Abstract
STING (stimulator of interferon genes) has been recognized as an important signaling molecule in the innate immune response to cytosolic nucleic acids. Although it has been proposed that STING signaling pathway may play a pathogenic role in developing autoimmune and autoinflammatory diseases, its involvement in rheumatic disease processes remains to be elucidated. Here, we evaluated STING protein levels, expression and relationship with inflammatory parameters in synovial fluid (SF) of patients with psoriatic arthritis (PsA), rheumatoid arthritis (RA), gout, calcium pyrophosphate crystal-induced arthritis (CPP-IA), osteoarthritis (OA), and OA with CPP crystals (OA + CPP). The correlation with its negative regulator, nuclear factor erythroid 2-related factor 2 (Nrf2), was also investigated. SFs from 72 patients were analyzed for white blood cell (WBC) count, polymorphonuclear cell percentage (PMN%), and IL-1β, IL-6, IL-8, extra- and intracellular STING levels. STING and Nrf2 expression was also determined. WBC count and PMN% were greater in SF from inflammatory arthritis, while they were lower in OA groups. RA and gouty SFs have the highest levels of IL-1β, IL-8, and IL-6; while OA and OA + CPP showed the lowest concentrations. Gout and RA had the highest intracellular STING levels, while extracellular STING was greater in CPP-IA and OA SFs. STING was not detectable in PsA. STING mRNA was lower in PsA than other arthritides. Nrf2 mRNA was not detectable in OA. This study determines the presence of STING in SF of different arthritides, except for PsA, and suggests that it may be involved in pathogenesis and progression of arthropathies.
Keywords: Arthritis, cytokines, inflammation, STING, synovial fluid
Impact Statement
The stimulator of interferon genes (STING) is a transmembrane protein of the endoplasmic reticulum that behaves as a sensor of cytosolic DNA and leads to the production of IFN-I and pro-inflammatory cytokines. Although it is well known that STING plays an important role in innate immune responses, its potential involvement in rheumatic disease processes remains to be elucidated. This study demonstrates that STING was contained at high concentrations within cells in inflammatory synovial fluids, and extracellularly in non-inflammatory synovial fluids or in those with calcium pyrophosphate crystals. STING has never been identified in synovial fluids from psoriatic arthritis and intracellularly in osteoarthritis. In these same samples, STING mRNA levels were extremely low. Our results suggest that STING may play a key role in the pathogenesis and progression of arthropathies.
Introduction
The stimulator of interferon genes (STING) is a 40-kDa evolutionary conserved transmembrane protein of the endoplasmic reticulum (ER) which is ubiquitously expressed in several cells and tissues where it mainly facilitates the innate immunity signaling. 1 STING expression is downregulated by nuclear factor erythroid 2-related factor 2 (Nrf2) which operates as transcription factor involved in the antioxidative stress response and, when overexpressed, inhibits type I IFN response. 2
STING behaves as a sensor of cytosolic DNA from bacteria and viruses and stimulates the production of type I interferon (IFN-α and IFN-β). 3 It has been demonstrated that STING recognizes and binds to cyclic dinucleotides: cyclic di-GMP (c-di-GMP), a second messenger released by bacteria, and cyclic GMP-AMP (cGAMP), a signaling molecule produced by cyclic GMP–AMP synthase (cGAS) in response to the presence of virus DNA in the cytosol.4,5 Upon binding, STING oligomerizes, translocates from the ER to perinuclear compartments and is phosphorylated by the kinase TBK1, leading to enrollment and activation of the transcription factor IRF3 to promote expression of type I interferon and pro-inflammatory cytokines, such as TNF-α and IL-6.6–8
Beside a mediated IFN I immune response, STING plays a direct role in autophagy and promotes cellular senescence and apoptosis after DNA damages or microbial infection. 6
Different studies have reported the presence of IFN I in the synovial fluid (SF) of patients with rheumatic diseases,9–11 thus suggesting a possible involvement of STING in the pathogenetic processes of these diseases. In particular, significant levels of INF-α have been detected in SF obtained from patients with rheumatoid arthritis (RA), 10 while INF-β was difficult to determine due to its high instability in this body fluid. 9 More recently, it has been found that STING may be involved in the development of autoimmune and autoinflammatory rheumatic diseases, such as systemic lupus erythematosus (SLE), and STING-associated vasculitis with onset in infancy (SAVI), and could be a central mediator of the downstream events leading to disease symptoms and inflammation.12–14 These findings have been corroborated in murine models showing how targeting STING proteins may reduce overexpression of pro-inflammatory cytokines and severe spontaneous autologous DNA-mediated polyarthritis. 15 More recently, Hwang et al. 16 observed an activation of the cGAS-STING pathway triggered by degradation fragments of the extracellular matrix of cartilage (e.g. fibronectin fragments) and an overexpression of pro-inflammatory cytokines in SF samples of osteoarthritis (OA) patients. No studies to date have investigated the role of the STING pathway in other rheumatic inflammatory diseases. Therefore, we endeavored to evaluate the concentrations and expression of STING, as well as its correlation with local inflammation in SF of patients with psoriatic arthritis (PsA), RA, gout, calcium pyrophosphate crystal-induced arthritis (CPP-IA), OA, and OA with CPP crystals (OA + CPP). The downregulation of STING by Nrf2 has also been investigated as a potential hallmark in the pathogenesis and progression of joint diseases.
Materials and methods
Collection and analysis of synovial fluids
Human SF samples from patients were collected with the approval of the Institutional Review Board of the Padova University Hospital. All participants gave written informed consent. SF was collected by arthrocentesis from the knees of 72 untreated patients: 12 with PsA, 12 with RA, 12 with gout, 12 with CPP-IA, 12 with OA, and 12 with OA + CPP. SF samples were examined under optical light microscopy to determine total white cell count (WBC) and differential cell count. Pathogenic crystals (monosodium urate (MSU) and CPP crystals) were identified using ordinary and polarized light microscopy. 17 Patients’ characteristics are outlined in Supplementary Table 1. SF cells and supernatants were stored at -80°C after centrifugation at 300g for 30 min as previously described. 18
Determination of STING and cytokine concentrations
SF supernatants were analyzed to determine extracellular concentrations of IL-8, IL-6, IL-1β (eBioscience, San Diego, CA, USA) and STING (MyBioSource, Inc., San Diego, CA, USA) levels by enzyme-linked immunosorbent assay (ELISA). Intracellular STING concentrations were determined in lysates obtained after three freeze–thaw cycles and resuspended in phosphate-buffered saline (PBS). 19
mRNA expression analysis
Total RNA was isolated with Total RNA Purification Kit (Norgen Biotek Corp., Canada) from human SF cells (six SFs per group). A pair of human STING primers, sense 5′-CCTGAGTCTCAGAACAACTGCC-3′ and anti-sense 5′-GGTCTTCAAGCTGCCCACAGTA-3′, of human Nrf2 primers, sense 5′-CTTTTGGCGCAGACATTCC-3′ and anti-sense 5′-AAGACTGGGCTCTCGATGTG-3′, and of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers, sense 5′-AGCCACATCGCTCAGACA-3′ and anti-sense 5′-GCCCAATACGACCAAATCC-3′, as housekeeping gene, were used for real-time reverse transcription polymerase chain reaction (RT-PCR) (ABI Prism 7900 HT, Thermo Fisher Scientific, Madison, WI, USA). iScript cDNA Synthesis Kit (Biorad, CA, USA) was used to convert 116 ng of total RNA into first-strand cDNA. Subsequently, RT-PCR reaction was run at the following amplification cycle: 95°C for 30 s, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min using iTaq Universal SYBR Green Supermix (BioRad, CA, USA). All molecular targets were analyzed in duplicates for each sample. Results were normalized to the housekeeping gene and evaluated using the 2-∆∆Ct method.
Statistical analysis
All values are expressed as mean ± SD. Data significance were evaluated by a one-way analysis of variance (ANOVA) followed by Dunnett’s test, where appropriate. Correlations between different variables were calculated by Spearman rank correlation test. A p-value < 0.05 is considered significant.
Results
SF characteristics and analysis
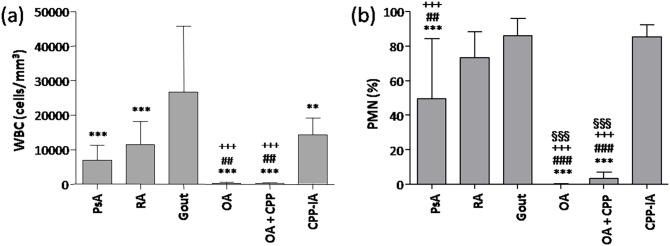
The results of SF analysis are reported in Figure 1. SF from patients with inflammatory arthritis showed WBC count greater than 5000 cells/mm3 with greater than 40% polymorphonuclear (PMN) cells. The highest concentrations of both parameters were observed in SFs from gout patients (WBC 26.74 ± 18.43 × 103/mm3; PMN 86% ± 9.86%). By contrast, WBC count and PMN% were lower in OA and OA + CPP patients (<500 cells/mm3; PMN < 4%).
Figure 1.
Cell counts in synovial fluids. (a) Number of white blood cells (WBC) and (b) percentage of polymorphonuclear cells (PMNs) were determined in synovial fluid (SF) from patients with psoriatic arthritis (PsA), rheumatoid arthritis (RA), gout, calcium pyrophosphate crystal-induced arthritis (CPP-IA), osteoarthritis (OA), and OA with CPP crystals (OA + CPP). Values are expressed as mean ± SD of 12 SF per group. **p < 0.01 versus gout, ***p < 0.001 versus gout, ##p < 0.01 versus RA, ###p < 0.001 versus RA, +++p < 0.001 versus CPP-IA, §§§p < 0.001 versus PsA.
SF concentrations of cytokines
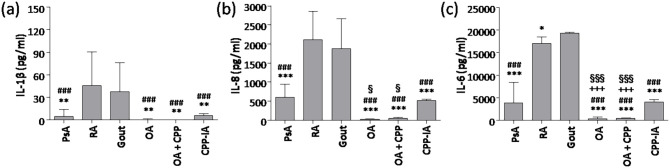
Similar cytokine profiles were observed in SF from patients with RA and gout, who showed the highest levels of IL-1β, IL-8, and IL-6, whereas patients with OA and OA + CPP showed the lowest concentrations of the same cytokines (Figure 2). SF concentration of IL-1β in gout and RA was eightfold to ninefold higher than in PsA, and sevenfold to eightfold higher than in CPP-IA. SF concentrations of IL-8 were threefold to fourfold higher in patients with RA and gout versus PsA and CPP-IA. SF concentrations of IL-8 in patients with OA + CPP were twofold that of patients with OA. Finally, SF concentration of IL-6 was fourfold to fivefold higher in patients with RA and gout versus PsA and CPP-IA.
Figure 2.
Cytokine concentrations in synovial fluids. (a) IL-1β, (b) IL-8, and (c) IL-6 concentrations were determined in synovial fluid (SF) from patients with psoriatic arthritis (PsA), rheumatoid arthritis (RA), gout, calcium pyrophosphate crystal-induced arthritis (CPP-IA), osteoarthritis (OA), and OA with CPP crystals (OA + CPP). Values are expressed as mean ± SD of 12 SF per group. *p < 0.05 versus gout, **p < 0.01 versus gout, ***p < 0.001 versus gout, ###p < 0.001 versus RA, +++p < 0.001 versus CPP-IA, §p < 0.05 versus PsA, §§§p < 0.001 versus PsA.
SF concentrations of STING
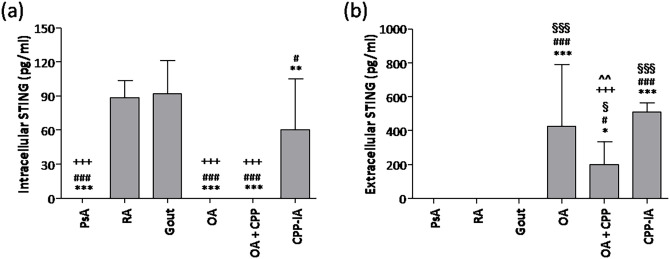
Patients with gout and RA had the highest concentrations of intracellular STING (Figure 3(a)). STING was not detectable in SF of PsA, OA, and OA + CPP patients. The highest concentrations of extracellular STING were found in SF of patients with CPP-IA and OA, which were twofold higher than in OA + CPP (Figure 3(b)). Extracellular STING remained under detection limit (50 pg/mL) in SF from RA, PsA, and gout.
Figure 3.
STING concentrations in synovial fluids. (a) intracellular and (b) extracellular STING concentrations were determined in synovial fluid (SF) from patients with psoriatic arthritis (PsA), rheumatoid arthritis (RA), gout, calcium pyrophosphate crystal-induced arthritis (CPP-IA), osteoarthritis (OA), and OA with CPP crystals (OA + CPP). Values are expressed as mean ± SD of 12 SF per group. *p < 0.05 versus gout, **p < 0.01 versus gout, ***p < 0.001 versus gout, #p < 0.05 versus RA, ###p < 0.001 versus RA, +++p < 0.001 versus CPP-IA, §p < 0.05 versus PsA, §§§p < 0.001 versus PsA, ^^p < 0.01 versus OA.
We found a positive correlation between intracellular STING and WBC (r = 0.631; p < 0.001), PMN (r = 0.703; p < 0.001), IL-1β (r = 0.784; p < 0.001), IL-8 (r = 0.657; p < 0.001) and IL-6 (r = 0.717; p < 0.001) in SF. By contrast, we found a negative correlation between extracellular STING and IL-1β (r = -0.454; p < 0.001), IL-8 (r = -0.640; p < 0.001), and IL-6 (r = 0.506; p < 0.001) in SF.
SF STING and Nrf2 expression
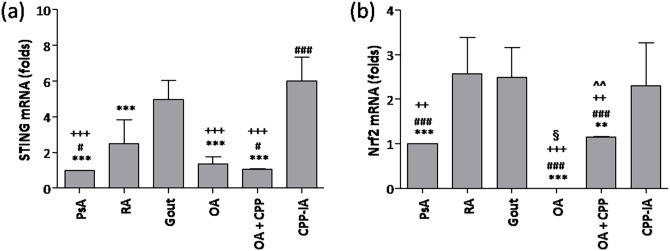
As shown in Figure 4(a), the mRNA levels of STING were twofold, fivefold, and sixfold lower in PsA than in RA, gout, and CPP-IA, respectively; though it was similar in OA groups. Nrf2 mRNA was 2.5-fold higher in cells from RA, gout, and CPP-IA SFs compared with PsA (Figure 4(b)). Nrf2 was not detectable in SF from patients with OA without CPP crystals.
Figure 4.
STING and Nrf2 expression in synovial fluids. (a) STING and (b) Nrf2 mRNA levels were determined in synovial fluid (SF) cells from patients with psoriatic arthritis (PsA), rheumatoid arthritis (RA), gout, calcium pyrophosphate crystal-induced arthritis (CPP-IA), osteoarthritis (OA), and OA with CPP crystals (OA + CPP). Values are expressed as mean ± SD of 12 SF per group. **p < 0.01 versus gout, ***p < 0.001 versus gout, #p < 0.05 versus RA, ###p < 0.001 versus RA, ++p < 0.01 versus CPP-IA, +++p < 0.001 versus CPP-IA, §p < 0.05 versus PsA, ^^p < 0.01 versus OA.
Discussion
Our results showed that STING was contained at high concentrations within cells in inflammatory SF, and extracellularly in non-inflammatory SF or in those with CPP crystals. STING has never been identified in SF from PsA and intracellularly in OA, which may explain the low expression of STING mRNA in SF of these patients, displaying a 2.5-fold downregulation versus RA, gout, and CPP-IA.
There is growing evidence that STING may play a relevant role in modulating inflammatory response and other pathophysiological processes involved in several diseases. 13 Elevated concentrations of STING were found in liver tissues of subjects with non-alcoholic fatty liver disease (NAFLD). 20 The increased expression of STING in peripheral blood mononuclear cells and intestinal biopsies of patients with abdominal sepsis, correlated with intestinal inflammation. 21 Elevated concentrations of STING were also found in endometrial epithelial cells of patients with endometriosis and adenomyosis, and correlated with inflammatory cell infiltration within the epithelium, thus indicating that STING signaling may play a role in initiating chronic inflammation. 22 Elevated STING concentrations were also found in colonic tissue of colitic mice as well as in murine or human M1-polarized THP-1 macrophages; 23 similarly, increased concentrations of STING mRNA and protein were found in leukocytes during acute pancreatitis in mice. 24 In line with the aforementioned findings, we demonstrated that elevated concentrations of intracellular STING in inflammatory SFs of patients with RA, gout, and CPP-IA patients, correlated with SF pro-inflammatory cytokine concentrations and with the cell infiltration. Conversely, intracellular STING remained under detection limits in SF from OA, which is associated with a lower degree of inflammation than other arthropathies considered. 25 STING plays a critical role in the release of Type I IFN in response to foreign pathogenic DNA and RNA, or mislocated self-DNA and mitochondrial DNA (mtDNA) in the cytosol.15,26 Recent reports have proposed intracellular cell-free DNA (cfDNA) species (mitochondrial or nuclear) as potential biomarkers for autoimmune rheumatic diseases, such as RA. This stems from the observation that elevated concentrations of cfDNA have been found in body fluids of patients affected by these conditions and appear to be associated with cell death and inflammatory mechanisms, including apoptosis, necrosis, NETosis, and pyroptosis.27,28 Increased concentrations of cfDNA in SF and serum of patients with RA, gout, and pseudogout may help explain our results.29–31 Indeed, it has been demonstrated that cytosolic double-stranded DNA accumulates in fibroblast-like synoviocytes in patients with active RA and promotes synovial inflammation via the cGAS/STING pathway. 32 It is entirely possible that phagocytosed pathogenic crystals might interact directly with STING.
However, in our study, STING remained under detection limits in SFs of patients with PsA, despite inflammatory features. This indicates that STING might not be involved in the pathogenetic processes of PsA or a downregulation of STING signaling. It has been reported that STING is tightly regulated by various control mechanisms, such as degradation, post-translational modifications, or overexpression of certain microRNAs that can target suppressor genes.13,33 In PsA patients, in addition to specific serological biomarkers, suppressor genes have also been suggested as possible diagnostic and prognostic biomarkers as there have been reports of altered miRNA in articular tissues and peripheral blood mononuclear cells.34,35
In our study, we found extracellular STING in SF of patients with OA or CPP-IA. This may be due to the activation of processes that reduce its interaction with the ER and appears to corroborate previous reports showing that STING can be released from infected cells in microvesicles (e.g. exosomes). 36 An imbalance in cytosolic calcium concentrations, as seen in the presence of CPP or basic calcium phosphate crystals that are frequently identified in SF of OA patients, could be involved in the translocation of STING from ER to the Golgi via vesicles. 37 Subsequently, other mechanisms involved in the pathogenesis of CPP-IA or OA, such as crystal-induced cell damage, senescence, apoptosis, or shedding of microvesicles could lead to the leakage of STING from the cells.
We also demonstrated high concentrations of STING in SF of patients with OA, even in presence of low-grade inflammation. These findings corroborate a previous study by Guo et al., 38 who reported an increased expression of STING in OA human and mice cartilage, where it promotes senescence and apoptosis in chondrocytes. In addition, in OA samples, we did not detect significant mRNA levels of Nrf2, a STING negative regulator. Indeed, it has been shown that increased expression of Nrf2 represses STING mRNA and protein levels in human cells, 2 thus suggesting that the high levels of STING in OA observed in our results could be due to the lack of regulation by Nrf2.
However, different from what was expected, an inverse correlation between STING and Nrf2 in inflammatory SFs was not found. This might be because other mechanisms may be involved in the pathogenesis of the diseases considered. For instance, although Nrf2 is recognized as a pivotal mediator in protecting against oxidative stress, it has been reported that it is necessary for the activation of the NLRP3 inflammasome and the IL-1β production induced by MSU and other types of microcrystals.39,40 This could explain the high Nrf2 mRNA levels in gout and CCP-IA.
Another study shows that, although Nrf2 maintains the cellular defense against oxidative stress in RA, it is expressed and active in synovial membrane of patients with this disease. 41 In our results, Nrf2 mRNA levels were similar in gout, CPP-IA, and RA, but those of STING were significantly lower in RA, thus revealing a reduction in this inflammatory condition.
In conclusion, our study highlights the expression of STING in SF of patients affected by the most common joint diseases. Further investigations are warranted to ascertain the role of STING in articular and bone tissues homeostasis and the possible presence of exogenous and damaged DNA/RNA in SF. Although the exact role of STING remains unknown, our results suggest that the c-GAS-STING pathway may be involved in the pathogenesis and progression of arthropathies.
Supplemental Material
Supplemental material, sj-doc-1-ebm-10.1177_15353702221087966 for Identification in synovial fluid of a new potential pathogenic player in arthropathies by Anna Scanu, Mariagrazia Lorenzin, Roberto Luisetto, Paola Galozzi, Augusta Ortolan, Francesca Oliviero, Andrea Doria and Roberta Ramonda in Experimental Biology and Medicine
Footnotes
Authors’ contributions: All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; AS, RL, and RR contributed to the conception and experimental design of the study; AS, PG, and FO performed the biological experiments, analyzed the data, and prepared the figures; AO and ML collected the biological samples and revised the manuscript for important intellectual content; AS, RL, and PG wrote and edited the manuscript; and AD and RR critically revised the manuscript. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The study procedures were approved by the Institutional Review Board of the University of Padua (Prot. N. 3304/AO/14). All patients signed a written consent form to participate in this study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Institutional Research Funds “DOR2020” from the University of Padova (DOR2082329/20) and grant from the Fondazione Italiana per la Ricerca sull’Artrite (FIRA).
ORCID iDs: Mariagrazia Lorenzin  https://orcid.org/0000-0002-5082-3043
https://orcid.org/0000-0002-5082-3043
Francesca Oliviero  https://orcid.org/0000-0002-4958-5105
https://orcid.org/0000-0002-4958-5105
Andrea Doria  https://orcid.org/0000-0003-0548-4983
https://orcid.org/0000-0003-0548-4983
Roberta Ramonda  https://orcid.org/0000-0002-9683-8873
https://orcid.org/0000-0002-9683-8873
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kawasaki T, Kawai T, Akira S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol Rev 2011;243:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olagnier D, Brandtoft AM, Gunderstofte C, Villadsen NjL Krapp C, Thielke AL, Laustsen A, Peri S, Hansen AL, Bonefeld L, Thyrsted J, Bruun V, Iversen MB, Lin L, Artegoitia VM, Su C, Yang L, Lin R, Balachandran S, Luo Y, Nyegaard M, Marrero B, Goldbach-Mansky R, Motwani M, Ryan DG, Fitzgerald KA, O’Neill LA, Hollensen AK, Damgaard CK, de Paoli FV, Bertram HC, Jakobsen MR, Poulsen TB, Holm CK. Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming. Nat Commun 2018;9:3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 2016;17:1142–9 [DOI] [PubMed] [Google Scholar]
- 4. Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011;478:515–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013;339:786–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang X, Bai XC, Chen ZJ. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity 2020;53:43–53 [DOI] [PubMed] [Google Scholar]
- 7. Thompson MR, Sharma S, Atianand M, Jensen SB, Carpenter S, Knipe DM, Fitzgerald KA, Kurt-Jones EA. Interferon γ-inducible protein (IFI) 16 transcriptionally regulates type I interferons and other interferon-stimulated genes and controls the interferon response to both DNA and RNA viruses. J Biol Chem 2014;289:23568–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ablasser A, Chen ZJ. cGAS in action: expanding roles in immunity and inflammation. Science 2019;363:eaat8657 [DOI] [PubMed] [Google Scholar]
- 9. O’Kelly P, Thomsen L, Tilles JG, Cesario T. Inactivation of interferon by serum and synovial fluids. Proc Soc Exp Biol Med 1985;178:407–11 [DOI] [PubMed] [Google Scholar]
- 10. Hopkins SJ, Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin Exp Immunol 1988;73:88–92 [PMC free article] [PubMed] [Google Scholar]
- 11. Westacott CI, Whicher JT, Barnes IC, Thompson D, Swan AJ, Dieppe PA. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis 1990;49:676–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol 2015;15:760–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Wilson HL, Kiss-Toth E. Regulating STING in health and disease. J Inflamm (Lond) 2017;14:11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Sanchez GAM, Tenbrock K, Wittkowski H, Jones OY, Kuehn HS, Lee CR, DiMattia MA, Cowen EW, Gonzalez B, Palmer I, DiGiovanna JJ, Biancotto A, Kim H, Tsai WL, Trier AM, Huang Y, Stone DL, Hill S, Kim HJ, St Hilaire C, Gurprasad S, Plass N, Chapelle D, Horkayne-Szakaly I, Foell D, Barysenka A, Candotti F, Holland SM, Hughes JD, Mehmet H, Issekutz AC, Raffeld M, McElwee J, Fontana JR, Minniti CP, Moir S, Kastner DL, Gadina M, Steven AC, Wingfield PT, Brooks SR, Rosenzweig SD, Fleisher TA, Deng Z, Boehm M, Paller AS, Goldbach-Mansky R. Activated STING in a vascular and pulmonary syndrome. N Engl J Med 2014;371:507–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci USA 2012;109:19386–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwang HS, Lee MH, Choi MH, Kim HA. Induction of pro-inflammatory cytokines by 29-kDa FN-f via cGAS/STING pathway. BMB Rep 2019;52:336–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scanu A, Oliviero F, Ramonda R, Frallonardo P, Dayer JM, Punzi L. Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor β1 in the resolution phase. Ann Rheum Dis 2012;71:621–4 [DOI] [PubMed] [Google Scholar]
- 18. Scanu A, Oliviero F, Gruaz L, Galozzi P, Luisetto R, Ramonda R, Burger D, Punzi L. Synovial fluid proteins are required for the induction of interleukin-1β production by monosodium urate crystals. Scand J Rheumatol 2016;45:384–93 [DOI] [PubMed] [Google Scholar]
- 19. Oliviero F, Zamudio-Cuevas Y, Belluzzi E, Andretto L, Scanu A, Favero M, Ramonda R, Ravagnan G, López-Reyes A, Spinella P, Punzi L. Polydatin and resveratrol inhibit the inflammatory process induced by urate and pyrophosphate crystals in THP-1 cells. Foods 2019;8:560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo X, Li H, Ma L, Zhou J, Guo X, Woo SL, Pei Y, Knight LR, Deveau M, Chen Y, Qian X, Xiao X, Li Q, Chen X, Huo Y, McDaniel K, Francis H, Glaser S, Meng F, Alpini G, Wu C. Expression of STING is increased in liver tissues from patients with NAFLD and promotes macrophage-mediated hepatic inflammation and fibrosis in mice. Gastroenterology 2018;155:1971–84.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu Q, Ren H, Li G, Wang D, Zhou Q, Wu J, Zheng J, Huang J, Slade DA, Wu X, Ren J. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis. Ebiomedicine 2019;41:497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qu H, Li L, Wang TL, Seckin T, Segars J, Shih IM. Epithelial cells in endometriosis and adenomyosis upregulate STING expression. Reprod Sci 2020;27:1276–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin GR, Blomquist CM, Henare KL, Jirik FR. Stimulator of interferon genes (STING) activation exacerbates experimental colitis in mice. Sci Rep 2019;9:14281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao Q, Wei Y, Pandol SJ, Li L, Habtezion A. STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology 2018;154:1822–35.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scanzello CR. Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol 2017;29:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015;520:553–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duvvuri B, Lood C. Cell-free DNA as a biomarker in autoimmune rheumatic diseases. Front Immunol 2019;10:502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mondelo-Macía P, Castro-Santos P, Castillo-García A, Muinelo-Romay L, Diaz-Peña R. Circulating free DNA and its emerging role in autoimmune diseases. J Pers Med 2021;11:151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leon SA, Revach M, Ehrlich GE, Adler R, Petersen V, Shapiro B. DNA in synovial fluid and the circulation of patients with arthritis. Arthritis Rheum 1981;24:1142–50 [DOI] [PubMed] [Google Scholar]
- 30. Barnett EV. Detection of nuclear antigens (DNA) in normal and pathologic human fluids by quantitative complement fixation. Arthritis Rheum 1968;11:407–17 [DOI] [PubMed] [Google Scholar]
- 31. Koffler D, Agnello V, Winchester R, Kunkel HG. The occurrence of single-stranded DNA in the serum of patients with systemic lupus erythematosus and other diseases. J Clin Invest 1973;52:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J, Li R, Lin H, Qiu Q, Lao M, Zeng S, Wang C, Xu S, Zou Y, Shi M, Liang L, Xu H, Xiao Y. Accumulation of cytosolic dsDNA contributes to fibroblast-like synoviocytes-mediated rheumatoid arthritis synovial inflammation. Int Immunopharmacol 2019;76:105791 [DOI] [PubMed] [Google Scholar]
- 33. Landman SL, Ressing ME, van der Veen AG. Balancing STING in antimicrobial defense and autoinflammation. Cytokine Growth Factor Rev 2020;55:1–14 [DOI] [PubMed] [Google Scholar]
- 34. Ramonda R, Modesti V, Ortolan A, Scanu A, Bassi N, Oliviero F, Punzi L. Serological markers in psoriatic arthritis: promising tools. Exp Biol Med (Maywood) 2013;238:1431–6 [DOI] [PubMed] [Google Scholar]
- 35. Wade SM, McGarry T, Wade SC, Fearon U, Veale DJ. Serum microRNA signature as a diagnostic and therapeutic marker in patients with psoriatic arthritis. J Rheumatol 2020;47:1760–7 [DOI] [PubMed] [Google Scholar]
- 36. Kalamvoki M, Du T, Roizman B. Cells infected with herpes simplex virus 1 export to uninfected cells exosomes containing STING, viral mRNAs, and microRNAs. Proc Natl Acad Sci USA 2014;111:E4991–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith JA. STING, the endoplasmic reticulum, and mitochondria: is three a crowd or a conversation? Front Immunol 2021;11:611347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo Q, Chen X, Chen J, Zheng G, Xie C, Wu H, Miao Z, Lin Y, Wang X, Gao W, Zheng X, Pan Z, Zhou Y, Wu Y, Zhang X. STING promotes senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the NF-κB signaling pathway. Cell Death Dis 2021;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jhang JJ, Cheng YT, Ho CY, Yen GC. Monosodium urate crystals trigger Nrf2- and heme oxygenase-1-dependent inflammation in THP-1 cells. Cell Mol Immunol 2015;12:424–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol 2011;41:2040–51 [DOI] [PubMed] [Google Scholar]
- 41. Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg LO, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S, Varoga D, Lippross S, Pufe T. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis 2011;70:844–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-ebm-10.1177_15353702221087966 for Identification in synovial fluid of a new potential pathogenic player in arthropathies by Anna Scanu, Mariagrazia Lorenzin, Roberto Luisetto, Paola Galozzi, Augusta Ortolan, Francesca Oliviero, Andrea Doria and Roberta Ramonda in Experimental Biology and Medicine