Abstract
BACKGROUND
Excess neurological stress by hemorrhagic stoke induces cardiomyopathy, namely takotsubo cardiomyopathy. Here, the authors report a case of takotsubo myopathy following mechanical thrombectomy for acute large vessel occlusion.
OBSERVATIONS
A 73-year-old man was emergently brought to the authors’ hospital because of left hemiparesis and consciousness disturbance. An ischemic lesion of the right cerebral hemisphere and the right internal carotid artery occlusion was revealed. Emergently, endovascular treatment was performed, and occlusion of the artery was reanalyzed. However, he suffered from hypotension with electrocardiogram abnormality. Subsequently, coronary angiography was performed, but the arteries were patent. The authors made a diagnosis of takotsubo cardiomyopathy.
LESSONS
Endovascular recanalization for large cerebral artery occlusion is so effective that it is becoming widely used. Even in the successful recanalization, we need to care for the takotsubo cardiomyopathy.
Keywords: acute ischemic stroke, mechanical thrombectomy, takotsubo cardiomyopathy
ABBREVIATIONS : ICA = internal carotid artery, MCA = middle cerebral artery, MR = magnetic resonance, MRI = magnetic resonance imaging, SAH = subarachnoid hemorrhage
Takotsubo cardiomyopathy is characterized by transient left ventricular apical ballooning that resembles a Japanese octopus catcher pot (takotsubo) with a short narrow neck and round bottom.1 Central nervous system disorders most frequently triggering takotsubo cardiomyopathy include subarachnoid hemorrhage (SAH), intracerebral bleeding, epilepsy, migraine, and ischemic stroke. Here, we report a case of takotsubo cardiomyopathy following mechanical thrombectomy for acute ischemic stroke.
Illustrative Case
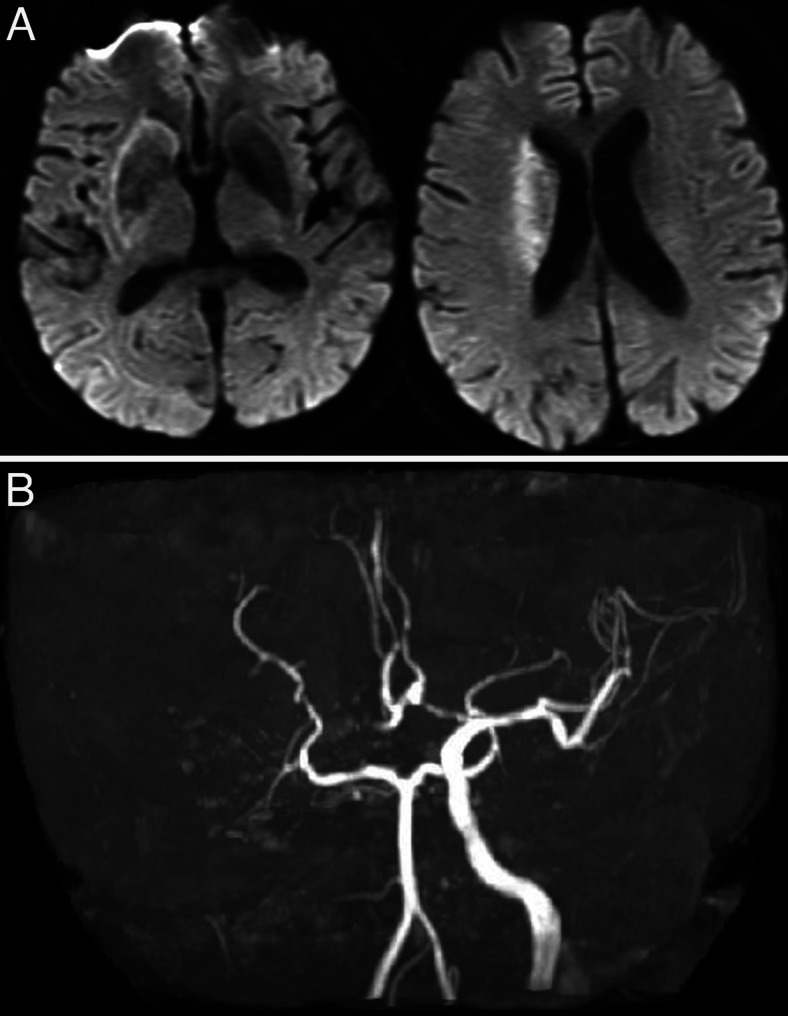
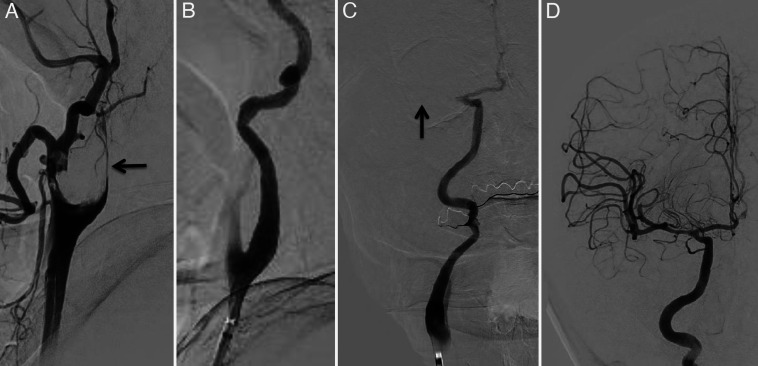
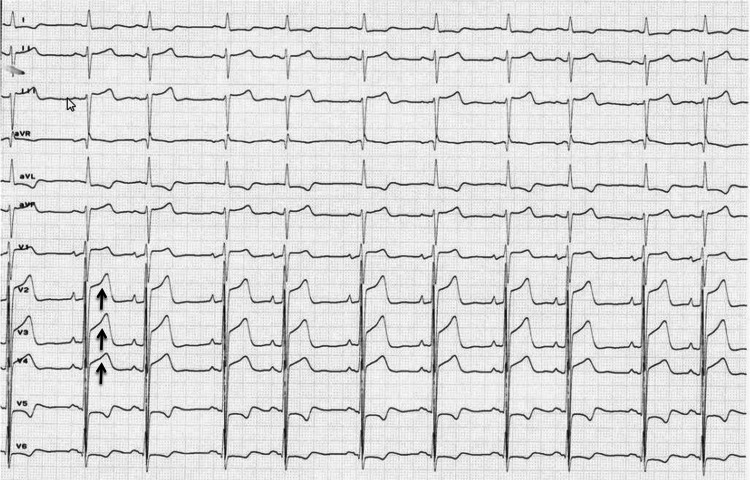
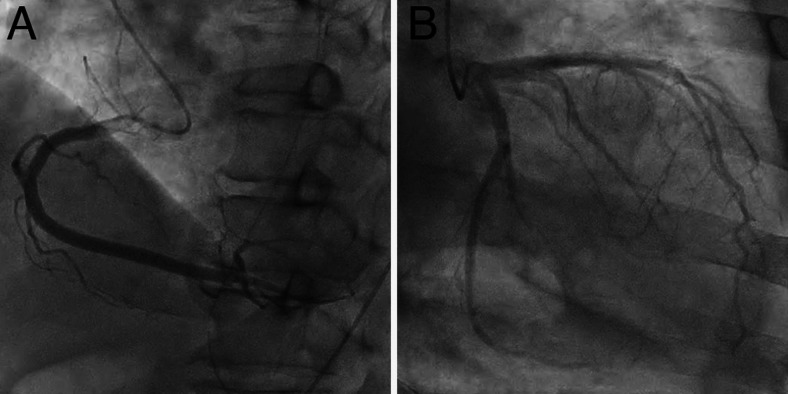
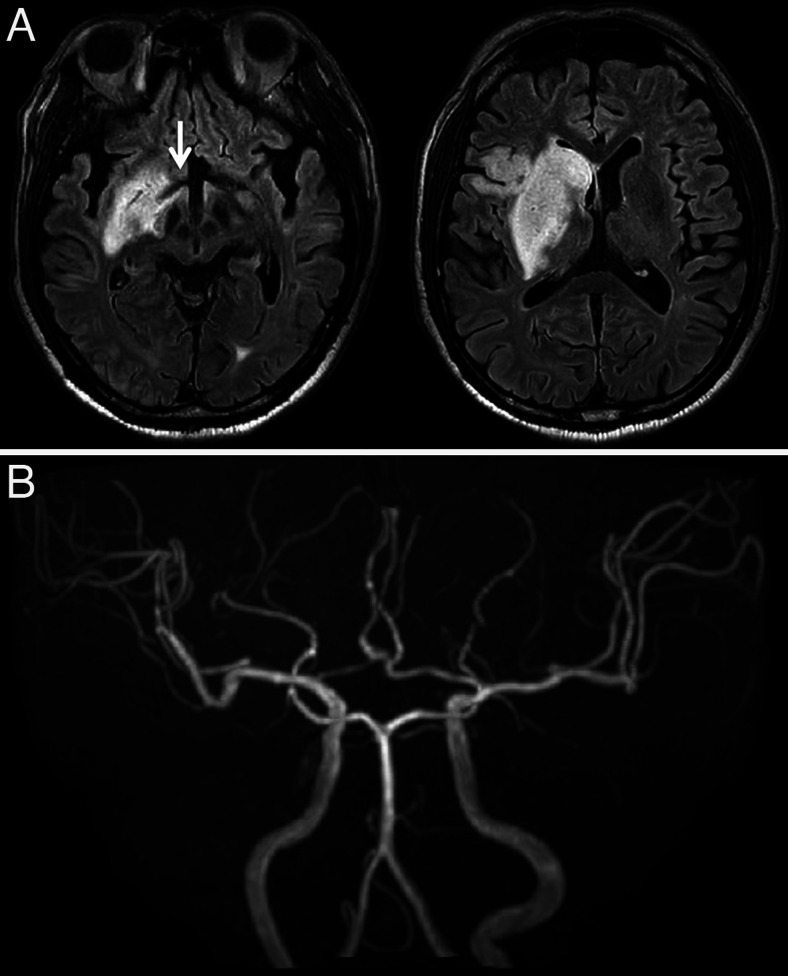
A 73-year-old man was emergently brought to our hospital because of left hemiparesis. The patient’s blood pressure was 152/86 mmHg, and his heart rate was 83 per minutes. Neurological examination showed mild consciousness disturbance, left hemiplegia, and conjugated deviation to the right. The National Institutes of Health stroke scale was 21 points. Brain magnetic resonance imaging (MRI) diffusion-weighted image showed a slightly high-intensity lesion in the right basal ganglia, indicating acute ischemic stroke (Fig. 1A). Neither the right internal carotid artery (ICA) nor the middle cerebral artery (MCA) was visualized with MR angiography (Fig. 1B). Following intravenous injection of tissue plasminogen activator, endovascular treatment was carried out. The right carotid angiography showed occlusion at the origin of the right ICA (Fig. 2A). The occlusion was reanalyzed with aspiration of the thrombus (Fig. 2B), and angiography revealed occlusion at the M1 portion of the right MCA (Fig. 2C). The thrombectomy was performed using stent-retriever, and occlusion was successfully reanalyzed (Fig. 2D). In each step, blood was aspirated from the guiding catheter to prevent distal thrombus migration, and a total of 100 mL of blood was aspirated. At the end of the procedures, the patient’s systolic blood pressure decreased to 70 mmHg, and an ST elevation in V2–4 was found on electrocardiogram (Fig. 3). Transthoracic echocardiogram revealed left ventricular apical hypokinesis. We suspected the cause as acute myocardial infarction by the emboli, and coronary angiography was performed subsequently. However, the coronary arteries were patent (Fig. 4), and serum troponin I was within normal range (0.06 ng/mL). We made a diagnosis of takotsubo cardiomyopathy and provided treatment with atropine sulfate, beta-blocker, and free radical scavenger. Cardiac function recovered 1 week later. Postoperative MRI showed infarction in the right hypothalamus as well as the right basal ganglia including insula cortex (Fig. 5A). Both MCA an ICA were visualized sufficiently (Fig. 5B). The left hemiparesis was improved after 3 months rehabilitation, and he returned to usual daily life.
FIG. 1.
MRI on admission. A: Diffusion-weighted image shows a slightly high-intensity lesion in the right basal ganglia. B: MR angiography shows an occlusion of the right ICA as well as MCA.
FIG. 2.
Endovascular treatment. A: Lateral view of the right carotid angiography shows occlusion of the ICA (arrow). B: Lateral view of the right carotid angiography shows recanalization of the ICA occlusion. C: A-P view of the right carotid angiography shows occlusion of the MCA (arrow). D: Postoperative right carotid angiography shows recanalization of the MCA as well as ICA.
FIG. 3.
Electrocardiogram at the end of thrombectomy. The ST-elevation was noted in V2–4 (arrows).
FIG. 4.
Coronary angiography. A: Right coronary angiography shows no apparent stenosis. B: Left coronary angiography shows no apparent stenosis.
FIG. 5.
Postoperative MRI. A: Fluid attenuated inversion recovery (FLAIR) image shows infarction in the right hypothalamus (arrow) as well as the right basal ganglia including insular cortex. B: MR angiography shows that both right ICA and MCA are patent.
Discussion
Observations
Clinical symptoms of takotsubo cardiomyopathy mimic those of acute myocardial infarction. However, coronary artery stenosis is not seen in takotsubo cardiomyopathy.2,3 In this case, firstly, we suspected acute myocardial infarction by the emboli as same etiology with cerebral infarction and evaluated coronary artery, but stenosis was not found.
Takotsubo cardiomyopathy is a well-known complication of SAH; massive catecholamine release caused by the hemorrhage may trigger the cardiomyopathy.4 It has been reported that takotsubo cardiomyopathy occurs in 4.4% of SAH.5 The predominant patient phenotype of cardiomyopathy was postmenopausal females and elders. Patients who developed takotsubo cardiomyopathy had a higher neurological severity grading on presentation. The worse grading of SAH might be a triggering factor for takotsubo cardiomyopathy occurrence in such patients through an increase in the catecholamine surge.5
Of the 569 consecutive patients who were admitted to the stroke center within 24 hours after onset of acute ischemic stroke, 7 patients (1.2%) were diagnosed as having takotsubo cardiomyopathy,6 which usually occurred soon after the onset of stroke and was mostly asymptomatic.6 Female sex and insular damage were predominant features of patients who complicated with takotsubo cardiomyopathy. Patients with an insular infarct (particularly right side) have been reported to show decreased heart rate variability and an increased incidence of complex arrhythmias and sudden death.7 Therefore, the insular cortex appears to play a major role in autonomic control of cardiac activity.8 In this case, perioperative imaging study demonstrated involvement of the right insula cortex (Fig. 5A). Furthermore, hypothalamic–pituitary–adrenal axis might link takotsubo cardiomyopathy.9 Hypothalamus is supplied from perforating arteries from A1 portion of the anterior cerebral artery. In this case, the right A1 portion was not visualized on MR angiography on admission, and follow-up MRI revealed involvement of hypothalamus (Fig. 5A).
In addition to intracranial large vessel occlusion, the origin of the ICA was occluded in this case, and the thrombus was firstly removed. Procedure in that region affected the carotid blub, known as autonomic pathway, might result in takotsubo cardiomyopathy. During the recanalization, a total of 100 mL of blood was aspirated to prevent thrombus migration, and the physical stress might have stimulated release of catecholamine.10
Another etiology is reperfusion injury. Mechanical thrombectomy for acute large vessel occlusion has recently become a standard procedure following the development of aspiration catheters and stent retrievers. Emergent recanalization rescues the ischemic penumbra. However, the basal ganglia is supplied by lenticulostriate arteries and is less tolerant for ischemia because of poor collateral circulation. In addition to parenchymal damage, cerebral vessels are also damaged, leading to vasoparalysis. Finally, recanalization of the occluded vessel results in hyperperfusion or reperfusion injury. We treated the patients with atropine sulfate, beta-blocker, and free radical scavenger, and cardiomyopathy improved soon thereafter. Usually, the prognosis of takotsubo cardiomyopathy is favorable.2
In the case of major stroke, patients become comatose and could not report their symptoms. Takotsubo cardiomyopathy may not be a rare complication of acute ischemic stroke. We need to be aware of the signs of cardiomyopathy, even after successful recanalization.
Lessons
A case of cardiomyopathy following mechanical thrombectomy for acute ischemic stroke has been reported. Even in successful recanalization, we need to assess for takotsubo cardiomyopathy.
Acknowledgments
We thank the individuals who contributed to the study or manuscript preparation but who do not fulfill all the criteria of authorship.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Yamasaki, Hayashi, Shibata, Furuta, Uchimura, Kambara, Nagai. Acquisition of data: Yamasaki, Hayashi, Furuta, Yamamoto, Nakagawa, Kambara, Yoshikane, J Tanabe. Analysis and interpretation of data: Yamasaki, Hayashi, Furuta, Uchimura, Akiyama, K Tanabe. Drafting the article: Yamasaki, Kambara. Critically revising the article: Yamasaki, Hayashi, Uchimura, Kambara, Nagai. Reviewed submitted version of manuscript: Yamasaki, Hayashi, Uchimura, Nagai. Approved the final version of the manuscript on behalf of all authors: Yamasaki. Administrative/technical/material support: Uchimura, Fujiwara, Nagai, Akiyama. Study supervision: Yoshikane.
References
- 1. Ishikawa K. “Takotsubo” cardiomyopathy A syndrome characterized by transient left ventricular apical ballooning that mimics the shape of a bottle used for trapping octopus in Japan. Intern Med. 2004;43(4):275–276. doi: 10.2169/internalmedicine.43.275. [DOI] [PubMed] [Google Scholar]
- 2. Dawson DK. Acute stress-induced (takotsubo) cardiomyopathy. Heart. 2018;104(2):96–102. doi: 10.1136/heartjnl-2017-311579. [DOI] [PubMed] [Google Scholar]
- 3. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 4. Kurisu S, Kihara Y. Tako-tsubo cardiomyopathy: clinical presentation and underlying mechanism. J Cardiol. 2012;60(6):429–437. doi: 10.1016/j.jjcc.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 5. Elgendy AY, Elgendy IY, Mansoor H, Mahmoud AN. Clinical presentations and outcomes of Takotsubo syndrome in the setting of subarachnoid hemorrhage: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2018;7(3):236–245. doi: 10.1177/2048872616679792. [DOI] [PubMed] [Google Scholar]
- 6. Yoshimura S, Toyoda K, Ohara T, et al. Takotsubo cardiomyopathy in acute ischemic stroke. Ann Neurol. 2008;64(5):547–554. doi: 10.1002/ana.21459. [DOI] [PubMed] [Google Scholar]
- 7. Tokgözoglu SL, Batur MK, Topçuoglu MA, Saribas O, Kes S, Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke. 1999;30(7):1307–1311. doi: 10.1161/01.str.30.7.1307. [DOI] [PubMed] [Google Scholar]
- 8. Sander D, Klingelhöfer J. Changes of circadian blood pressure patterns and cardiovascular parameters indicate lateralization of sympathetic activation following hemispheric brain infarction. J Neurol. 1995;242(5):313–318. doi: 10.1007/BF00878874. [DOI] [PubMed] [Google Scholar]
- 9. Schnabel RB, Hasenfuß G, Buchmann S, et al. Heart and brain interactions: pathophysiology and management of cardio-psycho-neurological disorders. Herz. 2021;46(2):138–149. doi: 10.1007/s00059-021-05022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finsterer J, Stöllberger C. Central nervous system (CNS) disease triggering Takotsubo syndrome. 2016;5 doi: 10.1016/j.ijcard.2016.08.185. DOI: 10.17987/icfj.v5i0.183. [DOI] [PubMed] [Google Scholar]