Abstract
Simple Summary
Ductal carcinoma in situ (DCIS) is a potential precursor to invasive breast cancer (IBC). Although in many women DCIS will never become breast cancer, almost all women diagnosed with DCIS undergo surgery with/without radiotherapy. Several studies are ongoing to de-escalate treatment for DCIS. Multiple decision support tools have been developed to aid women with DCIS in selecting the best treatment option for their specific goals. The aim of this study was to identify these decision support tools and evaluate their quality and clinical utility. Thirty-three studies were reviewed, in which four decision aids and six prediction models were described. While some of these models might be promising, most lacked important qualities such as tools to help women discuss their options or good quality validation studies. Therefore, the need for good quality, well validated decision support tools remains unmet.
Abstract
Even though Ductal Carcinoma in Situ (DCIS) can potentially be an invasive breast cancer (IBC) precursor, most DCIS lesions never will progress to IBC if left untreated. Because we cannot predict yet which DCIS lesions will and which will not progress, almost all women with DCIS are treated by breast-conserving surgery +/− radiotherapy, or even mastectomy. As a consequence, many women with non-progressive DCIS carry the burden of intensive treatment without any benefit. Multiple decision support tools have been developed to optimize DCIS management, aiming to find the balance between over- and undertreatment. In this systematic review, we evaluated the quality and added value of such tools. A systematic literature search was performed in Medline(ovid), Embase(ovid), Scopus and TRIP. Following the PRISMA guidelines, publications were selected. The CHARMS (prediction models) or IPDAS (decision aids) checklist were used to evaluate the tools’ methodological quality. Thirty-three publications describing four decision aids and six prediction models were included. The decision aids met at least 50% of the IPDAS criteria. However, most lacked tools to facilitate discussion of the information with healthcare providers. Five prediction models quantify the risk of an ipsilateral breast event after a primary DCIS, one estimates the risk of contralateral breast cancer, and none included active surveillance. Good quality and external validations were lacking for all prediction models. There remains an unmet clinical need for well-validated, good-quality DCIS risk prediction models and decision aids in which active surveillance is included as a management option for low-risk DCIS.
Keywords: ductal carcinoma in situ, decision support tool, decision aid, prediction model
1. Introduction
Ductal carcinoma in situ (DCIS) is a potential precursor lesion to invasive breast cancer (IBC). It accounts for approximately 20% of all newly screen-detected breast lesions [1,2]. It is mostly non-symptomatic, and detected through population-based screening. As DCIS is historically considered a potential precursor lesion for IBC, it is usually treated with breast-conserving surgery, often supplemented with radiotherapy, or even mastectomy. In several countries, endocrine treatment may also be prescribed. However, many DCIS lesions will never progress to IBC during the patient’s lifetime [3]. Biopsy review studies of patients where DCIS was initially misdiagnosed as benign and thus not treated after biopsy suggest that up to 85% of all DCIS will never progress into IBC [3,4,5,6]. Consequently, there is a growing concern about possible overtreatment for low-risk, with favorable characteristics, DCIS [3,7,8,9]. DCIS treatment de-escalation trials are being conducted to investigate the safety of active surveillance in women with low-risk DCIS [10,11,12].
Decision making about DCIS treatment is not straightforward. The key difference is the impact these treatments can have on patients’ quality of life [13]. Evidence on patient reported outcomes in women with DCIS is still lacking [14]. Active surveillance may potentially safeguard patients’ quality of life by allowing them to forego the invasive standard treatments, thereby avoiding potential harm (e.g., psychological stress and side effects and complications of surgery, radiotherapy and endocrine treatment) [15]. By not removing the DCIS lesion, however, there is a risk of progression to IBC; this knowledge might cause some women to experience elevated levels of anxiety. Patients (in consultation with their doctors) need to evaluate the risks and benefits of both options, and decide what is of most importance to them. lowering their risk of experiencing invasive breast cancer or avoiding potentially unnecessary treatments and their associated harms. This is a preference-sensitive decision that needs to be based on individual patients’ informed preferences.
Currently, women with DCIS and their clinicians already have a choice in type of surgery (i.e., breast conserving surgery or mastectomy) and the use of adjuvant treatment (i.e., radiotherapy and/or endocrine treatment). However, in the future, and also currently in the context of the ongoing DCIS treatment de-escalation trials, women with low-risk DCIS may also have the added alternative of active surveillance as a potential management strategy. Clinicians will therefore need tools to inform women with low-risk DCIS about the risks and benefits of active surveillance as a potential management strategy [16]. Patients need to receive clear and balanced information to enable them to make an informed decision.
Risk perception and views on the role of active surveillance in the management of DCIS differ among clinicians [17,18]. In addition, several studies have described overestimation of risk of recurrence/progression and lack of knowledge about available management strategies and their associated risks, both physical and psychological, amongst women diagnosed with DCIS [13,15,19,20]. Women searching for information may turn to the internet to meet their needs. However, Blackwood et al. reported that few of the plain language summaries available on the internet met quality criteria for consumer health information [21], thus highlighting the need for properly designed DCIS decision support tools.
Well-designed evidence-based decision support tools such as decision aids, communication tools or prediction models may help patients and clinicians make better informed and value-congruent decisions. Decision aids – tools developed to support patients facing preference-sensitive decisions – provide balanced and easily accessible plain language information about all viable options; they may contain value clarification exercises or question prompt lists to help patients discuss their options with medical professionals [22]. Patients who have used decision aids often report feeling better informed about DCIS; this may result in more accurate risk perception [23]. Communication aids are tools developed to help clinicians provide their patients with balanced, easy-to-understand information during the clinical encounter [24]; this may serve to increase effective patient participation in decision making. Finally, prediction models, statistical models used to quantify individualized risks of experiencing specific outcomes (e.g., a recurrence) during a certain period, could also help clinicians and patients to evaluate the risks and benefits of available treatment options. Women with low-risk DCIS, as well as healthcare professionals involved in their care, may therefore benefit from having access to decision support tools when deciding whether to undergo surgery or opt for active surveillance (if/when available). The aim of this study is to identify and evaluate the methodological quality of published decision support tools, developed to support decision making about the management of DCIS.
2. Methods
2.1. Literature Search
A systematic literature review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [25,26]. The protocol was published in the international prospective register of systematic reviews [27,28] (PROSPERO, CRD42020212297). The systematic search was designed and executed in collaboration with a medical information specialist (EAW). The databases Medline [29] (ovid), Embase [30] (ovid), Scopus [31] and TRIP [32] were initially searched up to 24 September 2020 (inclusive). A search update was performed on 22 February 2022. The following terms, including synonyms and closely related words, were used as index terms or free-text words: “DCIS” and “decision support systems” (Supplementary Table S1 provides the full search strategy). No limits were applied for date or study design.
2.2. Selection Criteria
For this study, the selection criteria were: (1) studies describing the development and/or evaluation of a decision support tool (e.g., a patient decision aid, communication tool or prediction model) aimed at women with DCIS; and (2) the article was written in English or Dutch. Decision aids aimed at women with invasive breast cancer were evaluated in a recently published systematic review by Vromans et al. [33]. Thus, decision aids designed for women with invasive breast cancer with a secondary focus on DCIS were not included in the current study.
2.3. Literature Screening
Papers were imported and duplicates were removed. The unique papers were screened based on title and abstract by two authors (RSJMS and EGE) independently using Rayyan QRCI [34,35]. Full-text versions were retrieved for the papers selected based on title and abstract. All full texts were screened by two authors independently (RSJMS and EGE), and disagreements were resolved through consensus. A cross-reference check and a search for papers cited by or citing the publications were selected based on the full-text performed in Scopus and Web of Science and this process was repeated until there were no more new relevant papers found (February 2021). Article selection was also performed by two researchers (RSJMS and EGE) independently, with any disagreement resolved through consensus.
2.4. Quality Assessment and Data Extraction
The (methodological) quality of the decision aids themselves and papers describing the development and/or evaluation of the decision aids were assessed using the IPDAS (International Patient Decision Aid Standards) checklist, which reflects the gold standard in the field of decision aid development [36]. The IPDAS checklist consists of 74 items distributed over three key domains: content (30 items); development process (36 items); and effectiveness (8 items) of the decision aid. The criteria were scored as either ‘met’ or ‘unmet’. For each decision aid, we calculated the percentage of criteria that had been met per IPDAS domain. A test set of three DA were scored by both reviewers (RSJMS and EGE) independently, and disagreement was resolved through consensus. Thereafter, the remaining decision aids were scored by one reviewer (RSJMS), as there was 97% congruence in scoring.
The methodological quality of the development and validation of the DCIS prediction models was assessed using the widely endorsed CHARMS (Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies) checklist [37,38]. The CHARMS-PF checklist (items to extract from the primary studies [38]) consists of 32 criteria spread across nine domains (Source of Data; Participants; Outcomes; Prognostic Factors; Sample Size; Missing Data; Analysis; Results; and Interpretation and Discussion). For presentation purposes, we grouped the nine CHARMS methodological quality domains into three overarching categories, namely: Participants (consisting of the CHARMS domains Source of Data, Participants and Sample Size), Methodology (consisting of the CHARMS domains Outcomes, Prognostic Factors, Missing Data and Analysis) and Results (consisting of the CHARMS domains Results and Interpretation and Discussion). For each overarching category, risk of bias is presented as low, moderate or high. All papers were double-coded, and thus individually scored by both reviewers (RSJMS and EGE), and disagreement was resolved through consensus.
3. Results
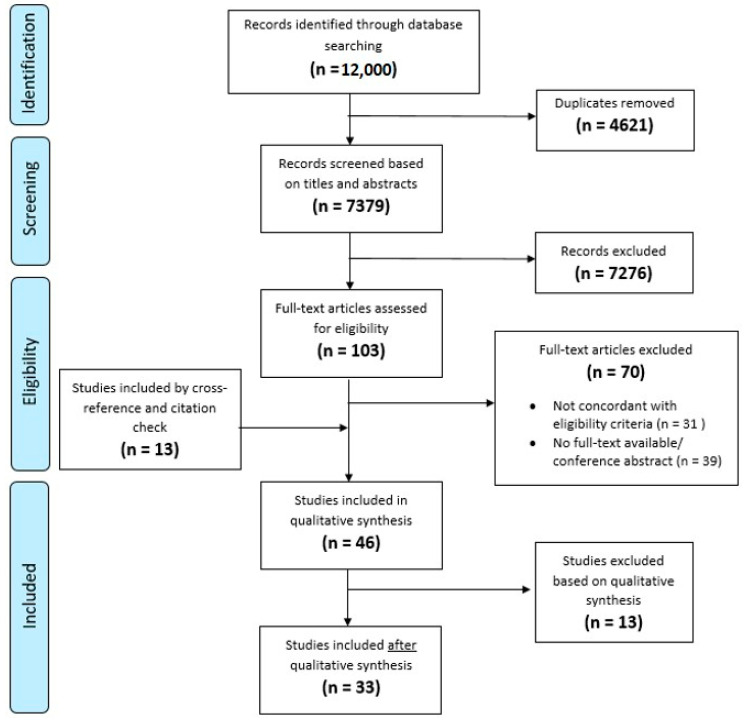
Of 12,000 papers screened, 33 were included in the final review (Flowchart, Figure 1). These papers described three decision aids, a communication aid and six prediction models.
Figure 1.
Flowchart inclusion of papers.
3.1. Decision Aids and Communication Tool
Three decision aids were retrieved (Table 1), specifically: a decision aid for the German context by Berger-Hoger et al. [39,40,41]; OnlineDeCISion.org by Ozanne et al. [42,43]; DCISoptions.org by the COMET research team [44]; and one communication aid by De Morgan et al. [24]. Two of the four decision aids were aimed solely at women with DCIS [41,44], whilst onlineDeCISion.org provides a separate interface for patients and clinicians. The communication aid by De Morgan et al. was aimed at clinicians, and intended for use during consultations with the patient. The onlineDeCISion.org tool contains a disease simulation model integrating data from the published literature to simulate clinical events [45]. Three of the tools provided information in English. The decision aid by Berger-Hoger et al. was developed for German patients, and therefore written in German.
Table 1.
Overview of patient decision aids on treatment decision for (low-risk) DCIS.
| Decision Aid Berger-Hoger et al. |
Communication Aid De Morgan et al. |
Online DeCISion.org Ozanne et al. |
DCISoptions.org COMET Trial Decision Aid |
|
|---|---|---|---|---|
| Last update | Not reported | Not reported | Not reported | Not reported |
| Language | German | English | English | English |
| Country | Germany | Australia | USA | USA |
| Format | On paper only | Online booklet * | Web-based ** | Web-based *** |
| Target audience | Women with DCIS | Healthcare professionals | Healthcare professionals and women with DCIS | Women with DCIS |
| Patients involved in development | Yes | Yes | Yes | Not reported |
| Healthcare professionals involved in development | Yes | Yes | Yes | Not reported |
| Evaluation study conducted | Yes | Yes | Not reported | Not reported |
| Design evaluation study | RCT | Interview | N.A. | N.A. |
| Sample size evaluation study | 64 | 25 | N.A. | N.A. |
| Main finding evaluation study | More patient involvement | Communication tool assists SDM | N.A. | N.A. |
| Implementation study conducted | Not reported | Not reported | Not reported | Not reported |
| IPDAS score for CONTENT (% criteria met) |
87% | 57% | 65% | 78% |
| IPDAS score for DEVELOPMENT (% criteria met) |
71% | 59% | 67% | 42% |
| IPDAS score for EFFECTIVENESS (% criteria met) |
100% | 50% | 75% | 75% |
Abbreviations: DCIS: Ductal Carcinoma in Situ, SDM: Shared decision making, IPDAS: Inter-national Patient Decision Aids Standard.
For three decision aids, both women with DCIS and healthcare professionals were included in the development process [24,39,42], while for DCISoptions.org, this was not reported. Both Berger-Hoger et al. and De Morgan et al. performed an evaluation study for their respective tools, which showed that using the tool stimulated patient involvement in decision making. The decision aid by Berger-Hoger et al. achieved the highest score for methodological quality, but all decision aids met at least 50% of the IPDAS criteria (Supplementary Table S2). Areas requiring improvement were: presentation of probability in more than one format (words, diagrams, etc.); inclusion of methods for clarifying and expressing patient’s values; guidance in deliberation and communication; and providing references to the evidence on which the decision aid was based.
3.2. Prediction Models
Six prediction models were described in 27 papers (Table 2). These include four classical prediction models using clinicopathological factors–two of which were designed to predict the risk of ipsilateral invasive breast cancer (iIBC) and one to predict the risk of contralateral breast cancer (CBC)–and two models including biomolecular factors.
Table 2.
Overview of prediction models predicting subsequent breast events after DCIS.
| Oncotype DCIS (Solin et al., (2013)) |
DCISionRT PreludeDX (Bremer et al., (2018)) |
Van Nuys Prognostic Index (Silverstein et al., (1995)) |
MSKCC DCIS Nomogram (Rudlof et al., (2010)) |
Patient Prognostic Score (Sagara et al., (2016)) |
CBC Risk Model (Chowdhury et al., (2017)) |
|
|---|---|---|---|---|---|---|
| Country | USA | Sweden | USA | USA | USA | USA |
| Format | On order * | On order ** | On paper | Web based *** | On paper | On paper |
| Predicted outcome | Ipsilateral in situ or invasive breast event | Ipsilateral in situ or invasive breast event | Disease-free survival | Ipsilateral in situ or invasive breast event | Breast cancer-specific death | Contralateral invasive breast cancer |
| Tool based on | Multigene assay | Clinicopathological factors + biomarkers |
Clinicopathological factors | Clinicopathological factors | Clinicopathological factors |
Clinicopathological factors |
| Type of data | Trial cohort | Multi center | Single center | Trial cohort | Population-based | Population-based |
| Number of patients | 327 | 526 | 238 | 1868 | 32,144 | 7684 |
| Number of events | 46 | Not reported | 31 | 202 | 304 | 1921 |
| Intended to support decision making about: | Adjuvant radiotherapy |
Adjuvant radiotherapy |
Type of surgery and adjuvant radiotherapy |
Adjuvant radiotherapy |
Adjuvant radiotherapy |
Screening or prophylactic mastectomy |
| Risk of bias based on CHARMS | Moderate | Moderate | Moderate/High | Moderate | Moderate | Moderate |
| Number of validation studies retrieved | 3 | 2 | 10 | 3 | 0 | 0 |
| Type of data validation studies | Trial and population-based | Trial and Single center |
Single- and Multi center |
Single center | N.A. | N.A. |
| Number of patients validation studies (range) | 718–1102 | 455–504 | 159–949 | 467–734 | N.A. | N.A. |
| Number of events validation studies (range) | 65–100 | 54–90 | 11–165 | 42–63 | N.A. | N.A. |
| C-index/AUC | 0.68 | None reported |
None reported |
0.61–0.68 | None reported |
None reported |
| Clinical utility | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
Abbreviations: DCIS: Ductal Carcinoma In Situ, CBC: Contralateral Breast Cancer, DFS: Disease Free Survival, CHARMS: Critical Appraisal and Data Extraction form Systematic Reviews of Prediction Modelling Studies, AUC: Area Under the Curve.
3.3. iIBC Models
Three classical prediction models, predicting the risk of iIBC after DCIS, were the first models to be developed for DCIS: the Van Nuys Prognostic Index (VNPI) [46,47,48,49,50,51,52,53,54,55,56,57]; Memorial Sloane Kettering Cancer Centre Nomogram (MSKCC nomogram) [58,59,60,61]; and the DCIS patient prognostic score [62]. The VNPI and MSKCC nomogram were developed on small (n= 238 to 202 patients), single-centre cohorts of patients diagnosed between 1979 and 2006, and treated with surgery with or without radiotherapy and endocrine treatment. The DCIS patient prognostic score was developed using 32,144 patients from the SEER database diagnosed between 1988 and 2007 and only included patients treated with BCS with or without radiotherapy. Measures widely recommended by experts for assessing model performance (e.g., C-index for discriminatory accuracy and calibration) were frequently not reported. For example, no C-index was reported in the studies evaluating the VNPI [46,47,48,49,50,51,52,53,54,55,56,57] and DCIS patient prognostic score [62]. The measures used to report discriminatory accuracy, e.g., Kaplan Meier curves and descriptive statistics, are suboptimal. For the MSKCC nomogram, calibration was reported to be imperfect to good, and the C-index varied from 0.61 in a validation study [61] to 0.69 in the development study [58]. The CHARMS risk of bias was high/moderate for the VNPI and moderate for the DCIS prognostic score and the MSKCC nomogram.
3.4. CBC Model
One model predicting risk of developing contralateral breast cancer after DCIS was retrieved, the CBC risk model [63], which was developed using the SEER database. The CBC risk model was developed for patients with all grades of DCIS, IBC, or a combination of DCIS/IBC. The type of treatment received and follow-up duration for the development set were not reported. C-index and calibration were also not reported. Furthermore, no external validation was reported. Risk of bias according to the CHARMS checklist was moderate.
3.5. Biomolecular Models
Two prediction models containing biomolecular features (such as immunohistochemistry markers and gene expression) were retrieved: Oncotype DCIS [64,65,66,67] (derived from Oncotype DX [68], developed for women with invasive breast cancer) and DCISionRT [69,70]. Oncotype DCIS was developed in a trial cohort of 327 patients. DCISionRT was developed in a multicentre cohort of 526 patients. Patients in both studies were treated with BCS with or without radiotherapy and endocrine treatment (DCISionRT). Diagnosis years varied from 1986 to 2004. The number of events was very low (n = 46) in the development study of Oncotype DCIS, and not reported for DCISionRT. Area under the curve (AUC), C-index and calibration were not reported for DCISionRT; for Oncotype DCIS, calibration was reported to be good, and AUC was 0.68 according to a validation study by Paszat et al. [67]. Both models were developed in highly selected and relatively small patient samples, with a modest number of events (N < 550). Consequently, the CHARMS risk of bias was moderate for both models.
3.6. Clinical Utility
For all six prediction models, clinical utility remains unclear due to the highly selective development and validation datasets used, and due to limited good quality validation studies. The number of validation studies varied from zero to nine. For the VNPI [46], for example, ten validation studies were published. However, most were performed in small, highly selected patient samples consisting partly of the development sample, with low numbers of events (range 11–165) (Table 2); only Kaplan Meier curves and descriptive statistics were used to report on the discriminatory value of the model. Thus, it is very difficult to evaluate clinical utility. Similarly, Oncotype DCIS [64] was validated in three separate papers, all using the same Ontario DCIS cohort [71]. For the DCIS RT score [62] and the CBC risk model [63], no validation studies were retrieved.
4. Discussion
By performing a systematic literature review, we inventoried and assessed the quality of available DCIS decision support tools. We retrieved three decision aids, one communication aid and six prediction models. All decision aids included the option of active surveillance; the communication aid by de Morgan et al. did not. There is room for improvement amongst all the decision aids, but they may serve as templates for the development of other novel aids for informing patients about their treatment options for DCIS. There are few decision aids available, but most are only available in English. None of the six published prediction models included the option of active surveillance, thus there is a need to extend existing models to include this option or develop new tools. Furthermore, assessment of and reporting on the performance of the models was generally suboptimal. The clinical utility of the available models will remain unclear until additional, good-quality external validations are performed in adequately sized cohorts with sufficient events.
Patient decision aids have been shown to effectively provide patients with balanced information on all available treatment strategies, and help them make value-congruent decisions [23]. Decision aids on surgery for women with early-stage breast cancer, for example, have been shown to improve patient involvement, patient knowledge and decision-related outcomes such as decisional conflict, satisfaction and overall quality of life [72]. Women with low-risk DCIS may face similar treatment decisions to women with early-stage breast cancer. However, they have a different prognosis and may in the future have the option to forego surgery and opt for active surveillance. Therefore, well-designed (according to the IPDAS criteria [36]) and properly evaluated decision aids, including the benefits and harms associated with active surveillance, need to be developed to support women with (low-risk) DCIS.
The literature shows that women diagnosed with DCIS have clear knowledge gaps. These misconceptions, particularly incorrect estimation of recurrence/progression risk, can cause increased worry and anxiety and impact negatively upon quality of life [19,73,74,75].
Prediction models and decision aids for DCIS are not widely used in daily practice. As a result, their applicability and impact are not well studied. For the prediction models reviewed, validation studies were limited in number and/or quality. Similarly, for the decision aids, no implementation studies were performed. A lack of validation and implementation studies might therefore hinder the decision support tools in being fully implemented within the clinic setting.
The limited availability of large, population-based datasets of women diagnosed with DCIS that include sufficient follow-up time and events is a barrier for the development of good prediction models. Consequently, many of the models we identified were developed and validated using small, highly selected populations (e.g., trial data and single centre cohorts) with limited follow-up and a small number of events. Thus, the clinical utility of these models for the general DCIS population remains unclear. For example, in a US-based general community cohort of 91 DCIS patients, there was limited agreement in local breast event risk estimates when comparing the VNPI, MSKCC nomogram, Oncotype DCIS and risk estimates from three radiation oncologists [76]. The predicted risks were so highly divergent that it is difficult to determine what should be the “gold standard”. This large divergence in risk estimates is not seen in all studies; Van Zee et al., for example, have reported a 92% concordance in the estimates of loco-regional recurrence risk generated by the MSKCC nomogram and Oncotype DCIS in a dataset with 59 US-based women with DCIS [77].
These examples stress the need for new prediction models to be designed specifically for women with DCIS, but also the need for rich datasets with information for large representative cohorts. Previous publications have shown that translating prediction models that focus on invasive breast cancer to DCIS might be challenging. For instance, a model designed to predict risk of developing contralateral breast cancer for women treated for invasive breast cancer (PredictCBC [78]) was applied to a cohort of Dutch DCIS patients [79]. This model did not perform well in the DCIS cohort, and one of the reasons for this was that many of the strong predictors in the model were not available in the dataset. In this model, hormone receptor status and BRCA status were important predictors, but as these variables are not routinely collected for women with DCIS, the model could not be applied optimally. Similarly, Oncotype DCIS was derived from Oncotype DX, the prediction model developed for women with invasive breast cancer, which had to be extensively adapted to apply to women with DCIS [64]. Thus, models that predict risk of developing invasive breast cancer for women without a history of breast cancer, such as the Breast Cancer Risk Assessment Tool (BCRAT) or Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), cannot simply be applied to a woman with a history of DCIS [80,81].
The prediction models described in our study used similar clinicopathological factors (such as size, DCIS grade and margin width). Model performance was rarely reported, but when available, most models showed modest performance at best. We also included more recently developed models containing biomolecular features and arrays. However, due to limited validation and reporting of performance measures, it is currently unclear if these models perform adequately for women with DCIS. A promising next step towards achieving improved models is moving into the realm of artificial intelligence and machine learning. Such techniques could provide interesting novel options to explore in the context of DCIS. For example, Klimov et al., developed a novel machine learning pipeline to predict risk of ipsilateral breast cancer after DCIS using digitized whole slide images and clinicopathologic long-term outcome data [82], thereby offering a promising new direction.
None of the prediction models we retrieved included the option of active surveillance. As clinical trials studying the safety of active surveillance for women with DCIS are still ongoing, and thus active surveillance is not yet a standard management strategy for DCIS, adequately predicting the outcome of active surveillance is challenging, as it is not yet offered regularly to women with low-risk DCIS. A promising new decision support tool combining a risk calculator with a decision aid that included almost 2000 patients receiving active surveillance in the United States was recently published by Fridman et al. [83]. However, the development paper of the risk calculator within this decision support tool has not yet been published.
A limitation of our study is that we used a search of scientific publications to identify decision support tools. We did not perform an extensive search for unpublished web-based decision support tools for women with DCIS. However, Blackwood et al. performed an extensive search to identify internet-based information resources for DCIS in which they retrieved mostly plain language summaries or informational websites. Only two decision aids were retrieved, neither of which was specifically for women with DCIS [21]. Therefore, we expect that it is unlikely that we have omitted unpublished DCIS decision aids available online.
5. Conclusions
To our knowledge, our systematic review is the first to provide an extensive overview of the available decision support tools for women with DCIS. From our study, we can conclude that there are only a few decision support tools available for women with DCIS, and these tools are mainly in English. The available decision aids are promising, but they do require improvement (e.g., addition of components to facilitate communication with healthcare professionals) to maximize their usefulness and clinical utility, and there is a need for clinical evaluation studies to establish their effectiveness. Based on the available evidence, none of the prediction models retrieved are ready to be implemented in daily clinical practice for women with DCIS. Additional validation studies in larger, more diverse populations are urgently needed to establish the clinical utility of these models. Furthermore, prediction models must be extended, or new models developed, to include the option of active surveillance for women diagnosed with low-risk DCIS to align this area of research with ongoing clinical developments.
Acknowledgments
Grand Challenge PRECISION Consortium Steering Group: Jelle Wesseling (Netherlands Cancer Institute, Amsterdam, the Netherlands), Alastair Thompson (Baylor College of Medicine, Houston, Texas, USA), Serena Nik-Zainal (University of Cambridge, Cambridge, UK), Elinor J. Sawyer (King’s College London, London, UK), Helen Davies (University of Cambridge, Cambridge, UK), Andrew Futreal (MD Anderson Cancer Center, Houston, USA), Nicholas Navin (MD Anderson Cancer Center, Houston, USA), E. Shelley Hwang (Duke University School of Medicine, Durham, NC, USA), Jos Jonkers (Netherlands Cancer Institute, Amsterdam, the Netherlands), Jacco van Rheenen (Netherlands Cancer Institute, Amsterdam, the Netherlands), Fariba Behbod (Kansas University Medical Center, Kansas, USA), Esther H. Lips (Netherlands Cancer Institute, Amsterdam, the Netherlands), Marjanka Schmidt (Netherlands Cancer Institute, Amsterdam, the Netherlands), Lodewyk F.A. Wessels (Netherlands Cancer Institute, Amsterdam, the Netherlands), Daniel Rea (University of Birmingham, Birmingham, UK), Proteeti Bhattacharjee (Netherlands Cancer Institute, Amsterdam, the Netherlands), Hilary Stobart (Independent Cancer Patients’ Voice, UK), Deborah Collyar (Patient Advocates in Research, USA), Donna Pinto (dcis411, USA), Ellen Verschuur (Borstkanker Vereniging Nederland, the Netherlands) and Marja van Oirsouw (Borstkanker Vereniging Nederland, the Netherlands).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14133259/s1, Table S1. Search strategy, Table S2. IPDAS score Decision Aids.
Author Contributions
Conceptualization: R.S.J.M.S., E.G.E. and E.M.A.B.; methodology, R.S.J.M.S., E.G.E. and E.A.W.; validation, R.S.J.M.S. and E.G.E.; formal analysis, R.S.J.M.S. and E.G.E.; investigation, R.S.J.M.S., E.G.E. and E.A.W.; data curation, R.S.J.M.S. and E.G.E.; writing—original draft preparation, R.S.J.M.S. and E.G.E.; writing—review & editing, E.A.W., F.v.D., M.v.O., E.V., T.L., R.S.P., E.S.H., J.W., M.K.S., G.C.P.C. and E.M.A.B.; visualization, R.S.J.M.S. and E.G.E.; supervision, J.W., M.K.S., E.M.A.B. and E.G.E.; project administration, R.S.J.M.S.; and funding acquisition, J.W. and G.C.P.C. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by Cancer Research UK and by KWF Dutch Cancer Society (ref.C38317/A24043).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Kankerbestrijding N. Incidentie— En Overlevingscijfers. [(accessed on 23 June 2021)]. Available online: https://iknl.nl/nkr-cijfers.
- 3.Ryser M.D., Weaver D.L., Zhao F., Worni M., Grimm L.J., Gulati R., Etzioni R., Hyslop T., Lee S.J., Hwang E.S. Cancer outcomes in DCIS patients without locoregional treatment. J. Natl. Cancer Inst. 2019;111:952–960. doi: 10.1093/jnci/djy220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erbas B., Provenzano E., Armes J., Gertig D. The natural history of ductal carcinoma in situ of the breast: A review. Breast Cancer Res. Treat. 2006;97:135–144. doi: 10.1007/s10549-005-9101-z. [DOI] [PubMed] [Google Scholar]
- 5.Ryser M.D., Worni M., Turner E.L., Marks J.R., Durrett R., Hwang E.S. Outcomes of active surveillance for ductal carcinoma in situ: A computational risk analysis. J. Natl. Cancer Inst. 2016;108:372. doi: 10.1093/jnci/djv372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders M.E., Schuyler P.A., Dupont W.D., Page D.L. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103:2481–2484. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 7.Van Seijen M., Precision on behalf of the PRECISION team. Lips E.H., Thompson A.M., Nik-Zainal S., Futreal A., Hwang E.S., Verschuur E., Lane J., Jonkers J., et al. Ductal carcinoma in situ: To treat or not to treat, that is the question. Br. J. Cancer. 2019;121:285–292. doi: 10.1038/s41416-019-0478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleyer A., Welch H.G. Effect of three decades of screening mammography on breast-cancer incidence. N. Engl. J. Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 9.Gierisch J.M., Myers E.R., Schmit K.M., Crowley M.J., McCrory D.C., Chatterjee R., Coeytaux R.R., Kendrick A., Sanders G.D. Prioritization of research addressing management strategies for ductal carcinoma in situ. Ann. Intern. Med. 2014;160:484–491. doi: 10.7326/M13-2548. [DOI] [PubMed] [Google Scholar]
- 10.Francis A., Thomas J., Fallowfield L., Wallis M., Bartlett J.M., Brookes C., Roberts T., Pirrie S., Gaunt C., Young J., et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur. J. Cancer. 2015;51:2296–2303. doi: 10.1016/j.ejca.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Hwang E.S., Hyslop T., Lynch T., Frank E., Pinto D., Basila D., Collyar D., Bennett A., Kaplan C., Rosenberg S., et al. The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: A phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS) BMJ Open. 2019;9:e026797. doi: 10.1136/bmjopen-2018-026797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elshof L.E., Tryfonidis K., Slaets L., Van Leeuwen-Stok A.E., Skinner V.P., Dif N., Pijnappel R.M., Bijker N., Rutgers E.J., Wesseling J. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—The LORD study. Eur. J. Cancer. 2015;51:1497–1510. doi: 10.1016/j.ejca.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 13.De Morgan S., Redman S., White K.J., Cakir B., Boyages J. ‘Well, have I got cancer or haven’t I?’ The psycho-social issues for women diagnosed with ductal carcinoma in situ. Health Expect. 2002;5:310–318. doi: 10.1046/j.1369-6513.2002.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King M.T., Winters Z.E., Olivotto I.A., Spillane A.J., Chua B.H., Saunders C., Westenberg A.H., Mann G.B., Burnett P., Butow P., et al. Patient-reported outcomes in ductal carcinoma in situ: A systematic review. Eur J. Cancer. 2017;71:95–108. doi: 10.1016/j.ejca.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 15.Ganz P.A. Quality-of-life issues in patients with ductal carcinoma in situ. J. Natl. Cancer Inst. Monogr. 2010;2010:218–222. doi: 10.1093/jncimonographs/lgq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallowfield L., Matthews L., Francis A., Jenkins V., Rea D. Low grade Ductal Carcinoma in situ (DCIS): How best to describe it? Breast. 2014;23:693–696. doi: 10.1016/j.breast.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Partridge A., Winer J.P., Golshan M., Bellon J.R., Blood E., Dees E.C., Sampson E., Emmons K.M., Winer E. Perceptions and management approaches of physicians who care for women with ductal carcinoma in situ. Clin. Breast Cancer. 2008;8:275–280. doi: 10.3816/CBC.2008.n.032. [DOI] [PubMed] [Google Scholar]
- 18.Nickel B., McCaffery K., Houssami N., Jansen J., Saunders C., Spillane A., Rutherford C., Dixon A., Barratt A., Stuart K., et al. Views of healthcare professionals about the role of active monitoring in the management of ductal carcinoma in situ (DCIS): Qualitative interview study. Breast. 2020;54:99–105. doi: 10.1016/j.breast.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutherford C., Mercieca-Bebber R., Butow P., Wu J.L., King M.T. Treatment decision-making in ductal carcinoma in situ: A mixed methods systematic review of women’s experiences and information needs. Patient Educ. Couns. 2017;100:1654–1666. doi: 10.1016/j.pec.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Nyhof B.B., Wright F.C., Hong N.J.L., Groot G., Helyer L., Meiers P., Quan M.L., Baxter N.N., Urquhart R., Warburton R., et al. Recommendations to improve patient-centred care for ductal carcinoma in situ: Qualitative focus groups with women. Health Expect. 2020;23:106–114. doi: 10.1111/hex.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackwood J., Wright F.C., Hong N.J.L., Gagliardi A.R. Quality of DCIS information on the internet: A content analysis. Breast Cancer Res. Treat. 2019;177:295–305. doi: 10.1007/s10549-019-05315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin R.W., Andersen S.B., O’Brien M.A., Bravo P., Hoffmann T., Olling K., Shepherd H.L., Dankl K., Stacey D., Steffensen K.D. Providing balanced information about options in patient decision aids: An update from the international patient decision aid standards. Med. Decis Making. 2021;41:780–800. doi: 10.1177/0272989X211021397. [DOI] [PubMed] [Google Scholar]
- 23.Stacey D., Légaré F., Lewis K., Barry M.J., Bennett C.L., Eden K.B., Holmes-Rovner M., Llewellyn-Thomas H., Lyddiatt A., Thomson R., et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Morgan S.E., Butow P.N., Lobb E.A., Price M.A., Nehill C. Development and pilot testing of a communication aid to assist clinicians to communicate with women diagnosed with ductal carcinoma in situ (DCIS) Support. Care Cancer. 2011;19:717–723. doi: 10.1007/s00520-010-1040-5. [DOI] [PubMed] [Google Scholar]
- 25.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booth A., Clarke M., Ghersi D., Moher D., Petticrew M., Stewart L. An international registry of systematic-review protocols. Lancet. 2011;377:108–109. doi: 10.1016/S0140-6736(10)60903-8. [DOI] [PubMed] [Google Scholar]
- 28.PROSPERO Database. [(accessed on 1 June 2020)]. Available online: https://www.crd.york.ac.uk/prospero/
- 29.Medline Database. [(accessed on 22 February 2022)]; Available online: https://www.nlm.nih.gov/medline/index.html.
- 30.Embase Database. [(accessed on 22 February 2022)]. Available online: https://www.embase.com.
- 31.Scopus Database. [(accessed on 22 February 2022)]. Available online: https://www.scopus.com/home.uri.
- 32.TRIP Medical Database. [(accessed on 22 February 2022)]. Available online: http://www.tripdatabase.com/
- 33.Vromans R., Tenfelde K., Pauws S., Van Eenbergen M., Mares-Engelberts I., Velikova G., Van de Poll-Franse L., Krahmer E. Assessing the quality and communicative aspects of patient decision aids for early-stage breast cancer treatment: A systematic review. Breast Cancer Res. Treat. 2019;178:1–15. doi: 10.1007/s10549-019-05351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rayyan Systematic Review Tool. [(accessed on 26 June 2020)]. Available online: https://www.rayyan.ai/
- 36.Elwyn G., O’Connor A., Stacey D., Volk R., Edwards A., Coulter A., Thomson R., Barratt A., Barry M., Bernstein S., et al. Developing a quality criteria framework for patient decision aids: Online international Delphi consensus process. BMJ. 2006;333:417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moons K.G.M., De Groot J.A.H., Bouwmeester W., Vergouwe Y., Mallett S., Altman D.G., Reitsma J.B., Collins G.S. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: The CHARMS checklist. PLoS Med. 2014;11:e1001744. doi: 10.1371/journal.pmed.1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riley R.D., Moons K.G.M., Snell K.I.E., Ensor J., Hooft L., Altman D.G., Hayden J., Collins G.S., Debray T.P.A. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. doi: 10.1136/bmj.k4597. [DOI] [PubMed] [Google Scholar]
- 39.Berger-Hoger B., Liethmann K., Muhlhauser I., Haastert B., Steckelberg A. Informed shared decision-making supported by decision coaches for women with ductal carcinoma in situ: Study protocol for a cluster randomized controlled trial. Trials. 2015;16:452. doi: 10.1186/s13063-015-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger-Hoger B., Liethmann K., Muhlhauser I., Steckelberg A. Implementation of shared decision-making in oncology: Development and pilot study of a nurse-led decision-coaching programme for women with ductal carcinoma in situ. BMC Med. Inform. Decis Mak. 2017;17:160. doi: 10.1186/s12911-017-0548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger-Höger B., Liethmann K., Mühlhauser I., Haastert B., Steckelberg A. Nurse-led coaching of shared decision-making for women with ductal carcinoma in situ in breast care centers: A cluster randomized controlled trial. Int J. Nurs Stud. 2019;93:141–152. doi: 10.1016/j.ijnurstu.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Ozanne E.M., Schneider K.H., Soeteman D., Stout N., Schrag D., Fordis M., Punglia R.S. OnlineDeCISion.org: A web-based decision aid for DCIS treatment. Breast Cancer Res. Treat. 2015;154:181–190. doi: 10.1007/s10549-015-3605-y. [DOI] [PubMed] [Google Scholar]
- 43.Ozanne E.M., Soeteman D.I., Frank E.S., Clarke J., Hassett M.J., Stout N.K., Punglia R.S. Commentary: Creating a patient-centered decision aid for ductal carcinoma in situ. Breast J. 2020;26:1498–1499. doi: 10.1111/tbj.13779. [DOI] [PubMed] [Google Scholar]
- 44.Dcisoptions.Org. [(accessed on 26 June 2020)]. Available online: https://dcisoptions.org/
- 45.Soeteman D.I., Stout N.K., Ozanne E.M., Greenberg C., Hassett M.J., Schrag D., Punglia R.S. Modeling the effectiveness of initial management strategies for ductal carcinoma in situ. J. Natl. Cancer Inst. 2013;105:774–781. doi: 10.1093/jnci/djt096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poller D., Barth A., Slamon D., Silverstein M., Gierson E., Coburn W., Waisman J., Gamagami P., Lewinsky B. Prognostic classification of breast ductal carcinoma-in-situ. Lancet. 1995;345:1154–1157. doi: 10.1016/S0140-6736(95)90982-6. [DOI] [PubMed] [Google Scholar]
- 47.Silverstein M.J., Lagios M.D., Craig P.H., Waisman J.R., Lewinsky B.S., Colburn W.J., Poller D.N. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77:2267–2274. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2267::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 48.De Mascarel I., Bonichon F., Macgrogan G., De Lara C.T., Avril A., Picot V., Durand M., Mauriac L., Trojani M., Coindre J.M. Application of the Van Nuys prognostic index in a retrospective series of 367 ductal carcinomas in situ of the breast examinated by serial macroscopic sectioning: Practical considerations. Breast Cancer Res. Treat. 2000;61:151–159. doi: 10.1023/A:1006437902770. [DOI] [PubMed] [Google Scholar]
- 49.Boland G.P., Chan K.C., Knox W.F., Roberts S.A., Bundred N.J. Value of the Van Nuys Prognostic Index in prediction of recurrence of ductal carcinoma in situ after breast-conserving surgery. Br. J. Surg. 2003;90:426–432. doi: 10.1002/bjs.4051. [DOI] [PubMed] [Google Scholar]
- 50.Silverstein M.J. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am. J. Surg. 2003;186:337–343. doi: 10.1016/S0002-9610(03)00265-4. [DOI] [PubMed] [Google Scholar]
- 51.MacAusland S.G., Hepel J.T., Chong F.K., Galper S.L., Gass J.S., Ruthazer R., Wazer D.E. An attempt to independently verify the utility of the Van Nuys Prognostic Index for ductal carcinoma in situ. Cancer. 2007;110:2648–2653. doi: 10.1002/cncr.23089. [DOI] [PubMed] [Google Scholar]
- 52.Di Saverio S., Catena F., Santini D., Ansaloni L., Fogacci T., Mignani S., Leone A., Gazzotti F., Gagliardi S., De Cataldis A., et al. 259 Patients with DCIS of the breast applying USC/Van Nuys prognostic index: A retrospective review with long term follow up. Breast Cancer Res. Treat. 2008;109:405–416. doi: 10.1007/s10549-007-9668-7. [DOI] [PubMed] [Google Scholar]
- 53.Altintas S., Lambein K., Huizing M.T., Braems G., Asjoe F.T., Hellemans H., Van Marck E., Weyler J., Praet M., Broecke R.V.D., et al. Prognostic significance of oncogenic markers in ductal carcinoma in situ of the breast: A clinicopathologic study. Breast J. 2009;15:120–132. doi: 10.1111/j.1524-4741.2009.00686.x. [DOI] [PubMed] [Google Scholar]
- 54.Silverstein M.J., Lagios M.D. Choosing treatment for patients with ductal carcinoma in situ: Fine tuning the University of Southern California/Van Nuys Prognostic Index. J. Natl Cancer Inst. Monogr. 2010;2010:193–196. doi: 10.1093/jncimonographs/lgq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelley L., Silverstein M., Guerra L. Analyzing the risk of recurrence after mastectomy for DCIS: A new use for the USC/Van Nuys Prognostic Index. Ann. Surg Oncol. 2011;18:459–462. doi: 10.1245/s10434-010-1335-2. [DOI] [PubMed] [Google Scholar]
- 56.Silverstein M.J., Lagios M.D. Treatment selection for patients with ductal carcinoma in situ (DCIS) of the breast using the University of Southern California/Van Nuys (USC/VNPI) prognostic index. Breast J. 2015;21:127–132. doi: 10.1111/tbj.12368. [DOI] [PubMed] [Google Scholar]
- 57.Kunkiel M., Niwińska A. Assessment of the usefulness of prognostic Van Nuys Prognostic Index in the treatment in ductal carcinoma in situ in 15-year observation. Sci Rep. 2021;11:22645. doi: 10.1038/s41598-021-02126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rudloff U., Jacks L.M., Goldberg J.I., Wynveen C.A., Brogi E., Patil S., Van Zee K.J. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J. Clin. Oncol. 2010;28:3762–3769. doi: 10.1200/JCO.2009.26.8847. [DOI] [PubMed] [Google Scholar]
- 59.Yi M., Meric-Bernstam F., Kuerer H.M., Mittendorf E.A., Bedrosian I., Lucci A., Hwang R.F., Crow J.R., Luo S., Hunt K.K. Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:600–607. doi: 10.1200/JCO.2011.36.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang F., Li H., Tan P., Chua E., Yeo R., Lim F., Kim S., Tan D., Wong F. Validation of a nomogram in the prediction of local recurrence risks after conserving surgery for Asian women with ductal carcinoma in situ of the breast. Clin. Oncol. 2014;26:684–691. doi: 10.1016/j.clon.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Sweldens C., Peeters S., van Limbergen E., Janssen H., Laenen A., Patil S., Van Zee K.J. Local relapse after breast-conserving therapy for ductal carcinoma in situ: A European single-center experience and external validation of the Memorial Sloan-Kettering Cancer Center DCIS nomogram. Cancer J. 2014;20:1–7. doi: 10.1097/PPO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 62.Sagara Y., Freedman R.A., Vaz-Luis I., Mallory M.A., Wong S.M., Aydogan F., DeSantis S., Barry W.T., Golshan M. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast-conserving surgery for ductal carcinoma in situ: A population-based longitudinal cohort study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016;34:1190–1196. doi: 10.1200/JCO.2015.65.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chowdhury M., Euhus D., Onega T., Biswas S., Choudhary P.K. A model for individualized risk prediction of contralateral breast cancer. Breast Cancer Res. Treat. 2017;161:153–160. doi: 10.1007/s10549-016-4039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solin L.J., Gray R., Baehner F.L., Butler S.M., Hughes L.L., Yoshizawa C., Cherbavaz D.B., Shak S., Page D.L., Sledge G.W., Jr., et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J. Natl. Cancer Inst. 2013;105:701–710. doi: 10.1093/jnci/djt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rakovitch E., Nofech-Mozes S., Hanna W., Baehner F.L., Saskin R., Butler S.M., Tuck A., Sengupta S., Elavathil L., Jani P.A., et al. A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res. Treat. 2015;152:389–398. doi: 10.1007/s10549-015-3464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rakovitch E., Gray R., Baehner F.L., Sutradhar R., Crager M., Gu S., Nofech-Mozes S., Badve S.S., Hanna W., Hughes L.L., et al. Refined estimates of local recurrence risks by DCIS score adjusting for clinicopathological features: A combined analysis of ECOG-ACRIN E5194 and Ontario DCIS cohort studies. Breast Cancer Res. Treat. 2018;169:359–369. doi: 10.1007/s10549-018-4693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paszat L., Sutradhar R., Zhou L., Nofech-Mozes S., Rakovitch E. Including the Ductal Carcinoma-In-Situ (DCIS) Score in the Development of a Multivariable Prediction Model for Recurrence After Excision of DCIS. Clin. Breast Cancer. 2019;19:35–46. doi: 10.1016/j.clbc.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 68.Paik S., Shak S., Tang G., Kim C., Baker J., Cronin M., Baehner F.L., Walker M.G., Watson D., Park T., et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 69.Bremer T., Whitworth P.W., Patel R., Savala J., Barry T., Lyle S., Leesman G., Linke S.P., Jirström K., Zhou W., et al. A biological signature for breast ductal carcinoma in situ to predict radiotherapy benefit and assess recurrence risk. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018;24:5895–5901. doi: 10.1158/1078-0432.CCR-18-0842. [DOI] [PubMed] [Google Scholar]
- 70.Weinmann S., Leo M.C., Francisco M., Jenkins C.L., Barry T., Leesman G., Linke S.P., Whitworth P.W., Patel R., Pellicane J., et al. Validation of a Ductal Carcinoma In Situ Biomarker Profile for Risk of Recurrence after Breast-Conserving Surgery with and without Radiotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020;26:4054–4063. doi: 10.1158/1078-0432.CCR-19-1152. [DOI] [PubMed] [Google Scholar]
- 71.Rakovitch E., Nofech-Mozes S., Narod S.A., Hanna W., Thiruchelvam D., Saskin R., Taylor C., Tuck A., Sengupta S., Elavathil L., et al. Can we select individuals with low risk ductal carcinoma in situ (DCIS)? A population-based outcomes analysis. Breast Cancer Res. Treat. 2013;138:581–590. doi: 10.1007/s10549-013-2455-8. [DOI] [PubMed] [Google Scholar]
- 72.Si J., Guo R., Lu X., Han C., Xue L., Xing D., Chen C. Decision aids on breast conserving surgery for early stage breast cancer patients: A systematic review. BMC Med. Inform. Decis Mak. 2020;20:275. doi: 10.1186/s12911-020-01295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y., Perez M.C.R., Schootman M., Aft R.L., Gillanders W.E., Ellis M.J., Jeffe D. A longitudinal study of factors associated with perceived risk of recurrence in women with ductal carcinoma in situ and early-stage invasive breast cancer. Breast Cancer Res. Treat. 2010;124:835–844. doi: 10.1007/s10549-010-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y., Pérez M., Schootman M., Aft R.L., Gillanders W.E., Jeffe D.B. Correlates of fear of cancer recurrence in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res. Treat. 2011;130:165–173. doi: 10.1007/s10549-011-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hawley S.T., Janz N.K., Griffith K.A., Jagsi R., Friese C., Kurian A.W., Hamilton A.S., Ward K.C., Morrow M., Wallner L.P., et al. Recurrence risk perception and quality of life following treatment of breast cancer. Breast Cancer Res. Treat. 2017;161:557–565. doi: 10.1007/s10549-016-4082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lei R.Y., Carter D.L., Antell A.G., Nowels M.A., Tole S.P., Bennett J.P., Turner M., Baehner F.L., Leonard C.E. A comparison of predicted ipsilateral tumor recurrence risks in patients with ductal carcinoma in situ of the breast after breast-conserving surgery by breast radiation oncologists, the van nuys prognostic index, the memorial sloan kettering cancer center dcis nomogram, and the 12-Gene DCIS score assay. Adv. Radiat Oncol. 2021;6:100607. doi: 10.1016/j.adro.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Zee K.J., Zabor E.C., Di Donato R., Harmon B., Fox J., Morrow M., Cody H.S., III, Fineberg S.A. Comparison of local recurrence risk estimates after breast-conserving surgery for dcis: Dcis nomogram versus refined oncotype dx breast dcis score. Ann. Surg. Oncol. 2019;26:3282–3288. doi: 10.1245/s10434-019-07537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giardiello D., Steyerberg E.W., Hauptmann M., Adank M.A., Akdeniz D., Blomqvist C., Bojesen S.E., Bolla M.K., Brinkhuis M., Chang-Claude J., et al. Prediction and clinical utility of a contralateral breast cancer risk model. Breast Cancer Res. 2019;21:144. doi: 10.1186/s13058-019-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giardiello D., Kramer I., Hooning M.J., Hauptmann M., Lips E.H., Sawyer E., Thompson A.M., De Munck L., Siesling S., Wesseling J., et al. Contralateral breast cancer risk in patients with ductal carcinoma in situ and invasive breast cancer. NPJ Breast Cancer. 2020;6:60. doi: 10.1038/s41523-020-00202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Louro J., Posso M., Boon M.H., Román M., Domingo L., Castells X., Sala M. A systematic review and quality assessment of individualised breast cancer risk prediction models. Br. J. Cancer. 2019;121:76–85. doi: 10.1038/s41416-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ståhlbom A.K., Johansson H., Liljegren A., Von Wachenfeldt A., Arver B. Evaluation of the BOADICEA risk assessment model in women with a family history of breast cancer. Fam. Cancer. 2012;11:33–40. doi: 10.1007/s10689-011-9495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klimov S., Miligy I.M., Gertych A., Jiang Y., Toss M.S., Rida P., Ellis I.O., Green A., Krishnamurti U., Rakha E.A., et al. A whole slide image-based machine learning approach to predict ductal carcinoma in situ (DCIS) recurrence risk. Breast Cancer Res. 2019;21:83. doi: 10.1186/s13058-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fridman I., Chan L., Thomas J., Fish L.J., Falkovic M., Brioux J., Hunter N., Ryser D.H., Hwang E.S., Pollak K.I., et al. A web-based personalized decision support tool for patients diagnosed with ductal carcinoma in situ: Development, content evaluation, and usability testing. Breast Cancer Res. Treat. 2022 doi: 10.1007/s10549-022-06512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.