Abstract
A substantial number of Bacillus species have been marketed for use in oral bacteriotherapy because of their purported ability to prevent or treat various gastrointestinal disorders. Recently, some of the Bacillus strains in Enterogermina, which is made up of aqueous suspensions of viable Bacillus spores, have been partially characterized and aligned with members of the Bacillus alcalophilus subgroup rather than with Bacillus subtilis, as previously reported. With a view toward verifying the original taxonomic position of the Enterogermina strains, we catalogued both phenotypic and genotypic traits exhibited by the four Bacillus strains isolated from the spore mixtures found in original commercial preparations dated 1975 and 1984 and commercial preparations now being propagated industrially. Analyses of physiological and biochemical traits, complete 16S rRNA gene sequences, DNA-DNA reassociation, tRNA intergenic spacer length polymorphism, single-strand conformation polymorphism of PCR-amplified spacer regions of tRNA genes, and randomly amplified polymorphic DNA led to the finding that all of the Enterogermina strains belong to a unique genospecies, which is unequivocally identified as the alkalitolerant species Bacillus clausii. Moreover, we provide evidence that in contrast to several reference strains of B. clausii, the strains constituting Enterogermina are characterized by a notable low level of intraspecific genome diversity and that each strain has remained the same for the last 25 years.
The spore-bearing alkaliphilic Bacillus species constitute a large, heterogeneous group of microorganisms which are now being investigated in order to better understand the physiology, biochemistry, and especially molecular genetics underlying the behavior of alkaliphilic bacteria (27, 28). Most of the studies have been performed to examine enzyme biotechnology, as alkaliphilic Bacillus strains produce enzymes, such as xylanases, cellulases, amylases, and proteases, that are very useful in industry and domestic life (15–17). Because of these relevant applications and commercial interest, more alkaliphilic Bacillus strains are being isolated from diverse alkaline environments. The newly recovered microorganisms are often described simply as Bacillus sp. (15) since a reliable taxonomic framework enabling species identification has not been completely defined yet (10). The few extensive studies undertaken to classify the truly alkaliphilic Bacillus strains (strains that grow at or above pH 9) and the alkalitolerant Bacillus strains (strains that also grow at pH 7) have led to the conclusion that these bacteria may be clustered into 11 different phena and 13 genospecies which are, with the exception of Bacillus alcalophilus and Bacillus cohnii, phylogenetically distinct from all validly described Bacillus species (10, 21, 22). However, some heterogeneity in both phenotypic and genetic characteristics has been found in strains putatively aligned with given phena, particularly strains assigned to the phenon encompassing the species Bacillus clausii (21), which indicates that there are intrinsic difficulties in identifying the alkaliphilic strains at the species level.
Our interest in the taxonomy of alkaliphilic Bacillus strains arose from the demonstration that some of the strains present in the pharmaceutical preparation Enterogermina (Sanofi-Synthelabo SpA, Milan, Italy) have been partially characterized and aligned with members of the B. alcalophilus subgroup rather than with Bacillus subtilis (12), as previously reported (3). Enterogermina, which is an aqueous suspension of viable Bacillus spores, has been marketed in Italy for more than 30 years because of its purported ability to prevent or treat infectious bacterial diarrhea (3). Although direct evidence supporting the claimed probiotic activity exhibited by Enterogermina is still lacking (25), some studies have suggested that the spores present in Enterogermina germinate and populate, albeit briefly, the intestinal tract (20). In addition, the ability of Enterogermina to stimulate production of secretory A immunoglobulins (9) and to display immunostimulatory activity (9, 23) is one of the mechanisms claimed to contribute to its therapeutic efficacy. The extensive use of Enterogermina in Italy and the remarkable industrial interest in this preparation prompted us to examine the taxonomic positions of the four Bacillus strains present in Enterogermina samples dated 1975 and 1984 and recent commercial samples. The usefulness of combining phenotypic characterization with different molecular typing methods (complete 16S rRNA gene sequencing, DNA-DNA hybridization, and PCR-based methods which sample the whole genome and the hypervariable parts of conserved genomic regions, such as the tRNA gene spacers) for identifying alkaliphilic and alkalitolerant Bacillus species is discussed below.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The four Bacillus strains now being propagated for production of Enterogermina, designated strains O/C, N/R, T, and SIN, were obtained from Sanofi-Synthelabo SpA as separate spore suspensions. The designations of these strains are derived from their resistance to diverse antibiotics: O/C is resistant to chloramphenicol, N/R is resistant to novobiocin and rifampin, T is resistant to tetracycline, and SIN is resistant to neomycin and streptomycin (3). From Enterogermina spore mixtures dated 1984 and 1975, four and two Bacillus strains, respectively, were isolated on Luria-Bertani medium (Fluka, Buchs, Switzerland) plates supplemented with chloramphenicol (100 μg/ml), tetracycline (100 μg/ml), rifampin (100 μg/ml) plus novobiocin (100 μg/ml), or neomycin (100 μg/ml) plus streptomycin (25 μg/ml). The strains isolated showed the same antibiotic resistance exhibited by the Bacillus strains that are currently propagated; therefore, they were designated O/C84, N/R84, T84, SIN84, T75, and SIN75. B. clausii DSM 8716 (= NCIMB 10309) and DSM 2515, Bacillus sp. strain ATCC 21536 (= Bacillus sp. strain DSM 2514), B. alcalophilus ATCC 21522 (= B. clausii DSM 2512), Bacillus amyloliquefaciens ATCC 23350, and B. subtilis ATCC 6633, ATCC 6051, and Bcv1 (a clinical isolate) were used as reference microorganisms. All strains were stored at 5°C on nutrient agar (Oxoid, Basingstoke, England) slopes.
Phenotypic characterization.
Phenotypic tests were performed by using the methods described by Gordon et al. (11). Growth at pH 5.7 was evaluated as described in Bergey's Manual of Systematic Bacteriology (4). Growth at pH 6, 7, 8, 9, and 10 was evaluated in either nutrient broth (Oxoid), Luria-Bertani broth (Fluka), or brain heart infusion broth (Biolife, Milan, Italy). The pH values of different media were adjusted as described by Nielsen et al. (21). Growth at different temperatures was quantified in liquid media (optical density at 560 nm) for temperatures ranging from 20 to 50°C and in solid media for temperatures of 45 and 55°C. Tolerance or susceptibility to concentrations of NaCl ranging from 5 to 12% was assayed by culturing bacterial cells on nutrient agar (Oxoid) buffered at pH 8.0 at 30°C. Reduction of nitrate, hydrolysis of casein, gelatin, starch, Tween 80 (Sigma, St. Louis, Mo.), and Tween 20 (Merck, Schuchardt, Germany), and phenylalanine deamination were determined as described by Fritze et al. (10). All the phenotypic assays were performed in triplicate for each strain studied.
DNA extraction and 16S rRNA gene sequencing.
Genomic DNA was extracted and purified from Bacillus strains as described by Celandroni et al. (2). PCR amplification of the 16S rRNA gene was performed as described by Rainey et al. (24) with a GeneAmp PCR 9600 thermal cycler (Perkin-Elmer, Norwalk, Conn.). PCR products were purified and concentrated by using a Prep-A-Gene kit (Bio-Rad, Hercules, Calif.) and were sequenced with a Ready Reaction dye terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.) and a 373A DNA sequencer (Applied Biosystems). Sequences were aligned with the ae2 editor (19) and were compared with representative 16S rRNA gene sequences of microorganisms belonging to the genus Bacillus. The 16S rRNA gene sequences used for comparison were obtained from the EMBL database and the Ribosomal Database Project (19). The results are presented below in a similarity matrix in which values were calculated by pairwise comparison of the sequences within the alignment.
DNA hybridization.
Probe DNA was prepared by randomly primed labeling with [3H]dCTP of total chromosomal DNA from strains O/C, N/R, T, and SIN by using a Megaprime kit (Amersham). Hybridization of the probed DNA was performed with B. clausii DSM 8716 genomic DNA. Quantitative DNA-DNA hybridization was performed as described by Grimont et al. (13). The percentage of similarity was calculated by dividing the counts per minute for a heterologous nuclease S1-resistant DNA by the counts per minute for the homologous S1-resistant DNA and multiplying by 100. The thermal stability of a DNA hybrid (ΔTm) was calculated by determining the difference between the denaturation temperature of the homologous reaction mixture and that of the heterologous reaction mixture.
tDNA-PCR and SSCP analysis.
tRNA intergenic regions were amplified with primers T3A (5′-GGGGGTTCGAATTCCCGCCGGCCCCA-3′) and T5B (5′-AATGCTCTACCAACTGAACT-3′) (5). Amplifications were performed in 50-μl reaction mixtures containing each primer at a concentration of 1 μM, each deoxynucleoside triphosphate at a concentration of 200 μM, 5 μl of MgCl2 reaction buffer (10 mM Tris-HCl [pH 8.8], 1.5 mM MgCl2, 50 mM KCl, 0.1% Triton X-100), 2.5 U of Taq polymerase (Pharmacia Biotech), and 0.05 μg of genomic DNA. A hot start protocol was used. The PCR mixture without Taq polymerase was incubated at 85°C for 3 min, the enzyme was then added, and the mixture was subjected to the following amplification conditions: denaturation at 94°C for 4 min, followed by 30 cycles consisting of 94°C for 1 min, 50°C for 1 min, and 72°C for 2 min and by a final extension step at 72°C for 10 min. PCR products were electrophoresed in 3% agarose gels in TBE buffer (89 mM Tris-boric acid, 2 mM EDTA). For single-strand conformation polymorphism (SSCP) analysis the amplified products were electrophoresed in 6% polyacrylamide (Pharmacia Biotech) gels as described by Borin et al. (1).
RAPD-PCR.
Randomly amplified polymorphic DNA (RAPD) fingerprinting of bacterial genomes was performed with primers RPO2 (5′-GCGATCCCCA-3′), M13 (5′-GAGGGTGGCGGCTCT-3′), and Pro-Up (5′-GCTGCTGGCGGTGG-3′). PCR were carried out in 50-μl reaction mixtures containing 1 μM primer, each deoxynucleoside triphosphate at a concentration of 200 μM, 5 μl of MgCl2 reaction buffer, 2.5 U of Taq polymerase (Pharmacia Biotech), and 0.1 μg of genomic DNA. The amplification conditions were as follows: 30 cycles consisting of 94°C for 1 min, 36°C for 1 min, and 72°C for 2 min, followed by one cycle consisting of 72°C for 10 min. The reproducibility of the RAPD profiles which we obtained was assessed in at least six separate experiments. PCR products were visualized after electrophoresis on 1% agarose gels containing 0.5 μg of ethidium bromide per ml. To better appreciate the differences and similarities in the electrophoretic patterns obtained by RAPD-PCR, the strain profiles were compared by using the Image Master 1D Elite software (Pharmacia Biotech), and dendrograms based on SAB values (similarity between the patterns for every pair of strains) were generated with the Image Master 1D Database software (Pharmacia Biotech) based on the unweighted pair group method with arithmetic means (26). An SAB value of 1.00 was considered to indicate complete identity between the patterns generated by two strains.
Nucleotide sequence accession numbers.
The sequence data determined in this study have been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession numbers AJ297491, AJ297492, AJ297493, AJ297494, AJ297495, AJ297496, AJ297497, and AJ297498 for the 16S rRNA genes of strains O/C, N/R, T, SIN, O/C84, N/R84, T84, and SIN84, respectively.
RESULTS AND DISCUSSION
Phenotypic characterization.
All of the Enterogermina strains which we analyzed form white, rough colonies with irregular edges. The growing cells are long rods (0.7 to 0.9 by 2.5 to 5.0 μm) and form ellipsoidal spores located subterminally in nonswollen sporangia. Bacterial growth did not occur at pH 5.7 or 6.0 in the liquid and solid media used. Optimal growth (generation time, 30 min) was obtained at pH 8.0, although each strain was able to grow at pH 7.0 (generation time, 39 min), pH 9.0 (generation time, 37 min), and pH 10.0 (generation time, 41 min). These results clearly indicate that the Bacillus strains constituting Enterogermina are alkalitolerant microorganisms, at least for Enterogermina produced from 1975 to the present. All the strains were able to grow at temperatures ranging from 20 to 45°C in liquid media and at temperatures up to 50°C in solid media. The optimal growth temperature was 35 to 40°C, while no growth occurred at 55°C. The strains showed salt tolerance at NaCl concentrations up to 10%. They hydrolyzed casein, gelatin, and starch and reduced nitrate; phenylalanine was not deaminated; and Tween 20 and Tween 80 were not hydrolyzed.
The overall biochemical and physiological traits suggest that all of the Enterogermina strains should be placed in alkalitolerant Bacillus phena and that the closest relative is the taxon referred to as phenon 6 by Nielsen et al. (21). The name B. clausii was proposed for bacteria assigned to phenon 6, and strain DSM 8716 was chosen as the type strain of this species.
Homology of 16S rRNA gene sequences.
Sequencing of the 16S rRNA genes was performed for the four strains now used to produce Enterogermina (O/C, N/R, T, and SIN; accession numbers AJ297491 to AJ297494, respectively) and for the strains isolated from the commercial preparation dated 1984 (O/C84, N/R84, T84, and SIN84; accession numbers AJ297495 to AJ297498, respectively). The sequence data revealed 100% identity among the strains analyzed (Table 1), and when the sequences were compared with the sequences available in databases, they were indistinguishable from the sequence reported for B. clausii DSM 8716 (99.8% homology; accession number X76440). Lower levels of homology were found with other alkalitolerant Bacillus strains, particularly DSM 8714 (96.9% homology; accession number X76438) and DSM 8717 (96.5% homology; accession number X76441), which still lack taxonomic standing. The levels of phylogenetic relatedness between the Enterogermina strains and strains of B. alcalophilus (95.6% homology; accession number X76436), Bacillus pseudofirmus (95.3% homology; accession number X76439), Bacillus pseudalcaliphilus (95.2% homology; accession number X76449), Bacillus halodurans (94.4% homology; accession number AB021187), Bacillus agaradhaerens (92.7% homology; accession number X76445), and Bacillus clarkii (92.2% homology; accession number X76444) were even lower (Table 1).
TABLE 1.
16S rRNA gene sequence similarity values for the Enterogermina strains and related taxa
| Organism
|
% 16S rDNA sequence similarity
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Strain or species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| 1 | O/C | |||||||||||||||||||
| 2 | N/R | 100 | ||||||||||||||||||
| 3 | SIN | 100 | 100 | |||||||||||||||||
| 4 | T | 100 | 100 | 100 | ||||||||||||||||
| 5 | O/C84 | 100 | 100 | 100 | 100 | |||||||||||||||
| 6 | N/R84 | 100 | 100 | 100 | 100 | 100 | ||||||||||||||
| 7 | SIN84 | 100 | 100 | 100 | 100 | 100 | 100 | |||||||||||||
| 8 | T84 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||||||||||||
| 9 | B. clausii DSM 8716 | 99.8 | 99.8 | 99.8 | 99.8 | 99.8 | 99.8 | 99.8 | 99.8 | |||||||||||
| 10 | B. agaradhaerens | 92.7 | 92.7 | 92.7 | 92.7 | 92.7 | 92.7 | 92.7 | 92.7 | 92.7 | ||||||||||
| 11 | B. clarkii | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 | |||||||||
| 12 | B. halodurans | 94.4 | 94.4 | 94.4 | 94.4 | 94.4 | 94.4 | 94.4 | 94.4 | 94.3 | 93.3 | 93.9 | ||||||||
| 13 | B. pseudofirmus | 95.3 | 95.3 | 95.3 | 95.3 | 95.3 | 95.3 | 95.3 | 95.3 | 95.1 | 94.3 | 94.4 | 96.5 | |||||||
| 14 | B. pseudalcaliphilus | 95.2 | 95.2 | 95.2 | 95.2 | 95.2 | 95.2 | 95.2 | 95.2 | 95.2 | 92.7 | 93.4 | 96.0 | 96.2 | ||||||
| 15 | B. alcalophilus | 95.6 | 95.6 | 95.6 | 95.6 | 95.6 | 95.6 | 95.6 | 95.6 | 95.5 | 93.8 | 93.6 | 96.5 | 96.5 | 98.5 | |||||
| 16 | DSM 8724 | 95.5 | 95.5 | 95.5 | 95.5 | 95.5 | 95.5 | 95.5 | 95.5 | 95.3 | 93.7 | 93.7 | 96.3 | 96.2 | 98.2 | 99.7 | ||||
| 17 | Bacillus gibsonii | 95.3 | 95.3 | 95.3 | 95.3 | 95.3 | 95.3 | 95.3 | 95.3 | 95.2 | 93.0 | 92.7 | 94.9 | 95.0 | 94.9 | 95.0 | 95.0 | |||
| 18 | DSM 8717 | 96.5 | 96.5 | 96.5 | 96.5 | 96.5 | 96.5 | 96.5 | 96.5 | 96.3 | 92.4 | 92.1 | 94.0 | 94.5 | 94.8 | 95.6 | 95.6 | 94.9 | ||
| 19 | DSM 8714 | 96.9 | 96.9 | 96.9 | 96.9 | 96.9 | 96.9 | 96.9 | 96.9 | 96.8 | 92.2 | 91.5 | 94.1 | 95.3 | 94.7 | 95.3 | 95.1 | 95.0 | 97.0 | |
| 20 | Brevibacterium brevis | 89.0 | 89.0 | 89.0 | 89.0 | 89.0 | 89.0 | 89.0 | 89.0 | 88.9 | 88.8 | 88.7 | 89.2 | 89.2 | 89.0 | 89.2 | 89.2 | 88.9 | 89.0 | 89.1 |
DNA-DNA hybridization analysis.
The chromosomal DNA extracted from the O/C, N/R, T, and SIN Enterogermina strains showed 75% reassociation (ΔTm, 3.5°C) with the chromosomal DNA extracted from B. clausii type strain DSM 8716. Therefore, since microorganisms showing more than 70% DNA reassociation with ΔTm of less than 5°C are considered members of the same species (29), the results obtained confirm that the four Enterogermina strains belong to a unique genospecies which can be unequivocally identified as B. clausii. This finding is of intrinsic value, since some bacterial strains described as B. clausii strains have been reported to exhibit levels of DNA hybridization with the reference type strain of less than 61% (21), thus emphasizing the great genomic heterogeneity of the strains placed in the species B. clausii.
tRNA intergenic length polymorphism (tDNA-PCR).
The rationale of the tRNA intergenic length polymorphism molecular strategy for taxonomic studies relies on the observation that bacterial tRNA genes contain sequence motifs that exhibit a high level of phylogenetic conservation, while tRNA intergenic regions exhibit a higher degree of variation (30). Indeed, the PCR fingerprints of tRNA intergenic regions generated by using consensus tRNA gene primers generally produce species-specific patterns which have been successfully used to discriminate species belonging to the same genus, as reported for Acinetobacter sp., Streptococcus sp., Staphylococcus sp., and Bacillus sp. (1, 5, 7, 8, 18). Moreover, application of tDNA-PCR analysis to strains validly placed in the same species has also allowed discrimination of strain clusters showing distinct PCR fingerprints, as demonstrated for Streptococcus mitis, Bacillus stearothermophilus, and Bacillus licheniformis (1, 5, 7).
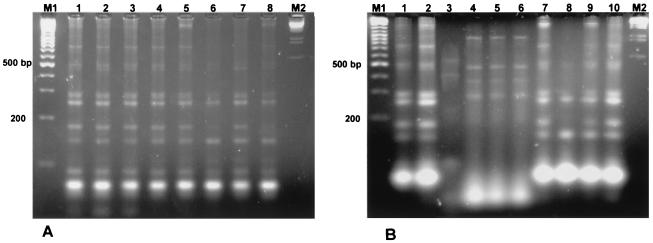
The tDNA-PCR technique with consensus primers T3A and T5B, which have been proven to amplify tDNA intergenic regions in several Bacillus species (1, 5), was used to evaluate whether there were differences in the amplification patterns of the B. clausii Enterogermina strains. The lengths of tRNA intergenic spacers derived from strains O/C, N/R, T, SIN, O/C84, N/R84, T84, SIN84, T75, and SIN75 were compared with those of B. clausii DSM 8716 and DSM 2515, Bacillus sp. strain ATCC 21536 (= Bacillus sp. strain DSM 2514), and B. alcalophilus ATCC 21522 (= B. clausii DSM 2512). All of the Enterogermina strains analyzed were very homogeneous, producing the same band pattern with amplicon lengths ranging from 850 to about 150 bp (Fig. 1). B. clausii DSM 8716 and DSM 2515 produced identical profiles (Fig. 1B), which were indistinguishable from the amplification patterns obtained from the Enterogermina strains. Both Bacillus sp. strain ATCC 21536 (= Bacillus sp. strain DSM 2514) and B. alcalophilus ATCC 21522 (= B. clausii DSM 2512) produced amplification patterns which were identical to that produced by the type strain of B. clausii (DSM 8716) (Fig. 1B), suggesting that they may be members of B. clausii. This observation is of particular interest since Bacillus sp. strain ATCC 21536 (= Bacillus sp. strain DSM 2514), identified as B. clausii on the basis of phenotypic characterization (21), exhibits a level of DNA-DNA reassociation as low as 61% with the type strain of B. clausii (DSM 8716). Strains of B. subtilis (ATCC 6633, ATCC 6051, and Bcv1) and B. amyloliquefaciens (ATCC 23350) were analyzed by the same procedure, since these species were proven to produce species-specific amplification patterns with the T3A and T5B consensus primers (1). Both these species can be distinguished from B. clausii by the presence of two signature double bands around 500 to 460 bp and around 290 to 240 bp which are peculiar to B. clausii (Fig. 1B).
FIG. 1.
DNA fragments obtained by amplification of the tRNA intergenic spacer sequences of Bacillus strains. (A) Enterogermina strains. Lane 1, O/C; lane 2, N/R; lane 3, SIN; lane 4, O/C84; lane 5, N/R84; lane 6, SIN84; lane 7, T84; lane 8, SIN75. (B) Lanes 1 and 2, Enterogermina strains (lane 1, T; lane 2, T75); lane 3, B. amyloliquefaciens ATCC 23350; lane 4, B. subtilis ATCC 6633; lane 5, B. subtilis ATCC 6051; lane 6, B. subtilis Bcv1; lane 7, B. clausii DSM 8716; lane 8, B. clausii DSM 2515; lane 9, Bacillus sp. strain ATCC 21536 (= Bacillus sp. strain DSM 2514); lane 10, B. alcalophilus ATCC 21522 (= B. clausii DSM 2512). Lane M1 contained a 100-bp DNA ladder, and lane M2 contained λEcoRI-HindIII DNA.
The overall results obtained by tDNA-PCR further support alignment of the Enterogermina strains with B. clausii and indicate that the amplification pattern obtained with B. clausii strains may be considered species specific.
SSCP analysis of tDNA-PCR products.
To further increase the power of resolution of tDNA-PCR fingerprinting, the tDNA-PCR amplified products were analyzed by the SSCP method. This method, which is commonly used to search for point mutations in DNA sequences consisting of few hundred base pairs (14), has also been applied to the taxonomy of certain bacteria, including Bacillus species (1), in order to efficiently resolve tDNA-PCR amplified products having molecular weights lower than 200 bp. We observed no differences between the profiles obtained from the tDNA-PCR products derived from the B. clausii Enterogermina strains and the profiles obtained from the B. clausii reference strains, and signature bands were detected at 170, 60, 45, and 25 bp (Fig. 2). Moreover, the differences between the tDNA-PCR fingerprints of B. subtilis and B. amyloliquefaciens were confirmed by the SSCP analysis, and signature bands were detected at 100 and 72 bp for the three B. subtilis strains and at 125, 80, and 40 bp for B. amyloliquefaciens (Fig. 2).
FIG. 2.
SSCP analysis of tDNA-PCR products derived from Bacillus strains. Lanes 1 and 2, representative Enterogermina strains (lane 1, T; lane 2, T75); lane 3, B. amyloliquefaciens ATCC 23350; lane 4, B. subtilis ATCC 6633; lane 5, B. subtilis ATCC 6051; lane 6, B. subtilis Bcv1; lane 7, B. clausii DSM 8716; lane 8, B. clausii DSM 2515; lane 9, Bacillus sp. strain ATCC 21536 (= Bacillus sp. strain DSM 2514); lane 10, B. alcalophilus ATCC 21522 (= B. clausii DSM 2512); lane M, 100-bp DNA ladder.
Genome polymorphism analysis by RAPD-PCR.
RAPD-PCR fingerprinting of whole genomes has been used successfully to differentiate species and strains belonging to the genus Bacillus using oligonucleotide primers having arbitrary sequences (5, 6). In this study, three primers (RPO2, M13, and Pro-Up) were used under low-stringency conditions to amplify the genomic DNA of B. clausii DSM 8716 and DSM 2515, Bacillus sp. strain ATCC 21536 (= Bacillus sp. strain DSM 2514), B. alcalophilus ATCC 21522 (= B. clausii DSM 2512), and the B. clausii strains present in Enterogermina produced from 1975 to the present. The amplification patterns generated with each of the primers were distinct for all of the B. clausii reference strains analyzed (Fig. 3B), as shown by the substantial differences in the similarity values recorded by the computer-aided analysis of RAPD fingerprints (SAB value range, 0.29 to 0.82). These results underline the notable intraspecific variability of the strains placed in B. clausii and thus confirm the genomic heterogeneity reported for this species (21). The electrophoretic profiles derived from amplification of the Enterogermina strains revealed identity or negligible differences among strains when M13 or RPO2 was used as the primer (Fig. 3A), as shown by the similarity values obtained (1.00 and 0.93, respectively). When Pro-Up was used (Fig. 3A), two major groups of strains were evident (SAB values, 1.00 and 0.90), indicating that Enterogermina includes two different strain clusters having a similarity value of 0.70; interestingly, while one of the clusters was found to comprise strains T and N/R, the other cluster comprised strains O/C and SIN in the preparations of Enterogermina produced from 1975 to the present.
FIG. 3.
RAPD fingerprinting of B. clausii strains obtained by amplifying total DNA with primers M13, RPO2, and Pro-Up. (A) Enterogermina strains. Lane 1, O/C; lane 2, N/R; lane 3, T; lane 4, SIN; lane 5, O/C84; lane 6, N/R84; lane 7, T84; lane 8, SIN84; lane 9, SIN75; lane 10, T75. (B) B. clausii strains. Lane 11, B. clausii DSM 8716; lane 12, Bacillus sp. strain ATCC 21536 (= Bacillus sp. strain DSM 2514); lane 13, B. alcalophilus ATCC 21522 (= B. clausii DSM 2512); lane 14, B. clausii DSM 2515. Lane M contained λEcoRI-HindIII DNA.
Conclusions.
Based on the main results described in the present report, we concluded that (i) the four Bacillus strains constituting Enterogermina belong to a unique genospecies identified as the alkalitolerant species B. clausii, (ii) the four B. clausii Enterogermina strains display a low level of intraspecific diversity compared with that observed for the reference strains of B. clausii, and (iii) each of the Enterogermina strains has exhibited a high degree of genomic conservation through time. These observations support the idea that the Enterogermina strains originated from closely related strains or even from a common ancestor through early selection of stable bacterial clones maintained during industrial propagation. An important finding that emerged from this study is that the spacers between tRNA genes are fully conserved in all of the strains of B. clausii studied, while heterogeneity among the strains can be detected by RAPD-PCR fingerprinting of the whole genomes. Therefore, we propose that tRNA intergenic spacer length polymorphism analysis, alone or coupled with SSCP analysis, is a rapid and accurate taxonomic system for validly identifying B. clausii strains at the species level, while RAPD-PCR analysis may be useful for delineating intraspecific differences among strains identified as B. clausii.
ACKNOWLEDGMENTS
We are very grateful to Attilia Brugo (Sanofi-Synthelabo SpA, OTC Division, Milan, Italy) for providing Enterogermina samples and for critical discussions.
This work was supported by grants 1998 to 2000 from the University of Pisa.
REFERENCES
- 1.Borin S, Daffonchio D, Sorlini S. Single strand conformation polymorphism analysis of PCR-tDNA fingerprinting to address the identification of Bacillus species. FEMS Microbiol Lett. 1997;157:87–93. doi: 10.1111/j.1574-6968.1997.tb12757.x. [DOI] [PubMed] [Google Scholar]
- 2.Celandroni F, Ghelardi E, Pastore M, Lupetti A, Kolstø A-B, Senesi S. Characterization of the chemotaxis fliY and cheA genes in Bacillus cereus. FEMS Microbiol Lett. 2000;190:247–253. doi: 10.1111/j.1574-6968.2000.tb09294.x. [DOI] [PubMed] [Google Scholar]
- 3.Ciffo F. Determination of the spectrum of antibiotic resistance of the Bacilus subtilis strains of Enterogermina. Chemioterapia. 1984;3:45–52. [PubMed] [Google Scholar]
- 4.Claus D, Berkeley R C W. Genus Bacillus Chon 1872. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1105–1139. [Google Scholar]
- 5.Daffonchio D, Borin S, Frova G, Manachini P L, Sorlini C. PCR fingerprinting of whole genomes: the spacer between the 16S and 23S rRNA genes and intergenic tRNA gene regions reveal a different intraspecific genomic variability of Bacillus cereus and Bacillus licheniformis. Int J Syst Bcteriol. 1998;48:107–116. doi: 10.1099/00207713-48-1-107. [DOI] [PubMed] [Google Scholar]
- 6.Daffonchio D, Borin S, Frova G, Gallo R, Mori E, Fani R, Sorlini C. A randomly amplified polymorphic DNA marker specific for the Bacillus cereus group is diagnostic for Bacillus anthracis. Appl Environ Microbiol. 1999;65:1298–1303. doi: 10.1128/aem.65.3.1298-1303.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Gheldre Y, Vandamme P, Goossens H, Strulens M J. Identification of clinically relevant viridans streptococci by analysis of transfer DNA intergenic spacer length polymorphism. Int J Syst Bacteriol. 1999;49:1591–1598. doi: 10.1099/00207713-49-4-1591. [DOI] [PubMed] [Google Scholar]
- 8.Ehrenstein B, Bernards A T, Dijkshoorn L, Gerner-Smidt P, Towner K J, Bouvet P J, Daschner F D, Grundmann H. Acinetobacter species identification by using tRNA spacer fingerprinting. J Clin Microbiol. 1996;34:2414–2420. doi: 10.1128/jcm.34.10.2414-2420.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorini G, Cimminiello C, Chianese R. Il B. subtilis come stimolatore selettivo delle IgA linfocitarie di membrana. Farmaci. 1985;9:331–334. [Google Scholar]
- 10.Fritze D, Flossdorf J, Claus D. Taxonomy of alkaliphilic Bacillus strains. Int J Syst Bacteriol. 1990;40:92–97. doi: 10.1099/00207713-40-1-92. [DOI] [PubMed] [Google Scholar]
- 11.Gordon R E, Haynes W C, Pang C H. The genus Bacillus. Agricultural handbook no. 427. U.S. Washington, D.C.: Department of Agriculture; 1973. [Google Scholar]
- 12.Green D H, Wakely P R, Page A, Barnes A, Baccigalupi L, Ricca E, Cutting S M. Characterization of two Bacillus probiotics. Appl Environ Microbiol. 1999;65:4288–4291. doi: 10.1128/aem.65.9.4288-4291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimont P A, Popoff M Y, Grimont F, Coynault C, Lemelin M. Reproducibility and correlation study of three deoxyribonucleic acid hybridization procedures. Curr Microbiol. 1980;4:325–330. [Google Scholar]
- 14.Grompe M. The rapid detection of unknown mutations in nucleic acids. Nature Genet. 1993;5:111–117. doi: 10.1038/ng1093-111. [DOI] [PubMed] [Google Scholar]
- 15.Ikawa K, Araki H, Tsujino Y, Hajashi Y, Igarashi K, Hatada Y, Hagihara H, Ozawa T, Ozaki K, Kobayashi T, Ito S. Hyperexpression of the gene for a Bacillus α-amylase in Bacillus subtilis cells: enzymatic properties and crystallization of the recombinant enzyme. Biosci Biotechnol Biochem. 1998;62:1720–1725. doi: 10.1271/bbb.62.1720. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Kobayashi T, Ara K, Kawai S, Hatada Y. Alkaline detergent enzymes from alkaliphiles: enzymatic properties, genetics, and structures. Extremophiles. 1998;2:185–190. doi: 10.1007/s007920050059. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni N, Lakshmikumaran M, Rao M. Xylanase II from an alkaliphilic thermophilic Bacillus with a distinctly different structure from other xylanases: evolutionary relationship to alkaliphilic xylanases. Biochem Biophys Res Commun. 1999;263:640–645. doi: 10.1006/bbrc.1999.1420. [DOI] [PubMed] [Google Scholar]
- 18.Maes N, De Gheldre Y, De Ryck R, Vaneechoutte M, Meugnier H, Etienne J, Struelens M J. Rapid and accurate identification of Staphylococcus species by tRNA intergenic spacer length polymorphism analysis. J Clin Microbiol. 1997;35:2477–2481. doi: 10.1128/jcm.35.10.2477-2481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maidak B L, Olsen G J, Larsen N, McCaughey M J, Woese C R. The Ribosomal Database Project (RDP) Nucleic Acids Res. 1996;24:82–85. doi: 10.1093/nar/24.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazza P. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll Chim Farm. 1994;133:3–18. [PubMed] [Google Scholar]
- 21.Nielsen P, Fritze D, Priest F G. Phenetic diversity of alkaliphilic Bacillus strains: proposal for nine new species. Microbiology. 1995;141:1745–1761. [Google Scholar]
- 22.Nielsen P, Rainey F A, Outtrup H, Priest F G, Fritze D. Comparative 16S rDNA sequence analysis of some alkaliphilic bacilli and the establishment of a sixth rRNA group within the genus Bacillus. FEMS Microbiol Lett. 1994;117:61–66. [Google Scholar]
- 23.Novelli A, Ulivelli A, Reali E F, Mannelli F, Trombi-Belcari L, Spezia R, Periti P. Bacillus subtilis spores as a natural pro-host oral agent. Preliminary data in children. Chemioterapia. 1984;3:152–155. [PubMed] [Google Scholar]
- 24.Rainey F A, Ward-Rainey N, Kroppenstedt R M, Stackebrandt E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage; proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol. 1996;46:1088–1092. doi: 10.1099/00207713-46-4-1088. [DOI] [PubMed] [Google Scholar]
- 25.Sanders M E. Probiotics. Food Technol. 1999;53:67–77. [Google Scholar]
- 26.Sneath P H A, Sokal R R. Numerical taxonomy. San Francisco, Calif: Freeman; 1973. [Google Scholar]
- 27.Takami H, Horikoshi K. Analysis of the genome of an alkaliphilic Bacillus strain from an industrial point of view. Extremophiles. 2000;4:99–108. doi: 10.1007/s007920050143. [DOI] [PubMed] [Google Scholar]
- 28.Takami H, Takaki Y, Nakasone K, Sakiyama T, Maeno G, Sasaki R, Hirama C, Fuji F, Masui N. Genetic analysis of the chromosome of alkaliphilic Bacillus halodurans C-125. Extremophiles. 1999;3:227–233. doi: 10.1007/s007920050120. [DOI] [PubMed] [Google Scholar]
- 29.Wayne L G, Brenner D J, Colwell R R, Grimont P A D, Kandler O, Krichevsky M I, Moore L H, Moore W E C, Murray R G E, Stackebrandt E, Starr M P, Trüper H G. Report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int J Syst Bacteriol. 1987;37:463–464. [Google Scholar]
- 30.Welsh J, McClelland M. Genomic fingerprints produced by PCR with consensus tRNA gene primers. Nucleic Acids Res. 1991;19:861–866. doi: 10.1093/nar/19.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]