Abstract
Relapsed or refractory (R/R) acute myeloid leukemia (AML) has a poor prognosis. In this study, we evaluated chimeric antigen receptor (CAR) T cell therapy targeting CLL-1 in adults with R/R AML patients. Patients received conditioning chemotherapy with cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) for 3 days and an infusion of a dose of 1–2 × 106 CAR-T cells/kg. The incidence of dose-limiting toxicity was the primary endpoint. Ten patients were treated, and all developed cytokine release syndrome (CRS); 4 cases were low-grade, while the remaining 6 were considered high-grade CRS. No patient developed CAR-T cell-related encephalopathy syndrome (CRES). Severe pancytopenia occurred in all patients. Two patients died of severe infection due to chronic agranulocytosis. The complete response (CR)/CR with incomplete hematologic recovery (CRi) rate was 70% (n = 7/10). The median follow-up time was 173 days (15–488), and 6 patients were alive at the end of the last follow-up. CAR-T cells showed peak expansion within 2 weeks. Notably, CLL-1 is also highly expressed in normal granulocytes, so bridging hematopoietic stem cell transplantation (HSCT) may be a viable strategy to rescue long-term agranulocytosis due to off-target toxicity. In conclusion, this study is the first to demonstrate the positive efficacy and tolerable safety of CLL-1 CAR-T cell therapy in adult R/R AML.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-022-01308-1.
Keywords: Chimeric antigen receptor, Acute myeloid leukemia, C-type lectin-like molecule 1
To the Editor,
R/R AML patients have poor long-term survival [1, 2]. T cells expressing CAR have been recognized as a promising approach for hematological malignancies [3], but the effects of CAR-T cell therapy in R/R AML are limited and need to be improved [4, 5]. Human C-type lectin-like molecule 1 (CLL-1) is expressed on malignant cells in more than 90% of AML patients, but is underexpressed in normal hematopoietic stem cells [6]. Previous studies have shown that targeting CLL-1 can treat AML in preclinical studies [7–9], and 3 of 4 children with refractory AML who received CLL-1 CAR-T cells achieved CR [10]. Here, we report the first clinical trial of CLL-1 CAR-T cells in adult patients with R/R AML, recruiting 10 patients with positive efficacy and tolerable safety after cell infusion.
Patient characteristics and CLL-1 CAR-T cell
Ten patients with relapsed/refractory AML were enrolled. The median age of all patients was 43.5 years (range 18–73), 8 of 10 patients had relapse, and 5 of them relapsed after transplantation. Three had MDS-to-AML transformation. The median of previous treatment lines was 5 (range 2–10), and all patients were resistant to the most recent chemotherapy before receiving CLL-1 CAR-T cell therapy (Table 1). The median of positive expression rate of CLL-1 in tumor cells of all patients was 85.2% (range 50.2%-97.6%). The median CAR-T cell infection efficiency was 50.53% (range 23.98%-73.14%). The median dose of infused CAR-T cells was 1.5 × 106/kg (range 1 × 106–2 × 106) (Additional file 1: Table S1).
Table 1.
Characteristics of patients before CAR-T cell treatment
| ID | Age/Sex | FAB subtype | Fusion gene | Gene mutation | Karyotype | History of MDS/MPN | Prior lines of treatment | Previous HSCT | Extramedullary invasion | Pre-infusion disease burden (%) | Post-infusion disease burden (%)/efficacy evaluation | Bridged HSCT | CLL-1 Positivity (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53/F | M5 | Negative | KRAS、TET2、ETV6 | 46, XX, -7, + mar | Yes | 6 | No | Yes | 16.43 | 71.78/NR | Yes | 50.2 |
| 2 | 44/M | M2 | N/A | NPM1、DNMT3A、IDH1、RUNX1、NRAS | N/A | No | 8 | No | No | 13.47 | 35.42/NR | Yes | 90.3 |
| 3 | 73/M | M5 | Negative | RUNX1、CEBPA、TET2、ASXL1、NRAS | 46, XY del (7), (q22q34) | Yes | 2 | No | No | 18.49 | 0.23/CRi | No | 82.8 |
| 4 | 29/M | M5 | Negative | CEBPA、FLT3、TET2 | N/A | No | 2 | No | Yes | 14.50 | 0/CRi | Yes | 92.3 |
| 5 | 47/F | M5 | Negative | Negative | Normal | No | 8 | Yes | No | 83.55 | 86.51/NR | No | 89.6 |
| 6 | 49/F | M5 | MLL-AF9 | ZRSR2 | N/A | No | 2 | No | No | 28.36 | 0/CR | No | 97.6 |
| 7 | 43/F | M2 | Negative | Negative | N/A | No | 10 | Yes | No | 7.12 | 0/CRi | No | 82.2 |
| 8 | 39/F | M5 | Negative | RUNX1, U2AF1 | 46, XX, + 1, der(1;7) | Yes | 4 | Yes | No | 10.24 | 2.11/CRi | Yes | 66.9 |
| 9 | 18/F | M2 | Negative | RUNX1、FLT3 | Normal | No | 6 | Yes | No | 3.09 | 0/CRi | Yes | 80.6 |
| 10 | 29/M | M5 | Negative | Negative | Normal | No | 3 | Yes | No | 22.80 | 3.02/CRi | Yes | 87.6 |
ID identification number, F female, M male, FAB French–American–British, N/A not available, MDS/MPN myelodysplastic syndrome/myeloproliferative neoplasm, HSCT hematopoietic stem cell transplantation, CR complete response, CRi CR with incomplete blood count recovery
Safety
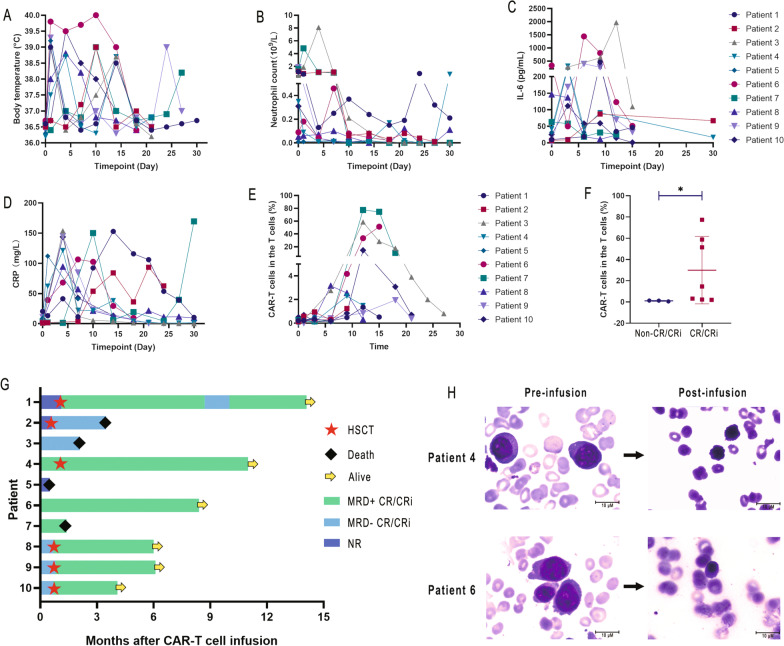
Most patients developed fever during the infusion, which we consider to be an infusion-related reaction not related to CRS (Fig. 1A). The patient's fever almost always occurred in the range of 4–14 days after the infusion, which is correlated with the period of neutropenia (Fig. 1B). All patients developed CRS (Fig. 1C, D; Additional file 1: Fig. S1 A–C; Additional file 1: Table S1). CRS was controlled after 6/10 patients and 3/10 patients had received corticosteroids and tocilizumab, respectively. None of the 10 patients developed CRES. However, all patients had severe pancytopenia, 9/10 had grade 3/4 agranulocytosis, 7/10 had grade 3/4 anemia, and 7/10 had grade 3/4 thrombocytopenia (Additional file 1: Tables S2, S3). Patient 2 underwent salvage hematopoietic stem cell transplantation (HSCT) after achieving partial response (PR) with infusion and died of disease progression 2 months later. Patients 3 and 7 died of severe infection due to chronic agranulocytosis despite achieving CRi after therapy. Patient 5 died due to a nonresponse (NR) to treatment and rapid disease progression. Among the 6 patients who received bridging haploidentical transplantation after infusion (Fig. 1G). Granulocytes, erythroid and platelets all engrafted normally, and no serious infection occurred. Therefore, while severe agranulocytosis occurs after infusion, bridging transplantation may reverse this toxicity.
Fig. 1.
Kinetics of peripheral blood biomarkers and clinical outcome after CLL-1 CAR-T cell infusion. (A, B) Changes in patient body temperature and peripheral blood neutrophil numbers after CAR-T cell infusion, respectively. (C, D) Peripheral blood serum levels of IL-6, C-reactive protein (CRP) and ferritin before and after CAR-T cell infusion. (E) The ratio of CAR-T cells (CAR-T cells did not specifically distinguish between CD4 and CD8) to T cells in peripheral blood at various time periods. (F) Comparison of the peak values of CAR-T cells (CAR-T cells did not specifically distinguish between CD4 and CD8) in complete response (CR)/CRi and nonresponse (NR) patients. (G) Duration of response and survival after infusion of CLL-1 CAR-T cells. (H) Bone marrow smears of patient 4 and patient 6 before and after infusion. The data are expressed as the mean ± standard deviation (*p < 0.05)
Efficacy
7/10 patients achieved CR/CRi (Fig. 1G, H). The median follow-up time was 173 days (15–488), and 6 patients were alive at the end of the last follow-up (Table S1). Patient 1 had no response (possibly related to lower CLL-1 expression on her tumor cells) after infusion followed by salvage HSCT and achieved CR. However, she was found to have minimal residual disease (MRD) 9 months later and became MRD negative after azacytidine and venetoclax treatment. Patient 6 did not undergo other treatments after infusion, and the patient was in continuous CR during the follow-up. Six patients underwent HSCT at a median of 20 days after infusion (range: 18–34), and four (50%) were still CR at the last follow-up.
Biomarker analysis
CLL-1 CAR-T cell expansion was assessed by flow cytometry. The median CAR-T cell expansion peaked at day 12 after reinfusion (range 6–18 days) (Fig. 1E). Comparing peak CAR-T cell expansion in CR/CRi and non-CR/CRi patients, the CR/CRi patients had significantly higher proportions of CAR-T cells (Fig. 1F). Cytokine levels were increased to varying degrees after infusion (Fig. 1C, D; Fig. S1 A-C). However, in non-CR/CRi patients, the detection values of cytokines and CAR-T cells were relatively low.
In conclusion, CLL-1 may be a potential therapeutic target for AML. Although severe agranulocytosis may occur, CLL-1 CAR-T cell can provide R/R AML patients with the chance to achieve CR/CRi before transplantation, which may reduce the risk of relapse and prolong patient survival. Our study found that granulocytes are difficult to recover after infusion, which is inconsistent with a previous study in children [10], which may be due to the repopulating potential of hematopoietic stem cells in childhood AML patients.
Supplementary Information
Additional file 1. Study methods and additional patient information.
Acknowledgements
Not applicable.
Abbreviations
- R/R
Relapsed or refractory
- AML
Acute myeloid leukemia
- CAR
Chimeric antigen receptor
- CRS
Cytokine release syndrome
- CRES
Chimeric antigen receptor T cell-related encephalopathy syndrome
- HSCT
Hematopoietic stem cell transplantation
- CR
Complete response
- CRi
CR with incomplete blood count recovery
- CLL-1
C-type lectin-like molecule 1 (CLL-1)
- MRD
Minimal residual disease
- PR
Partial response
Author contributions
XJ, WYL, HKZ and MFZ designed the research. XJ, MZ, RS, HRL, XX, XMZ, DNX, XX, JXW and MFZ performed the research. XJ, MZ, RS, FL and MFZ analyzed the data. XJ, MZ and RS wrote the manuscript. WYL, HKZ and MFZ revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the General Project of National Natural Science Foundation of China (81970180 to MZ) and the Key Science and Technology Support Project of Tianjin Science and Technology Bureau (20YFZCSY00800 to MZ), as well as Tianjin Key Medical Discipline (Specialty) Construction Project.
Availability of data and materials
All data generated or analyzed during this study are included in this published article or its supplementary information files. The raw datasets are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Tianjin First Central Hospital and was registered for clinical trials in the China Clinical Trial Registration Center (Trial registration: ChiCTR2000041054. Registered 17 December 2020, http://www.chictr.org.cn/showproj.aspx?proj=65781). All patients signed an informed consent form. The animal experiment protocol was approved by the Animal Care and Use Committee of Tianjin Medical University.
Consent for publication
All patients signed informed consent and also consented to the publication of these data.
Competing interests
All authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Jin, Meng Zhang and Rui Sun contributed equally to this work.
Contributor Information
Wenyi Lu, Email: luwenyi0323@163.com.
Hongkai Zhang, Email: hongkai@nankai.edu.cn.
Mingfeng Zhao, Email: mingfengzhao@sina.com.
References
- 1.Rollig C, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29:2758–2765. doi: 10.1200/JCO.2010.32.8500. [DOI] [PubMed] [Google Scholar]
- 2.Rubnitz JE, Kaspers GJL. How I treat pediatric acute myeloid leukemia. Blood. 2021;138:1009–1018. doi: 10.1182/blood.2021011694. [DOI] [PubMed] [Google Scholar]
- 3.Young RM, Engel NW, Uslu U, Wellhausen N, June CH. Next-generation CAR T-cell therapies. Cancer Discov. 2022 doi: 10.1158/2159-8290.CD-21-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mardiana S, Gill S. CAR T cells for acute Myeloid Leukemia: state of the art and future directions. Front Oncol. 2020;10:697. doi: 10.3389/fonc.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marvin-Peek J, Savani BN, Olalekan OO, Dholaria B. Challenges and advances in chimeric antigen receptor therapy for acute Myeloid Leukemia. Cancers (Basel) 2022 doi: 10.3390/cancers14030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morsink LM, Walter RB, Ossenkoppele GJ. Prognostic and therapeutic role of CLEC12A in acute myeloid leukemia. Blood Rev. 2019;34:26–33. doi: 10.1016/j.blre.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol. 2018;11:7. doi: 10.1186/s13045-017-0553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tashiro H, et al. Treatment of acute myeloid leukemia with T cells expressing chimeric antigen receptors directed to C-type lectin-like molecule 1. Mol Ther. 2017;25:2202–2213. doi: 10.1016/j.ymthe.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laborda E, et al. Development of A chimeric antigen receptor targeting C-type lectin-like molecule-1 for human acute myeloid leukemia. Int J Mol Sci. 2017 doi: 10.3390/ijms18112259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, et al. Anti-CLL1 chimeric antigen receptor T-cell therapy in children with relapsed/refractory acute myeloid leukemia. Clin Cancer Res. 2021;27:3549–3555. doi: 10.1158/1078-0432.CCR-20-4543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Study methods and additional patient information.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or its supplementary information files. The raw datasets are available from the corresponding authors on reasonable request.