Abstract
With the submersible JAGO and by scuba diving we discovered three remarkable geothermal cones, rising 33, 25, and 45 m from the seafloor at a depth of 65 m in Eyjafjordur, northern Iceland. The greatest geothermal activity was on the highest cone, which discharged up to 50 liters of freshwater per s at 72°C and pH 10.0. The cones were built up from precipitated smectite, formed by mixing of the hot SiO2-rich geothermal fluid with the cold Mg-rich seawater. By connecting a rubber hose to one outflow, about 240 liters of pure geothermal fluids was concentrated through a 0.2-μm-pore-size filter. Among 50 thermophilic isolates, we found members of Bacillus and Thermonema and a new unidentified low-G+C gram-positive member of the Bacteria as well as one member of the Archaea, Desulfurococcus mobilis. Analysis of small-subunit rRNA genes PCR amplified and cloned directly from environmental DNA showed that 41 out of 45 Bacteria sequences belonged to members of the Aquificales, whereas all of the 10 Archaea sequences belonged to the Korarchaeota. The physiological characteristics of isolates from different parts of the cones indicate a completely freshwater habitat, supporting the possibility of subterranean transmittance of terrestrial organisms.
Since the discovery of hydrothermal activity on oceanic spreading centers in the Eastern Pacific in 1977 and 1979, hot springs have been found at numerous locations on ridge systems (7, 24). The Mid-Atlantic ridge is one of these systems, and Iceland, with its volcanic activity, is the only place where it emerges from the sea. The Eyjafjordur region, in northern Iceland, is one of several localities in Iceland with known submarine geothermal activity. The geothermal activity is in basaltic lava 6 to 12 million years of age (5, 14) and seems to be related to either one or both of two NNE- and NW-trending fault zones with no known surface hot spring activity (4).
Oceanic hydrothermal vent fluids originate from seawater, which percolates into the oceanic crust and is heated at the top of magma chambers or in hot rock formations. The hot fluid, discharged from the seafloor, is anoxic and acidic, with salinity varying from 0.1 to 2 times that of seawater and with variable chemical composition (29). Elements dissolved in the hydrothermal fluid precipitate around the vents, commonly forming characteristic chimney-like structures (6, 9). In such an extreme environment, diverse types of thermophilic microorganisms have been detected and isolated (21).
Molecular phylogenetic studies on environmental DNA from hydrothermal vent samples have been limited due to low quantity and poor quality of the collected biomass. Part of the problem at submarine hot springs is that they are difficult to access and samples are therefore precious. A variety of sampling devices have been used to collect hydrothermal vent samples for chemical and microbiological analysis. These include samplers for collecting hot fluid, for collecting particles from both warm and hot fluids, and for measuring in situ microbial activity in hydrothermal fluids (3, 20).
We report here the discovery of a new type of microbial habitat, where hot fresh water discharges from hydrothermal vents on the seafloor, building up conical structures as high as 45 m above the seafloor. A new technique was developed for sampling biomass from such an environment, and we showed by cultivation and by molecular phylogenetic survey that a community of thermophilic microorganisms of terrestrial origin is thriving in this unique ecosystem.
MATERIALS AND METHODS
Study site and sample collection.
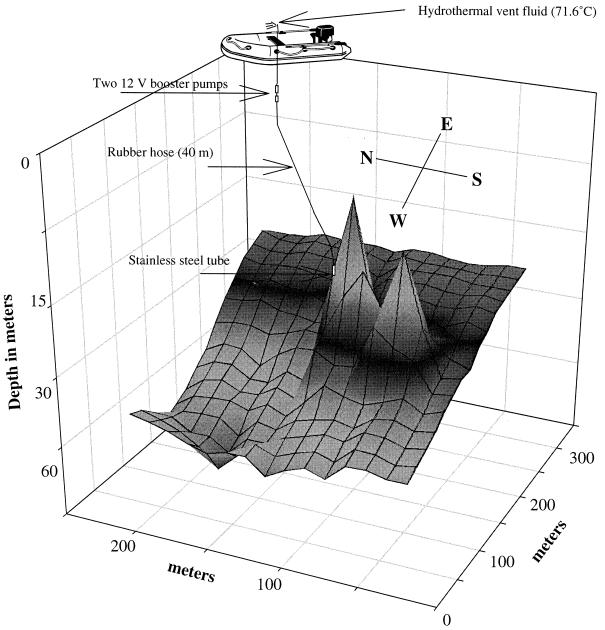
The bottom at the study site was surveyed with an echo sounder, and a contour map was constructed by using SigmaPlot (version 4.01). Solid samples were brought up to the surface in plastic bags, but hot fluid was collected in syringes and by pumping large volumes to the surface. A stainless steel tube (0.4 m by 10 mm) attached to a rubber hose was placed by a scuba diver in a discharge opening at a depth of 27.5 m. Two successive 12-V booster pumps were mounted inside the tubing a few meters below the sea surface. The other end of the tube was attached to a rubber dinghy (Fig. 1). The whole system was rinsed with the hot fluid (around 2 liters min−1) for 30 min before sampling hot fluid for chemical and microbial analysis. The vent fluid was concentrated directly by cross-flow filtration through sterile hollow fiber cartridges (0.2-μm-pore-size filter; Amicon). The cells retained inside the cartridge (600 ml) were concentrated further in the laboratory by centrifugation.
FIG. 1.
Contour map of the new vent site showing the giant cone structures and the sampling technique.
Chemical analysis.
The hydrothermal vent fluid was analyzed as described by Kristmannsdottir et al. (16), and the cone composition was determined by X-ray diffraction, X-ray fluorescence, and energy-dispersive X-ray analysis (EDX) electron microprobe.
Microscopy.
Cultivated and noncultivated cells were observed with a Leica DM LB light microscope equipped with a phase-contrast oil immersion objective (magnification, ×100). In situ hybridizations were performed as described by Harmsen et al. (12). Fixed cells were applied to gelatin-coated slides, dried, rinsed in a series of ethanol concentrations, and hybridized with ARC915 and EUB338 fluorescein-labeled probes. The cells were viewed in a Leica microscope equipped with a 100-W halogen light source for fluorescence and I3 blue BP (for fluorescein) and N2.1 green BP (for rhodamine) filter cubes.
Enrichments and isolations.
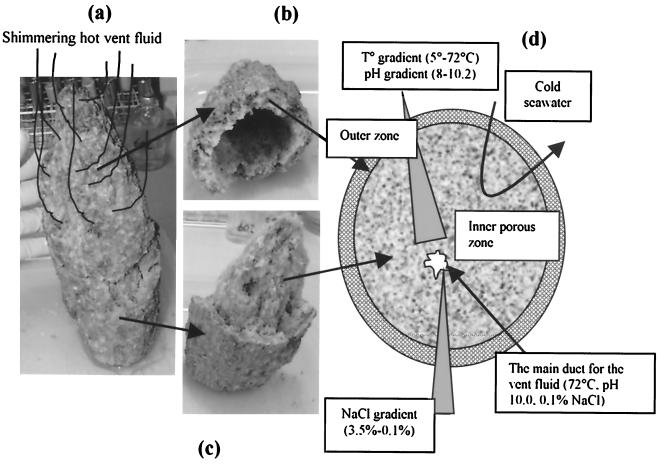
Rock samples and concentrated water samples were used to enrich for chemolithotrophic and chemorganotrophic organisms in different media under both aerobic and anaerobic conditions. A small chimney, approximately 40 cm high and 15 cm wide (Fig. 2), located close to the top of the main cone was brought to the surface in one piece. Subsamples across the chimney were inoculated into standard medium for aerobic thermophiles (8) but also in modified media with different salinities and pHs. The following agar and liquid media were used: marine broth (MB; Difco) diluted in 1/4 and 1/2 (designated MB4 and MB2) with 0 to 1% (wt/vol) NaCl, DT medium (19) with 0 to 2% (wt/vol) NaCl, R2A medium with 0 to 1% (wt/vol) NaCl (19), and 166 medium (22) with 0 to 2% (wt/vol) NaCl. All media were prepared with distilled water, but 166 medium and MB were also prepared with the vent fluid. Agar media had 28 g of agar per liter. The pH was also increased to 9.5 in 166 medium with 0 to 1% (wt/vol) NaCl. Aerobic enrichments were incubated at 60, 65, and 72°C until growth was observed. Colonies were selected and purified by streaking onto the same agar medium.
FIG. 2.
(a) Small chimney; (b) top part of the chimney; (c) bottom part of the chimney; (d) schematic diagram of the chimney showing the different sampling zones.
Anaerobic enrichments were incubated at 65 and 83°C. Anaerobiosis was achieved by applying a vacuum to the medium and saturating it with N2 or H2-CO2 (80% H2 and 20% CO2). Finally, the medium was reduced by adding a sterile solution of Na2S · 9H2O (final concentration, 0.025% [wt/vol]). The following media were used: medium prepared with the vent fluid, supplemented with 0.4% (wt/vol) yeast extract and S° (5 g liter −1), and cultivated with H2-CO2 as the gas phase; modified Thermotoga medium (20) with and without NaCl; and YPS medium (10).
Physiological tests.
The physiological characteristics of all aerobic isolates and of one anaerobic isolate were examined. Fresh cultures were used as inocula to test growth at different temperatures, pHs, and salinities. Aerobic strains were tested for growth at 60, 65, 72, and 76°C in liquid and on solid agar medium. Salinity testing was performed at 65°C on 166 agar medium and on MB2. The salinities were 0, 1, 2, 3, and 4% (wt/vol) NaCl. The pH was adjusted with NaOH and checked after sterilization. HEPES (Sigma) buffer (1 g liter −1) was used for pH tests at 9.0, and AMPSO (Sigma) buffer was used at pH 10.0. The anaerobic strain was tested for growth at 55, 65, 90, and 93°C and at 0, 0.5, and 1% (wt/vol) NaCl.
DNA extraction and PCR amplification.
DNA was extracted from the biomass obtained by filtration and from the isolated strains. The biomass pellet was homogenized by using a homogenizer before incubation at 50°C for 3 h in a lysis buffer (1% [wt/vol] sarcosyl, 1% [wt/vol] sodium dodecyl sulfate, proteinase K [1 mg/ml; Sigma], lysozyme [2 mg/ml; Sigma]). DNA was extracted with phenol–chloroform-isoamyl alcohol (24:1) and precipitated with ethanol. The PCR amplifications were performed as described by Skirnisdottir et al. (23). The oligonucleotide primers used for detection of Archaea were 1391R, 23FPL, and R1544; F9B was used for Bacteria.
Cloning and sequencing of 16S ribosomal DNA (rDNA).
The PCR products from the biomass were cloned directly by the TA cloning method by using the TOPO TA cloning kit according to the manufacturer's instructions (Invitrogen). Plasmid DNAs from single colonies were isolated and sequenced with universal reverse and forward M13 vector primers, with the reverse primer R805 (5′-GACTACCCGGGTATCTAATCC-3′) and with an ABI 377 DNA sequencer by using BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems). PCR products from isolates were sequenced with the ABI 377 sequencer by using the R805 sequencing primer. The 16S rRNA PCR products from strain SEA were cloned and 1,351 bp was sequenced. The following Archaea-specific sequencing primers were used: forward primers 23FPL, F515, and F23 and reverse primers R805 and R1391.
Phylogenetic analysis.
The sequences (400 to 500 bp) were analyzed with DNA Sequencing Analysis software, version 3.2 (PE Applied Biosystems). Sequences from isolates and environmental sequences were selected and identified with BLAST searches. The sequence from strain SEA was manually aligned (1,351 bp) with closely related sequences obtained from the Ribosomal Database Project. Phylogenetic analysis was performed with the ARB program (http://www.mikro.biologie.tu-muenchen.de), omitting regions of sequence ambiguity. A phylogenetic tree was constructed using the neighbor-joining algorithms with the Jukes and Cantor correction included in the ARB package and maximum-likelihood analyses of the data sets.
RESULTS
Discovery of the geothermal cones.
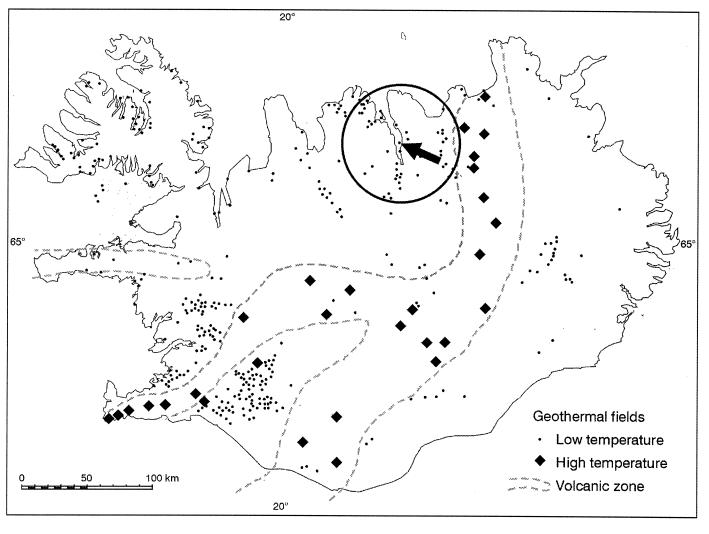
The new submarine hydrothermal field is located 1.8 km offshore in the northeastern part of the fjord Eyjafjordur, near 65°49.73′N latitude and 18°06.69′W longitude (Fig. 3). The vents occur on the east slope, which rises from a depth of 100 m from the center of the fjord. At about a depth of 65 m, three giant silicate cone structures have grown at the site to heights of 33, 25, and 45 m above the sea bottom (Fig. 1). The 33-m cone was first discovered in 1997 with the German research submersible JAGO (25), but the 45-m cone was unexpectedly discovered in 1998 during a scuba diving expedition. Most of the hydrothermal activity is in the highest cone, with fluid of 60 to 72°C flowing from small chimneys and fissures located all over it. Very little activity was found on the two smaller cones. Estimating the flow rate was difficult, but the cumulative flow could be on the order of 50 liters per s. The underlying mound is made of sediments, shells, and precipitates from the fluid. The cones contain a high proportion of poorly crystalline clay-like silicates. The vent system appears to be related to the intersection of two main fault zones, one NW trending and the other parallel to the predominant NE tectonic trend in the area and the main dyke direction. The chimneys follow the NE trend (Fig. 1).
FIG. 3.
Map showing the geothermal fields in Iceland. The arrow shows the location of the submarine hydrothermal vent field in the Eyjafjordur fjord.
Chemical analysis of the fluid.
The sampled hydrothermal fluid had only about 0.1% contamination by seawater and was very similar in composition to the geothermal waters in springs and wells onshore (26) (Table 1). Both the chemical properties and stable isotope ratios of the fluids were almost identical to those of waters of similar temperature from other geothermal fields in the Eyjafjordur region. The conductivity of the purest water obtained was 290 μS/cm, but the conductivity was 415 μS/cm in the sample used for chemical analysis. The pH was 10.0. The concentration of hydrogen sulfide was 0.3 mg liter −1, and concentrations of heavy metals were also low. Small volumes of the vent water taken in syringes were contaminated by 1.5% seawater.
TABLE 1.
Chemical composition of the hydrothermal vent fluid
| Parameter | Vent fluid | Onshore geothermal water (26) | Seawatera |
|---|---|---|---|
| Temp (°C) | 71.4 | 80 | |
| pH temp (°C) | 10.03/24 | 9.95/20 | 8.0/25 |
| H2S (mg/liter) | 0.32 | 0.09 | 0 |
| Carbonate (mg/liter) | 25 | 13 | 102 |
| Total dissolved (mg/liter) | 291 | 223 | |
| Silica (mg/liter) | 93.7 | 92.3 | 2.9 |
| Sodium (mg/liter) | 79.2 | 53 | 10,800 |
| Potassium (mg/liter) | 1.62 | 1.0 | 390 |
| Calcium (mg/liter) | 2.45 | 3.6 | 410 |
| Magnesium (mg/liter) | 2.59 | 0.006 | 1,290 |
| Sulfate (mg/liter) | 19.5 | 43 | 2,700 |
| Chloride (mg/liter) | 44.7 | 11 | 19,400 |
| Fluoride (mg/liter) | 0.86 | 0.47 | 1.3 |
| Bromide (mg/liter) | 0.19 | 67.3 | |
| Boron (mg/liter) | 0.19 | 0.16 | 4.45 |
| Aluminum (mg/liter) | 0.122 | 0.10 | 0.001 |
| Iron (mg/liter) | 0.0081 | 0.012 | 0.003 |
| Manganese (mg/liter) | 0.0001 | 0.002 | 0.0004 |
| δ 18O (‰) | −13.13 | −14.12 | 0 |
| δ D (‰) | −92.9 | −102.2 | 0 |
| δ 13C (‰) | −9.44 | ||
| Apparent 14C age (yrs) | 11,000 ± 90 | ||
| Mercury (mg/liter) | 0.000014 | 0.00015 | |
| Copper (mg/liter) | 0.0012 | 0.0009 | |
| Arsenic (mg/liter) | 0.0208 | 0.0026 | |
| Cadmium (mg/liter) | 0.00011 | 0.0001 | |
| Lead (mg/liter) | 0.0002 | 0.00003 | |
| Chromium (mg/liter) | 0.0010 | 0.0002 | |
| Zinc (mg/liter) | 0.0008 | 0.005 |
Seawater according to Turekian (28); not measured.
Chemical analysis of chimney material.
The solid samples were mainly composed of poorly crystalline clay minerals, amorphous silica, and minor carbonate. No metallic sulfides, typical of deep-sea hydrothermal chimneys, were observed, reflecting the low sulfide and metal content of the dilute hydrothermal fluid. A bulk sample from one chimney contained about 50% (by weight) SiO2 and up to 25% (by weight) MgO. The main Mg-bearing mineral was Mg-rich smectite (saponite), which has an approximate formula of (Ca, Na)0.2Mg6Si7.2Al0.8O20 (OH)4 · nH2O, based on electron microprobe and EDX analyses. Anhydrite (CaSO4) was not present.
Microscopic observation.
From a vent at a depth of 27.5 m, about 240 liters of hot vent fluid (71.6°C) was pumped; it was concentrated to 600 ml by filtration and then centrifuged and pelleted in an Eppendorf tube. In the concentrated fluid, coccoid and rod-shaped bacteria were detected by phase-contrast and epifluorescence microscopy (acridine orange staining). Specific-fluorescence oligonucleotide universal 16S rRNA probes for Bacteria (EUB338) and Archaea (ARC915) revealed coccoid and rod-shaped cells belonging to both domains (data not shown). rRNA detection by this technique indicated that the cells were still intact. Spores were detected in 44 out of 50 isolated aerobic strains.
Enrichments and isolations.
Growth was observed mainly on MB2 and 166 agar medium. The time of development of colonies varied from 2 days to 3 weeks. Only colonies from agar plates were selected and purified by streaking on the same agar media at least six times. A total of 50 aerobic strains whose colonies were gray, transparent, or pale yellow were isolated. Thirty strains were isolated from the inner zone of the chimney after enrichment at 65 to 72°C. Twenty-six were isolated on MB2 plates, one was isolated on 166 medium with 1% NaCl (pH 9.5), and one was isolated on R2A medium with 1% NaCl. Two strains were isolated on MB2 and on 166 agar medium from enrichment in complementary liquid media. Twelve strains from pure vent fluid were isolated on various agar media with low salt concentrations at 60 to 72°C. Five of the strains were enriched in liquid media and isolated on complementary agar media. Seven pale yellow strains were isolated from the outer part of the chimney where the vent fluid mixes with seawater. Only two anaerobic enrichments yielded growth. The enrichments, inoculated in medium that consisted of the hydrothermal fluid with 0.4% yeast extract and elemental sulfur (5 g liter −1), showed coccoid cells after incubation at 65 and 85°C. One strain (SEA) was isolated at 85°C after six successful serial dilutions.
Physiological characterization.
The isolates were characterized according to maximum growth temperature, salt tolerance, and pH (Table 2). All the aerobic strains grew at 60 and 65°C, and all except two grew in the presence of 1% NaCl. Forty isolates grew at 72°C and 25 grew at 76°C. At least 40 isolates grew at 2% NaCl, and 7 grew at 4% NaCl. All strains grew at pH 9.0 but only 20 grew at pH 10. The anaerobic strain SEA did not grow at 0.5% NaCl or above 93°C.
TABLE 2.
Origin and characterization of aerobic isolates
| Sample type or source | No. of growing isolates
|
Identificationa | Match (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Temp of 76°C | NaCl concn in medium of:
|
pH of 10.0 | ||||||
| 1.0% | 2.0% | 3.0% | 4.0% | |||||
| Concentrated fluid (total, 12 isolates) | 3 | 2 | 0 | 0 | 0 | 3 | Bacillus thermoleovorans | 95 |
| 1 | 1 | 1 | 1 | 0 | 1 | Bacillus caldotenax | 98 | |
| 0 | 8 | 6 | 0 | 0 | 8 | Bacillus flavothermus | 97–99 | |
| Total isolates (%) | 33 | 92 | 58 | 8 | 0 | 100 | ||
| Chimney inner zone (total, 30 isolates) | 14 | 15 | 14 | 14 | 0 | 3 | Bacillus thermoleovorans | 97–99 |
| 1 | 1 | 1 | 0 | 0 | 1 | Bacillus caldotenax | 98 | |
| 1 | 2 | 0 | 0 | 0 | 2 | Bacillus caldovelox | 96–97 | |
| 0 | 2 | 2 | 2 | 0 | 0 | Bacillus thermodenitrificans | 97–98 | |
| 2 | 2 | 2 | 2 | 0 | 0 | Bacillus caldoxylolyticus | 96 | |
| 3 | 7 | 7 | 5 | 0 | 2 | Gram+, low G+C content | 93–96 | |
| Total isolates (%) | 70 | 97 | 87 | 77 | 0 | 27 | ||
| Chimney outer zone (total, 7 isolates) | 0 | 7 | 7 | 7 | 7 | 0 | Thermonema sp. | 96–99 |
| Total isolates (%) | 0 | 100 | 100 | 100 | 100 | 0 | ||
Database (BLAST) match of the SSU rRNA gene.
Cloning and sequence analysis of SSU rRNA.
DNA was successfully extracted from environmental biomass and from isolated strains. Analysis of small-subunit (SSU) rRNA partial sequences (ca. 400 bp) revealed that all the aerobic isolates belonged to the Bacteria and that the anaerobic strain SEA belonged to the Archaea. Most of the Bacteria isolates belong to the genus Bacillus, but seven belong to Thermonema (Table 2). Total sequencing of the SSU rRNA gene (1,351 bp) from the Archaea strain SEA placed it in the genus Desulfurococcus, and the generated similarity matrix put it as 99.5% similar to D. mobilis. Sequencing of environmental clones revealed both Bacteria (45 clones) and Korarchaea (10 clones) sequences (Table 3). Most of the Bacteria clones were closest to the order Aquificales, and one was close to unidentified Nitrospira. Three clones were closest to the divisions Proteobacteria and Firmicutes. None of the isolated strains belonged to the genus Thermus, nor was this genus represented by any of the cloned sequences.
TABLE 3.
Molecular diversity analysis of environmental DNA from the Eyjafjordur cones
| Type sequence | No. of clones | Bacterial division | Closest database match (%) |
|---|---|---|---|
| Bacteria library | |||
| ST22 | 1 | Nitrospira group | Unidentified (OPB67A) (97) |
| ST56 | 15 | Aquificales | Hydrogenobacter thermophilus TK-6 (90) |
| ST10 | 26 | Aquificales | EM17 (97) |
| ST43 | 1 | Firmicutes | Propionibacterium acnes (96) |
| ST12 | 1 | α-Proteobacteria | Caulobacter crescentus (99) |
| ST50 | 1 | β-Proteobacteria | Alcaligens sp. (99) |
| Archaea library ST89 | 10 | Korarchaeota | Clone pJP78 (99) |
DISCUSSION
Our findings indicate that this freshwater hydrothermal vent system, located on the seafloor at a depth of 65 m, is a new kind of submarine geothermal vent system and a novel environment for thermophilic microorganisms that extends the known ecological habitats for thermophiles.
Sampling large quantities of hydrothermal vent fluid from submarine hot springs has been very difficult until now. Here we describe a simple technique that can provide large quantities of pure hydrothermal fluid from sites that can be reached by scuba diving. To our knowledge, such large volumes have never before been retrieved from submarine hydrothermal systems.
The location of the vent site places it within submarine hot springs, and its large precipitate cone structures resemble chimney structures in the deep-sea hydrothermal vents. However, the chemical composition of the cones and the discharging fluid was different from that of any previously described submarine hydrothermal vent system. The Mg-rich clays have formed as hot alkaline SiO2-rich, geothermal fluids have mixed with cold Mg-rich seawater. Anhydrite is the dominant chimney-building mineral in deep-sea hydrothermal vents but was not present in the Eyjafjordur vent. The cones were composed mainly of clay minerals that are rare in most seafloor hydrothermal chimneys and have never before been found as the dominant mineral phase. However, these minerals are commonly encountered as precipitates in geothermal wells in Iceland (18). The chemical and microbial analyses indicated that the fluid originates from the surrounding terrestrial groundwater that has been filtrated through the crust below the Eyjafjordur fjord. The apparent 14C age of the vent fluid is 11,000 years, similar to that of onshore geothermal water in the region (26). The stable isotopic ratios (δ D and δ 18O) suggest that the water has recharge, from the high inland mountains located about 100 km south of Eyjafjordur (17).
The maximum temperature measured in the system was about 72°C, but the reservoir temperature is unknown. However, the silica geothermometer (11) indicated reservoir temperatures of about 80°C. Moreover, the highest growth temperature of the isolates was approximately 92°C; this also indicates a higher reservoir temperature than 72°C.
The presence of apparently purely terrestrial thermophiles in the submarine hot hydrothermal fluid confirms the freshwater origin of the fluid and that the seawater does not penetrate the principal geothermal channels below the seafloor. However, small chimneys located on the giant cones have porous structures and are made of two mineralogical zones. Ambient aerobic seawater could possibly penetrate these zones, mix with the hot fluid, and form small niches for thermophilic microorganisms that are influenced by different environmental factors. The origin and the characterization of the strains (Table 2) isolated from small chimneys suggest habitats of various temperatures, pHs, and salinities. The most halotolerant strains were isolated from the outer zone of the chimney. They grew aerobically at pH 9.0 and in the presence of 4% (wt/vol) NaCl, but not at pH 10.0 or at 76°C. This suggests that cold seawater mixes into the vent fluid in the outer zone with increasing salinity and decreasing temperature. Strains isolated from the inner part had broader growth ranges. None of them grew at 4% NaCl, many grew at 3% NaCl (77%) and at 76°C (70%), but only a few grew at pH 10.0 (27%). This indicates decreasing seawater mixing in the inner part of the chimney. All 12 aerobic strains isolated from concentrated fluid grew at pH 10.0, but only 1 grew above 2.0% NaCl. Moreover, the anaerobic strain D. mobilis SEA was halosensitive and oxygen sensitive, indicating a very deep origin. Furthermore, molecular phylogenetic analysis of biomass from the pure vent fluid identified terrestrial microorganisms that have been detected in terrestrial hot springs in Iceland (23) and Yellowstone National Park in the United States (2). Most of the cloned sequences were closely related to either Aquificales or Korarchaeota (2).
Surprisingly, no Thermus strain was isolated from the samples, although this genus is widely distributed in terrestrial and coastal springs in Iceland (1, 15). Most of the isolates belonged to known Bacillus species widely found in Icelandic hot springs, but some new, unidentified species with a low G+C content were also isolated. This new group was more halotolerant than the Bacillus species and showed the closest database match to strains isolated from a marine environment in Japan (27). Seven marine species belonging to the genus Thermonema were isolated only from the outer zone of the chimney, but members of this genus have not been isolated before from Icelandic hot springs.
The origin of the microbial community in the submarine hot springs is at present unclear. Nevertheless, it is likely that the thermophilic marine species have disseminated in the sea to the vent fields (13). Spore-forming terrestrial thermophiles could also have disseminated by air or runoff water from land to the vent field at the sea bottom. However, it is more likely that terrestrial anaerobes, such as D. mobilis, have penetrated though dikes and faults at least 1.8 km from land to the vent site (Fig. 3). This hypothesis is further supported by the apparently complete absence of halotolerant Thermus species in this system.
ACKNOWLEDGMENTS
We thank Erlendur Bogason for scuba diving and Jon Benjamínsson for useful information. Thanks are also due to Solveig Olafsdottir for technical assistance. We are grateful to the JAGO team (J. Schauer and K. Hissmann) and the crew of RV Poseidon.
This work was supported by a grant from the Icelandic National Research Council.
REFERENCES
- 1.Alfredsson G A, Kristjánsson J K. Ecology, distribution, and isolation of Thermus. In: Sharp R, Williams R, editors. Thermus species. Biotechnology handbook. New York, N.Y: Plenum Press; 1995. pp. 43–66. [Google Scholar]
- 2.Barns S M, Fundyga R E, Jeffries M W, Pace N R. Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc Natl Acad Sci USA. 1994;91:1609–1613. doi: 10.1073/pnas.91.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baross J A, Deming J W. Growth at high temperatures: isolation and taxonomy, physiology and ecology. In: Karl D M, editor. The microbiology of deep-sea hydrothermal vents. Boca Raton, Fla: CRC Press; 1995. pp. 169–217. [Google Scholar]
- 4.Björnsson A. Exploration of low-temperature geothermal fields for district heating in Akureyri, North Iceland. Geothermal Resour Counc Trans. 1981;5:495–498. [Google Scholar]
- 5.Björnsson A, Saemundsson K. Orkustofnun report (OSJHD 7557). Reykjavík, Iceland: Orkustofnun; 1975. Geothermal activity in the vicinity of Akureyri; p. 53. [Google Scholar]
- 6.Converse D R, Holland H D, Edmond J M. Flow rates in the axial hot springs of the East Pacific Rise (21°N): implications for the heat budget and the formation of massive sulfide deposits. Earth Planet Sci Lett. 1984;69:159–175. [Google Scholar]
- 7.Corliss J B, Dymond J, Gordon L I, Edmond J M, von Herzen R P, Ballard R D, Green K, Williams D, Bainbridge A, Crane K, van Andel T H. Submarine thermal spring on the Galapagos Rift. Science. 1979;203:1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- 8.Degryse E, Glansdorff N, Pierard A. A comparative analysis of extreme thermophilic bacteria belonging to the genus Thermus. Arch Microbiol. 1978;117:189–196. doi: 10.1007/BF00402307. [DOI] [PubMed] [Google Scholar]
- 9.Edmond J M, Van Damm K L, MacDuff R E, Measures C I. Chemistry of hot springs on the East Pacific Rise and their effluent dispersal. Nature. 1982;297:187–191. [Google Scholar]
- 10.Erauso G, Reysenbach A-L, Godfroy A, Meunier J R, Crump B, Parensky F, Baross J A, Marteinsson V T, Barbier G, Pace N R, Prieur D. Pyrococcus abyssi sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Arch Microbiol. 1993;160:338–349. [Google Scholar]
- 11.Fournier R O. Chemical geothermometers and mixing models for geothermal systems. Geothermics. 1977;5:41–50. [Google Scholar]
- 12.Harmsen H J M, Prieur D, Jeanthon C. Group-specific 16S rRNA-targeted oligonucleotide probes to identify thermophilic bacteria in marine hydrothermal vents. Appl Environ Microbiol. 1997;63:4061–4068. doi: 10.1128/aem.63.10.4061-4068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber R, Stoffers P, Cheminee J L, Richnow H H, Stetter K O. Hyperthermophilic archaebacteria within the crater and open-sea plume of erupting Macdonald Seamount. Nature. 1990;345:179–181. [Google Scholar]
- 14.Johannesson H, Saemundsson K. Geological map of Iceland (1:500000). Reykjavík, Iceland: Icelandic Institute of Natural History; 1998. [Google Scholar]
- 15.Kristjansson J K, Hreggvidsson G O, Alfredsson G A. Isolation of halotolerant Thermus spp. from submarine hot springs in Iceland. Appl Environ Microbiol. 1986;52:1313–1316. doi: 10.1128/aem.52.6.1313-1316.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristmannsdottir H, Armannsson H, Sigurđsson K H, Björnsson J, Olafsson M, Hauksdottir S, Hardardottir V. Orkustofnun report (HK-HA-KHS-JÖB-MO-SH-VH 07). Reykjavík, Iceland: Orkustofnun; 1999. Sample treatment and analytical methods used at Orkustofnun laboratory; p. 2. [Google Scholar]
- 17.Kristmannsdottir H, Johnsson S. Chemistry and stable isotope composition of geothermal waters in the Eyjafjördur region, northern Iceland. Jökull. 1982;32:83–90. [Google Scholar]
- 18.Kristmannsdottir H. Magnesium silicate scaling in district heating systems in Iceland. Geothermics. 1989;18:191–198. [Google Scholar]
- 19.Marteinsson V T, Kristjansson J K. Bakterier i varmtvannssystemer. Report no. 544. Copenhagen, Denmark: Nordistk Mineterraad; 1991. Enumeration of thermophilic bacteria in district heating system in Iceland. [Google Scholar]
- 20.Marteinsson V T, Birrien J-L, Prieur D. In situ enrichment and isolation of thermophilic microorganisms from deep-sea environments. Can J Microbiol. 1997;43:694–697. [Google Scholar]
- 21.Prieur D, Erauso G, Jeanthon C. Hyperthermophilic life at deep-sea hydrothermal vents. Planet Space Sci. 1995;43:115–122. doi: 10.1016/0032-0633(94)00143-f. [DOI] [PubMed] [Google Scholar]
- 22.Skirnisdottir, S., G. O. Hreggvidsson, O. Holst, and J. K. Kristjansson. Isolation and characterization of a mixotrophic sulfur-oxidizing Thermus scotoductus. Extremophiles, in press. [DOI] [PubMed]
- 23.Skirnisdottir S, Hreggvidsson G O, Hjörleifsdottir S, Marteinsson V T, Petursdottir S, Holst O, Kristjansson J K. Influence of sulfide and temperature on species composition and community structure of hot spring microbial mats. Appl Environ Microbiol. 1999;66:2835–2841. doi: 10.1128/aem.66.7.2835-2841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiess F N, Macdonald K C, Atwater T, Ballard R, Carranza A, Cordoba D, Cox C, Diaz Garcia V M, Francheteau J, Guerrero J, Hawkins J, Haymon R, Hessler R, Juteau T, Kastner M, Larson R, Layendyk B, Mcdougall J D, Miller S, Normark W, Orcutt J, Rangin C. East Pacific Rise: hot springs and geophysical experiments. Science. 1980;207:1421–1433. doi: 10.1126/science.207.4438.1421. [DOI] [PubMed] [Google Scholar]
- 25.Stoffers P, Botz R, Garbe-Schönber D, Hannington M, Hauzel B, Herzig P, Hissman K, Huber R, Kristjansson J K, Petursdottir S K, Schauer J, Schmitt M, Zimmer M. Kolbeinsey Ridge, cruise report Poseidon 229a. Kiel, Germany: Geologisch Palaontologisches Institut; 1997. [Google Scholar]
- 26.Sveinbjörnsdottir A E, Arnorsson S, Heinemeier J, Boaretto E. Proceedings of the 9th International Symposium on Water-Rock Interaction. A. A. Rotterdam, The Netherlands: Balkema; 1998. Geochemistry of natural waters in Skagafjördur, N-Iceland. II. Isotopes; pp. 653–656. [Google Scholar]
- 27.Takami H, Inoue A, Fuji F, Horikoshi K. Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol Lett. 1997;152:279–285. doi: 10.1111/j.1574-6968.1997.tb10440.x. [DOI] [PubMed] [Google Scholar]
- 28.Turekian K K. The oceanic streams and atmosphere. In: Wedwpohl K H, editor. Handbook of geochemistry. New York, N.Y: Springer; 1969. pp. 297–323. [Google Scholar]
- 29.Von Damm K L. Seafloor hydrothermal activity: black smoker chemistry and chimneys. Annu Rev Earth Planet Sci. 1990;18:173–208. [Google Scholar]