually generate regular data reports on demand and then progresses to more automated systems with real-time data dashboards and a seamless experience navigating between legacy EHR data and CGM data. There was an emphasis on the fact that patients should maintain ownership of and primary decision-making about their data throughout the integration journey. Overall, the purpose of data integration is to drive better patient outcomes and improved care rather than amassing as much data as possible.
Session 5: What Are Examples of Successful Integration?
Moderator
Azhar Rafiq, MD, MBA, MEd
National Aeronautics and Space Administration, Washington, DC, USA
Speakers
Avinash Shanbhag, MS
US Department of Health and Human Services, Office of the National Coordinator for Health Information Technology, Washington, DC, USA
Amy B. Criego, MD, MS
International Diabetes Center at Park Nicollet, Minneapolis, MN, USA
Juan Espinoza, MD, FAAP
Children’s Hospital Los Angeles, University of Southern California, Los Angeles, CA, USA
Priya Prahalad, MD, PhD
Stanford University, Stanford, CA, USA
The HHS ONC HIT is tasked with improving health with accessible technology and associated health information. This agency coordinates with federal agencies in formulating federal health IT strategies that transform healthcare delivery and health technology infrastructure. Certification and adoption of technologies like the EHR are also driven by the ONC. The federal Health IT Strategic Plan was renewed by the ONC from 2015 to 2020 and from 2020 to 2025. 18 In addition, a standard for interoperability across the nation has been published with the Trusted Exchange Framework and Common Agreement which includes the “minimum data set” referred to as the US Core Data for Interoperability (USCDI). 19 The USCDI establishes a standard set of health data classes and data elements to ensure that data sets exchanged with the EHR include these parameters.
The International Diabetes Center at Park Nicollet’s recent efforts have been oriented toward best practices to standardize, organize, analyze, and utilize glucose data. To do so efficiently, they launched a program to integrate CGM data into the EHR. 20 The effort was coordinated in alignment with the Risk Management and Information System and Technology teams. The integration had a standard operational process in collaboration with Redox for all clinic facilities to participate. The CGM data from primary care, adult endocrine, and pediatric endocrine populations were imported as discrete glucose metrics for easy access for population health analysis and quality improvement. A crucial component for integration is the data sharing agreement to link the patient’s LibreView (Abbott Diabetes Care, Alameda, California, USA) record with their health record in the EHR. Once established, the CGM data is connected to the EHR, and data from varying times and in varying visual displays can be ordered and viewed by an HCP. Data from multiple time periods can be viewed simultaneously to see trends over time.
Prior to the integration of CGM data into the EHR, the diabetes clinic at Children’s Hospital Los Angeles had a workflow for patients with Dexcom (San Diego, California, USA) devices where (1) CGM data was uploaded into Clarity, (2) the nurse logged into Clarity and printed the report, (3) the physician reviewed the report, and (4) the report was scanned into the EHR. Their data integration effort created two new workflows for account linkage and data requests using the EHR computerized physician order entry (CPOE) interface to decrease the time and energy needed to access CGM data. 21 For account linkage, an HCP creates an account linkage request, which generates a Health Level 7 (HL7) message that is sent via Redox, an integration engine, to Dexcom. The manufacturer will then send an e-mail consent form to the patient, and once the patient authorizes data sharing, the manufacturer generates a status report that is sent via Redox back to the EHR. The data request workflow is similar; the HCP creates a CGM data request, which generates another HL7 message, and once received, Dexcom creates a report that is delivered to the EHR. This improved the clinical workflow but also created new issues such as account linkage and billing considerations. The overall process was also manufacturer specific.
A clinic at the Lucile Packard Children’s Hospital at Stanford achieved the integration of CGM data into the EHR through using Apple HealthKit (Apple, Cupertino, California, USA). 22 The data from a CGM is transferred to the Dexcom G6 application on the patient’s mobile device. Apple HealthKit enables sharing of this data into the Epic MyChart (Epic Systems, Verona, Wisconsin, USA) mobile application, ultimately making the data available in the EHR. The CGM data is available as discrete data points with timestamps that can be visualized by an HCP. The visualization of the CGM data was developed as a dashboard, GluVue (https://gluvue.stanfordchildrens.org/), and showed daily trends as well as trends over time. This integration was easy to build and leveraged existing infrastructure, but there were limitations such as the patient needing to have an iOS device, a lengthy setup time, a limit to the amount of data available that can be stored, and the types of devices that were supported.
Session 6: Which Privacy, Security, and Regulatory Issues Must Be Addressed to Integrate CGM Data into the EHR?
Moderator
Axel Wirth, CPHIMS, CISSP, HCISPP, AAMIF, FHIMSS
MedCrypt, San Diego, CA, USA
Speakers
Bryan Cunningham, JD
University of California, Irvine, Irvine, CA, USA
L. Reuven Pasternak, MD, MPH, MBA
US Department of Homeland Security, Arlington, VA, USA
Bakul Patel, MSc, MBA
US Food and Drug Administration, Silver Spring, MD, USA
Iliana L. Peters, JD, LLM, CISSP
Polsinelli, Washington, DC, USA
Privacy, security, and regulatory issues for medical devices create serious obligations for health sector entities and their business partners. If medical devices, especially implanted ones, are comprised, then there is a threat to human life and safety. There have already been attacks on oncology devices that compromised care for cancer patients or on the vaccine supply chain.23,24
Threat actors can target any software-based device, and such devices can be vulnerable to other data security issues as well, yet many devices do not have enough processing power to support sufficient security technology, or they do not have sufficient security controls. After many headline-grabbing cyber-attacks, the government is taking action by, for example, requiring zero trust cybersecurity principles to be used on government networks. Analogously, for CGMs, policy makers, healthcare entities, and patients should consider implementing robust technological, administrative, and physical safeguards to protect the health information flowing along the entire CGM supply and communications chain end to end. The US Food and Drug Administration (FDA) has recognized the need for strong safeguards with regard to the evolving Internet of Medical Things, making compliance with and implementation of such safeguards a necessary part of regulatory compliance.25,26 However, the security and privacy features of these consumer-oriented devices still need to be easy to use. As patients are becoming more involved in their own healthcare, patient and caregiver education is necessary in addition to the development of new technical features to allow patients to make decisions about sharing their health information.
Data security, like device quality, is not negotiable on a case-by-case basis. Barriers that still need to be overcome include a patchwork of state, federal, and international laws and regulations, the insufficiency of traditional security models and technologies, and the lack of open cybersecurity information sharing.
Sessions 7 and 8: Breakout Group Discussions and Presentation of Breakout Group Ideas
Moderators
David Kerr, MBChB, DM, FRCP, FRCPE
Sansum Diabetes Research Institute, Santa Barbara, CA, USA
Raman Khanna, MD, MS
University of California, San Francisco, San Francisco, CA, USA
Scott Weinstein, JD
McDermott Will & Emery, Washington, DC, USA
Breakout Group 1
Many existing barriers to integration of CGM data are based around complexities associated with “change management” for the various stakeholders including clinicians, patients, healthcare systems, CGM manufacturers, regulators, IT specialists, and EHR systems. To circumvent this, there is interest in using third-party aggregators to facilitate CGM data integration, but this will depend on the cost and time needed from stakeholders.
Proposed measures of CGM data to include in the data integration include values in the AGP and the GRI composite metric. Forward compatible features ideally should include easy access to insulin data as well as information on food choices and physical activity, with a natural progression to decision support features. The amount and duration of CGM data stored also require further assessment. One solution would be the creation of a “data lake” with clinicians and researchers being able to access specific amounts of data from it depending on need. Other desired key features include (1) patients being able to access their own data, (2) ensuring privacy and security, and (3) being able to auto-populate progress reports.
Breakout Group 2
To accommodate different workflows, CGM integration into the EHR should allow for different levels of granularity. Different HCPs, like inpatient versus outpatient, may want to visualize the data from various time intervals, whether it be several days, a week, or even weekdays versus weekends. It will be beneficial to think about how similar data, such as vital sign data, has been handled in the past. However, the solution for vital sign data was often to discard large amounts of data and to not set alarms given the reliability of repeated testing. This must be addressed to improve the usability of CGM data.
Data should be presented as discrete points to allow for medication adjustment at specific timepoints. The amount of data and the length of time for which it is available should also be standardized. It could be helpful to integrate other relevant data such as device metadata and vital signs. It will also be important to make sure the integration process is not manufacturer or vendor specific so that it can easily be adapted as medical technology continues to evolve.
Data privacy and security must also be considered throughout the process of developing the integration workflow. Identity matching of a patient’s medical device data with their EHR health data should be done through an equitable, standardized, and accurate means. Patients should have the ability to control their data sharing so that they can elect to go off the grid if desired. However, they should be reminded that if not enough data is shared, then this process may not be as helpful for disease management.
Breakout Group 3
There is a clear distinction between the outpatient and the inpatient use cases for continuous glucose monitoring—in terms of both the level of detail and the frequency of data that an HCP would want to observe from a CGM and the types of devices that would be used. There are also use cases for special populations, such as people with gestational diabetes and older adults, as well as the patient-driven data sharing where patients may want to share their CGM data with HCPs other than their endocrinologist.
It is important to determine the level of data granularity from a CGM that would be most useful in the EHR. In an outpatient setting, it would be helpful to define key metrics over a standard period of time (e.g., two weeks). It would also be helpful to have a one-page report template that would include the basic amount of information that an HCP is typically looking for when reviewing CGM tracings. While including common trend-based analytics with the data integration would be helpful, HCPs may also still want access to raw CGM data in the EHR. Technically, there was debate about whether data integration should be built around a “push” (CGM data automatically move into the EHR) or “pull” (EHR sends requests for CGM data) framework.
Conclusion
The January 27, 2022 meeting was the launch of the iCoDE project. This program is intended to create a consensus set of standards and policies to overcome current barriers that impede the flow of CGM data from siloed software and mobile applications into the EHR. The iCoDE project’s Steering Committee members consist of key stakeholders who will meet via videoconferencing. These stakeholders have the technical and clinical experience to develop data standards and implementation policies to integrate CGM data into the EHR. Once there, the liberated data can be combined with other health information to develop better treatment plans for managing the health and well-being of PWD. Following the initial meeting described in this report, iCoDE project members will move forward to make CGM technology more accessible to all patients and the healthcare community by distilling consensus and establishing a needed standard for stakeholders in the integration and use of CGM data.
Acknowledgments
The organizers of the iCoDE project thank Eileen Faulds, PhD, MS, RN, Jane Jeffrie Seley, DNP, MPH, MSN, GNP, BC-ADM, CDCES, CDTC, FADCES, Guillermo E. Umpierrez, MD, CDCES, FACE, MACP, Amisha Wallia, MD, MS, Wei-An (Andy) Lee, DO, Charlotte Niznik, APRN, CDCES, Maya Payne, MD, Viral N. Shah, MD, Jennifer L. Sherr, MD, PhD, Carole Carey, MEngSc (Computer Eng), Gora Datta, FHL7, VS, SMIEEE, SMACM, Tom Haskell, BS, Ed Deng, BS, Mark Clements, MD, PhD, CPI, FAAP, CaroLynn Brinckerhoff, BA, Yael Shtrit, MSc, MBA, Avinash Shanbhag, MS, Amy B. Criego, MD, MS, Priya Prahalad, MD, PhD, Bryan Cunningham, JD, L. Reuven Pasternak, MD, MPH, MBA, Bakul Patel, MSc, MBA, and Iliana L. Peters, JD, LLM, CISSP for their helpful advice in planning this meeting and preparing this report and Annamarie Sucher-Jones for her expert editorial assistance.
Footnotes
Abbreviations: AGP, ambulatory glucose profile; CGM, continuous glucose monitor; CPOE, computerized physician order entry; DTMoSt, DTS Mobile Platform Controlling a Diabetes Device Security and Safety Standard; DTS, Diabetes Technology Society; DTSec, DTS Cybersecurity Standard for Connected Diabetes Devices; EHR, electronic health record; FDA, Food and Drug Administration; GRADE, Glycemic Risk Assessment Diabetes Equation; GRI, Glycemia Risk Index; HCP, healthcare professional; HHS ONC HIT, US Department of Health and Human Services Office of the National Coordinator Health Information Technology; HL7, Health Level 7; iCoDE, Integration of Continuous Glucose Monitor Data into the Electronic Health Record; IEEE, Institute of Electrical and Electronics Engineers; IT, information technology; PDF, Portable Document Format; PWD, persons with diabetes; US, United States; USCDI, US Core Data for Interoperability.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: NYX is a consultant to Abbott Diabetes Care. KTN is a consultant to Abbott Diabetes Care. DCK is a consultant to AI Health, Dexcom, Eli Lilly, EOFlow, Integrity, Lifecare, Medtronic, Novo, Roche Diagnostics, Rockley Photonics, and Thirdwayv. EKS reports that this work was supported in part by a VA MERIT award from the US Department of Veterans Affairs Clinical Sciences Research and Development Service (1I01CX001825). He has received unrestricted research support from Dexcom (to the Baltimore VA Medical Center and to the University of Maryland) for the conduction of clinical trials. SS serves as a clinical director for the Digital Care Delivery Team at Anthem Inc. for which he is compensated with salary and other benefits. DK has received remuneration for participation in Advisory Boards from Sanofi, Novo Nordisk, and Abbott Diabetes Care. He also has received research support from Novo Nordisk and Abbott Diabetes Care and has financial interests in Glooko, Hi.Health, and SNAQ. JE is a paid consultant for AI Health. AI Health played no role in the design, execution, analysis, or write up of this work. AI Health did not play a role in the decision to publish this article and had no editorial input. JE is supported by the Food and Drug Administration (FDA) under award number P50FD006425 for The West Coast Consortium for Technology & Innovation in Pediatrics (PI: Espinoza). The funding sources had no involvement in the development of this article or in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the FDA. AYD, JMG, SNS, CMB, AR, AW, RK, and SW have nothing relevant to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: iCoDE is supported by grants from Abbott, AI Health, Ascensia Diabetes Care, Bigfoot Biomedical, Dexcom, Diabeloop, Eli Lilly, EOFlow, Glytec, LifeScan, Medtronic, Rockley Photonics, Sanofi, Terumo, and Welldoc.
ORCID iDs: Nicole Y. Xu  https://orcid.org/0000-0001-9353-8819
https://orcid.org/0000-0001-9353-8819
Kevin T. Nguyen  https://orcid.org/0000-0001-9102-6537
https://orcid.org/0000-0001-9102-6537
Ashley Y. DuBord  https://orcid.org/0000-0002-1478-7065
https://orcid.org/0000-0002-1478-7065
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
Julian M. Goldman  https://orcid.org/0000-0001-7740-0975
https://orcid.org/0000-0001-7740-0975
Shahid N. Shah  https://orcid.org/0000-0001-8481-6493
https://orcid.org/0000-0001-8481-6493
Elias K. Spanakis  https://orcid.org/0000-0002-9352-7172
https://orcid.org/0000-0002-9352-7172
Charisse Madlock-Brown  https://orcid.org/0000-0002-3647-1045
https://orcid.org/0000-0002-3647-1045
Siavash Sarlati  https://orcid.org/0000-0003-1023-9130
https://orcid.org/0000-0003-1023-9130
Azhar Rafiq  https://orcid.org/0000-0001-5618-5911
https://orcid.org/0000-0001-5618-5911
Axel Wirth  https://orcid.org/0000-0002-5724-8125
https://orcid.org/0000-0002-5724-8125
David Kerr  https://orcid.org/0000-0003-1335-1857
https://orcid.org/0000-0003-1335-1857
Raman Khanna  https://orcid.org/0000-0003-0117-075X
https://orcid.org/0000-0003-0117-075X
Scott Weinstein  https://orcid.org/0000-0003-0241-1551
https://orcid.org/0000-0003-0241-1551
Juan Espinoza  https://orcid.org/0000-0003-0513-588X
https://orcid.org/0000-0003-0513-588X
References
- 1. Espinoza J, Xu NY, Nguyen KT, Klonoff DC. The need for data standards and implementation policies to integrate CGM data into the electronic health record [published online ahead of print November 20, 2021]. J Diabetes Sci Technol. doi: 10.1177/19322968211058148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The Office of the National Coordinator for Health Information Technology. Patient engagement playbook. Health Information Technology. https://www.healthit.gov/playbook/pe/. Published May 31, 2019. Accessed March 4, 2022.
- 3. The Office of the National Coordinator for Health Information Technology. Integrate patient-generated health data and EHRs. Health Information Technology. https://www.healthit.gov/playbook/pe/chapter-5/. Published May 30, 2018. Accessed March 4, 2022.
- 4. Singh LG, Satyarengga M, Marcano I, et al. Reducing inpatient hypoglycemia in the general wards using real-time continuous glucose monitoring: the glucose telemetry system, a randomized clinical trial. Diabetes Care. 2020;43(11):2736-2743. doi: 10.2337/dc20-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis GM, Faulds E, Walker T, et al. Remote continuous glucose monitoring with a computerized insulin infusion protocol for critically ill patients in a COVID-19 medical ICU: proof of concept. Diabetes Care. 2021;44(4):1055-1058. doi: 10.2337/dc20-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Faulds ER, Jones L, McNett M, et al. Facilitators and barriers to nursing implementation of continuous glucose monitoring (CGM) in critically ill patients with COVID-19. Endocr Pract. 2021;27(4):354-361. doi: 10.1016/j.eprac.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chow KW, Kelly DJ, Rieff MC, et al. Outcomes and healthcare provider perceptions of real-time continuous glucose monitoring (rtCGM) in patients with diabetes and COVID-19 admitted to the ICU. J Diabetes Sci Technol. 2021;15(3):607-614. doi: 10.1177/1932296820985263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallia A, Umpierrez GE, Rushakoff RJ, et al. Consensus statement on inpatient use of continuous glucose monitoring. J Diabetes Sci Technol. 2017;11(5):1036-1044. doi: 10.1177/1932296817706151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umpierrez GE, Klonoff DC. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care. 2018;41(8):1579-1589. doi: 10.2337/dci18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallia A, Prince G, Touma E, El Muayed M, Seley JJ. Caring for hospitalized patients with diabetes mellitus, hyperglycemia, and COVID-19: bridging the remaining knowledge gaps. Curr Diab Rep. 2020;20(12):77. doi: 10.1007/s11892-020-01366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill NR, Hindmarsh PC, Stevens RJ, Stratton IM, Levy JC, Matthews DR. A method for assessing quality of control from glucose profiles. Diabet Med J Br Diabet Assoc. 2007;24(7):753-758. doi: 10.1111/j.1464-5491.2007.02119.x. [DOI] [PubMed] [Google Scholar]
- 12. Klonoff DC, Wang J, Rodbard D, et al. A glycemia risk index (GRI) of hypo- and hyperglycemia for continuous glucose monitoring validated by clinician ratings. J Diabetes Sci Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diabetes Technology Society. DTS cybersecurity standard for connected diabetes devices. Diabetes Technology Society. Date unknown. https://www.diabetestechnology.org/dtsec.shtml. Accessed March 4, 2022.
- 14. Diabetes Technology Society. Diabetes technology society mobile platform controlling a diabetes device security and safety standard (DTMoSt). Diabetes Technology Society. Date unknown. https://www.diabetestechnology.org/dtmost.shtml. Accessed March 4, 2022.
- 15. SA Main Site. Medical devices cybersecurity—IEEE P2621 series of standards. Date unknown. https://standards.ieee.org/products-services/icap/programs/p2621-series-of-standards/. Accessed March 4, 2022.
- 16. Olsen E. Digital health apps balloon to more than 350,000 available on the market, according to IQVIA report. MobiHealthNews. August 4, 2021. https://www.mobihealthnews.com/news/digital-health-apps-balloon-more-350000-available-market-according-iqvia-report. Accessed March 4, 2022.
- 17. OAuth. OAuth 2.0. Date unknown. https://oauth.net/2/. Accessed March 4, 2022.
- 18. HealthIT.gov. 2020-2025 federal health IT strategic plan. Date unknown. https://www.healthit.gov/topic/2020-2025-federal-health-it-strategic-plan. Accessed March 4, 2022.
- 19. Health information technology. United States Core Data for Interoperability (USCDI). Date unknown. https://www.healthit.gov/isa/united-states-core-data-interoperability-uscdi. Accessed March 4, 2022.
- 20. HealthPartners. First-of-its-kind model integrates continuous glucose monitoring data directly in electronic health record, allowing for easy access to valuable diabetes management information. HealthPartners. https://www.healthpartners.com/hp/about/press-releases/first-of-its-kind-model-integrates-continuous-glucose-monitoring-data.html. Published 2021. Accessed March 4, 2022.
- 21. Espinoza J, Shah P, Raymond J. Integrating continuous glucose monitor data directly into the electronic health record: proof of concept. Diabetes Technol Ther. 2020;22(8):570-576. doi: 10.1089/dia.2019.0377. [DOI] [PubMed] [Google Scholar]
- 22. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi: 10.1093/jamia/ocv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slabodkin G. Ransomware attacks put availability of medical devices at risk: FDA cyber chief. MedTech Dive. October 1, 2021. https://www.medtechdive.com/news/cyber-attacks-security-medical-devices-kevin-fu-advamed/607483/. Accessed March 4, 2022.
- 24. Vengattil M, Ganguli S. IBM flags more cyber attacks on COVID vaccine infrastructure. Reuters. April 14, 2021. https://www.reuters.com/article/us-health-coronavirus-vaccines-cyber-idUSKBN2C12EU. Accessed March 4, 2022.
- 25. Center for Devices and Radiological Health. Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions. U.S. Food and Drug Administration. Published April 6, 2022. Accessed April 19, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cybersecurity-medical-devices-quality-system-considerations-and-content-premarket-submissions
- 26. Center for Devices and Radiological Health. Postmarket management of cybersecurity in medical devices. US Food & Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/postmarket-management-cybersecurity-medical-devices. Published March 7, 2019. Accessed February 24, 2022.