Abstract
Background
Inflammation plays a critical role in acute myocardial infarction (AMI). Recent studies have shown the value of hematologic indicators in MI risk stratification and prognostic assessment. However, the association between lymphocyte-to-monocyte ratio (LMR) and the long-term mortality of critically ill MI patients remains unclear.
Methods
Clinical data were extracted from the Medical Information Mart for Intensive Care III database. Patients diagnosed with AMI on admission in the intensive care units were include. The optimal cutoff value of LMR was determined by X-tile software. The Cox proportional hazard model was applied for the identification of independent prognostic factors of 1-year mortality and survival curves were estimated using the Kaplan–Meier method. In order to reduce selection bias, a 1:1 propensity score matching (PSM) method was performed.
Results
A total of 1517 AMI patients were included in this study. The cutoff value for 1-year mortality of LMR determined by X-Tile software was 3.00. A total of 534 pairs of patients were matched after PSM. Multivariate analysis (HR = 1.369, 95%CI 1.110–1.687, P = 0.003) and PSM subgroups (HR = 1.299, 95%CI 1.032–1.634, P = 0.026) showed that 1-year mortality was significantly higher in patients with LMR < 3.00 than patients with LMR ≥ 3.00 in Cox proportional hazard models. The survival curves showed that patients with LMR < 3.00 had a significantly lower 1-year survival rate before (63.83 vs. 81.03%, Log rank P < 0.001) and after PSM (68.13 vs. 74.22%, Log rank P = 0.041).
Conclusion
In this retrospective cohort analysis, we demonstrated that a low admission LMR (< 3.00) was associated with a higher risk of 1-year mortality in critically ill patients with AMI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-022-02745-z.
Keywords: Lymphocyte-to-monocyte ratio (LMR), Acute myocardial infarction (AMI), Propensity score matching, Mortality, Intensive care unit (ICU)
Introduction
Cardiovascular diseases (CVDs) are the leading cause of global mortality and disability, bringing a great burden of disease to health expenditure [1]. The prevalence and death rate of ischemic heart disease (IHD) remain increasing during last decades [2]. There is an urgent need for health system to improve pre- and in-hospital care for acute coronary syndrome, so it appears to be particularly significant to identify patients at high risk for adverse outcomes of IHD.
The inflammation following acute myocardial infarction (AMI) plays a critical role in determining MI size and subsequent left ventricular remodeling [3, 4]. Immune cells such as neutrophils, monocytes/macrophages and lymphocytes are activated or recruited to the infarct area contributing to necrotic substance removal and tissue repair [5–7], which are considered new targets for myocardial protection [8] and prognostic prediction [9–11].
Hematological indices such as hemoglobin levels, serum albumin, white blood cells (WBC), platelet to lymphocyte ratio (PLR) and neutrophil to lymphocyte ratio (NLR) have gained attention because of their low cost and clinical accessibility, which has also been proved valuable for risk stratification and prognosis in IHD patients [12–18]. Lymphocyte to monocyte ratio (LMR), a novel predictor of inflammation, are concerned to be associated with the severity and outcomes of cardiovascular diseases [19–21]. Whereas, no researches demonstrate the relevance between LMR and long-term mortality of critically ill patients with AMI.
In this study, we aimed to determine the association between admission LMR and risk of long-term mortality in critically ill patients with AMI based on the Medical Information Mart for Intensive Care-III (MIMIC-III) database.
Methods
Data source
All the relevant data were obtained from the Medical Information Mart for Intensive Care-III (MIMIC-III) database (version 1.4). MIMIC-III is a freely available database containing the records of 46,520 critically ill patients admitted to intensive care units (ICUs) of the Beth Israel Deaconess Medical Center (Boston, Massachusetts) from 2001 to 2012 [22, 23], which contains dates of death up to 4 years. The establishment of the MIMIC-III database was approved by the Institutional Review Boards (IRB) of the Massachusetts Institute of Technology (MIT, Cambridge, MA, USA) and Beth Israel Deaconess Medical Center. The database documents included charted events such as demographics data, laboratory tests, vital signs, survival data and diagnostic information such as the International Classification of Diseases, Ninth Revision (ICD-9). We completed the National Institutes of Health online course and passed the exam named “Protecting Human Research Participants” (Record ID 36,331,340). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Population selection
We included all critically ill patients diagnosed with AMI using ICD-9 diagnosis codes at first ICU admission in MIMIC-III database. The exclusion criteria were as follows: (1) age less than 18 years old; (2) missing lymphocyte and monocyte counts values at first 24 h of admission.
Data extraction
All data were extracted from MIMIC-III database using structure query language (SQL) with PostgreSQL (version 9.6). The code that supports the MIMIC-III documentation and website is publicly available, and contributions from the community of users are encouraged (https://github.com/MIT-LCP/mimic-website). The extracted data included: (1) demographics: age, gender, ethnicity and body mass index (BMI); (2) vital signs: heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), respiratory rate (RR), temperature and percutaneous oxygen saturation (SpO2); (3) comorbidities: congestive heart failure (CHF), cardiac arrhythmias, hypertension, diabetes, chronic pulmonary disease, renal failure, liver disease, coagulopathy and elixhauser comorbidity index (ECI); (4) laboratory parameters: peripheral white blood cell count (WBC), neutrophil count, monocyte count, lymphocyte count, platelet count (PLT), hemoglobin (Hb), hematocrit (HCT), glucose (Glu), blood urea nitrogen (BUN), serum creatinine (Scr) and LMR; (5) scoring system: systemic inflammatory response syndrome (SIRS), simplified acute physiology score (SAPS) and sequential organ failure assessment (SOFA); (6) treatment information: percutaneous coronary intervention (PCI) and coronary bypass artery grafting (CABG); (7) outcomes: ICU length of stay, hospital length of stay, in-hospital mortality, 30-day mortality and 1-year mortality. Variables with less than 30% missing values were imputed using the multiple imputation method.
Statistical analysis
Continuous variables were presented as the mean ± SD or median (interquartile range) and compared by t-test or Mann–Whitney U test. Categorical data were presented as frequencies with percentages and analyzed by χ2 test. Skewness/Kurtosis test and histogram were adopted to assess the normality of variables. After propensity score matching analysis, the paired t-test and Wilcoxon rank sum test for continuous data and the McNemar test for categorical data was used for assessing the comparability of baseline characteristics in the matched groups. The optimal cutoff value of the LMR for 1-year mortality was determined by X-tile (Version 3.6.1, Yale University School of medicine) software [24]. The principle of the software is to enumerate continuous variables as cutoff values and log-rank tests were performed for all cases based on survival data separately, with the variable value corresponding to the smallest P-value being determined as the optimal cutoff value. Survival curves were estimated using the Kaplan–Meier method and compared by the log-rank test.
To reduce the selection bias between different LMR groups, propensity score matching analysis (PSM) was performed. The propensity score was calculated according to the following baseline characteristics: age, gender, congestive heart failure, hypertension, chronic pulmonary disease, renal failure, SpO2, WBC, Glu, Scr, BUN, SAPSII and SOFA scores. The psmatch2 package in STATA software was used to create matched sample. Propensity scores were estimated using logistic regression models. Patients were derived using 1:1 matching with a caliper of 0.02 and without replacement. A total of 1086 patients were propensity score-matched eventually.
The Cox proportional hazard model was applied for the univariate and multivariate analyses to identify independent prognostic factors of 1-year mortality. To evaluate the association between the LMR and mortality, model I was adjusted for age, gender and ethnicity; model II was adjusted for variables with P values less than 0.05 in the univariate regression analysis. The results are presented as hazard ratios (HR) and 95% confidence intervals (CI). Subgroup analysis were performed with Cox regression model according to age, gender, ethnicity, hypertension, CHF, cardiac arrhythmias, chronic pulmonary disease, renal failure, coagulopathy, PCI, CABG, SIRS, SAPS II, SOFA, HR, DBP, RR, SpO2, WBC, Hb, PLT, Glu, Scr and BUN. All tests were two-sided, and P values < 0.05 were considered significant. All statistical analyses in our study were performed using STATA V.16.0 and R version 4.1.0.
Results
Patient characteristics
A total of 1517 acute myocardial infarction patients were included in our study (Additional file 1: Fig. S1). The optimal cutoff value of admission LMR for 1-year mortality was 3.00 (with a sensitivity of 60.45% and a specificity of 62.06%) calculated by the X-tile software. Patients were divided into two groups according to the LMR: the low LMR group (LMR < 3.00, n = 647) and high LMR group (LMR ≥ 3.00, n = 870). The comparison of baseline characteristics between two LMR groups was summarized in Table 1. Before propensity score matching, there were significant differences in baseline data between the two groups. The low LMR group patients tended to be older with a lower DBP, SpO2 and higher HR, RR, WBC, PLT Glu, Scr, BUN, ECI, SIRS score, SOFA score and SAPSII score. Furthermore, patients with lower LMR had higher incidence of CHF, chronic pulmonary disease and renal failure while had a lower prevalence of hypertension and lower PCI or CABG treatment rate. With the use of propensity score matching (1:1 ratio), 543 pairs of patients were generated. The imbalance between patients with an LMR < 3.00 and an LMR ≥ 3.00 was significantly reduced (Additional file 1: Fig. S2), and almost all baseline characteristics were comparable between the two groups (Table 1).
Table 1.
Baseline characteristics before and after PSM matched
| Characteristics | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| LMR < 3.00 (n = 647) | LMR ≥ 3.00 (n = 870) | P value | LMR < 3.00 (n = 543) | LMR ≥ 3.00 (n = 543) | P value | |
| Demographics | ||||||
| Age, years | 71.14 (61.20–80.86) | 66.54 (56.60–78.66) | < 0.001 | 70.48 (59.99–80.26) | 72.08 (60.13–81.79) | 0.269 |
| Gender, male | 415 (64.14%) | 542 (62.30%) | 0.462 | 348 (64.09%) | 346 (63.72%) | 0.899 |
| Ethnicity, white | 423 (65.38%) | 584 (67.13%) | 0.476 | 354 (65.19%) | 381 (70.17%) | 0.080 |
| BMI, Kg/m2 | 27.16 ± 6.19 | 27.55 ± 5.77 | 0.205 | 26.94 ± 5.96 | 27.30 ± 5.85 | 0.297 |
| Comorbidities | ||||||
| Hypertension | 274 (42.35%) | 434 (49.89%) | 0.004 | 246 (45.30%) | 264 (48.62%) | 0.274 |
| Diabetes | 173 (26.74%) | 246 (28.28%) | 0.508 | 134 (24.68%) | 176 (32.41%) | 0.005 |
| Congestive heart failure | 95 (14.68%) | 59 (6.78%) | < 0.001 | 58 (10.68%) | 56 (10.31%) | 0.843 |
| Cardiac arrhythmias | 59 (9.12%) | 44 (5.06%) | 0.054 | 44 (8.10%) | 39 (7.18%) | 0.568 |
| Chronic pulmonary disease | 111 (17.16%) | 104 (11.95%) | 0.004 | 84 (15.47%) | 88 (16.21%) | 0.740 |
| Liver disease | 13 (2.01%) | 19 (2.18%) | 0.815 | 11 (2.03%) | 11 (2.03%) | > 0.999 |
| Renal failure | 101 (15.61%) | 92 (9.12%) | 0.004 | 70 (12.89%) | 75 (13.81%) | 0.656 |
| Coagulopathy | 65 (10.05%) | 63 (7.24%) | 0.052 | 50 (9.21%) | 43 (7.92%) | 0.448 |
| ECI | 0.00 (0.00–15.00) | 0.00 (0.00–11.00) | < 0.001 | 4.00 (0.00–12.00) | 3.00 (0.00–12.00) | 0.147 |
| Vital signs | ||||||
| HR, beats/min | 84.03 ± 15.31 | 81.06 ± 15.00 | < 0.001 | 83.91 ± 14.83 | 81.81 ± 15.66 | 0.029 |
| SBP, mmHg | 113.16 ± 16.46 | 113.41 ± 15.03 | 0.758 | 113.96 ± 16.53 | 113.15 ± 15.61 | 0.400 |
| DBP, mmHg | 59.41 ± 10.36 | 60.97 ± 9.68 | 0.001 | 59.83 ± 10.18 | 59.93 ± 9.73 | 0.855 |
| MBP, mmHg | 77.24 ± 10.54 | 78.07 ± 9.58 | 0.111 | 77.97 ± 10.45 | 77.41 ± 9.62 | 0.338 |
| RR, times/min | 19.27 ± 3.86 | 18.33 ± 3.28 | < 0.001 | 19.22 ± 3.82 | 18.64 ± 3.46 | 0.009 |
| Temperature, ℃ | 36.85 ± 0.71 | 36.80 ± 0.62 | 0.127 | 36.87 ± 0.68 | 36.78 ± 0.69 | 0.030 |
| SpO2, % | 97.04 ± 2.44 | 97.32 ± 2.72 | 0.039 | 97.19 ± 2.18 | 97.09 ± 3.24 | 0.541 |
| Laboratory parameters | ||||||
| WBC, 109/L | 13.97 ± 6.37 | 12.11 ± 5.33 | < 0.001 | 13.11 ± 5.08 | 13.30 ± 6.00 | 0.520 |
| PLT, 109/L | 240.35 ± 103.55 | 226.43 ± 95.44 | 0.007 | 238.25 ± 103.67 | 230.50 ± 89.77 | 0.177 |
| LMR | 1.88 (1.29–2.40) | 4.84 (3.81–6.50) | < 0.001 | 1.93 (1.36–2.44) | 4.75 (3.68–6.36) | < 0.001 |
| Hb, g/dL | 11.47 ± 2.18 | 11.68 ± 2.11 | 0.059 | 11.58 ± 2.18 | 11.63 ± 2.16 | 0.692 |
| HCT, % | 33.85 ± 6.34 | 34.18 ± 6.02 | 0.304 | 34.07 ± 6.33 | 34.16 ± 6.19 | 0.816 |
| Glu, mg/dL | 174.64 ± 91.80 | 164.88 ± 87.53 | 0.036 | 172.76 ± 91.90 | 176.47 ± 99.44 | 0.533 |
| Scr, mg/dL | 1.10 (0.90–1.70) | 0.90 (0.80–1.30) | < 0.001 | 1.00 (0.80–1.40) | 1.00 (0.80–1.40) | 0.977 |
| BUN, mg/dL | 24.00 (17.00–35.00) | 17.00 (13.00–25.00) | < 0.001 | 22.00 (16.00–30.00) | 21.00 (15.00–29.00) | 0.421 |
| Scoring system | ||||||
| SIRS | 3.00 (2.00–4.00) | 3.00 (2.00–3.00) | < 0.001 | 3.00 (2.00–4.00) | 3.00 (2.00–4.00) | 0.035 |
| SAPSII | 37.00 (28.00–48.00) | 30.00 (23.00–41.00) | < 0.001 | 35.00 (27.00–44.00) | 35.00 (26.00–47.00) | 0.465 |
| SOFA | 4.00 (2.00–7.00) | 2.00 (1.00–5.00) | < 0.001 | 3.00 (2.00–6.00) | 3.00 (1.00–6.00) | 0.628 |
| Treatment information | ||||||
| PCI | 296 (45.75%) | 491 (56.44%) | < 0.001 | 267 (49.17%) | 287 (52.85%) | 0.225 |
| CABG | 86 (13.29%) | 173 (19.89%) | 0.001 | 81 (14.92%) | 101 (18.60%) | 0.104 |
Data are presented as mean ± SD, median (interquartile range), or number of patients (%)
PSM propensity score matching, BMI body mass index, ECI elixhauser comorbidity index, HR heart rate, SBP systolic blood pressure, DBP diastolic blood pressure, MBP mean blood pressure, RR respiratory rate, SpO2, percutaneous oxygen saturation; WBC white blood cell, PLT platelet, LMR lymphocyte-to-monocyte ratio, Hb hemoglobin;, HCT hematocrit, Glu glucose, Scr serum creatinine, BUN blood urea nitrogen, SIRS systemic inflammatory response syndrome, SAPS simplified acute physiology score, SOFA sequential organ failure assessment, PCI percutaneous transluminal coronary intervention, CABG coronary artery bypass grafting
Outcomes
Patients in low LMR group had longer ICU length of stay (3.12 vs. 2.08 days, P < 0.001) and hospital length of stay (7.42 vs. 5.17 days P < 0.001) compared to high LMR group before PSM. Notably, the low LMR group had a high risk of hospital mortality (19.78 vs. 10.34%, P < 0.001), 30-day mortality (22.10 vs. 11.84%, P < 0.001) and 1-year mortality (36.32 vs. 19.20%, P < 0.001). After matching, ICU length of stay (3.01 vs. 2.38 days, P < 0.001), hospital length of stay (7.09 vs. 5.44 days P < 0.001) and 1-year mortality (32.04 vs. 26.15%, P < 0.001) remained significantly different between the two groups, while no differences were observed in hospital mortality (16.21 vs. 14.18%, P = 0.352) and 30-day mortality (18.60 vs. 16.02%, P = 0.261) (Table 2).
Table 2.
Outcomes of patients before and after PSM matched
| LMR < 3.00 | LMR ≥ 3.00 | P value | |
|---|---|---|---|
| Before PSM | N = 647 | N = 870 | |
| ICU length of stay, days | 3.12 (1.62–7.06) | 2.08 (1.24–3.90) | < 0.001 |
| Hospital length of stay, days | 7.42 (4.05–13.89) | 5.17 (3.21–9.23) | < 0.001 |
| Hospital mortality, n (%) | 128 (19.78%) | 90 (10.34%) | < 0.001 |
| 30-day mortality, n (%) | 143 (22.10%) | 103 (11.84%) | < 0.001 |
| 1-year mortality, n (%) | 235 (36.32%) | 167 (19.20%) | < 0.001 |
| After PSM | N = 543 | N = 543 | |
|---|---|---|---|
| ICU length of stay, days | 3.01 (1.55–6.85) | 2.38 (1.32–4.78) | < 0.001 |
| Hospital length of stay, days | 7.09 (4.03–13.68) | 5.44 (3.24–10.08) | < 0.001 |
| Hospital mortality, n (%) | 88 (16.21%) | 77 (14.18%) | 0.352 |
| 30-day mortality, n (%) | 101 (18.60%) | 87 (16.02%) | 0.261 |
| 1-year mortality, n (%) | 174 (32.04%) | 142 (26.15%) | 0.033 |
LMR lymphocyte-to-monocyte ratio, PSM propensity score matching
Survival analysis
The survival curves for patients of different LMR groups were shown in a Kaplan–Meier plot in Fig. 1. Patients with low LMR had a significant lower 1-year survival rate compared to high LMR group whether before (63.83 vs.81.03%, Log rank P < 0.001) or after (68.13 vs.74.22%, Log rank P = 0.041) PSM.
Fig. 1.
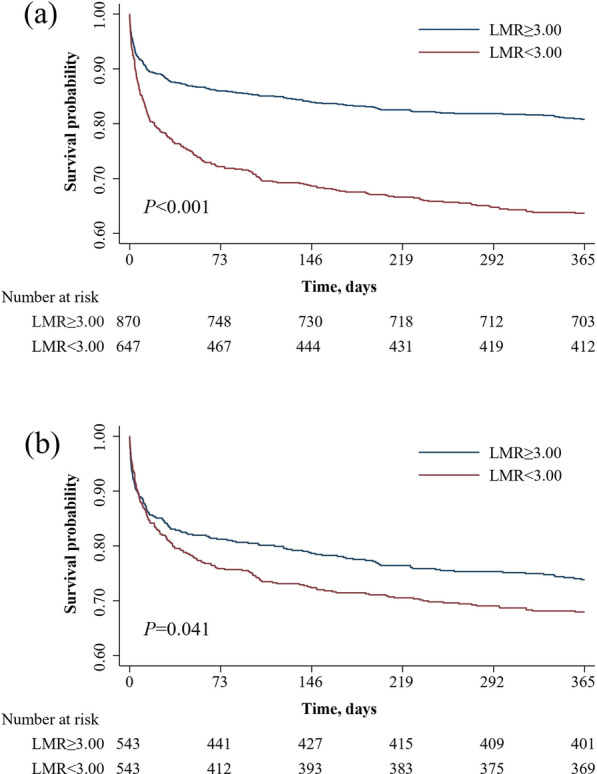
Kaplan–Meier survival analysis plot for 1-year survival in the high LMR group vs. low LMR group before a and after b propensity score matching. LMR lymphocyte-to-monocyte ratio
A Cox regression model was performed to determine the association between LMR and 1-year mortality of AMI patients. Variables with P values less than 0.05 in the univariate Cox regression analysis were included to be adjusted in model II for multivariate analysis, while model I was only adjusted by age, gender and ethnicity. The multivariate analysis showed that low LMR was associated with increased risk of 1-year mortality compared to high LMR (Model I: HR = 2.060, 95%CI 1.688–2.515, P < 0.001; Model II: HR = 1.369, 95%CI 1.110–1.687, P = 0.003) (Table 3). After matching, the higher risk of 1-year mortality remained significant in low LMR group (Model I: HR = 1.279, 95%CI 1.024–1.598, P = 0.030; Model II: HR = 1.299, 95%CI 1.032–1.634, P = 0.026) (Table 4).
Table 3.
Univariate and multivariate Cox regression analysis for 1-year mortality before PSM
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model I | Model II | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| LMR < 3.00 | 2.094 | 1.717–2.554 | 0.000 | 2.060 | 1.688–2.515 | 0.000 | 1.369 | 1.110–1.687 | 0.003 |
| Age, years | 1.004 | 1.002–1.005 | 0.000 | 1.003 | 1.002–1.004 | 0.000 | 1.002 | 1.001–1.003 | 0.004 |
| Gender, Male | 0.673 | 0.553–0.819 | 0.000 | 0.727 | 0.592–0.892 | 0.002 | 0.945 | 0.758–1.178 | 0.614 |
| Ethnicity, White | 0.739 | 0.605–0.902 | 0.003 | 0.733 | 0.600–0.896 | 0.002 | 0.760 | 0.618–0.935 | 0.010 |
| Hypertension | 0.680 | 0.556–0.831 | 0.000 | 1.077 | 0.859–1.351 | 0.519 | |||
| Congestive heart failure | 1.978 | 1.526–2.563 | 0.000 | 0.760 | 0.568–1.018 | 0.066 | |||
| Cardiac arrhythmias | 1.970 | 1.449–2.679 | 0.000 | 1.315 | 0.938–1.843 | 0.113 | |||
| Chronic pulmonary disease | 1.339 | 1.036–1.731 | 0.026 | 1.027 | 0.787–1.341 | 0.844 | |||
| Renal failure | 1.800 | 1.407–2.302 | 0.000 | 1.159 | 0.859–1.562 | 0.334 | |||
| Coagulopathy | 2.012 | 1.523–2.658 | 0.000 | 1.104 | 0.816–1.494 | 0.521 | |||
| HR, beats/min | 1.022 | 1.016–1.028 | 0.000 | 1.006 | 0.999–1.014 | 0.091 | |||
| DBP, mmHg | 0.964 | 0.955–0.975 | 0.000 | 0.991 | 0.979–1.002 | 0.124 | |||
| RR, times/min | 1.100 | 1.073–1.128 | 0.000 | 1.041 | 1.014–1.070 | 0.003 | |||
| SpO2, % | 0.915 | 0.895–0.935 | 0.000 | 0.948 | 0.927–0.969 | 0.000 | |||
| WBC, 109/L | 1.046 | 1.033–1.059 | 0.000 | 1.000 | 0.985–1.015 | 0.992 | |||
| PLT, 109/L | 1.000 | 0.999–1.001 | 0.833 | – | – | – | |||
| Hb, g/dL | 0.867 | 0.829–0.907 | 0.000 | 0.957 | 0.905–1.012 | 0.126 | |||
| Glu, mg/dL | 1.003 | 1.002–1.003 | 0.000 | 1.001 | 1.000–1.002 | 0.013 | |||
| Scr, mg/dL | 1.193 | 1.143–1.245 | 0.000 | 1.060 | 0.968–1.159 | 0.208 | |||
| BUN, mg/dL | 1.019 | 1.016–1.022 | 0.000 | 0.999 | 0.992–1.005 | 0.643 | |||
| SIRS | 1.506 | 1.360–1.669 | 0.000 | 1.069 | 0.940–1.216 | 0.307 | |||
| SAPSII | 1.058 | 1.052–1.064 | 0.000 | 1.045 | 1.034–1.056 | 0.000 | |||
| SOFA | 1.228 | 1.199–1.259 | 0.000 | 0.997 | 0.952–1.045 | 0.914 | |||
| PCI | 0.526 | 0.430–0.643 | 0.000 | 0.695 | 0.551–0.878 | 0.002 | |||
| CABG | 0.405 | 0.285–0.575 | 0.000 | 0.394 | 0.266–0.582 | 0.000 | |||
PSM propensity score matching, LMR lymphocyte-to-monocyte ratio, HR heart rate, DBP diastolic blood pressure, RR respiratory rate, SpO2, percutaneous oxygen saturation; WBC white blood cell, PLT platelet, Hb hemoglobin, Glu glucose, Scr serum creatinine, BUN blood urea nitrogen, SIRS systemic inflammatory response syndrome, SAPS simplified acute physiology score, SOFA Sequential organ failure assessment, PCI percutaneous transluminal coronary intervention, CABG coronary artery bypass grafting
Table 4.
Univariate and multivariate Cox regression analysis for 1-year mortality after PSM
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model I | Model II | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| LMR < 3.00 | 1.260 | 1.009–1.572 | 0.041 | 1.279 | 1.024–1.598 | 0.030 | 1.299 | 1.032–1.634 | 0.026 |
| Age, years | 1.033 | 1.024–1.042 | 0.000 | 1.033 | 1.023–1.043 | 0.000 | 1.025 | 1.014–1.036 | 0.000 |
| Gender, Male | 0.719 | 0.575–0.898 | 0.004 | 0.933 | 0.738–1.180 | 0.564 | 1.096 | 0.855–1.405 | 0.470 |
| Ethnicity, White | 0.648 | 0.517–0.811 | 0.000 | 0.642 | 0.513–0.805 | 0.000 | 0.651 | 0.515–0.823 | 0.000 |
| Hypertension | 0.629 | 0.501–0.791 | 0.000 | 0.888 | 0.688–1.146 | 0.360 | |||
| Congestive heart failure | 1.612 | 1.188–2.186 | 0.002 | 0.876 | 0.620–1.236 | 0.451 | |||
| Cardiac arrhythmias | 1.567 | 1.097–2.236 | 0.013 | 0.926 | 0.624–1.374 | 0.702 | |||
| Chronic pulmonary disease | 1.167 | 0.874–1.557 | 0.295 | – | – | – | |||
| Renal failure | 1.580 | 1.192–2.093 | 0.001 | 0.908 | 0.638–1.292 | 0.591 | |||
| Coagulopathy | 2.088 | 1.532–2.848 | 0.000 | 1.325 | 0.944–1.861 | 0.104 | |||
| HR, beats/min | 1.020 | 1.013–1.027 | 0.000 | 1.009 | 1.001–1.018 | 0.034 | |||
| DBP, mmHg | 0.970 | 0.959–0.982 | 0.000 | 0.998 | 0.984–1.011 | 0.726 | |||
| RR, times/min | 1.087 | 1.057–1.118 | 0.000 | 1.053 | 1.022–1.085 | 0.001 | |||
| SpO2, % | 0.928 | 0.902–0.954 | 0.000 | 0.953 | 0.930–0.977 | 0.000 | |||
| WBC, 109/L | 1.024 | 1.005–1.043 | 0.012 | 1.003 | 0.982–1.024 | 0.805 | |||
| PLT, 109/L | 0.999 | 0.998–1.001 | 0.299 | – | – | – | |||
| Hb, g/dL | 0.870 | 0.827–0.915 | 0.000 | 0.962 | 0.903–1.024 | 0.223 | |||
| Glu, mg/dL | 1.002 | 1.002–1.003 | 0.000 | 1.001 | 1.000–1.002 | 0.060 | |||
| Scr, mg/dL | 1.173 | 1.116–1.233 | 0.000 | 1.090 | 0.989–1.201 | 0.082 | |||
| BUN, mg/dL | 1.021 | 1.017–1.026 | 0.000 | 0.997 | 0.989–1.004 | 0.389 | |||
| SIRS | 1.372 | 1.220–1.542 | 0.000 | 0.937 | 0.804–1.092 | 0.405 | |||
| SAPSII | 1.056 | 1.049–1.063 | 0.000 | 1.034 | 1.021–1.047 | 0.000 | |||
| SOFA | 1.222 | 1.187–1.257 | 0.000 | 1.067 | 1.010–1.126 | 0.020 | |||
| PCI | 0.637 | 0.509–0.796 | 0.000 | 0.721 | 0.559–0.930 | 0.012 | |||
| CABG | 0.433 | 0.294–0.639 | 0.000 | 0.414 | 0.270–0.633 | 0.000 | |||
PSM propensity score matching, LMR lymphocyte-to-monocyte ratio, HR heart rate, DBP diastolic blood pressure, RR respiratory rate, SpO2, percutaneous oxygen saturation; WBC white blood cell, PLT platelet, Hb hemoglobin, Glu glucose, Scr serum creatinine, BUN blood urea nitrogen, SIRS systemic inflammatory response syndrome, SAPS simplified acute physiology score, SOFA sequential organ failure assessment, PCI percutaneous transluminal coronary intervention, CABG coronary artery bypass grafting
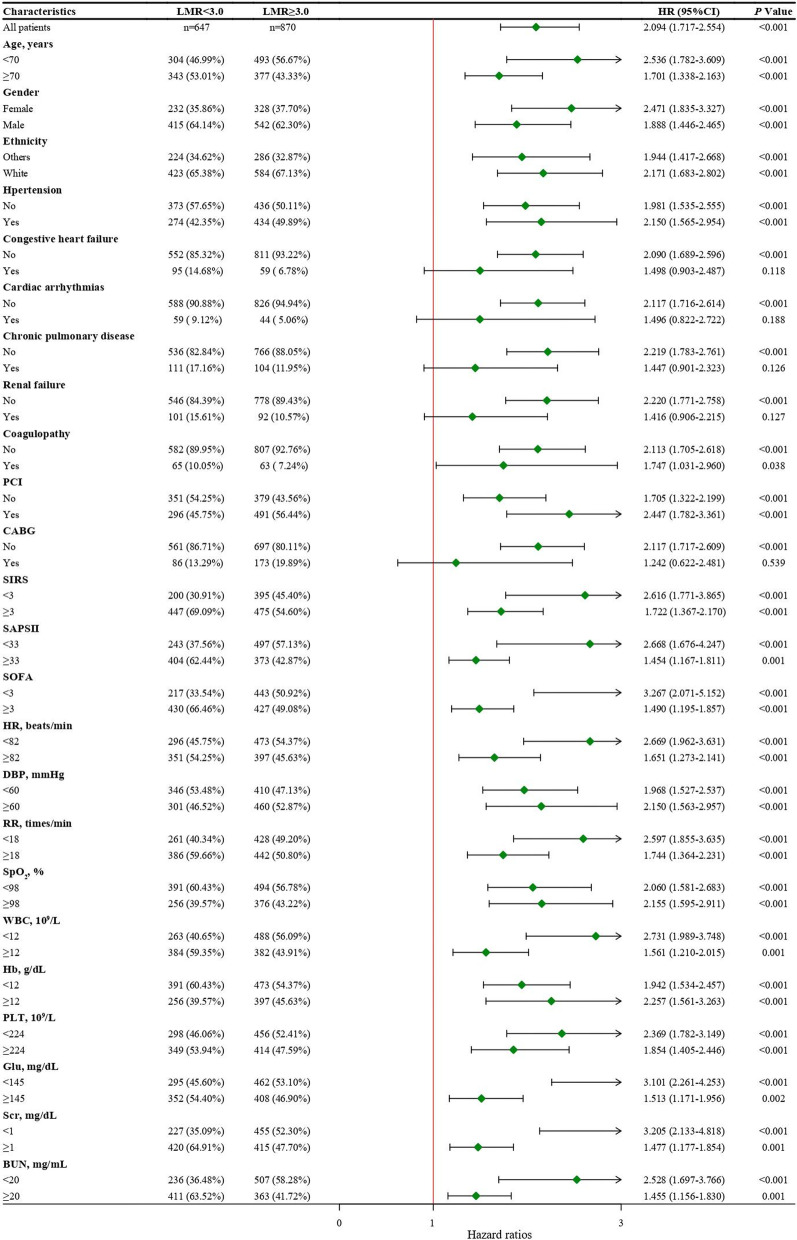
Subgroup analysis
Subgroup analysis of variables with significant differences in baseline characteristics was performed to verify the stability of the Cox regression results. As shown in the Fig. 2, AMI patients with an LMR < 3.00 had higher risk of 1-year mortality than those with an LMR ≥ 3.00 in subgroups except for the patients with congestive heart failure (HR = 1.498, 95%CI 0.903–2.487, P = 0.118), cardiac arrhythmias (HR = 1.496, 95%CI 0.822–2.722, P = 0.188), chronic pulmonary disease (HR = 1.447, 95%CI 0.901–2.323, P = 0.126), renal failure (HR = 1.416, 95%CI: 1.416, 95%CI 0.906–2.215, P = 0.127) and CABG treatment (HR = 1.242, 95%CI 0.622–2.481, P = 0.539).
Fig. 2.
Association between LMR group and 1-year mortality of AMI patients in different subgroups. LMR lymphocyte-to-monocyte ratio, PCI percutaneous transluminal coronary intervention, CABG coronary artery bypass grafting, SIRS systemic inflammatory response syndrome, SAPS simplified acute physiology score, SOFA sequential organ failure assessment, HR heart rate, DBP diastolic blood pressure, RR respiratory rate, SpO2, percutaneous oxygen saturation; WBC white blood cell, PLT platelet, Hb hemoglobin, Glu glucose, Scr serum creatinine, BUN blood urea nitrogen
Discussion
Circulating leukocyte subtype counts is considered to be of great value for disease evaluation and prognosis prediction of inflammatory injury patients. In the present study, we demonstrated an independent relationship between admission LMR and 1-year mortality in AMI patients. To our knowledge, this is the first study focusing on the association between the measurement of LMR and long-term prognosis of critically ill AMI patients.
Acute myocardial infarction, usually caused by plaque rupture and interruption of coronary blood flow, triggers an intense inflammatory response which is essential for myocardium repair. Alternatively, hyperactivity and prolonged inflammation after infarction may lead to myocardial dysfunction [25]. The critical role of immune cells in the pathophysiological process after myocardial infarction is now being deeply revealed [4]. Each component of immune cells plays a dynamic role in pro-inflammation response and anti-inflammation repair stage after AMI [3, 26].
As indicators of systemic inflammatory status, the role of WBC and its subtypes in the diagnosis, risk stratification and prognostic prediction of AMI has also been demonstrated in various clinical trials and practices [9–11, 27]. In previous studies, both a low lymphocyte counts and high monocyte counts were associated with an increased risk for major adverse cardiovascular events [28, 29]. Although the role of macrophages in inflammatory response after IHD has been widely recognized [6], the potential mechanisms of low lymphocyte level and its predictive value are not fully understood. Acute lymphocytopenia is generally considered to be part of the stress response and is associated with increased cortisol and sympathetic activation [30]. The increased lymphocytes apoptosis could also explain the association with adverse outcomes [31].
Unlike simple cell counts, ratios between different WBC subtypes, such as NLR, can fully combine the prognostic information of different components to provide greater predictive abilities [9]. LMR, as a novel hematologic indicator which is calculated by dividing lymphocyte count by monocyte count, has shown great prognostic value in cardiovascular diseases such as heart failure [19] and acute coronary syndrome [20, 21, 32–34]. To further investigate the relationship between LMR and long-term prognosis in critically ill AMI patients, we constructed a retrospective cohort in MIMIC-III database using cut-off values generated by X-tile software, and showed that low admission LMR levels were independently associated with higher risk for 1-year mortality.
In this study we performed PSM analysis, which helps to balance confounding factors in baseline characteristics. Although there were no significant differences in hospital mortality and 30-day mortality between the LMR groups after PSM, the main outcomes we focused on remained consistent before (36.32% vs. 19.20%, P < 0.001) and after matching (32.04% vs. 26.15%, P = 0.033). The HRs of 1-year mortality with an LMR < 3.00 were changed before and after PSM (Model I:2.060 vs. 1.279; Model II:1.369 vs. 1.299), which may be the result of the equilibrium of baseline characteristics, or related to the change of optimal cutoff values after PSM. Furthermore, procedure events during hospitalization were also included in the Cox regression model. The results showed that PCI and CABG were both protective factors for 1-year mortality before and after matching, suggesting that MI patients may benefit from aggressive coronary revascularization, which is consistent with the recommendations of guideline [35]. Due to the relatively small sample size and single-center-based cohort of the current study, further studies based on larger populations with external validation are warranted.
To validate the robustness of the regression results, subgroup analysis which containing variable with significant differences in baseline characteristics was performed to examine the statistical potency of LMR under different conditions. LMR maintained its predictive ability in most subgroups except in patients with CHF, cardiac arrhythmias, chronic pulmonary disease, renal failure, or CABG treatment. On the one hand, underlying disease such as heart failure usually suggest worse pathophysiological conditions and thus interfere with the long-term prognosis of LMR. On the other hand, CABG treatment which is a powerful protection factors shown in Cox regression (HR = 0.394, 95%CI 0.266–0.582, P < 0.001) could balance the mortality between two groups. Generally, LMR demonstrated its excellent predictive value and stability in our research.
Several limitations of our study should be noted. Firstly, patients with myocardial infarction were identified using ICD-9 codes rather than clinical diagnostic criteria, and few patients were inevitably ignored. Secondly, the information of admission WBC subtype was missing in some patients. Therefore, they were excluded from our case cohort, which may lead to selection bias. Thirdly, since our sample size is relatively small and the cohort is single-center, the possibility that the optimal cut-off value may vary with different study populations. Fourthly, factors such as hematological, inflammatory and infectious diseases that may affect the counts of circulating immune cells were not excluded as exclusion criteria because of the difficulty in obtaining accurate and detailed information from the database. Further studies and external validation based on large multicenter prospective cohorts are needed to determine the most appropriate LMR cut-off value for different populations.
Conclusions
In this retrospective cohort analysis, we demonstrated that a low admission LMR (< 3.00) was associated with a higher risk of 1-year mortality in critical ill AMI patients. Our findings provide an affordable, convenient, and reliable tool for clinical prediction of long-term adverse outcomes in MI patients.
Supplementary Information
Additional file 1. Figure S1: Flow chart: the inclusion of the study population. ICU intensive care units, AMI acute myocardial infarction. Figure S2: Propensity score matching graph between two LMR groups. LMR lymphocyte-to-monocyte ratio.
Acknowledgements
None.
Abbreviations
- AMI
Acute myocardial infarction
- LMR
Lymphocyte-to-monocyte ratio
- MIMIC-III
Medical Information Mart for Intensive Care III
- ICUs
Intensive care units
- PSM
Propensity score matching
- CVDs
Cardiovascular diseases
- IHD
Ischemic heart disease
- WBC
White blood cells
- PLR
Platelet to lymphocyte ratio
- NLR
Neutrophil to lymphocyte ratio
- ICD-9
International Classification of Diseases, Ninth Revision
- BMI
Body mass index
- HR
Heart rate
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- RR
Respiratory rate
- SpO2
Percutaneous oxygen saturation
- CHF
Congestive heart failure
- ECI
Elixhauser comorbidity index
- PLT
Platelet count
- Hb
Hemoglobin
- HCT
Hematocrit
- Glu
Glucose
- BUN
Blood urea nitrogen
- Scr
Serum creatinine
- SIRS
Systemic inflammatory response syndrome
- SAPS
Simplified acute physiology score
- SOFA
Sequential organ failure assessment
- PCI
Percutaneous coronary intervention
- CABG
Coronary bypass artery grafting
Author contributions
YZ, CH and LC had full access to all data. YZ and CH curated the data, performed the data and produced an initial draft of the manuscript. LC, XB, ZL and HQ participated in critically revising the manuscript. All authors participated in concept and design of the present study. All authors read and approved the final manuscript.
Funding
This study was supported by the National Nature Science Foundation of China (Grant No. 81270203 and 81770231), the Natural Science Foundation of Jiangsu (Grant No. BK20161436), the Jiangsu Provincial Key Medical Discipline (Laboratory No. ZDXKA2016023), the Jiangsu Provincial Key Research and Development Program (Grant No. BE2016785) and the Medical Scientific Research Key Project of Jiangsu Commission of Health (Grant No. K2019006).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Access to the database for research was approved by the Institutional Review Boards of the Massachusetts Institute of Technology (Cambridge, MA, USA) and the Beth Israel Deaconess Medical Center. The requirement for written informed consent was waived due to the retrospective study design. The identification information of the participants was hidden to protect their privacy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuanyuan Zhao and Chunshu Hao contributed equally to this work and share first authorship
References
- 1.Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong SB, Hernandez-Resendiz S, Crespo-Avilan GE, et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87. doi: 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kologrivova I, Shtatolkina M, Suslova T, et al. Cells of the Immune system in cardiac remodeling: main players in resolution of inflammation and repair after myocardial infarction. Front Immunol. 2021;12:664457. doi: 10.3389/fimmu.2021.664457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Y. Role of neutrophils in cardiac injury and repair following myocardial infarction. Cells. 2021;10(7):1676. doi: 10.3390/cells10071676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peet C, Ivetic A, Bromage DI, et al. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. 2020;116(6):1101–1112. doi: 10.1093/cvr/cvz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Duan Y, Sluijter JP, et al. Lymphocytic subsets play distinct roles in heart diseases. Theranostics. 2019;9(14):4030–4046. doi: 10.7150/thno.33112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreadou I, Cabrera-Fuentes HA, Devaux Y, et al. Immune cells as targets for cardioprotection: new players and novel therapeutic opportunities. Cardiovasc Res. 2019;115(7):1117–1130. doi: 10.1093/cvr/cvz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto E, Sugiyama S, Hirata Y, et al. Prognostic significance of circulating leukocyte subtype counts in patients with coronary artery disease. Atherosclerosis. 2016;255:210–216. doi: 10.1016/j.atherosclerosis.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Jiang L, Xu L, et al. Predictive value of in-hospital white blood cell count in Chinese patients with triple-vessel coronary disease. Eur J Prev Cardiol. 2019;26(8):872–882. doi: 10.1177/2047487319826398. [DOI] [PubMed] [Google Scholar]
- 12.Leonardi S, Gragnano F, Carrara G, et al. Prognostic implications of declining hemoglobin content in patients hospitalized with acute coronary syndromes. J Am Coll Cardiol. 2021;77(4):375–388. doi: 10.1016/j.jacc.2020.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polat N, Oylumlu M, Işik MA, et al. Prognostic significance of serum albumin in patients with acute coronary syndrome. Angiology. 2020;71(10):903–908. doi: 10.1177/0003319720941747. [DOI] [PubMed] [Google Scholar]
- 14.Madjid M, Awan I, Willerson JT, et al. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44(10):1945–1956. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 15.Cho KH, Jeong MH, Ahmed K, et al. Value of early risk stratification using hemoglobin level and neutrophil-to-lymphocyte ratio in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2011;107(6):849–856. doi: 10.1016/j.amjcard.2010.10.067. [DOI] [PubMed] [Google Scholar]
- 16.Cicek G, Acikgoz SK, Bozbay M, et al. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio combination can predict prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2015;66(5):441–447. doi: 10.1177/0003319714535970. [DOI] [PubMed] [Google Scholar]
- 17.Dong G, Huang A, Liu L. Platelet-to-lymphocyte ratio and prognosis in STEMI: a meta-analysis. Eur J Clin Invest. 2021;51(3):e13386. doi: 10.1111/eci.13386. [DOI] [PubMed] [Google Scholar]
- 18.Oylumlu M, Oylumlu M, Arslan B, et al. Platelet-to-lymphocyte ratio is a predictor of long-term mortality in patients with acute coronary syndrome. Postepy Kardiol Interwencyjnej. 2020;16(2):170–176. doi: 10.5114/aic.2020.95859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva N, Bettencourt P, Guimaraes JT. The lymphocyte-to-monocyte ratio: an added value for death prediction in heart failure. Nutr Metab Cardiovasc Dis. 2015;25(11):1033–1040. doi: 10.1016/j.numecd.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Lin Y, Zhang H, et al. Relationship of platelet counts and inflammatory markers to 30-day mortality risk in patients with acute type a aortic dissection. Biomed Res Int. 2020;2020:1057496. doi: 10.1155/2020/1057496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiris T, Celik A, Varis E, et al. Association of Lymphocyte-to-monocyte ratio with the mortality in patients with ST-elevation myocardial infarction who underwent primary percutaneous coronary intervention. Angiology. 2017;68(8):707–715. doi: 10.1177/0003319716685480. [DOI] [PubMed] [Google Scholar]
- 22.Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–220. doi: 10.1161/01.CIR.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 23.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 25.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Xu J, Wu M, et al. The effector cells and cellular mediators of immune system involved in cardiac inflammation and fibrosis after myocardial infarction. J Cell Physiol. 2020;235(12):8996–9004. doi: 10.1002/jcp.29732. [DOI] [PubMed] [Google Scholar]
- 27.Sabatine MS, Morrow DA, Cannon CP, et al. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 (Treat angina with aggrastat and determine cost of therapy with an invasive or conservative strategy- thrombolysis in myocardial infarction 18 trial)substudy. J Am Coll Cardiol. 2002;40(10):1761–1768. doi: 10.1016/S0735-1097(02)02484-1. [DOI] [PubMed] [Google Scholar]
- 28.Boag SE, Das R, Shmeleva EV, et al. T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. J Clin Invest. 2015;125(8):3063–3076. doi: 10.1172/JCI80055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Laan AM, Hirsch A, Robbers LF, et al. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: monocytes and myocardial infarction. Am Heart J. 2012;163(1):57–65 e52. doi: 10.1016/j.ahj.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Onsrud M. Influence of in vivo hydrocortisone on some human blood leucocyte sub-populations. II. Effects on T cell-monocyte cooperation. Acta Pathol Microbiol Scand C. 1981;89(5):321–327. doi: 10.1111/j.1699-0463.1981.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 31.Forteza MJ, Trapero I, Hervas A, et al. Apoptosis and mobilization of lymphocytes to cardiac tissue is associated with myocardial infarction in a reperfused porcine model and infarct size in post-PCI patients. Oxid Med Cell Longev. 2018;2018:1975167. doi: 10.1155/2018/1975167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtul A, Yarlioglues M, Celik IE, et al. Association of lymphocyte-to-monocyte ratio with the no-reflow phenomenon in patients who underwent a primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis. 2015;26(8):706–712. doi: 10.1097/MCA.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 33.Shah B, Baber U, Pocock SJ, et al. White blood cell count and major adverse cardiovascular events after percutaneous coronary intervention in the contemporary era: insights from the PARIS Study (patterns of non-adherence to anti-platelet regimens in stented patients registry) Circ Cardiovasc Interv. 2017;10(9):e004981. doi: 10.1161/CIRCINTERVENTIONS.117.004981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalyoncuoglu M, Biter HI, Ozturk S, et al. Predictive accuracy of lymphocyte-to-monocyte ratio and monocyte-to-high-density-lipoprotein-cholesterol ratio in determining the slow flow/no-reflow phenomenon in patients with non-ST-elevated myocardial infarction. Coron Artery Dis. 2020;31(6):518–526. doi: 10.1097/MCA.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 35.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Figure S1: Flow chart: the inclusion of the study population. ICU intensive care units, AMI acute myocardial infarction. Figure S2: Propensity score matching graph between two LMR groups. LMR lymphocyte-to-monocyte ratio.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.