Abstract
3-Hydroxy-2-formylbenzothiophene (HFBT) is a metabolite found in many bacterial cultures that degrade dibenzothiophene (DBT) via the Kodama pathway. The fate of HFBT in cultures and in the environment is unknown. In this study, HFBT was produced by a DBT-degrading bacterium and purified by sublimation. When stored in organic solvent or as a crystal, the HFBT slowly decomposed, yielding colored products. Two of these were identified as thioindigo and cis-thioindigo. The supernatant of the DBT-degrading culture contained thioindigo, which has not been reported previously as a product of DBT biodegradation. In mineral salts medium, HFBT was sufficiently stable to allow biodegradation studies with a mixed microbial culture over a 3- to 4-week period. High-performance liquid chromatography analyses showed that HFBT was removed from the medium. 2-Mercaptophenylglyoxalate, detected as benzothiophene-2,3-dione, was found in an HFBT-degrading mixed culture, and the former appears to be a metabolite of HFBT. This mixed culture also mineralized HFBT to CO2.
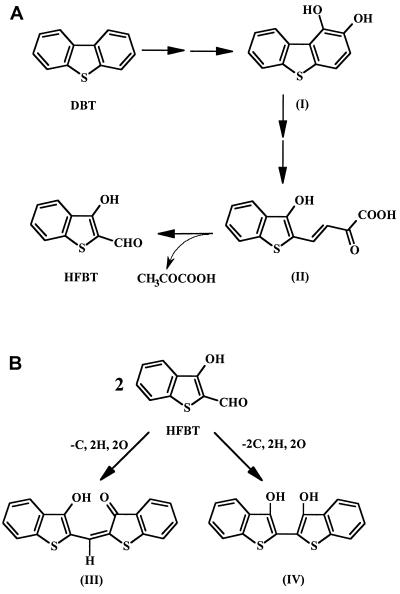
For more than 4 decades, dibenzothiophene (DBT) has been the model compound for studying the biodegradation or biodesulfurization of sulfur heterocycles. 3-Hydroxy-2-formylbenzothiophene (HFBT) has often been found as a metabolite of bacterial degradation of DBT (1, 9, 11, 12, 19). Yamada et al. (27) reported that two Pseudomonas isolates produced a metabolite that upon purification yielded yellow crystal with a melting point of 107°C. Kodama et al. (11) conclusively identified this metabolite as HFBT (Fig. 1A).
FIG. 1.
Abbreviated Kodama pathway for DBT biodegradation to HFBT (A) and proposed products from HFBT (B). Compounds and references in which they were reported include compounds I (1,2-dihydroxydibenzothiophene) (19), II (12), III (11), and IV (8).
Figure 1B shows that two molecules of HFBT can undergo a spontaneous abiotic reaction forming a red dyestuff, 3-oxo-(3′-hydroxythianaphthenyl-2-methylene)-dihydrothianaphthene (compound III) (11, 12). Finkelstein et al. (8) described a different product of two molecules of HFBT spontaneously reacting to give compound IV (Fig. 1B). However, they based this tentative identification solely on the interpretation of a mass spectrum.
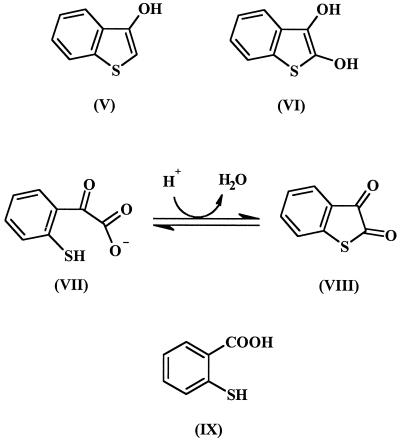
No biodegradation products of HFBT have been conclusively identified, but compounds that may originate from HFBT have been detected in cultures biodegrading DBT. Examples of these are shown in Fig. 2. Bohonos et al. (1) found 3-hydroxybenzothiophene (compound V) and benzothiophene-2,3-dione (compound VIII). Eaton and Nitterauer (4) showed that when acidic conditions are used to extract culture supernatants containing 2-mercaptophenylglyoxalate (compound VII), an acid-catalyzed dehydration occurs to form compound VIII. Finkelstein et al. (8) detected 2,3-dihydroxybenzothiophene (compound VI) and 2-mercaptobenzoic acid (or thiosalicylic acid; compound IX). However, no one has conclusively shown that these metabolites result from further catabolism of HFBT and are not products of a concurrent metabolic pathway.
FIG. 2.
Some possible biodegradation products of HFBT and references in which they were reported include compounds V (1), VI (8), VII (4), VIII (1), and IX (8).
To date, purified HFBT has not been used as a carbon and energy source for biodegradation studies. The only reported investigation into the biodegradation of HFBT is by Mormile and Atlas (21) who used filter-sterilized culture supernatants containing HFBT, trans-4[2-(3-hydroxy)-thianaphthenyl]-2-oxo-3-butenoic acid (compound II) (Fig. 1A), and presumably other metabolites, produced by a strain of Pseudomonas putida growing on HFBT. These were added to enrichment cultures inoculated with soil or creek sediment samples, and the loss of HFBT was monitored spectrophotometrically at 390 nm and by measuring CO2 production. The absorbance at 390 nm decreased to about 40% of its initial value over a 21-day incubation period. CO2 release was observed, but sulfate release was not detected.
The fate of HFBT in cultures or in the environment is unknown. Laboratory studies to address its fate have been hampered because HFBT is not commercially available, and it has not been produced in sufficient quantities for such investigations. This paper describes our production of HFBT by chemical and microbiological methods, the chemical stability of HFBT, and the mineralization of HFBT by a mixed microbial culture.
MATERIALS AND METHODS
Preparation of HFBT by microbiological methods.
Pseudomonas strain BT1d grows on DBT as its sole carbon and energy source, resulting in the conversion of about 50% of this substrate to HFBT (16). For production of HFBT, this strain was inoculated into several 2-liter Erlenmeyer flasks that each contained 1.5 liters of mineral medium (13) and 2 g of DBT (most of which remained as crystals). These cultures were incubated at 28°C with shaking for 2 months. Then the culture supernatant was acidified with citric acid (Aldrich Chemical Company, Milwaukee, Wis.) to pH ∼2 and extracted three times with 200-ml portions of ethyl acetate. Citric acid was used because it would not be extracted into the ethyl acetate and because it was found that mineral acids such as HCl or H2SO4 accelerated the loss of HFBT. It was also found that the use of dichloromethane as an extraction solvent increased the loss of HFBT by an unknown mechanism. The ethyl acetate extracts were combined, and all the solvent was removed at 50°C under vacuum in a rotary evaporator.
The resulting HFBT-containing residue was immediately purified by sublimation, yielding yellow HFBT crystals. The sublimation apparatus consisted of a 1-liter, three-necked, round-bottom flask which contained the dried crude HFBT preparation. This flask was connected to a vacuum pump through one neck of the flask. A Dewar condenser was then connected to the center neck of the flask and filled with a dry ice-ethanol mixture. The third flask opening was then sealed. The flask was pumped to vacuum and submerged in an paraffin oil bath which was heated to 80°C by a hot plate with stirring. The HFBT was allowed to sublime for approximately 4 h, which was sufficient to achieve near-maximum yield of yellow HFBT crystals.
Steam distillation was also evaluated as a method for HFBT purification from the culture supernatant after it was acidified it to pH 2 with HCl. Approximately 200 ml of the steam distillate was collected in a round-bottom flask which contained 10 ml of 0.1 mM phosphate buffer (pH 7).
Chemical synthesis of HFBT.
The chemical synthesis of HFBT was conducted as described by Smiles and McClelland (24). The synthesis involved heating a mixture of malic acid, thiosalicylic acid (both obtained from Aldrich), and concentrated sulfuric acid to 90°C for 30 min. Unfortunately, HFBT was not the major product. Purification of the HFBT was attempted by sublimation and by steam distillation.
Abiotic reactions of HFBT in crystalline form.
Crystals of HFBT slowly turned a reddish purple color when stored in a sealed vial with air in the headspace. To examine these spontaneous reaction products, crystals that had been stored for 1 year were dissolved in dichloromethane. A portion of this solution was analyzed by gas chromatography-mass spectrometry (GC-MS), and another portion was analyzed by preparative thin-layer chromatography (TLC) on 20- by 20-cm plates with a 250-μm layer of 60-Å K6F silica gel (Whatman Inc., Clifton, N.J.). The plate was developed with a mixture of hexane and ethyl acetate (70:30). Then two pink bands were scraped off the plate and washed from the silica gel with dichloromethane, and these were then analyzed by low- and high-resolution GC-MS. One of the pink compounds, with an Rf of 0.82, was analyzed by Fourier transformed infrared spectroscopy.
A purple band remained at the origin of the TLC plate. A solvent containing dichloromethane, methanol, and hexane (45:10:45) was required to move this compound from the origin.
Stability of HFBT in sterile aqueous solutions.
Aerobic shake-flask incubations were conducted in the dark at 28°C with 0.07 mM HFBT in a variety of different aqueous solutions (200 ml) in 500-ml Erlenmeyer flasks. One set of three flasks contained Milli-Q water (pH ∼7), dilute HCl (pH 2), and dilute NaOH (pH 11). Another set of three flasks contained sulfate-free medium (3) adjusted to pH 7, 2, or 11. Another aerobic shake flask contained HFBT in sulfate-free medium (pH 7) and was incubated with constant direct light (100-W light bulb from a 0.2-m distance). Finally, an anaerobic incubation was done in a sealed flask with a N2 headspace and 1 mM sodium sulfide added to the sulfate-free medium. These were all incubated for 55 days, with samples being removed at various times for high-performance liquid chromatography (HPLC) analyses for residual HFBT.
Biodegradation and mineralization of HFBT.
A mixed culture, designated SLPB, that has been maintained in liquid culture on Prudhoe Bay crude oil since 1983 (6) was used to enrich the HFBT-degrading culture. Twenty milliliters of SLPB was transferred to 200 ml of mineral medium with 10 mg of HFBT as the sole carbon and energy source. This shake-flask culture was incubated at 28°C for 3 weeks. Then 20 ml of this enrichment culture was transferred to fresh mineral medium with HFBT and incubated under the same conditions. This was repeated five times over a 4-month period. At various times, these enrichment cultures were analyzed for the decrease in HFBT content by HPLC.
The final enrichment culture was used for the mineralization study, in which 10 ml of the culture was added to 70 ml of mineral medium with 7 mg (0.039 mmol) of HFBT in sealed 160-ml serum bottles. Triplicate viable cultures and triplicate heat-killed, sterile controls were prepared. These cultures were incubated at 28°C and shaken at 200 rpm. The headspace gas contained sufficient O2 for complete mineralization of the HFBT. After 14 days of incubation, 2 ml of 5 M HCl was injected into each culture to release dissolved CO2 into the headspace, which was analyzed for CO2 by the GC method described previously (3).
Analytical methods.
HFBT in aqueous samples was analyzed by HPLC using a Hewlett Packard model 1050 chromatography system and a 125- by 4-mm (5 μm) LiChrospher 100 RP-18 column (Hewlett Packard) with a UV detector at 394 nm. The mobile phase was acetonitrile–0.01 M K2HPO4 buffer, pH 9 (15:85), with a flow rate of 1 ml/min. The retention time for HFBT was 3.5 min.
A culture supernatant was analyzed for thioindigo using the same HPLC column with an acetonitrile-water (70:30) mobile phase flowing at 3 ml/min and a detector wavelength of 536 nm. The retention time for thioindigo (TCI America, Portland, Oreg.) was 2.8 min.
Organic extracts containing HFBT or other sulfur heterocycles were analyzed by GC with a sulfur-selective detector (3). Some extracts were analyzed by low-resolution GC-MS using a Hewlett Packard 5890 series II gas chromatograph with a 5970 series mass selective detector and a 30-m DB-5 capillary column (J&W Scientific, Folsom, Calif.). High-resolution GC-MS was done as outlined previously (7). The GC temperature program used for all of these analyses was 90°C for 1 min and then an increase of 5°C/min to 280°C for 21 min.
The molar extinction coefficient of HFBT in water at 394 nm was determined at three concentrations (84, 56, and 28 μM) using a Unicam UV3 spectrophotometer (ATI Unicam).
RESULTS
Chemical synthesis of HFBT.
The chemical synthesis of HFBT gave unsatisfactory results because HFBT was only a minor product. A red product was formed from HFBT, through the action of sulfuric acid in the reaction mixture. This was the major product, and HFBT could not be separated from it. Thus, the use of the chemical synthesis method was abandoned.
HFBT from culture extracts.
The method for the production of HFBT from DBT by Pseudomonas strain BT1d was not optimized. At the end of the 2-month incubation period, the cultures were a dark reddish purple and there were always crystals of unused DBT in the culture flasks. After sublimation, the maximum HFBT yield from a 1.5-liter culture was 80 mg.
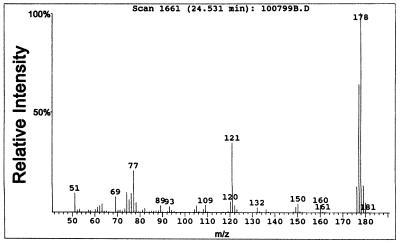
The freshly collected yellow crystals had a melting point of 106 to 107°C, which matched the value in the literature of 107°C (24, 27). GC analysis showed the purity to be greater than 99%. HPLC analysis showed only one peak. Figure 3 shows the mass spectrum of HFBT. The UV spectrum of HFBT in water showed a strong absorption at 394 nm, and the molar extinction coefficient was determined to be 12,400 liters/mol·cm.
FIG. 3.
Mass spectrum of HFBT.
Steam distillation was used in an attempt to improve the recovery of HFBT. However, during this procedure the HFBT appeared to undergo a reaction inside the condenser, leaving a pink material coating the condenser walls. The addition of the buffer at pH 7 to the collection flask stabilized the collected HFBT in an aqueous solution. Since we knew the molar extinction coefficient of HFBT, desired amounts of HFBT from the collected condensate could be dispensed into cultures by adding the correct volumes of this aqueous solution. However, in most cases, the crystals collected by sublimation were used for our studies.
Abiotic transformations of HFBT in crystalline form.
When HFBT was stored as crystals in sealed vials, a reddish purple color appeared on the crystals. This change also occurred when the crystals were stored under a N2 atmosphere, in the dark, and at −20°C, but it was accelerated by the presence of O2. Crystals that had been exposed to air for 1 year were examined to determine the product(s) of this reaction. TLC revealed at least four distinct colored compounds, one of which was HFBT.
Two dark pink bands with Rf values of 0.82 and 0.75 were observed when developed in the mixture of hexane and ethyl acetate. GC-MS analysis of the compound with an Rf of 0.75 showed a strong molecular ion at m/z 296 (with a relative intensity of 100%), with weak ions at m/z 298 (11), 297 (21) 240 (14), 120 (10), and 76 (8). This compound gave the same GC retention time (43.8 min) and mass spectrum as authentic thioindigo.
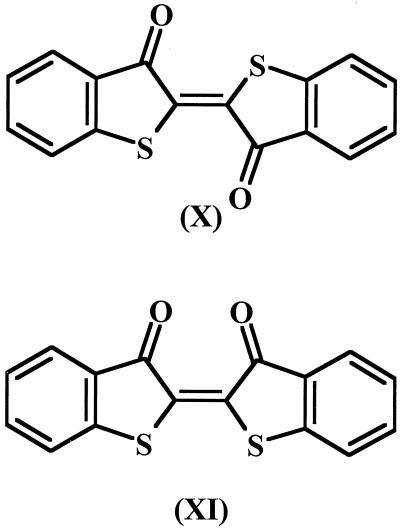
The GC retention time of the other pink compound, with an Rf of 0.82, was 40.2 min. Its mass spectrum also gave a molecular ion of m/z 296 but a markedly different fragmentation pattern from that of thioindigo. The most abundant ions and their relative intensities (in percent) were 298 (15), 297 (26), 296 (100), 295 (70), 268 (94), 240 (76), 236 (16), 208 (14), 195 (17), 164 (13), and 120 (24). The abundant fragments at m/z 268 and 240 suggested two sequential losses of C⩵O (M+ −28 and M+ −56, respectively). The Fourier transformed infrared spectrum of this compound revealed a strong absorption at 1,716 cm−1 and a weaker absorption at 1,283 cm−1. These are characteristic of C⩵O stretching and bending vibrations in ketones. These results were consistent with this compound being cis-thioindigo. Figure 4 shows the structures of thioindigo and cis-thioindigo.
FIG. 4.
Structures of the pink products found with HFBT crystals after 1 year of storage: thioindigo (compound X) and cis-thioindigo (compound XI).
A purple compound remained at the origin of the TLC plate when the hexane-ethyl acetate solvent was used. With a more polar solvent system, the purple compound had an Rf of 0.69. After this compound was recovered from the silica gel with methanol, attempts to analyze it by GC-MS analysis and by probe electron impact ionization mass spectrometry were unsuccessful because of the low volatility of the compound. Therefore, the identity of this compound remains unknown.
When purified HFBT was dissolved into organic solvents such as dichloromethane or ethyl acetate, the loss of HFBT was much more rapid than when the HFBT was stored as crystals. In solutions of dichloromethane, close to 10% of the HFBT was lost after only 5 days. Thus, HFBT was produced and purified in small batches and stored as crystals.
Thioindigo in the supernatant of strain BT1d grown on HFBT.
When thioindigo and cis-thioindigo were identified as abiotic reaction products from HFBT, efforts were made to detect these compounds in cultures of strain BT1d, which was used to produce HFBT. After a neutral extraction with dichloromethane and concentration of the solution, GC-MS analysis showed the presence of thioindigo (retention time, 43.8 min) but not cis-thioindigo (retention time, 40.2 min). HFBT, DBT sulfoxide, DBT sulfone, and residual DBT were also found in this extract.
It was possible the thioindigo detected in the dichloromethane extract actually formed in the organic solvent, rather than in the culture of strain BT1d. Thus, an HPLC method was used for the direct analysis of the culture supernatant for thioindigo. This analysis showed the presence of a compound with an absorbance at 536 nm and the same HPLC retention time as authentic thioindigo. Therefore, the culture supernatant did contain thioindigo.
Stability of HFBT in sterile aqueous solutions.
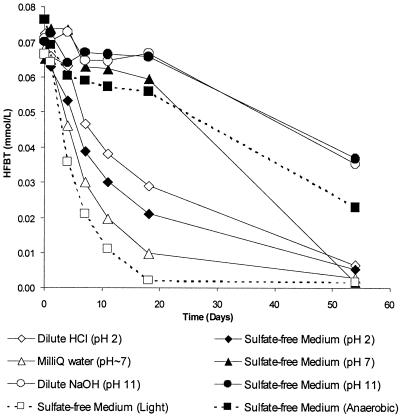
Freshly prepared aqueous solutions of HFBT were bright yellow, but these became a much lighter yellow color over time. In preparation for biodegradation studies, experiments were conducted to determine under which conditions HFBT was stable in sterile aqueous solutions in shake flasks. Figure 5 summarizes the results from the eight different conditions that were tested. The abiotic loss of HFBT occurred most rapidly in the shake flask that was exposed to light, with essentially all of the HFBT being gone by day 19. The HFBT was most stable at pH 11, whether in dilute NaOH or sulfate-free medium. At pH 11, less than 5% loss occurred over 19 days and about 50% loss was observed over 55 days. At pH 7, HBFT was more stable in the sulfate-free medium than in the Milli-Q water (Fig. 5). Over 19 days of incubation, HFBT was essentially as stable in the sodium sulfide-reduced medium under a N2 atmosphere as it was in the aerated sulfate-free medium at pH 7. However, after 55 days, the HFBT was completely depleted in the latter medium but about 30% remained in the reduced medium.
FIG. 5.
Stability of HFBT in various aqueous solutions at 28°C. For all but two cases, all the shake flasks were incubated aerobically in the dark. In one case the solution was incubated aerobically with illumination, and in another case, the solution was incubated in the dark under a N2 atmosphere after being reduced with sodium sulfide.
Biodegradation and mineralization of HFBT.
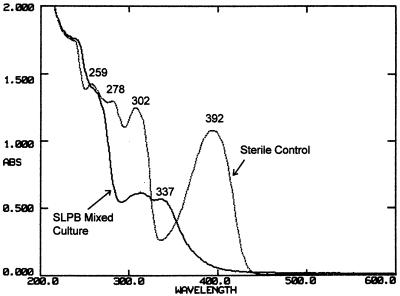
During the enrichment of HFBT degraders from the mixed culture SLPB, portions of the cultures were transferred at 3-week intervals. According to the data in Fig. 5, little abiotic loss of HFBT would be expected in mineral medium at pH 7 during this time. HPLC analyses showed that after 20 days of incubation, the concentration of HFBT in the viable culture was 16% of that in the sterile control. Figure 6 compares the UV-visible spectra of the supernatants from a viable enrichment culture and a sterile control after 1 month of incubation. The strong absorption of HFBT at 392 nm was essentially removed by the viable culture.
FIG. 6.
UV-visible spectra of the supernatant from a viable enrichment culture growing on HFBT for 1 month (A) and a sterile control (B).
Compound VIII was often detected by GC-MS analysis of the dichloromethane extracts of acidified culture supernatants from SLPB cultures maintained on HFBT. Smaller amounts of the dione were detected in the corresponding sterile controls. This compound was never detected in the crystals of HFBT collected by sublimation. At neutral pH, benzothiophene-2,3-dione exists as compound VII (4). Thus, the detection of the 2,3-dione in our culture extracts implies that 2-mercaptophenylglyoxalate was formed from HFBT in the sterile controls and to a greater extent in the viable cultures.
To determine if HFBT could be mineralized by the SLPB enrichment cultures, 0.039 mmol of this compound was added to each of three viable cultures incubated in sulfate-containing mineral medium in sealed serum bottles. After 14 days of incubation, 50% ± 5.6% of the carbon from the HFBT was recovered as CO2. In the heat-killed controls, the amount of CO2 detected was equivalent to 6.9% ± 0.3% of the carbon added as HFBT. These results clearly demonstrated the mineralization of HFBT.
DISCUSSION
These investigations show that HFBT is a rather unstable intermediate of DBT biodegradation. In its crystal form and in dichloromethane solutions, it reacts to form thioindigo, cis-thioindigo, and a purple compound which we could not identify. HFBT is fairly stable at pH 11, and it is stable enough in neutral sulfate-free medium (Fig. 5) to allow culture studies over a 3-week incubation period.
The chemical synthesis method for HFBT was unsatisfactory, and Smiles and McClelland (24) stated that the presence of mineral acids (H2SO4 was used during the synthesis) resulted in a red condensation product, with the same chemical structure as the diketo form of compound III in Fig. 1. Kodama et al. (11) described compound III as violet compound that decomposed between 271 and 274°C, and they based their identification on elemental analysis and on the work of Smiles and McClelland (24). The purple compound that we detected as a contaminant of HFBT may have been compound III, because it was not sufficiently volatile for GC-MS or probe mass spectrometry.
The microbiological method of producing HFBT yielded a mixture of metabolites from which HFBT was quite easily purified by sublimation. The maximum yield of 80 mg of HFBT was only 4% of the theoretical yield from 2 g of DBT added to the culture. However, not all of the added DBT was consumed in the cultures, and there were other metabolites generated from DBT. GC confirmed that the sulfoxide and sulfone of DBT were produced by isolate BT1d. Kropp and Fedorak (16) reported that 9% of the DBT added to cultures of BT1d was converted to the sulfoxide and/or sulfone of DBT, which have been observed in the extracts of other bacterial cultures that degrade DBT by via the Kodama pathway (8, 9, 11, 17, 19).
GC also revealed the presence of thioindigo in the neutral extract of the isolate BT1d. This is the first report of thioindigo being found in a DBT-degrading culture. In order to detect this compound, the GC temperature program had to be modified so that a higher final temperature of 280°C was reached. In our previous work with DBT-degrading cultures (17), the final temperature was only 250°C, which presumably precluded the detection of thioindigo. We are not aware of any studies that have shown the biodegradation of thioindigo or its cis isomer.
In our analyses of the extracts of acidified culture supernatants from strain BT1d grown on DBT, we found no evidence of the presence of compounds IV (Fig. 1B), V, or VI (Fig. 2), which were reported by other investigators. However, we frequently identified compound VIII, indicating the presence of compound VII in the neutral medium. Because the 2,3-dione was found in sterile controls, it appears that HFBT is abiotically transformed to 2-mercaptophenylglyoxalate in mineral medium. However, this process appears to be accelerated by microbial activity because the peak corresponding to the 2,3-dione in the GC analyses was always substantially larger in the viable cultures than that in the sterile controls.
The biodegradation of compound VII has not been reported. Based on the numerous reports that have detected benzothiophene-2,3-dione and its methyl analogs in acidified culture extracts, these 2-mercaptophenylglyoxalates must be important intermediates in the biodegradation of benzothiophenes and dibenzothiophenes. For example, benzothiophene-2,3-dione has been found in cultures degrading benzothiophene (1, 4, 5) and DBT (1, 17). In addition, methylbenzothiophene-2,3-diones have been detected in cultures degrading isomers of methylbenzothiophene (14, 22) and isomers of methyldibenzothiophene (23) and 2,8-dimethyldibenzothiophene (18). Even dimethylbenzothiophene-2,3-diones have been observed in cultures degrading certain isomers of dimethylbenzothiophenes (15) and 3,4-dimethyldibenzothiophene (18).
The study by Mormile and Atlas (21) used a mixture of metabolites from DBT biodegradation and provided preliminary evidence that HFBT can be mineralized. However, our investigations using pure HFBT unequivocally demonstrated the release of CO2 from HFBT. Subtracting the amount of CO2 found in the sterile control indicates that approximately 43% of the carbon in HFBT was released as CO2. This is consistent with the proportion of carbon released from other sulfur heterocycles. Bressler et al. (3) observed that 57 and 62% of the carbon from benzothiophene sulfone and 3-methylbenzothiophene sulfone, respectively, were released as CO2 by a Pseudonocardia sp. growing on these compounds. Three bacterial isolates that grow on sulfolane (tetrahydrothiophene-1,1-dioxide) liberated 40 to 42% of the sulfolane C as CO2 (10).
Because of the limited supply of purified HFBT, we chose to use a medium that contained 7 mM sulfate for the growth of the HFBT-degrading mixed culture, rather than supplying HFBT as the sole sulfur source in sulfate-free medium. If all of the sulfur from HFBT had been released, this would have increased the total sulfate concentration by 0.49 mM. The high sulfate concentration in the medium precluded the detection of any sulfate that might have been formed by the release of the sulfur atom from HFBT. Other studies have shown that sulfur from thiophene rings can be used as the sole sulfur source for bacterial growth (3, 10, 25), so it is likely that HFBT would serve as a sole sulfur source. However, it has yet to be determined whether this is true.
There are three different modes of microbial attack on DBT (see reference 2 for a review). Two of these involve the initial oxidation of the sulfur atom, yielding DBT sulfone, and the third, the Kodama pathway, begins with the oxidation of one of the homocyclic rings (Fig. 1A). The so-called 4S pathway leads to the selective removal of the sulfur atom, leaving a 2-hydroxybiphenyl which is not used as a carbon source by Rhodococcus strain IGTS8, the most extensively studied bacterium that carries out this biodesulfurization. This mode of degradation occurs only in the absence of sulfate in the medium. Just one research group has reported the initial oxidation of the sulfur atom of DBT releasing sulfate followed by the cleavage of the homocyclic rings (25, 26). Brevibacterium sp. strain DO is the only bacterium described that attacks DBT in this manner, leaving 9% of the carbon from DBT as dissolved organic carbon in the medium (25). Based on the larger number of reports of bacteria producing HFBT (1, 9, 11, 12, 19), it appears that in an environment that is not sulfate limited, the Kodama pathway is likely the most common method for microbial degradation of DBT.
In addition, the Kodama pathway (Fig. 1A) is commonly used by bacteria to degrade substituted DBTs, and the formation of methyl-HFBTs has been observed in cultures degrading methyl-DBTs and dimethyl-DBTs. For example, the degradation of each of the four isomers of methyl-DBT yielded the corresponding methyl-HFBT (23). Lu et al. (20) demonstrated that 4,6-dimethyl-DBT could also be biodegraded to produce 7-methyl-HFBT, and Kropp et al. (18) observed that 3,4-dimethyl-DBT yielded 6,7-dimethyl-HFBT.
Thus, a better understanding of the biodegradation of HFBT will lead to an understanding of the biodegradation of the methyl-HFBTs produced from methyl-substituted DBTs. Our finding that benzothiophene-2,3-dione (arising from 2-mercaptophenylglyoxalate) originates from HFBT suggests that the methyl-substituted benzothiophene-2,3-diones observed in culture extracts likely arise from methyl-substituted HFBTs.
Our results suggest that the accumulation of HFBT in the environment would probably not occur because it is chemically quite unstable and because it is susceptible to mineralization. Now that it has clearly been shown that HFBT can be biodegraded and mineralized by mixed bacterial cultures, it can be hypothesized that the methyl-HFBTs may also be biodegraded and mineralized. The testing of this hypothesis awaits the purification of some methyl-HFBTs to use as substrates for microbial cultures.
ACKNOWLEDGMENT
This work was funded by the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Bohonos N, Chou T-W, Spanggord R J. Some observations on biodegradation of pollutants in aquatic systems. Jpn J Antibiot. 1977;30(Suppl.):275–285. [PubMed] [Google Scholar]
- 2.Bressler D C, Fedorak P M. Bacterial metabolism of fluorene, dibenzofuran, dibenzothiophene and carbazole. Can J Microbiol. 2000;46:397–409. [PubMed] [Google Scholar]
- 3.Bressler D C, Leskiw B K, Fedorak P M. Biodegradation of benzothiophene sulfones by a filamentous bacterium. Can J Microbiol. 1999;45:360–368. [PubMed] [Google Scholar]
- 4.Eaton R W, Nitterauer J D. Biotransformation of benzothiophene by isopropylbenzene-degrading bacteria. J Bacteriol. 1994;176:3992–4002. doi: 10.1128/jb.176.13.3992-4002.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedorak P M, Grbić-Galić D. Aerobic microbial cometabolism of benzothiophene and 3-methylbenzothiophene. Appl Environ Microbiol. 1991;57:932–940. doi: 10.1128/aem.57.4.932-940.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedorak P M, Peakman T M. Aerobic microbial metabolism of some alkylthiophenes found in petroleum. Biodegradation. 1992;2:223–236. [Google Scholar]
- 7.Fedorak P M, Westlake D W S. Fungal metabolism of n-alkylbenzenes. Appl Environ Microbiol. 1986;51:435–437. doi: 10.1128/aem.51.2.435-437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelstein Z I, Baskunov B P, Vavilova L N, Golovleva L A. Microbial transformation of dibenzothiophene and 4,6-dimethyldibenzothiophene. Microbiologiya. 1997;66:481–487. [Google Scholar]
- 9.Frassinetti S, Setti L, Corti A, Farrinelli P, Montevecchi P, Vallini G. Biodegradation of dibenzothiophene by a nodulating isolate of Rhizobium meliloti. Can J Microbiol. 1998;44:289–297. doi: 10.1139/w97-155. [DOI] [PubMed] [Google Scholar]
- 10.Greene E A, Beatty P H, Fedorak P M. Sulfolane degradation by mixed cultures and a bacterial isolate identified as a Variovorax sp. Arch Microbiol. 2000;174:111–119. doi: 10.1007/s002030000184. [DOI] [PubMed] [Google Scholar]
- 11.Kodama K, Nakatani S, Umehara K, Shimizu K, Minoda Y, Yamada K. Microbial conversion of petro-sulfur compounds. Part III. Isolation and identification of products from dibenzothiophene. Agric Biol Chem. 1970;34:1320–1324. [Google Scholar]
- 12.Kodama K, Umehara K, Shimizu K, Nakatani S, Minoda Y, Yamada K. Identification of microbial products from dibenzothiophene and its proposed oxidation pathway. Agric Biol Chem. 1973;37:45–50. [Google Scholar]
- 13.Kropp K G, Gonçalves J A, Andersson J T, Fedorak P M. Microbially mediated formation of benzonaphthothiophenes from benzo[b]thiophenes. Appl Environ Microbiol. 1994;60:3624–3631. doi: 10.1128/aem.60.10.3624-3631.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kropp K G, Gonçalves J A, Andersson J T, Fedorak P M. Microbial transformations of benzothiophene and methylbenzothiophenes. Environ Sci Technol. 1994;28:1348–1356. doi: 10.1021/es00056a025. [DOI] [PubMed] [Google Scholar]
- 15.Kropp K G, Saftić S, Andersson J T, Fedorak P M. Transformations of six isomers of dimethylbenzothiophenes by three Pseudomonas strains. Biodegradation. 1996;7:203–221. doi: 10.1007/BF00058180. [DOI] [PubMed] [Google Scholar]
- 16.Kropp K G, Fedorak P M. A review of the occurrence, toxicity and biodegradation of condensed thiophenes found in petroleum. Can J Microbiol. 1998;44:605–622. doi: 10.1139/cjm-44-7-605. [DOI] [PubMed] [Google Scholar]
- 17.Kropp K G, Anderson J T, Fedorak P M. Bacterial transformations of 1,2,3,4-tetrahydrodibenzothiophene and dibenzothiophene. Appl Environ Microbiol. 1997;63:3032–3042. doi: 10.1128/aem.63.8.3032-3042.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kropp K G, Anderson J T, Fedorak P M. Biotransformations of three dimethyldibenzothiophenes by pure and mixed bacterial cultures. Environ Sci Technol. 1997;31:1547–1554. [Google Scholar]
- 19.Laborde A L, Gibson D T. Metabolism of dibenzothiophene by a Beijerinckia sp. Appl Environ Microbiol. 1977;34:783–790. doi: 10.1128/aem.34.6.783-790.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, Nakajima-Kambe T, Shigeno T, Ohbo A, Nomura N, Nakahara T. Biodegradation of dibenzothiophene and 4,6-dimethyldibenzothiophene by Sphingomonas paucimobilis strain TZS-7. J Biosci Bioeng. 1999;88:293–299. doi: 10.1016/s1389-1723(00)80012-2. [DOI] [PubMed] [Google Scholar]
- 21.Mormile M R, Atlas R M. Mineralization of the dibenzothiophene biodegradation products 3-hydroxy-2-formylbenzothiophene and dibenzothiophene sulfone. Appl Environ Microbiol. 1988;54:3183–3184. doi: 10.1128/aem.54.12.3183-3184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saftić S, Fedorak P M, Andersson J T. Diones, sulfoxides, and sulfones from the aerobic cometabolism of methylbenzothiophenes by Pseudomonas strain BT1. Environ Sci Technol. 1992;26:1759–1764. [Google Scholar]
- 23.Saftić S, Fedorak P M, Andersson J T. Transformations of methyldibenzothiophenes by three Pseudomonas isolates. Environ Sci Technol. 1993;27:2577–2584. [Google Scholar]
- 24.Smiles S, McClelland E W. Derivatives of 3-oxy(1)thionaphthene. J Chem Soc. 1921;119:1810–1816. [Google Scholar]
- 25.van Afferden M, Schacht S, Klein J, Trüper H G. Degradation of dibenzothiophene by Brevibacterium sp. DO. Arch Microbiol. 1990;153:324–328. [Google Scholar]
- 26.van Afferden M, Tappe D, Beyer M, Trüper H G, Klein J. Biochemical mechanisms for the desulfurization of coal-relevant organic sulfur compounds. Fuel. 1993;72:1635–1643. [Google Scholar]
- 27.Yamada K, Minoda Y, Kodama K, Nakatani S, Akasaki T. Microbial conversion of petro-sulfur compounds. Part I. Isolation and identification of dibenzothiophene-utilizing bacteria. Agric Biol Chem. 1968;32:840–845. [Google Scholar]