We carefully read the comment by Serrano et al. [1] discussing the recent published article entitled “Prevalence and Management of Cancer of the Rectal Stump after Total Colectomy and Rectal Sparing in Patients with Familial Polyposis: Results from a Registry-Based Study” [2].
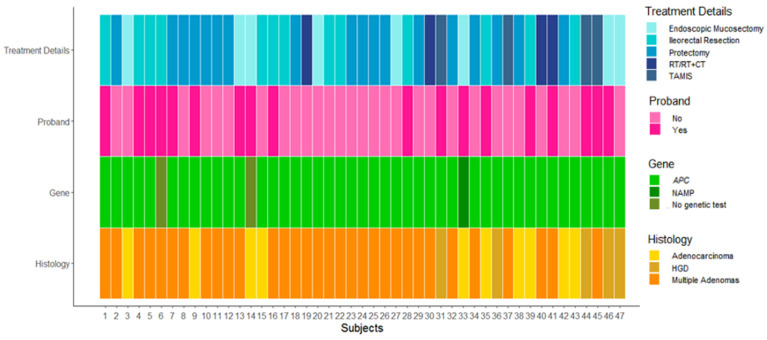
We thank them for the interest they have shown regarding some aspects of the study and the FAP patients’ management following prophylactic surgery with rectal sparing. They would like to know further genotype data about patients who developed cancer in the rectal stump during follow-up. We are pleased to list the genotype information of each of the 47 patients with rectal cancer in Table 1 and the patient-level heat map with the main clinical and genetic data in Figure 1. Regarding the comparison of our results with the literature, neither Colletti et al. [2] nor this author’s reply aim to act as a systematic review of the literature; thus, we do not exclude the possibility that some studies on the same topic may differ from our data. However, we still believe this to be in keeping with the current literature. Serrano et al. argued that the median interval of diagnosis of rectal cancer from primary surgery (i.e., 13 years) was consistently low compared with those in the literature. Particularly, they cited studies by Bulow [3] and Koskenvuo [4] which showed a median interval of 11 and 14 years, respectively. Despite the absence of the datasets of the aforementioned studies, our results seem to be in line with them. Moreover, Serrano et al. deem that 6.57% of patients developing rectal cancer following IRA is a very good result compared to the literature. Our results are fully in line with those reported in three studies [3,5,6], while they are quite a bit lower compared to Koskenvuo et al. [4].
Table 1.
Genotype information of the 47 patients who developed a rectal cancer.
| Pts Code | Protein-Coding Variants | Single Nucleotide Variants |
|---|---|---|
| 1 | p.Asp842Argfs*2 | c.2523dup |
| 2 | p.Leu629* | c.18886T>A |
| 3 | p.Arg216* | c.646C>T |
| 4 | p.Arg976Lysfs*9 | c.2926dup |
| 5 | p.Tyr935* | c.2805C>A |
| 6 | NO | NO |
| 7 | p.Q1294* | c.3880C>T |
| 8 | p.Gln1328* | c.3982C>T |
| 9 | p.Tyr1376Cysfs*9 | c.4127_4128del |
| 10 | p.Glu1538Ilefs*5 | c.4612_4613delGA |
| 11 | p.Glu1309Aspfs*4 | c.3927_3931delAAAGA |
| 12 | p.Arg213* | c.637C>T |
| 13 | p.Gln1062* | c.3183_3187del |
| 14 | NO | NO |
| 15 | p.Arg640Thrfs*11 | c.1917dup |
| 16 | p.Glu1309Aspfs*4 | c.3927_3931del |
| 17 | p.Glu1309Aspfs*4 | c.3927_3931delAAAGA |
| 18 | p.Glu1309Aspfs*4 | c.3927_3931del |
| 19 | p.Thr1301Asnfs*14 | c.3901dup |
| 20 | p.Glu1309Aspfs*4 | c.3927_3931delAAAGA |
| 21 | p.Gln181* | c.541C>T |
| 22 | p.Glu1309Aspfs*4 | c.3927_3931delAAAGA |
| 23 | p.Lys1061Asnfs*65 | c.3183del |
| 24 | p.Glu1309Aspfs*4 | c.3927_3931del |
| 25 | p.Glu1309Aspfs*4 | c.3927_3931del |
| 26 | p.Ser1110* | c.3329C>G |
| 27 | p.Glu1309Aspfs*4 | c.3927_3931delAAAGA |
| 28 | p.Ser1276* | c.3827C>G |
| 29 | p.Glu1309Aspfs*4 | c.3927_3931delAAAGA |
| 30 | p.Gly471Aspfs*27 | c.1409del |
| 31 | p.Arg1114* | c.3340C>T |
| 32 | p.Arg213* | c.637C>T |
| 33 | NO | NO |
| 34 | p.Asn936Lysfs*7 | c.2808_2815del |
| 35 | p.Thr1556Leufs*9 | c.4666delA |
| 36 | p.Glu1157Aspfs*7 | c.3471_3474del |
| 37 | p.Val312Cisysfs*16 | c.1312+5G>T |
| 38 | p.Lys455Glufs*5 | c.1362dupG |
| 39 | p. Glu1157Aspfs*7 | c.3471_3474del |
| 40 | p. Glu1157Aspfs*7 | c.3471_3474del |
| 41 | p. Arg1450* | c.4348C>T |
| 42 | p.Ile544Leufs*5 | c.1629delT |
| 43 | p. Glu1157Aspfs*7 | c.3471_3474del |
| 44 | p.Asp1266* | c.3795_3796InsT |
| 45 | p.Lys1061Lysfs*2 | c.3183_3187delACAAA |
| 46 | p.EX 11_EX 15del | Genomic reference g.(112157642_112162832)_(112179726_?)del |
| 47 | p.Gly972Valfs*4 | c.2915_2916delinsTAAA |
Figure 1.
Patient-level heatmap. Representation of the genetic, baseline, and rectal stump main surgical details of the considered 47 patients.
Reading the comment, we had the feeling that Serrano et al. strongly seek a relation between genotype variant and surveillance following IRA. Thanks to a number of authors who investigated the relation between genotype and phenotype, it has been well established that number of polyps, age of onset of symptoms, colonic cancer, or extracolonic manifestations correlate with some APC mutations [7]. In fact, the aim of those studies was to categorize a subgroup of FAP patients according to genotype variant in the attempt to design better management. However, at the moment, the only significant and independent risk factor for rectal cancer following IRA is chronological age. Years after colectomy, sex, proband/call-up status, familial/isolated case, colon cancer at IRA, or location of mutation did not show enough statistical significance [3]. Based on these data, the patients undergoing IRA at the National Cancer Institute of Milan are scheduled for an endoscopic surveillance every 6–12 months, as we mentioned in the article [2]. Lastly, Serrano et al. questioned that, despite strict endoscopic surveillance, the conservative treatment was feasible only in 25pts (53%). As we stated in the article [2], strict endoscopic surveillance allows detection of rectal cancer at an early stage in the majority of patients. However, we are analyzing the data of patients who have been treated over the last 45 years in a single center, and we undoubtedly need to consider some bias. First, we need to consider that the surgical treatment has substantially shifted towards a minimally invasive approach (TAMIS) over the last two decades, and it always depends on the surgeon’s expertise and skills [8]. Moreover, in our series, some patients underwent a proctectomy because of a carpet-like rectal polyposis, although the tumor was at an early stage. However, we feel that the key perspective which should emerge is that the majority of our patients had rectal cancer detected at an early stage and were promptly treated; this scheme should dramatically improve their oncological outcomes and strengthen the IRA indication as preventive surgery [9].
Author Contributions
Conceptualization: G.C., M.V., S.S., E.R., C.M.C. and P.V.; methodology: P.V., M.V. and C.M.C.; validation: P.V., C.M.C., M.V., L.C., M.M. and M.T.R.; formal analysis: P.V., C.M.C.; investigation: M.V., S.S., G.C., A.M. (Andrea Mancini), A.M. (Andrea Magarotto), C.M.C., P.V., M.M., F.C. and M.T.R.; resources: M.V., P.V.; data curation: C.M.C., P.V., G.C. and M.V.; writing—original draft preparation: G.C., M.V., C.M.C., S.S., P.V. and E.R.; writing—review and editing: E.R., C.M.C., S.S., F.C., I.M.F.C., C.B., M.T.R. and P.V.; supervision: M.V., P.V. and M.M.; project administration: M.V. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Serrano D., Feroce I., Bonanni B., Bertario L. Comment on Colletti et al. Prevalence and Management of Cancer of the Rectal Stump after Total Colectomy and Rectal Sparing in Patients with Familial Polyposis: Results from a Registry-Based Study. Cancers 2022, 14, 298. Cancers. 2022;14:2650. doi: 10.3390/cancers14112650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colletti G., Ciniselli C.M., Signoroni S., Cocco I.M.F., Magarotto A., Ricci M.T., Brignola C., Bagatin C., Cattaneo L., Mancini A., et al. Prevalence and Management of Cancer of the Rectal Stump after Total Colectomy and Rectal Sparing in Patients with Familial Polyposis: Results from a Registry-Based Study. Cancers. 2022;14:298. doi: 10.3390/cancers14020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bülow C., Vasen H., Järvinen H., Björk J., Bisgaard M.L., Bülow S. Ileorectal anastomosis is appropriate for a subset of patients with familial adenomatous polyposis. Gastroenterology. 2000;119:1454–1460. doi: 10.1053/gast.2000.20180. [DOI] [PubMed] [Google Scholar]
- 4.Koskenvuo L., Mustonen H., Renkonen-Sinisalo L., Jarvinen H.J., Lepistö A. Comparison of proctocolectomy and ileal pouch-anal anastomosis to colectomy and ileorectal anastomosis in familial adenomatous polyposis. Fam. Cancer. 2014;14:221–227. doi: 10.1007/s10689-014-9773-9. [DOI] [PubMed] [Google Scholar]
- 5.A Vasen H.F., van Duijvendijk P., Buskens E., Bülow C., Björk J., Järvinen H.J., Bülow S. Decision analysis in the surgical treatment of patients with familial adenomatous polyposis: A Dutch-Scandinavian collaborative study including 659 patients. Gut. 2001;49:231–235. doi: 10.1136/gut.49.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasquer A., Benech N., Pioche M., Breton A., Rivory J., Vinet O., Poncet G., Saurin J.C. Prophylactic colectomy and rectal preservation in FAP: Systematic endoscopic follow-up and adenoma destruction changes natural history of polyposis. Endosc. Int. Open. 2021;9:E1014–E1022. doi: 10.1055/a-1467-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieuwenhuis M.H., Vasen H.F.A. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): A review of the literature. Crit. Rev. Oncol. 2007;61:153–161. doi: 10.1016/j.critrevonc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Leo E., Audisio R., Belli F., Vitellaro M., Baldini M., Mascheroni L., Patuzzo R., Rigillo G., Rebuffoni G., Filiberti A., et al. Total rectal resection and colo-anal anastomosis for low rectal tumours: Comparative results in a group of young and old patients. Eur. J. Cancer. 1994;30:1092–1095. doi: 10.1016/0959-8049(94)90463-4. [DOI] [PubMed] [Google Scholar]
- 9.Ardoino I., Signoroni S., Malvicini E., Ricci M.T., Biganzoli E.M., Bertario L., Occhionorelli S., Vitellaro M. Long-term survival between total colectomy versus proctocolectomy in patients with FAP: A registry-based, observational cohort study. Tumori J. 2019;106:139–148. doi: 10.1177/0300891619868019. [DOI] [PubMed] [Google Scholar]