Abstract
Methadone (MTD) is a commonly prescribed treatment for opioid use disorder in pregnancy, despite limited information on the effects of passive exposure on fetal brain development. Animal studies suggest a link between perinatal MTD exposure and impaired white matter development. In this study, we characterized the effect of perinatal MTD exposure through the evaluation of oligodendrocyte development and glial cell activation in the neonatal rat brain. Six pregnant Sprague Dawley rat dams were randomized to MTD (0.2 mL/L) or untreated drinking water from embryonic day 7. Pups were terminated at postnatal day 7 and tissue sections were harvested from six randomly selected pups (one male and one female per litter) of each experimental group for immunohistochemistry in areas of corpus callosum (CC), lateral CC, external capsule (EC), and cerebellar white matter. In the MTD-exposed rat pups, myelination was significantly decreased in the CC, lateral CC, EC, and arbor vitae compared with the controls. The increased density and percentage of oligodendrocyte precursor cells (OPCs) were observed in the CC and cerebellar white matter. The highly active proliferation of OPCs as well as decreased density and percentage of differentiated oligodendrocytes were found in the cerebellum but no differences in the cerebrum. Apoptotic activities of both differentiated oligodendrocytes and myelinating oligodendrocytes were significantly increased in all regions of the cerebrum and cerebellum after MTD exposure. There was no quantitative difference in astrocyte, however, cell density and/or morphologic difference consistent with activation were observed in microglia throughout MTD-exposed CC and cerebellum. Taken together, perinatal MTD exposure reveals global attenuation of myelination, accelerated apoptosis of both differentiated and myelinating oligodendrocytes, and microglia activation, supporting an association between antenatal MTD exposure and impaired myelination in the developing brain.
Keywords: Oligodendrocyte, myelination, microglia, methadone, perinatal opioid exposure, neonatal opioid withdrawal syndrome, brain, neonatal abstinence syndrome, opioid use disorder
Impact Statement
Methadone (MTD) is a common medication treatment for opioid use disorder (OUD) during pregnancy. Recent studies support the impairment of myelination in the fetal rat brain after antenatal exposure to MTD. In this study, pregnant rats were administrated a dosing approximately equivalent to the OUD treatment and the effects of passive in utero and postnatal MTD exposure on myelin development was investigated in neonatal rat brain. The findings reveal the potential mechanism(s) underlying the association between myelin impairment and antenatal opioid exposure. Our study alongside others concerns for fetal brain development when using MTD to manage the OUD during pregnancy. Improved understanding of the effects of opioid exposure will allow us to design treatments for OUD during pregnancy that minimize effects on the fetal brain or target postnatal treatment to mitigate the effects of antenatal opioid exposure.
Introduction
The opioid crisis continues to be a substantial public health concern and its impact has been noted during pregnancy with a doubling of diagnoses related to opioid misuse from 2010 to 2017 and a parallel increase in incidence of neonatal opioid withdrawal syndrome (NOWS). 1 An increase in maternal diagnosis rates may be related to heightened recognition and treatment of opioid use disorder (OUD) in pregnancy. 1 Medication for opioid use disorder (MOUD) with methadone (MTD) has been the standard of care for OUD in pregnancy since the 1970s and has been shown to improve maternal and neonatal outcomes compared with illicit substance use. 2 However, maternal MTD use during pregnancy does expose the developing fetus to exogenous opioids which may negatively influence neurological development. Understanding the mechanisms through which MTD impacts fetal brain development is crucial to evaluating treatment choices for OUD during pregnancy and designing novel treatments that minimize adverse effects on the fetus.
A growing body of evidence shows that prenatal opioid exposure associates with altered brain development and functional outcomes. Observational clinical studies suggest perinatal exposure to exogenous opioids may negatively influence neurodevelopmental outcomes including cognitive, psychomotor, and emotional development.3–6 In utero opioid exposure has been associated with neuroanatomical changes including decreased head circumference and decreased brain volume.7–9 Neuroimaging studies reveal decreased white matter volume and altered myelination patterns in the brains of prenatal opioid exposed children.10–14 Although observational clinical studies in this patient population have many limitations and are prone to confounding, when coupled with evidence from in vitro studies the current evidence supports an association between MTD exposure and altered fetal brain development.
Oligodendrocyte precursor cells (OPCs) express Mu opioid receptors during proliferation and maturation, then downregulate Mu expression and develop Kappa receptors once differentiated.15–17 In-vitro studies show a mitogenic effect of Mu opioid receptor stimulation on immature oligodendrocytes, but little in vivo data exists.15,16 Further studies are required to fully understand the effect of Mu opioid receptor stimulation on oligodendrocyte development in vivo. The aim of this study is to determine the impact of antenatal MTD exposure on the development of oligodendrocyte-lineage cells and myelination in neonatal rat brains.
Materials and methods
Animals
Animal use in this study was approved by the University of Louisville Research Resources Center and the Association for Assessment and Accreditation of Laboratory Animal Care approved facility (AAALAC), and all procedures were performed in accordance with the guidelines of the Animal Care and Use Committee of University of Louisville School of Medicine and National Institutes of Health (NIH) requirements for care and use of laboratory animals. Twelve-week-old male and female Sprague Dawley rats from Charles River Laboratories (Wilmington, MA) were housed in an animal facility maintained at a temperature (22 ± 2°C) and in a light-controlled environment (12-h light/dark cycle) with ad libitum supply of standard chow and water.
Rat model of antenatal MTD exposure
The rat model is used to mimic the antenatal MTD exposure in human. Neural development and myelination of the rat brain at postnatal day 7 (P7) is roughly equal developmentally to a full-term human newborn.18,19 Timed pregnant rats were achieved by mating female rats with a male rat and checking for insemination the next morning. Six pregnant Sprague Dawley rat dams were randomized at embryonic day 7 (E7) to MTD or control group (CTR), as described in our prior publication. 20 Pregnant dams in the treatment group were dosed with 0.2 mL MTD (150 mg/mL) diluted in 1 L drinking water according to the reported daily ad libitum water consumption of 40 mL 21 (3 mg/kg bodyweight/day). This dose is to approximate average weight-based dosing of an adult woman on opioid replacement therapy (roughly equivalent to the maximum day dose of MAT (40 mg) for 75 kg American women) with interspecies adjustment.20,22 Pups in the treatment group received MTD via placenta antenatally and via breast milk postnatally. 23 The litter size was recorded at birth. Eight pups were culled for each litter to minimize the within-litter variation and their body weights were weighed at P7.
Tissue processing
Tissue processing was performed as previously described with minor modifications. 24 Three male and three female rats (one male and one female pups per litter to minimize the litter effects) 25 in each experimental group were anesthetized with isoflurane (Covetrus) and terminated by intracardiac perfusion with cold phosphate-buffered saline (PBS) (Gibco) at P7. Whole brain was removed, and its size was assessed by wet weight and length (Table 1). The brain was separated into two halves by a mid-sagittal cut, followed by overnight fixation in 4% (w/v) paraformaldehyde (Sigma-Aldrich) at 4°C. Brain tissues were cryoprotected in 30% (w/v) sucrose (Research Products International (RPI)) for 48 h at 4°C and embedded in Tissue-Tek OCT Compound (Sakura Finetek) for cryostat. Coronal sections of cerebrum and sagittal sections of cerebellum were collected on a cryostat (Leica Biosystems Inc.) with a thickness of 20 μm for immunohistochemistry.
Table 1.
Measurements of litter size at birth and body weight and brain size at P7.
| Treatment | Litter N | Litter size | Sex | Animal N | Body weight (g) | Wet brain weight (g) | Brain length (cm) |
|---|---|---|---|---|---|---|---|
| Control | 3 | 11.33 ± 2.08 | Female | 10 | 16.30 ± 2.69 | 0.65 ± 0.08 | 1.39 ± 0.04 |
| Male | 14 | 17.52 ± 2.47 | 0.67 ± 0.09 | 1.39 ± 0.05 | |||
| Methadone | 3 | 10.67 ± 1.53 | Female | 11 | 15.80 ± 2.11 | 0.65 ± 0.08 | 1.38 ± 0.06 |
| Male | 13 | 17.09 ± 2.10 | 0.68 ± 0.08 | 1.40 ± 0.06 |
Male versus female and CTR versus MTD do not show a statistical difference.
Immunohistochemistry and quantification
Double immunohistochemistry was performed on cryostat sections as detailed in our previous reports.24,26,27 In brief, heat-induced epitope retrieval was performed in citric acid-based antigen unmasking solution (Vector Laboratories). Slides were blocked in solution containing 5% goat or horse serum (Gibco) and 0.3% Triton X-100 (Sigma-Aldrich) for 1 h followed by overnight incubation in a PBS-based antibody solution containing 1% goat or horse serum and primary antibodies as listed in Table 2. Following washes with PBS, slides were incubated in secondary antibodies congregated to Alexa Fluor 488 or 594 (Table 2) for 2 h at room temperature. All tissue sections were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma) to visualize nuclei and mounted with Mowiol® 4-88 (Sigma) for microscopic visualization. For the staining of MBP, Iba1, and GFAP, positive staining area divided by respective region of interest (ROI) (corpus callosum (CC), lateral corpus callosum (Lateral CC), external capsule (EC), or cerebellar white matter – arbor vitae) was quantified automatically with ImageJ software (http://imagej.nih.gov/ij/; NIH) and reported as positive-labeling area fraction. Mean values from three rat pups was calculated to examine the effects of perinatal MTD exposure on each rat brain region. For the other co-staining, positive cells within each ROI were manually counted via ImageJ. Cells in the oligodendrocyte lineage were identified by Olig2+ staining. They were further characterized and categorized as follows: OPCs (Olig2+/Pdgfrα+), apoptotic OPC (Pdgfrα+/cleaved Caspase-3+), proliferative OPCs (Ki67+/Pdgfrα+ cells), differentiated oligodendrocytes (CC1+/cleaved Caspase-3+), and myelinating oligodendrocytes (MBP+/cleaved Caspase-3+). Each stage of oligodendrocyte development was analyzed both by density in each ROI (cells/mm) and as a percentage of the total oligodendrocyte CC1+ population. Mean values from three male and three female rat pups were used to analyze the changes after perinatal MTD exposure in each rat brain region. To limit the experimental bias and improve the consistency and optimization in image, tissue-section of interest was chosen at the same anatomical level based on the hallmarks shown in rat brain atlas diagram. The immunostaining was run at the same condition and images were acquired using the same parameters for uniform illumination. For image quantitation, image size was uniformed, and uniform background subtraction was applied to reduce noise. Blinded experiments were performed for immunostaining, data acquisition, and analysis.
Table 2.
Antibodies for Immunohistochemistry.
| Antibody | Manufacturer | Dilution | References |
|---|---|---|---|
| Primary antibodies | |||
| Oligodendrocyte-lineage cells | |||
| Olig2 (pAb) | Millipore/MAB9610 | 1:800 | Cai et al.
24
Cai et al. 39 Chu et al. 40 |
| Pdgfrα (pAb) | Novus Biologicals/AF1062 | 1:50 | Chu et al. 24 |
| APC (CC-1; mAb) | Calbiochem/OP80 | 1:100 | Cai et al.
39
Chu et al. 24 |
| MBP (mAb) | Neuromics/MO22121 | 1:2000 | Tian et al. 26 |
| Axon | |||
| NF-H (pAb) | Abcam/ab8135 | 1:1000 | Cai et al.
26
Wang et al. 27 Tian et al. 41 |
| Microglia/macrophage | |||
| IbaI (pAb) | Wako/019-19741 | 1:500 | Wang et al.
24
Chu et al. 26 Tian et al. 27 |
| Astrocyte | |||
| GFAP (GA5; mAb) | Millipore/MAB3402 | 1:1000 | Wang et al.
24
Chu et al. 27 |
| Proliferation | |||
| Ki67 (SP6; mAb) | Thermo Scientific/ RM-9106 |
1:200 | Cai et al.
24
Chu et al. 41 |
| Apoptosis | |||
| Cleaved-caspase 3 (Asp175; pAb) | CellSignaling/9661 | 1:400 | Cai et al. 41 |
| Secondary antibodies | |||
| Goat anti-Rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | Invitrogen/A-11012 |
1:1000 |
|
| Donkey anti-Rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | Invitrogen/A-21207 | 1:1000 | |
| Donkey anti-Goat IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Invitrogen/A-11055 | 1:1000 | |
| Goat anti-Mouse IgG1 Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Invitrogen/A-21121 | 1:1000 | |
| Goat anti-Mouse IgG2b Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Invitrogen/A-21141 | 1:1000 | |
| Nucleic acid staining | |||
| DAPI | Sigma/D9542 | 1µ g/ml |
pAb: polyclonal antibody; mAb: monoclonal antibody; DAPI: 4,’6-diamidino-2-phenylindole.
Statistics
Data are expressed as mean ± standard deviation (SD). Two-way ANOVA was performed, with one factor being the combination of MTD exposure, the other being sex, along with their interactions. It was examined whether the interaction and the main effect for sex are significant. Sexual dimorphisms of oligodendrocyte and white matter development were reported in rodents28,29 due to sex hormones including estradiol, progesterone, and testosterone. 29 However, endogenous sex hormones are maintained at a very low level and no significant difference of estradiol and progesterone between sexes in neonatal rats. 30 Furthermore, the linear mixed model which included each litter as a random effect to capture the “intralitter likeness,” and which also included sex and group as fixed effect to capture the sex effect and group effect. 31 No significant sex effects were found for all endpoints (p > 0.05). Since the effects for sex were not significant in this study, the data were pooled together to examine the MTD effect on oligodendrocytes and myelination. Comparisons between CTR and MTD groups were conducted by linear mixed models including group as fixed effect and litter as a random effect to control intralitter likeness. 32 The analyses for linear mixed-effect models were carried out via IBM SPSS Statistical software for Mac (Version 28.0). The significant level was set at 0.05 for all comparisons.
Results
Measurements of litter size and neonatal body weight and brain size
The high dose of maternal MTD exposure (8–16 mg/kg/day) to mimic the OUD in utero significantly reduces litter size 33 and neonatal body weight.23,33 In the current study, the pregnant dams were exposed to 3 mg/kg/day of MTD. Compared with the controls, the litter size at birth and pup’s body weight at P7 were slightly reduced without statistical significance when exposed to MTD. Brain wet weight and length were not changed in either the male or the female offspring (Table 1).
Perinatal MTD exposure inhibits myelination in neonatal rat brains
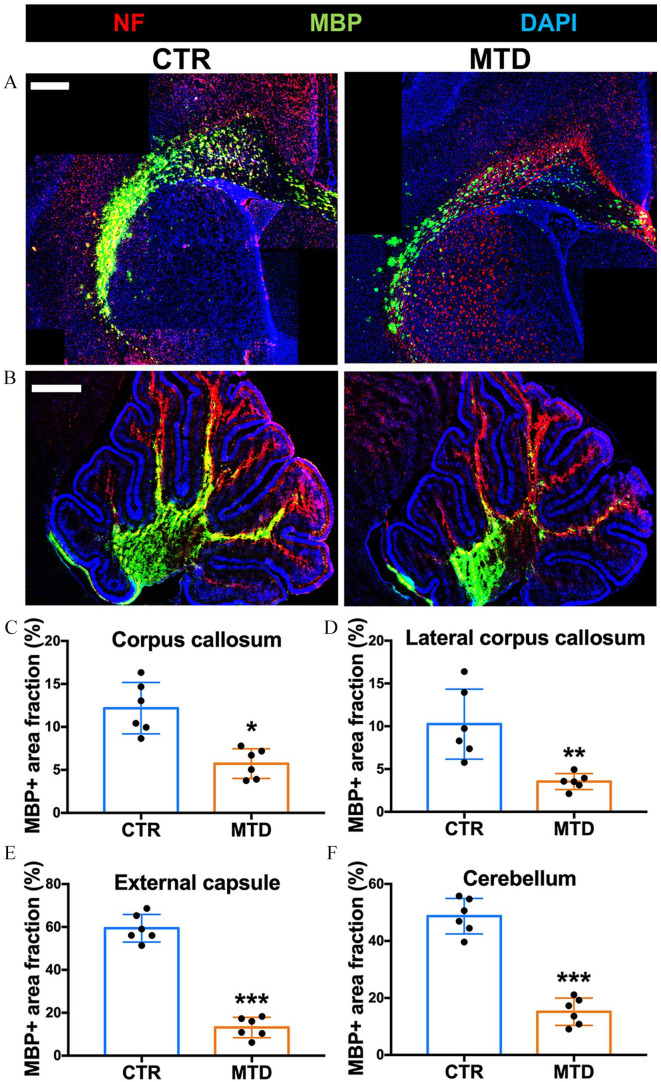
Myelination, measured by area labeled with myelin basic protein (MBP), was observed in both cerebral and cerebellum (Figure 1(A) and (B)) of the rat pups exposed to MTD from E7 to P7. Myelination was attenuated in the CC (Figure 1(C); p < 0.05), lateral CC (Figure 1(D); p < 0.01), and EC (Figure 1(E); p < 0.001) of the cerebrum and arbor vitae (Figure 1(F); p < 0.001) of the cerebellum of MTD exposed rat pups.
Figure 1.
Perinatal MTD exposure attenuates myelin development. (A, B) Representative photomicrographs of neurofilament (NF, red) and myelin basic protein (MBP, green) in (A) cerebrum and (B) cerebellum from control and MTD-exposed rat pups. Scale bar: 500 µm. (C–F) Quantification of MBP+ area fraction (%) in corpus callosum, lateral corpus callosum, external capsule, and cerebellar white matter. The area fraction was compared with the CTR group of each brain region (*p < 0.05, **p < 0.01, ***p < 0.001, n = 6). (A color version of this figure is available in the online journal.)
Effects of MTD exposure on OPC generation, proliferation, and differentiation
OPC density (cells/mm2) and proportion of OPCs relative to total oligodendrocyte population were significantly increased within the corpus callosum (Figure 2(A), (C), and (F); p < 0.01 for OPC density, p < 0.05 for OPC proportion) and cerebellar white matter of MTD exposed pups (Figure 2(B), (E) and (H); p < 0.01 for OPC density, p < 0.001 for OPC proportion). There were no significant differences in OPC density or proportion of oligodendrocyte population in the lateral CC (Figure 2(D) and (G)).
Figure 2.
Perinatal MTD exposure tends to increase OPCs in corpus callosum and cerebellum. (A, B) Representative photomicrographs of OPCs (Olig2+ Pdgfrα+) in (A) corpus callosum, lateral corpus callosum, and (B) cerebellar white matter from control and MTD-exposed rat pups. Dashed white line indicates the margins of region. Scale bar: (A) 100 µm (B) 50 µm. Representative double labeling is indicated by white arrowhead in the inset. (C–H) Quantification of OPC density (C–E, Olig2+ Pdgfrα+ cells/mm2) and OPC proportion in oligodendrocyte-lineage cells (F–H, Olig2+ Pdgfrα+/Olig2+%). The cell density and proportion were compared with the CTR group of each brain region (*p < 0.05, **p < 0.01, ***p < 0.001, n = 6). (A color version of this figure is available in the online journal.)
OPC proliferation was increased in the cerebellum (Figure 3(B) and (E); p < 0.05), but not the CC and lateral CC of the MTD group (Figure 3(A), (C), and (D)).
Figure 3.
Changes in OPC proliferation after perinatal MTD exposure. (A, B) Representative photomicrographs of proliferative OPCs (Ki67+ Pdgfrα+) in (A) corpus callosum, lateral corpus callosum, and (b) cerebellar white matter from control and MTD-exposed rat pups. Dashed white line indicates the margins of region. Scale bar: (A) 100 µm (B) 50 µm. Representative double labeling is indicated by white arrowhead in the inset. (C–E) Quantification of proliferative OPC density (Ki67+ Pdgfrα+ cells/mm2). The cell density was compared with the CTR group of each brain region (*p < 0.05, n = 6). (A color version of this figure is available in the online journal.)
Reduced density of differentiated oligodendrocytes and significantly decreased proportion of differentiated oligodendrocytes relative to the total oligodendrocyte population were noted in the cerebellum of the MTD exposed group (Figure 4(B) and (E)), and H; p = 0.056 for cell density, p < 0.05 for cell proportion. There were no differences in differentiated oligodendrocyte population or density in the cerebrum (Figure 4(A), (C), (D), (F), and (G)).
Figure 4.
Changes in oligodendrocyte differentiation after perinatal MTD exposure. (A, B) Representative photomicrographs of mature oligodendrocytes (Olig2+ CC1+) in (A) corpus callosum, lateral corpus callosum, and (B) cerebellar white matter from control and MTD-exposed rat pups. Dashed white line indicates the margins of region. Scale bar: (A) 100 µm (B) 50 µm. Representative double labeling is indicated by white arrowhead in the inset. (C–H) Quantification of mature oligodendrocyte density (C–E, Olig2+ CC1+ cells/mm2) and its proportion in oligodendrocyte-lineage cells (F–H, Olig2+ CC1+/Olig2+%). The cell density and proportion were compared with the CTR group of each brain region (*p < 0.05, n = 6). (A color version of this figure is available in the online journal.)
MTD exposure causes divergent apoptosis in oligodendrocyte-lineage cells
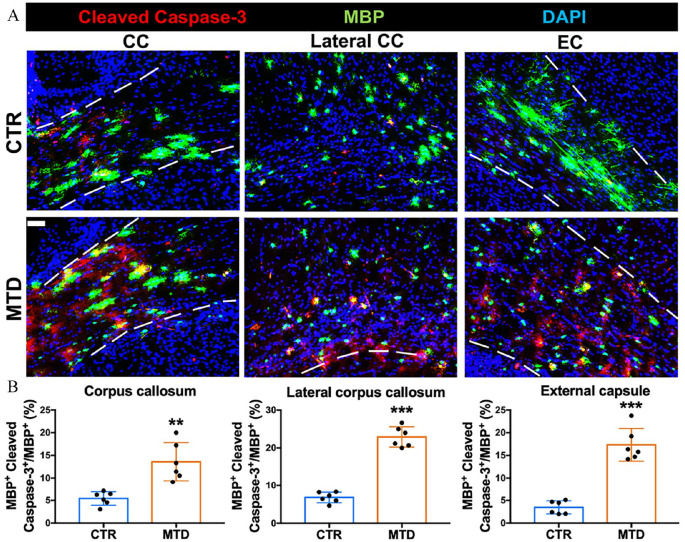
The apoptotic OPCs in the CC and lateral CC were not increased when exposed to MTD (Figure 5(A) and (B)). Increased apoptosis of differentiated oligodendrocytes in the CC (Figure 5(D) and (E); p < 0.001), lateral CC (Figure 5(D) and (E); p < 0.001), and EC (Figure 5(D) and (E); p < 0.001) was found without a change in overall oligodendrocyte density (Figure 5(C)) after MTD exposure. There was also an increase in apoptosis of myelinating oligodendrocytes in the CC (Figure 6(A) and (B); p < 0.01), lateral CC (Figure 6(A) and (B); p < 0.001), and EC (Figure 6(A) and (B); p < 0.001) of the MTD group. Apoptosis was also increased in the overall oligodendrocyte population of the cerebellum in the MTD group (Figure 7(A) and (B); p < 0.01).
Figure 5.
Divergent apoptotic activities of OPCs and mature oligodendrocytes after perinatal MTD exposure. (A) Representative photomicrographs of apoptotic OPCs (Pdgfrα+ Cleaved Caspase-3 +) in corpus callosum and lateral corpus callosum. (B) Quantification of the apoptosis ratio of OPCs (Pdgfrα+ Cleaved Caspase-3+/Pdgfrα+%) in corpus callosum and lateral corpus callosum. (C) Quantification of oligodendrocyte-lineage cell density (Olig2+ cells/mm2) in corpus callosum and lateral corpus callosum. (D) Representative photomicrographs of apoptotic mature oligodendrocytes (CC1+ Cleaved Caspase-3+) in corpus callosum, lateral corpus callosum, and external capsule. (E) Quantification of the apoptosis ratio of mature oligodendrocytes (CC1+ Cleaved Caspase-3+/CC1+%) in corpus callosum, lateral corpus callosum and external capsule. Scale bar: 50 µm. The cell density and ratio were compared with the CTR group of each brain region (***p < 0.001, n = 6). (A color version of this figure is available in the online journal.)
Figure 6.
Divergent apoptotic activities of myelinating oligodendrocytes after perinatal MTD exposure. (A) Representative photomicrographs of apoptotic myelinated oligodendrocytes (MBP+ Cleaved Caspase-3+) in corpus callosum, lateral corpus callosum, and external capsule. (B) Quantification of the apoptosis ratio of myelinated oligodendrocytes (MBP+ Cleaved Caspase-3+/MBP+%) in corpus callosum, lateral corpus callosum and external capsule. Dashed white line indicates the margins of region. Scale bar: 50 µm. The cell density and ratio were compared with the CTR group of each brain region (**p < 0.01, ***p < 0.001, n = 6). (A color version of this figure is available in the online journal.)
Figure 7.
Perinatal MTD exposure exacerbates the apoptosis of oligodendrocyte-lineage cells in cerebellum. (A) Representative photomicrographs of apoptotic activity of oligodendrocyte-lineage cells (Olig2+ Cleaved Caspase-3+) in cerebellar white matter. Scale bar: 50 µm. (B) Quantification of the density of apoptotic oligodendrocyte-lineage cells (Olig2+ Cleaved Caspase-3+/mm2) in cerebellar white matter. (C) Quantification of oligodendrocyte-lineage cell density (Olig2+ cells/mm2) in cerebellar white matter. The cell density was compared with the CTR group of each brain region (**p < 0.01, n = 6). (A color version of this figure is available in the online journal.)
MTD exposure triggers the microglial response but not astrocytic activation
Compared with the control group, the MTD group had an increased microglia density only in the CC (Figure 8(A) and (C); p < 0.05), however there were morphological changes including reduced cellular processes and diminished degree of branching in all targeted brain areas (Figure 8(B), inserts). There were no differences in astrocyte activation between groups (Figure 8(A) and (D)).
Figure 8.
Divergent responses of astrocyte and microglia after perinatal MTD exposure. (A) Representative photomicrographs of astrocyte (GFAP+) and microglia (Iba1+) in corpus callosum, lateral corpus callosum, external capsule, and cerebellum. (B) MTD exposure stimulates the activation of microglia with morphological change. Dashed white line indicates the margins of region. Scale bar: 100 µm for cerebral regions, 500 µm for cerebellum. Representative labeling is displayed in the inset. (C, D) Quantification of microglial response (C, Iba1+ area fraction%) and astrocytic response (D, GFAP+ area fraction%) in corpus callosum, lateral corpus callosum, external capsule, and cerebellum. The area fraction was compared with the CTR group of each brain region (*p < 0.05, n = 6). (A color version of this figure is available in the online journal.)
Discussion
Our animal model demonstrates disruption of fetal brain development with perinatal MTD exposure in neonatal rat pups. This finding may provide additional insight into the influence of MTD on human fetal brain development and functional outcomes.
In this study we found attenuation of myelination and increased apoptosis of mature and myelinating oligodendrocytes in brains of MTD exposed rat pups. These findings add to our previous work which noted decreased myelin basic protein (MBP) and proteolipid protein (PLP) expression in the hindbrains of the MTD exposed rats20,34 and regional differences in the effect of MTD on expression of myelin-related proteins in the cerebral cortex and hippocampus. 20 Altered myelination in cerebrum and cerebellum reported in this study (Figure 1) are consistent with our prior findings. The combination of decreased myelin protein expression and myelin tissue staining corroborate our hypothesis that antenatal MTD exposure alters myelination in neonatal rat pups and lends further support to alteration in myelination as the etiology of decreased white matter volume in neonates with antenatal opioid exposure.9–12
The mechanism by which opioid exposure leads to altered myelination is still unclear. Our results suggest that these differences are unlikely due to changes in proliferation or differentiation and possibly due to increased apoptosis or impaired migration in cerebral white matter. We found a slight increase of OPCs only in the CC area (Figure 2(A), (C) and (F)), but no significant change in proliferation (Figure 3(A), (C) and (D)) and differentiation (Figure 4(A), (C), (D), (F), and (G)) in the cerebrum, which suggests that there is not an arrest of development nor is there accelerated terminal differentiation. There was a significant global increase in apoptosis of differentiated and myelinating oligodendrocyte, but not OPCs in the cerebrum (Figures 5 and 6). This suggests that the developmental process from mature to myelinating oligodendrocytes was disturbed by MTD exposure and resulted in the programmed death of differentiated/myelinating oligodendrocyte.
Interestingly, MTD appeared to have a mitogenic effect on OPCs in the cerebellum as evidenced by increased density and percentage of OPCs (Figure 2(B), (E), and (H)) and increased proliferative oligodendrocytes (Figure 3(B) and (E)). There was a parallel decrease in the density and proportion of mature oligodendrocytes (Figure 4(B), (E), and (H)) which may represent an arrest of oligodendrocyte differentiation in response to MTD exposure. Similar to cerebrum, increased apoptosis also occurred in oligodendrocyte-lineage cells in cerebellar white matter (Figure 7). Taken together, these findings suggest that perinatal MTD exposure may affect the proliferation and differentiation of OPCs in a region-dependent manner followed by increased oligodendroglial apoptosis, eventually leading to compromised myelination (Figure 9). Oligodendrocytes in cerebellar white matter are more vulnerable to MTD than those in cerebral white matter probably due to arrest of oligodendrocyte differentiation, which supports the observation of significantly reduced volume of white matter in cerebellum but not in cerebrum in school-aged children with prenatal opioid exposure. 14 It may provide a therapeutic basis for early-stage interventions to prevent the regional diversity of brain white matter deficits in children with prenatal opioid exposure.
Figure 9.

A hypothetical hierarchy of white matter injury in antenatal exposure to MTD. MTD, methadone; OPC, oligodendrocyte progenitor cell; OL, oligodendrocyte; WM, white matter. (A color version of this figure is available in the online journal.)
There was also qualitative activation in microglia but not astrocytes (Figure 8(A) and (B)), which could suggest a pro-inflammatory state in the brains of MTD exposed pups. Activated microglia with reduced cellular processes and branching following perinatal MTD exposure is consistent with previously reported alternations in microglial morphology and activation in the cortex from MTD-exposed P10 rat pups. 23 It is possible that increased neuroinflammation leads to increased apoptosis of mature and myelinating oligodendrocytes, resulting in overall decreased myelination. Alternatively, MTD-induced oligodendrocyte injury and apoptosis may prime microglia and neuroinflammation. These findings also suggest the divergent neuroinflammatory responses of astrocyte and microglia to MTD in cerebrum and cerebellum.
Our results are concordant with the work from Jantzie et al, who report decreased levels of MBP and neurofilament proteins in the cortex of MTD exposed pups at P21, suggestive of impaired myelination. They also showed poorly organized white matter tracks in the corpus callosum or MTD exposed rat pups via magnetic resonance imaging (MRI) with fractional anisotropy, which mirrors data from clinical studies and our work. 23 Conversely, Vestal-Labor et al. found increased expression of myelin-related proteins myelin basic protein (MBP), myelin proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG) in the MTD exposed brains compared with controls at P11 and P19. 35 Notable differences exist between these groups’ work and ours. Both Jantzie et al. and Vestal-Laborde et al. dosed pregnant rats using an implanted medication pump and targeted higher doses, from 8 mg/kg/day to 16 mg/kg/day.23,35,36 The higher doses investigated seem to mimic opioid abuse in contrast to our dose, which was chosen to approximate dosing for OUD 3 mg/kg/day when interspecies differences are accounted for.20,34 Taking all of the differences into account, our findings do seem concordant with the findings from Jantzie et al. and corroborate clinical studies which suggest impaired myelination in infants with in utero MTD exposure.
Additional studies should build upon this relationship and explore regional differences in the effect of MTD at different doses. Using implanted pumps may provide more precise dosing of MTD and measuring the urine MTD levels in rat dams provides an additional level of precision. The corpus callosum and peri corpus callosum are areas densely populated with oligodendrocytes and contain the highest level of myelin; however, the peri-ventricular white matter should be studied because this is where OPCs originate and migrate from. 37 The OPCs in the periventricular white matter were too densely populated to accurately perform counts at E7, however differences might be detected at later time points after a larger proportion of cells have migrated, or by a different methodology. The methods used in this study to analyze the changes in cell density and immunoreactive tissue area fraction are standard but limited in accuracy, stereological estimation may provide non-biased and more objective quantification of immunohistochemistry. Clinical imaging studies showed both diffusive and punctate brain white matter lesions in infants with prenatal opioid exposure,10,11,38 which were correlated with cognitive function. 11 Thus, the neurofunctional consequences relating to the region-different oligodendrocyte impairment need a further investigation on this model.
Our work suggests direct effects of perinatal MTD exposure on oligodendrocyte development and function in neonatal rat pups, which may be a possible etiology for clinical differences observed in opioid exposed neonates if findings translate across species. Improved understanding of the effects of opioid exposure will allow us to design treatments for OUD during pregnancy that minimize effects on the fetal brain or target postnatal treatment to mitigate the effects of antenatal opioid exposure.
Footnotes
Authors’ Contributions: LADP and JC designed the study, interpreted the data, and coordinated the project. WZ and NM performed the animal treatments. WZ and JC dissected and collected brain tissues. JMG, TC, and ACW conducted immunofluorescence staining, data collection, and statistical analysis. MK provided consultation and statistical review. The manuscript was written by JMG and TC, and revised by LADP and JC. The submitted version was approved by all the authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by a grant from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (R21 NS098170, JC), a pilot grant from the National Institute of Environmental Health Sciences of the National Institutes of Health (P30ES030283, JC), and a trainee research grant from Norton Children’s Hospital (JMG).
ORCID iD: Jun Cai  https://orcid.org/0000-0003-1721-7786
https://orcid.org/0000-0003-1721-7786
References
- 1. Hirai AH, Ko JY, Owens PL, Stocks C, Patrick SW. Neonatal abstinence syndrome and maternal opioid-related diagnoses in the US 2010–2017. JAMA 2021;325:146–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Minozzi S, Amato L, Jahanfar S, Bellisario C, Ferri M, Davoli M. Maintenance agonist treatments for opiate-dependent pregnant women. Cochrane Database Syst Rev 2020;11:CD006318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baldacchino A, Arbuckle K, Petrie DJ, McCowan C. Neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: a systematic review and meta-analysis. BMC Psychiat 2014;14:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Konijnenberg C, Melinder A. Prenatal exposure to methadone and buprenorphine: a review of the potential effects on cognitive development. Child Neuropsychol 2011;17:495–519 [DOI] [PubMed] [Google Scholar]
- 5. Lee SJ, Bora S, Austin NC, Westerman A, Henderson JMT. Neurodevelopmental outcomes of children born to opioid-dependent mothers: a systematic review and meta-analysis. Acad Pediatr 2020;20:308–18 [DOI] [PubMed] [Google Scholar]
- 6. Yeoh SL, Eastwood J, Wright IM, Morton R, Melhuish E, Ward M, Oei JL. Cognitive and motor outcomes of children with prenatal opioid exposure: a systematic review and meta-analysis. JAMA Netw Open 2019;2:e197025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Towers CV, Hyatt BW, Visconti KC, Chernicky L, Chattin K, Fortner KB. Neonatal head circumference in newborns with neonatal abstinence syndrome. Pediatrics 2019;143:e20180541 [DOI] [PubMed] [Google Scholar]
- 8. Yuan Q, Rubic M, Seah J, Rae C, Wright IM, Kaltenbach K, Feller JM, Abdel-Latif ME, Chu C, Oei JL, BOB (Brains Opioids and Babies) Collaborative Group. Do maternal opioids reduce neonatal regional brain volumes? A pilot study. J Perinatol 2014;34:909–13 [DOI] [PubMed] [Google Scholar]
- 9. Walhovd KB, Moe V, Slinning K, Due-Tonnessen P, Bjornerud A, Dale AM, van der Kouwe A, Quinn BT, Kosofsky B, Greve D, Fischl B. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. NeuroImage 2007;36:1331–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walhovd KB, Watts R, Amlien I, Woodward LJ. Neural tract development of infants born to methadone-maintained mothers. Pediatr Neurol 2012;47:16. [DOI] [PubMed] [Google Scholar]
- 11. Walhovd KB, Westlye LT, Moe V, Slinning K, Due-Tønnessen P, Bjørnerud A, van der Kouwe A, Dale AM, Fjell AM. White matter characteristics and cognition in prenatally opiate- and polysubstance-exposed children: a diffusion tensor imaging study. AJNR Am J Neuroradiol 2010;31:894–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monnelly VJ, Anblagan D, Quigley A, Cabez MB, Cooper ES, Mactier H, Semple SI, Bastin ME, Boardman JP. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin 2018;18:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merhar SL, Parikh NA, Braimah A, Poindexter BB, Tkach J, Kline-Fath B. White matter injury and structural anomalies in infants with prenatal opioid exposure. AJNR Am J Neuroradiol 2019;40:2161–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sirnes E, Oltedal L, Bartsch H, Eide GE, Elgen IB, Aukland SM. Brain morphology in school-aged children with prenatal opioid exposure: a structural MRI study. Early Hum Dev 2017;106-107:33–9 [DOI] [PubMed] [Google Scholar]
- 15. Hauser KF, Knapp PE. Opiate drugs with abuse liability hijack the endogenous opioid system to disrupt neuronal and glial maturation in the central nervous system. Front Pediatr 2017;5:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knapp PE, Hauser KF. mu-Opioid receptor activation enhances DNA synthesis in immature oligodendrocytes. Brain Res 1996;743:341–5 [DOI] [PubMed] [Google Scholar]
- 17. Tryoen-Toth P, Gaveriaux-Ruff C, Labourdette G. Down-regulation of mu-opioid receptor expression in rat oligodendrocytes during their development in vitro. J Neurosci Res 2000;60:10–20 [DOI] [PubMed] [Google Scholar]
- 18. Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience 2001;105:7–17 [DOI] [PubMed] [Google Scholar]
- 19. Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology 2007;28:931–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oberoi R, Chu T, Mellen N, Jagadapillai R, Ouyang H, Devlin LA, Cai J. Diverse changes in myelin protein expression in rat brain after perinatal methadone exposure. Acta Neurobiol Exp 2019;79:367–73 [PubMed] [Google Scholar]
- 21. Atherton JC, Dark JM, Garland HO, Morgan MR, Pidgeon J, Soni S. Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J Physiol 1982;330:81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016;7:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jantzie LL, Maxwell JR, Newville JC, Yellowhair TR, Kitase Y, Madurai N, Ramachandra S, Bakhireva LN, Northington FJ, Gerner G, Tekes A, Milio LA, Brigman JL, Robinson S, Allan A. Prenatal opioid exposure: the next neonatal neuroinflammatory disease. Brain Behav Immun 2020;84:45–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chu T, Zhang YP, Tian Z, Ye C, Zhu M, Shields LBE, Kong M, Barnes GN, Shields CB, Cai J. Dynamic response of microglia/macrophage polarization following demyelination in mice. J Neuroinflam 2019;16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiménez JA, Zylka MJ. Controlling litter effects to enhance rigor and reproducibility with rodent models of neurodevelopmental disorders. J Neurodev Dis 2021;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tian Z, Chu T, Shields LBE, Zhu Q, Zhang YP, Kong M, Barnes GN, Wang Y, Shields CB, Cai J. Platelet-activating factor deteriorates lysophosphatidylcholine-induced demyelination via its receptor-dependent and -independent effects. Mol Neurobiol 2020;57:4069–81 [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Gao Z, Zhang Y, Feng S-Q, Liu Y, Shields LBE, Zhao Y-Z, Zhu Q, Gozal D, Shields CB, Cai J. Attenuated reactive gliosis and enhanced functional recovery following spinal cord injury in null mutant mice of platelet-activating factor receptor. Mol Neurobiol 2015;53:3448–61 [DOI] [PubMed] [Google Scholar]
- 28. Cerghet M, Skoff RP, Swamydas M, Bessert D. Sexual dimorphism in the white matter of rodents. J Neurol Sci 2009;286:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swamydas M, Bessert D, Skoff R. Sexual dimorphism of oligodendrocytes is mediated by differential regulation of signaling pathways. J Neurosci Res 2009;87:3306–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bell MR. Comparing postnatal development of gonadal hormones and associated social behaviors in rats, mice, and humans. Endocrinology 2018;159:2596–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown H, Prescott R. Applied mixed models in medicine. 3rd ed. New York: John Wiley & Sons, 2014. [Google Scholar]
- 32. Golub MS, Sobin CA. Statistical modeling with litter as a random effect in mixed models to manage “intralitter likeness.” Neurotoxicology and Teratology 2020;77:106841. [DOI] [PubMed] [Google Scholar]
- 33. Kunko PM, Smith JA, Wallace MJ, Maher JR, Saady JJ, Robinson SE. Perinatal methadone exposure produces physical dependence and altered behavioral development in the rat. J Pharmacol Exp Ther 1996;277:1344–51 [PubMed] [Google Scholar]
- 34. Gourevitch B, Cai J, Mellen N. Cellular and network-level adaptations to in utero methadone exposure along the ventral respiratory column in the neonate rat. Exp Neurol. Epub ahead of print 20 March 2016. DOI: 10.1016/j.expneurol.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 35. Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci 2014;36:409–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salihu HM, Salinas A, Medina I, Krishnaswami J, Aliyu MH. Biopsychosocial determinants of opioid use disorder (OUD) and implications for maternal and child health research: A scoping review. J Opioid Manag 2019;15:77–91 [DOI] [PubMed] [Google Scholar]
- 37. Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 2001;81:871–927 [DOI] [PubMed] [Google Scholar]
- 38. Merhar SL, McAllister JM, Wedig-Stevie KE, Klein AC, Meinzen-Derr J, Poindexter BB. Retrospective review of neurodevelopmental outcomes in infants treated for neonatal abstinence syndrome. J Perinatol 2018;38:587–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cai J, Zhu Q, Zheng K, Li H, Qi Y, Cao Q, Qiu M. Co-localization of Nkx6. 2 and Nkx2. 2 homeodomain proteins in differentiated myelinating oligodendrocytes. Glia 2010;58:458–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cai J, Qi Y, Hu X, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron 2005;45: 41–53 [DOI] [PubMed] [Google Scholar]
- 41. Cai J, Tuong C, Zhang YP, Shields CB, Guo G, Fu H, Gozal D. Mouse intermittent hypoxia mimicking apnoea of prematurity: Effects on myelinogenesis and axonal maturation. J Pathol 2012;226:495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]