Abstract
Simple Summary
Ticks transmit different disease-causing agents to humans and animals. Pakistan is an agricultural country, the rural economy mainly relies on livestock farming, and tick infestation is a severe constraint to its livelihood. The genus Anaplasma comprises obligate Gram-negative intracellular bacteria multiplying within the host cells and can be transmitted to humans and animals through the tick vector. The current study aimed to molecularly characterize the Anaplasma spp. in hard ticks infesting livestock in different districts of Khyber Pakhtunkhwa, Pakistan. The present study reported nine species of hard ticks infesting different hosts. The most prevalent tick life stage was adult females, followed by nymphs and adult males. In the phylogenetic tree, 16S rDNA sequences of Anaplasma spp. clustered with sequences of A. marginale. The hard ticks act as a carrier for the transmission of A. marginale. Further extensive country-wide research is required to explore the diverse tick species and the associated pathogens in Pakistan.
Abstract
Ticks transmit pathogens to animals and humans more often than any other arthropod vector. The rural economy of Pakistan mainly depends on livestock farming, and tick infestations cause severe problems in this sector. The present study aimed to molecularly characterize the Anaplasma spp. in hard ticks collected from six districts of Khyber Pakhtunkhwa, Pakistan. Ticks were collected from various livestock hosts, including cattle breeds (Holstein-Friesian, Jersey, Sahiwal, and Achai), Asian water buffaloes, sheep, and goats from March 2018 to February 2019. Collected ticks were morphologically identified and subjected to molecular screening of Anaplasma spp. by amplifying 16S rDNA sequences. Six hundred seventy-six ticks were collected from infested hosts (224/350, 64%). Among the nine morphologically identified tick species, the highest occurrence was noted for Rhipicephalus microplus (254, 37.6%), followed by Hyalomma anatolicum (136, 20.1%), Rhipicephalus haemaphysaloides (119, 17.6%), Rhipicephalus turanicus (116, 17.1%), Haemaphysalis montgomeryi (14, 2.1%), Hyalomma dromedarii (11, 1.6%), Haemaphysalis bispinosa (10, 1.5%), Hyalomma scupense (8, 1.2%), and Haemaphysalis kashmirensis (8, 1.2%). The occurrence of tick females was highest (260, 38.5%), followed by nymphs (246, 36.4%) and males (170, 25.1%). Overall, the highest occurrence of ticks was recorded in the Peshawar district (239, 35.3%), followed by Mardan (183, 27.1%), Charsadda (110, 16.3%), Swat (52, 7.7%), Shangla (48, 7.1%), and Chitral (44, 6.5%). Among these ticks, Anaplasma marginale was detected in R. microplus, R. turanicus, and R. haemaphysaloides. The 16S rDNA sequences showed high identity (98–100%) with A. marginale reported from Australia, China, Japan, Pakistan, Thailand, Uganda, and the USA. In phylogenetic analysis, the sequence of A. marginale clustered with the same species reported from Australia, China, Pakistan, Thailand, Uruguay, and the USA. Further molecular work regarding the diversity of tick species and associated pathogens is essential across the country.
Keywords: Anaplasma marginale, cattle, livestock, Pakistan, ticks
1. Introduction
Ticks (Acari: Ixodoidea) are most prevalent in the tropical and subtropical regions of the world, parasitizing almost all terrestrial and semi-aquatic vertebrates [1]. Ticks can transmit various pathogens to their vertebrate hosts, including viruses, bacteria, and protozoans [2,3]. Tick-borne pathogens (TBPs) that often infect animals include Babesia spp., Anaplasma spp., Theileria spp., Ehrlichia spp., and Rickettsia spp. [2,4,5]. Anaplasmosis, theileriosis, babesiosis, and cowdriosis are the leading tick-borne diseases (TBDs). They affect bovines and small ruminants. Among these, the first three diseases have a prime impact on the economy of Pakistan [6,7].
Anaplasma species (Anaplasmataceae: Rickettsiales) are obligate intracellular Gram-negative bacteria transmitted by ticks to animal hosts, including humans, that can propagate and survive within the host cells [8,9,10]. Tick genera such as Rhipicephalus, Haemaphysalis, Dermacentor, Amblyomma, and Ixodes transmit Anaplasma spp. to different hosts [11]. Anaplasma marginale is an important bacterium of public and veterinary health that is mainly distributed in tropical, subtropical, and temperate regions [11,12]. The tick genera known for transmitting various TBPs in Pakistan are Rhipicephalus, Hyalomma, Haemaphysalis, Ixodes, Ornithodoros, and Argas [5,13,14,15,16,17,18]. Anaplasmosis is one of the most common hemo-rickettsiales diseases of cattle and Asian water buffaloes [19], and various Rhipicephalus and Hyalomma species transmit Anaplasma spp., such as Anaplasma centrale, Anaplasma ovis, Anaplasma platys-like organism, and A. marginale in Pakistan [6,19,20].
In Pakistan, the Khyber Pakhtunkhwa (KP) province is a hotspot for developing and reoccurring TBDs of veterinary and public health relevance [5,6,15,16,18,21]. Due to the extension in dairy cattle industries and the introduction of exotic cattle breeds, the surveillance of ticks and TBPs in indigenous and exotic cattle breeds is essential. There is a scarcity of available information regarding the detection of Anaplasma spp. in ticks [5,19]. The current study aimed to investigate different tick species to detect Anaplasma spp. throughout KP.
2. Materials and Methods
2.1. Ethical Approval
The research study was approved by the Advanced Studies Research Board (ASRB) members of Abdul Wali Khan University Mardan, Pakistan (Dir/A&R/AWKUM/2018/1410). Verbal/oral permission was taken from the livestock owners and farmers during tick collection.
2.2. Study Area
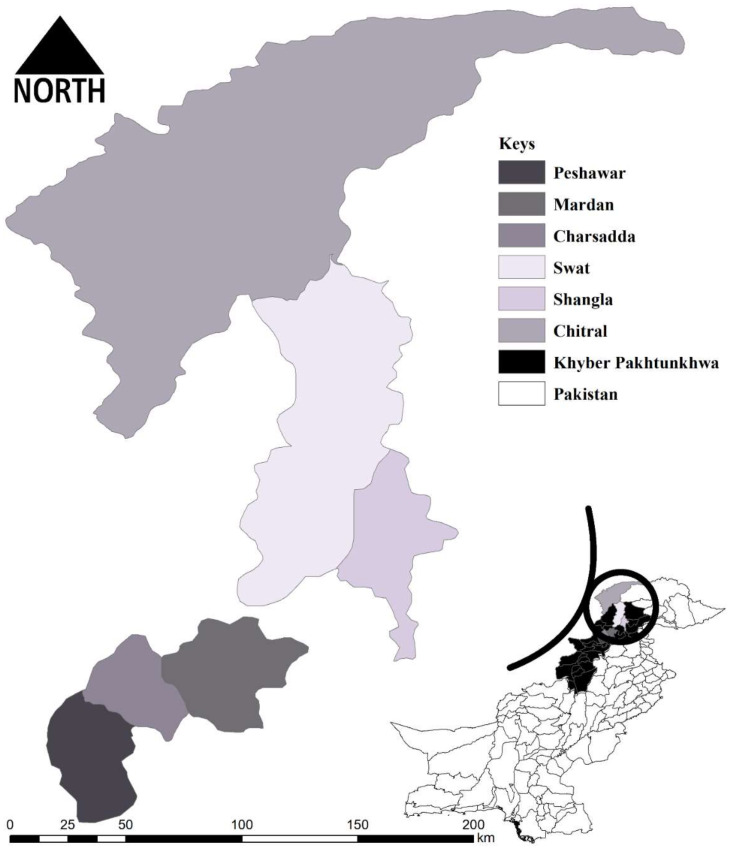
Tick specimens were collected from various herds randomly in six districts, including Peshawar (34.039825° N, 71.566832° E), Mardan (34.194697° N, 72.050557° E), Charsadda (34.161297° N, 71.753660° E), Swat (35.2227° N, 72.4258° E), Shangla (34.8883° N, 72.6003° E), and Chitral (35.7699° N, 71.7741° E) of KP, Pakistan. Ticks collected from hosts in the northern three districts, including Chitral, Shangla, and Swat, were associated with transhumant herds. The average temperature of northern districts (Chitral, Shangla, and Swat) are 0–15 °C and 15–35 °C, while the southern districts (Peshawar, Mardan, and Charsadda) are 10–28 °C and 30–43 °C in winter and summer seasons, respectively (climate-data.org) (accessed on 26 April 2022). Tick specimens were collected from various hosts available in accessible regions of the study area. The geographical coordinates of each collection site were obtained using a Global Positioning System (GPS) and processed in Microsoft Excel V. 2016 (Microsoft 365®), and then imported to ArcGIS V. 10.3.1 (ESRI, Redlands, CA, USA) to design the map (Figure 1).
Figure 1.
Map showing study districts where tick specimens were collected.
2.3. Ticks Collection and Preservation
Tick specimens were randomly collected from March 2018 to February 2019 during four seasons (spring, summer, fall, and winter). These specimens were collected from livestock hosts having different ages, genders, and host types reared at houses (herd size at house was 1–4 animals) and animal farms (herd size at farm was 20–80 animals). The collections were randomly done from different cattle breeds (Holstein-Friesian, Jersey, Sahiwal, and Achai), Asian water buffaloes, sheep, and goats at various collection sites in the six districts of KP, Pakistan. Ticks were washed with distilled water followed by 70% ethanol to remove the contaminants and tissues from the tick’s body. Finally, the specimens were preserved in 100% ethanol for further experimental work.
2.4. Morphological Identification of Ticks
The collected tick specimens were identified under a stereo zoom microscope (SZ61, Olympus, Tokyo, Japan) using standard taxonomic keys based on morphological features [22,23,24,25,26,27].
2.5. DNA Extraction and PCR
Genomic DNA was extracted from 268 selected ticks (Table 1) using the phenol-chloroform method [28]. The extracted genomic DNA samples were quantified via NanoQ (Optizen, Daejeon, South Korea) and stored at −20 °C for further analysis. A conventional PCR (GE-96G, BIOER, Hangzhou, China) was performed to amplify the 16S rRNA gene for Anaplasma spp. using a pair of primers (Ehr-F2, 5′-AGA GTT TGA TCC TGG CTC AG-3′ and Ehr-R, 5′-GAG TTT GCC GGG ACT TYT TCT-3′) [29]. PCR reaction mixture of 25 µL was comprised of 2 µL genomic DNA (50 ng), 1 µL each forward and reverse primer (10 µM), 8.5 µL PCR water, and 12.5 µL DreamTaq PCR Master Mix (2×) (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Thermocycling conditions were: 95 °C for 3 min, followed by 35 cycles at 95 °C for 30 s, 50 °C for 30 s, 72 °C for 1 min, and final extensions at 72 °C for 7 min. In each PCR reaction, PCR water and Rickettsia massiliae DNA were taken as negative and positive controls, respectively. The PCR products were run on a 2% agarose gel electrophoresis and observed via Gel Documentation (BioDoc-It™ Imaging Systems UVP, LLC, Upland, CA, USA). The amplicons were purified through the DNA Clean Kit and Concentrator (Zymo Research, Irvine, CA, USA).
Table 1.
Occurrence of ticks in various hosts and molecular detection of Anaplasma marginale in different districts of Khyber Pakhtunkhwa, Pakistan.
| District | Host | Observed Hosts | Infested Hosts (%) | Ticks | Tick Life Stages | Molecularly Screened Ticks | Anaplasma Positive Ticks |
|---|---|---|---|---|---|---|---|
| Peshawar | Holstein-Friesian | 19 | 18 (94.7) | R. microplus | 43 (19N, 16M, 8F) | 15N, 8F | 5N |
| R. turanicus | 21 (7N, 9M, 5F) | 5N, 5F | 2N | ||||
| R. haemaphysaloides | 16 (8N, 3M, 5F) | 3N, 5F | 1N | ||||
| Hy. scupense | 8 (6N, 2F) | 1N, 1F | - | ||||
| Jersey | 12 | 11 (91.6) | R. microplus | 32 (15N, 10M, 7F) | 9N, 7F | 1F | |
| Hy. anatolicum | 28 (4N, 4M, 20F) | 1F | - | ||||
| Sahiwal | 3 | 3 (100) | R. microplus | 4 (2N, 1M, 1F) | 1N, 1F | - | |
| Hy. anatolicum | 21 (1N, 20F) | 1F | - | ||||
| Achai | 2 | - | - | - | - | - | |
| Asian water buffaloes | 21 | 16 (76) | R. microplus | 12 (4N, 5M, 3F) | 4N, 3F | - | |
| R. turanicus | 7 (3N, 2M, 2F) | 2N, 2F | - | ||||
| R. haemaphysaloides | 33 (14N, 13M, 6F) | 9N, 6F | - | ||||
| Sheep | 13 | 7 (58.3) | R. turanicus | 3 (1N, 1M, 1F) | 1N, 1F | - | |
| R. haemaphysaloides | 3 (1N, 1M, 1F) | 1N, 1F | - | ||||
| Goats | 11 | 7 (63.6) | R. turanicus | 8 (3N, 4M, 1F) | 3N, 1F | - | |
| Mardan | Holstein-Friesian | 16 | 15 (93.7) | R. microplus | 8 (4N, 2M, 2F) | 2N, 2F | 2N |
| R. haemaphysaloides | 23 (11N, 7M, 5F) | 7N, 5F | 1N | ||||
| Jersey | 10 | 9 (90) | R. microplus | 8 (1N, 2M, 5F) | 1N, 1F | - | |
| R. turanicus | 21 (8N, 7M, 6F) | 7N, 6F | 1N | ||||
| R. haemaphysaloides | 9 (4N, 3M, 2F) | 2N, 2F | - | ||||
| Sahiwal | 4 | 3 (75) | R. microplus | 2 (1N, 1F) | 1N, 1F | - | |
| Hy. anatolicum | 16 (1M, I5F) | 1F | - | ||||
| Achai | 2 | - | - | - | - | - | |
| Asian water buffaloes | 21 | 14 (66.7) | R. microplus | 31 (14N, 10M, 7F) | 6N, 7F | - | |
| R. turanicus | 21 (9N, 8M, 4F) | 8N, 4F | 2F | ||||
| R. haemaphysaloides | 4 (2N, 1M, 1F) | 1N, 1F | - | ||||
| Hy. anatolicum | 26 (1N, 1M, 24F) | 1F | - | ||||
| Sheep | 10 | 5 (50) | R. turanicus | 4 (2N, 1M, 1F) | 1N, 1F | - | |
| R. haemaphysaloides | 2 (1N, 1F) | 1F | - | ||||
| Goats | 10 | 7 (70) | R. turanicus | 2 (1N, 1F) | 1F | - | |
| R. haemaphysaloides | 6 (2N, 2M, 2F) | 2N, 2F | - | ||||
| Charsadda | Holstein-Friesian | 15 | 14 (93.3) | R. microplus | 22 (10N, 7M, 5F) | 8N, 5F | 2N |
| Hy. dromedarii | 11 (6N, 4M, 1F) | 1F | - | ||||
| R. haemaphysaloides | 14 (6N, 3M, 5F) | 5N, 3F | - | ||||
| Jersey | 10 | 9 (90) | R. microplus | 11 (5N, 3M, 3F) | 5N, 1F | - | |
| R. turanicus | 11 (4N, 3M, 4F) | 3N, 1F | 1F | ||||
| Hy. anatolicum | 6 (2N, 2M, 2F) | 1F | |||||
| Sahiwal | 4 | 2 (50) | R. microplus | 2 (1N, 1F) | 1F | - | |
| Hy. anatolicum | 11F | 1F | - | ||||
| Achai | 2 | - | - | - | - | - | |
| Asian water buffaloes | 22 | 13 (59) | R. microplus | 11 (5N, 3M, 3F) | 4N, 2F | 1F | |
| Sheep | 8 | 2 (25) | R. microplus | 2 (1N, 1F) | 1F | - | |
| Goats | 9 | 5 (55.5) | R. microplus | 2 (1N, 1F) | 1F | - | |
| R. turanicus | 7 (3N, 2M, 2F) | 2N, 1F | - | ||||
| Swat | Holstein-Friesian | 2 | 2 (100) | R. microplus | 9 (4N, 3M, 2F) | 2N, 1F | 1N |
| R. haemaphysaloides | 2 (1N, 1F) | 1F | - | ||||
| Jersey | 3 | 2 (66.7) | R. microplus | 4 (2N, 1M, 1F) | 1N, 1F | - | |
| R. haemaphysaloides | 2 (1N, 1F) | 1F | - | ||||
| Sahiwal | 1 | 1 (100) | R. microplus | 3 (2N, 1F) | 1F | - | |
| Achai | 8 | 1 (12.5) | R. microplus | 2(1N, 1F) | 1F | - | |
| Asian water buffaloes | 17 | 10 (58.8) | R. microplus | 5 (2N, 1M, 2F) | 2N, 1F | - | |
| R. turanicus | 2 (1N, 1F) | 1N, 1F | - | ||||
| Hy. anatolicum | 9 (1N, 1M, 7F) | 1F | - | ||||
| Sheep | 4 | 1 (25) | Ha. montgomeryi | 1F | 1F | - | |
| Ha. kashmirensis | 1F | 1F | - | ||||
| Goats | 9 | 6 (66.7) | Ha. montgomeryi | 7 (3N, 3M, 1F) | 1F | - | |
| Ha. bispinosa | 5 (2N, 2M, 1F) | 1F | - | ||||
| Shangla | Holstein-Friesian | 3 | 3 (100) | R. microplus | 4 (2N, 1M, 1F) | 1N, 1F | 1N |
| Hy. anatolicum | 7F | - | - | ||||
| R. turanicus | 7 (4N, 2M, 1F) | 1N, 1F | - | ||||
| Jersey | 2 | 2 (100) | R. microplus | 3 (1N, 1M, 1F) | 1F | - | |
| R. turanicus | 2 (1M, 1F) | 1F | - | ||||
| Sahiwal | 3 | 2 (66.6) | R. microplus | 2 (1N, 1F) | 1F | - | |
| Achai | 7 | 1 (14.3) | R. microplus | 2 (1N, 1F) | 1F | - | |
| Asian water buffaloes | 16 | 10 (62.5) | R. microplus | 14 (8N, 4M, 2F) | 2N, 1F | - | |
| R. haemaphysaloides | 2 (1N, 1F) | 1F | - | ||||
| Sheep | 3 | 1 (33.3) | Ha. kashmirensis | 1F | 1F | - | |
| Goats | 9 | 4 (44.4) | Ha. montgomeryi | 2 (1N, 1F) | 1N | - | |
| Ha. kashmirensis | 2 (1N, 1F) | 1N | - | ||||
| Chitral | Holstein-Friesian | 2 | 2 (100) | R. microplus | 4 (1N, 2M, 1F) | 1N, 1F | 1N |
| Jersey | 2 | 2 (100) | R. microplus | 2 (1M, 1F) | 1F | - | |
| R. haemaphysaloides | 3 (1N, 2F) | 1N | - | ||||
| Sahiwal | 2 | 1 (50) | R. microplus | 2 (1M, 1F) | 1F | - | |
| Achai | 7 | 1 (14.3) | R. microplus | 2 (1N, 1F) | 1N | - | |
| Asian water buffaloes | 13 | 5 (38.46) | Hy. anatolicum | 12 (1N, 11F) | 1N | - | |
| R. microplus | 6 (3N, 2M, 1F) | 2N, 1F | - | ||||
| Sheep | 5 | 2 (50) | Ha. montgomeryi | 2 (1N, 1F) | 1N | - | |
| Ha. bispinosa | 5 (2N, 2M, 1F) | 1N, 1F | - | ||||
| Goats | 8 | 5 (62.5) | Ha. montgomeryi | 2 (1M, 1F) | 1F | - | |
| Ha. kashmirensis | 4 (3N, 1F) | 3N | - | ||||
| Overall total | 350 | 224 (64) | 676 (246N, 170M, 260F) | 268 (142N, 126F) | 22 (8.2%) | ||
2.6. DNA Sequencing and Phylogenetic Analysis
Amplified amplicons of 16S rDNA of Anaplasma spp. from different tick species infesting Holstein-Friesian, Jersey, and Asian water buffaloes were sequenced in both directions (Macrogen, Inc., Seoul, South Korea). The obtained sequences were trimmed to remove the contaminated and poor reading regions via SeqMan V. 5 (DNASTAR, Inc., Madison, WI, USA). The obtained sequences were subjected to the Basic Local Alignment Search Tool (BLAST) [30] at National Center for Biotechnology Information (NCBI). The homologous sequences were downloaded in FASTA format from NCBI based on their high percentage identity. The downloaded sequences were aligned with the obtained sequences and an outgroup sequence using ClustalW multiple alignments [31] in BioEdit alignment editor V.7.0.5 (Raleigh, NC, USA) [32]. The phylogenetic tree was constructed through the Maximum-Likelihood statistical method and Tamura-Nei model [33] with a 1000 bootstrapping value in Molecular evolutionary genetics analysis (MEGA-X) [34].
2.7. Statistical Analyses
The descriptive statistical analyses were performed in Microsoft Excel V. 2016 (Microsoft 365®). Chi-square tests were performed in the GraphPad Prism V. 5 (GraphPad Software, Inc., San Diego, CA, USA), and the analysis was considered significant at a p-value < 0.05.
3. Results
3.1. Ticks Description
A total of 350 hosts, including 141 cattle breeds, 110 Asian water buffaloes, 43 sheep, and 56 goats, were observed during tick collection. The overall recorded tick occurrence was 64% (224/350). Based on the hosts, the highest occurrence of ticks was recorded in cattle breeds (104/141, 73.8%: Holstein-Friesian 54/57, 94.7%; Jersey 35/39, 89.7%; Sahiwal 12/17, 70.6%; and Achai 3/28, 10.7%) followed by Asian water buffaloes (68/110, 61.8%), goats (34/56, 60.7%), and sheep (18/43, 41.9%). The highest tick occurrence was recorded in the Peshawar district (239, 35.3%), followed by the Mardan district (183, 27.1%), Charsadda district (110, 16.3%), Swat district (52, 7.7%), Shangla district (48, 7.1%), and Chitral district (44, 6.5%) (Table 1). Different tick species, including Rhipicephalus microplus, Rhipicephalus turanicus, Rhipicephalus haemaphysaloides, Hyalomma anatolicum, Hyalomma dromedarii, Hyalomma scupense, Haemaphysalis bispinosa, Haemaphysalis montgomeryi, and Haemaphysalis kashmirensis were morphologically identified. Out of the total percentage composition for each tick species in all districts, the highest occurrence was observed for R. microplus (254, 37.6%), followed by Hy. anatolicum (136, 20.1%), R. haemaphysaloides (119, 17.6%), R. turanicus (116, 17.1%), Ha. montgomeryi (14, 2.1%), Hy. dromedarii (11, 1.6%), Ha. bispinosa (10, 1.5%), Hy. scupense (8, 1.2%), and Ha. kashmirensis (8, 1.2%). The most prevalent life stage of ticks was adult females (260, 38.5%), followed by nymphs (246, 36.4%) and adult males (170, 25.1%) (Table 1).
3.2. Variables Associated with a Tick Infestation
A total of 350 hosts of different ages, genders, and host types were observed for the presence of ticks in different seasons. Based on the hosts’ gender, the highest tick occurrence was noted in female hosts compared to male hosts. Based on age, hosts were stratified into three age groups; the highest tick occurrence was recorded in the <3 years age group, followed by the 1–3 years age group and >1-year age group. Among the observed animals, the highest occurrence was recorded in Holstein-Friesian, followed by Jersey, Sahiwal, Asian water buffaloes, goats, sheep, and Achai. The occurrence of ticks was highest in summer, followed by spring, fall, and winter seasons. District-wise, the occurrence of ticks was highest in the Peshawar district and lowest in Chitral. All variables, including genders, ages, hosts, seasons, and areas associated with tick occurrence, were highly significant (Table 2).
Table 2.
Tick infestation on the hosts’ gender, ages, hosts, year-round, and collection sites.
| Variables | Levels | Total | Positive | X² | p-Value | |
|---|---|---|---|---|---|---|
| Genders | Female | 250 | 190 | 3.00 | 0.0125 | |
| Male | 100 | 34 | ||||
| Ages | ≤1 years | 59 | 20 | 8.22 | 0.0164 | |
| 1–3 years | 118 | 75 | ||||
| >3 years | 173 | 129 | ||||
| Hosts | Cattle Breeds | Holstein-Friesian | 57 | 54 | 3.382 | 0.0024 |
| Jersey | 39 | 35 | ||||
| Sahiwal | 17 | 12 | ||||
| Achai | 28 | 3 | ||||
| Total | 141 | 104 | ||||
| Asian water buffaloes | 110 | 68 | ||||
| Sheep | 43 | 18 | ||||
| Goats | 56 | 34 | ||||
| Seasons | Spring | 105 | 71 | 3.227 | 0.0009 | |
| Summer | 140 | 101 | ||||
| Fall | 55 | 31 | ||||
| Winter | 50 | 21 | ||||
| Areas | Peshawar | 81 | 62 | 0.8165 | 0.0001 | |
| Mardan | 73 | 53 | ||||
| Charsadda | 70 | 45 | ||||
| Swat | 44 | 23 | ||||
| Shangla | 43 | 23 | ||||
| Chitral | 39 | 18 | ||||
3.3. Detection of Anaplasma spp. in Ticks
The genomic DNA of 268 ticks (142N, 126F) was subjected to PCR to amplify the 16S rRNA gene of Anaplasma spp. Out of 268 ticks, 22 (8.2%) (9/97 (9.2%) Peshawar, 6/74 (8.1%) Mardan, 4/46 (8.7%) Charsadda, 1/19 (5.2%) Swat, 1/15 (6.7%) Shangla, and 1/17 (5.9%) Chitral) were found positive for Anaplasma spp. Among various tick species, R. microplus, R. turanicus, and R. haemaphysaloides were found positive for Anaplasma spp. Rhipicephalus microplus ticks showed the highest occurrence for Anaplasma spp. (14, 63.6%), followed by R. turanicus (6, 27.3%), and R. haemaphysaloides (2, 9.1%). The district-wise occurrence of Anaplasma spp. was highest in Peshawar (9/97, 9.2%), followed by Charsadda (4/46, 8.7%), Mardan (6/74, 8.1%), Shangla (1/15. 6.7%), Chitral (1/17, 5.9%), and lowest in Swat (1/19, 5.2%). Positive R. microplus, R. turanicus, and R. haemaphysaloides infesting Holstein-Friesian and Jersey were reported from the Peshawar district, whereas positive R. microplus, R. turanicus, and R. haemaphysaloides infesting Holstein-Friesian, Jersey, and Asian water buffaloes were reported from the Mardan district. Furthermore, positive R. microplus infesting cattle breeds (Holstein-Friesian and Jersey) and Asian water buffaloes were reported from the Charsadda district. Positive R. microplus infesting Holstein-Friesian were reported from the Swat, Shangla, and Chitral districts (Table 1).
3.4. Phylogenetic Analysis of Anaplasma marginale
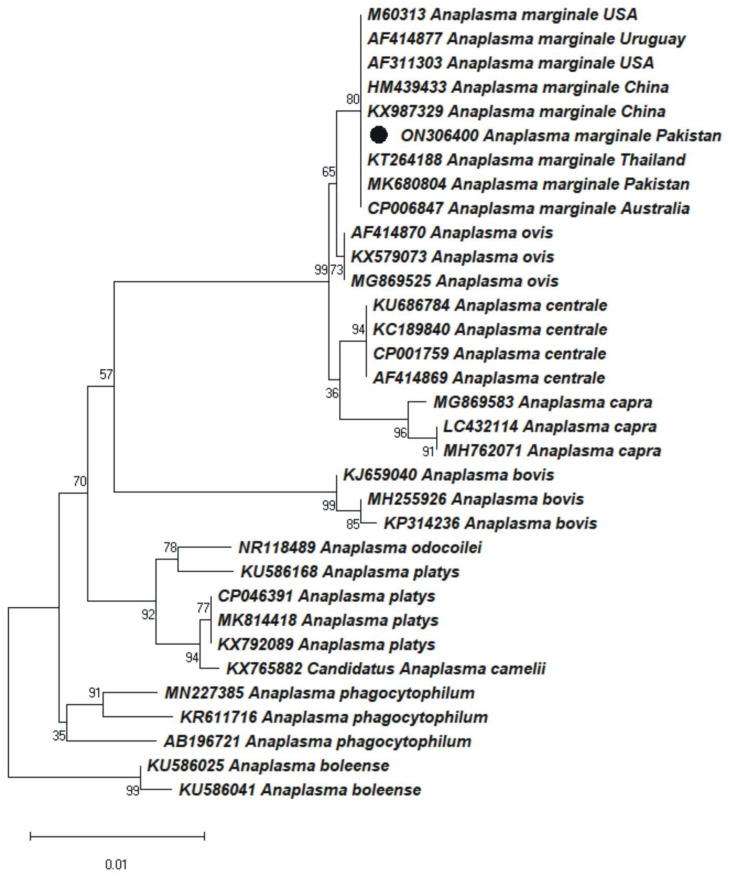
The multiple obtained identical sequences were considered as a consensus sequence. The BLAST results of the obtained 16S rDNA (1109 bp) sequences showed 98–100% identity with the A. marginale reported from Australia (CP006847 and AF414874), China (KX987330, DQ341369, FJ389579, and HM439433), Japan (FJ226454), Pakistan (MK680804, MK680806, and MK680807), Thailand (KT264188), Uganda (KU686785, KU686774, and KU686775), and the USA (AF311303, CP000030, and AF309866). Thirty-two sequences were downloaded for 16S rDNA of A. marginale from NCBI. The phylogenetic tree obtained a 16S rDNA sequence of A. marginale clustered with the identical species sequences reported from Australia, China, Thailand, Pakistan, Uruguay, and the USA (Figure 2). The 16S rDNA sequence of A. marginale was uploaded to NCBI under the accession number ON306400.
Figure 2.
The maximum likelihood phylogenetic tree of Anaplasma marginale was constructed based on a partial 16S rDNA sequence. Anaplasma boleense 16S rDNA sequences were used as an outgroup. The obtained sequence is represented with a black dot (ON306400).
4. Discussion
Pakistan’s hot and humid environment is ideal for the growth of ticks and their associated pathogens [5,21]. Previous studies have reported numerous tick species infesting diverse hosts in different areas of the country [5,15,18,20,35]. More than twenty different hard tick species are biological vectors of A. marginale, causing bovine anaplasmosis [36]. In Pakistan, there is a scarcity of molecular approaches to detecting A. marginale in ticks, primarily identified through microscopy of blood smear analysis [37,38]. During this study, A. marginale was genetically characterized in collected ticks from diverse livestock hosts in Pakistan. The current study identified hard ticks comprised of nine medically essential tick species infesting cattle breeds (Holstein-Friesian, Jersey, Sahiwal, and Achai), Asian water buffaloes, sheep, and goats. Among these nine tick species, three species in the genus Rhipicephalus were found positive for A. marginale, with A. marginale being dominant in R. microplus. This tick has been identified as a significant vector of A. marginale in several tropical and subtropical countries [39].
A low tick occurrence was recorded in local cattle breeds (Achai), which may be due to the natural resistance of Achai towards the tick infestation. The female hosts were found to have a significantly higher occurrence of ticks than male hosts, which is consistent with the previous findings [20]. Female hosts may have a high occurrence due to hormonal changes, because the high level of prolactin and progesterone hormone in female hosts make them more vulnerable to tick infestation [40]. A high tick occurrence has been recorded in adult hosts compared to the younger ones, which is in accordance with the findings of previous reports [17,35]. Free grazing practices and large surfaces of the adult hosts make them more susceptible to tick attachment, in contrast to the younger ones that get less of a tick burden due to less grazing practices, the low surface area of their bodies, and their strong immune system [41]. Annual patterns of tick activity are influenced by seasonal temperature variations, affecting the dynamics of ticks and TBDs. Fluctuation in different seasons may result in varying tick occurrence in the same region [42]. The summer season provides the best conditions for developing and expanding ticks. The winter season hampers the infestation of ticks because all stages of ticks hibernate in cold climatic conditions, and these findings support the previous reports in the region [15,35]. The highest tick occurrence was recorded in the Peshawar district, followed by Mardan, and the least was recorded from the Chitral district. These results may correspond to favorable environmental conditions associated with ticks rearing [18,35,43].
For identifying Anaplasma spp., molecular techniques such as PCR have significant advantages over traditional serological and blood smear testing because PCR is the most sensitive and reliable diagnostic method [44]. The molecular phylogeny of A. marginale from the study area was developed by amplifying 16S rDNA sequences, as this marker is of prime importance in characterizing Anaplasma spp. [40]. The sequence obtained in this study shared a 98–100% identity with available sequences in GenBank. Phylogenetic analysis revealed that A. marginale from the northern regions of Pakistan clustered with related isolates reported from Australia, China, Thailand, Pakistan, Uruguay, and the USA (Figure 2). Previous research on the molecular phylogeny of A. marginale based on 16S rDNA sequences from Pakistan validates our findings [20,45].
Ticks infected with A. marginale may be of significant concern to both animals and humans due to the increased risk of infection, complicating clinical care. These findings highlight the need for a larger-scale tick surveillance program in understanding various TBDs, and their zoonotic and pathogenic potential.
5. Conclusions
The present study provides information regarding the occurrence of hard ticks as carriers for A. marginale in Pakistan. Anaplasma marginale was detected in three tick species: R. microplus, R. turanicus, and R. haemaphysaloides. These findings will inform the veterinary and livestock community regarding the diversity of tick species and associated A. marginale. Further research is needed to explore the variety of ticks and tick-associated pathogens in Pakistan.
Acknowledgments
This study was carried out under the financial support given by the Pakistan Science Foundation and Higher Education Commission of Pakistan.
Author Contributions
A.A. (Abid Ali), Z.K., A.A. (Abdulaziz Alouffi), M.M.A., T.T. and S.U. carried out the experimental design of the study. Z.K., S.U., S.S., M.K.O., A.Z.K., M.N., S.A. and O.A. collected the tick samples. A.A. (Abid Ali), S.S., A.Z.K., S.Z.S., A.A. (Abdulaziz Alouffi), M.M.A., M.K.O., T.T., M.N. and S.U. performed the experiments. S.U., A.A. (Abid Ali) and M.N. performed the phylogenetic and statistical analysis. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The research study was approved by the Advanced Studies Research Board (ASRB) members of Abdul Wali Khan University Mardan, Pakistan (Dir/A&R/AWKUM/2018/1410).
Data Availability Statement
Details regarding data supporting reported results can be found https://www.ncbi.nlm.nih.gov/nuccore/?term= (accessed on 18 March 2022).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by JSPS KAKENHI Grant Number JP22H02522 and the Heiwa Nakajima Foundation.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guglielmone A.A., Petney T.N., Robbins R.G. Ixodidae (Acari: Ixodoidea): Descriptions and redescriptions of all known species from 1758 to 31 December 2019. Zootaxa. 2020;4871:1–322. doi: 10.11646/zootaxa.4871.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Parola P., Paddock C.D., Socolovschi C., Labruna M.B., Mediannikov O., Kernif T., Abdad M.Y., Stenos J., Bitam I., Fournier P.E., et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013;26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rego R.O., Trentelman J.J., Anguita J., Nijhof A.M., Sprong H., Klempa B., Hajdusek O., Tomás-Cortázar J., Azagi T., Strnad M. Counterattacking the tick bite: Towards a rational design of anti-tick vaccines targeting pathogen transmission. Parasit. Vectors. 2019;12:229. doi: 10.1186/s13071-019-3468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labruna M.B., Whitworth T., Horta M.C., Bouyer D.H., McBride J.W., Pinter A., Popov V., Gennari S.M., Walker D.H. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004;42:90–98. doi: 10.1128/JCM.42.1.90-98.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karim S., Budachetri K., Mukherjee N., Williams J., Kausar A., Hassan M.J., Adamson S., Dowd S.E., Apanskevich D., Arijo A., et al. A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Negl. Trop. Dis. 2017;11:e0005681. doi: 10.1371/journal.pntd.0005681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabbar A., Abbas T., Saddiqi H.A., Qamar M.F., Gasser R.B. Tick-borne diseases of bovines in Pakistan: Major scope for future research and improved control. Parasit. Vectors. 2015;8:283. doi: 10.1186/s13071-015-0894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghafar A., Abbas T., Rehman A., Sandhu Z.U.D., Cabezas-Cruz A., Jabbar A. Systematic review of ticks and tick-borne pathogens of small ruminants in Pakistan. Pathogens. 2020;9:937. doi: 10.3390/pathogens9110937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ybañez A.P., Inokuma H. Anaplasma species of veterinary importance in Japan. Vet. World. 2016;9:1190. doi: 10.14202/vetworld.2016.1190-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neal A.J., Singh N., Mendes M.T., Pedra J.H. The genus Anaplasma: Drawing back the curtain on tick–pathogen interactions. Pathog. Dis. 2021;79:ftab022. doi: 10.1093/femspd/ftab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnittger L., Ganzinelli S., Bhoora R., Omondi D., Nijhof A.M., Florin-Christensen M. The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: Species compilation, molecular phylogeny, and evolutionary insights. Parasitol. Res. 2022;121:1207–1245. doi: 10.1007/s00436-022-07424-8. [DOI] [PubMed] [Google Scholar]
- 11.Jongejan F., Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- 12.Choubdar N., Karimian F., Koosha M., Nejati J., Oshaghi M.A. Hyalomma spp. ticks and associated Anaplasma spp. and Ehrlichia spp. on the Iran-Pakistan border. Parasit. Vectors. 2021;14:469. doi: 10.1186/s13071-021-04956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begum F., Wisseman C.L., Jr., Traub R. Tick-borne viruses of West Pakistan: I. Isolation and general characteristics. Am. J. Epidemiol. 1970;92:180–191. doi: 10.1093/oxfordjournals.aje.a121196. [DOI] [PubMed] [Google Scholar]
- 14.Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 1979;15:307–417. doi: 10.1093/jmedent/15.4.307. [DOI] [PubMed] [Google Scholar]
- 15.Ali A., Khan M.A., Zahid H., Yaseen P.M., Khan M.Q., Nawab J., Rehman Z.U., Ateeq M., Khan S., Ibrahim M. Seasonal dynamics, record of ticks infesting humans, wild and domestic animals and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front. Physiol. 2019;10:793. doi: 10.3389/fphys.2019.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahid H., Muñoz-Leal S., Khan M.Q., Alouffi A.S., Labruna M.B., Ali A. Life Cycle and Genetic Identification of Argas persicus Infesting Domestic Fowl in Khyber Pakhtunkhwa, Pakistan. Front. Vet. Sci. 2021;8:302. doi: 10.3389/fvets.2021.664731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamran K., Ali A., Villagra C., Siddiqui S., Alouffi A.S., Iqbal A. A cross-sectional study of hard ticks (acari: Ixodidae) on horse farms to assess the risk factors associated with tick-borne diseases. Zoonoses Public Health. 2021;68:247–262. doi: 10.1111/zph.12809. [DOI] [PubMed] [Google Scholar]
- 18.Ali A., Shehla S., Zahid H., Ullah F., Zeb I., Ahmed H., Vaz I., Jr., Tanaka T. Molecular survey and spatial distribution of Rickettsia spp. in ticks infesting free-ranging wild animals in Pakistan (2017–2021) Patogens. 2022;11:162. doi: 10.3390/pathogens11020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghafar A., Cabezas-Cruz A., Galon C., Obregon D., Gasser R.B., Moutailler S., Jabbar A. Bovine ticks harbour a diverse array of microorganisms in Pakistan. Parasit. Vectors. 2020;13:1. doi: 10.1186/s13071-019-3862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehman A., Conraths F.J., Sauter-Louis C., Krücken J., Nijhof A.M. Epidemiology of tick-borne pathogens in the semi-arid and the arid agro-ecological zones of Punjab province, Pakistan. Transbound. Emerg. Dis. 2019;66:526–536. doi: 10.1111/tbed.13059. [DOI] [PubMed] [Google Scholar]
- 21.Ali A., Mulenga A., Vaz I., Jr. Tick and Tick-Borne Pathogens: Molecular and Immune Targets for Control Strategies. Front. Physiol. 2020;11:744. doi: 10.3389/fphys.2020.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoogstraal H., Varma M.G.R. Haemaphysalis cornupunctata sp. n. and H. kashmirensis sp. n. from Kashmir, with Notes on H. sundrai Sharif and H. sewelli Sharif of India and Pakistan (Ixodoidea, Ixodidae) J. Parasitol. 1962;48:185–194. doi: 10.2307/3275561. [DOI] [PubMed] [Google Scholar]
- 23.Hoogstraal H., Trapido H. Redescription of the type materials of Haemaphysalis (Kaiseriana) bispinosa Neumann (India), H. (K.) neumanni Dönitz (Japan), H. (K.) lagrangei Larrousse (Vietnam), and H. (K.) yeni Touma off (Vietnam) (Ixodoidea, Ixodidae) J. Parasitol. 1966;52:1188–1198. doi: 10.2307/3276366. [DOI] [PubMed] [Google Scholar]
- 24.Hoogstraal H., Trapido H., Kohls G.M. Studies on southeast Asian Haemaphysalis ticks (Ixodoidea, Ixodidae). Speciation in the H. (Kaiseriana) obesa group: H. semermis Neumann, H. obesa Larrousse, H. roubaudi Toumanoff, H. montgomeryi Nuttall, and H. hirsuta sp. n. J. Parasitol. 1966;52:169–191. doi: 10.2307/3276410. [DOI] [PubMed] [Google Scholar]
- 25.Walker J.B., Keirans J.E., Horak I.G. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World. Cambridge University Press; Cambridge, UK: 2005. [Google Scholar]
- 26.Apanaskevich D.A., Schuster A.L., Horak I.G. The genus Hyalomma: VII. Redescription of all parasitic stages of Hy. (Euhyalomma) dromedarii and Hy. (E.) schulzei (Acari: Ixodidae) J. Med. Entomol. 2008;45:817–831. doi: 10.1093/jmedent/45.5.817. [DOI] [PubMed] [Google Scholar]
- 27.Apanaskevich D.A., Filippova N.A., Horak I.G. The genus Hyalomma Koch, 1844. X. Redescription of all parasitic stages of Hy. (Euhyalomma) scupense Schulze, 1919 (Hy. detritum Schulze) (Acari: Ixodidae) and notes on its biology. Folia Parasitol. 2010;57:69–78. doi: 10.14411/fp.2010.009. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J., Fritsch E.F., Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press; New York, NY, USA: 1989. [Google Scholar]
- 29.Hailemariam Z., Ahmed J.S., Clausen P.H., Nijhof A.M. A comparison of DNA extraction protocols from blood spotted on FTA cards for the detection of tick-borne pathogens by Reverse Line Blot hybridization. Ticks Tick-Borne Dis. 2017;8:185–189. doi: 10.1016/j.ttbdis.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 31.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall T., Biosciences I., Carlsbad C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011;2:60–61. doi: 10.1016/j.compbiolchem.2019.02.002. [DOI] [Google Scholar]
- 33.Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali A., Zahid H., Zeb I., Tufail M., Khan S., Haroon M., Bilal M., Hussain M., Alouffi A.S., Muñoz-Leal S. Risk factors associated with tick infestations on equids in Khyber Pakhtunkhwa, Pakistan, with notes on Rickettsia massiliae detection. Parasit. Vectors. 2021;14:363. doi: 10.1186/s13071-021-04836-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pothmann D., Poppert S., Rakotozandrindrainy R., Hogan B., Mastropaolo M., Thiel C., Silaghi C. Prevalence and genetic characterization of Anaplasma marginale in zebu cattle (Bos indicus) and their ticks (Amblyomma variegatum, Rhipicephalus microplus) from Madagascar. Ticks Tick-Borne Dis. 2016;7:1116–1123. doi: 10.1016/j.ttbdis.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Bhutto B., Gadahi J.A., Khuhro A., Rajput H.M., Bhutto F., Rajput M.A., Talpur A.R. A survey on haemo-protozoan parasites in buffaloes of Landhi Dairy Colony, Karachi-Pakistan. Int. J. Agro Vet. Med. Sci. 2012;6:73–76. doi: 10.5455/ijavms.150. [DOI] [Google Scholar]
- 38.Khan M.I., Shah S.S.A., Khan H., Irshad M., Aziz U. Determination of parasitic load in government cattle breeding and dairy farm, Charsadda, Khyber Pakhtunkhwa-Pakistan. Adv. Anim. Vet. Sci. 2017;5:174–178. doi: 10.14737/journal.aavs/2017/5.4.174.178. [DOI] [Google Scholar]
- 39.Kocan K.M., de la Fuente J., Blouin E.F., Coetzee J.F., Ewing S.A. The natural history of Anaplasma marginale. Vet. Parasitol. 2010;167:95–107. doi: 10.1016/j.vetpar.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh S., Patra G., Borthakur S.K., Behera P., Tolenkhomba T.C., Das M., Lalnunpuia C. Prevalence of hard tick infestations in cattle of Mizoram, India. Biol. Rhythm Res. 2019;50:564–574. doi: 10.1080/09291016.2018.1474988. [DOI] [Google Scholar]
- 41.Swai E.S., Karimuribo E.D., Ogden N.H., French N.P., Fitzpatrick J.L., Bryant M.J., Kambarage D.M. Seroprevalence estimation and risk factors for A. marginale on smallholder dairy farms in Tanzania. Trop. Anim. Health Prod. 2005;37:599–610. doi: 10.1007/s11250-005-4307-y. [DOI] [PubMed] [Google Scholar]
- 42.Hancock P.A., Brackley R., Palmer S.C. Modelling the effect of temperature variation on the seasonal dynamics of Ixodes ricinus tick populations. Int. J. Parasitol. 2011;41:513–522. doi: 10.1016/j.ijpara.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Aiman O., Ullah S., Chitimia-Dobler L., Nijhof A.M., Ali A. First report of Nosomma monstrosum ticks infesting Asian water buffaloes (Bubalus bubalis) in Pakistan. Ticks Tick-Borne Dis. 2022;13:101899. doi: 10.1016/j.ttbdis.2022.101899. [DOI] [PubMed] [Google Scholar]
- 44.Shabana I.I., Alhadlag N.M., Zaraket H. Diagnostic tools of caprine and ovine anaplasmosis: A direct comparative study. BMC Vet. Res. 2018;14:165. doi: 10.1186/s12917-018-1489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeb J., Shams S., Din I.U., Ayaz S., Khan A., Nasreen N., Khan H., Khan M.A., Senbill H. Molecular epidemiology and associated risk factors of Anaplasma marginale and Theileria annulata in cattle from North-western Pakistan. Vet. Parasitol. 2020;279:109044. doi: 10.1016/j.vetpar.2020.109044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Details regarding data supporting reported results can be found https://www.ncbi.nlm.nih.gov/nuccore/?term= (accessed on 18 March 2022).