Abstract
Objective
To examine outpatient care fragmentation and its association with future hospitalization among patients at high risk for hospitalization.
Data Sources
Veterans Affairs (VA) and Medicare data.
Study Design
We conducted a longitudinal study, using logistic regression to examine how outpatient care fragmentation in FY14 (as measured by number of unique providers, Breslau's Usual Provider of Care (UPC), Bice‐Boxerman's Continuity of Care Index (COCI), and Modified Modified Continuity Index (MMCI)) was associated with all‐cause hospitalizations and hospitalizations related to ambulatory care sensitive conditions (ACSC) in FY15. We also examined how fragmentation varied by patient's age, gender, race, ethnicity, marital status, rural status, history of homelessness, number of chronic conditions, Medicare utilization, and mental health care utilization.
Data Extraction Methods
We extracted data for 130,704 VA patients ≥65 years old with a hospitalization risk ≥90th percentile and ≥ four outpatient visits in the baseline year.
Principal Findings
The mean (SD) of FY14 outpatient visits was 13.2 (8.6). Fragmented care (more providers, less care with a usual provider, more dispersed care based on COCI) was more common among patients with more chronic conditions and those receiving mental health care. In adjusted models, most fragmentation measures were not associated with all‐cause hospitalization, and patients with low levels of fragmentation (more concentrated care based on UPC, COCI, and MMCI) had a higher likelihood of an ACSC‐related hospitalization (AOR, 95% CI = 1.21 (1.09‐1.35), 1.27 (1.14‐1.42), and 1.28 (1.18‐1.40), respectively).
Conclusions
Contrary to expectations, outpatient care fragmentation was not associated with elevated all‐cause hospitalization rates among VA patients in the top 10th percentile for risk of admission; in fact, fragmented care was linked to lower rates of hospitalization for ACSCs. In integrated settings such as the VA, multiple providers, and dispersed care might offer access to timely or specialized care that offsets risks of fragmentation, particularly for conditions that are sensitive to ambulatory care.
Keywords: care fragmentation, continuity of care, multimorbidity, health system outcome models, care coordination
What is known on this topic
Care fragmentation (dispersion of a patient's care across clinicians and health care settings) is a common challenge for patients, particularly for those with multiple chronic conditions.
Fragmentation within primary care and across multiple prescribers and settings has been associated with higher rates of hospitalization and emergency department visits.
What this study adds
In this study of Veterans Affairs patients at high‐risk for hospitalization, fragmented care (more providers, less care with a usual provider, more dispersed care) was more common among patients with more chronic conditions and those receiving mental health care.
Contrary to expectations, we found that fragmented outpatient care did not increase risk of future all‐cause hospitalization among patients in the top 10th percentile for the VA patient population, and in fact was associated with a lower likelihood of hospitalization for ambulatory care‐sensitive conditions.
1. INTRODUCTION
Care fragmentation—dispersion of a patient's care across clinicians and health care settings—is a common challenge, particularly for patients with multiple chronic conditions. 1 , 2 , 3 , 4 , 5 One study found that Medicare beneficiaries with ≥7 chronic conditions (38% of those enrolled) saw a median of eight specialists working in seven different practices. 6 Given the prevalence of this issue, there is a need for a greater understanding of fragmentation patterns and their consequences across different settings.
System‐level care fragmentation is a relatively new area of inquiry, however primary care fragmentation (and the related concept of continuity) has been the focus of investigation for decades. 7 , 8 , 9 , 10 , 11 Systematic reviews of continuity measures 12 , 13 suggest that the most common measures of primary care continuity focus on density or concentration of care with a single provider (e.g., Breslau's Usual Provider of Care (UPC) measure) 14 or dispersion of care across multiple providers (e.g., Bice‐Boxerman's Continuity of Care Index (COCI)). 15 In recent years, several studies have adapted these and other measures to examine care fragmentation across all outpatient providers, 16 , 17 and have also developed and evaluated a number of measures that reflect the fragmentation of care across health systems, 18 , 19 although additional validation of outpatient care fragmentation measures is needed to inform policy and practice.
Substantial literature has documented the potential risks associated with fragmented care, including information loss, adverse medication interactions, duplicative tests, and unwieldy self‐care regimens. 20 , 21 , 22 , 23 Fragmentation within primary care and across multiple prescribers has been associated with higher rates of hospitalization and emergency department (ED) visits. 24 , 25 , 26 , 27 , 28 Fragmentation across health systems and/or payers has also been linked to increased risk of acute care utilization, as well as poor clinical outcomes. 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 One recent study found that Veterans Affairs (VA) patients who receive opioid prescriptions from both VA and Medicare participating providers are at higher risk for opioid overdose. 38
Despite the literature suggesting that fragmented care is associated with negative consequences, many patients require care from multiple providers and across multiple settings. This is particularly true for individuals with complex medical issues, many of whom are at the most elevated risk for poor outcomes such as hospitalization. Understanding the consequences of fragmented care in these patients could inform system‐level risk‐reduction interventions. In this study, we sought to advance understanding of the consequences of care fragmentation among patients within an integrated delivery system who were at high‐risk for hospitalization. Using national VA data, we characterized outpatient care fragmentation patterns among VA patients whose risk of one‐year hospitalization was in the top 10th percentile for the VA system. We then evaluated the relationship between fragmented care and hospitalizations in the following year.
2. METHODS
2.1. Study population and data sources
The study cohort comprised individuals aged 65 and older who received VA care in fiscal year 2014 (FY14) and were continuously enrolled in Medicare Fee‐for‐Service (FFS) Part A & Part B in FY14‐15. Analyses focused on patients who were at high‐risk for one‐year hospitalization based on an established VA Care Assessment Need (CAN) risk score 39 that is calculated weekly for all VA outpatients; patients were included if their last recorded CAN score in FY14 was ≥90, indicating a risk that was in or above the 90th percentile for the VA patient population, and if they were alive at the end of that year. The study used a cut‐point of 90th percentile because this is the cut‐point used operationally by VA program offices such as VA's Office of Primary Care to identify patients who are eligible for intensive outpatient programs and other services. 40 , 41 This cut‐point identifies patients with a range of risk for one‐year hospitalization, from 20 to 30% probability among those with a CAN score of 90, to 58 to 95% probability among those with a CAN score of 99. Previous studies suggest that there is minimal variation in care fragmentation for patients with three or fewer visits, 42 , 43 , 44 so we excluded patients with less than four outpatient visits (Appendix S2). For all included patients, we analyzed data for VA care, VA‐purchased community care (hereafter referred to as “Community Care”), and care covered by Medicare for FY14‐15. 45
2.2. Care fragmentation measures
Analyses focused on measures that reflect fragmentation of outpatient visit encounters, in VA, Community Care and Medicare. To combine care use across these systems, we used a modification of the classification algorithm presented in Burgess, et al. 46 We defined an eligible encounter as a clinic‐ or home‐based visit for evaluation and management (E&M), care coordination, or psychotherapy and other mental health services (as defined by Current Procedural Terminology [CPT] and Health care Common Procedure Coding System [HCPCS] codes). Using provider specialty codes, we included medical care visits conducted by a physician, nurse practitioner (NP), or physician assistant (PA); for mental health care visits, we included visits with a psychiatrist, psychologist, or licensed clinical social worker. Within VA only, residents were identified using provider taxonomy code. Outpatient visits were categorized as primary care, mental health care, or specialty medical and surgical care based on VA Stop Code (VA care) and provider specialty (Community Care and Medicare). 46 Due to limited detail about NP and PA specialty in Community Care and Medicare, these NP/PA claims were grouped as a category without specialty. National Provider Index (NPI) was used to identify unique providers and calculate all measures. Urgent care and ED visits were not included in outpatient care visits given that emergency care is often a precursor to hospitalization, the study outcome. Appendix S1 describes additional information about the specific data sources and identification of encounters and providers used in constructing care fragmentation measures.
We constructed four outpatient care fragmentation measures for FY14 (Table 1): provider count (number of unique providers), concentration of care with an empirically defined “usual provider” using Breslau's Usual Provider of Care (UPC), care dispersion across providers using Bice‐Boxerman's Continuity of Care Index (COCI), and an adaption of this measure, the Modified Modified Continuity Index (MMCI). We also used the UPC measure to identify the “usual provider” (i.e., the clinician who the patient saw most frequently), and categorized these clinicians as primary care, mental health care, specialty/surgical care, and NP/PA (in Community Care and Medicare). In cases of a tie for UPC, we assigned provider type in order of primary care, NP/PA, mental health, and specialty/surgical.
TABLE 1.
Fragmentation measures
| Measure | Formula | Definition | Range | |
|---|---|---|---|---|
| Provider Count | p | Number of unique providers | 0 to p (higher number = greater fragmentation across outpatient providers) | |
| UPC |
|
Density of care with the most frequently seen provider | 0 to 1 (higher number = greater proportion of visits with one provider) | |
| COCI |
|
Dispersion of care across providers | 0 to 1 (higher number = care more concentrated among providers) | |
| MMCI |
|
Dispersion of care across providers | 0 to 1 (higher number = care more concentrated among providers) |
Note: p, total number of providers; n, total visits to outpatient care; ni, number of visits to pi. UPC, Usual Provider of Care; COCI, Bice‐Boxerman's Continuity of Care Index; MMCI Modified Modified Continuity Index.
2.3. Outcome measures and covariates
The primary dependent variable of interest was the occurrence of an all‐cause VA, Community Care, or Medicare hospitalization in FY15. Secondary analyses examined hospitalizations for ambulatory care sensitive conditions (ACSCs), admissions that some consider to be more sensitive to optimal ambulatory care and high‐quality care coordination (Appendix S2). 47
We measured patient characteristics potentially related to fragmentation and hospitalization including demographic characteristics, clinical characteristics, and health care utilization. Data from VA's Observation Medical Outcomes Partnership (OMOP) was used for gender, race, ethnicity, and date of death. The transformation relies on available VA race and ethnicity data following VA‐defined best practices. Marital status was derived from VA enrollment data reflecting the last recorded status in FY14. Geocoded enrollment data provided information on rurality, based on Rural Urban Community Area 48 and patient's nearest VA facility providing ambulatory and inpatient care. History of homelessness was computed using the presence of the International Classification of Diseases, Ninth Revision (ICD‐9) V60.0 code. Chronic conditions were identified using ICD‐9 diagnosis codes for 47 conditions as categorized by the Agency for Healthcare Research and Quality 49 and VA's Women's Health Evaluation Initiative. 50 Conditions were coded as present if they occurred in two outpatient or one inpatient visit in FY14 in VA, Community Care or Medicare. Total number of FY14 visits and any mental health visit were calculated based on care in VA, Community Care and Medicare. Medicare enrollment in FFS Parts A & B was assessed using the Master Beneficiary Summary File.
2.4. Statistical analysis
We examined the properties of the care fragmentation measures by generating histograms and scatterplots for each measure and calculating Pearson correlation coefficients for each pair of measures. We used nonparametric tests (Wilcoxon for binary and Kruskal‐Wallis for multi‐category characteristics) to examine variation in each care fragmentation measure by patient characteristics, including age, gender, marital status, race, ethnicity, rurality, history of homelessness, number of chronic conditions, number of visits, Medicare visits, and indicator for any mental health visit.
We used mixed effects logistic models (using the glmer function from the lmerTest package in R 51 ) to test the association between care fragmentation in FY14 and (1) any hospitalization, and (2) ACSC hospitalization in FY15. In post‐hoc secondary analyses for models with the primary outcome, we examined whether fragmentation interacted with clinical characteristics such as number of chronic conditions and use of mental health care, and ran exploratory models stratified by these factors. Because of the competing risk of death, we also ran models excluding those who died in the follow‐up year as a sensitivity analysis. In addition, for models with the primary outcome, we fit competing risk survival models with hospitalization as the outcome of interest and death treated as a competing risk (using the coxph function from the survival package in R). In the development of the models, we fit restricted cubic splines with the rcspline.plot function of the Hmisc package 52 in R to test the linearity of the care fragmentation measure terms in the logistic models. With the exception of number of providers for ACSC hospitalizations, the models indicated that fragmentation measures could be treated as linear. Therefore, for the majority of models we included all measures as linear terms; the only exception was the inclusion of a linear spline with a knot at seven for number of providers in models for ACSC hospitalization. The patient's nearest VA facility providing ambulatory and inpatient care was included as a random effect (n = 165).
We conducted multiple imputation by chained equations with the mice package 53 in R to address missing data in race, ethnicity, marital status, and rurality. We used five imputed data sets as only a small proportion (<4%; Table 2) of Veterans had any missing values, and we used all default options of the mice function. All analyses were performed using SAS for Windows version (9.4) and R version (3.6). 54 All tests were two‐sided and p ≤ 0.05 was considered statistically significant.
TABLE 2.
Study population baseline characteristics (N = 130,704)
| N | % | |
|---|---|---|
| Gender | ||
| Female | 3035 | 2.3 |
| Male | 127,669 | 97.7 |
| Age, Mean (SD) | 73.7(7.9) | — |
| 65–74 | 83,106 | 63.6 |
| 75–84 | 31,113 | 23.8 |
| 85+ | 16,485 | 12.6 |
| Marital status | ||
| Married | 60,846 | 46.6 |
| Not Married | 69,334 | 53.0 |
| Missing | 524 | 0.4 |
| Race | ||
| White | 102,955 | 78.8 |
| Non‐white | 22,877 | 17.5 |
| Missing | 4872 | 3.7 |
| Ethnicity | ||
| Hispanic or Latino | 4793 | 3.7 |
| Not Hispanic or Latino | 121,943 | 93.3 |
| Missing | 3968 | 3.0 |
| Geographic location/rurality | ||
| Highly Rural/ Rural | 48,919 | 37.4 |
| Urban | 81,784 | 62.6 |
| Missing | 1 | 0.0 |
| History of Homelessness | 4356 | 3.3 |
| Chronic Conditions, Mean (SD) | 9.0 (4.0) | |
| <=5 | 24,867 | 19.03 |
| 6–7 | 25,899 | 19.82 |
| 8–9 | 26,704 | 20.43 |
| 10–12 | 28,919 | 22.13 |
| 13+ | 24,315 | 18.60 |
| VA, Community Care and Medicare Outpatient Visits, Mean (SD) | 13.2 (8.6) | |
| 4–6 | 24,786 | 19.0 |
| 7–9 | 27,575 | 21.1 |
| 10–12 | 23,606 | 18.1 |
| 13–17 | 26,228 | 20.1 |
| 18+ | 28,509 | 21.8 |
| Any Medicare Visit | 42,820 | 32.8 |
| Any Mental Health Visit | 50,003 | 38.3 |
This study was approved by the VA Palo Alto Health Care System Research & Development Committee and Stanford University Institutional Review Board.
3. RESULTS
3.1. Patient characteristics
For the 130,704 patients who met study criteria (Appendix S1), 97.7% were male, 78.8% were White, and the mean (SD) number of conditions was 9.0 (4.0) (Table 2). The most common chronic conditions were hypertension (84.2%), lipid disorders (64.8%), diabetes (52.1%), joint disorders (51.4%), and depression (31.0%) (Appendix S3).
The mean (standard deviation [SD]) number of total outpatient visits during FY14 was 13.2 (8.6); most patients in the sample (81.1%) had six or more visits, and 38.3% had a mental health visit. Approximately one‐third of the sample (32.8%) had at least one non‐VA outpatient visit covered by Medicare in FY14 (Table 2); 9.9% (12,897) had a VA‐purchased Community Care visit. More than half (57.8%) of the study cohort were hospitalized in the baseline year.
3.2. Care fragmentation measures: distribution and characteristics
The distribution of measures and their correlations are presented in Figure 1; median (IQR) values for each measure are in Appendix S4. Based on the UPC measure, the most common clinician identified as the “usual provider” was a primary care clinician (57.7%) followed by a specialist (26.7%), and a mental health care provider (14.8%). For the vast majority of patients (88.7%), the usual provider was located in the VA health system. COCI and MMCI were strongly correlated (r = 0.77), which reflects the fact that they measure the same dimension of fragmentation. UPC was correlated strongly with COCI (r = 0.93) and moderately with MMCI (r = 0.64). The number of providers was negatively correlated with UPC (r = −0.63), COCI (r = −0.51), and MMCI (r = −0.25) (Figure 1).
FIGURE 1.
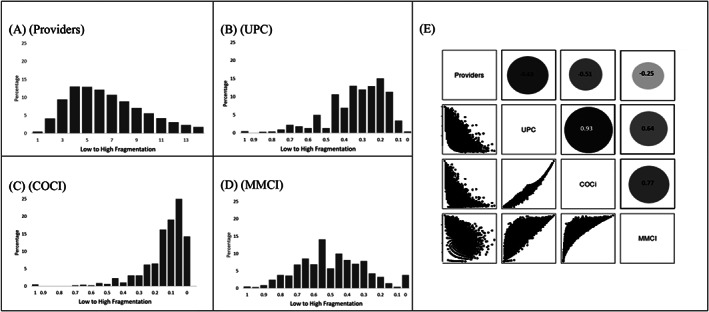
Fragmentation measure distributions and correlations. Panels A‐D illustrate the study population's fragmentation by (A) number of providers, (B) Usual Provider of Care (UPC), (C) Bice‐Boxerman's Continuity of Care Index (COCI), (D) Modified Modified Continuity Index (MMCI). Panel E illustrates the correlation among fragmentation measures using scatter plots, with the strength of correlation reflected by the corresponding circle size and density
Mental health care utilization, Medicare outpatient utilization, and the number of chronic conditions were associated with slightly more fragmented care across most measures (see Appendices S4 and S5). Although statistically significant differences were observed for other characteristics, most differences were not clinically meaningful. The associations between patient characteristics and fragmentation that were observed for number of providers, UPC, and COCI were not observed for MMCI, and in fact, the relationship between several characteristics (including chronic conditions and number of visits) was reversed (Appendices S4 and S5).
3.3. Association between fragmentation and future hospitalization risk
Close to half (46.1%, 60,262) of the observed patients were hospitalized in FY15, 14.5% (19,020) had an ACSC hospitalization, and 14.2% (18,564) died. In unadjusted analyses, patients with a greater number of providers at baseline had a higher rate of hospitalization the following year (Figure 2); the unadjusted relationships with hospitalization were modest for most other fragmentation measures (Appendix S6).
FIGURE 2.
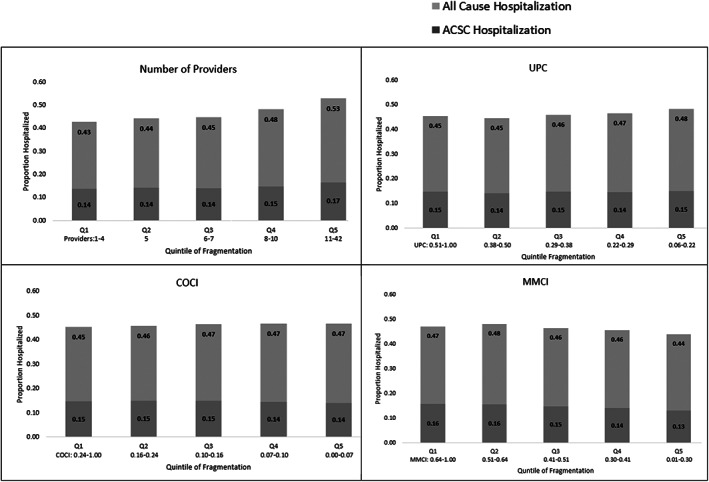
Unadjusted relationship between 12‐month outpatient care fragmentation in FY14 and hospitalizations in FY15. UPC Usual Provider of Care; COCI Bice‐Boxerman's Continuity of Care Index; MMCI Modified Modified Continuity Index. For all fragmentation measures, the panels depict the lowest quintile (most consolidated care) on the left, and the highest quintile (most dispersed care) on the right
In adjusted analyses, there was a relationship between provider count in FY14 and all‐cause hospitalization in FY15, with 1% higher odds of hospitalization per provider (AOR 1.01 [1.00, 1.01]); there were no statistically significant independent relationships between UPC, COCI, and MMCI and occurrence of all‐cause hospitalization in FY15 (Table 3). For ACSC hospitalization, among those with fewer than seven providers, the odds of ACSC hospitalization decreased for each additional provider; among those with seven or more providers, there was no relationship with ACSC hospitalization. UPC, COCI, and MMCI all had a positive association with ACSC hospitalization, such that those with less fragmented care had higher odds of an ACSC hospitalization: (UPC AOR 1.21, (1.09, 1.35), COCI AOR 1.27 (1.14, 1.42), MMCI AOR 1.28 (1.18, 1.40) (Table 3). Because a difference of 1 is the entire range for these outcomes, the effect for a smaller difference is more meaningful. For a difference of 0.2 on UPC, COCI, and MMCI these odds ratios correspond to point estimates of 3.9%, 4.9%, and 5.1% higher odds of hospitalization. Odds ratios for the full models are available in Appendix S7.
TABLE 3.
Adjusted relationship between outpatient care fragmentation in FY14 and hospitalization in FY15
| Outcome | Measure a | Adjusted odds ratio b (95% confidence interval) | |
|---|---|---|---|
| Full cohort | Sensitivity analysis c | ||
| Any Hospitalization | Provider count | 1.01 (1.00–1.01) | 1.02 (1.01–1.02) |
| UPC | 1.06 (0.98–1.14) | 0.97 (0.90–1.06) | |
| COCI | 1.04 (0.96–1.13) | 0.96 (0.88–1.05) | |
| MMCI | 0.98 (0.92–1.04) | 0.91 (0.85–0.97) | |
| Any ACSC Hospitalization | Provider count <7 | 0.96 (0.95–0.98) | 0.97 (0.95–0.98) |
| Provider count ≥7 | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | |
| UPC | 1.21 (1.09–1.35) | 1.20 (1.06–1.36) | |
| COCI | 1.27 (1.14–1.42) | 1.28 (1.12–1.45) | |
| MMCI | 1.28 (1.18–1.40) | 1.27 (1.15–1.40) | |
| N | 130,704 | 112,140 | |
| Number of hospitalizations | 60,262 | 46,587 | |
Note: Bolded values indicate statistically significant effects (p‐value ≤ 0.05). UPC, Usual Provider of Care; COCI, Bice‐Boxerman's Continuity of Care Index; MMCI, Modified Modified Continuity Index.
UPC, COCI and MMCI range from 0 to 1, where 0 is the most fragmented and 1 is least fragmented. Provider count for ACSC hospitalizations was modeled as a linear spline with a knot at 7.
Models mutually adjusted for demographics and clinical characteristics described in Table 2.
Sensitivity analyses exclude those died in FY15.
In sensitivity analyses excluding those who died in FY15, provider count remained significant for any hospitalization, and patients with more fragmented care as measured by MMCI were more likely to be hospitalized (Table 3). UPC and COCI were not significant in these models. In sensitivity analyses for the primary outcome that used a competing risks survival model, the hazard ratios yielded similar patterns to the original models (Appendix S8). For ACSC hospitalizations, relationships were similar to those observed in the full cohort. In post‐hoc exploratory models stratifying by number of chronic conditions, no significant differences were found for those with 6 to 10 chronic conditions. For patients with ≤5 conditions, having more concentrated care as measured by higher UPC and COCI was associated with a higher likelihood of hospitalization. For those with 10 or more conditions, having more concentrated care as measured by having fewer providers and higher MMCI was associated with a lower likelihood of hospitalization. Analyses stratified by the presence of any mental health care utilization suggest that patients with such use have a higher likelihood of hospitalization when their care is more fragmented. These exploratory analyses are presented in Appendix S8.
4. DISCUSSION
This study of VA patients at high‐risk for hospitalization offers novel insights about fragmentation measures and associated outcomes in patients with high levels of health care utilization. We found that individuals with greater medical complexity and mental health care utilization experienced more outpatient care fragmentation, but the association between this fragmentation and all‐cause hospitalization was close to zero after adjusting for clinical and sociodemographic factors. Surprisingly, our findings suggest that among VA patients in the top 10th percentile for hospitalization risk, having multiple providers and dispersed care was associated with a lower likelihood of ACSC hospitalization.
Our findings that most outpatient care fragmentation measures were not associated with all‐cause hospitalization in this high‐risk patient population calls into question the general assumption that fragmented care is always a problem. Previous research in the VA health care system found that fragmentation across multiple prescribers was associated with increased hospitalization and ED visit rates, 28 and that fragmentation across health systems (e.g., dual use of VA and Medicare) was linked to poor clinical outcomes for a range of conditions and increased all‐cause hospitalization. 24 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 Our study adds to this literature by suggesting that in VA patients who are at particularly high‐risk for hospitalization, care fragmentation might not be a major independent contributor to all‐cause hospitalization. This might be due to other factors driving hospitalization outcomes in these patients, or it might reflect the fact that many patients who are at extremely high risk for hospitalization require dispersed care to meet their needs. There may also be organizational factors specific to the VA, including its robust interoperable electronic health record, well‐established patient centered medical home program, 55 and care coordination programs such as home telehealth, 56 that mitigate the risks of fragmented care in this setting. Outside the VA, fragmentation patterns vary substantially across physicians and regions, 57 suggesting important contributors related to organizational structure and behavior.
The finding that patients with less fragmented outpatient care were more likely to experience an ACSC hospitalization suggests that dispersed care may be appropriate or even beneficial for certain conditions. For example, in a patient with heart failure, visits with multiple providers could indicate better access to timely or specialty care that contributes to better management in the ambulatory care setting. This explanation aligns with the view that ACSC hospitalizations are less a marker of ambulatory care quality and more an indication of access 58 and in some cases intensive case management. Despite the risks that are inherent in fragmented care, when more clinicians are involved in a patient's care, it could increase the chances that someone catches warning signs at an earlier stage, that a patient receives an appropriate diagnostic test or referral, or that necessary problem‐solving occurs when management challenges arise. Importantly, the same may not be true when the dispersed care is occurring within a single service such as primary care; a previous study found that older Veterans had lower rates of all‐cause and ACSC hospitalization when they had extremely high continuity (≥90% visits) with a single primary care provider. 26
Many patients in this study had a mental health diagnosis, a scenario in which having additional providers and visits across clinical specialties including mental health might be important to reduce risk of hospitalization. Post‐hoc exploratory analyses, however, found that patients with one or more mental health visits appeared to have a higher likelihood of all‐cause hospitalization when their care was more fragmented (Appendix S8). Additional exploratory analyses suggested that the association between outpatient fragmentation and hospitalization was influenced by a patient's number of chronic conditions; most notably, for the measures where there was a statistically significant odds ratio, patients with five or fewer chronic conditions whose care was concentrated had a higher likelihood of hospitalization, whereas in patients with 10 or more chronic conditions, patients with concentrated care had a lower likelihood of hospitalization (Appendix S8). Previous analyses of Medicare patients similarly found variation by number of chronic conditions and determined that fragmented care was associated with fewer hospitalization among patients with a high burden of comorbidities. 59 Together, these findings suggest that the consequences of outpatient care fragmentation may depend in part on a patient's number and type of comorbid conditions.
This study also offers some useful information about properties of claims‐based outpatient fragmentation measures among patients at high‐risk for hospitalization. Like previous analyses of fragmentation measures, we found a strong correlation between UPC and COCI. 60 , 61 , 62 This is logical, as patients whose care is dispersed across multiple providers (generating a low COCI) are less likely to have care highly concentrated with any one provider and therefore also likely to have a low UPC. 44 Our study adds to this literature by also investigating number of unique providers, which has not been studied as extensively as COCI, UPC, or MMCI. We found that number of providers had a more moderate correlation with UPC, and a distinct relationship with hospitalization, suggesting that number of providers and UPC might be a better choice when considering a pair of complementary fragmentation measures. Reporting number of providers with UPC could provide a more holistic picture of a patient's care pattern, as concentration of care with a single provider could help offset the overall fragmentation that is reflected by provider count. Finally, we found that while MMCI had a more normal distribution curve (by design), it varies more from the other measures (i.e., COCI and UPC) as number of visits increase, and has a qualitatively different unadjusted relationship with hospitalization. Although the formulae for both COCI and MMCI include a term for number of unique providers, COCI has the advantage of incorporating the number of visits with each provider and the number of providers, which might account for the differences observed between these measures and hospitalization (Appendix S9).
Our findings have several implications for policy and health care system innovation. Veteran care fragmentation may become more pronounced as legislation such as the 2014 Veterans Access, Choice and Accountability Act (Choice Act) 63 and the 2018 Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act 64 expand access to care from a wider range of sources. There are tradeoffs between timely access to care and fragmentation that result from accessing multiple providers. A recent study suggested that VA patients with diabetes whose care is highly fragmented and who have a non‐VA clinician as their usual provider of ambulatory care have a 19% increased odds of hospitalization. 17 Care coordination for these patients will be paramount – however, primary care management of fragmentation can be time‐ and resource‐intensive. VA has implemented interventions to coordinate VA and community care 65 and has tested a number of programs that focus on high‐risk patient populations, including Veterans with serious mental illness, 66 those who are home‐bound, 67 and those who are at high‐risk for hospitalization. 40 , 68 Additional research is needed to understand how these specialized programs influence fragmentation and the effects of fragmented care on outcomes.
There are several limitations to this study. First, our study was limited to patients in the top 10th percentile for one‐year hospitalization risk, so these results cannot be generalized to all patients at risk for fragmentation; future work could consider alternate cut‐points in identifying high‐risk patients, as well as a longer follow‐up period. Second, the observational design of this study is subject to bias from residual confounding on account of unmeasured covariates that might influence fragmentation patterns and hospitalization, such as a patient's perceived mental health or health status, and also does not account for potential endogeneity from factors that can simultaneously influence both their use of outpatient care and their risk of hospitalization. Failing to take these into account may result in biased estimates of the effect size of fragmentation and continuity measures. 69 Third, the period of investigation preceded the Choice and MISSION Acts that expanded access to community care for Veterans, so evaluations of fragmentation in the current climate will be critical. Fourth, we focused our analyses on VA and Medicare‐covered care; we did not have access to a reliable source of insurance coverage for those under 65, or utilization information for Veterans with Medicare Advantage. To focus on a cohort whose data were as complete as possible, we excluded Veterans with partial coverage in the study years and those without Medicare A and B coverage. We explored incorporating Medicaid coverage for the study years, but at the time of analysis only 17 states reported Medicaid coverage, so we could not reliably look at Medicaid coverage in the study population. These decisions limit information on fragmentation between health care systems. This study was also not designed to assess the effects of different types of care fragmentation, such as fragmentation across different service lines within VA. A more nuanced evaluation of the impact of different types of fragmentation merits future research. Finally, we limited the study to patients who were alive at the end of the baseline year, so fragmentation patterns in the baseline year should be interpreted in this context.
5. CONCLUSION
In this cohort of VA patients at high‐risk for hospitalization, we found that fragmented care did not increase the risk of future all‐cause hospitalization, and in fact was associated with a lower likelihood of hospitalization for ambulatory care‐sensitive conditions. These findings suggest that dispersed care might not be problematic for patients with high‐levels of need, especially when they are receiving care within an integrated health care system. These findings were robust to multiple measures of fragmentation. Additional research is needed to examine whether this relationship is due to the benefits of multiple providers and dispersed care, or whether it could be related to underuse of inpatient services or other factors. Our findings highlight the complexity of measuring care fragmentation, and the value of utilizing multiple measures that examine more than one aspect of fragmented care.
Supporting information
Appendix Supporting information.
ACKNOWLEDGMENTS
We greatly appreciate the contributions of Camila Chaudhary, Jiaqi Hu, and Amy Gregory to literature review, data analysis support, and manuscript preparation. Views expressed do not represent the views of VA or the United States Government.
Zulman DM, Greene L, Slightam C, et al. Outpatient care fragmentation in Veterans Affairs patients at high‐risk for hospitalization. Health Serv Res. 2022;57(4):764‐774. doi: 10.1111/1475-6773.13956
Funding information Funding for this work was provided by VA HSR&D (IIR 15‐316, PI: Zulman; RCS 10‐391, PI: Maciejewski; CDA 15‐259, PI: Vanneman). This work was supported using resources and facilities at the VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13‐457. Support for VA/CMS Data provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (Project Numbers SDR 02‐237 and 98‐004).
REFERENCES
- 1. Centers for Medicare and Medicaid Services . Chronic Conditions among Medicare Beneficiaries, Chartbook. 2012th ed. Baltimore; 2012. https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/2012ChartBook. Accessed February 22, 2022. [Google Scholar]
- 2. Zulman DM, Jenchura EC, Cohen DM, Lewis ET, Houston TK, Asch SM. How can eHealth technology address challenges related to multimorbidity? Perspectives from patients with multiple chronic conditions. J Gen Intern med. 2015;30(8):1063‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rouse WB, Johns MM, Pepe KM. Service supply chains for population health: overcoming fragmentation of service delivery ecosystems. Learn Health Syst. 2019;3(2):e10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parekh AK, Goodman RA, Gordon C, Koh HK, The HHS Interagency Workgroup on Multiple Chronic Conditions . Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep. 2011;126(4):460‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maciejewski ML, Hammill BG, Bayliss EA, et al. Prescriber continuity and disease control of older adults. Med Care. 2017;55(4):405‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pham HH, Schrag D, O'Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J med. 2007;356(11):1130‐1139. [DOI] [PubMed] [Google Scholar]
- 7. Shortell SM. Continuity of medical care: conceptualization and measurement. Med Care. 1976;14(5):377‐391. [DOI] [PubMed] [Google Scholar]
- 8. Mindlin RL, Densen PM. Medical care of urban infants: continuity of care. Am J Public Health Nations Health. 1969;59(8):1294‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005;3(2):159‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Starfield BH, Simborg DW, Horn SD, Yourtee SA. Continuity and coordination in primary care: their achievement and utility. Med Care. 1976;14(7):625‐636. [DOI] [PubMed] [Google Scholar]
- 11. Eriksson EA, Mattsson LG. Quantitative measurement of continuity of care. Measures in use and an alternative approach. Med Care. 1983;21(9):858‐875. [DOI] [PubMed] [Google Scholar]
- 12. Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Med Care Res Rev. 2006;63(2):158‐188. [DOI] [PubMed] [Google Scholar]
- 13. van Walraven C, Oake N, Jennings A, Forster AJ. The association between continuity of care and outcomes: a systematic and critical review. J Eval Clin Pract. 2010;16(5):947‐956. [DOI] [PubMed] [Google Scholar]
- 14. Breslau N, Haug MR. Service delivery structure and continuity of care: a case study of a pediatric practice in process of reorganization. J Health Soc Behav. 1976;17(4):339‐352. [PubMed] [Google Scholar]
- 15. Bice TW, Boxerman SB. A quantitative measure of continuity of care. Med Care. 1977;15(4):347‐349. [DOI] [PubMed] [Google Scholar]
- 16. Liu CF, Chapko M, Bryson CL, et al. Use of outpatient care in Veterans Health Administration and Medicare among veterans receiving primary care in community‐based and hospital outpatient clinics. Health Serv res. 2010;45(5 Pt 1):1268‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajan M, Helmer D, Rowneki M, Fried D, Kern LM. Ambulatory care fragmentation and hospitalization among veterans with diabetes. Am J Manag Care. 2021;27(4):155‐160. [DOI] [PubMed] [Google Scholar]
- 18. Liu CF, Manning WG, Burgess JF Jr, et al. Reliance on veterans affairs outpatient care by Medicare‐eligible veterans. Med Care. 2011;49(10):911‐917. [DOI] [PubMed] [Google Scholar]
- 19. Mattocks KM, Cunningham K, Elwy AR, et al. Recommendations for the evaluation of cross‐system care coordination from the VA state‐of‐the‐art working group on VA/non‐VA care. J Gen Intern med. 2019;34(1):18‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716‐724. [DOI] [PubMed] [Google Scholar]
- 21. Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease‐specific guidelines for patients with multiple conditions. N Engl J med. 2004;351(27):2870‐2874. [DOI] [PubMed] [Google Scholar]
- 22. Kizer KW. Veterans and the affordable care act. JAMA. 2012;307(8):789‐790. [DOI] [PubMed] [Google Scholar]
- 23. Kern LM, Seirup JK, Casalino LP, Safford MM. Healthcare fragmentation and the frequency of radiology and other diagnostic tests: a cross‐sectional study. J Gen Intern med. 2017;32(2):175‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaiyachati KH, Gordon K, Long T, et al. Continuity in a VA patient‐centered medical home reduces emergency department visits. PLoS One. 2014;9(5):e96356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nelson K, Sun H, Dolan E, et al. Elements of the patient‐centered medical home associated with health outcomes among veterans: the role of primary care continuity, expanded access, and care coordination. J Ambul Care Manage. 2014;37(4):331‐338. [DOI] [PubMed] [Google Scholar]
- 26. Katz DA, McCoy KD, Vaughan‐Sarrazin MS. Does greater continuity of veterans administration primary care reduce emergency department visits and hospitalization in older veterans? J Am Geriatr Soc. 2015;63(12):2510‐2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wasson JH, Sauvigne AE, Mogielnicki RP, et al. Continuity of outpatient medical care in elderly men. A randomized trial. JAMA. 1984;252(17):2413‐2417. [PubMed] [Google Scholar]
- 28. Maciejewski ML, Powers BJ, Sanders LL, et al. The intersection of patient complexity, prescriber continuity and acute care utilization. J Gen Intern Med. 2014;29(4):594‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jia H, Zheng Y, Reker DM, et al. Multiple system utilization and mortality for veterans with stroke. Stroke. 2007;38(2):355‐360. [DOI] [PubMed] [Google Scholar]
- 30. Tarlov E, Lee TA, Weichle TW, et al. Reduced overall and event‐free survival among colon cancer patients using dual system care. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2231‐2241. [DOI] [PubMed] [Google Scholar]
- 31. Axon RN, Gebregziabher M, Everett CJ, Heidenreich P, Hunt KJ. Dual health care system use is associated with higher rates of hospitalization and hospital readmission among veterans with heart failure. Am Heart J. 2016;174:157‐163. [DOI] [PubMed] [Google Scholar]
- 32. Maciejewski ML, Wang V, Burgess JF Jr, Bryson CL, Perkins M, Liu CF. The continuity and quality of primary care. Med Care Res Rev. 2013;70(5):497‐513. [DOI] [PubMed] [Google Scholar]
- 33. Cooper AL, Jiang L, Yoon J, et al. Dual‐system use and intermediate health outcomes among veterans enrolled in Medicare advantage plans. Health Serv Res. 2015;50:1868‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pizer SD, Gardner JA. Is fragmented financing bad for your health? Inquiry. 2011;48(2):109‐122. [DOI] [PubMed] [Google Scholar]
- 35. Ajmera M, Wilkins TL, Sambamoorthi U. Dual Medicare and veteran health administration use and ambulatory care sensitive hospitalizations. J Gen Intern Med. 2011;26(Suppl 2):669‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolinsky FD, Miller TR, An H, Brezinski PR, Vaughn TE, Rosenthal GE. Dual use of Medicare and the Veterans Health Administration: are there adverse health outcomes? BMC Health Serv Res. 2006;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hester EJ, Cook DJ, Robbins LJ. The VA and Medicare HMOs — complementary or redundant? New England J Med. 2005;353(12):1302‐1303. 10.1056/nejmc051890 [DOI] [PubMed] [Google Scholar]
- 38. Moyo P, Zhao X, Thorpe CT, et al. Dual receipt of prescription opioids from the Department of Veterans Affairs and Medicare part D and prescription opioid overdose death among veterans: a nested case–control study. Ann Intern med. 2019;170(7):433‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368‐373. [DOI] [PubMed] [Google Scholar]
- 40. Yoon J, Chang E, Rubenstein LV, Zulman DM, Asch SM. Impact of primary care intensive management on high‐risk Veterans' costs and utilization. Ann Intern Med. 2018;169(7):515‐516. [DOI] [PubMed] [Google Scholar]
- 41. Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthcare (Amsterdam, Netherlands). 2018;6(4):231‐237. [DOI] [PubMed] [Google Scholar]
- 42. Kern LM, Seirup JK, Rajan M, Jawahar R, Stuard SS. Fragmented ambulatory care and subsequent emergency department visits and hospital admissions among Medicaid beneficiaries. Am J Manag Care. 2019;25(3):107‐112. [PubMed] [Google Scholar]
- 43. Nyweide DJ, Anthony DL, Bynum JP, et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013;173(20):1879‐1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosenberg NA, Zulman DM. Measures of care fragmentation: mathematical insights from population genetics. Health Serv Res. 2020;55(2):318‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. System of Records Notice 97VA10P1 : Consolidated Data Information System‐VA. US Department of Veterans Affairs 2015.
- 46. Burgess JF Jr, Maciejewski ML, Bryson CL, et al. Importance of health system context for evaluating utilization patterns across systems. Health Econ. 2011;20(2):239‐225. [DOI] [PubMed] [Google Scholar]
- 47. National Committee for Quality Assurance . Hospitalization for Potentially Preventable Complications (HPC). https://www.ncqa.org/hedis/measures/hospitalization-for-potentially-preventable-complications/. Published 2020. Accessed December 30, 2020.
- 48. US Department of Veterans Affairs Information Resource Center . VIReC Research User Guide: PSSG Geocoded Enrollee Files, 2015 Edition Hines IL2016.
- 49. Agency for Healthcare Research and Quality HCUP Chronic Condition Indicator. Healthcare Cost and Utilization Project (HCUP). http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Published 2011. Accessed July 17, 2013.
- 50. Frayne S, Phibbs C, Saechao F, et al. Sourcebook: Women veterans in the veterans health administration. Volume 3. Sociodemographics, utilization, costs of care, and health profile. In: Women's Health Evaluation Initiative, Women's Health Services, Veterans Health Administration, Department of Veterans Affairs; 2014. https://www.womenshealth.va.gov/docs/Sourcebook_Vol_3_FINAL.pdf. Accessed February 22, 2022.
- 51. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82(13):1‐26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- 52. Harrell FE Jr, Hmisc Dupont C. Harrell miscellaneous. R package version 4.0‐3. Online Publication. 2017.
- 53. Groothuis‐Oudshoorn K, Van Buuren S. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1‐67. [Google Scholar]
- 54. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria; 2019. https://www.R-project.org/. Accessed February 22, 2022. [Google Scholar]
- 55. Rosland AM, Nelson K, Sun H, et al. The patient‐centered medical home in the veterans health administration. Am J Manag Care. 2013;19(7):e263‐e272. [PubMed] [Google Scholar]
- 56. Darkins A, Kendall S, Edmonson E, Young M, Stressel P. Reduced cost and mortality using home telehealth to promote self‐management of complex chronic conditions: a retrospective matched cohort study of 4,999 veteran patients. Telemed J E Health. 2015;21(1):70‐76. [DOI] [PubMed] [Google Scholar]
- 57. Agha L, Erickson KM, Zhao X. The impact of organizational boundaries on healthcare coordination and utilization. Natl Bure Econ Res Work Paper Series. 2020;28179. https://www.nber.org/system/files/working_papers/w28179/w28179.pdf. Accessed February 22, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Busby J, Purdy S, Hollingworth W. A systematic review of the magnitude and cause of geographic variation in unplanned hospital admission rates and length of stay for ambulatory care sensitive conditions. BMC Health Serv res. 2015;15(1):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kern LM, Seirup JK, Rajan M, Jawahar R, Stuard SS. Fragmented ambulatory care and subsequent healthcare utilization among Medicare beneficiaries. Am J Manag Care. 2018;24(9):e278‐e284. [PubMed] [Google Scholar]
- 60. Chan CL, You HJ, Huang HT, Ting HW. Using an integrated COC index and multilevel measurements to verify the care outcome of patients with multiple chronic conditions. BMC Health Serv Res. 2012;12:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pollack CE, Hussey PS, Rudin RS, Fox DS, Lai J, Schneider EC. Measuring care continuity: a comparison of claims‐based methods. Med Care. 2016;54(5):e30‐e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ejlertsson G, Berg S. Continuity‐of‐care measures. An analytic and empirical comparison. Med Care. 1984;22(3):231‐239. [DOI] [PubMed] [Google Scholar]
- 63. Veterans Access, Choice, and Accountability Act of 2014, (2014).
- 64. S.2372 VA MISSION Act. In. 115 ed2018.
- 65. Greenstone CL, Peppiatt J, Cunningham K, et al. Standardizing care coordination within the Department of Veterans Affairs. J Gen Intern med. 2019;34(1):4‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mohamed S, Neale M, Rosenheck RA. VA intensive mental health case management in urban and rural areas: veteran characteristics and service delivery. Psychiatr Serv. 2009;60(7):914‐921. 10.1176/ps.2009.60.7.914 [DOI] [PubMed] [Google Scholar]
- 67. Edwards ST, Prentice JC, Simon SR, Pizer SD. Home‐based primary care and the risk of ambulatory care–sensitive condition hospitalization among older veterans with diabetes mellitus. JAMA Intern med. 2014;174(11):1796. 10.1001/jamainternmed.2014.4327 [DOI] [PubMed] [Google Scholar]
- 68. Zulman DM, Chee CP, Ezeji‐Okoye SC, et al. Effect of an intensive outpatient program to augment primary care for high‐need veterans affairs patients: a randomized clinical trial. JAMA Intern Med. 2017;177(2):166‐175. [DOI] [PubMed] [Google Scholar]
- 69. Pu C, Chou YJ. The impact of continuity of care on emergency room use in a health care system without referral management: an instrumental variable approach. Ann Epidemiol. 2016;26(3):183‐188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Supporting information.


