Abstract
Background
Kidney donation after circulatory death (DCD) follows confirmation of death using cardiorespiratory criteria, while donation after brain death (DBD) uses neurological criteria. DBD and DCD donors are the main sources of grafts for transplantation. This retrospective cohort study from a single center in the Czech Republic aimed to compare 5-year post-transplantation outcomes after DCD and DBD transplantation without pre-mortem heparin administration.
Material/Methods
A total of 227 recipients with matched donors enrolled in the transplantation program at our institution between 2015 and 2019 were analyzed. Following the application of the inclusion criteria, 99 recipients and 94 matched donors were finally included in the study.
Results
The duration of cold ischemia (median 961 vs 1100 min, P=0.028) and the perfusion with the preservation solution (median 11 vs 22 min, P<0.001) was statistically significantly shorter in DBD than in DCD grafts. The 1-year survival rates were 97.5% (95% CI 94.1–100.0%) and 90.0% (95% CI: 77.8–100.0%) for DBD and DCD recipients, respectively. The 3-year survival rates were 91.9 (95% CI: 86.0–98.4) and 90.0 (95% CI: 77.8–100.0) for the DBD and DCD groups, respectively. The overall difference in survival between the 2 groups of patients was not statistically significant (P=0.750) nor was disease-free survival (P=0.370).
Conclusions
This retrospective study from a single center showed similar 5-year results after kidney transplantation for DCD and DBD donors without pre-mortem heparin administration, including the time to graft failure and patient survival.
Keywords: Allografts, Kidney Diseases, Kidney Transplantation, Organ Transplantation, Renal Insufficiency
Background
Kidney transplantation is an integral part of the care of patients with chronic renal failure. Kidney graft transplantation substantially improves the quality of life by eliminating the need for regular dialysis. Given the prevalence of arterial hypertension, diabetes mellitus, or chronic glomerulopathies in the population, the number of patients potentially indicated for renal transplantation is quite high. Finding a suitable kidney donor is therefore often a lengthy process that significantly prolongs the time to transplantation for potential recipients, despite a good national transplant policy and effective coordination of the transplantation program. Although in the past only donors after brain death (DBD) were considered for inclusion in the transplantation program, efforts to expand the donor pool have also led to the inclusion of donors with circulatory failure (donors after circulatory death, DCD) [1] or those with a combination of both conditions (donor after brain death followed by circulatory death, DBCD) [2]. Although the latter is rarely used in the Czech Republic, both DBD and DCD kidney transplants are routinely used in national transplant centers. Kidney donation after circulatory death (DCD) follows confirmation of death using cardiorespiratory criteria, and donation after brain death (DBD) uses neurological criteria. The delayed onset of graft function (delayed graft function) in DCD is the main difference between the methods [3]; other studies reported comparable results for both methods [4,5]. Therefore, this retrospective study from a single center in the Czech Republic aimed to compare 5-year post-transplantation results after DCD and DBD, without pre-mortem administration of heparin.
Material and Methods
This study was conducted according to the principles of the Declaration of Helsinki. Informed consent to participate in the study was obtained from all study participants. This study was approved by a local ethics committee of the University Hospital of Ostrava.
Data from the 227 kidney recipients enrolled in the kidney transplantation program at the University Hospital of Ostrava in 2015–2019, as well as those of donors, were retrospectively analyzed. To eliminate confounding factors, only recipients (and relevant donors) who received a graft harvested at Ostrava University Hospital were included in the study, and the remaining patients (grafts harvested or transplantations performed in institutions) were excluded. After this selection, a total of 99 transplant recipients and 94 matched donors (in 5 cases, both kidneys of the same donor were used for different patients) were included in the study. During the 5-year follow-up period, 1 or 2 kidneys were harvested from 77 donors after brain death (DBD group) and 17 donors after circulatory death (DCD group).
Donors After Brain Death
In donors with DBD, brain death is determined primarily by scintigraphy. In several cases, in our study, angiography or transcranial Doppler assessment was used, according to current national recommendations. Kidneys are then harvested traditionally – the donor is transported to the operating room and the laparotomy is performed. Depending on the evaluation made by the transplant coordinator, suitable organs are harvested for transplant purposes, kidneys usually being the last organ system to be harvested. In situ kidney perfusion with 10 L of Custodiol preservation solution (Dr. Franz Köhler Chemie GMBH, Bensheim, GER) is performed. The appropriate kidney grafts are then harvested in a standard surgical manner and placed in a transport box, connected to pulsatile perfusion.
Donors After Circulatory Death
The DCD donor selection process begins with the decision of the attending intensive care unit staff to withdraw futile treatment, followed by the initiation of terminal weaning. Once blood oxygen saturation falls below 70% or means arterial pressure (MAP) below 50 mmHg (whichever comes first), a period of functional warm ischemia begins. In our department, no heparin is administered to patients before cardiac arrest, so the method of perfusion and subsequent harvesting is fully comparable to those used when harvesting grafts from DBD donors (except for the period of warm ischemia).
The cardiac arrest of DCD donors is verified by simultaneous analysis of electrocardiography (ECG), echocardiography, and arterial pulse waveform followed by a 5-min no-touch interval. After this interval, the donor is immediately transported to the operating room. Modified in situ organ preservation is preferred versus emergency laparotomy at our department: On arrival in the operating room, the femoral artery and vein are cannulated, and after placement of obturator catheters, in situ organ perfusion is initiated with 10 L of Custodiol preservation solution. The beginning of the administration of the preservation solution ends the warm ischemia interval, and the cold ischemia interval begins. This is followed by an urgent laparotomy with an application of crushed ice to the retroperitoneum. Simultaneously with organ preservation, the inferior vena cava and aorta are being prepared; mesenteric arteries are ligated to prevent the leaching of catabolites into the circulation. Once the perfusion is completed, organ harvesting continues using standard techniques. After the kidneys from the retroperitoneum, they are connected to pulsatile perfusion.
Data Collected
Long-term outcomes of the patients were followed up at our institution until 31 December 2020. In addition to basic descriptive parameters, data on comorbidities and infection detection and selected laboratory parameters in both groups were analyzed (laboratory parameters were measured preoperatively and postoperatively on days 1, 3, and 7, and then 1 month and 1 year after transplantation). In donors, cause of death, method of verification, need for circulatory support, and donor marginality were also analyzed (all kidney donors over 60 years of age and donors in the age group 50–59 years with at least 2 of the following comorbidities: arterial hypertension, serum creatinine level above 133 mmol/l, or cerebrovascular cause of death were considered marginal/expanded-criteria donors, ECD). This rule was applied to both DBD and DCD donors according to the purposes of the study.
The suitability for a particular recipient was always evaluated by both the transplant surgeon and the nephrologist based on medical criteria (body surface area, blood group, comorbidities, serum creatinine levels and estimated glomerular filtration rate, preoperative and maximal reactive antibodies, and compatibility index) and individual evaluation. The induction and maintenance of immunosuppressive therapy were supervised by transplant nephrologists according to the internal guidelines of the transplant center based on generally accepted guidelines, including the Kidney Disease Improving Global Outcomes (KDIGO) recommendations for the care of kidney transplant recipients and current medical knowledge [6]. In addition, records on causes of kidney failure, the number of previous transplants and data from the dialysis program were also evaluated, as well as data on the surgical procedure itself and transplant immunology. Disease-free survival was defined as the time from surgery to graft failure.
Statistical Analysis
Numerical variables are expressed as the median and the interquartile range (lower and upper quartiles). Categorical variables are presented as absolute frequencies and relative frequencies in percentages. The defined groups were compared using the Mann-Whitney test or the chi-square independence test for contingency tables. Changes in selected parameters over time were visualized using paired boxplots. The Kaplan-Meier curves and the log-rank test were used for the analysis of overall survival and disease-free survival. All statistical analyses were performed with maximum available data and the significance level was set to 0.05. Data were analyzed in R (version 4.1.1, www.r-project.org).
Results
Detailed descriptive donor data are presented in Table 1. The most common blood group of donors with DBD was O (46%), and the least common was AB (6%). In donors with DCD, blood group A (47%) was the most common group and AB (6%) was the least common group, with no statistically significant differences (P=0.441). In terms of comorbidities, the 2 groups were fully comparable both in general and when comparing individual comorbidities (Table 1). As many donors were admitted to the hospital through the A&E department while unconscious, personal history data was often incomplete.
Table 1.
Basic descriptive parameters of kidney donors; please note that for some donors, it was not possible to acquire the data; hence, only donors in whom these parameters were known are presented in the table and used for statistical testing.
| Parameter | Median (IQR) or n (%)i | P-valueiv | |
|---|---|---|---|
| DBDii (n=77) | DCDiii (n=17) | ||
| Age (years) | 51 (41–58) | 51 (43–53) | 0.791 |
| Weight (kg) | 75 (70–85) | 80 (65–90) | 0.396 |
| Body mass index (BMI) | 24.7 (23.1–27.5) | 24.7 (23.5–27.8) | 0.879 |
| Body surface area (BSA)v | 1.89 (1.78–2.00) | 1.97 (1.73–2.08) | 0.382 |
| Sex | 0.592 | ||
| Male | 37 (48) | 10 (59) | |
| Female | 40 (52) | 7 (41) | |
| Arterial hypertension | 0.923 | ||
| Yes | 30 (39) | 6 (35) | |
| No | 33 (43) | 5 (29) | |
| Diabetes mellitus | >0.999 | ||
| Yes | 5 (6) | 1 (6) | |
| No | 57 (74) | 8 (47) | |
| Ischemic heart disease | >0.999 | ||
| Yes | 6 (8) | 1 (6) | |
| No | 56 (73) | 8 (47) | |
| COPDvi | >0.999 | ||
| Yes | 3 (4) | 0 (0) | |
| No | 59 (77) | 9 (53) | |
| Smoking | 0.320 | ||
| Yes | 7 (9) | 4 (24) | |
| No | 14 (18) | 2 (12) | |
The median with the interquartile range or the absolute frequency and relative frequency in percentages;
DBD – Donors after Brain Death;
DCD – Donors after Circulatory Death;
The p-value of the Mann-Whitney test or the Chi-square test of independence;
BSA according to the Mosteller calculation method;
COPD – Chronic Obstructive Pulmonary Disease.
Intracerebral hemorrhage was the most common cause of death in donors with DBD (n=26; 34%) followed by trauma (n=21; 27%) and subarachnoid hemorrhage (n=18; 23%). Less common causes included conditions after cardiac arrest, subdural hematomas, ischemic stroke, anaphylaxis, thromboembolism, and hypoxia. In the DCD donor group, intracerebral hemorrhage (n=7; 41%) and trauma (n=5; 29%) were the most common, followed by acute myocardial infarction, hypoxia, and subarachnoid hemorrhage.
Of all donors to DBD, 50 (65%) were standard criteria donors (SCD), and the rest were classified as expanded-criteria donors (ECD) [7]. Of the DCD donors, 2 donors (12%) were in the SCD group and 15 donors (88%) were in the ECD group (P<0.001). There were no statistically significant differences in other clinical and laboratory parameters between the 2 groups at baseline (Table 2). Of DCD donors, only those who meet the Maastricht 3 Group criteria [8] are considered for inclusion in the transplantation program in our department (planned withdrawal of circulatory support), which falls into the controlled DCD transplant group. Detailed descriptive data on donors are presented in Table 2.
Table 2.
Paraclinical parameters of kidney donors.
| Parameter | Median (IQR) or n (%)i | P-valueiv | |
|---|---|---|---|
| DBDii (n=77) | DCDiii (n=17) | ||
| Serum creatinine (μmol/l) | 88 (65–115) | 85 (68–99) | 0.713 |
| eGFRv (ml/s/1,73 m2) | 1.33 (0.93–1.72) | 1.13 (1.04–1.63) | 0.643 |
| Norepinephrine (mg/kg/min) | 0.18 (0.09–0.51) | 0.11 (0.00–0.36) | 0.079 |
| Hourly urine output (ml) | 200 (133–261) | 135 (103–210) | 0.139 |
| Time from insult to transplantation (days) | – | 4 (2–6) | – |
| Time from WLSTvi to meeting fWITvii criteriaviii (minutes) | – | 4 (1–6) | – |
| Time from therapy withdrawal to cardiac arrest (minutes) | – | 11 (8–16) | – |
| No-touch interval duration (minutes) | – | 5 (5–5) | – |
| Time from beginning of WIT to ORix arrival (minutes) | – | 17 (15–20) | – |
| Time from beginning of WIT to the beginning of perfusion (minutes) | – | 21 (18–25) | – |
The median with the interquartile range or the absolute frequency and relative frequency in percentages;
DBD – Donors after Brain Death;
DCD – Donors after Circulatory Death;
The p-value of the Mann-Whitney test;
eGFR – Estimated Glomerular Filtration Rate from the creatinine concentration;
WLST – withdrawal of life-sustaining therapy;
fWIT – functional warm ischemia time;
The criteria for reaching the start of WIT are described in the Material and Methods section;
OR – Operating Room.
The representation of blood groups in recipients is similar to that of donors (O is the most common group for DBD and A for DCD, with AB the rarest group), with no statistically significant differences (P=0.396). The 2 groups were also fully comparable in terms of comorbidities and results of serological analyses.
Chronic glomerulonephritis (n=38; 38%) was the most common cause of chronic renal failure in our cohort. Other causes included chronic tubulointerstitial nephritis (n=15; 15%), diabetic kidney disease (n=11; 11%), and hypertension (n=7; 7%). Other diagnoses, such as Alport syndrome, polycystic kidney disease, vasculitis associated with ANCA (anti-neutrophil cytoplasmic antibodies), monoclonal gammopathy, systemic lupus erythematosus, and others, occurred more rarely. The vast majority of patients required long-term dialysis, usually hemodialysis, with a minority undergoing peritoneal and combined dialysis (ie, both methods are used sequentially). To optimize renal parameters, 21 DBD recipients (27%) underwent preoperative hemodialysis on the day of transplantation, compared to 6 DCD recipients (30%); the remaining patients did not require preoperative hemodialysis. Table 3 also shows detailed pre-transplant immunocompatibility parameters.
Table 3.
Basic descriptive parameters of kidney recipients.
| Median (IQR) or n (%)i | P-valueiv | ||
|---|---|---|---|
| DBDii (n=79) | DCDiii (n=20) | ||
| Numerical parameters | |||
| Age (years) | 55 (46–62) | 48 (42–63) | 0.467 |
| Weight (kg) | 82 (74–93) | 82 (74–88) | 0.972 |
| Body mass index (BMI) | 26.7 (24.4–30.0) | 26.5 (25.0–28.1) | 0.708 |
| Body surface area (BSA)v | 1.98 (1.86–2.15) | 1.99 (1.87–2.07) | 0.913 |
| BSA donor/recipient ratio | 0.97 (0.85–1.08) | 0.95 (0.89–1.07) | 0.705 |
| Duration of chronic hemodialysis (months) | 23 (13–29) | 21 (12–46) | 0.844 |
| Compatibility index | 13 (12–16) | 14 (7–17) | 0.819 |
| Preopreative PRAvi (%) | 0 (0-0) | 0 (0-0) | 0.097 |
| Maximal PRAvi (%) | 0 (0–3) | 0 (0-0) | 0.213 |
| Categorical parameters | |||
| Sex (Male) | 59 (75) | 14 (70) | 0.888 |
| Arterial hypertension (Yes) | 75 (95) | 17 (85) | 0.289 |
| Diabetes mellitus (Yes) | 18 (23) | 6 (30) | 0.704 |
| Ischemic heart disease (Yes) | 22 (28) | 3 (15) | 0.372 |
| COPDvii | 8 (10) | 0 (0) | 0.327 |
| Smoking (Yes) | 15 (19) | 4 (20) | 0.610 |
| Number of prior kidney transplantations | 0.116 | ||
| 0 | 58 (73) | 19 (95) | |
| 1 | 20 (25) | 1 (5) | |
| 2 | 1 (2) | 0 (0) | |
| Chronic hemodialysis (Yes) | 76 (96) | 20 (100) | 0.877 |
| Dialysis preoperatively (Yes) | 21 (27) | 6 (30) | 0.980 |
| Chronic dialysis | <0.001 | ||
| Chronic hemodialysis | 71 (90) | 12 (60) | |
| Chronic peritoneal dialysis | 4 (5) | 7 (35) | |
| Chronic combined dialysis | 1 (1) | 1 (5) | |
| No | 3 (4) | 0 (0) | |
| Induction | <0.001 | ||
| Anti-CD25 | 21 (27) | 3 (15) | |
| ATGviii | 14 (18) | 14 (70) | |
| Anti-CD25 + ATGviii | 1 (1) | 0 (0) | |
| No | 43 (54) | 3 (15) | |
The median with the interquartile range or the absolute frequency and relative frequency in percentages;
DBD – Donors after Brain Death;
DCD – Donors after Circulatory Death;
The p-value of the Mann-Whitney test or the Chi-square test of independence;
BSA according to the Mosteller calculation method;
PRA – Panel-reactive antibodies;
COPD – Chronic Obstructive Pulmonary Disease;
ATG – anti-thymocyte globulin.
Table 4 shows the perioperative and postoperative parameters. Transplantation was performed in the vast majority of patients with prophylactic antibiotics, most notably cefuroxime (n=90; 91%), typically at a dose of 750 mg. In 4 patients (4%), ciprofloxacin was used; Cefotaxime, ceftazidime, meropenem and piperacillin/tazobactam were administered in individual cases and in 1 case no antibiotic prophylaxis was administered.
Table 4.
Parameters of the transplantation procedure and post-transplantation graft function.
| Parameter | Median (IQR) or n (%)i | P-valueiv | |
|---|---|---|---|
| DBDii (n=79) | DCDiii (n=20) | ||
| Duration of surgery (minutes) | 125 (114–146) | 126 (112–150) | 0.972 |
| Cold ischemia (CIT, minutes) | 961 (856–1076) | 1100 (946–1244) | 0.028 |
| Warm ischemia (WIT, minutes) | – | 21 (19–25) | – |
| Duration of perfusion (minutes) | 11 (10–13) | 22 (19–31) | <0.001 |
| Pigtail catheter implantation (Yes) | 14 (18) | 5 (25) | 0.674 |
| Acute rejection (Yes) | 5 (6) | 0 (0) | 0.560 |
| Graft function | >0.999 | ||
| Delayed graft function (DGF) | 18 (23) | 4 (20) | |
| Immediate graft function (IGF) | 61 (77) | 16 (80) | |
| Primary graft dysfunction (PGD, Yes) | 1 (1) | 0 (0) | – |
The median with the interquartile range or the absolute frequency and relative frequency in percentages;
DBD – Donors after Brain Death;
DCD – Donors after Circulatory Death;
The p-value of the Mann-Whitney test or the Chi-square test of independence.
Of the variations in the number of individual anatomical structures, the abnormal number of renal arteries was the most common non-standard anatomical feature (double arteries in 18 cases [18%], triple arteries in 3 cases [3%]). A smaller number of anomalies were observed in the veins (double vein in only 2 cases [2%]), and a double ureter was observed in a single kidney (1%). Postoperatively, most of the patients (with no statistically significant differences between groups) received the immunosuppressant combination tacrolimus/mycophenolate mofetil/prednisone (n=95; 96%). In individual cases (ie, in which intolerance to that combination of immunosuppressants was observed), combinations tacrolimus/azathioprine/prednisone, tacrolimus/mycophenolate, mofetil/methylprednisolone can be used or tacrolimus/prednisone alone.
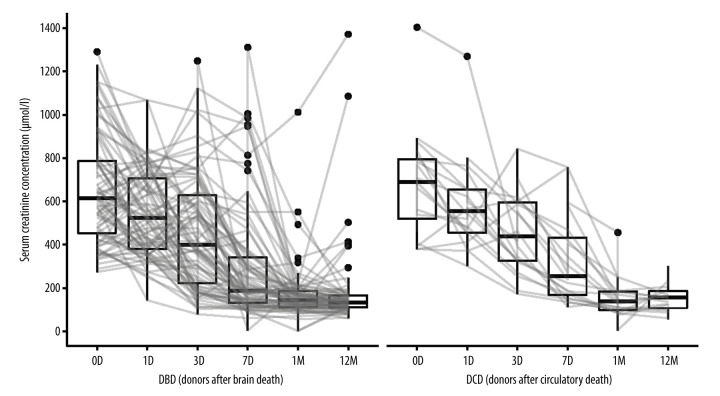
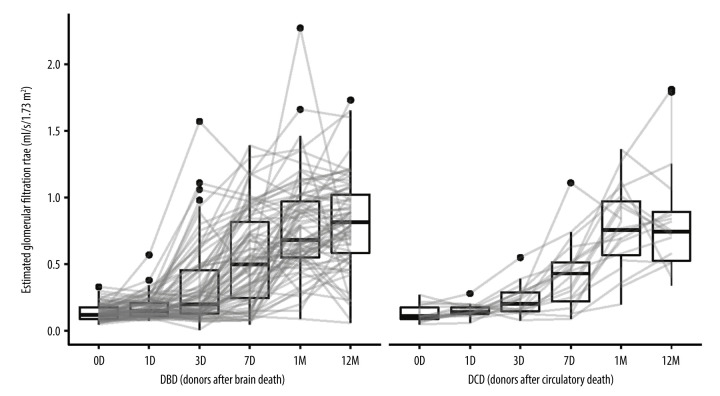
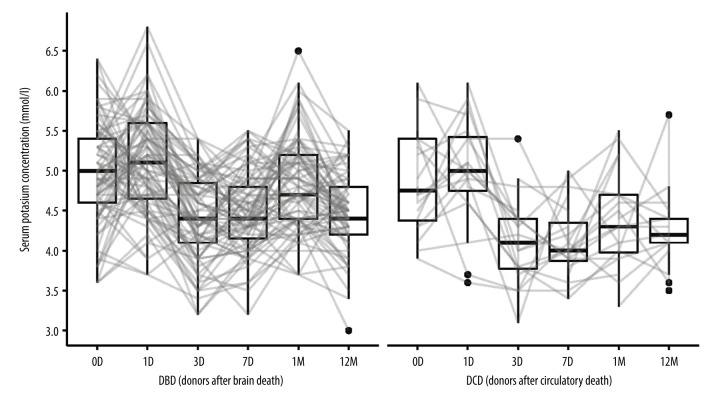
The results of laboratory tests of creatinine levels, eGFR (estimated glomerular filtration rate calculated from creatinine levels) and kalemia are presented in Figures 1–3.
Figure 1.
Serum creatinine concentrations in kidney recipients over the study period. D (day), M (month), DBD (donors after brain death), DCD (donors after circulatory death). Image created in R statistical software (version 4.1.1, The R Foundation).
Figure 2.
Estimated glomerular filtration rate (eGFR) calculated from serum creatinine in kidney recipients over the study period. D (day), M (month), DBD (donors after brain death), DCD (donors after circulatory death). Image created in R statistical software (version 4.1.1, The R Foundation).
Figure 3.
Potassium concentrations in kidney recipients over the study period. D (day), M (month), DBD (donors after brain death), DCD (donors after circulatory death). Image created in R statistical software (version 4.1.1, The R Foundation).
In the DCD group, serum creatinine levels decreased by a median of 364 (IQR [interquartile range]: 176.5; 579.5) μmol/L and 469.5 (IQR: 307.2; 618.2) μmol/l after 7 days and 1 year, respectively. Similarly, in the DBD group, creatinine levels dropped by a median of 405.5 (IQR: 163.2; 494.0) μmol/l and 518 (IQR: 354.2; 627.2) μmol/l.
Seven days after transplantation, the eGFR increased by 0.36 (IQR: 0.10; 0.66) ml/s/1.73 m2 in the DBD group and by 0.30 (IQR: 0.04; 0.42) ml/s/1.73 m2 in the DCD group. Similarly, after 1 year, the eGFR increased by 0.65 (IQR: 0.46; 0.89) ml/s/1.73 m2 and 0.65 (IQR: 0.39; 0.81) ml/s/1.73 m2, respectively, in both groups.
Kalemia decreased by a median of 0.40 (IQR: −0.05; 1.00) mmol/l after 7 days in DBD recipients, while in DCD recipients, the decrease observed in the same period was 0.80 mmol/l (IQR: 0.35; 1.30). After 1 year, the observed decrease in kalemia was 0.45 (IQR: −0.10; 1.00) in the DBD group and 0.30 (IQR: 0.00; 1.30) in the DCD group. None of these parameters differed significantly between the DBD and DCD groups.
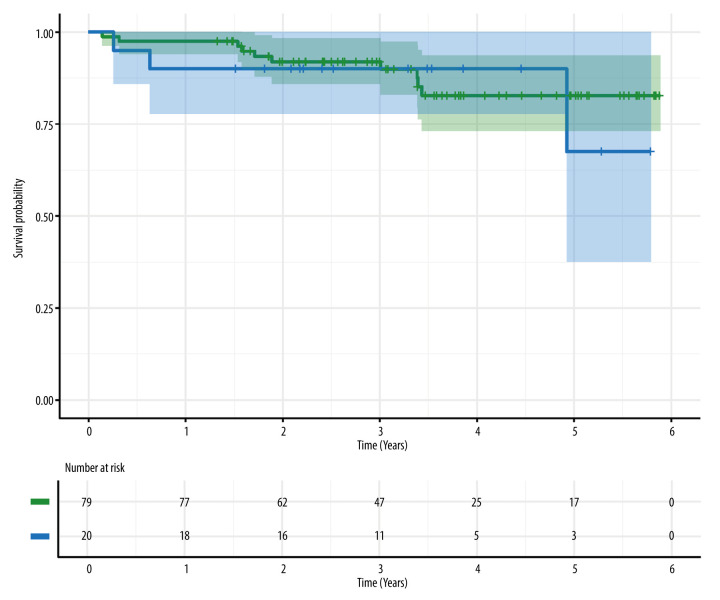
Of the 79 kidneys transplanted to DBD recipients, a total of 10 grafts (13%) failed during the follow-up period, while only 1 graft (5%) failed in DCD recipients (P=0.565). At 1 year, disease-free survival was 94.9% (95% CI [confidence interval] 90.1–99.9) and 100% (95% CI 100.0–100.0) in the DBD and DCD groups, respectively. The 3-year disease-free survival rate was 88.8% (95% CI 81.7–96.5) and 90.9% (95% CI 75.4–100.0) in these groups, while 5-year disease-free survival was 84.1% (95% CI 73.5–96.3) and 90.9% (95% CI 75.4–100.0), respectively (however, please note that the results for the DCD group are potentially overly optimistic, as reflected in the width of the confidence intervals; this is due to the small number of patients, only a single event in this group, and differences in the follow-up period). Kaplan-Meier analysis did not show statistically significant differences in disease-free survival between the 2 groups (P=0.370). A total of 13 patients died during the follow-up period - 10 in the DBD group (13%) and 3 in the DCD group (15%) – without statistically significant differences in overall survival (P=0. 750). The overall 1-, 3- and 5-year survival rates are shown in Table 5. The Kaplan-Meier analysis of overall survival is shown in Figure 4.
Table 5.
Results of Kaplan-Meier analysis of the overall survival of kidney recipients (survival rates with 95% confidence intervals).
| DBDi (n=79) | DCDii (n=20) | |
|---|---|---|
| 1-year | 97.5 (94.1–100.0) | 90.0 (77.8–100.0) |
| 3-year | 91.9 (86.0–98.4) | 90.0 (77.8–100.0) |
| 5-year | 82.7 (73.1–93.6) | 67.5 (37.6–100.0) |
Median survival time could not be determined;
DBD – Donors after Brain Death;
DCD – Donors after Circulatory Death.
Figure 4.
Results of Kaplan-Meier analysis of overall survival of kidney recipients (donors after brain death – green, donors after circulatory death – blue). No significant difference was found in overall survival between groups (log-rank test, P=0.750). Image created in R statistical software (version 4.1.1, The R Foundation).
Discussion
The introduction of DCD transplants was intended to expand the pool of potential cadaver kidney donors. In combination with living donors, less common types of transplantation such as DBCD (donation after brain death followed by circulatory death), and the individual use of expanded-criteria donors, the pool of organ donors is currently used to an acceptable maximum. Today, DCD donors account for about 20% of the total number of grafts in Europe [9]. However, currently there are no widely accepted European standards for DCD transplantation available, although individual countries often issue their recommendations adapted to local practices and legal standards. Compared to DBD transplantation, typically fewer organs are harvested from DCD donors (2.8 vs 3.8), but the frequency of kidney graft utilization from such donors is comparable (83% vs 87%) [10]. Recently, the use of DBD harvesting for kidney transplantation in pediatric recipients has also attracted interest [11]. As kidney grafts are in high demand, optimal graft allocation is necessary. To help with the allocation process, the Kidney Donor Profile Index (KDPI) was proposed in 2014 [12], using several donor parameters that affect the quality of the kidney graft (age, height, weight, cause of death, serum creatinine, history of diabetes and hypertension, HCV infection, ethnicity, and discrimination between DBD/DCD) to calculate the index. The index value then predicts the allograft function and survival. However, it should be noted that the index is well correlated with patient age, which is the most common predictor of short- and long-term results [13].
Effect of Ischemia
Although the negative effect of the warm ischemia time on recovery of graft function has been demonstrated [14], calculation of the duration of warm ischemia remains controversial. More recent publications have distinguished between periods of total warm ischemia (from withdrawal of life support to perfusion) and functional warm ischemia (lasting from a drop in pressure or saturation below a defined level and ending with perfusion). The median duration of total warm ischemia reported in the literature is 26 min (10 to 174 min) [15], which is consistent with our experience. Of this, the time from withdrawal of therapy to patient death from cardiac arrest constitutes approximately two-thirds.
No-Touch Interval
A consensus on the length of the no-touch interval has also not been reached. There is controversy over the typically used 5-min interval, which has been shown in some animal experiments to be too short to ensure complete loss of donor physiological functions [16]. Although the use of the no-touch interval is not required by law in the Czech Republic, the 5-minute limit is generally accepted; however, some countries use a longer no-touch interval of up to 30 min [10]. Pre-mortem administration of heparin is, in some countries, illegal. However, the effect of pre-mortem heparin administration on DCD donors on the course of transplantation and postoperative graft function has not yet been compared in the literature versus the procedure without its application [10].
Delayed Graft Function and Primary Non-Function
The short-term results of DCD transplants compared to DBD transplants have been discussed in the literature, with a significantly higher incidence of delayed graft function (DGF) reported in DCD than in DBD transplants (17–80% vs 8–50%) [1–5, 17–19]. The short period of warm ischemia in DCD transplantations is the most common explanation for this [18]. However, in our study, the representation of DGF was not significantly different between the groups (20% DCD vs 23% DBD). Based on creatinine concentrations that remained comparable between groups over the long-term, DGF can be successfully managed with supportive therapy regardless of the type of transplantation and does not affect overall graft survival. The inevitability of warm ischemia is another likely cause of the inferior laboratory parameters reported immediately after DCD transplantation [3]; these differences gradually level off. In our study, the results were even comparable at all time points. Primary non-function (PNF) is generally a rare event [19,20], which was also confirmed in our study. In our opinion, a technical error during transplantation is probably the most likely cause of this event; prolonged warm graft ischemia or perivascular capillary edema may also play a role [2]. Large European studies report that the frequency of PNF in patients after DCD transplantation is as high as 5.0%, which is much higher than the 0.7–0.9% reported for DBD grafts [21]. Acute rejection of the graft is also relatively rare, with reported frequencies of 0.0–8.5% for DBD and 5.5–8.0% for DCD in the case of kidney transplantation [3–5]. Furthermore, a recent (2021) meta-analysis by Rijkse et al concluded that the risk of PNF (RR 1.43; 95% CI 1.26; 1.62) and DGF (RR 2.02; 95% CI 1.88; 2.16) is higher in patients in the DCD group [22].
Graft Function
In our study, the difference in time to graft failure was not statistically significant between the groups. This is in accordance with studies reporting 1-year graft function rates of 87.0 to 98.3% for the DBD group and 85.0 to 100% for the DCD group [2,3,19,21]. In the aforementioned meta-analysis, a 13% higher risk of graft loss was reported for the DCD group compared to DBD (RR 1.13; 95% CI 1.08; 1.19). These results are in agreement with the literature, which shows a 1-year graft function of 87.0 to 100% for DBD grafts and 85.0 to 100% for DCD grafts. In the literature, the 5-year graft function rate is reported to be 80.0 to 100.0% for DBD and 75.8 to 85.9% for DCD kidney transplants, respectively [1–3,5,10,21,23]. Nakamura et al reported that the graft origin of the standard or expanded-criteria donor graft affected graft function to a greater degree than the origin of the DCD/DBD graft [5], and some authors also reported differences between controlled and uncontrolled kidney transplantation [10,24].
Overall Mortality
The overall mortality in kidney transplantation studies is 92.8–98.3% in DBD and 94.9–100% in DCD grafts [2,3,19,21], which agrees well with our results.
The 3-year mortality observed in our study is in good accordance with large studies. Summers et al reported a 3-year survival of 91.4% in DBD patients and 92.2% in DCD patients [1], while Buxeda et al reported the 3-year survival of elderly DBD donors at 87% and 78–97% in DCD donors, depending on the age of the recipient [25]. The latter study also showed that donor age is not relevant to the overall survival of the recipients.
After 5 years, disease-free survival in our patient cohort was 84.1% (95% CI 73.5–96.3) and 90.9% (95% CI: 75.4–100.0) in the DBD and DCD groups, respectively. As mentioned above, 5-year survival in the DCD group in our study comes with a wide confidence interval due to the very small number of patients who were observed for that long. This is a clear limitation of our study; the rates in the literature are 89.0–93.0% in the DBD group and 86.5–88.0% in the DCD group [1,23,26].
Extended-Criteria Donors
The results of our study agree with others concluding that the outcomes of DCD transplantations are fully comparable to those of DBD in the controlled transplantation regimen, even if older donors to ECD donors were included [27]. The high percentage of ECD (marginal) donors in the DCD group can be explained by the generally higher occurrence of physiological and laboratory pathologies in this type of donor, while in DBD donors, functions other than cerebral are often physiological.
It should be noted that although the percentage representation of ECD donors was significantly higher in the DCD group, there was no statistically significant difference between the groups in transplant outcomes, which is quite surprising (since the expectation that ECD donors would perform worse). This interesting result of our study could be explained by the careful selection of patients and the well-executed coordination of withdrawal from therapy and transplantation itself. In Maastricht 3 donors (potential donors from other Maastricht categories are not considered for inclusion in the transplantation program in our department), the process of kidney harvesting and transplantation process is highly controlled, limiting the duration of warm ischemia to a minimum.
Limitations of the Study
The main limitation of this study is the small number of DCD donors, resulting in wide confidence intervals (especially at the longest follow-up period of 5 years). The difference in the representation of ECD donors between groups could also be considered a limitation; however, in this case, it underlines the successful DCD program in our department, as despite the significantly higher representation of marginal donors (ECD) in the DCD group, the results remained comparable.
Conclusions
Our results indicate that the short- and long-term outcomes of kidney transplants from donors after circulatory death and after brain death, both without heparin administration, are fully comparable. Despite the minor differences between the 2 groups (in type of dialysis, induction, cold ischemia, perfusion time, and donor marginality), graft function at 1 year is fully sufficient, with a significant decrease in kalemia and serum creatinine levels and an improvement in eGFR after transplantation in both groups. The fact that the time to graft failure and patient survival did not differ significantly allows us to recommend kidney transplantation from all eligible donors with DCD, even without pre-mortem heparin administration.
Footnotes
Conflict of interest: None declared
Statement
The study was performed at the Clinic of Surgery and Transplantation Center of the University Hospital Ostrava.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: Supported by Ministry of Health, Czech Republic – Conceptual Development of Research Organization RVO-FNOs/2012)
References
- 1.Summers DM, Watson CJ, Pettigrew GJ, et al. Kidney donation after circulatory death (DCD): State of the art. Kidney Int. 2015;88(2):241–49. doi: 10.1038/ki.2015.88. [DOI] [PubMed] [Google Scholar]
- 2.Sun Q, Zhou H, Cao R, et al. Donation after brain death followed by circulatory death, a novel donation pattern, confers comparable renal allograft outcomes with donation after brain death. BMC Nephrol. 2018;19(1):164. doi: 10.1186/s12882-018-0972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G, Wang C, Ko DS-C, et al. Comparison of outcomes of kidney transplantation from donation after brain death, donation after circulatory death, and donation after brain death followed by circulatory death donors. Clin Transplant. 2017;31(11):e13110. doi: 10.1111/ctr.13110. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Lyu J, Yu X, et al. Comparison of graft outcome between donation after circulatory death and living-donor kidney transplantation. Transplant Proc. 2020;52(1):111–18. doi: 10.1016/j.transproceed.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y, Kihara Y, Yokoyama T, et al. Similar outcomes of kidney transplantations using organs from donors after cardiac death and donors after brain death. Transplant Proc. 2018;50(8):2404–11. doi: 10.1016/j.transproceed.2018.03.088. [DOI] [PubMed] [Google Scholar]
- 6.Special issue: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 7.Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3:114–25. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 8.Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 2016;29(7):749–59. doi: 10.1111/tri.12776. [DOI] [PubMed] [Google Scholar]
- 9.Global Observatory on Donation and Transplantation. Organ donation and transplantation activities. Published 2016 http://www.transplant-observatory.org/download/2016-activity-data-report/
- 10.Lomero M, Gardiner D, Coll E, et al. Donation after circulatory death today: An updated overview of the European landscape. Transpl Int. 2020;33(1):76–88. doi: 10.1111/tri.13506. [DOI] [PubMed] [Google Scholar]
- 11.Marlais M, Callaghan C, Marks SD. Kidney donation after circulatory death: Current evidence and opportunities for pediatric recipients. Pediatr Nephrol. 2016;31(7):1039–45. doi: 10.1007/s00467-015-3175-6. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. Kidney Donor Profile Index (KDPI) – guide for clinicians. Published 2017 https://optn.transplant.hrsa.gov/resources/guidance/kidney-donor-profile-index-kdpi-guide-for-clinicians.
- 13.Dahmen M, Becker F, Pavenstädt H, et al. Validation of the Kidney Donor Profile Index (KDPI) to assess a deceased donor’s kidneys’ outcome in a European cohort. Sci Rep. 2019;9(1):11234. doi: 10.1038/s41598-019-47772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill J, Rose C, Lesage J, et al. Use and outcomes of kidneys from donation after circulatory death donors in the United States. J Am Soc Nephrol. 2017;28(12):3647–57. doi: 10.1681/ASN.2017030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill J, Rose C, Lesage J, et al. Use and outcomes of kidneys from donation after circulatory death donors in the United States. J Am Soc Nephrol. 2017;28(12):3647–57. doi: 10.1681/ASN.2017030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rady MY, Verheijde JL. No-touch time in donors after cardiac death (nonheart-beating organ donation) Curr Opin Organ Transplant. 2013;18(2):140–47. doi: 10.1097/MOT.0b013e32835e29a8. [DOI] [PubMed] [Google Scholar]
- 17.de Vries EE, Hoogland PER, Wind J, et al. Transplantation of kidneys from paediatric DCD donors: A comparison with DBD donors. Nephrol Dial Transplant. 2013;28(1):220–26. doi: 10.1093/ndt/gfs464. [DOI] [PubMed] [Google Scholar]
- 18.Shao M-J, Ye Q-F, Ming Y-Z, et al. Risk factors for delayed graft function in cardiac death donor renal transplants. Chin Med J (Engl) 2012;125(21):3782–85. [PubMed] [Google Scholar]
- 19.Mori G, Solazzo A, Tonelli L, et al. Comparison between kidney transplantation after circulatory death and after brain death: A monocentric retrospective study after 1 year of follow-up. Transplant Proc. 2020;52(5):1536–38. doi: 10.1016/j.transproceed.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 20.Hamed MO, Chen Y, Pasea L, et al. Early graft loss after kidney transplantation: Risk factors and consequences. Am J Transplant. 2015;15(6):1632–43. doi: 10.1111/ajt.13162. [DOI] [PubMed] [Google Scholar]
- 21.Domínguez-Gil B, Haase-Kromwijk B, Van Leiden H, et al. Current situation of donation after circulatory death in European countries. Transpl Int. 2011;24(7):676–86. doi: 10.1111/j.1432-2277.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 22.Rijkse E, Ceuppens S, Qi H, IJzermans JNM, et al. Implementation of donation after circulatory death kidney transplantation can safely enlarge the donor pool: A systematic review and meta-analysis. Int J Surg. 2021;92:106021. doi: 10.1016/j.ijsu.2021.106021. [DOI] [PubMed] [Google Scholar]
- 23.Bell R, Farid S, Pandanaboyana S, et al. The evolution of donation after circulatory death renal transplantation: a decade of experience. Nephrol Dial Transplant. 2019;34(10):1788–98. doi: 10.1093/ndt/gfy160. [DOI] [PubMed] [Google Scholar]
- 24.Roldán-Reina ÁJ, Egea-Guerrero JJ, Palomo-López N, et al. Postoperative care in kidney transplantation: A comparison between controlled and uncontrolled donation after circulatory death. Transplant Proc. 2018;50(2):533–35. doi: 10.1016/j.transproceed.2017.11.058. [DOI] [PubMed] [Google Scholar]
- 25.Buxeda A, Velis G, Arias-Cabrales C, et al. Kidney transplantation outcomes from elderly donors after circulatory death: A comparison with elderly brain-dead donors. Clin Kidney J. 2021;14(4):1181–89. doi: 10.1093/ckj/sfaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jochmans I, Darius T, Kuypers D, et al. Kidney donation after circulatory death in a country with a high number of brain dead donors: 10-year experience in Belgium. Transpl Int. 2012;25(8):857–66. doi: 10.1111/j.1432-2277.2012.01510.x. [DOI] [PubMed] [Google Scholar]
- 27.Walls DO, Lee-Riddle GS, Bover Manderski M, et al. Kidney transplant outcomes from donation after circulatory death donors of advanced age. Clin Transplant. 2020;34(7):e13881. doi: 10.1111/ctr.13881. [DOI] [PubMed] [Google Scholar]