Abstract
The influence of cell surface hydrophobicity and electrostatic charge of bacteria on grazing rates of three common species of interception-feeding nanoflagellates was examined. The hydrophobicity of bacteria isolated from freshwater plankton was assessed by using two different methods (bacterial adhesion to hydrocarbon and hydrophobic interaction chromatography). The electrostatic charge of the cell surface (measured as zeta potential) was analyzed by microelectrophoresis. Bacterial ingestion rates were determined by enumerating bacteria in food vacuoles by immunofluorescence labelling via strain-specific antibodies. Feeding rates varied about twofold for each flagellate species but showed no significant dependence on prey hydrophobicity or surface charge. Further evidence was provided by an experiment involving flagellate grazing on complex bacterial communities in a two-stage continuous culture system. The hydrophobicity values of bacteria that survived protozoan grazing were variable, but the bacteria did not tend to become more hydrophilic. We concluded that variability in bacterial cell hydrophobicity and variability in surface charge do not severely affect uptake rates of suspended bacteria or food selection by interception-feeding flagellates.
Grazing by phagotrophic protists can be considered a major source of mortality for suspended bacteria in marine and freshwater systems. In particular, heterotrophic nanoflagellates, which feed mostly by direct interception of single bacterial cells, have been identified as the main consumers of planktonic bacteria (12, 40, 46). There is increasing interest in qualitative aspects of this important selective agent for bacterial communities, such as selective grazing and adaptation of bacteria to predation. Recent studies have revealed that grazing is an important force shaping the structure and composition of communities of planktonic bacteria (17, 20, 28, 51). Bacterial cell size is one of the major phenotypic traits which influence the predator-prey relationship between bacteria and heterotrophic nanoflagellates. Grazing rates increase with bacterial size (15, 48) due to increased encounter rates (11). On the other hand, bacteria reach a predation refuge when they grow beyond a certain size as they become morphologically inedible for nanoflagellates (21, 22). In field and laboratory studies it has been shown that size-related differences in predation vulnerability can result in shifts in bacterial species composition during enhanced protozoan grazing (28, 49).
It has been speculated that properties of bacteria other than size might also be relevant for vulnerability to grazers (27). There is, however, little evidence of effects of other phenotypic traits, such as motility (14) or the chemical surface composition (26, 31, 35, 45) of bacterial cells, on protist grazing rates. Studies of bacterial ingestion (phagocytosis) by phagocytes of mammalian immune systems have revealed that bacterial surface properties, especially hydrophobicity, strongly affect the contact probability and the ingestion process (1, 54). It has been shown that the efficiency of phagocytosis increases with the hydrophobicity of bacterial cells and that hydrophilic bacteria resist ingestion by phagocytes. It is tempting to assume that some similar principles affect the uptake of free-living bacteria by heterotrophic protists (27, 36).
On physicochemical grounds, cellular interactions between flagellates and bacterial prey are assumed to be subject to forces similar to those ruling colloidal aggregation between surfaces or particles in liquids (36). Adhesion depends on the balance of electrostatic and hydrophobic interactions that result in either attraction or repulsion between particles. The main repulsive effect often results from electrostatic interactions (24). On the other hand, the hydrophobic interaction forces are strongly attractive (25) and are determined by the ratio of hydrophilic and hydrophobic surface components. Thermodynamic approaches describe hydrophobic interactions as Helmholtz free energy relationships at the phagocyte-bacterium interface with the aqueous surroundings (55). According to these approaches, the probability that phagocytosis will be averted increases when bacteria are more hydrophilic than phagocytes as the overall free energy change becomes positive for the process of phagocytosis.
It was the aim of this study to examine the effects of electrostatic and hydrophobic cell surface properties of freshwater bacteria on the ingestion rates by several species of interception-feeding nanoflagellates. The specific hypotheses that we wanted to test were as follows: (i) electrostatic repulsion prevents grazing of bacteria with a high surface charge by making it difficult for flagellates to make surface contact; (ii) bacteria with weak surface hydrophobicity are ingested at a lower rate than bacteria with high surface hydrophobicity; and (iii) the presence of flagellate grazers selects for low overall surface hydrophobicity values in mixed bacterial assemblages.
In order to overcome the inconsistencies involved in bacterial hydrophobicity measurement (41), we used two different techniques to assess bacterial surface hydrophobicity and minimize cell manipulation prior to measurement. The effective charge of a bacterial surface was characterized by determining its zeta potential. In addition, precise measurement of bacterial ingestion rates was achieved by using an immunofluorescence technique which avoided any alteration of bacterial surface properties prior to ingestion.
MATERIALS AND METHODS
Microorganisms and cultivation.
The three nanoflagellate species used in this study were Bodo saltans, Spumella pudica, and the mixotroph Ochromonas sp., all of which are known to be interception feeders of freely suspended bacteria. B. saltans was isolated from the plankton of a mesotrophic lake (Schöhsee in northern Germany), S. pudica CCAP 930/1 was obtained from the Culture Collection of Algae and Protozoa in Windermere, United Kingdom, and Ochromonas sp. was isolated from Lake Constance in southern Germany by D. Springmann. All flagellate stock cultures were maintained in WC medium (18) supplemented with wheat grain.
Forty-one bacterial strains were isolated from different chemostat systems by using inocula obtained from plankton samples from Schöhsee. Twenty-seven of these strains were isolated from a bacterium-flagellate chemostat, in which it was assumed that there was high selection pressure from flagellate grazing. Fourteen strains were isolated from C-limited chemostats without bacterial grazers, in which it was assumed that substrate competition was the main selective force (the strains were provided by K. Beck, Plön, Germany) (2). One strain (Pseudomonas aeruginosa SG81) was isolated from the biofilm of a technical water system (provided by J. Wingender, Duisburg, Germany) (16). All isolates were stored at −70°C until they were used for cultivation.
In order to assess cell surface hydrophobicity (CSH) and electrostatic charge, bacteria were first grown in nutrient broth medium for 24 h; then they were transferred to duplicate Erlenmeyer flasks containing WC medium plus 100 mg of glucose per liter and grown to the stationary phase. All cultures were incubated on an orbital shaker at 17°C. Two subsamples of each replicate culture were subjected to the bacterial adhesion to hydrocarbon (BATH) assay. The bacteria selected for ingestion experiments were monitored by using BATH and hydrophobic interaction chromatography (HIC) procedures simultaneously. The electrostatic net charge of the cell surface (measured as zeta potential) was determined for the same bacteria.
BATH test.
The BATH assay was performed largely as described previously (43); however, the washing steps and centrifugation were omitted to avoid damaging cell surfaces (41). Briefly, 4 ml of a bacterial suspension (106 to 107 cells ml−1) was vortexed with 1 ml of n-hexadecane for at least 2 min. The aqueous and hydrocarbon phases were allowed to separate for 15 min. One milliliter of the aqueous phase was sampled carefully with a Pasteur pipette and preserved with 2% formaldehyde. The concentration of cells left in the water phase was determined by epifluorescence microscopy using DAPI (4′,6′-diamidino-2-phenylindole) staining (42). Hydrophobicity (expressed as a percentage) was calculated as follows: [(a − b)/a] × 100, where a is the initial cell concentration in the aqueous phase and b is the cell concentration in the aqueous phase after partitioning. Duplicate samples from two parallel bacterial cultures were examined. For various bacterial strains, the precision of BATH values for the stationary growth phase was tested by using two subsamples and two replicate cultures. Growth experiments were repeated three times.
HIC.
Generally following the technique developed by Smyth et al. (50), we filled Pasteur pipettes (diameter, 5 mm) plugged with glass wool with 1 ml of either Sepharose CL-4B (nonhydrophobic control) or octyl-Sepharose CL-4B (Pharmacia, Uppsala, Sweden) (hydrophobic). The resulting columns were washed with 8 ml of filter-sterilized WC medium, and replicates were used for each bacterial culture. Bacterial suspensions were diluted in WC medium to 5 × 107 cells ml−1, and subsequently, 1 ml was applied to each column. The gels were eluted with 1 ml of phosphate-buffered saline, and the eluates were fixed with formalin. Direct DAPI cell counts were used to compute a relative HIC index as described by Clark et al. (7): HIC index = (NSeph − NOctyl)/NSeph, where NSeph is the number of cells in eluates from the Sepharose column and NOctyl is the number of cells in eluates from the octyl-Sepharose column.
Determination of zeta potentials.
Zeta potentials of 14 bacterial strains were determined with a Zetasizer 3000 (Malvern Instruments Ltd., Malvern, United Kingdom), which measured electrophoretic mobility by laser Doppler velocimetry. Prior to the measurements, the Zetasizer 3000 was calibrated with a DTS5050 zeta potential standard (Malvern Instruments Ltd.). Measurements were made at a modulator frequency of 1,000 Hz at room temperature. Bacteria from stationary-phase cultures were suspended at a concentration of 106 cells ml−1 in 10−3 M KCl in order to avoid nonspecific adsorption of ions on cell surfaces. Samples were injected directly into the quartz capillary with 10-ml disposable syringes. Between sample measurements, 20 ml of a 10−3 M KCl solution was passed through the capillary cell to rinse it. Electrophoretic mobilities were determined by obtaining five readings per replicate culture at the pH of the prepared bacterial suspension (pH 5.5 ± 0.5) and were converted to zeta potential values by the Smoluchowski equation (24). The experimental reproducibility was tested three times with a randomly selected strain. After microelectrophoresis, bacterial suspensions were fixed with 2% formaldehyde and cell size and morphology were controlled by epifluorescence microscopy.
Chemostat culture.
The three nanoflagellates were grown separately in the second stage of a two-stage chemostat system. The initial bacterial inoculum for the first stage originated from prefiltered (pore size, 0.8 μm) water from Schöhsee and was cultured in a 5-liter vessel on WC medium plus 10 mg of glucose per liter. The second stage consisted of three parallel 500-ml reactors, each containing one of the three nanoflagellate species, and was fed with the bacterial suspension from the first stage at a dilution rate of 0.02 h−1. Cell abundance and overall cell hydrophobicity were measured in all four reactors at 7-day intervals over a 6-week period. Hydrophobicity was monitored by the BATH procedure mentioned above.
Ingestion experiments.
In order to test the effects of bacterial CSH and surface charge on flagellate feeding rates, 14 bacterial strains having comparable cell sizes within the flagellate prey spectrum, as well as a wide range of hydrophobicities and zeta potentials, were selected from a collection of 41 isolates. Polyclonal antibodies against these strains were developed by immunizing rabbits (Eurogentec, Herstal, Belgium).
Prior to the ingestion experiments, the bacterial strains were grown to the stationary phase (24 h), in which the bacterial cells were considered to be less variable in terms of hydrophobicity and size. The CSHs of these cultures were determined by the BATH assay and the HIC technique. Four bacterial strains representing the full range of BATH values and HIC indices available were tested to determine effects on flagellate feeding rates. Five-milliliter subsamples of the flagellate continuous culture were transferred to triplicate bottles, and the flagellates were then allowed to adapt to the experimental conditions for 5 h and to reduce the numbers of indigenous bacteria to less than 106 bacteria ml−1. The bacteria that were to be examined were added to a final concentration of 107 cells ml−1 immediately after the CSH was measured. The flagellates were incubated for 15 min at room temperature and fixed with 5 ml of ice-cold glutaraldehyde (final concentration, 2%) (47). A control bottle was fixed before the bacterial suspension was added. The ingested bacteria in 100 flagellate cells per replicate were detected directly by using strain-specific antibodies as described for a modification of the protocol of Christoffersen et al. (6). Visualization was performed by binding of Cy3-conjugated goat anti-rabbit immunoglobulin G (Jackson ImmunoResearch Laboratories, West Grove, Pa.) and by nonspecific staining with DAPI. Bacterial cell sizes were measured by using DAPI-stained preparations and an automated image analysis system (SIS GmbH, Münster, Germany).
RESULTS
BATH and HIC assays.
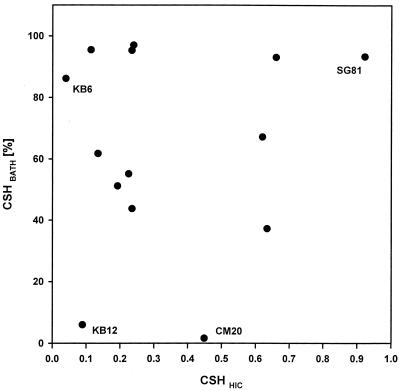
A dual hydrophobicity assay, comprising the BATH assay and the HIC technique, was conducted with 14 bacterial strains. As determined by both methods, the CSH of the bacterial strains spanned a wide range; the values ranged from 1.6 to 97.0% on the BATH scale and from 0.039 to 0.922 for HIC indices (Fig. 1). The lack of a significant correlation between the hydrophobicity values obtained with the BATH assay and HIC (r = 0.21 and P = 0.449) suggested that the data obtained by the two procedures should be treated independently. From each data set, two weakly hydrophobic bacterial strains and two strongly hydrophobic strains were selected for ingestion experiments. As determined by the BATH assay, 6% of the cells of strain KB12 and less than 2% of the strain CM20 cells adhered to the hydrocarbon; thus, these strains were the most hydrophilic bacterial strains in the set of strains used. In contrast, strains KB6 and SG81 were the most hydrophobic prey as more than 85% of the cells were retained by the hydrocarbons. The levels of hydrophobicity of strains KB12 and SG81 were confirmed by the HIC assay, whereas strain KB6 was classified as rather hydrophobic and CM20 exhibited an intermediate value. The growth experiments were repeated for the four strains, and the results did not reveal significant variability in the CSH of any bacterial strain during the stationary phase (P ≥ 0.646, as determined by t tests). The bacterial cell morphologies were within the range of those of particles that could be readily ingested by nanoflagellates; the cells were rod shaped with average lengths of 2.0 ± 0.8 μm for KB6, 2.1 ± 0.8 μm for KB12, 1.3 ± 0.3 μm for CM20, and 1.8 ± 0.6 μm for SG81 (Table 1).
FIG. 1.
Relationship between CSH assessed by the BATH assay (CSHBATH) and CSH assessed by the HIC technique (CSHHIC) for 14 freshwater bacterial isolates. All data points represent means based on duplicate measurements for two replicate cultures. The labeled data points are the data points for the four strains selected for ingestion experiments.
TABLE 1.
Zeta potentials and cell volumes of 14 bacterial isolates measured in 10−3 M KCl
| Bacterial strain | Zeta potential (mV)a | Cell vol (μm3)b |
|---|---|---|
| KB6 | −23.4 ± 1.63 | 0.35 ± 0.157 |
| KB9 | 3.2 ± 0.71 | 0.24 ± 0.067 |
| KB10 | −25.7 ± 1.34 | 0.44 ± 0.171 |
| KB12 | −36.1 ± 2.76 | 0.32 ± 0.082 |
| KB16 | 3.1 ± 0.23 | 0.12 ± 0.049 |
| KB20 | −7.3 ± 0.11 | 0.26 ± 0.057 |
| KB23 | −29.3 ± 1.84 | 0.29 ± 0.071 |
| KB27 | −6.8 ± 2.86 | 0.52 ± 0.230 |
| SG81 | −20.0 ± 1.13 | 0.32 ± 0.081 |
| SG81R1 | −24.7 ± 1.09 | 0.23 ± 0.085 |
| CM10 | −20.9 ± 0.03 | 0.29 ± 0.058 |
| CM20 | −16.6 ± 3.04 | 0.26 ± 0.057 |
| CM28 | −24.9 ± 0.54 | 0.17 ± 0.051 |
| MM1 | −15.6 ± 1.13 | 0.50 ± 0.221 |
Mean ± standard deviation based on five measurements for duplicate cultures.
Mean ± standard deviation (n ≥ 100).
Zeta potential.
The electrostatic surface charges (measured as zeta potentials) of the 14 bacterial strains also differed, ranging from 3.2 mV for strain KB9 to −36.1 mV for KB12 (Table 1). The four strains used for the ingestion experiments not only represented different categories of surface hydrophobicity but also covered a wide range of the zeta potential data set (from −16.6 to −36.1 mV), including the extremely negatively charged strain KB12. As the cell sizes of the four strains were similar, the zeta potentials were not corrected. Correlations between zeta potentials and the BATH test results (r = −0.021 and P = 0.942) or the HIC assay results (r = −0.031 and P = 0.917) were not observed.
Ingestion experiments.
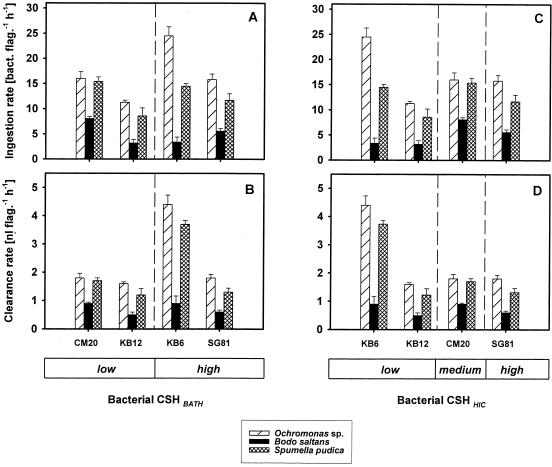
The ingestion rates determined for the four bacterial strains varied from 11.3 to 24.5 bacteria flagellate−1 h−1 for Ochromonas sp., from 8.6 to 15.4 bacteria flagellate−1 h−1 for S. pudica, and from 3.2 to 8.1 bacteria flagellate−1 h−1 for B. saltans (Fig. 2A and C). The low feeding rates for B. saltans were probably due to the larger number of indigenous bacteria in the flagellate culture, which was two times higher than the numbers of indigenous bacteria in the Ochromonas and Spumella cultures. The flagellate species and the bacterial strains had significant effects on the ingestion rate (F = 299.27 and P < 0.001 and F = 49.41 and P < 0.001, respectively, as determined by two-way analysis of variance). Clearance rates take into account slight variations in initial bacterial concentrations. Ochromonas sp. exhibited clearance rates between 1.6 and 4.4 nl flagellate−1 h−1, and S. pudica exhibited clearance rates between 1.2 and 3.7 nl flagellate−1 h−1, whereas the values for B. saltans ranged from 0.5 to 0.9 nl flagellate−1 h−1 (Fig. 2B and D).
FIG. 2.
Ingestion experiments: feeding rates of three bacterivorous flagellates on four bacterial strains having different CSHs as measured by the BATH assay (CSHBATH) (A and B) and the HIC assay (CSHHIC) (C and D). Bacterial strains KB6, KB12, CM20, and SG81 were classified into two CSH categories based on the BATH values (low and high) and into three CSH categories based on the HIC values (low, medium, and high). The ingestion and feeding rates are means based on 300 flagellate cells in three replicates. The error bars indicate standard deviations.
Due to the poor correlation of the results of the two CSH techniques, the ingestion rates measured were plotted independently for the two hydrophobicity measurements (Fig. 2). Pooled data for hydrophilic and hydrophobic strains from the BATH assay (Fig. 2A and B) revealed a significant difference only for ingestion rates and clearance rates of Ochromonas sp. (t = 2.789 and P = 0.019 and t = 2.391 and P = 0.038, respectively, as determined by t tests). On the HIC index scale, bacterial prey were classified into three categories of CSH (Fig. 2C and D). For these three categories significant differences between feeding rates were not observed for any of the flagellate species.
A relationship between the net surface electrostatic charges of the four bacterial strains and the flagellate feeding rates was not evident. The ingestion and clearance rates obtained with the most negatively charged strain, strain KB12 (zeta potential, −36.6 mV), did not differ significantly from the rates obtained with CM20 (−16.6 mV) and SG81 (−20.0 mV). The twofold-higher feeding rates obtained with Ochromonas sp. and S. pudica on strain KB6 were not related to any of the surface parameters measured.
Screening of bacterial isolates.
The CSHs of 27 strains isolated in the presence of high grazing pressure and 14 strains isolated in the absence of flagellate predators were determined in the stationary growth phase by the BATH assay. In both sets of strains a higher proportion of strongly hydrophobic bacterial strains was evident; the hydrophobicity values were 75.1% ± 26.04% and 72.6% ± 28.16%, respectively. There was no significant dependence of CSH on the presence or absence of grazing in the original enrichment culture (t = 0.279 and P = 0.782, as determined by t tests).
Chemostat cultures.
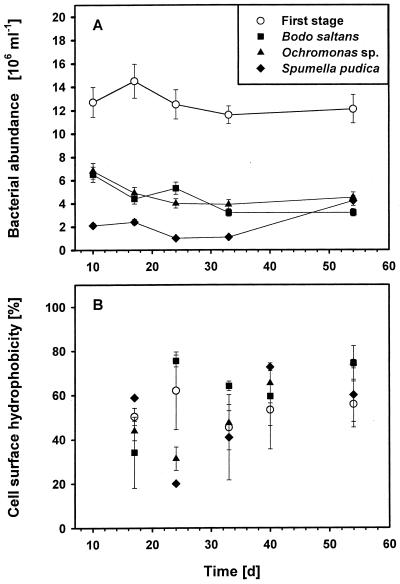
In all chemostat vessels stable steady-state populations of bacteria and the flagellate species were established for at least a 5-week period (Fig. 3A). The CSH of the bacterial community in the first-stage reservoir was compared with the CSH of the bacterial communities in the second stages with the flagellates Ochromonas sp., S. pudica, and B. saltans. CSH measurements provided no evidence that there was a shift towards a less hydrophobic bacterial community with any flagellate species (Fig. 3B). The CSHs of the bacterial communities were, however, rather variable in all reactors (coefficients of variation, >10%) and ranged from 20 to 75% during a 6-week period, but there was no significant trend. The mean hydrophobicities between the first and second stages did not differ significantly (F = 0.032 and P = 0.861, as determined by two-way analysis of variance), and those between the parallel reactors of the second stage also did not differ significantly (F = 0.450 and P = 0.721). However, the coefficients of variation for the flagellate-containing reactors (27 to 40%) indicated that there was a broader range of hydrophobicities in a grazed bacterial community.
FIG. 3.
Chemostat experiment: bacterial abundance (A) and overall CSH (B) in a two-stage chemostat (means ± standard deviations). Bacteria in the first stage were cultured without flagellate grazers, and the second-stage vessels contained B. saltans, Ochromonas sp., or S. pudica. The hydrophobicity values are means based on duplicate measurements obtained by using the BATH assay.
DISCUSSION
Methodological aspects.
Use of the BATH test and the HIC technique with subsamples of identical cultures revealed no consistent relationship between the two methods of CSH measurement. Although both procedures are most commonly employed to assess microbial CSH and both are thought to measure actual binding to hydrophobic ligands (52), a lack of correlation has been reported by other workers (9, 10, 13, 39, 52). Dillon et al. (9) and Van der Mei et al. (52) found better correspondence between the tests when they were applied to closely related strains, which suggests that the assays may measure essentially different cell properties. Hence, general conclusions regarding the impact of CSH on biotic interactions should be drawn very carefully.
Similar to findings obtained with other bacteria (44), we found no clear relationship between CSH and electrostatic charge, which might have been due to the compensating effect of positively charged moieties on the cell surface.
Flagellate grazing on bacteria with different surface properties.
The present study demonstrated that three common species of freshwater nanoflagellates fed on bacteria having very different physicochemical surface properties at comparable rates. Differences in the electrostatic surface charges of the bacteria did not influence the flagellate feeding rates. Moreover, we found no evidence that less hydrophobic bacteria are less vunerable to ingestion by flagellates. The twofold variation in the feeding rates for each flagellate species was related neither to CSH values measured by the BATH assay nor to the HIC index. Bacterial cell size did not influence the outcome.
Zeta potentials revealed that all of the test bacteria had negative net charges, and the values were within the range of values found for other bacterial isolates (13, 52). Lower ingestion rates for highly negatively charged particles were not observed. To date, this phenomenon has been described only by Hammer et al. (23), who compared the effects of artificial and natural food items with different surface charges on feeding rates of the heterotrophic dinoflagellate Oxyrrhis marina. The zeta potentials of these particles, however, were probably outside the range found in bacteria.
Our findings on hydrophobic interactions do not seem to conform to data from studies on bacterial uptake by mammalian leukocytes (1, 54) nor to theoretical considerations concerning the underlying thermodynamic mechanism of nonspecific phagocytosis (36, 55), which suggest that more hydrophilic food items are discriminated. However, they are consistent with the study of Lock et al. (33), which showed that a hydrophilic strain of Escherichia coli resisted ingestion by leukocytes but not by amoebae. There are several factors that make comparisons of studies of different organisms more difficult. One major component determining the extent of bacterial ingestion is the balance between the relative surface tension of the phagocytic cell and the surface tensions of the bacteria and the suspending medium (1). For instance, bacteria exhibiting low CSH values still might be more hydrophobic than the flagellate cell surface, so that engulfment of these bacteria is thermodynamically favored. In addition, the plasticity of protozoan surface properties should be taken into consideration as a function of the physiological state or the life cycle. The ciliate Paramecium caudatum has been reported to alter its CSH during the early stage of reproduction (29). In our study, we assumed that the chemostat cultures of B. saltans, S. pudica, and Ochromonas sp. provided flagellate cells of constant CSH variability, although currently there is no technique available to measure CSH in flagellates. The fact that starved flagellates did not differ in bacterial CSH preferences from growing cells (Matz, unpublished data) indicates, however, that there is little variation in flagellate hydrophobicity.
Apart from merely thermodynamic considerations, attention also has to be paid to the different feeding types found in phagotrophic protists and in other phagocytic cells. Studies on the role of prey hydrophobicity in food selectivity (1, 19, 33, 37, 54) have revealed at least three different feeding types and have led to rather heterogeneous results. For example, Gurijala and Alexander (19) described better survival of highly hydrophobic bacteria in the presence of the ciliate Tetrahymena thermophila. Different feeding types may imply that there are profound differences in interaction forces between predator and prey. In Spumella, Ochromonas, and Bodo food capture is mediated by folding over at least one of the two flagella immediately after prey contact (4). Hence, it is conceivable that the contact and capture rate may depend not only on hydrophobic or electrostatic interaction forces but also on rather mechanical processes.
The observation that severe flagellate grazing in the second stage of the chemostat system did not result in a shift in the bacterial community towards lower levels of hydrophobicity also provided indirect evidence that CSH plays a minor role as a mechanism of resistance against flagellates. Higher coefficients of variation in flagellate treatments, however, reflected stronger community dynamics. Whether this variation is causally linked to factors other than grazing pressure, such as recycled nutrients (30, 32, 34), or to an increase in bacterial growth rates (53) remains unclear so far. A wide range of CSH was recorded for isolates from freshwater bacterioplankton, which is in accordance with studies of other aquatic habitats (8, 30) and might also explain the community scatter found in our chemostat culture. The mean CSH for bacterial isolates and chemostat data may provide evidence that the majority of freshwater bacteria are rather hydrophobic.
The study of Monger et al. (37) is the only investigation so far that has dealt with the impact of prey hydrophobicity in heterotrophic nanoflagellates. In addition to a positive correlation between clearance rates of the marine nanoflagellate Paraphysomonas bandiensis and the hydrophobicities of Prochlorococcus prey, these authors found a considerable scatter of clearance rates for Prochlorococcus cells exhibiting the same hydrophobicity. This indicates that Prochlorococcus cell size had an impact but might also have been the result of interfering cell surface properties that were not investigated. It has been shown that the use of surface hydrophobicity and zeta potential as overall cell surface parameters can mask substantial differences in the chemical compositions of cell surfaces (5, 38). Since chemically mediated prey selection has been observed in bacterivorous protists (3, 26, 31, 56), more specific selection behavior might account for the inconsistent findings for the role of CSH in protozoan-bacterium interactions.
In summary, our data provided no evidence that low surface hydrophobicity (or at least surface characteristics measured by the BATH and HIC methods) and highly negative zeta potentials of bacterial cells substantially reduce mortality due to interception-feeding freshwater flagellates. Since differences in bacterial CSH and electrostatic charge could not account for the variation in feeding rates, other factors, such as the specific surface composition, might be more important than nonspecific hydrophobicity in determining food selection by bacterivorous flagellates and require further investigation.
ACKNOWLEDGMENTS
This work was supported by grant Ju 367/2-1 from the Deutsche Forschungsgemeinschaft.
We thank Cordula Grüttner (micromod Partikeltechnologie GmbH, Rostock-Warnemünde, Germany) and Alessandra Montorro-Wilck for technical assistance during this study.
REFERENCES
- 1.Absolom D R. The role of bacterial hydrophobicity in infection: bacterial adhesion and phagocytic ingestion. Can J Microbiol. 1988;34:287–298. doi: 10.1139/m88-054. [DOI] [PubMed] [Google Scholar]
- 2.Beck K. Experimentelle Überprüfung der “Intermediate disturbance hypothesis” (Connell 1978) an Modell-Lebensgemeinschaften planktischer Bakterien. Ph.D. dissertation. Kiel, Germany: University of Kiel; 2000. [Google Scholar]
- 3.Bennett S J, Sanders R W, Porter K G. Chemosensory responses of heterotrophic and mixotrophic flagellates to potential food sources. Bull Mar Sci. 1988;43:764–771. [Google Scholar]
- 4.Boenigk J, Arndt H. Particle handling during interception feeding by four species of heterotrophic nanoflagellates. J Eukaryot Microbiol. 2000;47:350–358. doi: 10.1111/j.1550-7408.2000.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 5.Boonaert C J P, Rouxhet P G. Surface of lactic acid bacteria: relationships between chemical composition and physicochemical properties. Appl Environ Microbiol. 2000;66:2548–2554. doi: 10.1128/aem.66.6.2548-2554.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christoffersen K, Nybroe O, Jürgens K, Hansen M. Measurement of bacterivory by heterotrophic nanoflagellates using immunofluorescence labelling of ingested cells. Aquat Microb Ecol. 1997;13:127–134. [Google Scholar]
- 7.Clark W B, Lane M D, Beem J E, Bragg S L, Wheeler T T. Relative hydrophobicities of Actinomyces viscosus and Actinomyces naeslundii strains and their adsorption to saliva-treated hydroxyapatite. Infect Immun. 1985;47:730–736. doi: 10.1128/iai.47.3.730-736.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahlbäck B, Hermansson M, Kjelleberg S, Norkrans B. The hydrophobicity of bacteria—an important factor in their initial adhesion at the air-water interface. Arch Microbiol. 1981;128:267–270. doi: 10.1007/BF00422527. [DOI] [PubMed] [Google Scholar]
- 9.Dillon J K, Fuerst J A, Hayward A C, Davis G H G. A comparison of five methods for assaying bacterial hydrophobicity. J Microbiol Methods. 1986;6:13–19. [Google Scholar]
- 10.Donlon B, Collaran E. A comparison of different methods to determine the hydrophobicity of acetogenic bacteria. J Microbiol Methods. 1993;17:27–37. [Google Scholar]
- 11.Fenchel T. Ecology of heterotrophic microflagellates. I. Some important forms and their functional morphology. Mar Ecol Prog Ser. 1982;8:211–223. [Google Scholar]
- 12.Fenchel T. Ecology of heterotrophic microflagellates. IV. Quantitative occurrence and importance as bacterial consumers. Mar Ecol Prog Ser. 1982;9:35–42. [Google Scholar]
- 13.Gannon J T, Manilal V B, Alexander M. Relationship between cell surface properties and transport of bacteria through soil. Appl Environ Microbiol. 1991;57:190–193. doi: 10.1128/aem.57.1.190-193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González J M, Sherr E B, Sherr B F. Differential feeding by marine flagellates on growing versus starving, and on motile versus nonmotile, bacterial prey. Mar Ecol Prog Ser. 1993;102:257–267. [Google Scholar]
- 15.González J M, Sherr E B, Sherr B F. Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl Environ Microbiol. 1990;56:583–589. doi: 10.1128/aem.56.3.583-589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grobe S, Wingender J, Trüper H G. Characterization of mucoid Pseudomonas aeruginosa strains isolated from technical water systems. J Appl Bacteriol. 1995;79:94–102. doi: 10.1111/j.1365-2672.1995.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 17.Güde H. The role of grazing on bacteria in plankton succession. In: Sommer U, editor. Plankton ecology: succession in plankton communities. Berlin, Germany: Springer; 1989. pp. 337–364. [Google Scholar]
- 18.Guillard R R L, Lorenzen C J. Yellow-green algae with chlorophyllide c. J Phycol. 1972;8:10–14. [Google Scholar]
- 19.Gurijala K R, Alexander M. Effect of growth rate and hydrophobicity on bacteria surviving protozoan grazing. Appl Environ Microbiol. 1990;56:1631–1635. doi: 10.1128/aem.56.6.1631-1635.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn M W, Höfle M G. Flagellate predation on a bacterial model community: interplay of size-selective grazing, specific bacterial cell size, and bacterial community composition. Appl Environ Microbiol. 1999;65:4863–4872. doi: 10.1128/aem.65.11.4863-4872.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn M W, Höfle M G. Grazing pressure by a bacterivorous flagellate reverses the relative abundance of Comamonas acidovorans PX54 and Vibrio strain CB5 in chemostat cocultures. Appl Environ Microbiol. 1998;64:1910–1918. doi: 10.1128/aem.64.5.1910-1918.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn M W, Moore E R B, Höfle M G. Bacterial filament formation, a defense mechanism against flagellate grazing, is growth rate controlled in bacteria of different phyla. Appl Environ Microbiol. 1999;65:25–35. doi: 10.1128/aem.65.1.25-35.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammer A, Grüttner C, Schumann R. The effect of electrostatic charge of food particles on capture efficiency by Oxyrrhis marina Dujardin (dinoflagellate) Protist. 1999;150:375–382. doi: 10.1016/S1434-4610(99)70039-8. [DOI] [PubMed] [Google Scholar]
- 24.Hunter R J. Introduction to modern colloid science. Oxford, United Kingdom: Oxford University Press; 1993. [Google Scholar]
- 25.Israelachvili J N, McGuiggan P M. Forces between surfaces in liquids. Science. 1988;241:795–800. doi: 10.1126/science.241.4867.795. [DOI] [PubMed] [Google Scholar]
- 26.Jürgens K, De Mott W R. Behavioral flexibility in prey selection by bacterivorous nanoflagellates. Limnol Oceanogr. 1995;40:1503–1507. [Google Scholar]
- 27.Jürgens K, Güde H. The potential importance of grazing-resistant bacteria in planktonic systems. Mar Ecol Prog Ser. 1994;112:169–188. [Google Scholar]
- 28.Jürgens K, Pernthaler J, Schalla S, Amann R. Morphological and compositional changes in a planktonic bacterial community in response to enhanced protozoan grazing. Appl Environ Microbiol. 1999;65:1241–1250. doi: 10.1128/aem.65.3.1241-1250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamura A. Evidence for an increase in the hydrophobicity of the cell surface during sexual interactions of Paramecium. Cell Struct Funct. 1984;9:91–95. [Google Scholar]
- 30.Kjelleberg S, Hermansson M. Starvation-induced effects on bacterial surface characteristics. Appl Environ Microbiol. 1984;48:497–503. doi: 10.1128/aem.48.3.497-503.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landry M R, Lehner-Fournier J M, Sundstrom J A, Fagerness L, Selph K E. Discrimination between living and heat-killed prey by a marine zooflagellate, Paraphysomonas vestita (Stokes) J Exp Mar Biol Ecol. 1991;146:139–151. [Google Scholar]
- 32.Lemke M J, Churchill P F, Wetzel R G. Effect of substrate and cell surface hydrophobicity on phosphate utilization in bacteria. Appl Environ Microbiol. 1995;61:913–919. doi: 10.1128/aem.61.3.913-919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lock R, Öhman L, Dahlgren C. Phagocytic recognition mechanisms in human granulocytes and Acanthamoeba castellani using type 1 fimbriated Escherichia coli as phagocytic prey. FEMS Microbiol Lett. 1987;44:135–140. [Google Scholar]
- 34.McEldowney S, Fletcher M. Effect of growth conditions and surface characteristics of aquatic bacteria on their attachment to solid surfaces. J Gen Microbiol. 1986;132:513–523. [Google Scholar]
- 35.Mischke U. Influence of food quality and quantity on ingestion and growth rates of three omnivorous heterotrophic flagellates. Mar Microb Food Webs. 1994;8:125–143. [Google Scholar]
- 36.Monger B C, Landry M R. Direct-interception feeding by marine zooflagellates: the importance of surface and hydrodynamic forces. Mar Ecol Prog Ser. 1990;65:123–140. [Google Scholar]
- 37.Monger B C, Landry M R, Brown S L. Feeding selection of heterotrophic marine nanoflagellates based on the surface hydrophobicity of their picoplankton prey. Limnol Oceanogr. 1999;44:1917–1927. [Google Scholar]
- 38.Mozes N, Léonard A J, Rouxhet P G. On the relations between the elemental surface composition of yeasts and bacteria and their charge and hydrophobicity. Biochim Biophys Acta. 1988;945:324–334. doi: 10.1016/0005-2736(88)90495-6. [DOI] [PubMed] [Google Scholar]
- 39.Mozes N, Rouxhet P G. Methods for measuring hydrophobicity of microorganisms. J Microbiol Methods. 1987;6:99–112. [Google Scholar]
- 40.Pace M L. Bacterial mortality and the fate of bacterial production. Hydrobiologia. 1988;159:41–49. [Google Scholar]
- 41.Pembrey R S, Marshall K C, Schneider R P. Cell surface analysis techniques: what do cell preparation protocols do to cell surface properties? Appl Environ Microbiol. 1999;65:2877–2894. doi: 10.1128/aem.65.7.2877-2894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porter K G, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 43.Rosenberg M. Bacterial adherence to hydrocarbons: a useful technique for studying cell surface hydrophobicity. FEMS Microbiol Lett. 1984;22:289–295. [Google Scholar]
- 44.Rosenberg M, Doyle R J. Microbial cell surface hydrophobicity: history, measurement, and significance. In: Doyle R J, Rosenberg M, editors. Microbial cell surface hydrophobicity. Washington, D.C.: American Society for Microbiology; 1990. pp. 1–37. [Google Scholar]
- 45.Sanders R W. Feeding by Cyclidium sp. (Ciliophora, Scuticociliatida) on particles of different sizes and surface properties. Bull Mar Sci. 1988;43:446–457. [Google Scholar]
- 46.Sanders R W, Caron D A, Berninger U-G. Relationships between bacteria and heterotrophic nanoplankton in marine and fresh waters: an inter-system comparison. Mar Ecol Prog Ser. 1992;86:1–14. [Google Scholar]
- 47.Sanders R W, Porter K G, Bennett S J, DeBiase A E. Seasonal patterns of bacterivory by flagellates, cilliates, rotifers, and cladocerans in a freshwater plankton community. Limnol Oceanogr. 1989;34:673–687. [Google Scholar]
- 48.Šimek K, Chrzanowski T H. Direct and indirect evidence of size-selective grazing on pelagic bacteria by freshwater nanoflagellates. Appl Environ Microbiol. 1992;58:3715–3720. doi: 10.1128/aem.58.11.3715-3720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Šimek K, Vrba J, Pernthaler J, Posch T, Hartman P, Nedoma J, Psenner R. Morphological and compositional shifts in an experimental bacterial community influenced by protists with contrasting feeding modes. Appl Environ Microbiol. 1997;63:587–595. doi: 10.1128/aem.63.2.587-595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smyth C J, Jonsson P, Olsson E, Söderlind O, Hjertén S, Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978;22:462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sommaruga R, Psenner R. Permanent presence of grazing-resistant bacteria in a hypertrophic lake. Appl Environ Microbiol. 1995;61:3457–3459. doi: 10.1128/aem.61.9.3457-3459.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van der Mei H C, Weerkamp A H, Busscher H J. A comparison of various methods to determine hydrophobic properties of streptococcal cell surfaces. J Microbiol Methods. 1987;6:277–287. [Google Scholar]
- 53.Van Loosdrecht M C M, Lyklema J, Norde W, Schraa G, Zehnder A J B. Electrophoretic mobility and hydrophobicity as a measure to predict the intial steps of bacterial adhesion. Appl Environ Microbiol. 1987;53:1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Oss C J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]
- 55.Van Oss C J, Gillman C F, Neumann A W. Phagocytic engulfment and cell adhesiveness as cellular surface phenomena. New York, N.Y: Marcel Dekker, Inc; 1975. [Google Scholar]
- 56.Verity P G. Feeding in planktonic protozoans: evidence for non-random acquisition of prey. J Protozool. 1991;38:69–76. [Google Scholar]