Abstract
Objective
To examine the associations of primary care physician (PCP) care continuity with cancer‐specific survival and end‐of‐life care intensity.
Data Sources
Surveillance, epidemiology, and end results linked to Medicare claims data from 2001 to 2015.
Study Design
Cox proportional hazards models with mixed effects and hierarchical generalized logistic models were used to examine the associations of PCP care continuity with cancer‐specific survival and end‐of‐life care intensity, respectively. PCP care continuity, defined as having visited the predominant PCP (who saw the patient most frequently before diagnosis) within 6 months of diagnosis.
Data Extraction Methods
We identified Medicare patients diagnosed at age 66.5–94 years with stage‐III or IV poor‐prognosis cancer during 2001–2012 and followed them up until 2015. Patients who died within 6 months after diagnosis were excluded.
Principal Findings
Primary study cohort consisted of 85,467 patients (median survival 22 months), 71.7% of whom had PCP care continuity. Patients with PCP care continuity tended to be older, married, nonblack, non‐Hispanic, and to have fewer comorbid conditions (p < 0.001 for all). Patients with PCP care continuity had lower cancer‐specific mortality (adjusted hazard ratio: 0.93; 95% confidence interval [CI]: 0.91 to 0.95; p = 0.001) than did those without PCP care continuity. Findings of the 2001–2003 cohorts (nearly all of whom died by 2015) show no associations of overall end‐of‐life care intensity measures with PCP care continuity (adjusted marginal effects: 0.005; 95% CI: −0.016 to 0.026; p = 0.264).
Conclusions
Among Medicare beneficiaries with advanced poor‐prognosis cancer, PCP continuity was associated with modestly improved survival without raising overall aggressive end‐of‐life care.
Keywords: cancer/oncology, care continuity, end of life, primary care physician, prospective cohort study, survival analysis
What is known on this topic
End‐of‐life care for patients diagnosed with advanced cancer is often aggressive, without aligned patient preferences.
Continuity of primary care physician (PCP) care may facilitate information exchange and patient‐centered decision making across PCPs, cancer specialists, and other care providers.
Among older persons with cancer, continuity with their primary care physician has been expected to improve survival and reduce the intensity of end‐of‐life care, but evidence to date has been inconclusive.
What this study adds
Medicare beneficiaries with stage‐III or IV poor‐prognosis cancer have a modest survival benefits but no associations with aggressive end‐of‐life care from following up with their predominant PCPs within 6 months following diagnosis.
Efforts to improve PCP continuity of care over time may benefit patients with poor‐prognosis cancer without increasing their end‐of‐life care burdens.
1. INTRODUCTION
With the increasing number of cancer survivors, 1 smooth care transition before and after cancer diagnosis becomes vital for patients' long‐term survival. 2 , 3 Continuity of care with one's primary care physician (PCP) is advocated as a way of not only improving cancer outcomes but also decreasing the intensity of end‐of‐life care. 4 , 5 , 6 For instance, patients with cancer who have a close relationship with their PCP may be more likely to share their values, including preferences of end‐of‐life care and lifestyle behaviors, with their PCP. 7 Continuity of PCP care may facilitate patients' information exchange between their PCPs, cancer specialists, and other care providers, 7 , 8 , 9 , 10 , 11 thus improving survival and reducing the need for aggressive care at the end‐of‐life period.
Evidence regarding associations between continuity of PCP care and survival benefits for cancer patients is limited. One study of Medicare beneficiaries, who had at least one PCP during the 2‐year baseline period in 1991–1993, found a 16% reduction in mortality risk associated with continuous visits to the same PCPs. 4 This sample, however, was not restricted to cancer patients. Follow‐up management of cancer survivors requires not only routine monitoring of cancer recurrence 12 but also active, systematic planning for additional cancer prevention and patient‐centered monitoring based on their personal risks, cancer treatments, family histories, lifestyle behaviors, and other comorbid clinical conditions. 13 Yet, little is known whether PCP care continuity would improve cancer survival of patients diagnosed with poor‐prognosis cancers.
Concerns have also been expressed about aggressive end‐of‐life cancer care, which often conflicts with patient preferences. 14 , 15 For various clinical conditions, PCP continuity is associated with lower use of health care services, 6 , 16 but evidence on end‐of‐life care in cancer has been mixed. 10 , 17 For example, in a Medicare decedent cohort with advanced lung cancer, PCP involvement prior to the diagnosis was associated with increased aggressive end‐of‐life care, including hospitalization, admission to intensive care units, and chemotherapy during the last month of life. 10 That study, however, was limited to prediagnosis PCP involvement, 18 and, as patients with longer survival were less likely to receive intensive end‐of‐life care, 19 studies using a decedent cohort may lead to biased conclusions. 20
To address these important knowledge gaps, we assessed continuity of PCP care prospectively in a cohort of patients with poor‐prognosis cancer. We present here the first study that examined the role of care continuity with the same PCP from the year prior to diagnosis through 6 months after diagnosis played in cancer‐specific survival and end‐of‐life care intensity. Findings from this study may aid in facilitating PCP care continuity for patients with advanced cancer, to maximize their survival while minimizing end‐of‐life burdens.
2. METHODS
2.1. Data source and study population
Using the Surveillance, Epidemiology, and End Results (SEER)—Medicare database, we constructed a historic cohort. The SEER registries, which currently cover approximately 30% of the US population, 21 collect information on patient demographics, tumor characteristics, month and year of diagnosis, and date and cause of death across 12 states. 21 Data on the use of health services were derived from Medicare claims. We identified patients diagnosed at age 66.5–94 years with stage‐III or IV poor‐prognosis cancer (Table S1), including lung, colorectal, kidney, esophagus, bladder, and brain and nervous system (hereafter designated brain) cancers between January 1, 2001 and December 31, 2012. These patients were followed up until December 31, 2015. We limited the study to patients who were alive 6 months after the diagnosis, so we would have a complete 6‐months' follow‐up for defining PCP care continuity. Patients with these poor‐prognosis cancers were chosen because the trajectory of disease progression for these advanced cancers tends to be homogeneous and PCP care continuity before and after cancer diagnosis might vary treatment compliance and prognosis management. To identify each patient's predominant PCP (the one who was visited the most during the year prior to cancer diagnosis), we limited our cohort to patients who had at least one PCP visit in the year prior to diagnosis. On average, the final sample patients visited their predominant PCPs eight times the year prior to diagnosis (M = 7.52; SD = 5.46). Detailed sample cohort selection is described in the supplementary material and illustrated in Figure S1.
To observe a cohort from diagnosis to deaths, we further analyzed survival and end‐of‐life intensity among the patients diagnosed with stage‐III or IV poor‐prognosis cancer between January 1, 2001, and December 31, 2003, as nearly all of these cohorts died during the study period. Focusing on this, 2001–2003 cohort will allow us to track recovering or improvements prior to dying, 20 as all patients diagnosed in these same years with or without PCP care continuity were observed from cancer diagnosis to deaths.
Yale University Human Investigation Committee determined that this study did not directly involve human subjects.
2.2. Measurement
Continuity of PCP care was defined as having at least one outpatient visit within 6 months of cancer diagnosis by the predominant PCP who saw the patient most frequently in the year before cancer diagnosis. To identify provider interactions, we used unique provider identification number (UPIN) and national provider identifier (NPI) variables from carrier and outpatient claims. If an NPI is missing, the NPI is assigned based on the National Cancer Institute–provided NPI–UPIN crosswalk. 22 Because this study focuses on PCP care continuity, we identified specialists in general practice (01), family practice (08), internal medicine (11), obstetrics and gynecology (16), or geriatric medicine (38), using Health Care Finance Administration provider specialty codes shown in the parenthesis. 23 The number of PCP visits was counted across outpatient visits for which multiple visits in the same day with the same PCP were counted as one visit. Outcomes of interest consisted of 3‐year cancer‐specific mortality and intensive end‐of‐life care, including chemotherapy received within 14 days of death, >1 emergency department visit, >1 hospitalization, or ≥1 intensive care unit admission within 30 days of death, and in‐hospital death. 24 , 25 , 26
We included the following potential confounders in our analysis: patient age at diagnosis (66.5–69, 70–74, 75–79, 80–84, 85–94), sex, race (white, black, or other), Hispanic, rurality of patient residence (metro or nonmetro), marital status, residential ZIP‐code income quintile, residential ZIP‐code educational attainment, number of providers visits within 1 year prior to cancer diagnosis, stage‐IV versus stage‐III cancer, number of comorbid conditions, tumor site, multiple cancer diagnosis, disability (whether an individual had a disability status scale greater than 2, calculated from the Eastern Cooperative Oncology Group [ECOG] performance status scale where a disability scale is equivalent of having either an ECOG of 3 or 4), 27 SEER registry sites, and year of diagnosis. 28 , 29 , 30 Information on patients' income and education was derived from linked ZIP‐code level data. The number of comorbid conditions was calculated according to Elixhauser comorbidity conditions in the year prior to cancer diagnosis, based on a previously documented approach. 31 We also included an indicator of any treatment (chemotherapy, radiation, or surgery) within 6 months after cancer diagnosis and its interaction with tumor sites, as the incidence of receiving treatments varied significantly by tumor site. Chemotherapy‐, radiation‐, and surgery‐related claims were identified from Medicare claims files, based on the previously documented International Classification of Diseases, 9th Revision, Current Procedural Terminology codes, and Healthcare Common Procedure Coding System codes. 32 , 33 , 34 , 35 We also included follow‐up duration between cancer diagnosis and death or the end of the study period (December 31, 2015) when modeling intensity of care.
2.3. Statistical analysis
We first compared the patient and clinical characteristics of patients who had or did not have PCP care continuity by Chi‐square tests for categorical variables and two‐group t‐tests for number of provider visits. Patients who survived were censored on December 31, 2015. Survival curves for those who had or did not have care continuity were calculated from the date of poor‐prognosis cancer diagnosis with the Kaplan–Meier method. We further stratified survival curves and Kaplan–Meier analyses according to primary cancer type. The log‐rank test was used to compare survival curves for each analysis.
Cox proportional hazards models with mixed effects on patient‐, PCP‐, and hospital/practice‐levels were used to estimate the association between care continuity and cancer mortality. We adjusted for sociodemographic characteristics, clinical factors, and socioeconomic factors in the residential ZIP‐code areas. In addition to control for tumor site, we included an interaction term between care continuity indicator and tumor site for the proportional hazards model to account for the differential variations of care continuity by the type of cancer. The proportional hazards assumption was checked by including a time‐dependent covariate representing the interaction between care continuity and survival time. Significant results showed the violation of proportional hazards assumption, implying various time effects on cancer survival between patients with and without PCP care continuity. Therefore, we stratified the models by tumor site, in which the proportional hazards tests resulted in nonsignificant results.
Based on the cohorts who were diagnosed with poor‐prognosis cancers between January 1, 2001, and December 31, 2003—nearly all (99.9%) of these patients died as of December 2015, we estimated the associations between early PCP follow‐up and end‐of‐life care intensity. This approach allowed us to identify actual end‐of‐life patterns for the cohorts diagnosed in the same years, thus eliminating immortal time biases from which cohorts were followed during which outcomes could not occur. A separate model was estimated for each of the five end‐of‐life care intensity outcomes, including chemotherapy received within 14 days of death, >1 emergency department visit, >1 hospitalization, or ≥1 intensive care unit admission within 30 days of death, and in‐hospital death. Because of the nested nature of the data, with multiple patients associated with a PCP and multiple PCPs serving in a hospital or a freestanding clinic, we conducted hierarchical generalized linear models with a logit link function. All models controlled for confounding variables related to the likelihood of PCP follow‐up and end‐of‐life care intensity. These variables include patient age at diagnosis (66–69, 70–74, 75–79, 80–84, 85–94 years), sex (female and male), race (white, black, and others), Hispanic, rurality of patient residence (metro versus nonmetro), marital status, residential ZIP‐code income quintile, residential ZIP‐code educational attainment, number of total provider (both PCP and non‐PCP) visits within 1 year prior to cancer diagnosis, number of PCP visits within 6 months of cancer diagnosis, stage IV versus stage III, number of comorbid conditions (none, 1–2, 3, or more), tumor site, multiple cancer diagnosis, disability, number of months since diagnosis, indicator of any treatment (chemotherapy, radiation, or surgery) within 6 months following diagnosis, SEER registry sites, and year of diagnosis. We then derived marginal percentage changes in the end‐of‐life care use by PCP continuity, accounting for clustering effects of residential ZIP‐codes. We used the variance inflation factor (VIF) calculation to confirm no multicollinearity issues in the final models (mean VIF = 2.16). 36
We performed sensitivity analyses by separately including patients with at least two and three PCP visits the year prior to diagnosis. In addition to control for primary tumor sites, we examined differential associations between PCP care continuity and survival by primary tumor sites. All statistical analyses in this section were performed in Stata 14 (College Station, TX) and SAS 9.4 (SAS Institute, Cary, NC).
3. RESULTS
The sample consisted of 85,467 patients with mean age at diagnosis of 76.3 years. Overall, 61,318 (71.7%) patients had PCP care continuity after their cancer diagnosis. Patients with PCP care continuity tended to be older, nonblack, married, and living in areas with higher educational attainment. They also had fewer comorbid conditions, had stage‐III cancer, were less likely to receive chemotherapy and radiotherapy within 6 months after cancer diagnosis, and visited PCPs more frequently the years before and after cancer diagnosis (p < 0.001 for all; Table 1). The median follow‐up was 22 months. Of decedents who were diagnosed during 2001–2003, the median follow‐up was 21 months. Those with PCP care continuity also tended to be older, nonblack, married, and had more stage‐II cancer, as well as more frequently visited PCPs than their peers without PCP care continuity. Yet, PCP care continuity was more prevalent among these decedents who lived in areas with lower income levels and had more comorbid conditions (Table S2).
TABLE 1.
Patient and clinical characteristics by primary care physician continuity a (N = 85,467)
| No care continuity (N = 24,149) | Care continuity (N = 61,318) | p values | |
|---|---|---|---|
| Age (years) | <0.001 | ||
| 66.5–69 | 5250 (21.7%) | 11,475 (18.7%) | |
| 70–74 | 7035 (29.1%) | 16,935 (27.6%) | |
| 75–79 | 5892 (24.4%) | 15,377 (25.1%) | |
| 80–84 | 3887 (16.1%) | 11,093 (18.1%) | |
| 85–94 | 2085 (8.6%) | 6438 (10.5%) | |
| Sex | 0.850 | ||
| Male | 11,254 (46.6%) | 28,530 (46.5%) | |
| Female | 12,895 (53.4%) | 32,788 (53.5%) | |
| Race | <0.001 | ||
| White | 21,006 (87.0%) | 53,408 (87.1%) | |
| Black | 1999 (8.3%) | 4503 (7.3%) | |
| Other | 1144 (4.7%) | 3407 (5.6%) | |
| Hispanic | 1145 (4.7%) | 2602 (4.2%) | 0.001 |
| Marital status | <0.001 | ||
| Married | 12,796 (53.0%) | 33,666 (54.9%) | |
| Unmarried | 10,573 (43.8%) | 25,608 (41.8%) | |
| Unknown | 780 (3.2%) | 2044 (3.3%) | |
| Nonmetro residence location | 4053 (16.8%) | 10,598 (17.3%) | 0.156 |
| Residential ZIP‐code income quintiles | <0.001 | ||
| <$33,000 | 5401 (22.4%) | 13,356 (21.8%) | |
| $33,000–$39,999 | 3644 (15.1%) | 9594 (15.7%) | |
| $40,000–$49,999 | 4705 (19.5%) | 12,846 (21.0%) | |
| $50,000–$62,999 | 4663 (19.3%) | 12,012 (19.6%) | |
| ≥$63,000 | 5702 (23.6%) | 13,441 (21.9%) | |
| Unknown | 34 (0.1%) | 69 (0.1%) | |
| Residential ZIP‐code high school education | <0.001 | ||
| <30% | 5641 (23.4%) | 13,168 (21.5%) | |
| 30%–39% | 3848 (15.9%) | 9530 (15.5%) | |
| 40%–49% | 3901 (16.2%) | 10,905 (17.8%) | |
| 50%–59% | 4415 (18.3%) | 11,332 (18.5%) | |
| ≥60% | 6310 (26.1%) | 16,314 (26.6%) | |
| Unknown | 34 (0.1%) | 69 (0.1%) | |
| Comorbid conditions | <0.001 | ||
| None | 10,725 (44.4%) | 24,036 (39.2%) | |
| 1 to 2 | 9429 (39.1%) | 27,005 (44.0%) | |
| 3 or more | 3995 (16.5%) | 10,277 (16.8%) | |
| Tumor site | <0.001 | ||
| Lung | 11,991 (49.7%) | 28,979 (47.3%) | |
| Colorectal | 6822 (28.3%) | 17,838 (29.1%) | |
| Kidney | 1012 (4.2%) | 3097 (5.1%) | |
| Esophagus | 455 (1.9%) | 1026 (1.7%) | |
| Bladder | 969 (4.0%) | 2273 (3.7%) | |
| Brain and nerve | 2900 (12.0%) | 8105 (13.2%) | |
| Any direct cancer treatments b | 19,064 (78.9%) | 47,826 (78.0%) | <0.001 |
| Chemotherapy | 13,928 (57.7%) | 34,164 (55.7%) | |
| Radiotherapy | 8268 (34.2%) | 19,622 (32.0%) | |
| Surgery | 7707 (31.9%) | 20,399 (33.3%) | |
| Stage IV c | 12,380 (51.3%) | 30,926 (50.4%) | 0.006 |
| Disability | 2020 (8.4%) | 3714 (6.1%) | <0.001 |
| Multiple cancer | 2170 (9.0%) | 5573 (9.1%) | 0.637 |
| Months from diagnosis to death/censored d | |||
| 7–12 months | 7120 (29.5%) | 17,214 (28.1%) | |
| 13–24 months | 6025 (25.0%) | 15,030 (24.5%) | |
| 25–36 months | 3406 (14.1%) | 8791 (14.3%) | |
| >36 months | 7598 (31.5%) | 20,283 (33.1%) | |
| Year of diagnosis | <0.001 | ||
| 2001–2003 | 5159 (21.4%) | 11,758 (19.2%) | |
| 2004–2006 | 6323 (26.2%) | 16,336 (26.6%) | |
| 2007–2009 | 6325 (26.2%) | 16,324 (26.6%) | |
| 2010–2012 | 6342 (26.3%) | 16,900 (27.6%) | |
| Site of region | 0.004 | ||
| California | 6737 (27.9%) | 17,046 (27.8%) | |
| Connecticut | 1499 (6.2%) | 3901 (6.4%) | |
| Detroit | 1676 (6.9%) | 4855 (7.9%) | |
| Georgia | 3027 (12.5%) | 6693 (10.9%) | |
| Hawaii | 188 (0.8%) | 780 (1.3%) | |
| Iowa | 1388 (5.8%) | 4090 (6.7%) | |
| Kentucky | 2075 (8.6%) | 5683 (9.3%) | |
| Louisiana | 1587 (6.6%) | 3680 (6.0%) | |
| New Jersey | 3431 (14.2%) | 9222 (15.0%) | |
| New Mexico | 574 (2.4%) | 1049 (1.7%) | |
| Seattle | 1507 (6.2%) | 3398 (5.5%) | |
| Utah | 460 (1.9%) | 921 (1.5%) |
| Mean (SD) | p values | ||
|---|---|---|---|
| Number of primary care physician visits the year prior to diagnosis | 6.9 (8.2) | 7.8 (7.1) | <0.001 |
| Number of primary care physician visits the year after diagnosis | 8.9 (10.1) | 10.5 (10.2) | <0.001 |
Note: p values for having primary care physician (PCP) continuity and patient characteristics were calculated with Pearson's Chi‐square tests for categorical variables and two‐group t‐tests for PCP visits.
PCP care continuity was defined as having visited the predominant PCP—who saw a patient the most frequently during the year prior to cancer diagnosis—within 6 months after cancer diagnosis.
An indicator of patients who underwent any direct cancer treatments, including chemotherapy, radiation therapy, and surgical procedures for the primary tumor sites within 6 months following a diagnosis of poor‐prognosis cancers. Chemotherapy‐, radiation‐, and surgical‐related claims were identified using codes from the International Classification of Diseases, 9th Revision, Current Procedural Terminology codes, and Healthcare Common Procedure Coding System codes.
Brain cancer (n = 11,005) is unspecified in the staging system.
Follow‐up time since diagnosis is the duration from cancer diagnosis to death among decedents and to December 31, 2015 among nondecedents.
3.1. Cancer‐specific survival
Continuity of PCP care for patients with poor‐prognosis cancer was associated with improved cancer‐specific survival. Three‐year cancer‐specific cumulative survival rates were higher among patients with PCP care continuity than among those without care continuity (37.5% vs. 34.5%; p < 0.001). Median cancer‐specific survival was 22 months (interquartile range [IQR]: 12–46 months) for patients with care continuity and 20 months (IQR, 10–44) for patients without care continuity. The results of survival analyses were consistent, with a significantly longer survival time among patients with PCP care continuity (p value of log‐rank test < 0.001, Figure 1). Cox proportional hazard models confirmed that PCP care continuity was independently associated with a reduced risk of cancer mortality (adjusted hazard ratio [AHR] = 0.93; 95% confidence interval [CI]: 0.91 to 0.95; p < 0.001).
FIGURE 1.
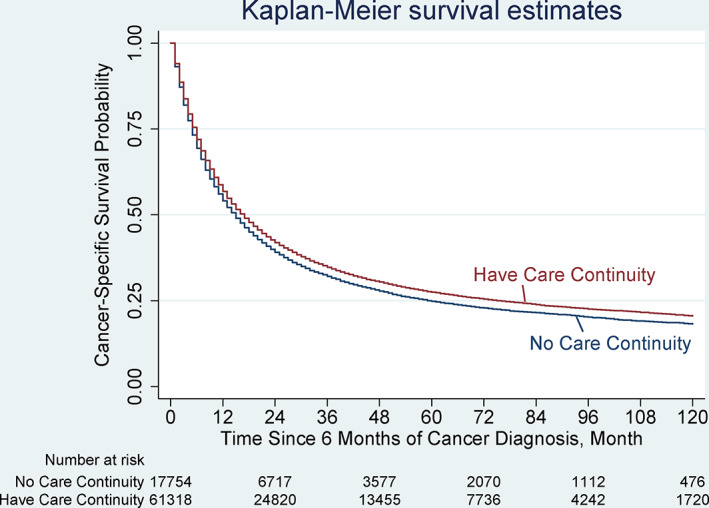
Kaplan–Meier Survival Curves for cancer‐specific survival. Overall cancer‐specific survival was analyzed based on whether a patient followed‐up with their primary care physician within the 6 months after a diagnosis of poor‐prognosis cancer, including lung, colorectal, kidney, esophagus, bladder, and brain cancers. Survival was compared using Kaplan–Meier analysis (p < 0.001, log‐rank test). The cancer‐specific survival was significantly different between patients who had primary care physician continuity (median cancer‐specific survival, 22 months [interquartile range, 12–47]) and patients who had no care continuity (20 months [11–44]; p < 0.001). Tumor‐specific survival curves can be found in Figure 2 [Color figure can be viewed at wileyonlinelibrary.com]
By tumor site, the associations between PCP care continuity and survival varied (Figure 2). Cancer‐specific survival was significantly higher with care continuity for patients with lung (p = 0.001), colorectal (p = 0.005), kidney (p = 0.038), bladder (p = 0.002), and brain cancer (p < 0.001) but not for patients with esophagus (p = 0.42; Figure 2). The Cox proportional hazard model looking at tumor‐site specific associations between PCP care continuity and survival had similar results (Table S3). For patients diagnosed with brain cancer, care continuity was associated with 27% lower hazards of mortality (AHR = 0.73; 95% CI: 0.67 to 0.79; p < 0.001). The associations between care continuity and higher mortality for patients with lung (AHR = 0.96; 95% CI: 0.93 to 0.98; p = 0.001), colorectal (AHR = 0.95; 95% CI: 0.91 to 0.98; p = 0.005), kidney (AHR = 0.89; 95% CI: 0.81 to 0.99; p = 0.038), and bladder cancers (AHR = 0.86; 95% CI: 0.78 to 0.94; p = 0.002) were less profound than their counterparts with brain cancer. To assess the issues with lag times for survival, we further limited our sample to those who were diagnosed with cancer during 2001–2003 (Table S4). Patients with and without PCP continuity had similar survival for an average group of patients with poor‐prognosis cancers, but better survival with care continuity were found among patients with bladder cancer (AHR = 0.74; 95% CI: 0.62 to 0.89; p = 0.002).
FIGURE 2.
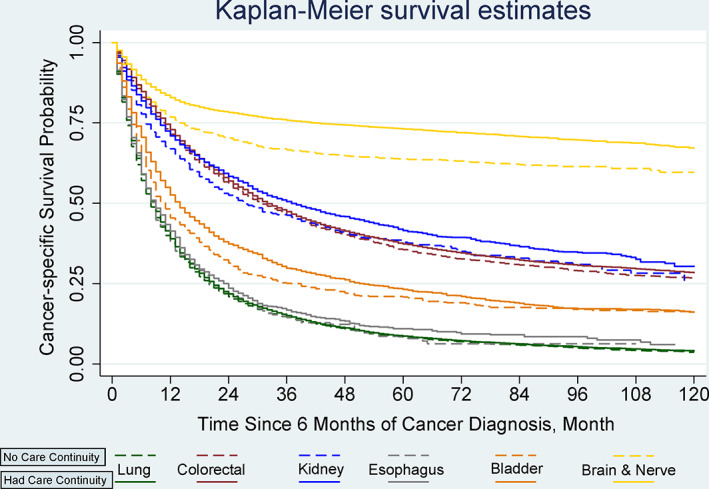
Kaplan–Meier Survival Curves for cancer‐specific survival by primary care physician continuity and primary tumor type. Overall cancer‐specific survival was analyzed based on primary tumor sites by whether a patient followed up with their primary care physician within the 6 months after a diagnosis of poor‐prognosis cancer. Survival was compared using Kaplan–Meier analysis (log‐rank tests). The cancer‐specific survival was significantly different between patients who had primary care physician continuity and patients who had no care continuity for patients diagnosed with lung (p = 0.001), colorectal (p = 0.005), kidney cancer (p = 0.038), bladder cancer (p = 0.002), and brain cancer(p < 0.001), but not different for esophagus (p = 0.420) [Color figure can be viewed at wileyonlinelibrary.com]
3.2. End‐of‐life care intensity by care continuity
Among patients who were diagnosed with poor prognosis during 2001–2003 and died within during the study period, PCP care continuity was not associated with overall end‐of‐life care intensity but significantly associated with lower hospitalization rates within 30 days of death (Table 2). In this prospective cohort, no significant differences in overall aggressive end‐of‐life care were observed with PCP care continuity (0.015 [−0.01 to 0.04]; p = 0.23; Table 3), representing the marginal incidence rate of any aggressive end‐of‐life care at 42.7% and 42.8% for decedents with and without PCP continuity, respectively (Table S5).
TABLE 2.
End‐of‐life care intensity by primary care physician continuity among decedents (N = 15,272)
| No care continuity (N = 4674) | Care continuity (N = 10,598) | p values | |
|---|---|---|---|
| Any of aggressive end‐of‐life care | 45.3% | 45.9% | 0.612 |
| Chemotherapy received within 14 days of death | 4.2% | 3.8% | 0.564 |
| More than one emergency department visit within 30 days of death | 30.5% | 31.7% | 0.304 |
| More than one hospitalization within 30 days of death | 12.3% | 12.7% | 0.485 |
| Any intensive care unit admission in last 30 days of life | 12.8% | 12.9% | 0.671 |
| In‐hospital deaths | 22.7% | 22.7% | 0.997 |
Note: All end‐of‐life outcomes were estimated among from 15,272 patients ages 66.5–94, who were diagnosed with poor‐prognosis cancers in 2001–2003, alive at the 6 months following a cancer diagnosis, had at least one primary care physician visit 12 months prior to cancer diagnosis, who died (99%) as of December 31, 2012. p values for having primary care physician continuity and outcomes were calculated with Pearson's Chi‐square tests.
TABLE 3.
Multivariate analysis of end‐of‐life outcomes by primary care physician care continuity for 2001–2003 cohorts a (N = 15,272)
| No care continuity (N = 4674) | Care continuity (N = 10,598) | ||
|---|---|---|---|
| Average percentage differences (95% CI) | p Values | ||
| Any of aggressive end‐of‐life care | Ref | 0.005 (−0.016, 0.026) | 0.638 |
| Chemotherapy received within 14 days of death | Ref | −0.007 (−0.038, 0.024) | 0.669 |
| More than one emergency department visit within 30 days of death | Ref | −0.005 (−0.031, 0.021) | 0.699 |
| More than one hospitalization within 30 days of death | Ref | −0.044 (−0.077, −0.010) | 0.010 |
| Any ICU admission in last 30 days of life | Ref | 0.008 (−0.058, 0.073) | 0.819 |
| In‐hospital deaths | Ref | −0.038 (−0.086, 0.010) | 0.123 |
Note: All models controlled for age at diagnosis, sex, race, Hispanic, metro/nonmetro, marital status, residential ZIP‐code income quintile, residential ZIP‐code educational attainment, number of provider visits prior to cancer diagnosis, stage‐IV indicator, number of comorbid conditions, disability, tumor site, multiple cancer diagnosis, months since diagnosis, Surveillance, Epidemiology, and End Results (SEER) registry sites, year of diagnosis, an indicator of any treatment (chemotherapy, radiation, or surgery) within 6 months following cancer diagnosis and its interaction with tumor sites, and hospital‐fixed effects. Bold texts indicate the statistically significant result with a p value less than 0.05.
Abbreviation: ICU, intensive care unit.
Estimates were from 15,272 patients who were diagnosed with poor‐prognosis cancers in 2001–2003 and who died (99%) as of December 31, 2014, using generalized logistic regressions with clustered standard errors at the residential ZIP‐code level.
4. DISCUSSION
We found that PCP continuity in older persons with poor‐prognosis cancer had modest survival benefits for some cancers but not all, without an increase in overall end‐of‐life care intensity. While PCP continuity and survival were significantly associated for all poor‐prognosis cancers, except esophagus cancer, the increased survival duration with PCP continuity was more prominent in patients with brain cancer.
To our knowledge, this study is the first investigating the prognostic impact of same‐PCP care continuity on survival and end‐of‐life care intensity in a cohort of poor‐prognosis cancer patients. It extends prior work on PCP involvement in the postdiagnosis period. 5 Previous research compared patients who had and did not have PCP care after cancer diagnosis but did not consider access to PCP care before the diagnosis. 5 We studied patients with primary care visits before cancer diagnosis and confirmed slightly longer survival of those following up with the same PCP. However, it is important to note that this study measures PCP care continuity by at least one postdiagnosis follow‐up with one's predominant PCP. By looking at one single continuity visit, our survival benefits might be underestimated. Patients with PCP continuity by at least one PCP visit during cancer diagnosis might have benefited from improved patient compliance with treatments, health information continuity, patient‐centered prognosis management, thereby improving their survival outcomes. Our results along with these previously documented benefits suggest that cancer survivorship might be directly or indirectly improved with the assurance of resuming a patient's PCP care following cancer diagnosis.
Nonetheless, we uncovered differential associations of PCP continuity with survival by tumor site among poor‐prognosis cancers. These results are concordant with the mortality rates by tumor site. For example, of all included tumor sites, the 3‐year mortality rates are lowest among patients with brain and nervous cancers (24.8%), followed by kidney (74.5%), bladder (76.4%), colorectal (76.4%), lung (86.9%), and esophagus (88.9%). The role of PCP continuity in cancer survival reduction is much stronger among patients with brain cancers than those with any other primary tumor sites, with no survival benefits for patients with esophagus. These results suggest that PCP continuity benefits some poor‐prognostic cancers in terms of their survival but not as helpful for those with extremely high mortality risk as for those with lower mortality risk. The underlying mechanisms warrant further investigation.
Diagnosis with stage‐III or IV poor‐prognosis cancer can be a life‐changing event. Beginning at diagnosis, patients may face a trade‐off between longer survival and less aggressive care. We found that PCP care continuity was associated with survival but not with end‐of‐life care intensity. Potential mechanisms driving such survival differences may include patient compliance with PCPs' recommendations, 37 , 38 improved exchange of health information, 7 , 38 and PCPs' individualized prognosis management. 8 , 11 , 39 , 40 In prior studies, physician continuity and patient compliance with care management and medication plans were positively associated. 18 , 37 , 41 As PCPs play a role in managing chronic pain, emotional issues, and care referral, continual care with the same PCPs might improve patients' medical compliance and facilitate individualized care, with better cancer survival without using aggressive end‐of‐life care. Also, interpersonal relationships with a patient's PCP can facilitate informational continuity. With the personal and clinical knowledge of patients diagnosed with advanced cancer, the PCP can plan with patients, their oncologists, and other care providers to align care with patients' personal needs and preferences. 17 Constant psychosocial support in cancer care might improve patients' satisfaction and symptom management, ultimately with better survival outcomes.
Smooth transitioning between primary and oncology care requires multifaceted coordination, as care for patients with poor‐prognosis cancer always involves multiple health care components. 42 , 43 Ensuring one's PCP involvement in cancer care in the early phase would help in sharing responsibilities between PCPs and oncologists. Our finding that patients with PCP care continuity were less likely to receive cancer‐directed treatments within 6 months of diagnosis suggests a functioning network of communications between PCPs and cancer care specialists is critical. Despite this, after adjusting for the variations in the receipt of any treatment, our results help inform the national debate on PCP care continuity. We demonstrated survival benefits without aggressive end‐of‐life care by having the continuity of primary care transition from cancer providers back to the PCPs who were actively involved in patient care before cancer diagnosis within 6 months after diagnosis.
Several limitations should be noted. First, as an observational study, our estimates on the associations between PCP continuity and outcomes are not causal. Unmeasured confounders may include patient preferences, appropriateness of care, patients' social capital (caregivers), and PCPs' group‐practice characteristics. We also relied on claims data for the markers of intensive end‐of‐life care, and these outcomes may be coarse. Additionally, this study is limited to fee‐for‐service Medicare beneficiaries with at least four PCP visits prior to diagnosis of stage‐III or IV poor‐prognosis cancers. The results are not generalizable to other populations, especially patients who died within 6 months and those with other cancers or early‐stage cancer. With the median survival of 22 months in our cohort, we recognize the importance of evaluating whether the PCP remains involved beyond 6 months but, to reduce bias in exposure assessment, were not able to do so in the current study. This study employed a population‐based cohort study design to examine the association between PCP care continuity and EOL care; future studies might use a case–control study design to identify differences in PCP care continuity between homogeneous patients with and without EOL care intensity. Given the multiple cancer types in the analysis, the indicator of any treatment was across different treatment; specific treatments may be an important mechanism with survival for different cancer types. We addressed this heterogeneity by including the interactions between any treatment indicator and cancer type. The additional differential associations between PCP continuity and survival by primary tumor site also underscore the heterogeneity of service line effects across tumor sites.
5. CONCLUSION
Our findings shed light on the role of early PCP care continuity within 6 months of diagnosis over the course of cancer diagnosis and enrich the national conversations regarding cancer survivorship for patients diagnosed with poor‐prognosis cancer. Medicare beneficiaries with stage‐III or IV poor‐prognosis cancer who followed‐up with their PCPs within 6 months after diagnosis had somewhat survival benefits without increased overall aggressive end‐of‐life care. Understanding how PCP continuity of care benefited patients with poor‐prognosis cancer in the small margins of survival outcomes is essential to better inform the developments of cancer care workforce collaboration model between PCP and cancer care clinicians.
Supporting information
Data S1. Supporting information.
ACKNOWLEDGMENTS
This study was supported by Yale University Cancer Center pilot grant and a P30 Cancer Center Support Grant from Yale University Comprehensive Cancer Center through National Cancer Institute (Grant No. P30 CA01635933). All authors have completed the International Committee of Medical Journal Editors (ICMJE) uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that there are no financial relationships with any organizations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Hung P, Cramer LD, Pollack CE, Gross CP, Wang S‐Y. Primary care physician continuity, survival, and end‐of‐life care intensity. Health Serv Res. 2022;57(4):853‐862. doi: 10.1111/1475-6773.13869
Funding information Division of Cancer Prevention, National Cancer Institute, Grant/Award Number: P30 CA01635933
REFERENCES
- 1. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Oncol Pract. 2007;3(2):79‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayer DK, Shapiro CL, Jacobson P, McCabe MS. Assuring quality cancer survivorship care: we've only just begun. Am Soc Clin Oncol Educ Book. 2015;35:e583‐e591. 10.14694/EdBook_AM.2015.35.e583 [DOI] [PubMed] [Google Scholar]
- 3. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24(9):1029‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolinsky FD, Bentler SE, Liu L, et al. Continuity of care with a primary care physician and mortality in older adults. J Gerontol Ser A Biol Sci Med Sci. 2010;65A(4):421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones LE, Doebbeling CC. Beyond the traditional prognostic indicators: the impact of primary care utilization on cancer survival. J Clin Oncol. 2007;25(36):5793‐5799. [DOI] [PubMed] [Google Scholar]
- 6. Sharma G, Freeman J, Zhang D, Goodwin JS. Continuity of care and intensive care unit use at the end of life. Arch Intern Med. 2009;169(1):81‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katz DA, McCoy K, Sarrazin MV. Does improved continuity of primary care affect clinician–patient communication in VA? J Gen Intern Med. 2014;29(S2):682‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michiels E, Deschepper R, Van Der Kelen G, et al. The role of general practitioners in continuity of care at the end of life: a qualitative study of terminally ill patients and their next of kin. Palliat Med. 2007;21(5):409‐415. [DOI] [PubMed] [Google Scholar]
- 9. Mack JW, Cronin A, Taback N, et al. End‐of‐life care discussions among patients with advanced cancer: a cohort study. Ann Intern Med. 2012;156(3):204‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma G, Wang Y, Graham JE, Kuo YF, Goodwin JS. Provider continuity prior to the diagnosis of advanced lung cancer and end‐of‐life care. PLoS One. 2013;8(9):e74690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aubin M, Vézina L, Verreault R, et al. Patient, primary care physician and specialist expectations of primary care physician involvement in cancer care. J Gen Intern Med. 2012;27(1):8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hudson SV, Miller SM, Hemler J, et al. Adult cancer survivors discuss follow‐up in primary care: “Not what I want, but maybe what I need”. Ann Fam Med. 2012;10(5):418‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Institute of Medicine and National Research Council . From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 14. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor‐prognosis cancer. JAMA. 2014;312(18):1888‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pourat N, Davis AC, Chen X, Vrungos S, Kominski GF. In California, primary care continuity was associated with reduced emergency department use and fewer hospitalizations. Health Aff. 2015;34(7):1113‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005;3(2):159‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nyweide DJ, Anthony DL, Bynum JPW, et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013;173(20):1879‐1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Accordino MK, Wright JD, Vasan S, et al. Association between survival time with metastatic breast cancer and aggressive end‐of‐life care. Breast Cancer Res Treat. 2017;166(2):549‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bach PB, Schrag D, Begg CB. Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA. 2004;292(22):2765‐2770. [DOI] [PubMed] [Google Scholar]
- 21. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER‐Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV‐3‐IV‐18. [DOI] [PubMed] [Google Scholar]
- 22. Parsons HM, Enewold LR, Banks R, Barrett MJ, Warren JL. Creating a National Provider Identifier (NPI) to unique physician identification number (UPIN) crosswalk for Medicare data. Med Care. 2017;55(12):e113‐e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Cancer Institute . Appendix for SEER‐Medicare 10/2018 Claims Files. National Cancer Institute Division of Cancer Control and Population Sciences. February 22, 2019. Accessed February 9, 2021. https://healthcaredelivery.cancer.gov/seermedicare/program/docs/appendix.versionk.pdf
- 24. Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end‐of‐life cancer care from administrative data. J Clin Oncol. 2003;21(6):1133‐1138. [DOI] [PubMed] [Google Scholar]
- 25. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315‐321. [DOI] [PubMed] [Google Scholar]
- 26. Wang SY, Hall J, Pollack CE, et al. Trends in end‐of‐life cancer care in the Medicare program. J Geriatr Oncol. 2016;7(2):116‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims‐based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4(2):157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burge F, Lawson B, Johnston G. Family physician continuity of care and emergency department use in end‐of‐life cancer care. Med Care. 2003;41(8):992‐1001. [DOI] [PubMed] [Google Scholar]
- 29. Hussey PS, Schneider EC, Rudin RS, Fox DS, Lai J, Pollack CE. Continuity and the costs of care for chronic disease. JAMA Intern Med. 2014;174(5):742‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang S‐Y, Aldridge MD, Gross CP, et al. Geographic variation of hospice use patterns at the end of life. J Palliat Med. 2015;18(9):771‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8‐27. [DOI] [PubMed] [Google Scholar]
- 32. Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER‐Medicare data to identify chemotherapy use. Med Care. 2002;40(8 Suppl):IV‐55‐IV‐61. [DOI] [PubMed] [Google Scholar]
- 33. Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER‐Medicare‐linked data. Med Care. 2002;40(8 Suppl):IV‐49‐IV‐54. [DOI] [PubMed] [Google Scholar]
- 34. Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER‐Medicare data for measuring cancer surgery. Med Care. 2002;40(8 Suppl):IV‐43‐IV‐48. [DOI] [PubMed] [Google Scholar]
- 35. Clarke CA, Asch SM, Baker L, et al. Public reporting of hospital‐level cancer surgical volumes in California: an opportunity to inform decision making and improve quality. J Oncol Pract. 2016;12(10):e944‐e948. [DOI] [PubMed] [Google Scholar]
- 36. O'Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41(5):673‐690. [Google Scholar]
- 37. Egerton NJ. In‐office dispensing of oral oncolytics: a continuity of care and cost mitigation model for cancer patients. Am J Manag Care. 2016;22(4 Suppl):s99‐s103. [PubMed] [Google Scholar]
- 38. Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. Br Med J. 2003;327(7425):1219‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haggerty JL, Roberge D, Freeman GK, Beaulieu C. Experienced continuity of care when patients see multiple clinicians: a qualitative metasummary. Ann Fam Med. 2013;11(3):262‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pandhi N, Saultz JW. Patients' perceptions of interpersonal continuity of care. J Am Board Fam Med. 2006;19(4):390‐397. [DOI] [PubMed] [Google Scholar]
- 41. Warren JR, Falster MO, Tran B, Jorm L. Association of continuity of primary care and statin adherence. PLoS One. 2015;10(10):e0140008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han PKJ, Rayson D. The coordination of primary and oncology specialty care at the end of life. J Natl Cancer Inst Monogr. 2010;2010(40):31‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taplin SH, Clauser S, Rodgers AB, Breslau E, Rayson D. Interfaces across the cancer continuum offer opportunities to improve the process of care. J Natl Cancer Inst Monogr. 2010;2010(40):104‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.


