Abstract
We herein report the synthesis of poly (9-decenoic acid-1-vinylimidazole-N-isopropylacrylamide) nanoparticles containing indocyanine green (ICG) in one pot and in water phase throughout the reaction. We have shown that copolymers of 9-decenoic acid and 1-vinylimidazole, or 9-decenoic acid alone, have an enhanced sensitivity to pH values between 7.4 and 6.8 and are superior to the widely used acrylic acid. We have also shown that incorporation of acidic comonomers leads to the favorable outcome of a higher fluorescence signal intensity in lower pH values, whereas the opposite is true of basic comonomers, where the fluorescence signal intensity is lower at low pH values. It was shown that to keep the pH response favorable the molar ratio of basic comonomers to acidic comonomers should roughly equal 1:4. We controlled the lower critical solution temperature (LCST) of the nanoparticles from around 30 to 38°C for different applications by adding acrylamide comonomers. Finally, the nanoparticles at varying pH values, when imaged by an ultrasound switchable fluorescence (USF) imaging system, showed pH sensitivity and thermosensitivity at physiological and tumor pH.
Keywords: pH sensitive polymer, acrylic acid, 9-decenoic acid, 8-nonenoic acid, 1-vinylimidazole, near-infrared (NIR) fluorescence imaging
Impact Statement
We synthesized pH sensitive and thermosensitive nanoparticles for ultrasound switchable fluorescence imaging. Despite the need for acidic comonomers with pKa near physiological pH, acrylic acid has been widely used as a pH-sensitive conferring agent. The pKa of acrylic acid is 4.3 (at 25°C), thus not capable of granting substantial pH sensitivity. We used 9-decenoic acid in this study, which has been overlooked in the field owing to concerns about its solubility. We have successfully used 9-decenoic acid as the pH sensitivity conferring agent in polymeric nanoparticles with near physiological pKa, alone and in combination with 1-vinylimidazole, to confer enhanced sensitivity near physiological pH (7.4 and 6.8). This is the first time such monomers have been used in the nanoparticles of LCST polymers such as poly(N-isopropylacrylamide), pNIPAm. The method we are proposing is facile and fast and can be used in the synthesis of contrast agents and drug delivery vehicles.
Introduction
Early cancer detection can increase the chance of survival and reduce undue suffering and costs.1,2 Developing imaging modalities capable of early detection by reaching high sensitivities remains the goal in the development of imaging systems and probes. 3 Near-infrared (NIR) fluorescence imaging is a sensitive and noninvasive method for investigating physiological and biomolecular processes. 4 Although NIR fluorescence can penetrate biological tissue several centimeters deep, it suffers from poor spatial resolution owing to the highly scattering nature of tissue. 4 Hybrid ultrasound/optical methods are limited by acoustic diffraction. To address this issue, we have developed ultrasound switchable fluorescence imaging (USF).4–7 USF adopts a thermosensitive contrast agent and uses a highly focused ultrasound beam (HIFU) to slightly heat tissue in its focal zone to switch on the fluorescence emission from the probes in the focal zone.4,5 Because only a fraction of the probes inside the focal zone that reach the lower critical solution temperature (LCST) switch on, the spatial resolution can be improved beyond the acoustic diffraction limit. 6 This imaging modality can achieve high resolution and high sensitivity in tissue at a depth of several centimeters.
Low pH is a hallmark of many cancers and has been used as an indicator for cancer detection and surgery guidance. 8 Despite heterogeneity in tumor cells, dysregulated pH is a hallmark of most cancers, irrespective of their genetic origin. 9 Whereas normal cells have an intracellular pH (pHi) of about 7.2 and extracellular pH (pHe) of 7.4, cancer cells have an elevated pHi > 7.4 and a low pHe of 6.5–6.9. 8
To further improve the switching efficiency of the contrast agent in USF imaging, therefore, the sensitivity, or signal-to-noise ratio (SNR), we speculate to take advantage of the pH difference between cancerous and normal tissues. As such, because USF contrast agents until now have only been thermosensitive, we investigated contrast agents with pH-sensitivity and thermosensitivity in this work. A contrast agent that is pH sensitive and thermosensitive may potentially be switched on via both the internal cancerous lower pH environment and the externally applied ultrasound-induced thermos energy to achieve higher switching efficiency and SNR.
Because of the narrow difference between the pH of tumors and healthy tissue (pH = ~7.4), there is a need for acidic comonomers with pKa values near the physiological pH. Although there are amines with pKa near 7.4, their effect is not favorable in nanoparticles of LCST polymers, as fluorescence signal intensity is lower at low pH values.
Despite the need for acidic comonomers with higher pKa, the literature is rife with synthesis methods of pH-sensitive nanoparticles used for imaging and drug delivery in which acrylic acid has been used as a pH-sensitive conferring agent. This is despite the fact that the pKa of acrylic acid is 4.3 (at 25°C) 10 and most carboxyl groups are deprotonated within a pH of 3 to 5.
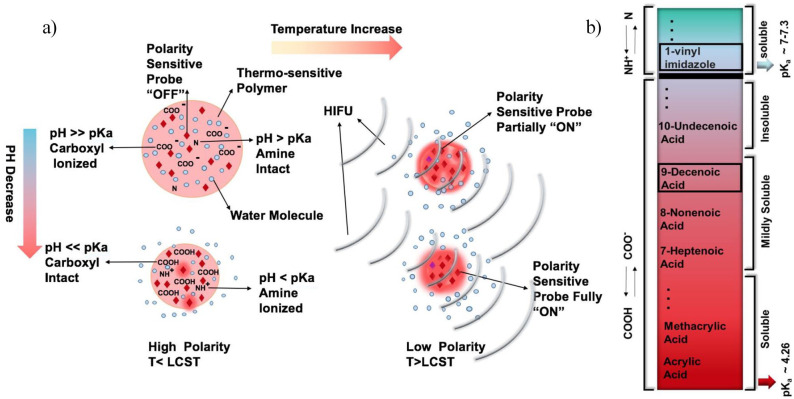
In this work, a facile and fast method for the synthesis of a pH-sensitive contrast agent of poly (N-isopropylacrylamide), PNIPAm, for USF imaging in one pot has been developed. We propose the application of acidic comonomers with longer carbon chains and, consequently, higher pKa, such as 8-nonenoic acid (NAc) and 9-decenoic acid (DAc) as well as the combination of acidic and basic comonomers such as 1-vinylimidazole to achieve near physiological pH sensitivity (Figure 1).
Figure 1.
(a) A schematic showing how the polarity-sensitive fluorophores encapsulated in the pH- and thermo-sensitive nanoparticle are switched on in an environment with a low pH value and a high temperature in USF imaging. When a nanoparticle is in an environment with a pH value lower than the pKa of acidic comonomer (such as in the tumor environment), the particle shrinks and water molecules are expelled. The polarity in the nanoparticle reduces, which leads to the fluorophores partially switching on. Upon applying the HIFU, the temperature increases above the LCST of the particle within the ultrasound focal zone. This further expels water molecules and reduces the polarity in the particle and, therefore, more fluorophores are switched on. (b) A schematic of the pKa of the comonomers. 9-decenoic acid has the highest pKa among the partially soluble carboxylic acids. (A color version of this figure is available in the online journal.)
Materials and methods
Materials
N-Isopropylacrylamide (NIPAm), 4-4′-Azobis (4-cyanopentanoic acid) (ACA), NAc and DAc, 1-vinylimidazole and acrylamide (AAm), indocyanine green (ICG) were obtained from Sigma-Aldrich Corporate (St. Louis, MO, USA). Acrylic acid (AAc) was obtained from Acros Organics. N, N′-Methylenebis (acrylamide) (BIS) was obtained from Alfa Aesar Co., Inc. Sodium dodecyl sulfate (SDS) was obtained from Fisher Scientific (Pittsburgh, PA, USA).
Contrast agent development (ICG-encapsulated pH-sensitive PNIPAm nanoparticles)
A revised protocol7,11–14 was used for the synthesis of NIPAm-based nanoparticles with an acidic comonomer, in which NIPAm (1 mol % based on NIPAm) of BIS and a variable amount of SDS (~5 mol % in [Generation 1] G1, ~3 mol % in G2) were dissolved in 60 ml of deionized water (DI water) in a 250 ml Schlenk tube, followed by nitrogen purging for 30 min. ICG (0.038 mol %), ACA (4 mol %), and the corresponding amount of carboxylic acid and/or 1-vynilimidazole (as declared in each experiment) were dissolved in 1 ml of DI water and added to the Schlenk tube. For controlling the LCST, Aam was also used in the corresponding experiments 7 (Table 1). The tube was sealed and vacuumed until most bubbles were drawn out and then purged with nitrogen (three rounds). The reaction was carried out at 70°C while stirring for 2 h. G1 samples were dialyzed by a 10-kDa molecular weight cut-off (MWCO) membrane for 3 days. G2 (#1 and #2) samples were also filtered by 10-kDa Amicon ultra centrifugal filters (Merck Millipore, Billericia, MA, USA) and G2 (#3 to #6) were only filtered (we found filtering superior to dialysis in efficiency and time). Samples were lyophilized and rehydrated in BupHTM Modified Dulbecco’s phosphate-buffered saline (PBS) from Thermo Scientific (Rockford, IL, USA). G1 samples had a concentration of 20 mg/mL and G2 samples 3.3 mg/mL for cuvette study. For USF imaging, the samples had a concentration of 20 mg/mL because higher concentrations were needed. The samples were then filtered by 0.45 µm membrane filters (Fisher Scientific). The pH was adjusted by adding different concentrations of acrylic acid and allylamine to keep constant the total concentration, and the salt concentration, of all the samples.
Table 1.
Components and weight of the ICG-encapsulated pH and thermosensitive nanoparticles.
| ICG (2 mg) |
NIPAm (mg) |
BIS (mg) |
SDS (mg) |
ACA (mg) |
acrylic acid (µL) | 8-nonenoic acid (µL) | 9-decenoic acid (µL) | |
|---|---|---|---|---|---|---|---|---|
|
G1 |
#1 pNIPAm-co-AAc (0.3 mmol) | 780 | 15 | 134 | 78 | 20.6 | – | – |
| #2 pNIPAm-co-NAc (0.3 mmol) | 780 | 15 | 134 | 78 | – | 51.6 | – | |
| #3 pNIPAm-co-DAc (0.3 mmol) | 780 | 15 | 134 | 78 | – | – | 59.4 | |
| ICG (2 mg) |
NIPAm (mg) |
BIS (mg) |
SDS (mg) |
ACA (mg) |
9-decenoic acid (µL) | 1-Vynil imidazole (µL) | Aam (mg) |
|
|
G2 |
#1 pNIPAm-co-DAc (0.1 mmol) | 780 | 15 | 75 | 78 | 19.8 | – | – |
| #2 pNIPAm-co-DAc (0.2 mmol) | 780 | 15 | 75 | 78 | 39.6 | – | – | |
| #3 pNIPAm-co-DAc (0.1 mmol)-co-1-vynilimidazole (0.025 mmol) | 780 | 15 | 75 | 78 | 19.8 | 2.26 | – | |
| #4 pNIPAm-co-DAc (0.15 mmol)-co-1-vynilimidazole (0.0375 mmol) | 780 | 15 | 75 | 78 | 29.7 | 3.39 | – | |
| #5 pNIPAm-co-DAc (0.1 mmol)-co-1-vynilimidazole (0.05 mmol) | 780 | 15 | 75 | 78 | 19.8 | 4.52 | – | |
| #6 pNIPAm-co-DAc (0.1 mmol)-co-1-vynilimidazole (0.025 mmol)-co-Aam (0.15 mmol) | 780 | 15 | 75 | 78 | 19.8 | 2.26 | 10.9 | |
ICG: indocyanine green; pNIPAm: poly(N-isopropylacrylamide); BIS: N, N′-Methylenebis (acrylamide); SDS: sodium dodecyl sulfate; ACA: 4-4′-Azobis (4-cyanopentanoic acid); G: generation; AAc: acrylic acid; NAc: 8-nonenoic acid; DAc: 9-decenoic acid.
Fluorescent intensity measurement in a cuvette system
A diode laser (MLL-FN-808, 808 nm, Dragon Lasers, Changchun, Jilin, China) was used to illuminate the sample. The laser light was driven by an Agilent function generator (33220A, Santa Clara, CA, USA). A qpod 2e temperature-controlled sample compartment with an internal temperature controller from Quantum Northwest (Liberty Lake, WA) was used to hold the sample and regulate the temperature. A Q-Blue program from the same company was used for controlling qpod 2e. A Quiet One® Pro Series Aquarium Pump model 100 from Lifegard Aquatics (Cerritos, CA) was used for circulating water inside the temperature-controlled sample compartment. A quartz cuvette containing 3 mL of the sample was inserted inside the compartment, and the fluorescence emission light passed through a 830 nm longpass filter (BLP01-830R-25, Semrock, New York) before being detected by the spectrometer (USB2000+, Ocean Insight, Dunedin, FL, USA). For each sample, fluorescence was measured twice and a two-tailed paired t-test was conducted for comparison of the fluorescence strength at specific temperatures.
Fluorescent spectra measurement in a cuvette system
The sample was illuminated by a diode laser (MLL-FN-671-500 mW, 671 nm, Dragon Lasers, Changchun, Jilin, China). The light passed through a 715 longpass filter (715LP: FF01-715/LP-25, Semrock, Rochester, NY, USA) before being detected by a spectrometer (USB2000+, Ocean Insight, Dunedin, FL, USA).
Hydrodynamic size measurement
The nanoparticles’ sizes were measured using dynamic light scattering (ZetaPLUS, Brookhaven Instruments, NY) at 25°C. The run duration was 1 min per reading.
The USF imaging system: Our previous article described the USF imaging system in detail. 6 Briefly, the excitation light with a wavelength of 808 nm passed through an excitation filter (785/62 nm bandpass) and illuminated the sample from two directions. The emitted fluorescent light was filtered by a set of 830 nm longpass emission filters and collected by an electron multiplying charge-coupled device (EMCCD) camera with a field of view of 3×3 cm. An HIFU transducer with a center frequency of 2.5 MHz was used to heat the sample and switch on the USF contrast agents inside the focus. In this study, the ultrasound exposure time was kept at 400 ms and the estimated ultrasound power changed with the different samples (i.e. low-LCST: 0.44 W; high-LCST: 1.21 W). The background temperature of the sample (i.e. 30°C for low-LCST and 37°C for high-LCST) was controlled by a water bath via a temperature controller system. A silicone tube with an inner diameter of 0.76 mm and an outer diameter of 1.65 mm (ST 60-011-04, Helix Medical, USA) was inserted into a piece of chicken breast tissue at a height of ~1 cm from the bottom surface (Figure 4(e) and (f)). The tissue was inserted into a plastic box with a window that was covered with parafilm. Ultrasound gel (Aquasonic 100, Parker Laboratories Inc., USA) was applied to fill the gap between the parafilm and the tissue (Figure 4(e) and (f)).
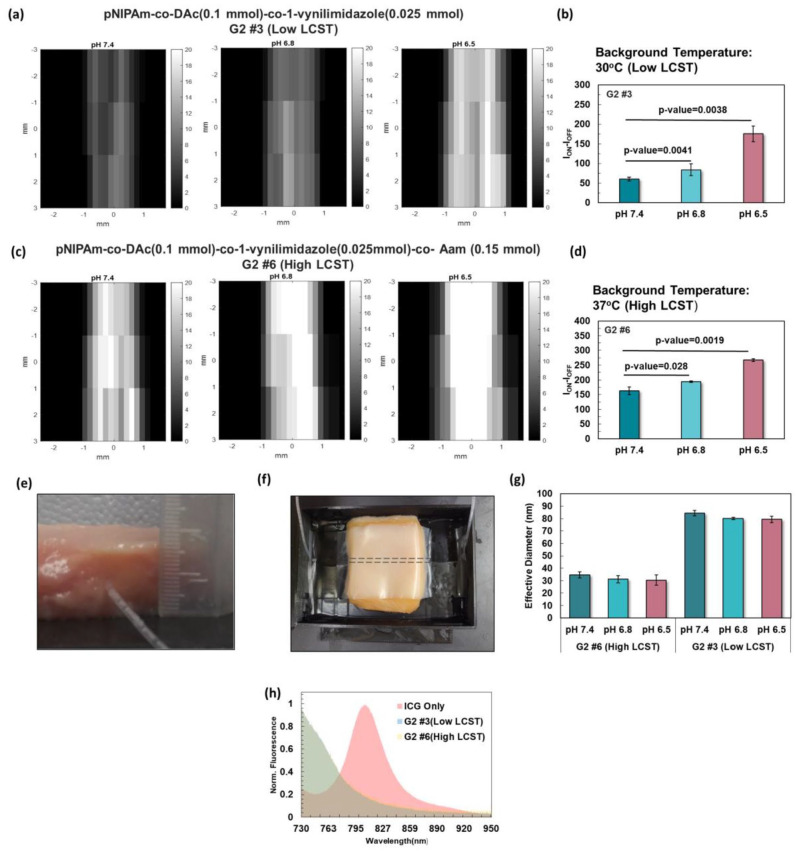
Figure 4.
(a) Imaging of the low LCST (30°C) polymeric nanoparticle of pNIPAm-co-DAc (0.1 mmol)-co-1-vynilimidazole (0.025 mmol), G2 #3, inside a silicone tube at the depth of ~1 cm in chicken tissue. (b) USF signal significantly increased from pH 7.4 (physiological pH), to 6.8 and 6.5 (higher and lower limits of tumor pH). (c) Imaging of pNIPAm-co-DAc (0.1 mmol)-co-1-vynilimidazole (0.025 mmol)-co-Aam (0.15 mmol) with high LCST of 38°C. (d) There was a significant difference in USF signal between pH 7.4 (physiological), compared to 6.8, and 6.5 (tumor pH). (e) and (f) Silicone tube inserted at the depth of ~1 cm in chicken tissue for USF imaging. (g) Effective diameter of samples G #3(Low LCST) and G #6 (High LCST) at 25°C.(h) Emission spectra of ICG and samples G #3 and G #6 at the excitation of 671 nm with an emission filter of 715 long-pass. (A color version of this figure is available in the online journal.)
USF image processing
The tube inserted into a piece of chicken breast tissue was scanned by the HIFU transducer in three lines (with each line containing 41 scan points). Two images were taken at each scan position. The background taken before the HIFU was fired was subtracted from the image taken after the HIFU was applied. A two-dimensional moving average filter was used to smooth the image, and the noise was further minimized through pattern filtering. The USF signal strength was then calculated by summing all the signals of the area showing the silicone tube. A two-tail t-test with unequal variance was conducted for the USF image analysis.
Results
Nanoparticle testing
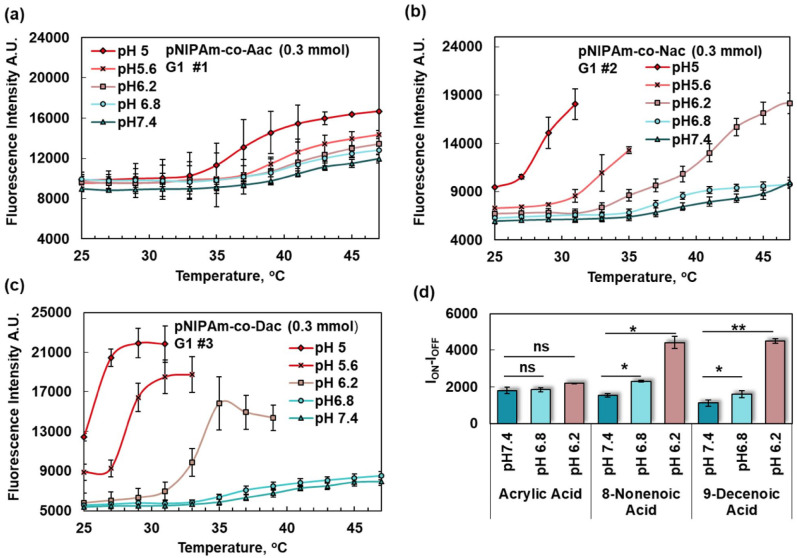
To find the most sensitive pH-conferring comonomer, fluorescence intensity was studied versus pH in G1 samples by increasing the temperature. The addition of comonomers of acrylic acid, NAc, and DAc to the PNIPAm nanoparticles and the degree of acquired sensitivity to the pH change was investigated (Figure 2(a) to (c), respectively). For comparing the pH sensitivity of the acidic comonomers, ION − IOFF, was defined as the fluorescence intensity when the probe was switched “On” minus the fluorescence strength when the probe was switched “Off” (Figure 2(e)).
Figure 2.
Nanoparticle response to temperature at different pH values (G1) when (a) acrylic acid, (b) 8-nonenoic acid and (c) 9-decenoic acid were used as comonomers with NIPAm. (d) ION − IOFF was calculated at for (a), (b), and (c) at (43–37°C), (41–35°C) and (39–33°C), respectively. ION − IOFF was significantly increased between pH 7.4 and 6.8 for (b) and (c) (p-values 0.0399 and 0.0218) as well as 7.4 and 6.2 (p-values 0.0364, 0.0048, respectively.) (A color version of this figure is available in the online journal.)
Based on our data, nanoparticles with comonomers of NAc and DAc were more sensitive to pH changes in the near physiological pH than the comonomers of acrylic acid. As such, comonomers of acrylic acid increased the switching ratio by 2.24% (pH 6.8 versus 7.4) and 18.78% (pH 6.2 versus 6.8), NAc increased the switching ratio by 49.63% (pH 6.8 versus 7.4) and 90.96% (pH 6.2 versus 6.8), and DAc increased the switching ratio by 43.69% (pH 6.8 versus 7.4) and 179.97% (pH 6.2 versus 6.8). Accordingly, DAc was used as the pH-conferring agent for the rest of the experiments because it had the highest pKa.
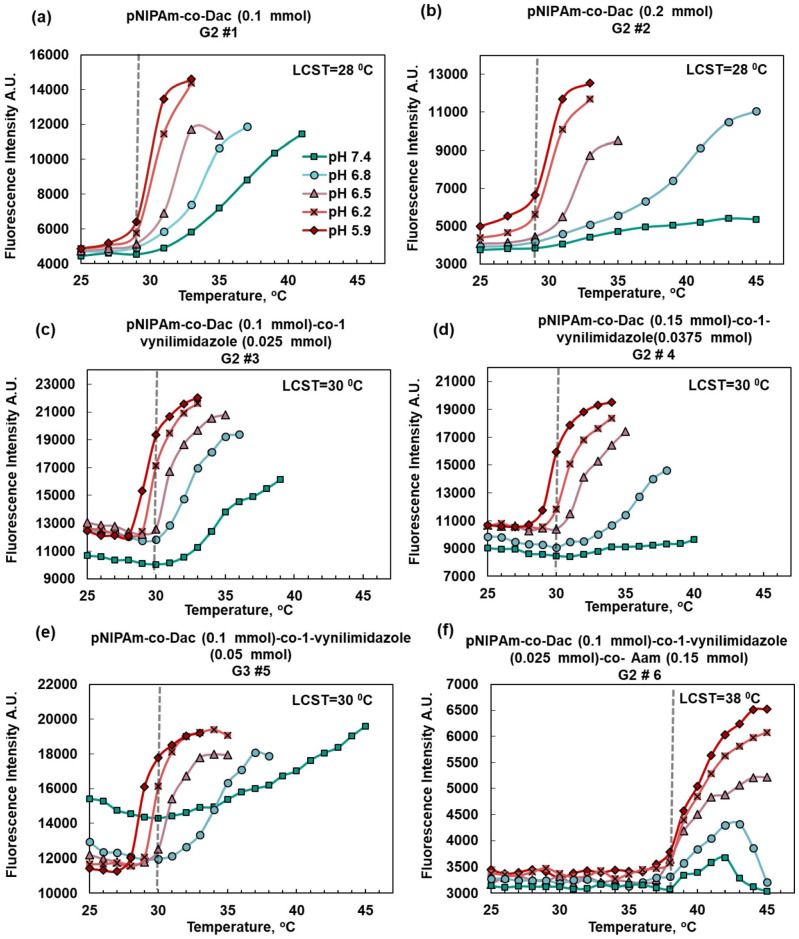
An important factor to study was the ratio of the carboxylic acid comonomer to the NIPAm monomers. USF contrast agents rely on thermosensitivity of the nanoparticles for the switching mechanism. The swelling, owing to the repulsion caused by the deprotonation of the acidic comonomers, is the heart of pH-sensitivity. However, it weakens the thermosensitivity of the nanoparticle and further decreases the USF signal. We have tried to optimize the amount of the acidic comonomer to compromise between pH-sensitivity and thermosensitivity. The amount of DAc comonomers was set to 0.1 mmol or ~1.5 mol % (of NIPAm) and to 0.2 mmol or ~3 mol % (Figure 3(a) and (b)). Based on these results for USF imaging, 0.2 mmol of acid was excessive and undermined thermosensitivity. For this particular application, we kept the acidic monomer below 0.2 mmol based on the need for a strong switching mechanism for USF imaging. This is despite the fact that slightly increasing the acidic comonomer to 0.15 mmol (2.2 mol %) is still applicable and might be superior in some applications such as drug delivery owing to stronger pH sensitivity (Figure 3(c)).
Figure 3.
(a and b) Response of pH and thermosensitive contrast agents to temperature when (0.1 mmol) 1.5 mol % (of NIPAm) and (0.2 mmol) 3 mol % were used, respectively. Thermosensitivity is preserved with the lower percentage of 9-decenoic acid comonomers (1.5 mol %). (c) 1-Vynilimidazole can increase pH sensitivity at pH near 7.4 when used at a molar ratio of 1:4 of the acid comonomers. (d) The concentration of acidic comonomer slightly increased while the basic comonomer was preserved at a 1:4 ratio of the acidic comonomer. (e) When the ratio of basic comonomer to acidic comonomer was higher than 1:4 (1:2 here), the fluorescence signal intensity unfavorably increased at higher pH values (f) The LCST was increased to 38°C from about 30°C by addition of 0.15 mmol of Aam (2.2 mol % of NIPAm). (A color version of this figure is available in the online journal.)
Although DAc can be used on its own as a pH sensitivity conferring agent without any other comonomers, we took benefit from the 1-vynilimidazole with its much desired pKa near physiological pH. As discussed before, using an amine as the sole pH-sensitive comonomer is not possible in LCST polymers such as pNIPAm owing to the unfavorable outcome of a stronger signal in higher pH. Our strategy has been to use 1-vynilimidazole in lower concentrations compared with DAc. Based on our data, the ratio in mmol of 1-vynilimidazole to DAc that yields a favorable strong signal in lower pH is 1:4 of the acid (Figure 3(c)). At the ratio of 1:2, the basic comonomer dominates and the unfavorable outcome is the stronger signal at a higher pH (Figure 3(e)). By keeping the ratio of 1:4 (moles basic comonomer/moles acidic comonomer), it was possible to increase the amount of acidic comonomers to 0.15 mmol (Figure 3(d)) and still have the favorable outcome, which highlights the importance of preserving the ratio.
LCST design and control
LCST is increased by increasing the hydrophilicity of the polymeric nanoparticles. Hydrophilicity can be controlled by increasing the polymer’s ion content. For achieving a high pH sensitivity, acidic DAc and basic comonomers 1-vynilimidazole are already present in high-LCST samples. However, for the high-LCST samples, the addition of ionic monomers is needed. Different strategies such as the addition of poly (ethylene glycol) methacrylate (PEG) and sulfobetaine, (2-(methacryloyloxy) ethyl) dimethyl-(3-sulfopropyl) ammonium hydroxide (DMAPS) were tried, but in the end only the addition of small molecules turned out to be feasible. Adding 0.15 mmol (2.2 mol %) of acrylamide made it possible to increase the LCST from 30 to 38°C (Figure 3(f)).
USF imaging
Based on the performance results in the previous section, USF imaging was performed on pNIPAm-co-DAc (0.1 mmol)-co-1-vynilimidazole (0.025 mmol) (G2 #3) and pNIPAm-co-DAc (0.1 mmol)-co-1-vynilimidazole (0.025 mmol)-co-Aam (0.15 mmol) (G2 #6; Figure 4). Sample G2 #3 was chosen because it had the best pH-sensitivity along with a strong thermosensitivity suitable for USF imaging. The LCST of sample G2 #3 was 30°C, which was increased to 38°C by the addition of Aam in sample G2 #6 for certain applications. The ICG-encapsulated contrast agents were injected into the silicone tube that was embedded in the tissue imaged at a depth of 1 cm.
The USF signal obtained from the low LCST sample was increased by 40% from pH 7.4 to 6.8 and 192.9% from pH 7.4 to 6.5. Similarly, an increase of 25% and 72% was observed for the high LCST sample. Based on our data, both contrast agents were sensitive to small changes in pH between the physiological and tumor environments.
Discussion
The sensitivity required for imaging solid tumors or for drug delivery is over pH 6.5. Although the pKa of carboxylic acids increases as the number of carbons increases, the solubility of the carboxylic acids nonetheless decreases as the number of carbons increases. From the members of the carboxylic acids having a carbon–carbon double bond, NAc and DAc were still soluble for water-based reactions, whereas 10-undecenoic was insoluble at room temperature (the solubility of octanoic, nonanoic, and decanoic acids has been previously discussed). 15 With a relatively high pKa, 10-undecenoic has been used previously in organic phase reactions. 16 However, because many fluorescent probes and drugs are water soluble, 10-undecenoic has not been as widely used as acrylic acid. In this work, we have proposed the application of 9-DAc with the highest pKa among the soluble and partially soluble carboxylic acids with a double bond for incorporation into pNIPAm nanoparticles. Although there are basic comonomers with a carbon–carbon double bond with near physiological pKa that are capable of conferring pH sensitivity, they are incompatible with thermosensitive polymers with LCST such as pNIPAm. In this work, we took advantage of the higher sensitivity of basic comonomer (1-vynilimidazole) by adding a roughly 1:4 molar ratio of 9-DAc. As such, we achieved the following two goals: first, because acidic comonomer was dominant, we had the favorable strong signal in lower pH in tumors; second, we reached better pH sensitivity at near physiological pH.
As such, in the USF pH-sensitive and thermosensitive probe containing comonomers of carboxylic acid, the carboxyl groups are protonated and neutral when the pH is below the pKa of the carboxylic acid. When above the pKa, they are charged and cause swelling. Upon applying an HIFU, the temperature increases above the LCST of the nanoparticles inside the focal zone of the ultrasound transducer, and shrinkage ensues. If the pH is above the pKa of the acid, nanoparticles that have already swelled in response to the pH shrink less in response to the increase in temperature than when the pH is below the pKa. When shrinkage occurs and water is expelled, owing to the decrease in polarity, the polarity sensitive dye, ICG, that had a weak fluorescence in the highly polar environment now turns “ON” and emits a strong signal in the low-polarity environment. The higher the pH is above pKa, the less shrinkage occurs and the polarity-sensitive dye turns partially “ON.” The result of thermosensitivity combined with pH sensitivity in the case of acidic comonomers is favorable for USF imaging in lower pH environments (i.e. the outcome (shrinkage) results in a stronger signal when the temperature increases in lower pH environments). In the case of basic comonomers, however, shrinkage occurs when pH increases, resulting in a weaker USF signal in lower pH environments. This hinders the application of basic comonomers as the sole pH-sensitive agent with LCST polymer.
Conclusions
To increase the pH-sensitivity of NIPAm nanoparticles while preserving the thermosensitivity, we used acidic and basic comonomers with pKa values near physiological pH (7.4). As such, we looked for carboxylic acid comonomers with higher pKa than that of the acrylic acid that is widely used as a pH-sensitive granting agent (pKa = 4.26). Although carboxylic acids with longer chains have favorable higher pKa values, they are generally insoluble in water. However, we found that DAc is the last soluble carboxylic acid in water. Although basic comonomers such as amines have pKa values near physiological pH, they are not suitable as the sole pH-sensitive agent in thermosensitive LCST polymers. We synthesized nanoparticles of poly (9-decenoic acid-1-Vinylimidazole-N-isopropylacrylamide) containing ICG in one pot and in water phase for USF imaging. We have shown that basic comonomers, such as amines, can be used along with acidic comonomers as long as the molar ratio of the basic comonomer to the acidic comonomer is roughly 1:4. The feasibility of using these pH-sensitive and thermosensitive nanoparticles for USF imaging has been demonstrated in tissue samples at background temperatures of 30 and 38°C with pH values between pH 7.4 and 6.5–6.8. A significant increase in the USF signal was observed in tumor pH (6.5–6.8) in contrast to physiological pH (7.4).
Footnotes
Authors’ Contributions: Conceptualization and writing – original draft preparation was done by BY and BS; contrast agent synthesis, signal processing, and data analysis were carried out by BS; contrast agent testing was done by BS and TY; writing – review and editing was done by all the authors; supervision, project administration, and funding acquisition was carried out by BY. All authors have read and agreed to the published version of the article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by funding from the NIH/NIBIB 1R15EB030809-01 (Baohong Yuan), the REP 270089 (Baohong Yuan), and the CPRIT RP170564 (Baohong Yuan).
ORCID iD: Baohong Yuan  https://orcid.org/0000-0001-6046-6580
https://orcid.org/0000-0001-6046-6580
References
- 1. Smith RA, Cokkinides V, von Eschenbach AC, Levin B, Cohen C, Runowicz CD, Sener S, Saslow D, Eyre HJ. American Cancer Society guidelines for the early detection of cancer. CA: A Cancer Journal for Clinicians 2002;52:8–22 [DOI] [PubMed] [Google Scholar]
- 2. Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. The case for early detection. Nature Reviews Cancer 2003;3:243–52 [DOI] [PubMed] [Google Scholar]
- 3. Ling D, Hackett MJ, Hyeon T. Lighting up tumours. Nat Mater 2014;13:122–4 [DOI] [PubMed] [Google Scholar]
- 4. Saremi B, Bandi V, Kazemi S, Hong Y, D’Souza F, Yuan B. Exploring NIR aza-BODIPY-based polarity sensitive probes with ON-and-OFF fluorescence switching in Pluronic nanoparticles. Polymers 2020;12:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yao T, Liu Y, Ren L, Yuan B. Improving sensitivity and imaging depth of ultrasound-switchable fluorescence via an EMCCD-gain-controlled system and a liposome-based contrast agent. Quant Imaging Med Surg 2021;11:957–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuan B, Pei Y, Kandukuri J. Breaking the acoustic diffraction limit via nonlinear effect and thermal confinement for potential deep-tissue high-resolution imaging. Appl Phys Lett 2013;102:063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu S, Cheng B, Yao T, Xu C, Nguyen KT, Hong Y, Yuan B. New generation ICG-based contrast agents for ultrasound-switchable fluorescence imaging. Sci Rep 2016;6:35942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou K, Liu H, Zhang S, Huang X, Wang Y, Huang G, Sumer BD, Gao J. Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. J Am Chem Soc 2012;134:7803–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nature Rev Cancer 2011;11:671–7 [DOI] [PubMed] [Google Scholar]
- 10. Charman WN, Christy DP, Geunin EP, Monkhouse DC. Interaction between calcium, a model divalent cation, and a range of poly (Acrylic Acid) resins as a function of solution pH. Drug Develop Ind Pharm 1991;17:271–80 [Google Scholar]
- 11. Jiang L, Zhou Q, Mu K, Xie H, Zhu Y, Zhu W, Zhao Y, Xu H-X, Yang X. pH/temperature sensitive magnetic nanogels conjugated with Cy5.5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials 2013;34:7418–28 [DOI] [PubMed] [Google Scholar]
- 12. Gan D, Lyon LA. Synthesis and protein adsorption resistance of PEG-modified poly(N-isopropylacrylamide) core/shell microgels. Macromolecules 2002;35:9634–9 [Google Scholar]
- 13. Liu Q, Zhang P, Qing A, Lan Y, Lu M. Poly (N-isopropylacrylamide) hydrogels with improved shrinking kinetics by RAFT polymerization. Polymer 2006;47:2330–6 [Google Scholar]
- 14. Han HD, Shin BC, Choi HS. Doxorubicin-encapsulated thermosensitive liposomes modified with poly (N-isopropylacrylamide-co-acrylamide): drug release behavior and stability in the presence of serum. Eur J Pharm Biopharm 2006;62:110–6 [DOI] [PubMed] [Google Scholar]
- 15. Wellen BA, Lach EA, Allen HC. Surface pKa of octanoic, nonanoic, and decanoic fatty acids at the air–water interface: applications to atmospheric aerosol chemistry. Phys Chem Chem Phys 2017;19:26551–8 [DOI] [PubMed] [Google Scholar]
- 16. Wei H, Zhang X-Z, Cheng H, Chen W-Q, Cheng S-X, Zhuo R-X. Self-assembled thermo- and pH responsive micelles of poly(10-undecenoic acid-b-N-isopropylacrylamide) for drug delivery. J Contr Release 2006;116:266–74 [DOI] [PubMed] [Google Scholar]