Abstract
In broiler breeders, background mortality is rarely addressed, however, it represents the death of a vast number of birds, a constant productivity loss, welfare concerns and it might affect chick quality. The study aimed to unveil lesions leading to mortality in a study population perceived as healthy, combined with whole-genome sequencing (WGS) of Escherichia coli, a well-known contributor to disease problems in poultry. Broiler breeders (n = 340) originating from three distinct, putative healthy flocks and their progeny (n = 154) were subjected to a comprehensive post-mortem examination, bacteriological sampling, and sequencing of 77 E. coli isolates. Productivity data confirmed an exemplary health status of the enrolled flocks, and post-mortem examination further verified the absence of general disease problems. Among the submitted broiler breeders, exudative peritonitis (31.2%) was the most frequent lesion linked to infectious disease, whereas airsacculitis, pericarditis, perihepatitis, and salpingitis occurred in 18.5%, 3.5%, 3.8% and 17%, respectively. Yolksacculitis occurred in 15.6% of the broilers, whilst pericarditis, perihepatitis and peritonitis were diagnosed in 9.7%, 7.1% and 9.1%, respectively. WGS revealed a diverse population where ST95 dominated the population retrieved from broiler breeders, whereas ST10 was highly prevalent among broilers. Both lineages could be isolated from extraintestinal sites of birds without lesions indicative of infection. In general, the genetic diversity within flocks was comparable to the diversity between farms, and the overall occurrence of resistance markers was low. In conclusion, a comprehensive insight into lesions associated with background mortality is presented, together with a vast diversity of E. coli isolated from extraintestinal sites during a non-outbreak situation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13567-022-01064-7.
Keywords: Mortality, pathology, antimicrobial resistance, Escherichia coli, colibacillosis, whole-genome sequencing, APEC, avian pathogenic E. coli, surveillance
Introduction
In broiler breeders, a mortality as high as approximately 8% can be expected throughout a production period in non-outbreak situations [1, 2]—i.e., when only the “background” mortality contributes to death and culling. Despite this represents a vast number of birds, lowered productivity and animal welfare concerns, background mortality is only rarely addressed [1].
Contributing further to the importance of underlying disease problems in broiler breeders is the established link between the parent stock and the quality and health status of the broilers [2–4].
A well-known contributor to morbidity and mortality in poultry is Escherichia coli, which has for long been considered the most important bacterial pathogen in poultry production [5–7] being a leading cause not only of mortality (death/culling) but also the main cause of carcass condemnation in broilers and broiler breeders [8–11]. Colibacillosis in poultry has formerly been viewed as an opportunistic disease secondary to other factors such as viral or Mycoplasma infections, high ammonia levels or stress [5, 12, 13]. However, more recently it has been speculated that high-virulent E. coli strains may be primary pathogens in case of “true” avian pathogenic E. coli (APEC) [5, 14]. Efforts have been made to unveil the transmission and describe APEC in-depth, especially in situations with elevated E. coli-related problems and/or across wide timespans [15–17]. In recent years, high-resolution techniques, such as whole-genome sequencing (WGS), have been applied with success and the method holds great potential in the quest to portray and define the APEC pathotype [18, 19]. Yet, the ability to genomically define this pathotype has been highly obscured by the lack of differentiation between APEC-driven and opportunistic colibacillosis, thereby, rendering the term APEC almost meaningless [18]. Therefore, it is essential to view E. coli, isolated from lesions within poultry, in a larger context taking flock mortality, morbidity, productivity and, especially, necropsy findings into consideration when designating strains as APEC.
The current study aimed to unveil the overall pathology present in a study population unaffected by general disease problems, i.e., the lesions leading to background mortality, and further characterise the isolated E. coli-types through genomic analyses.
Materials and methods
Study design
From September 2019 to August 2020, a total of 360 birds were scheduled to be collected from three distinct Ross 308 broiler breeder farms (A, B and C). Appointment to the study was based on historical productivity data provided by DanHatch Denmark A/S and all flocks had adhered to a standardised vaccination program during rearing containing Poulvac E coli® and an autogenous vaccine targeting E. coli. The selection criteria included cumulated mortality, disease history, and production data (e.g., hatchability, chick quality, farmer compliance etc.). From all farms, the birds were collected at two distinct stages of the production period, i.e., during week 27–34 and 45–60 of life, respectively. These time periods encompassed different yearly seasons, the farms were not synchronised, and each received birds originating from separate rearing farms. Farmers collected the birds following specific instruction from the field veterinarian, and euthanised birds were immediately placed at −20 °C until the time of necropsy. Cervical dislocation was applied in cases of euthanasia. Collection criteria were as follows: birds exhibiting depression, soiled cloacal areas, lacerations (mating injuries), lameness, or other clear signs of disease were gathered, and the field veterinarian visited the farms regularly during the study period. Birds with obvious lesions consistent with cannibalism and spontaneously dead birds with clear signs of cadaverosis were not collected. In the rare case that birds with any signs of cadaverosis were still submitted to the study, the birds were excluded prior to necropsy due to a high probability of bacterial overgrowth and the inability to evaluate organs and identify lesions in such animals. From each collection period, the farmer was told to euthanise a minimum of 10 clinically healthy birds to serve as controls.
In addition to broiler breeders, progeny broilers (age 4–11 days) from each of the included parent farms, hatched from eggs laid during week 27–34 and showing signs of disease and/or weakness, were collected.
Post-mortem examination
All birds were subjected to a thorough necropsy [20], and lesions were registered according to a standardised scheme (Additional files 1 and 2). Briefly, the surface of the animal was inspected, and lesions on skin, plumage and footpads were registered. The entire respiratory system, including sinus infraorbitalis, were examined on the surface as well as the larger luminal areas, content of the crop was noted, whilst the remaining intestinal tract was inspected on the surface and opened upon indication. However, in young progeny the ventriculus was routinely cut open and inspected. In broiler breeders, the salpinx was opened, and the reproductive status was evaluated with registration of the presence of a developing egg, fully developed egg, or absence of the former. Follicular status was also noted. The hip-, knee- and hock joints were opened and inspected, whilst other joints and parts of the locomotion system were examined in-depth upon indication. Musculus pectoralis superficialis and profundus were incised routinely for inspection. The kidneys were examined in situ, whilst the lungs, heart, liver, and spleen were removed from the carcass for inspection. In the broilers, the navel area, and the yolk sac, if still present, were carefully evaluated.
Microbiology
From all broiler breeders, bacteriological sampling from the right lung, liver and salpinx was performed using sterile wooden cotton swabs or steel cotton swabs, depending on the organ sampled with subsequent streak-plating on blood agar base supplemented with 5% bovine blood. Additional samples were collected upon indication. Prior to sampling, the organ surfaces were sterilised with a burning hot iron spatula in order to reduce contamination during necropsy. The plates were incubated at 37 °C overnight and examined for growth. Tentative E. coli in pure growth were sub-cultured using MacConkey agar, and freeze stock was prepared for storage at −80 °C until further analysis. From the progeny, swabs were collected from the liver and yolk sac, or the liver and left lung if the yolk sac had been completely resorbed. Classification of isolate-origin into birds suffering from colibacillosis or not was based on nearly indisputable signs of this disease, i.e., presence of fibrinous, purulent or fibrinopurulent exudate [5], concurrent with the recovery of abundant pure growth of E. coli.
Whole-genome sequencing
A subset of the collected E. coli isolates was selected for WGS with an aim of ensuring representation of multiple organs and birds from all farms. Genomic DNA was extracted using an enzymatic pre-lysis step before automated purification using the MagNA Pure 96 DNA and Viral NA Small Volume Kit and the DNA Blood ds SV 2.0 protocol (Roche Diagnostics) and quantified using the Qubit fluorometer (Invitrogen, Waltham, MA, USA). Subsequently, library construction and sequencing were performed using the Nextera XT Kit (Illumina, Little Chesterford, UK) and 300-cycle kits on the NextSeq 550 (Illumina) platform according to the manufacturer’s instructions. Quality control of the sequencing data was performed using bifrost [21] to ensure adequate sequencing depth, species verification and contamination issues of isolates prior to assembly using SPAdes v3.9.0.
For analysis into the relatedness of the isolates, the raw sequencing data were aligned to the joined contigs of E. coli E51 (GenBank accession number LYPJ00000000) [22] using NASP v1.0.0 [23] with BWA-MEM [24] and subsequent single nucleotide polymorphisms (SNPs) were called using GATK [25]. If a variant was present in <90% of the base calls per site across any individual isolate or a minimum coverage of 10 was not met, the position was excluded across the collection to retain only high-quality variant callings. The relatedness of the isolates was inferred utilising IQ-TREE v1.6.9 [26] using ModelFinder and bootstrap analysis using 100 replicates. The tree was mid-point rooted. For visualisation of the phylogeny and key genetic characteristics, iTol v6.4.2 was employed. Serotype prediction was performed in silico on assemblies utilising ECTyper v1.0 [27]. Sequence types (ST) were assigned using MLST [28] based on implementation of the Wirth et al. [29] typing scheme at PubMLST. To detect acquired resistance genes, ARIBA [30] was run on raw reads on the ResFinder database (accessed 5th December 2021). Only hits with minimum 90% sequence similarity and 90% coverage of the reference genes were considered present. Additionally, PointFinder [31] was used to detect chromosomal point mutations linked to resistance.
Data management
Registration of flock, house, age, weight, macroscopic lesions, and bacterial growth was conducted at animal level, whilst flock productivity and mortality data were provided by DanHatch Denmark A/S upon request.
Results
Study population
A total of 340 broiler breeders were collected. Farm A contributed with 140 birds, whilst Farm B and C delivered 96 and 104, respectively. From Farm A, nine birds (14.3%) aged 27–34 weeks were marked as putative healthy, i.e., controls, with this number being 14 (18.2%) in the birds aged 45–60 weeks. Farm B delivered three (7%) control birds in the young age category (27–34 weeks) and nine (17%) in the 45–60 weeks category. From Farm C, birds marked as healthy were only received among the older (45–60) birds (n = 9 (15.5%)).
Fifty-four broilers, hatched from eggs laid on Farm A aged 27–34 weeks, were collected, whilst this number was 52 and 48 from Farms B and C, respectively.
None of the flocks included in this study received any antibiotic treatment. Tables 1 and 2 present an overview of the study population, mortality, and productivity.
Table 1.
Overview of mortality, productivity, and the study population of broiler breeders
| General | Flock mortality | Productivity | Birds included in the study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farm | Flock size | Bird/m2 | Mortality, total (%) | Total no. of dead birds | Eggs/hen | Hatching (%) | No. of chicks delivered/ hen | Chick mortality, first week (%) | Chick mortality, total, average (%) | Chick slaughter condemnation, average (%) | No. of birds for post-mortem examination | No. of birds sampled for bacteriology | No. of Escherichia coli isolates subjected to genome sequencing |
| Farm A | 45,759 | 6.65 | 8.21 | 3757 | 168 | 86.7 | 135.3 | 0.91 | 2.90 | 1.50 | 140 | 120 | 22 |
| Farm B | 23,487 | 6.74 | 5.12 | 1203 | 173 | 86.7 | 144.1 | 0.79 | 2.88 | 1.34 | 96 | 74 | 10 |
| Farm C | 39,383 | 6.68 | 7.35 | 2895 | 174 | 86.7 | 144.7 | 0.86 | 3.66 | 1.33 | 104 | 87 | 17 |
Table 2.
Overview of the mortality, productivity, and study population of the progeny
| General | Flock mortality | Productivity | Birds included in the study | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Farm | Flock size | Bird/m2 | Mortality, total (%) | Mortality, first week (%) | Total no. of dead birds | Feed conversion | Slaughter weight, average (g) | Slaughter condemnation (%) | Total no. of birds for post-mortem examination | No. of birds sampled for bacteriology | No. of Escherichia coli isolates subjected to genome sequencing |
| Progeny A | 31,700 | 41.2 | 1.50 | 0.55 | 476 | 1.50 | 2140 | 0.7 | 54 | 54 | 5 |
| Progeny B | 27,000 | 41.7 | 1.31 | 0.52 | 415 | 1.47 | 2193 | 0.6 | 52 | 51 | 11 |
| Progeny C | 30,800 | 40.2 | 1.84 | 0.42 | 583 | 1.52 | 2148 | 0.6 | 48 | 48 | 12 |
Post-mortem findings
A comprehensive overview of the lesions in the broiler breeders is presented in Table 3, whereas the gross pathology identified in the progeny is presented in Table 4.
Table 3.
Overview of gross lesions in broiler breeders from Farms A, B and C
| Farm A (n = 140) |
Farm B (n = 96) |
Farm C (n = 104) |
Total (n = 340) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 27–34 weeks (n = 63) |
45–60 weeks (n = 77) |
Total | 27–34 weeks (n = 43) |
45–60 weeks (n = 53) |
Total | 27–34 weeks (n = 46) |
45–60 weeks (n = 58) |
Total | 27–34 weeks (n = 152) |
45–60 weeks (n = 188) |
Total | |
| General | ||||||||||||
| Euthanised | 34 (54%) | 52 (67.5%) | 86 (61.4%) | 23 (53.5%) | 21 (39.6%) | 44 (45.8%) | 10 (21.7%) | 21 (36.2%) | 31 (29.8%) | 67 (44.1%) | 94 (50%) | 161 (47.4%) |
| BW (kg) | 3.3 ± 0.5 | 3.3 ± 0.8 | 3.3 ± 0.6 | 3.0 ± 0.6 | 3.2 ± 0.8 | 3.1 ± 0.7 | 2.8 ± 0.6 | 3.4 ± 0.8 | 3.1 ± 0.8 | 3.1 ± 0.6 | 3.3 ± 0.8 | 3.2 ± 0.7 |
| No. of males | 0 (0%) | 4 (5.2%) | 4 (2.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (6.5%) | 3 (5.2%) | 6 (5.8%) | 3 (2%) | 7 (3.7%) | 10 (2.9%) |
| Dehydration | 19 (30.2%) | 24 (31.2%) | 43 (30.7%) | 9 (20.9%) | 27 (50.9%) | 36 (37.5%) | 15 (32.6%) | 25 (43.1%) | 40 (35.5%) | 43 (28.3%) | 76 (40.4%) | 119 (35%) |
| Emaciation | 2 (3.2%) | 3 (3.9%) | 5 (3.6%) | 2 (4.7%) | 5 (9.4%) | 7 (7.3%) | 7 (15.2%) | 2 (3.4%) | 9 (8.7%) | 11 (7.2%) | 10 (5.3%) | 21 (6.2%) |
| Skin, subcutis and foot pads | ||||||||||||
| Laceration (mating injuries)a | 17 (27%) | 19 (25.7%) | 36 (25.7%) | 10 (23.3%) | 3 (5.7%) | 13 (13.5%) | 2 (4.3%) | 2 (3.4%) | 4 (3.8%) | 29 (19.1%) | 24 (12.8%) | 53 (15.6%) |
| Bumblefootb | 1 (1.6%) | 3 (3.9%) | 4 (2.9%) | 0 (0%) | 2 (3.8%) | 2 (2.1%) | 1 (2.2%) | 0 (0%) | 1 (1%) | 2 (1.3%) | 5 (2.7%) | 7 (2.1%) |
| Pododermatitisc | 40 (63.5%) | 23 (29.9%) | 63 (45%) | 13 (30.2%) | 17 (32.1%) | 30 (31.3%) | 22 (47.8%) | 36 (62.1%) | 58 (55.8%) | 75 (49.3%) | 76 (40.4%) | 151 (44.4%) |
| Bursitis presternalis | 8 (12.7%) | 8 (10.4%) | 16 (11.4%) | 15 (34.9%) | 14 (26.4%) | 29 (30.2%) | 6 (13%) | 14 (24.1%) | 20 (19.2%) | 29 (19.1%) | 36 (19.1%) | 65 (19.1%) |
| Skeletal system and joints | ||||||||||||
| Fracture (long bones) | 1 (1.6%) | 4 (5.2%) | 5 (3.1%) | 0 (0%) | 4 (7.5%) | 4 (4.2%) | 0 (0%) | 2 (3.4%) | 2 (1.9%) | 1 (0.7%) | 10 (5.3%) | 11 (3.2%) |
| Arthritisd | 2 (3.2%) | 6 (7.8%) | 8 (5.7%) | 3 (7%) | 7 (13.2%) | 10 (10.4%) | 8 (17.4%) | 2 (3.4%) | 10 (9.6%) | 13 (8.5%) | 15 (8%) | 28 (8.2%) |
| Sternal fracture(s) | ||||||||||||
| Total | 2 (3.2%) | 25 (32.5%) | 27 (19.3%) | 4 (9.3%) | 31 (58.5%) | 35 (36.5%) | 2 (4.3%) | 29 (50%) | 31 (29.8%) | 8 (5.3%) | 85 (45.2%) | 93 (27.4%) |
| 0 | 61 (96.8%) | 52 (67.5%) | 113 (80.7%) | 38 (88.4%) | 22 (41.5%) | 60 (62.5%) | 45 (97.8%) | 28 (48.3%) | 73 (70.2%) | 144 (94.7%) | 102 (54.3%) | 246 (72.4%) |
| 1 | 2 (3.8%) | 15 (19.5%) | 17 (12.1%) | 4 (9.3%) | 19 (35.8%) | 23 (24%) | 2 (4.3%) | 18 (31%) | 20 (19.2%) | 8 (5.3%) | 52 (27.7%) | 60 (17.6%) |
| 2 | 0 (0%) | 6 (7.8%) | 6 (4.3%) | 0 (0%) | 4 (7.5%) | 4 (4.2%) | 0 (0%) | 7 (12.1%) | 7 (6.7%) | 0 (0%) | 17 (9%) | 17 (5%) |
| 3 | 0 (0%) | 3 (3.9%) | 3 (2.1%) | 0 (0%) | 6 (11.3%) | 6 (6.3%) | 0 (0%) | 3 (5.2%) | 3 (2.9%) | 0 (0%) | 12 (6.4%) | 12 (3.5%) |
| > 4 | 0 (0%) | 1 (1.3%) | 1 (0.7%) | 0 (0%) | 2 (3.8%) | 2 (2.1%) | 0 (0%) | 1 (1.7%) | 1 (1%) | 0 (0%) | 4 (2.1%) | 4 (1.2%) |
| Respiratory system | ||||||||||||
| Tracheal changese | 21 (33.3%) | 18 (23.4%) | 39 (27.9%) | 11 (25.6%) | 9 (17%) | 20 (20.8%) | 22 (47.9%) | 23 (39.7%) | 45 (43.3%) | 54 (35.5%) | 50 (26.6%) | 104 (30.6%) |
| Pulmonary changesf | 25 (39.7%) | 27 (35.1%) | 52 (37.1%) | 16 (37.2%) | 18 (34%) | 34 (35.4%) | 21 (45.7%) | 22 (37.9%) | 43 (41.3%) | 62 (40.8%) | 67 (35.6%) | 129 (38%) |
| Airsacculitisg | 14 (22.2%) | 14 (18.2%) | 28 (20%) | 6 (14%) | 4 (7.5%) | 10 (10.4%) | 12 (26.1%) | 13 (22.4%) | 25 (24%) | 32 (21.1%) | 31 (16.5%) | 63 (18.5%) |
| Coelomic cavity | ||||||||||||
| Pericarditish | 6 (9.5%) | 3 (3.9%) | 9 (6.4%) | 1 (2.3%) | 2 (3.8%) | 3 (3.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (4.6%) | 5 (2.7%) | 12 (3.5%) |
| Perihepatitish | 6 (9.5%) | 3 (3.9%) | 9 (6.4%) | 3 (7%) | 1 (1.9%) | 4 (4.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 9 (5.9%) | 4 (2.1%) | 13 (3.8%) |
| Fatty liver | 15 (23.8%) | 9 (11.7%) | 24 (17.1%) | 5 (11.6%) | 5 (9.4%) | 10 (10.4%) | 0 (0%) | 5 (8.6%) | 5 (4.8%) | 20 (13.2%) | 19 (10.1%) | 39 (11.5%) |
| Hepatic rupture | 2 (3.2%) | 2 (2.6%) | 4 (2.9%) | 0 (0%) | 3 (5.7%) | 3 (3.1%) | 1 (2.2%) | 5 (8.6%) | 6 (5.8%) | 3 (2%) | 10 (5.3%) | 13 (3.8%) |
| Peritonitish | 23 (36.5%) | 28 (36.4%) | 51 (36.4%) | 10 (23.3%) | 15 (28.3%) | 25 (26%) | 11 (23.9%) | 19 (32.8%) | 30 (28.8%) | 44 (28.4%) | 62 (33%) | 106 (31.2%) |
| Renal changesi | 17 (27%) | 24 (31.2%) | 41 (29.3%) | 11 (25.6%) | 19 (35.8%) | 30 (31.3%) | 27 (58.7%) | 23 (39.7%) | 50 (48.1%) | 55 (36.2%) | 66 (35.1%) | 121 (35.6%) |
| Reproductive system | ||||||||||||
| In layj | 39 (61.9%) | 20 (27.4%) | 59 (43.4%) | 16 (37.2%) | 21 (39.6%) | 37 (38.5%) | 12 (27.9%) | 27 (49.1%) | 39 (39.8%) | 67 (44.1%) | 68 (36.2%) | 135 (40.9%) |
| Egg bound | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (3.8%) | 2 (2.1%) | 3 (7%) | 1 (1.8%) | 4 (4.1%) | 3 (2%) | 3 (1.6%) | 6 (1.8%) |
| Perioophoritish | 8 (12.7%) | 17 (23.3%) | 25 (18.4%) | 4 (9.3%) | 12 (22.6%) | 16 (16.7%) | 5 (11.6%) | 10 (18.2%) | 15 (15.3%) | 17 (11.9%) | 39 (20.7%) | 56 (17%) |
| Ovarian regressionk | 14 (22.2%) | 28 (38.4%) | 42 (30.9%) | 15 (34.9%) | 21 (39.6%) | 36 (37.5%) | 20 (46.5%) | 28 (50.9%) | 48 (49%) | 49 (32.2%) | 77 (41%) | 126 (38.2%) |
| Salpingitisl | 14 (22.2%) | 10 (13.7%) | 24 (17.6%) | 8 (18.6%) | 10 (18.9%) | 18 (18.8%) | 5 (11.6%) | 9 (16.4%) | 14 (14.3%) | 27 (17.8%) | 29 (15.4%) | 56 (17%) |
| Regressed salpinxm | 6 (9.5%) | 16 (21.9%) | 22 (16.2%) | 7 (16.3%) | 18 (34%) | 25 (26%) | 15 (34.9%) | 20 (36.4%) | 35 (35.7%) | 28 (18.4%) | 54 (28.7%) | 82 (24.8%) |
| Cystic right oviduct/reminiscence | 9 (14.3%) | 14 (19.2%) | 23 (16.9%) | 8 (18.6%) | 17 (32.1%) | 25 (26%) | 0 (0%) | 6 (10.9%) | 6 (6.1%) | 17 (11.2%) | 37 (19.7%) | 54 (16.4%) |
| Miscellaneous | ||||||||||||
| Cannibalism | 4 (6.3%) | 3 (3.9%) | 7 (5%) | 0 (0%) | 1 (1.9%) | 1 (1%) | 1 (2.2%) | 0 (0%) | 1 (1%) | 5 (3.3%) | 4 (2.1%) | 9 (2.6%) |
| Neoplasia | 1 (1.6%) | 0 (0%) | 1 (0.7%) | 0 (0%) | 3 (5.7%) | 3 (3.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.7%) | 3 (1.6%) | 4 (1.2%) |
Continuous data is presented with mean ± standard deviation
BW, bodyweight; n, number
aCaudolaterodorsally located lacerations involving skin and muscle often associated with substantial tissue necrosis, “pocket formation” and fibrinopurulent cellulitis
bPododermatitis with profound swelling due to purulent material and/or fibrosis
cFootpad lesions consisting of discoloration, hyperkeratosis and/or ulceration
dExudate present within any of the major joints
ePresence of hyperaemia, haemorrhage, exudate, or excessive amounts of mucus with a turbid appearance
fOedema, consolidation and/or exudate
gPresence of fibrinous, purulent or fibrinopurulent exudate and/or thickening and opaqueness of the air sac
hPresence of fibrinous, purulent or fibrinopurulent exudate or fibrotic repair
iIncreased tubular pattern, urate deposition in ureters and/or swelling of the kidneys
jIn lay defined as hens having a developing- or fully developed egg present within the salpinx
kComplete absence of mature follicles or presence of atretic follicles indicating an early state of regression
lPresence of fibrinous, purulent, fibrinopurulent or mucopurulent exudate
mPale salpinx containing no developing egg and being less than half the expected size
Table 4.
Overview of gross lesions in broilers from Progenies A, B and C
| Progeny A (n = 54) |
Progeny B (n = 52) |
Progeny C (n = 48) |
Total (n = 154) |
|
|---|---|---|---|---|
| General | ||||
| Euthanised | 44 (81.5%) | 47 (90.4%) | 30 (62.5%) | 121 (78.6%) |
| Age (days) | ||||
| Mean | 8 ± 1.4 | 8.1 ± 2.4 | 6.1 ± 1.5 | 7.4 ± 2 |
| Min | 5 | 5 | 4 | 4 |
| Median | 8 | 8 | 7 | 7 |
| Max | 10 | 11 | 8 | 11 |
| BW (g), day | ||||
| 4 | – | – | 50 ± 19 | 50 ± 19 |
| 5 | 97 ± 27 | 65 ± 18 | – | 72 ± 24 |
| 6 | 156 ± 13 | 135 ± 10 | 80 ± 32 | 119 ± 39 |
| 7 | 133 ± 44 | – | 144 ± 48 | 140 ± 46 |
| 8 | 147 ± 55 | 193 ± 56 | 164 ± 20 | 166 ± 50 |
| 9 | 139 ± 61 | – | – | 139 ± 61 |
| 10 | 187 ± 91 | 193 ± 58 | – | 191 ± 68 |
| 11 | – | 253 ± 31 | – | 253 ± 31 |
| Gender | ||||
| Male | 22 (40.7%) | 20 (38.5%) | 19 (39.6%) | 61 (39.6%) |
| Female | 32 (59.3%) | 32 (61.5%) | 29 (60.4%) | 93 (60.4%) |
| Dehydration | 15 (27.8%) | 12 (23.1%) | 17 (35.4%) | 44 (28.6%) |
| Peri-cloacal urate | 2 (3.7%) | 6 (11.5%) | 7 (14.6%) | 15 (9.7%) |
| Umbilicus and yolk sac | ||||
| Unhealed umbilicus | ||||
| Total | 14 (25%) | 15 (28.8%) | 23 (47.9%) | 52 (33.8%) |
| Open | 1 (1.9%) | 0 (0%) | 1 (2.1%) | 2 (1.3%) |
| String | 11 (20.4%) | 13 (25%) | 16 (33.3%) | 40 (26%) |
| Button | 2 (3.7%) | 2 (3.8%) | 6 (12.5%) | 10 (6.5%) |
| Omphalitisb | 3 (5.6%) | 2 (3.8%) | 7 (14.6%) | 12 (7.8%) |
| Yolksacculitisc | 4 (7.4%) | 9 (17.3%) | 11 (22.9%) | 24 (15.6%) |
| Retained yolk sacd | 3 (8.6%) | 5 (15.6%) | 1 (2.1%) | 9 (11.8%) |
| Respiratory tract | ||||
| Tracheal changese | 3 (5.6%) | 1 (1.9%) | 2 (4.2%) | 6 (3.9%) |
| Pulmonary changesf | 38 (70.4%) | 39 (75%) | 34 (70.8%) | 111 (72.1%) |
| Airsacculitis | 3 (5.6%) | 1 (1.9%) | 1 (2.1%) | 4 (2.6%) |
| Coelomic cavity | ||||
| Pericarditisg | 3 (5.6%) | 6 (11.5%) | 6 (12.5%) | 15 (9.7%) |
| Perihepatitisg | 2 (3.7%) | 2 (3.8%) | 7 (14.6%) | 11 (7.1%) |
| Peritonitisg | 3 (5.6%) | 5 (9.6%) | 6 (12.5%) | 14 (9.1%) |
| Ascites | 4 (7.4%) | 4 (7.7%) | 2 (4.2%) | 10 (6.5%) |
| Renal changesh | 20 (37%) | 14 (26.9%) | 17 (35.4%) | 51 (33.1%) |
| Gastrointestinal tract | ||||
| Feed in crop | 51 (94.4%) | 44 (84.6%) | 33 (68.8%) | 128 (83.1%) |
| Empty/sparse GI content | 5 (9.3%) | 8 (15.4%) | 15 (31.3%) | 28 (18.2%) |
| Ulcus ventriculi | 1 (1.6%) | 3 (5.8%) | 5 (10.4%) | 9 (5.8%) |
Continuous data are presented with mean ± standard deviation
BW, bodyweight; GI, gastrointestinal; n, number
aPatent opening of the umbilicus without complications, or with entrapment of tissue forming either a string or a button
bPresence of hyperaemia and/or oedema in the umbilical area
cAlterations to the yolk consistency, e.g., watery, lumpy, thickened or inspissated yolk possibly with changes to the colour or odour as well as excessive hyperaemia
dPresence of the yolk sac in chickens older than seven days
ePresence of hyperaemia, haemorrhage, exudate, or excessive amounts of mucus with a turbid appearance
fOedema, consolidation and/or exudate
gPresence of fibrinous, purulent or fibrinopurulent exudate or fibrotic repair
hSwelling of the kidneys and/or increased tubular pattern
iPresence of exudate
Briefly, exudative peritonitis was present in 31.2% of the birds with perihepatitis and pericarditis being considerably less represented in only 3.8% and 3.5%, respectively. In females, salpingitis and perioophoritis both occurred in 17% of the birds, yet without any strict tendency to necessarily occur simultaneously in the same bird. Airsacculitis were present in 18.5% of the hens. The fractured long bones (n = 11 equalling 3.2%) included eight femoral and three tibial bones. Four birds from Farm C had an acute fracture of the beak. Two of these had additional injuries to the head area with extensive subcutaneous haemorrhage. Four additional birds from Farm C had similar trauma to the head area without any damage to the beak. These lesion types were not represented in animals from Farms A and B. The presence of purulent, fibrinous or fibrinopurulent arthritis identified in 8.2% of the birds was almost exclusively restricted to the intertarsal joints (n = 26), with the hip joint accounting for only two cases of arthritis. In adult females, 15.6% of the collected birds had caudolaterodorsal lacerations (mating injuries) (Figures 1D–F) ranging in sizes from 2 to 20 cm in length (mean 7.5 ± 3.5 cm). All revealed varying degree of associated profound tissue damage in relation to the laceration, i.e., muscle necrosis, fibrinopurulent cellulitis and/or pocket formation, and the lesions often contained bedding material. Registration on the reproductive status revealed that 64.2% of the birds with lacerations were not in active lay. Pododermatitis lesions, defined as the presence of discolouration, hyperkeratosis and/or ulceration, ranged in size from 2 to 40 mm (mean 9.4 ± 7 mm). Only seven birds had additional profound swelling with purulent material and/or fibrosis, i.e., bumblefoot.
Figure 1.

Lesions observed at post-mortem examination. A Chronic perihepatitis. The bird had multiple chronic lesions consistent with polyserositis, e.g., opaque and thickened thoracic air sacs, chronic adhesive pericarditis, and numerous adhesions between several parts of the intestines, the mesovarium and salpinx. The ovary of the hen was inactive. B Deposition of urate in the conjunctiva of a hen. Throughout the coelomic cavity and major joints urate deposition was present. The ureters were occluded by urate and the kidneys were swollen and showed an increased tubular pattern. The ovary was in regression and no egg was present within the oviduct. C Massive cystic enlargement of the oviduct containing fluid with lumps of fibrinopurulent exudate. The ovary was inactive and the remaining coelomic organs were all cranially displaced. D A massive skin laceration with a caudolaterodorsal location consistent with a mating injury. Externally, the wound measured approximately 9 × 5 cm, with multiple internal pockets with presence of necrotic tissue, fibrinopurulent material and bedding. E Fibrinopurulent exudate extending from the wound in D. The hen was not in lay , and the ovary had completely regressed. F A lesion similar to D–E with fibrinopurulent cellulitis extending from the caudodorsal part of the bird to cranial part of the leg. The external measurement of the wound was approximately 5 × 8 cm. The bird was not in lay , and the ovary was in regression with a few atretic follicles present. Fibrinopurulent peritonitis was present. G Purulent peritonitis in a young chicken. The yolk sac appeared grossly normal though a “button” was present in the unhealed umbilicus, and the yolk sac and liver yielded pure growth of E. coli. H Hyperaemia of the yolk sac which contained a partly inspissated content (yolksacculitis). The umbilical area was hyperaemic (omphalitis) extending to the abdominal wall, fibrinous pericarditis and perihepatitis was present as well as fibrinopurulent airsacculitis and peritonitis. I Ulcus ventriculi in a young chicken adjacent to the gastric isthmus.
All birds submitted as presumed clinically healthy were females, and 86.4% were in active lay, whereas this was the case in 40.9% of the remaining hens. In the putative healthy birds, 43.8% were graded as obese, 38.6% as above average and 18.2% as having a normal body condition, whilst these figures were 16.9%, 31.8% and 36.4%, respectively, among the remaining birds. In the latter, 18.5% were graded as having a body condition below average and 6.2% were cachectic. Two (4.5%) of the putative healthy hens had purulent peritonitis, one (2.3%) had purulent perioophoritis, one (2.3%) purulent salpingitis, and six (13.6%) had pulmonary changes.
A total of 10 roosters were submitted to post-mortem examination, of which Farm A contributed with four and Farm C with six, and all were euthanised except two males from Farm C. One of these suffered from a complete intestinal obstruction caused by Ascaridia galli, whereas the other was cachectic and dehydrated with empty intestines and had bilateral hypoplasia of the kidneys. The remaining male birds showed various lesions (purulent arthritis, tibial fracture, deformity to a toe and foot pads most likely as a consequence of a healed fracture, chronic pododermatitis, acute fracture of the beak and necrosis of the comb).
Broilers gathered from Progeny C were generally younger than those from Progeny A and Progeny B, and seemed to excel on some parameters, e.g., unhealed umbilicus, omphalitis, perihepatitis, absence of feed within the crop and empty/sparse content in the gastrointestinal tract (Table 4).
Escherichia coli diversity
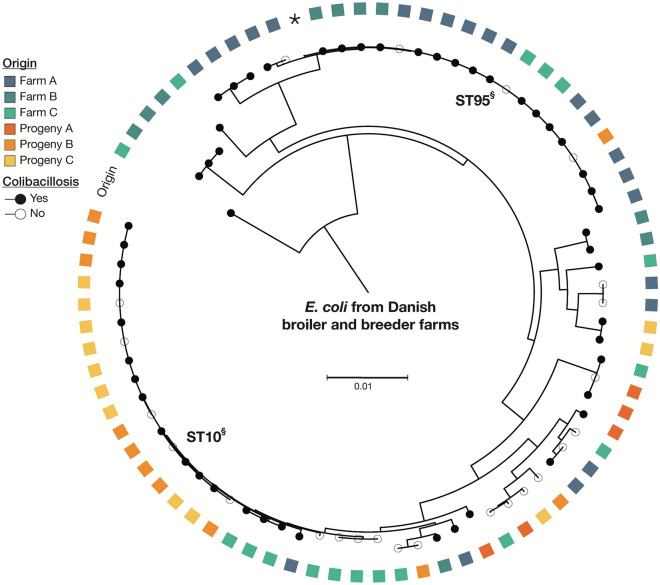
In total, 77 E. coli isolates obtained from extraintestinal organs in birds during a non-outbreak situation were selected for sequencing. This revealed a diverse range of sequence types (n = 23) (Table 5 and Figure 2). Overall, ST95 and ST10 were the most prevalent sequence types (27.3% and 23.4%, respectively) and birds lacking signs of colibacillosis were represented among both these sequence types.
Table 5.
Characteristics of the detected sequence types
| Sequence type | Prevalence within farma | No. of positive birds/farm | Total no | Serotype(s) | SNP-distance within farmb | SNP-distance between farms |
|---|---|---|---|---|---|---|
| ST10 | Farm A1 (4.5%) | 1 | 18 (23.4%) | O71:H40, –:H40 | – | 0–7038 |
| Farm C3 (17.6%) | 2 | 7 | ||||
| Progeny B5 (45.5%) | 4 | 1–960 | ||||
| Progeny C9 (75%) | 7 | 0–959 | ||||
| ST23 | Farm C1 (5.9%) | 1 | 1 (1.3%) | O78:H17 | – | – |
| ST38 | Farm C1 (5.9%) | 1 | 1 (1.3%) | O7:H15 | – | – |
| ST58 | Progeny C1 (8.3%) | 1 | 1 (1.3%) | O86:H30 | – | – |
| ST69 | Farm B2 (20%) | 2 | 2 (2.6%) | O15:H6 | 1861 | – |
| ST95 | Farm A13 (59.1%) | 11 | 21 (27.3%) | O2:H5, O1:H7, -:H5 | 0–3691 | 0–3691 |
| Farm B 4 (40%) | 2 | 39 | ||||
| Farm C3 (17.6%) | 3 | 0–1 | ||||
| Progeny B1 (9.1%) | 1 | – | ||||
| ST117 | Farm C1 (5.9%) | 1 | 1 (1.3%) | -:H4 | – | – |
| ST155 | Farm C1 (5.9%) | 1 | 2 (2.6%) | O8:H20, O37:H10 | – | 2526 |
| Progeny A1 (20%) | 1 | |||||
| ST219 | Farm C1 (5.9%) | 1 | 3 (3.9%) | O138:H48, –:H48 | – | 29–30 |
| Progeny A2 (40%) | 2 | 1 | ||||
| ST362 | Farm A2 (9.1%) | 1 | 2 (2.6%) | O7:H6 | – | – |
| ST442 | Progeny B1 (9.1%) | 1 | 1 (1.3%) | O91:H21 | – | – |
| ST457 | Farm B3 (30%) | 1 | 3 (3.9%) | O11:H25 | – | – |
| ST746 | Farm B1 (10%) | 1 | 3 (3.9%) | O100:H30, –:H18 | – | 10–7545 |
| Farm C1 (5.9%) | 1 | – | ||||
| Progeny B1 (9.1%) | 1 | – | ||||
| ST770 | Farm C1 (5.9%) | 1 | 1 (1.3%) | O15:H16 | – | – |
| ST1141 | Farm C1 (5.9%) | 1 | 1 (1.3%) | O113:H4 | – | – |
| ST1426 | Progeny A1 (20%) | 1 | 1 (1.3%) | O8:H16 | – | – |
| ST1564 | Farm A1 (4.5%) | 1 | 1 (1.3%) | –:H21 | – | – |
| ST1882 | Progeny C2 (16.7%) | 2 | 2 | O23:H4, –:H4 | 0 | – |
| ST3270 | Progeny A1 (20%) | 1 | 1 (1.3%) | O8:H16 | – | – |
| ST6665 | Farm C3 (17.6%) | 1 | 3 (3.9%) | O8:H30 | – | – |
| ST7321 | Progeny B3 (27.8%) | 2 | 3 (3.9%) | O71:H40 | 0 | – |
| ST7614 | Farm A2 (9.1%) | 2 | 2 (2.6%) | O9:H19 | 4 | – |
| ST11774 | Farm A3 (13.6%) | 3 | 3 (3.9%) | O46:H31, –:H31 | 1–4 | – |
SNP, single nucleotide polymorphism; ST, sequence type
aOnly farms positive for the given ST are listed
bOnly between bird comparison
Figure 2.
Phylogenetic tree presenting Escherichia coli obtained during a non-outbreak period. Midpoint rooted phylogenetic tree based on core genome single-nucleotide polymorphisms (SNPs) of the 77 E. coli isolated from Danish broiler breeder and broiler farms. Farm/progeny origin is indicated on the phylogeny, as is colibacillosis status of the host. Colibacillosis defined as presence of fibrinous-, purulent-, fibrinopurulent or mucopurulent exudate inflammation is presented with black circles, whereas the absence is denoted with open circles. §Single-locus variants are observed within the ST10 cluster (ST7321 (n = 3), ST6665 (n = 3) and ST1141 (n = 1)). The tree is based on 222 391 SNPs detected within a ~3.03 Mbp conserved core genome across the collection. *Depicts E. coli isolate E51 used as reference. Scale bar indicates substitutions per site. Among the 77 isolates, 24 were obtained from the liver, 16 from the salpinx, 22 from the lung, and 12 from the yolk sac. The spleen, umbilical area, and a case of arthritis each contributed with a single isolate. Isolates from birds with an absence of lesions consistent with colibacillosis represented isolation sites: liver, lung, salpinx, yolk sac and joint (n = 6, 11, 2, 4 and 1, respectively).
In four birds, contributing with multiple isolates obtained from different organs, different sequence types were found depending on the organ of isolation. There was no tendency of a particular organ to differ more from the rest and the birds were all classified as having colibacillosis. The variation between isolates of similar sequence type, isolated from the same bird, showed a SNP-distance of 0–2 across a core genome of 58% (3.03 Mb). From a single bird, E. coli isolated from the magnum was sequenced twice (different colonies) revealing a single SNP between the two colonies. Curiously, when comparing the ST95 isolates from Progeny B to Farm B, a variation between 14 and 39 SNPs was observed, whereas the SNP-based distances from Progeny B to Farm A and Farm C were between 0-3686 and 10–11, respectively. Likewise, comparing the diversity among the ST10 isolates from Farm C and Progeny C, the SNP-distances were between 4419 and 4428, whilst the SNP-distance between Progeny B and Progeny C varied from 0 to 958, which was comparable with the within-farm variation on the farms. For ST746, the genetic diversity was larger between Farm B and Progeny B than between Farm C and Progeny B with 7545 versus 10 SNPs, respectively. Overall, the presence of resistance markers was low with mdfA and sitABCD (multidrug transporter and hydrogen peroxide resistance, respectively) being the primary findings, except in one isolate (ST1564) additionally containing blaTEM1B, sul2, drfA1, tet(A), aadA1, and qnrS1, thereby, conferring resistance towards beta-lactams, sulphonamides, trimethoprim, tetracycline, aminoglycosides, and quinolones, respectively. Resistance towards quinolones was detected in one more isolate (ST770) containing the parE (I355T) mutation. Generally, ST95 seemed to harbour sul2 to a great extent with 19/21 carrying this gene. Detailed information on resistance markers is available in Additional file 3.
Discussion
In the present study, a comprehensive characterisation of lesions within three broiler breeder farms, as well as related progeny, all having acceptable levels of background mortality, is presented. Furthermore, E. coli isolates obtained from the farms were characterised by WGS.
All farms exhibited exemplary production results with an absence of general disease problems and a low mortality confirming a non-outbreak status. A wide range of infection-related lesions was registered within the broiler breeders, with peritonitis being the most prevalent, whereas yolksacculitis was the most prominent in the broilers. Both lesion-types are characteristic of E. coli infection [1, 5, 32].
Mating injuries were a common finding among the hens and must be considered a possible portal of entrance for bacteria such as E. coli. These lacerations also represent a major welfare concern [33] as the lesions are of considerable size and often associated with severe damage to the surrounding tissues. The lower level of mating injuries in hens from Farm C is most likely attributable to farm management on this location, as animals exhibiting this lesion-type are only rarely culled due to a belief in their ability to produce eggs despite their injuries (personal communication). Yet, in the current study, it was revealed that the majority of these birds were not in active lay.
Pododermatitis is another well-known factor impairing the welfare of poultry [34], and especially in broilers, footpad lesions have received marked attention and is routinely evaluated upon slaughter and used as a welfare parameter [34, 35]. However, in broiler breeders, less is known about the prevalence of footpad lesions [1]. An increased frequency as the birds ages has been reported previously but was not apparent throughout the farms in the current study [1, 36]. The prevalence both differed between farms and time periods, likely reflecting the condition of the bedding material— a well-known risk factor [34, 36, 37]. The occurrence of sternal fractures increased with age, which is in accordance with previous reports [38], and seemed to differ in prevalence between the farms in the current study.
A total of 23 different sequence types were represented among the 77 sequenced E. coli isolates. Among these, 16 different O-antigens were identified, including O78, O1 and O2 commonly associated with APEC [39, 40], whilst 17 different H-antigens were demonstrated. In the Danish broiler breeder production, a vaccine strategy targeting E. coli was implemented in 2016 following a devastating clonal E. coli outbreak [22]. The strategy was based on the commercial vaccine Poulvac® E. coli and an autogenous vaccine containing the outbreak isolates ST140 O1:H7 (clonal complex 95) and ST117 O78:H4 with subsequent modifications to the vaccine rendering broiler breeders in the current study to be vaccinated with ST117 O78:H4, ST117 O53:H4 and ST95 O1:H7 (personal communication). Prior to vaccine implementation, particularly ST117 had been identified as the primary agent in the aforementioned Danish outbreak [15]. Intriguingly, ST117 was only isolated from a single adult hen in the present study despite its former role as a major contributor to E. coli-related mortality in Denmark [15]. Though it is tempting to contribute this almost negligible occurrence of ST117 to the effects of the autogenous vaccine—especially considering the support from recent studies of their protective effect and ability to alter the E. coli population on farms [41–43], the relative absence of ST117 might simply reflect the study population, as the current study presents sporadic cases of colibacillosis on non-outbreak farms and, therefore, could be dominated by opportunistic E. coli strains and not “true” APEC.
On the contrary to ST117, ST95, likewise known to have previously been a prominent sequence type involved in outbreaks of colibacillosis [18, 44], was greatly represented amongst the broiler breeders but only occurred in a single isolate from the progeny. In fact, a dissimilarity between sequence types prevalent in the adult broiler breeders suffering from colibacillosis and the types found in their progeny flocks was apparent, with ST10 being a main finding within the broilers. A noteworthy finding is also the presence of both ST95 and ST10 in birds without lesions consistent with colibacillosis, suggesting the ability to cope with their extraintestinal presence under some circumstances. This could indicate a lack of a contributing factor in these animals, e.g., stress or comorbidities providing an opportunity to act as a pathogen.
Interestingly, isolates from epidemiologically linked parent and progeny farms were not necessarily more genetically similar than those from unrelated farms. Also, in several cases, there were an absence or only a few SNPs between isolates from unrelated farms, and the within-farm variation and between-farm variation were often similar, thus indicating a common or recent source across farms.
Generally, the occurrence of resistance markers was low especially within isolates obtained from the progeny, and the findings were comparable to those previously reported in Denmark [45, 46]. Among ST95, the sequence type mainly obtained from broiler breeders, the presence of the sul2 gene entailing resistance towards sulphonamides was common. This finding corresponds to previous observations in Danish broiler breeders, likewise reporting ST95 to often contain genes linked to sulphonamide resistance [47]. A single isolate exhibited multidrug-resistance with a presence of genes conferring resistance towards beta-lactams, sulphonamides, trimethoprim, tetracycline, aminoglycosides, and quinolones.
In conclusion, a comprehensive insight into the occurrence of lesions in broiler breeders and young broilers from farms without general disease problems is for the first time presented together with whole-genome sequencing of E. coli isolates. This revealed a diverse collection of E. coli, including several sequence types previously described as APEC, which could even be isolated from birds lacking colibacillosis-like lesions. The study emphasizes the elusive nature of the APEC pathotype and the great diversity of E. coli isolated from extraintestinal sites in poultry during non-outbreak situations.
Supplementary Information
Additional file 1. Standardised necropsy scheme for broiler breeders.
Additional file 2. Standardised necropsy scheme for broilers.
Additional file 3. Phylogenetic tree with presentation of resistance markers.
Acknowledgements
The authors would like to thank Katrine Aagaard, Toloe Allahghadry and Dennis Brok for technical assistance. Also, special thanks are extended to the farm personnel at all the farms contributing to this study, as well as to Johanne Juul and Hanne Skaarup for expert advice and support.
Authors' contributions
SK, HEJ, AMB, RHO, IT: study conception; IT, AMB, RHO, HEJ, SK, MS: study design and project managing; IT, AMB, RHO, HEJ, SK: practical execution; SK, IT, HEJ, AMB, RHO: necropsies; AMB, RHO: microbiology; SB, MS, SK: bioinformatic analysis and interpretation; SK: analysis and interpretation of results; SK, MS, HEJ, IT, AMB, RHO: Supervision; RHO and AMB: Funding acquisition; SK: writing—original draft. All authors read and approved the final manuscript.
Funding
This project was supported by the Innovation Fund of Denmark.
Availability of data and materials
Data, upon which the conclusions in this manuscript relies, are presented within the paper and the supplementary material. Bacterial isolates and whole genome sequences are available from the corresponding author upon request.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thofner ICN, Poulsen LL, Bisgaard M, Christensen H, Olsen RH, Christensen JP. Longitudinal study on causes of mortality in Danish broiler breeders. Avian Dis. 2019;63:400–410. doi: 10.1637/12006-113018-Reg.1. [DOI] [PubMed] [Google Scholar]
- 2.Poulsen LL, Thofner I, Bisgaard M, Christensen JP, Olsen RH, Christensen H. Longitudinal study of transmission of Escherichia coli from broiler breeders to broilers. Vet Microbiol. 2017;207:13–18. doi: 10.1016/j.vetmic.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Grafl B, Aigner F, Liebhart D, Marek A, Prokofieva I, Bachmeier J, Hess M. Vertical transmission and clinical signs in broiler breeders and broilers experiencing adenoviral gizzard erosion. Avian Pathol. 2012;41:599–604. doi: 10.1080/03079457.2012.740614. [DOI] [PubMed] [Google Scholar]
- 4.Liljebjelke KA, Hofacre CL, Liu T, White DG, Ayers S, Young S, Maurer JJ. Vertical and horizontal transmission of Salmonella within integrated broiler production system. Foodborne Pathog Dis. 2005;2:90–102. doi: 10.1089/fpd.2005.2.90. [DOI] [PubMed] [Google Scholar]
- 5.Nolan LK, Vaillancourt J-P, Barbieri NL, Logue CM. Colibacillosis. In: Swayne DE, Boulianne M, Logue CM, McDougald LR, Nair V, Suarez DL, Wit S, Grimes T, Johnson D, Kromm M, Prajitno TY, Rubinoff I, Zavala G, editors. Diseases of Poultry. Hoboken: Wiley; 2020. pp. 770–830. [Google Scholar]
- 6.Grafl B, Gaussmann B, Sulejmanovic T, Hess C, Hess M. Risks and disease aetiologies of compromised performance in commercial broilers kept at lower stocking density and limited antimicrobial use. Avian Pathol. 2020;49:621–630. doi: 10.1080/03079457.2020.1805411. [DOI] [PubMed] [Google Scholar]
- 7.Landman WJM, van Eck JHH. The incidence and economic impact of the Escherichia coli peritonitis syndrome in Dutch poultry farming. Avian Pathol. 2015;44:370–378. doi: 10.1080/03079457.2015.1060584. [DOI] [PubMed] [Google Scholar]
- 8.Yogaratnam V. Analysis of the causes of high-rates of carcass rejection at a poultry-processing plant. Vet Rec. 1995;137:215–217. doi: 10.1136/vr.137.9.215. [DOI] [PubMed] [Google Scholar]
- 9.Vandemaele F, Vereecken M, Derijcke J, Goddeeris BM. Incidence and antibiotic resistance of pathogenic Escherichia coli among poultry in Belgium. Vet Rec. 2002;151:355–356. doi: 10.1136/vr.151.12.355. [DOI] [PubMed] [Google Scholar]
- 10.Stokholm NM, Permin A, Bisgaard M, Christensen JP. Causes of mortality in commercial organic layers in Denmark. Avian Dis. 2010;54:1241–1250. doi: 10.1637/9375-041910-Reg.1. [DOI] [PubMed] [Google Scholar]
- 11.Guabiraba R, Schouler C. Avian colibacillosis: still many black holes. Fems Mic Let. 2015 doi: 10.1093/femsle/fnv118. [DOI] [PubMed] [Google Scholar]
- 12.Dho-Moulin M, Fairbrother JM. Avian pathogenic Escherichia coli (APEC) Vet Res. 1999;30:299–316. [PubMed] [Google Scholar]
- 13.Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog Dis. 2013;10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collingwood C, Kemmett K, Williams N, Wigley P. Is the concept of avian pathogenic Escherichia coli as a single pathotype fundamentally flawed? Front Vet Sci. 2014;1:5. doi: 10.3389/fvets.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronco T, Stegger M, Olsen RH, Sekse C, Nordstoga AB, Pohjanvirta T, Lilje B, Lyhs U, Andersen PS, Pedersen K. Spread of avian pathogenic Escherichia coli ST117 O78:H4 in Nordic broiler production. BMC Genomics. 2017 doi: 10.1186/s12864-016-3415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papouskova A, Masarikova M, Valcek A, Senk D, Cejkova D, Jahodarova E, Cizek A. Genomic analysis of Escherichia coli strains isolated from diseased chicken in the Czech Republic. BMC Vet Res. 2020;16:189. doi: 10.1186/s12917-020-02407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordoni G, Woodward MJ, Wu HH, Alanazi M, Wallis T, La Ragione RM. Comparative genomics of European avian pathogenic E. coli (APEC) BMC Genomics. 2016;17:960. doi: 10.1186/s12864-016-3289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehat JW, van Vliet AHM, La Ragione RM. The avian pathogenic Escherichia coli (APEC) pathotype is comprised of multiple distinct, independent genotypes. Avian Pathol. 2021;50:402–416. doi: 10.1080/03079457.2021.1915960. [DOI] [PubMed] [Google Scholar]
- 19.Mageiros L, Meric G, Bayliss SC, Pensar J, Pascoe B, Mourkas E, Calland JK, Yahara K, Murray S, Wilkinson TS, Williams LK, Hitchings MD, Porter J, Kemmett K, Feil EJ, Jolley KA, Williams NJ, Corander J, Sheppard SK. Genome evolution and the emergence of pathogenicity in avian Escherichia coli. Nat Commun. 2021;12:765. doi: 10.1038/s41467-021-20988-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen HE (2011) Necropsy - a handbook and atlas. Biofolia, Denmark
- 21.Bifrost. https://github.com/ssi-dk/bifrost.
- 22.Ronco T, Stegger M, Andersen PS, Pedersen K, Li L, Thofner ICN, Olsen RH. Draft genome sequences of two avian pathogenic Escherichia coli strains of clinical importance, E44 and E51. Genome Announc. 2016;4:e00768–e816. doi: 10.1128/genomeA.00768-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahl JW, Lemmer D, Travis J, Schupp JM, Gillece JD, Aziz M, Driebe EM, Drees KP, Hicks ND, Williamson CHD, Hepp CM, Smith DE, Roe C, Engelthaler DM, Wagner DM, Keim P. NASP: an accurate, rapid method for the identification of SNPs in WGS datasets that supports flexible input and output formats. Microb Genom. 2016;2:000074. doi: 10.1099/mgen.0.000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bessonov K, Laing C, Robertson J, Yong I, Ziebell K, Gannon VPJ, Nichani A, Arya G, Nash JHE, Christianson S. ECTyper: in silico Escherichia coli serotype and species prediction from raw and assembled whole- genome sequence data. Microb Genom. 2021;7:000728. doi: 10.1099/mgen.0.000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MLST. https://github.com/tseemann/mlst.
- 29.Wirth T, Falush D, Lan RT, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MCJ, Ochman H, Achtman M. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006;60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt M, Mather AE, Sanchez-Buso L, Page AJ, Parkhill J, Keane JA, Harris SR. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb Genom. 2017;3:000131. doi: 10.1099/mgen.0.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, Philippon A, Allesoe RL, Rebelo AR, Florensa AF, Fagelhauer L, Chakraborty T, Neumann B, Werner G, Bender JK, Stingl K, Nguyen M, Coppens J, Xavier BB, Malhotra-Kumar S, Westh H, Pinholt M, Anjum MF, Duggett NA, Kempf I, Nykasenoja S, Olkkola S, Wieczorek K, Amaro A, Clemente L, Mossong J, Losch S, Ragimbeau C, Lund O, Aarestrup FM. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75:3491–3500. doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen RH, Frantzen C, Christensen H, Bisgaard M. An investigation on first-week mortality in layers. Avian Dis. 2012;56:51–57. doi: 10.1637/9777-051011-Reg.1. [DOI] [PubMed] [Google Scholar]
- 33.De Jong IC, Guemene D. Major welfare issues in broiler breeders. World's Poult Sci J. 2011;67:73–81. doi: 10.1017/s0043933911000067. [DOI] [Google Scholar]
- 34.Shepherd EM, Fairchild BD. Footpad dermatitis in poultry. Poult Sci. 2010;89:2043–2051. doi: 10.3382/ps.2010-00770. [DOI] [PubMed] [Google Scholar]
- 35.Kyvsgaard NC, Jensen HB, Ambrosen T, Toft N. Temporal changes and risk factors for foot-pad dermatitis in Danish broilers. Poult Sci. 2013;92:26–32. doi: 10.3382/ps.2012-02433. [DOI] [PubMed] [Google Scholar]
- 36.Kaukonen E, Norring M, Valros A. Effect of litter quality on foot pad dermatitis, hock burns and breast blisters in broiler breeders during the production period. Avian Pathol. 2016;45:667–673. doi: 10.1080/03079457.2016.1197377. [DOI] [PubMed] [Google Scholar]
- 37.Ekstrand C, Algers B, Svedberg J. Rearing conditions and foot-pad dermatitis in Swedish broiler chickens. Prev Vet Med. 1997;31:167–174. doi: 10.1016/s0167-5877(96)01145-2. [DOI] [PubMed] [Google Scholar]
- 38.Rufener C, Makagon MM. Keel bone fractures in laying hens: a systematic review of prevalence across age, housing systems, and strains. J Anim Sci. 2020;98:S36–S51. doi: 10.1093/jas/skaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dziva F, Stevens MP. Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008;37:355–366. doi: 10.1080/03079450802216652. [DOI] [PubMed] [Google Scholar]
- 40.Mellata M, Dho-Moulin M, Dozois CM, Curtiss R, Lehoux B, Fairbrother JM. Role of avian pathogenic Escherichia coli virulence factors in bacterial interaction with chicken heterophils and macrophages. Infect Immun. 2003;71:494–503. doi: 10.1128/iai.71.1.494-503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koutsianos D, Gantelet H, Franzo G, Lecoupeur M, Thibault E, Cecchinato M, Koutoulis KC. An assessment of the level of protection against colibacillosis conferred by several autogenous and/or commercial vaccination programs in conventional pullets upon experimental challenge. Vet Sci. 2020;7:80. doi: 10.3390/vetsci7030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kromann S, Olsen RH, Bojesen AM, Jensen HE, Thofner I. Protective potential of an autogenous vaccine in an aerogenous model of Escherichia coli infection in broiler breeders. Vaccines. 2021;9:1233. doi: 10.3390/vaccines9111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lozica L, Repar J, Gottstein Z. Longitudinal study on the effect of autogenous vaccine application on the sequence type and virulence profiles of Escherichia coli in broiler breeder flocks. Vet Microbil. 2021;259:109159. doi: 10.1016/j.vetmic.2021.109159. [DOI] [PubMed] [Google Scholar]
- 44.Christensen H, Bachmeier J, Bisgaard M. New strategies to prevent and control avian pathogenic Escherichia coli (APEC) Avian Pathol. 2021;50:370–381. doi: 10.1080/03079457.2020.1845300. [DOI] [PubMed] [Google Scholar]
- 45.Bortolaia V, Bisgaard M, Bojesen AM. Distribution and possible transmission of ampicillin- and nalidixic acid-resistant Escherichia coli within the broiler industry. Vet Microbiol. 2010;142:379–386. doi: 10.1016/j.vetmic.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 46.DANMAP . Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Statens Serum Institut: National Food Institute, Technical University of Denmark; 2020. [Google Scholar]
- 47.Poulsen LL, Kudirkiene E, Jorgensen SL, Djordjevic SP, Cummins ML, Christensen JP, Christensen H, Bisgaard M, Thofner I. Whole genome sequence comparison of avian pathogenic Escherichia coli from acute and chronic salpingitis of egg laying hens. BMC Vet Res. 2020;16:148. doi: 10.1186/s12917-020-02369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Standardised necropsy scheme for broiler breeders.
Additional file 2. Standardised necropsy scheme for broilers.
Additional file 3. Phylogenetic tree with presentation of resistance markers.
Data Availability Statement
Data, upon which the conclusions in this manuscript relies, are presented within the paper and the supplementary material. Bacterial isolates and whole genome sequences are available from the corresponding author upon request.